Submitted:
05 August 2025
Posted:
20 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. AEB Structure
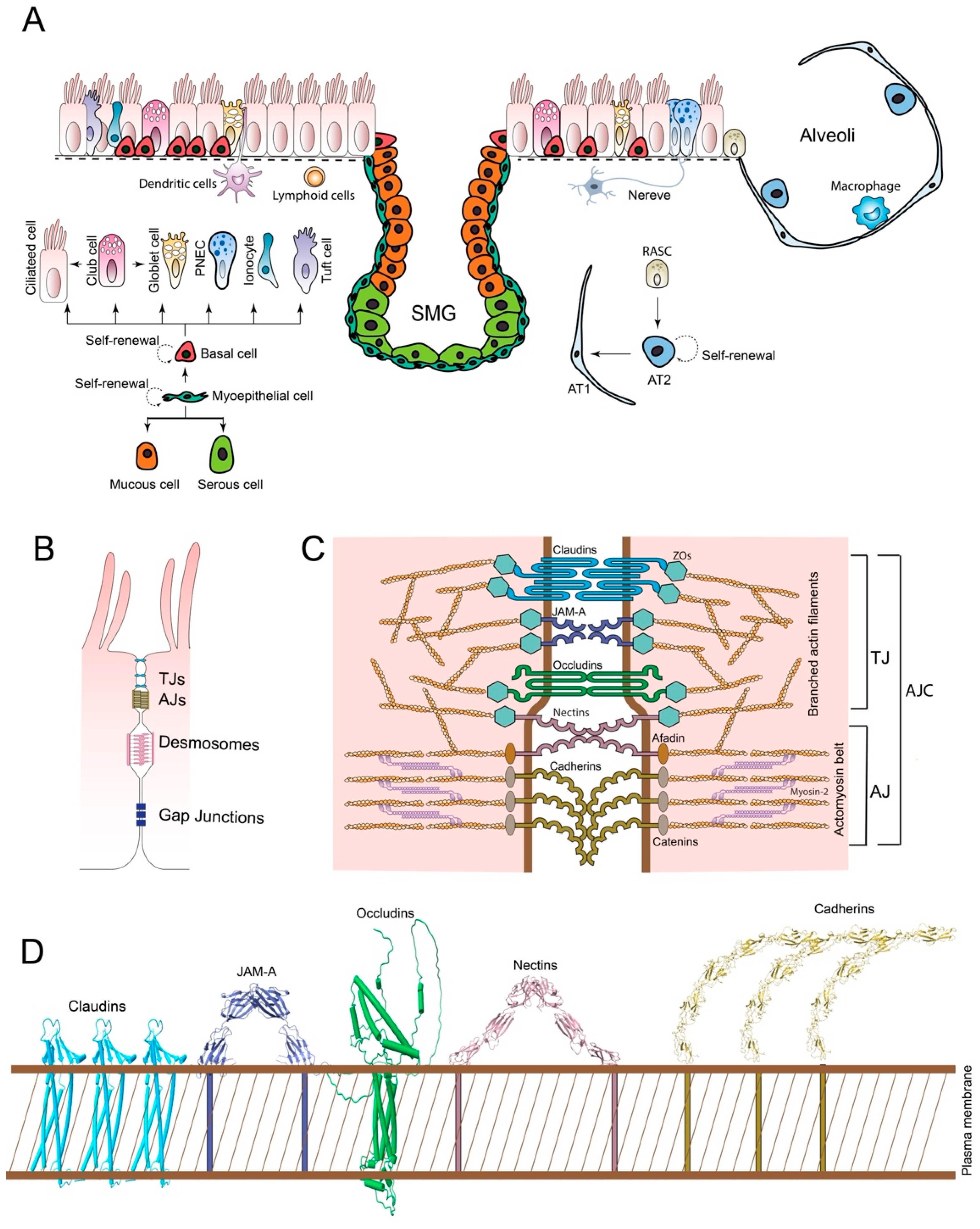
2.1. Airway Epithelial Cells (AECs) Landscape
2.1. Intercellular Junction Complexes
3. AEB Functions
3.1. Physical Barrier
3.2. Chemical Barrier
3.3. Immunological Barrier
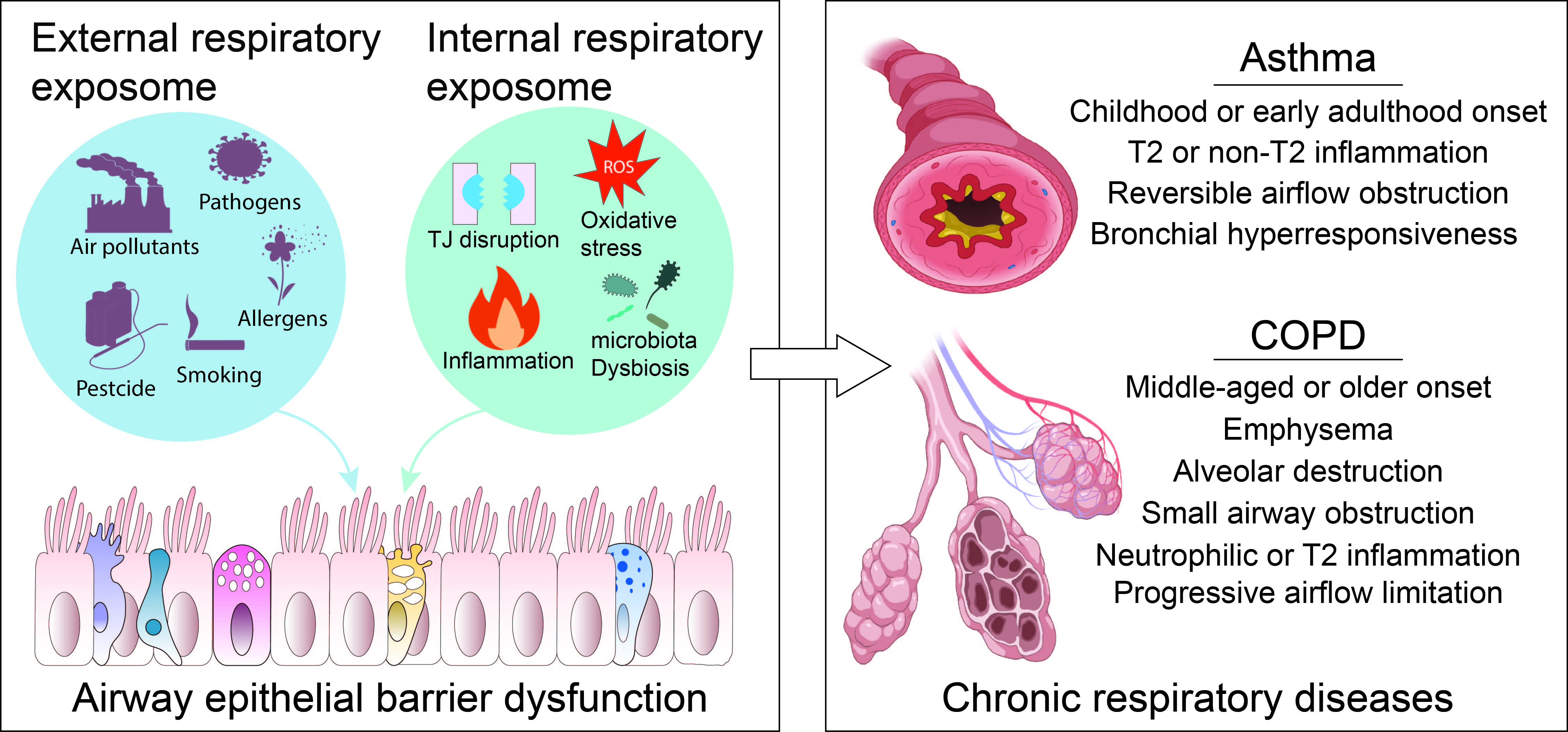
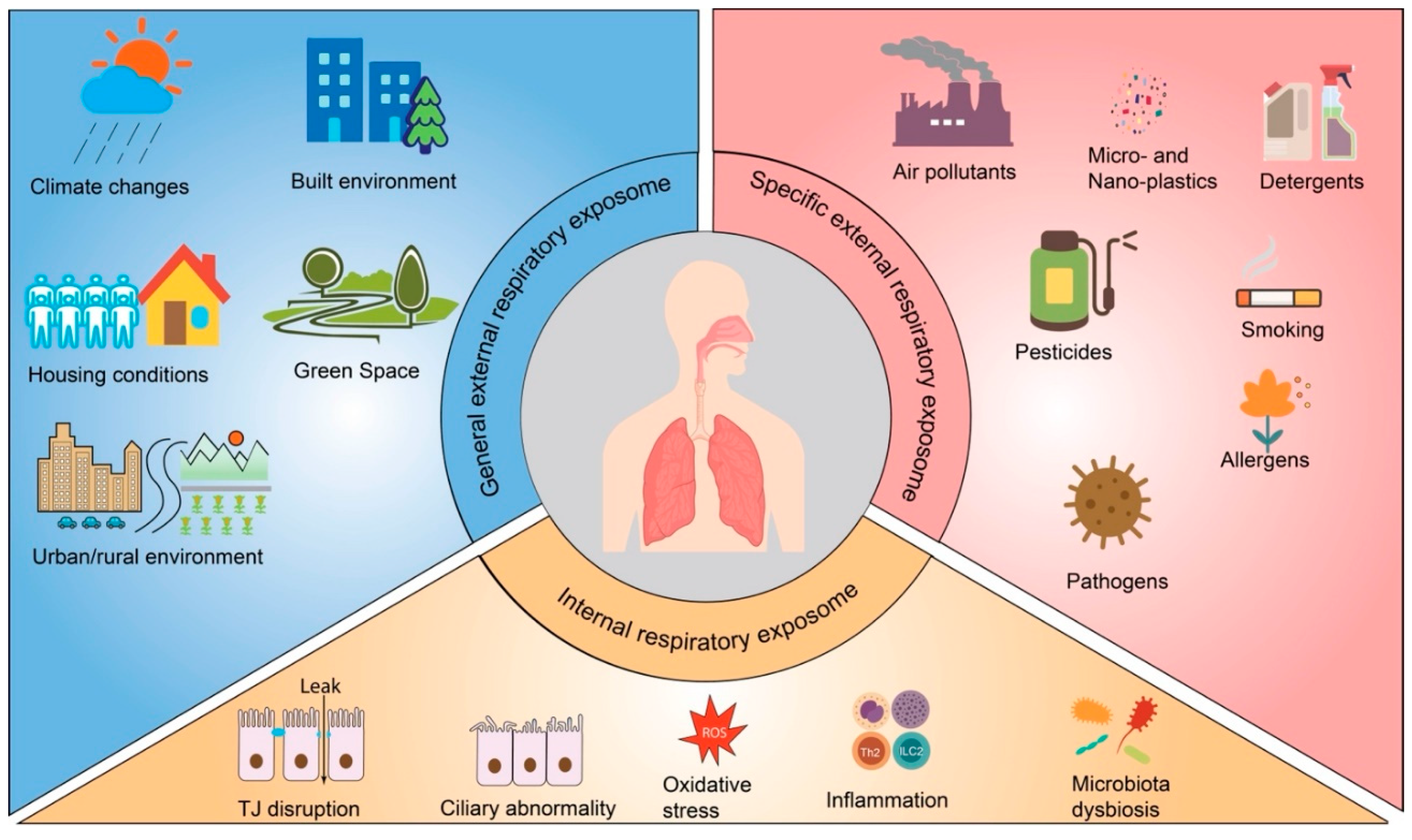
4. The Respiratory Exposome
5. The AEB Damage Involved in the Development of CRDs
5.1. Asthma
5.2. COPD
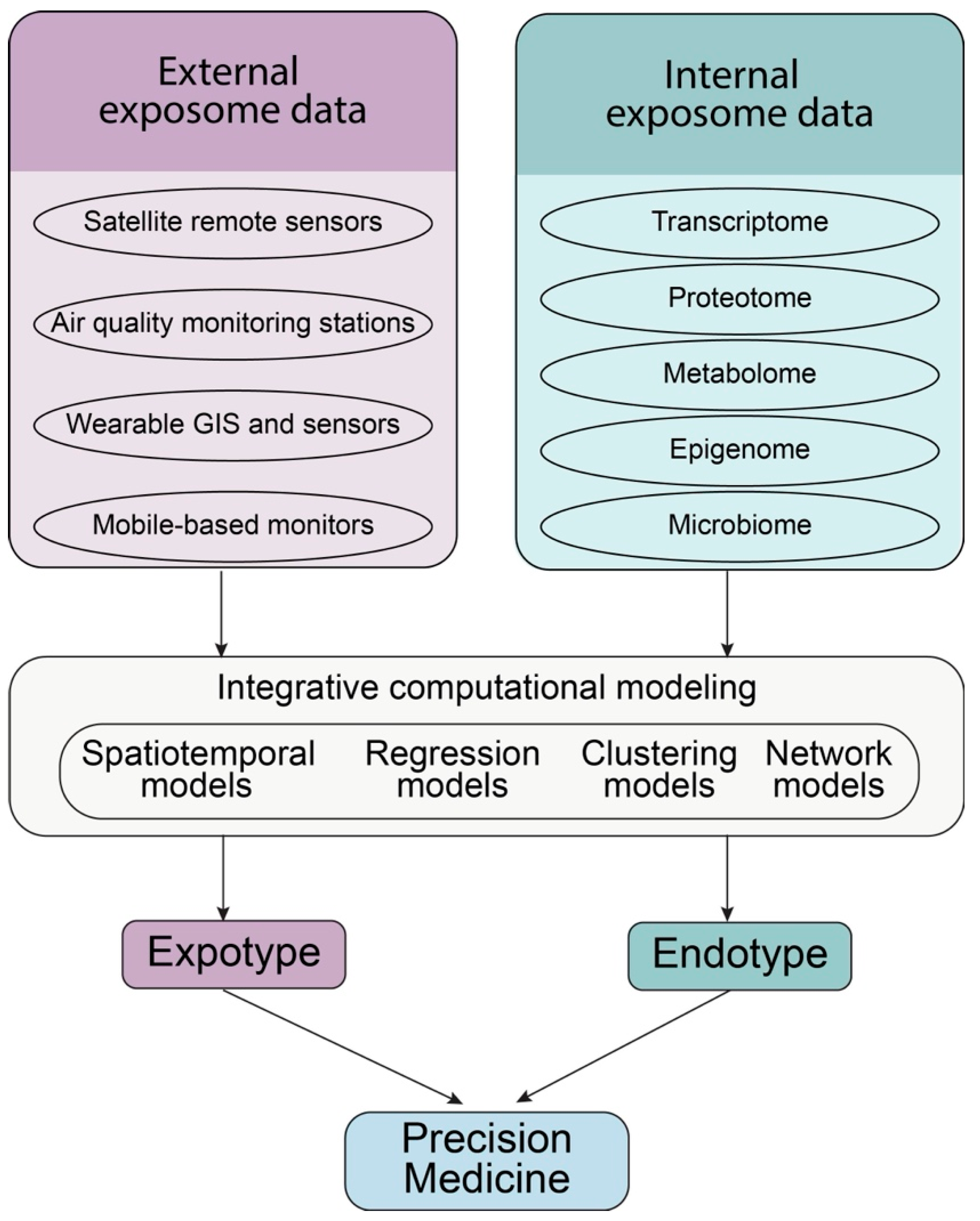
6. CRD Precision Medicine by Targeting Airway Epithelial Barrier
7. Conclusive Remarks and Perspectives
References
- Adrish, M., and P. Akuthota. 2023. Approach to non-type 2 asthma. Respir Med 216: 107327. [Google Scholar] [CrossRef]
- Agache, I., I. M. Adcock, F. Baraldi, K. F. Chung, I. Eguiluz-Gracia, S. L. Johnston, M. Jutel, P. Nair, A. Papi, C. Porsbjerg, O. S. Usmani, D. A. Meyers, M. Zemelka-Wiacek, and E. R. Bleecker. 2025. Personalised therapeutic approaches for asthma. J Allergy Clin Immunol. [Google Scholar] [CrossRef] [PubMed]
- Aghapour, M., P. Raee, S. J. Moghaddam, P. S. Hiemstra, and I. H. Heijink. 2018. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am J Respir Cell Mol Biol 58: 157–169. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S., R. Srivastava, F. Rahmatpanah, C. Madiraju, L. BenMohamed, and A. Agrawal. 2017. Airway epithelial cells enhance the immunogenicity of human myeloid dendritic cells under steady state. Clin Exp Immunol 189: 279–289. [Google Scholar] [CrossRef]
- Agusti, A., and J. C. Hogg. 2019. Update on the Pathogenesis of Chronic Obstructive Pulmonary Disease. N Engl J Med 381: 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A., M. Bafadhel, R. Beasley, E. H. Bel, R. Faner, P. G. Gibson, R. Louis, V. M. McDonald, P. J. Sterk, M. Thomas, C. Vogelmeier, and I. D. Pavord. 2017. on behalf of all participants in the, seminar Precision medicine in airway diseases: Moving to clinical practice. Eur Respir J 50. [Google Scholar] [CrossRef]
- Akdis, C. A. 2021. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol 21: 739–751. [Google Scholar] [CrossRef]
- Akenroye, A., J. A. Boyce, and H. Kita. 2025. Targeting alarmins in asthma: From bench to clinic. J Allergy Clin Immunol 155: 1133–1148. [Google Scholar] [CrossRef]
- Almuntashiri, S., Y. Zhu, Y. Han, X. Wang, P. R. Somanath, and D. Zhang. 2020. Club Cell Secreted Protein CC16: Potential Applications in Prognosis and Therapy for Pulmonary Diseases. J Clin Med 9. [Google Scholar] [CrossRef]
- Alvarez, F., G. D. De Melo, F. Larrous, L. Kergoat, B. Boëda, V. Michel, D. Seilhean, M. Tichit, D. Hing, D. Hardy, E. Kornobis, H. Bourhy, N. Wolff, and C. Caillet-Saguy. 2025. The SARS-CoV-2 envelope PDZ binding motif acts as a virulence factor disrupting host’s epithelial cell–cell junctions. Cell. Mol. Biol. Lett. 30. [Google Scholar] [CrossRef]
- Arbore, C., M. Sergides, L. Gardini, G. Bianchi, A. V. Kashchuk, I. Pertici, P. Bianco, F. S. Pavone, and M. Capitanio. 2022. alpha-catenin switches between a slip and an asymmetric catch bond with F-actin to cooperatively regulate cell junction fluidity. Nat Commun 13: 1146. [Google Scholar] [CrossRef]
- Baldassi, D., B. Gabold, and O. Merkel. 2021. Air-liquid interface cultures of the healthy and diseased human respiratory tract: Promises, challenges and future directions. Adv Nanobiomed Res 1. [Google Scholar] [CrossRef] [PubMed]
- Barbier, E., J. Carpentier, O. Simonin, P. Gosset, A. Platel, M. Happillon, L. Y. Alleman, E. Perdrix, V. Riffault, T. Chassat, J.-M. Lo Guidice, S. Anthérieu, and G. Garçon. 2023. Oxidative stress and inflammation induced by air pollution-derived PM2. 5 persist in the lungs of mice after cessation of their sub-chronic exposure. Environ. Int. 181: 108248. [Google Scholar] [CrossRef] [PubMed]
- Barr, J., M. E. Gentile, S. Lee, M. E. Kotas, M. Fernanda de Mello Costa, N. P. Holcomb, A. Jaquish, G. Palashikar, M. Soewignjo, M. McDaniel, I. Matsumoto, R. Margolskee, J. Von Moltke, N. A. Cohen, X. Sun, and A. E. Vaughan. 2022. Injury-induced pulmonary tuft cells are heterogenous, arise independent of key Type 2 cytokines, and are dispensable for dysplastic repair. Elife 11. [Google Scholar] [CrossRef]
- Basil, M. C., F. L. Cardenas-Diaz, J. J. Kathiriya, M. P. Morley, J. Carl, A. N. Brumwell, J. Katzen, K. J. Slovik, A. Babu, S. Zhou, M. M. Kremp, K. B. McCauley, S. Li, J. D. Planer, S. S. Hussain, X. Liu, R. Windmueller, Y. Ying, K. M. Stewart, M. Oyster, J. D. Christie, J. M. Diamond, J. F. Engelhardt, E. Cantu, S. M. Rowe, D. N. Kotton, H. A. Chapman, and E. E. Morrisey. 2022. Human distal airways contain a multipotent secretory cell that can regenerate alveoli. Nature 604: 120–126. [Google Scholar] [CrossRef] [PubMed]
- Beech, A., A. Higham, S. Booth, V. Tejwani, F. Trinkmann, and D. Singh. 2024. Type 2 inflammation in COPD: Is it just asthma? Breathe Sheff 20: 230229. [Google Scholar] [CrossRef]
- Bhatt, S. P., A. Agusti, M. Bafadhel, S. A. Christenson, J. Bon, G. C. Donaldson, D. D. Sin, J. A. Wedzicha, and F. J. Martinez. 2023. Phenotypes, Etiotypes, and Endotypes of Exacerbations of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 208: 1026–1041. [Google Scholar] [CrossRef]
- Bissonnette, E. Y., J. F. Lauzon-Joset, J. S. Debley, and S. F. Ziegler. 2020. Cross-Talk Between Alveolar Macrophages and Lung Epithelial Cells is Essential to Maintain Lung Homeostasis. Front Immunol 11: 583042. [Google Scholar] [CrossRef]
- Blackburn, J. B., N. F. Li, N. W. Bartlett, and B. W. Richmond. 2023. An update in club cell biology and its potential relevance to chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 324: L652–L665. [Google Scholar] [CrossRef]
- Boggon, T. J., J. Murray, S. Chappuis-Flament, E. Wong, B. M. Gumbiner, and L. Shapiro. 2002. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science 296: 1308–1313. [Google Scholar] [CrossRef]
- Bole, A., A. Bernstein, M. J. White, A. Bole, S. J. Balk, L. G. Byron, G. M. Huerta-Montañez, P. J. Landrigan, S. M. Marcus, A. L. Nerlinger, L. H. Patel, R. Philipsborn, A. D. Woolf, L. Zajac, K. A. Gray, J. Briskin, N. G. DeNicola, M. Karwowski, M. H. Ward, and COUNCIL ON ENVIRONMENTAL HEALTH AND CLIMATE CHANGE. 2024. SECTION ON MINORITY HEALTH, EQUITY, AND INCLUSION The Built Environment and Pediatric Health. Pediatrics 153. [Google Scholar] [CrossRef] [PubMed]
- Bray, S. J., and A. Bigas. 2025. Modes of Notch signalling in development and disease. Nat. Rev. Mol. Cell Biol. 26: 522–537. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, R. A., A. J. Fisher, and L. A. Borthwick. 2021. The Role of Epithelial Damage in the Pulmonary Immune Response. Cells 10. [Google Scholar] [CrossRef] [PubMed]
- Calderon, A. A., C. Dimond, D. F. Choy, R. Pappu, M. A. Grimbaldeston, D. Mohan, and K. F. Chung. 2023. Targeting interleukin-33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD. Eur Respir Rev 32. [Google Scholar] [CrossRef]
- Calven, J., E. Ax, and M. Radinger. 2020. The Airway Epithelium-A Central Player in Asthma Pathogenesis. Int J Mol Sci 21. [Google Scholar] [CrossRef]
- Campas, O., I. Noordstra, and A. S. Yap. 2024. Adherens junctions as molecular regulators of emergent tissue mechanics. Nat Rev Mol Cell Biol 25: 252–269. [Google Scholar] [CrossRef]
- Carlier, F. M., C. de Fays, and C. Pilette. 2021. Epithelial Barrier Dysfunction in Chronic Respiratory Diseases. Front Physiol 12: 691227. [Google Scholar] [CrossRef]
- Celli, B. R., and A. Agusti. 2018. COPD: Time to improve its taxonomy? ERJ Open Res 4. [Google Scholar] [CrossRef]
- Chapman, K. R., J. G. Burdon, E. Piitulainen, R. A. Sandhaus, N. Seersholm, J. M. Stocks, B. C. Stoel, L. Huang, Z. Yao, J. M. Edelman, N. G. McElvaney, and Rapid Trial Study Group. 2015. Intravenous augmentation treatment and lung density in severe alpha1 antitrypsin deficiency (RAPID): A randomised, double-blind, placebo-controlled trial. Lancet 386: 360–368. [Google Scholar] [CrossRef]
- Christenson, S. A., B. M. Smith, M. Bafadhel, and N. Putcha. 2022. Chronic obstructive pulmonary disease. Lancet 399: 2227–2242. [Google Scholar] [CrossRef]
- Chung, K. F., and I. M. Adcock. 2013. How variability in clinical phenotypes should guide research into disease mechanisms in asthma. Ann Am Thorac Soc 10: S109–17. [Google Scholar] [CrossRef] [PubMed]
- Citi, S., M. Fromm, M. Furuse, L. Gonzalez-Mariscal, A. Nusrat, S. Tsukita, and J. R. Turner. 2024. A short guide to the tight junction. J Cell Sci 137. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M. L., G. Barrientos, X. Cai, S. Mukherjee, M. Das, E. Stephen-Victor, and H. Harb. 2025. Regulatory T cells and their role in allergic disease. Allergy 80: 77–93. [Google Scholar] [CrossRef]
- Corren, J., A. Menzies-Gow, G. Chupp, E. Israel, S. Korn, B. Cook, C. S. Ambrose, A. Hellqvist, S. L. Roseti, N. A. Molfino, J. P. Llanos, N. Martin, K. Bowen, J. M. Griffiths, J. R. Parnes, and G. Colice. 2023. Efficacy of Tezepelumab in Severe, Uncontrolled Asthma: Pooled Analysis of the PATHWAY and NAVIGATOR Clinical Trials. Am J Respir Crit Care Med 208: 13–24. [Google Scholar] [CrossRef] [PubMed]
- Cortez, V., and S. Schultz-Cherry. 2021. The role of goblet cells in viral pathogenesis. FEBS J 288: 7060–7072. [Google Scholar] [CrossRef]
- Cummins, P. M. 2012. Occludin: One protein, many forms. Mol Cell Biol 32: 242–250. [Google Scholar] [CrossRef]
- Cumplido-Laso, G., D. A. Benitez, S. Mulero-Navarro, and J. M. Carvajal-Gonzalez. 2023. Transcriptional Regulation of Airway Epithelial Cell Differentiation: Insights into the Notch Pathway and Beyond. Int J Mol Sci 24. [Google Scholar] [CrossRef]
- Davis, J. D., and T. P. Wypych. 2021. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol 14: 978–990. [Google Scholar] [CrossRef]
- Di, Y. P., and H. Mou. 2024. Airway Serous Cells: A Comparative Study of Spatial Distribution and Abundance among Species. J Respir Biol Transl Med 1. [Google Scholar] [CrossRef]
- Di, Y. P., J. M. Kuhn, and M. L. Mangoni. 2024. Lung antimicrobial proteins and peptides: From host defense to therapeutic strategies. Physiol Rev 104: 1643–1677. [Google Scholar] [CrossRef]
- Duval, C., M. Watanabe, and G. Donati. 2018. Buried myoepithelial stem cells as a reservoir for repairing the exposed airway epithelium. Stem Cell Investig 5: 45. [Google Scholar] [CrossRef]
- Engelhardt, J. F., J. R. Yankaskas, S. A. Ernst, Y. Yang, C. R. Marino, R. C. Boucher, J. A. Cohn, and J. M. Wilson. 1992. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 2: 240–248. [Google Scholar] [CrossRef]
- England, E., D. G. Rees, I. C. Scott, S. Carmen, D. T. Y. Chan, C. E. Chaillan Huntington, K. F. Houslay, T. Erngren, M. Penney, J. B. Majithiya, L. Rapley, D. A. Sims, C. Hollins, E. C. Hinchy, M. D. Strain, B. P. Kemp, D. J. Corkill, R. D. May, K. A. Vousden, R. J. Butler, T. Mustelin, T. J. Vaughan, D. C. Lowe, C. Colley, and E. S. Cohen. 2023. Tozorakimab (MEDI3506): An anti-IL-33 antibody that inhibits IL-33 signalling via ST2 and RAGE/EGFR to reduce inflammation and epithelial dysfunction. Sci Rep 13: 9825. [Google Scholar] [CrossRef]
- Evans, C. M., K. Kim, M. J. Tuvim, and B. F. Dickey. 2009. Mucus hypersecretion in asthma: Causes and effects. Curr Opin Pulm Med 15: 4–11. [Google Scholar] [CrossRef]
- Farquhar, M. G., and G. E. Palade. 1963. Junctional complexes in various epithelia. J Cell Biol 17: 375–412. [Google Scholar] [CrossRef]
- Fawcett, L. K., N. Turgutoglu, K. M. Allan, Y. Belessis, J. Widger, A. Jaffe, and S. A. Waters. 2023. Comparing Cytology Brushes for Optimal Human Nasal Epithelial Cell Collection: Implications for Airway Disease Diagnosis and Research. J Med 13. [Google Scholar] [CrossRef] [PubMed]
- Feng, H., Z. Li, and R. Zheng. 2025. The global burden of chronic respiratory diseases attributable to tobacco from 1990 to 2021: A global burden of disease study 2021. BMC Public Health 25: 456. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y., X. Tang, H. Fu, X. Fan, J. Wei, J. Liu, H. Wang, H. Bi, Z. Chen, X. Wei, and Y. Zheng. 2025. Mechanistic insights into carbon black-activated AKT/TMEM175 cascade impairing macrophage-epithelial cross-talk and airway epithelial proliferation. Env. Pollut 372: 126076. [Google Scholar] [CrossRef] [PubMed]
- Fu, R., X. Jiang, G. Li, Y. Zhu, and H. Zhang. 2022. Junctional complexes in epithelial cells: Sentinels for extracellular insults and intracellular homeostasis. FEBS J 289: 7314–7333. [Google Scholar] [CrossRef]
- Furuse, M., H. Sasaki, K. Fujimoto, and S. Tsukita. 1998. A single gene product, claudin-1 or-2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol 143: 391–401. [Google Scholar] [CrossRef]
- Ganesan, S., A. T. Comstock, and U. S. Sajjan. 2013. Barrier function of airway tract epithelium. Tissue Barriers 1: e24997. [Google Scholar] [CrossRef] [PubMed]
- Gao, N., A. Raduka, and F. Rezaee. 2022. Respiratory syncytial virus disrupts the airway epithelial barrier by decreasing cortactin and destabilizing F-actin. J Cell Sci 135. [Google Scholar] [CrossRef] [PubMed]
- Gao, W., K. R. Kanagarajah, E. Graham, K. Soon, T. Veres, T. J. Moraes, C. E. Bear, R. A. Veldhuizen, A. P. Wong, and A. Günther. 2024. Collagen Tubular Airway-on-Chip for Extended Epithelial Culture and Investigation of Ventilation Dynamics. Small 20. [Google Scholar] [CrossRef] [PubMed]
- Garner, J. L., P. L. Shah, F. Herth, and D. J. Slebos. 2024. ERJ Advances: Interventional bronchoscopy. Eur Respir J 64. [Google Scholar] [CrossRef]
- Gauvreau, G. M., J. M. Hohlfeld, J. M. FitzGerald, L. P. Boulet, D. W. Cockcroft, B. E. Davis, S. Korn, O. Kornmann, R. Leigh, I. Mayers, H. Watz, S. S. Grant, M. Jain, M. Cabanski, P. E. Pertel, I. Jones, J. R. Lecot, H. Cao, and P. M. O’Byrne. 2023. Inhaled anti-TSLP antibody fragment, ecleralimab, blocks responses to allergen in mild asthma. Eur Respir J 61. [Google Scholar] [CrossRef]
- GBD Chronic Respiratory Disease Collaborators. 2020. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: A systematic analysis for the Global Burden of Disease Study. (2017). Lancet Respir Med 8: 585–596. [Google Scholar] [CrossRef]
- GBD Chronic Respiratory Disease Collaborators. 2023. Global burden of chronic respiratory diseases and risk factors, 1990-2019: An update from the Global Burden of Disease Study 2019. EClinicalMedicine 59: 101936. [Google Scholar] [CrossRef]
- Gerayeli, F. V., H. Y. Park, S. Milne, X. Li, C. X. Yang, J. Tuong, R. L. Eddy, S. M. Vahedi, E. Guinto, C. Y. Cheung, J. S. W. Yang, C. Gilchrist, D. Yehia, T. Stach, H. Dang, C. Leung, T. Shaipanich, J. Leipsic, G. J. Koelwyn, J. M. Leung, and D. D. Sin. 2024. Single-cell sequencing reveals cellular landscape alterations in the airway mucosa of patients with pulmonary long COVID. Eur. Respir. J. 64: 2301947. [Google Scholar] [CrossRef]
- Ghosh, A. J., B. D. Hobbs, M. Moll, A. Saferali, A. Boueiz, J. H. Yun, F. Sciurba, L. Barwick, A. H. Limper, K. Flaherty, G. Criner, K. K. Brown, R. Wise, F. J. Martinez, D. Lomas, P. J. Castaldi, V. J. Carey, D. L. DeMeo, M. H. Cho, E. K. Silverman, C. P. Hersh, and COPDGene Investigators. 2022. Alpha-1 Antitrypsin MZ Heterozygosity Is an Endotype of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 205: 313–323. [Google Scholar] [CrossRef]
- Godbole, N. M., A. A. Chowdhury, N. Chataut, and S. Awasthi. 2022. Tight Junctions, the Epithelial Barrier, and Toll-like Receptor-4 During Lung Injury. Inflammation 45: 2142–2162. [Google Scholar] [CrossRef]
- Gon, Y., and S. Hashimoto. 2018. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol Int 67: 12–17. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, L., A. Betanzos, P. Nava, and B. E. Jaramillo. 2003. Tight junction proteins. Prog Biophys Mol Biol 81: 1–44. [Google Scholar] [CrossRef] [PubMed]
- Gooptu, B., U. I. Ekeowa, and D. A. Lomas. 2009. Mechanisms of emphysema in alpha1-antitrypsin deficiency: Molecular and cellular insights. Eur Respir J 34: 475–488. [Google Scholar] [CrossRef] [PubMed]
- Greenan, G. A., R. D. Vale, and D. A. Agard. 2020. Electron cryotomography of intact motile cilia defines the basal body to axoneme transition. J Cell Biol 219. [Google Scholar] [CrossRef]
- Gui, M., H. Farley, P. Anujan, J. R. Anderson, D. W. Maxwell, J. B. Whitchurch, J. J. Botsch, T. Qiu, S. Meleppattu, S. K. Singh, Q. Zhang, J. Thompson, J. S. Lucas, C. D. Bingle, D. P. Norris, S. Roy, and A. Brown. 2021. De novo identification of mammalian ciliary motility proteins using cryo-EM. Cell 184: 5791–5806 e19. [Google Scholar] [CrossRef]
- Gunzel, D., and A. S. Yu. 2013. Claudins and the modulation of tight junction permeability. Physiol Rev 93: 525–569. [Google Scholar] [CrossRef]
- Guo, X., S. Yang, H. Zhu, F. Liu, K. Li, G. Li, Y. Lin, H. Yu, W. Qiu, H. Xu, Q. Liu, X. Xie, Y. Sun, P. Zheng, B. Chen, Z. Liu, X. Yuan, S. Peng, X. Bi, J. Yang, N. Y. Shao, and J. Dai. 2024. Involvement of M2 macrophages polarization in PM2. 5-induced COPD by upregulating MMP12 via IL4/STAT6 pathway. Ecotoxicol Env. Saf 283: 116793. [Google Scholar] [CrossRef]
- He, Y., W. J. Liu, N. Jia, S. Richardson, and C. Huang. 2023. Viral respiratory infections in a rapidly changing climate: The need to prepare for the next pandemic. eBioMedicine 93: 104593. [Google Scholar] [CrossRef]
- Hedstrom, U., L. Oberg, O. Vaarala, G. Dellgren, M. Silverborn, L. Bjermer, G. Westergren-Thorsson, O. Hallgren, and X. Zhou. 2021. Impaired Differentiation of Chronic Obstructive Pulmonary Disease Bronchial Epithelial Cells Grown on Bronchial Scaffolds. Am J Respir Cell Mol Biol 65: 201–213. [Google Scholar] [CrossRef]
- Heijink, I. H., P. M. Kies, H. F. Kauffman, D. S. Postma, A. J. van Oosterhout, and E. Vellenga. 2007. Down-regulation of E-cadherin in human bronchial epithelial cells leads to epidermal growth factor receptor-dependent Th2 cell-promoting activity. J Immunol 178: 7678–7685. [Google Scholar] [CrossRef]
- Heijink, I. H., V. N. S. Kuchibhotla, M. P. Roffel, T. Maes, D. A. Knight, I. Sayers, and M. C. Nawijn. 2020. Epithelial cell dysfunction, a major driver of asthma development. Allergy 75: 1902–1917. [Google Scholar] [CrossRef]
- Hessel, J., J. Heldrich, J. Fuller, M. R. Staudt, S. Radisch, C. Hollmann, B. G. Harvey, R. J. Kaner, J. Salit, J. Yee-Levin, S. Sridhar, S. Pillai, H. Hilton, G. Wolff, H. Bitter, S. Visvanathan, J. Fine, C. S. Stevenson, R. G. Crystal, and A. E. Tilley. 2014. Intraflagellar transport gene expression associated with short cilia in smoking and COPD. PLoS ONE 9: e85453. [Google Scholar] [CrossRef]
- Hewitt, R. J., and C. M. Lloyd. 2021. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 21: 347–362. [Google Scholar] [CrossRef]
- Hoagland, D. A., P. Rodríguez-Morales, A. O. Mann, A. Y. Baez Vazquez, S. Yu, A. Lai, H. Kane, S. M. Dang, Y. Lin, L. Thorens, S. Begum, M. A. Castro, S. D. Pope, J. Lim, S. Li, X. Zhang, M. O. Li, C. F. Kim, R. Jackson, R. Medzhitov, and R. A. Franklin. 2025. Macrophage-derived oncostatin M repairs the lung epithelial barrier during inflammatory damage. Science 389: 169–175. [Google Scholar] [CrossRef]
- Hogan, B., and P. R. Tata. 2019. Cellular organization and biology of the respiratory system. Nat Cell Biol. [Google Scholar] [CrossRef] [PubMed]
- Holtjer, J. C. S., L. D. Bloemsma, R. Beijers, M. E. B. Cornelissen, B. Hilvering, L. Houweling, R. C. H. Vermeulen, G. S. Downward, A. H. Maitland-Van der Zee, and P. O. consortium. 2023. Identifying risk factors for COPD and adult-onset asthma: An umbrella review. Eur Respir Rev 32. [Google Scholar] [CrossRef] [PubMed]
- Horton, K., P. A. C. Wing, C. L. Jackson, C. J. McCormick, M. P. Carroll, and J. S. Lucas. 2025. Interplay between respiratory viruses and cilia in the airways. Eur Respir Rev 34. [Google Scholar] [CrossRef] [PubMed]
- Howell, I., A. Howell, and I. D. Pavord. 2023. Type 2 inflammation and biological therapies in asthma: Targeted medicine taking flight. J Exp Med 220. [Google Scholar] [CrossRef]
- Hu, X. 2025. The lung exposome: Accelerating precision respiratory health. The Lung: pp. 629–645. [Google Scholar]
- Huff, R. D., C. Carlsten, and J. A. Hirota. 2019. An update on immunologic mechanisms in the respiratory mucosa in response to air pollutants. J Allergy Clin Immunol 143: 1989–2001. [Google Scholar] [CrossRef]
- Jacquet, A. 2011. Interactions of airway epithelium with protease allergens in the allergic response. Clin Exp Allergy 41: 305–311. [Google Scholar] [CrossRef]
- Jagielnicki, M., I. Kucharska, B. C. Bennett, A. L. Harris, and M. Yeager. 2024. Connexin Gap Junction Channels and Hemichannels: Insights from High-Resolution Structures. Biol. Basel 13. [Google Scholar] [CrossRef]
- Jameson, J. L., and D. L. Longo. 2015. Precision medicine--personalized, problematic, and promising. N Engl J Med 372: 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Jang, A. S. 2014. The apical junctional complex in respiratory diseases. Chonnam Med J 50: 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jia, J., J. Xia, R. Zhang, Y. Bai, S. Liu, M. Dan, T. Li, T. Yan, L. Chen, S. Gong, P. Niu, and T. Chen. 2019. Investigation of the impact of PM2. 5 on the ciliary motion of human nasal epithelial cells. Chemosphere 233: 309–318. [Google Scholar] [CrossRef] [PubMed]
- Jing, J. C., J. J. Chen, L. Chou, B. J. F. Wong, and Z. Chen. 2017. Visualization and Detection of Ciliary Beating Pattern and Frequency in the Upper Airway using Phase Resolved Doppler Optical Coherence Tomography. Sci Rep 7: 8522. [Google Scholar] [CrossRef]
- Joo, H., S. Min, and S. W. Cho. 2024. Advanced lung organoids for respiratory system and pulmonary disease modeling. J Tissue Eng 15: 20417314241232502. [Google Scholar] [CrossRef]
- Kageyama, T., T. Ito, S. Tanaka, and H. Nakajima. 2024. Physiological and immunological barriers in the lung. Semin Immunopathol 45: 533–547. [Google Scholar] [CrossRef]
- Kato, A., and H. Kita. 2025. The immunology of asthma and chronic rhinosinusitis. Nat Rev Immunol. [Google Scholar] [CrossRef]
- Kelsen, S. G., I. O. Agache, W. Soong, E. Israel, G. L. Chupp, D. S. Cheung, W. Theess, X. Yang, T. L. Staton, D. F. Choy, A. Fong, A. Dash, M. Dolton, R. Pappu, and C. E. Brightling. 2021. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: A randomized clinical trial. J Allergy Clin Immunol 148: 790–798. [Google Scholar] [CrossRef]
- Kim, K. A., J. H. Jung, Y. S. Choi, and S. T. Kim. 2024. Ginsenoside Re protects rhinovirus-induced disruption of tight junction through inhibition of ROS-mediated phosphatases inactivation in human nasal epithelial cells. Heliyon 10: e27688. [Google Scholar] [CrossRef]
- Knight, D. A., and S. T. Holgate. 2003. The airway epithelium: Structural and functional properties in health and disease. Respirology 8: 432–446. [Google Scholar] [CrossRef] [PubMed]
- Koch, C. M., A. D. Prigge, L. Setar, K. R. Anekalla, H. C. Do-Umehara, H. Abdala-Valencia, Y. Politanska, A. Shukla, J. Chavez, G. R. Hahn, and B. M. Coates. 2022. Cilia-related gene signature in the nasal mucosa correlates with disease severity and outcomes in critical respiratory syncytial virus bronchiolitis. Front Immunol 13: 924792. [Google Scholar] [CrossRef] [PubMed]
- Kostrewa, D., M. Brockhaus, A. D’Arcy, G. E. Dale, P. Nelboeck, G. Schmid, F. Mueller, G. Bazzoni, E. Dejana, T. Bartfai, F. K. Winkler, and M. Hennig. 2001. X-ray structure of junctional adhesion molecule: Structural basis for homophilic adhesion via a novel dimerization motif. EMBO J 20: 4391–4398. [Google Scholar] [CrossRef] [PubMed]
- Kovesi, T., G. Mallach, Y. Schreiber, M. McKay, G. Lawlor, N. Barrowman, A. Tsampalieros, R. Kulka, A. Root, L. Kelly, M. Kirlew, and J. D. Miller. 2022. Housing conditions and respiratory morbidity in Indigenous children in remote communities in Northwestern Ontario, Canada. CMAJ 194: E80–E88. [Google Scholar] [CrossRef]
- Kuek, L. E., and R. J. Lee. 2020. First contact: The role of respiratory cilia in host-pathogen interactions in the airways. Am. J. Physiol.-Lung Cell. Mol. Physiol. 319: L603–L619. [Google Scholar] [CrossRef]
- Kuruvilla, M. E., F. E. Lee, and G. B. Lee. 2019. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin Rev Allergy Immunol 56: 219–233. [Google Scholar] [CrossRef]
- Lee, D. C., H. Choi, J.-M. Oh, Junuk Lee, Joohyung Lee, H. Y. Lee, and J. Y. Kang. 2020. Urban particulate matter regulates tight junction proteins by inducing oxidative stress via the Akt signal pathway in human nasal epithelial cells. Toxicol. Lett. 333: 33–41. [Google Scholar] [CrossRef]
- Lee, P. H., S. Park, Y. G. Lee, S. M. Choi, M. H. An, and A. S. Jang. 2021. The Impact of Environmental Pollutants on Barrier Dysfunction in Respiratory Disease. Allergy Asthma Immunol Res 13: 850–862. [Google Scholar] [CrossRef]
- Lee, R. E., B. Reidel, M. R. Nelson, J. K. Macdonald, M. Kesimer, and S. H. Randell. 2023. Air-Liquid interface cultures to model drug delivery through the mucociliary epithelial barrier. Adv. Drug Deliv. Rev. 198: 114866. [Google Scholar] [CrossRef]
- Lee, Y., M. K. Lee, H.-R. Lee, B. Kim, M. Kim, and S. Jung. 2024. 3D-printed airway model as a platform for SARS-CoV-2 infection and antiviral drug testing. Biomaterials 311: 122689. [Google Scholar] [CrossRef]
- Lei, L., S. Traore, G. S. Romano Ibarra, P. H. Karp, T. Rehman, D. K. Meyerholz, J. Zabner, D. A. Stoltz, P. L. Sinn, M. J. Welsh, P. B. McCray, and I. M. Thornell. 2023. CFTR-rich ionocytes mediate chloride absorption across airway epithelia. J Clin Invest 133. [Google Scholar] [CrossRef]
- Lima, C., M. A. P. Falcao, J. G. S. Rosa, G. R. Disner, and M. Lopes-Ferreira. 2022. Pesticides and Their Impairing Effects on Epithelial Barrier Integrity, Dysbiosis, Disruption of the AhR Signaling Pathway and Development of Immune-Mediated Inflammatory Diseases. Int J Mol Sci 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, X., X. Zhao, X. Li, S. Lv, R. Ma, Y. Qi, A. Abulikemu, H. Duan, C. Guo, Y. Li, and Z. Sun. 2020. PM(2. 5) triggered apoptosis in lung epithelial cells through the mitochondrial apoptotic way mediated by a ROS-DRP1-mitochondrial fission axis. J Hazard Mater 397: 122608. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y., Y. Huang, J. Li, J. Huang, L. Zhang, J. Feng, J. Li, Q. Xia, Q. Zhao, L. Huang, S. Jiang, and S. Su. 2021. Eosinophil extracellular traps drive asthma progression through neuro-immune signals. Nat Cell Biol 23: 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Luan, X., N. Henao Romero, V. A. Campanucci, Y. Le, J. Mustofa, J. S. Tam, and J. P. Ianowski. 2024. Pulmonary Ionocytes Regulate Airway Surface Liquid pH in Primary Human Bronchial Epithelial Cells. Am J Respir Crit Care Med 210: 788–800. [Google Scholar] [CrossRef]
- Lynch, T. J., P. J. Anderson, P. G. Rotti, S. R. Tyler, A. K. Crooke, S. H. Choi, D. T. Montoro, C. L. Silverman, W. Shahin, R. Zhao, C. W. Jensen-Cody, A. Adamcakova-Dodd, T. I. A. Evans, W. Xie, Y. Zhang, H. Mou, B. P. Herring, P. S. Thorne, J. Rajagopal, C. Yeaman, K. R. Parekh, and J. F. Engelhardt. 2018. Submucosal Gland Myoepithelial Cells Are Reserve Stem Cells That Can Regenerate Mouse Tracheal Epithelium. Cell Stem Cell 22: 653–667 e5. [Google Scholar] [CrossRef]
- Martin-Sanchez, F., M. Atienza-Maderuelo, G. Lopez-Campos, and P. Collado. 2021. Use of informatics to characterise the exposome of COVID-19. BMJ Health Care Inf 28. [Google Scholar] [CrossRef]
- Matkovic Leko, I., R. T. Schneider, T. A. Thimraj, N. Schrode, D. Beitler, H.-Y. Liu, K. Beaumont, Y.-W. Chen, and H.-W. Snoeck. 2023. A distal lung organoid model to study interstitial lung disease, viral infection and human lung development. Nat. Protoc. 18: 2283–2312. [Google Scholar] [CrossRef]
- McBrien, C. N., and A. Menzies-Gow. 2017. The Biology of Eosinophils and Their Role in Asthma. Front Med Lausanne 4: 93. [Google Scholar] [CrossRef]
- Montgomery, M. T., S. P. Sajuthi, S.-H. Cho, J. L. Everman, C. L. Rios, K. C. Goldfarbmuren, N. D. Jackson, B. Saef, M. Cromie, C. Eng, V. Medina, J. R. Elhawary, S. S. Oh, J. Rodriguez-Santana, E. K. Vladar, E. G. Burchard, and M. A. Seibold. 2020. Genome-Wide Analysis Reveals Mucociliary Remodeling of the Nasal Airway Epithelium Induced by Urban PM2. 5. Am. J. Respir. Cell Mol. Biol. 63: 172–184. [Google Scholar] [CrossRef]
- Moratin, H., J. Lang, M. S. Picker, A. Rossi, C. Wilhelm, A. von Fournier, M. Stoth, M. Goncalves, N. Kleinsasser, S. Hackenberg, A. Scherzad, and T. J. Meyer. 2025. The Impact of NO(2) on Epithelial Barrier Integrity of a Primary Cell-Based Air-Liquid Interface Model of the Nasal Respiratory Epithelium. J Appl Toxicol 45: 482–491. [Google Scholar] [CrossRef]
- Narita, H., Y. Yamamoto, M. Suzuki, N. Miyazaki, A. Yoshida, K. Kawai, K. Iwasaki, A. Nakagawa, Y. Takai, and T. Sakisaka. 2011. Crystal Structure of the cis-Dimer of Nectin-1: Implications for the architecture of cell-cell junctions. J Biol Chem 286: 12659–12669. [Google Scholar] [CrossRef]
- Naser, A. N., Q. Lu, and Y. H. Chen. 2023. Trans-Compartmental Regulation of Tight Junction Barrier Function. Tissue Barriers 11: 2133880. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijsen, M., A. De Nazelle, J. Garcia-Aymerich, H. Khreis, and B. Hoffmann. 2024. Shaping urban environments to improve respiratory health: Recommendations for research, planning, and policy. Lancet Respir. Med. 12: 247–254. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K., K. A. Brune, N. Putcha, P. Mandke, W. K. O’Neal, D. Shade, V. Srivastava, M. Wang, H. Lam, S. S. An, M. B. Drummond, N. N. Hansel, D. N. Robinson, and V. K. Sidhaye. 2017. Cigarette smoke disrupts monolayer integrity by altering epithelial cell-cell adhesion and cortical tension. Am. J. Physiol. Lung Cell. Mol. Physiol. 313: L581–L591. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M., K. T. Furukawa, and M. Morimoto. 2020. Pulmonary neuroendocrine cells: Physiology, tissue homeostasis and disease. Model Mech 13. [Google Scholar] [CrossRef]
- Okuda, K., and M. Gentzsch. 2024. Pulmonary Ionocytes: What Are They Transporting and Which Way? Am J Respir Crit Care Med 210: 705–707. [Google Scholar] [CrossRef]
- Onufer, A. P., J. C. Mell, L. Cort, A. Rao, N. V. Mdluli, and A. J. Carey. 2025. Influenza virus-induced type I interferons disrupt alveolar epithelial repair and tight junction integrity in the developing lung. Mucosal Immunol. 18: 607–619. [Google Scholar] [CrossRef]
- Ortiz-Quintero, B., I. Martinez-Espinosa, and R. Perez-Padilla. 2022. Mechanisms of Lung Damage and Development of COPD Due to Household Biomass-Smoke Exposure: Inflammation, Oxidative Stress, MicroRNAs, and Gene Polymorphisms. Cells 12. [Google Scholar] [CrossRef]
- Otani, T., and M. Furuse. 2020. Tight Junction Structure and Function Revisited. Trends Cell Biol 30: 805–817. [Google Scholar] [CrossRef]
- Otani, T., T. P. Nguyen, S. Tokuda, K. Sugihara, T. Sugawara, K. Furuse, T. Miura, K. Ebnet, and M. Furuse. 2019. Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity. J Cell Biol 218: 3372–3396. [Google Scholar] [CrossRef]
- Owen, C. E. 2007. Immunoglobulin E: Role in asthma and allergic disease: Lessons from the clinic. Pharmacol Ther 113: 121–133. [Google Scholar] [CrossRef]
- Pellicano, C., L. Vantaggio, A. Colalillo, K. Pocino, V. Basile, M. Marino, U. Basile, and E. Rosato. 2023. Type 2 cytokines and scleroderma interstitial lung disease. Clin Exp Med 23: 3517–3525. [Google Scholar] [CrossRef]
- Perl, A. L., J. L. Pokorny, and K. J. Green. 2024. Desmosomes at a glance. J Cell Sci 137. [Google Scholar] [CrossRef] [PubMed]
- Pijnenburg, M. W., and R. Nantanda. 2021. Rising and falling prevalence of asthma symptoms. Lancet 398: 1542–1543. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K. F., B. R. Celli, M. E. Wechsler, R. M. Abdulai, X. Luo, M. M. Boomsma, H. Staudinger, J. E. Horowitz, A. Baras, M. A. Ferreira, M. K. Ruddy, M. C. Nivens, N. Amin, D. M. Weinreich, G. D. Yancopoulos, and H. Goulaouic. 2021. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: A genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med 9: 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Rackley, C. R., and B. R. Stripp. 2012. Building and maintaining the epithelium of the lung. J Clin Invest 122: 2724–2730. [Google Scholar] [CrossRef]
- Ramirez, S. I., F. Faraji, L. B. Hills, P. G. Lopez, B. Goodwin, H. D. Stacey, H. J. Sutton, K. M. Hastie, E. O. Saphire, H. J. Kim, S. Mashoof, C. H. Yan, A. S. DeConde, G. Levi, and S. Crotty. 2024. Immunological memory diversity in the human upper airway. Nature 632: 630–636. [Google Scholar] [CrossRef]
- Rao, W., S. Wang, M. Duleba, S. Niroula, K. Goller, J. Xie, R. Mahalingam, R. Neupane, A. A. Liew, M. Vincent, K. Okuda, W. K. O’Neal, R. C. Boucher, B. F. Dickey, M. E. Wechsler, O. Ibrahim, J. F. Engelhardt, T. C. J. Mertens, W. Wang, S. S. K. Jyothula, C. P. Crum, H. Karmouty-Quintana, K. R. Parekh, M. L. Metersky, F. D. McKeon, and W. Xian. 2020. Regenerative Metaplastic Clones in COPD Lung Drive Inflammation and Fibrosis. Cell 181: 848–864 e18. [Google Scholar] [CrossRef]
- Ray, A., M. Camiolo, A. Fitzpatrick, M. Gauthier, and S. E. Wenzel. 2020. Are We Meeting the Promise of Endotypes and Precision Medicine in Asthma? Physiol Rev 100: 983–1017. [Google Scholar] [CrossRef]
- Reyfman, P. A., B. Khuder, J. B. Pierce, D. A. Meza, M. R. Carnethon, R. Kalhan, and S. S. Khan. 2021. Urban–Rural Mortality Disparities from Chronic Lower Respiratory Diseases in the United States, 1999–2019. Am. J. Respir. Crit. Care Med. 203: 1435–1437. [Google Scholar] [CrossRef]
- Rhodin, J. 1959. LXVII ultrastructure of the tracheal ciliated mucosa in rat and man. Ann. Otol. Rhinol. Laryngol. 68: 964–974. [Google Scholar] [CrossRef]
- Rhodin, J., and T. Dalhamn. 1956. Electron microscopy of the tracheal ciliated mucosa in rat. Z Zellforsch Mikrosk Anat 44: 345–412. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, W., M. Rokicki, J. Wojtacha, and A. Dzeljijli. 2016. The role and importance of club cells (Clara cells) in the pathogenesis of some respiratory diseases. Kardiochir Torakochirurgia Pol 13: 26–30. [Google Scholar] [CrossRef] [PubMed]
- Russo, R. C., D. Togbe, I. Couillin, N. Segueni, L. Han, V. F. J. Quesniaux, T. Stoeger, and B. Ryffel. 2025. Ozone-induced lung injury and inflammation: Pathways and therapeutic targets for pulmonary diseases caused by air pollutants. Environ. Int. 198: 109391. [Google Scholar] [CrossRef] [PubMed]
- Ruysseveldt, E., K. Martens, and B. Steelant. 2021. Airway Basal Cells, Protectors of Epithelial Walls in Health and Respiratory Diseases. Front Allergy 2: 787128. [Google Scholar] [CrossRef]
- Saito, A. C., C. Endo, Y. Fukazawa, T. Higashi, and H. Chiba. 2022. Effects of TAMP family on the tight junction strand network and barrier function in epithelial cells. Ann N Acad Sci 1517: 234–250. [Google Scholar] [CrossRef]
- Sajjan, U., Q. Wang, Y. Zhao, D. C. Gruenert, and M. B. Hershenson. 2008. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med 178: 1271–1281. [Google Scholar] [CrossRef]
- Salvati, L., L. Maggi, F. Annunziato, and L. Cosmi. 2021. Thymic stromal lymphopoietin and alarmins as possible therapeutical targets for asthma. Curr Opin Allergy Clin Immunol 21: 590–596. [Google Scholar] [CrossRef]
- Sayers, I., C. John, J. Chen, and I. P. Hall. 2024. Genetics of chronic respiratory disease. Nat Rev Genet 25: 534–547. [Google Scholar] [CrossRef]
- Schreiner, T., L. Allnoch, G. Beythien, K. Marek, K. Becker, D. Schaudien, S. Stanelle-Bertram, B. Schaumburg, N. Mounogou Kouassi, S. Beck, M. Zickler, G. Gabriel, W. Baumgartner, F. Armando, and M. Ciurkiewicz. 2022. SARS-CoV-2 Infection Dysregulates Cilia and Basal Cell Homeostasis in the Respiratory Epithelium of Hamsters. Int J Mol Sci 23. [Google Scholar] [CrossRef] [PubMed]
- Shah, A. S., Y. Ben-Shahar, T. O. Moninger, J. N. Kline, and M. J. Welsh. 2009. Motile cilia of human airway epithelia are chemosensory. Science 325: 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Short, K. R., J. Kasper, S. van der Aa, A. C. Andeweg, F. Zaaraoui-Boutahar, M. Goeijenbier, M. Richard, S. Herold, C. Becker, D. P. Scott, R. W. Limpens, A. J. Koster, M. Barcena, R. A. Fouchier, C. J. Kirkpatrick, and T. Kuiken. 2016. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur Respir J 47: 954–966. [Google Scholar] [CrossRef] [PubMed]
- Singh, D., P. Guller, F. Reid, S. Doffman, U. Seppala, I. Psallidas, R. Moate, R. Smith, J. Kiraga, E. Jimenez, D. Brooks, A. Kelly, L. H. Nordenmark, M. Waqas Sadiq, L. Mateos Caballero, C. Kell, M. G. Belvisi, and H. Pandya. 2025. A phase 2a trial of the IL-33 mAb tozorakimab in patients with COPD: FRONTIER-4. Eur Respir J. [Google Scholar] [CrossRef]
- Smallcombe, C. C., D. T. Linfield, T. J. Harford, V. Bokun, A. I. Ivanov, G. Piedimonte, and F. Rezaee. 2019. Disruption of the airway epithelial barrier in a murine model of respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 316: L358–L368. [Google Scholar] [CrossRef]
- Srisomboon, Y., K. Iijima, M. Colwell, P. J. Maniak, M. Macchietto, C. Faulk, H. Kita, and S. M. O’Grady. 2023. Allergen-induced DNA release by the airway epithelium amplifies type 2 immunity. J Allergy Clin Immunol 151: 494–508 e6. [Google Scholar] [CrossRef]
- Su, X., W. Wu, Z. Zhu, X. Lin, and Y. Zeng. 2022. The effects of epithelial-mesenchymal transitions in COPD induced by cigarette smoke: An update. Respir Res 23: 225. [Google Scholar] [CrossRef]
- Sun, D., I. LuValle-Burke, K. Pombo-Garcia, and A. Honigmann. 2022. Biomolecular condensates in epithelial junctions. Curr Opin Cell Biol 77: 102089. [Google Scholar] [CrossRef]
- Sun, S., C. Wang, J. Hu, P. Zhao, X. Wang, and W. E. Balch. 2025. Spatial covariance reveals isothiocyanate natural products adjust redox stress to restore function in alpha-1-antitrypsin deficiency. Cell Rep Med 6: 101917. [Google Scholar] [CrossRef]
- Suzuki, H., T. Nishizawa, K. Tani, Y. Yamazaki, A. Tamura, R. Ishitani, N. Dohmae, S. Tsukita, O. Nureki, and Y. Fujiyoshi. 2014. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science 344: 304–307. [Google Scholar] [CrossRef]
- Sweerus, K., M. Lachowicz-Scroggins, E. Gordon, M. LaFemina, X. Huang, M. Parikh, C. Kanegai, J. V. Fahy, and J. A. Frank. 2017. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol 139: 72–81 e1. [Google Scholar] [CrossRef]
- Taylor-Blair, H. C., A. C. W. Siu, A. Haysom-McDowell, S. Kokkinis, A. Bani Saeid, D. K. Chellappan, B. G. G. Oliver, K. R. Paudel, G. De Rubis, and K. Dua. 2024. The impact of airborne particulate matter-based pollution on the cellular and molecular mechanisms in chronic obstructive pulmonary disease (COPD). Sci Total Env. 954: 176413. [Google Scholar] [CrossRef]
- Thakur, A., S. Mei, N. Zhang, K. Zhang, B. Taslakjian, J. Lian, S. Wu, B. Chen, J. Solway, and H. J. Chen. 2024. Pulmonary neuroendocrine cells: Crucial players in respiratory function and airway-nerve communication. Front Neurosci 18: 1438188. [Google Scholar] [CrossRef]
- Thomas, B., M. S. Koh, C. O’Callaghan, J. C. Allen, A. Rutman, R. A. Hirst, J. Connolly, S. Y. Low, O. Thun How, L. Chian Min, W. T. Lim, L. Lin Ean Oon, Q. He, O. H. Teoh, and T. S. Lapperre. 2021. Dysfunctional Bronchial Cilia Are a Feature of Chronic Obstructive Pulmonary Disease (COPD). COPD 18: 657–663. [Google Scholar] [CrossRef]
- Travaglini, K. J., A. N. Nabhan, L. Penland, R. Sinha, A. Gillich, R. V. Sit, S. Chang, S. D. Conley, Y. Mori, J. Seita, G. J. Berry, J. B. Shrager, R. J. Metzger, C. S. Kuo, N. Neff, I. L. Weissman, S. R. Quake, and M. A. Krasnow. 2020. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 587: 619–625. [Google Scholar] [CrossRef] [PubMed]
- Troyanovsky, S. M. 2023. Adherens junction: The ensemble of specialized cadherin clusters. Trends Cell Biol 33: 374–387. [Google Scholar] [CrossRef] [PubMed]
- Turner, M. C., M. Nieuwenhuijsen, K. Anderson, D. Balshaw, Y. Cui, G. Dunton, J. A. Hoppin, P. Koutrakis, and M. Jerrett. 2017. Assessing the Exposome with External Measures: Commentary on the State of the Science and Research Recommendations. Annu Rev Public Health 38: 215–239. [Google Scholar] [CrossRef]
- Varricchi, G., R. Poto, G. Criscuolo, C. Strisciuglio, P. Nair, and G. Marone. 2025. TL1A, a novel alarmin in airway, intestinal, and autoimmune disorders. J. Allergy Clin. Immunol. 155: 1420–1434. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, R., E. L. Schymanski, A. L. Barabasi, and G. W. Miller. 2020. The exposome and health: Where chemistry meets biology. Science 367: 392–396. [Google Scholar] [CrossRef]
- Vila-Gonzalez, M., L. Pinte, R. Fradique, E. Causa, H. Kool, M. Rodrat, C. M. Morell, M. Al-Thani, L. Porter, W. Guo, R. Maeshima, S. L. Hart, F. McCaughan, A. Granata, D. N. Sheppard, R. A. Floto, E. L. Rawlins, P. Cicuta, and L. Vallier. 2024. In vitro platform to model the function of ionocytes in the human airway epithelium. Respir Res 25: 180. [Google Scholar] [CrossRef]
- Walton, T., M. Gui, S. Velkova, M. R. Fassad, R. A. Hirst, E. Haarman, C. O’Callaghan, M. Bottier, T. Burgoyne, H. M. Mitchison, and A. Brown. 2023. Axonemal structures reveal mechanoregulatory and disease mechanisms. Nature 618: 625–633. [Google Scholar] [CrossRef]
- Wan, M., E. M. Simonin, M. M. Johnson, X. Zhang, X. Lin, P. Gao, C. J. Patel, A. Yousuf, M. P. Snyder, X. Hong, X. Wang, V. Sampath, and K. C. Nadeau. 2025. Exposomics: A review of methodologies, applications, and future directions in molecular medicine. EMBO Mol Med 17: 599–608. [Google Scholar] [CrossRef]
- Wang, M., G. Tan, A. Eljaszewicz, Y. Meng, P. Wawrzyniak, S. Acharya, C. Altunbulakli, P. Westermann, A. Dreher, L. Yan, C. Wang, M. Akdis, L. Zhang, K. C. Nadeau, and C. A. Akdis. 2019. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol 143: 1892–1903. [Google Scholar] [CrossRef]
- Wechsler, M. E., M. K. Ruddy, I. D. Pavord, E. Israel, K. F. Rabe, L. B. Ford, J. F. Maspero, R. M. Abdulai, C. C. Hu, R. Martincova, A. Jessel, M. C. Nivens, N. Amin, D. M. Weinreich, G. D. Yancopoulos, and H. Goulaouic. 2021. Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma. N Engl J Med 385: 1656–1668. [Google Scholar] [CrossRef]
- Widdicombe, J. H. 2019a. Early studies on the surface epithelium of mammalian airways. Am J Physiol Lung Cell Mol Physiol 317: L486–L495. [Google Scholar] [CrossRef]
- Widdicombe, J. H. 2019b. Early studies of airway submucosal glands. Am J Physiol Lung Cell Mol Physiol 316: L990–L998. [Google Scholar] [CrossRef] [PubMed]
- Wild, C. P. 2005. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomark. Prev 14: 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Wild, C. P. 2012. The exposome: From concept to utility. Int J Epidemiol 41: 24–32. [Google Scholar] [CrossRef] [PubMed]
- Witherden, D. A., and W. L. Havran. 2013. Cross-talk between intraepithelial gammadelta T cells and epithelial cells. J Leukoc Biol 94: 69–76. [Google Scholar] [CrossRef]
- Wittekindt, O. H. 2017. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch 469: 135–147. [Google Scholar] [CrossRef]
- Yang, J., and R. Shaykhiev. 2017. Reply: Epithelial-Mesenchymal Transition: A Necessary New Therapeutic Target in Chronic Obstructive Pulmonary Disease? Am J Respir Crit Care Med 196: 394–395. [Google Scholar] [CrossRef]
- Yi, X., J. Gao, and Z. Wang. 2022. The human lung microbiome-A hidden link between microbes and human health and diseases. Imeta 1: e33. [Google Scholar] [CrossRef]
- Yoshida, M., R. Arzili, and M. Z. Nikolic. 2025. Immune-epithelial cell interactions in lung development, homeostasis and disease. Int J Biochem Cell Biol 178: 106703. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, A. J., S. Mohammed, L. Carr, M. Yavari Ramsheh, C. Micieli, V. Mistry, K. Haldar, A. Wright, P. Novotny, S. Parker, S. Glover, J. Finch, N. Quann, C. L. Brookes, R. Hobson, W. Ibrahim, R. J. Russell, C. John, M. A. Grimbaldeston, D. F. Choy, D. Cheung, M. Steiner, N. J. Greening, and C. E. Brightling. 2022. Astegolimab, an anti-ST2, in chronic obstructive pulmonary disease (COPD-ST2OP): A phase 2a, placebo-controlled trial. Lancet Respir Med 10: 469–477. [Google Scholar] [CrossRef]
- Yu, W., T. O. Moninger, A. L. Thurman, Y. Xie, A. Jain, K. Zarei, L. S. Powers, A. A. Pezzulo, D. A. Stoltz, and M. J. Welsh. 2022. Cellular and molecular architecture of submucosal glands in wild-type and cystic fibrosis pigs. Proc Natl Acad Sci U A 119. [Google Scholar] [CrossRef]
- Zhang, B., X. Feng, L. Tian, B. Xiao, L. Hou, B. Mo, and D. Yao. 2025. Epithelial-mesenchymal transition in asthma: Its role and underlying regulatory mechanisms. Front Immunol 16: 1519998. [Google Scholar] [CrossRef]
- Zimmer, S. E., and A. P. Kowalczyk. 2024. The desmosome as a dynamic membrane domain. Curr. Opin. Cell Biol. 90: 102403. [Google Scholar] [CrossRef]
| Exposure factors | Mechanisms |
| Air pollution | |
| Particle matters (PMs) | Induce oxidative stress and inflammation (Barbier et al., 2023). |
| Lead to airway remodeling through oxidative stress, inflammation, and altered epithelial plasticity (Taylor-Blair et al., 2024). | |
| Cause mucociliary dysfunction by activating mucus secretory expression gene (Montgomery et al., 2020) and impacting the ciliary motion (Jia et al., 2019). | |
| Downregulate the level of Occludin, Claudin-1, E-cadherin, and ZO-1 (Lee et al., 2020). | |
| Ozone | Induced respiratory epithelial cell death, oxidative stress, inflammation, and barrier damage (Russo et al., 2025). |
| Nitrogen dioxide (NO2) | Decrease TJ protein expression and induce inflammation (Moratin et al., 2025). |
| Tobacco smoke | Reduce E-cadherin and ß-catenin expression and further destabilize cell adhesion by reducing the tension between epithelial cells via increasing actin polymer levels (Nishida et al., 2017). |
| Pesticides | Induce oxidative stress, and alter or disrupt of apical cell-cell junctions via decrease in the expression of proteins like E-cadherin, β-catenin, Occludin, and ZOs (Lima et al., 2022). |
| Laundry detergents | Disrupt epithelial barrier function with decreased transepithelial electrical resistance, increased paracellular flux, and irregular tight TJ structure (Wang et al., 2019). |
| Allergens | Induce mitochondrial or nuclear DNA release and nuclear DNA fragmentation in human bronchial epithelial cells (Srisomboon et al., 2023). |
| Directly digest Occludin and zonula occludens-1 (ZO-1) in airway epithelium (Jacquet, 2011). | |
| Viruses | |
| Respiratory syncytial virus (RSV) | Disrupt AJC by decrease the expression of ZO-1, Occludin, Claudin-1, Cleaves extracellular fragments of E-cadherin, depolymerizes F-actin and decreases cortactin, an actin-binding protein crucial for barrier stability (Gao et al., 2022; Smallcombe et al., 2019). |
| Lead to cilia loss and impaired mucociliary clearance (Koch et al., 2022). | |
| Influenza A viruses (IAV) | Causes significant damage to the alveolar epithelial barrier, leading to loss of tight junction integrity primarily through reduction or loss of tight junction proteins such as claudin-4 (Short et al., 2016). |
| Induce production of IFN-I, disrupting alveolar epithelial repair and tight junction integrity (Onufer et al., 2025), and causing the AEC death (Hoagland et al., 2025). | |
| Coronaviruses (CoV) | SARS-CoV-2 Disrupts TJ by the viral E protein-ZO-1 interaction (Alvarez et al., 2025). |
| SARS-CoV-2 infection led to cilia loss in hamsters (Schreiner et al., 2022). | |
| In bronchoscopy samples from long COVID patients, genes related to AEB dysfunction and mucus production were up-regulated (Gerayeli et al., 2024) | |
| Rhinovirus (RV) | Generate ROS and decrease Claudin, Occludin, E-cadherin and ZO-1 (Kim et al., 2024; Sajjan et al., 2008). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




