1. Introduction
Nanotechnology can be described as one of the future sciences and the progression of this science is an endless affair in terms of improvements in the enhancement of human lives [
1]. However, the liberal and unjustified usage of antibiotics and other various drugs is the primary cause of an increased bacterial resistance to these compounds [
2]. This more and more makes the scientists concerned and continue to work hard and find newer and different ways of combating the by now altered bacteria to the antibiotic drugs and find newer and better alternatives of combating life destroying diseases so as we may be there to live a quality life[
3]. All the solutions to all the problems may well be available because of the broader prospects with the help of nanotechnology. It has been disclosed in the literature brought forth by the preceding studies that CuO NP is used in various areas of biology and that it is less toxic as compared to other metal oxide nanoparticles. Since (CuO) NP is the most manufactured nano-product in the consumer products: toxicity and efficacy tests are on a regular basis, however, there exist minimal information about the particular toxicity in the event of intensive quantity of CuO NPs [
4]. Therefore, biocompatible and biodegradable polymers are used so as to reduce the toxicity of this compound. Materials that are widely used are referred to as Aspartic acid, PEG, albumin, plastic and dextran[
5]. The use of Nickel in the present research was justified by the fact that Nickel-environmental species have been found to increase the functionality of the NPs in biologic conditions; they also penetrate tissues and are taken up by the cell[
6]. They also increase encapsulation of Nanoparticle in the living organism and lower toxicity and thus making the naps of nanosize to be friendlier to the biological organism. The results of the current study were to synthesize CuO NPs by Hydrothermal method followed by Nickel doping and lastly, to determine the biological application of the same [
4].
2. Materials and Methods
2.1. Synthesis of (CuO) Nanoparticles
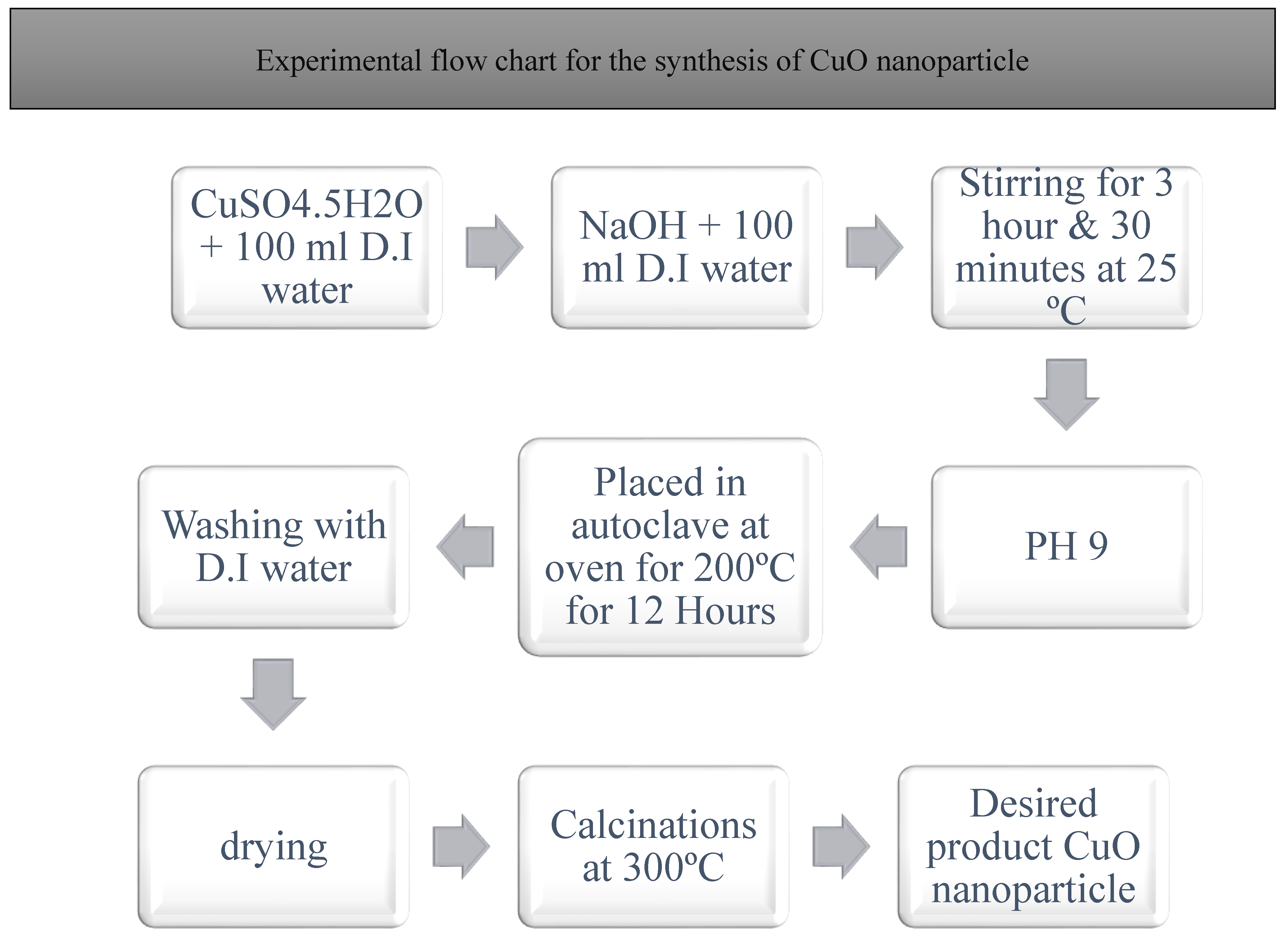
The nano sized of copper oxide nanoparticle was synthesized by the aid of hydrothermal chemical route. It can be used to deliver adequate quantity of as well as the required shape of the required material. It is also a single-step process, and the presence of the surfactant had no effect on regulating morphology of CuO NPs. What is shown in the
Figure 1 is known as the hydrothermal chemical method of production of the CuO nanoparticles. In the first place the definite number of 6 profitable means is required to arrange the work and create the good conditions to students. Copper sulphate pentahydrate purchased at the market was weighed thirty times in a highly sensitive digital weighing scale of 25g. The obtained potassium iodide was then dissolved in 100 millilitre’s of two-stage its double decomposition of water in the fire-polished round-bottom flask 500 ml. Thereafter, test tubes were used. The solution that is prepared is stirred at the rate of 8000 RPM in an (oil bath) until the CuSO
4 5/2 O dissolves in the mixture of double deionised water. This solution is called Solution-1 like shown under the Fig A above. Likewise, final volume of 0 is in the following formula: 1M of sodium hydroxide (NaOH) solution is then sequentially set in another flask. The 0.3g of this sample is completely melted in 50ml of the double deionized water, and the sounding of the solution occurs approximately 30 min in an ultrasound bath. The final-ready clear solution of sodium hydroxide, NaOH is identified as the Solution-2 as per the Fig. A as it has been above mentioned. This fitted burette has a double walled to allow efficient heating and it is taped to a round bottom flask, to which solution-2 is pipette into. To accomplish the reaction, an argon gas filled hot-air balloon was attached on top of the burette to seal the interior ambiance. A solution of NaOH that is concentrated was prepared and then by slow and drop wise manner it was added to the ready copper sulphate solution. To this end the colour of the reaction mixture was noted to turn to a dark brown solution and subsequently becomes transparent when further amounts of NaOH solution are added.[
7].
2.2. Doping of CuO NPs
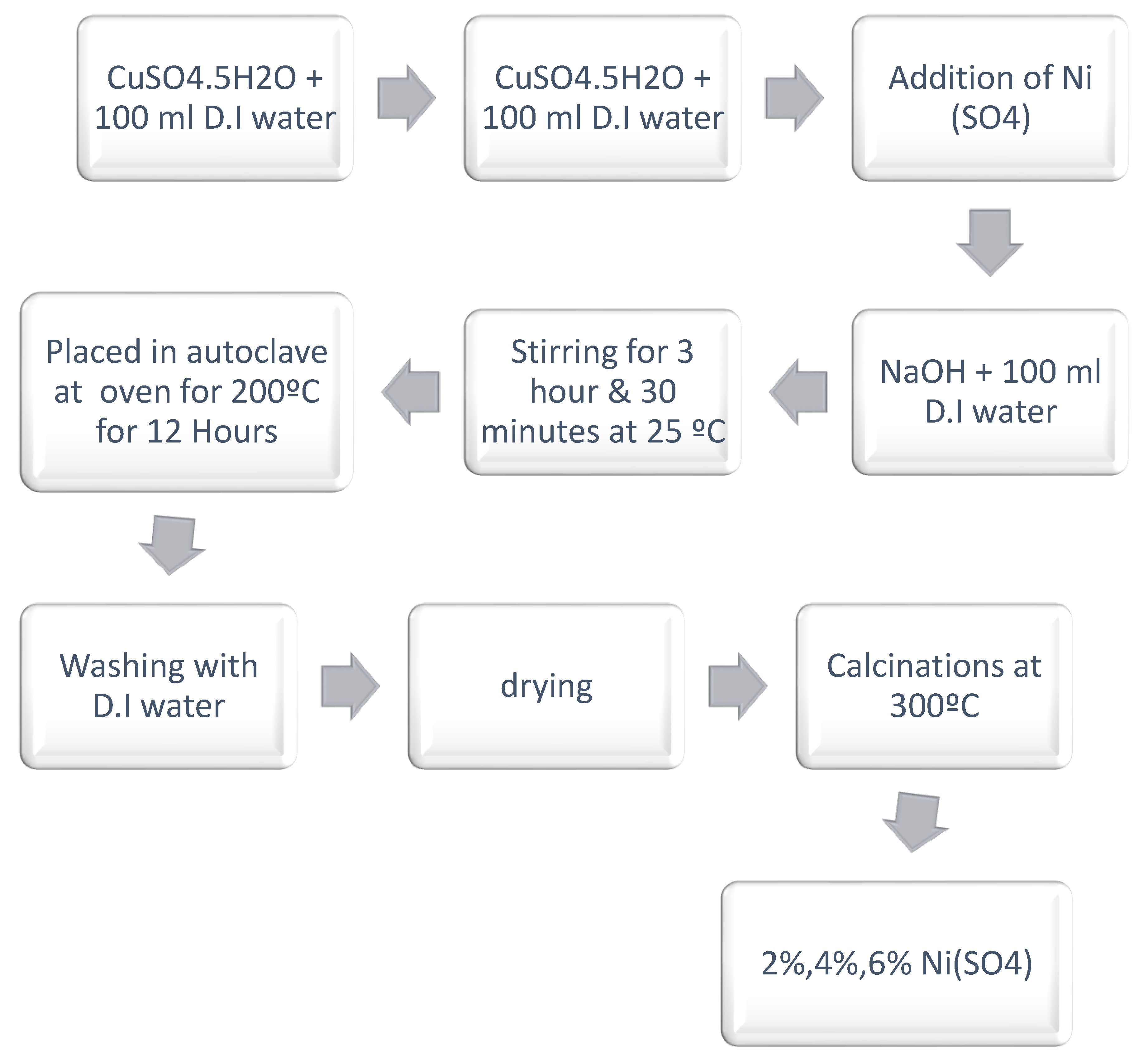
The design of the doping of copper oxide nano scale particles is provided in
Figure 2. In the case of Doping, CuO NPs in the nano form were dispersed in distilled water (labeled Solution A). Also, in another flask Solution B that has Nickel sulphate (MW of 4000) in it was dissolved using distilled water. Then, two solutions were combined, and final mixture was transferred magnetically into same occasion and was stirred at room temperature 48 hours and at medium pace. The solutions thus obtained were then placed in autoclaves oven and allowed to stay till it dried up at 200 , this assisted in Nickel doped CuO NPs formation[
8].
Figure 2.
Represent schematic’s presentation of (CuO) NPs synthesis via hydrothermal method.
Figure 2.
Represent schematic’s presentation of (CuO) NPs synthesis via hydrothermal method.
Figure 3.
Represent schematic’s presentation doping of Nickel.
Figure 3.
Represent schematic’s presentation doping of Nickel.
2.3. Characterization of (CuO) NPs
The last step of (CuO) NPs synthesis procedure was identified by X-ray diffractometer basically known as the XRD system. A Copper CuKalpha1 source was used as an X-ray source that releases radiations at 25 o C (wavelength, lambda = 1. 5406A). The results of the particle size of copper oxide under transmission electron microscope (TEM) were investigated using the parameters below: nanoparticles. These are Model JEM-2100 of JEOL who is based in Japan. Optical band gap was calculated by recording UV/ Vis spectrum of doped and non-doped CuO NPs using a range based UV / Vis spectrometer (200 to 800 nanometer.). Using a spectroscopic approach characterized as Fourier transforms infra-red (FTIR), the functional coordination of Cu with O that comprises the synthetic groups of the CuO NPs was found out[
9].
2.3.1. X-Ray Diffraction (XRD) Analysis
In this JDX-3532 monochromatic Cu ka radiation (wavelength=1.5418Aº), our sample was examined utilizing the XRD technique. At 2θ angel range, 0° to 160º pattern, it functions at 20–40kV and 30 mA. The scanning ended in this range, which was 0° to 160°[
10].
2.3.2. Transmission Electron Microscopy (TEM) Analysis2.3.3. Fourier Transform Infrared (FTIR) Spectroscopy
The functional group of CuONPs as well as Ni(CuO) NPs were detected through by using FTIR Cary630.Here the range of wavelength was 4000-350 cm
-1.Spectra of emission was obtained through by using lf-45 fluorescence spectrophotometer[
11].
2.3.4. UV–Visible Spectroscopy
With the help of UV-Vis spectrometer (Shimadzu UV-1601), absorbance spectrum of CuONPs as well as Ni(CuO) were achieved . Uv machine range of absorbance spectrum was 200-800nm[
12].
2.4. Antibacterial Activity Assay
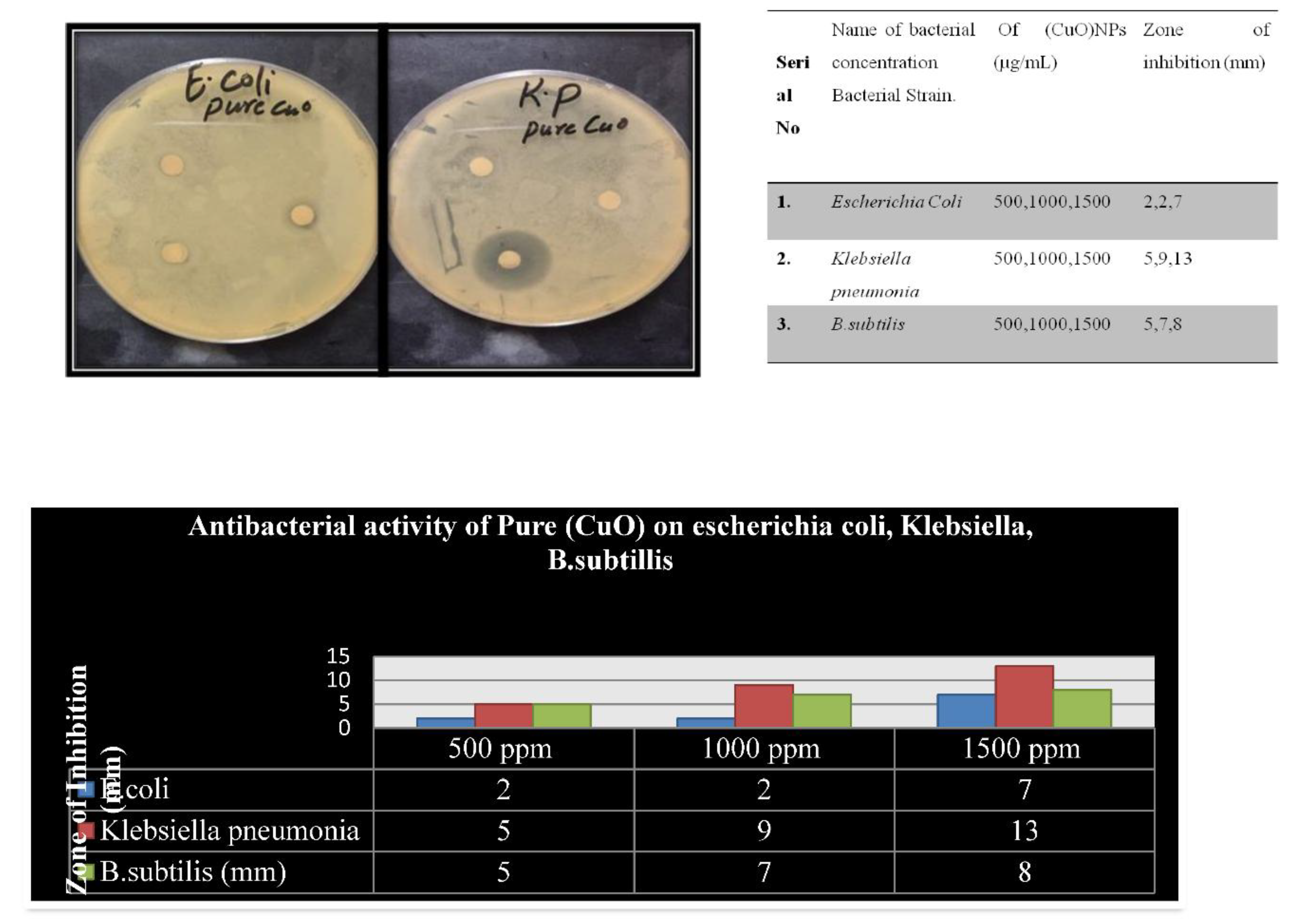
Procedures of antibacterial activity were employed to ascertain the ability of the synthesized NPs in reducing pathogenic microorganisms. Three concentrations of achieved nanoparticles at 500μg/ml, 1000μg/ml, and 1500μg/ml were inspected in the agar well diffusion method. With respect to region of Antibacterial activity procedures were used to determine the capacity of the synthesized NPs in the pathogenic microorganism reduction. Agar well diffusion method was used to inspect three concentrations of achieved nanoparticles that were 500lugr/ml, 1000lugr/ml and 1500lugr/ml. Additionally, as far as region of inhibition is concerned, there was the identification of antibacterial activity. However, as shown in
Figure 4(c), the antibacterial activity seems to show the highest act against the Klebsiella pneumonia using both the natural NPs and the doped (CuO) NPs compared to the other remaining bacteria[
13].
2.5. Hemolysis Assay
The pure and the doped-CuO did not cause any harm on the doses of 30, 50 and 70 ug/ml, whereas on plain CuO NPs, hemolysis was 1.8, 2 and 4 at 30, 50 and 70 ug/ml respectively and on Ni(CuO) NPs, the percent hemolysis was 0.79, 1.1 and 1.5 respectively[
14].
2.6. Brine Shrimp Cytotoxicity Bioassay
The mortality rate differed with the concentration of the pure CuO NPs at (500, 1000, and 1500 mu g/ml) and Ni(CuO) NPs at (500, 1000, and 1500 mu g/ml)[
14].
3. Results
3.1. XRD Analysis
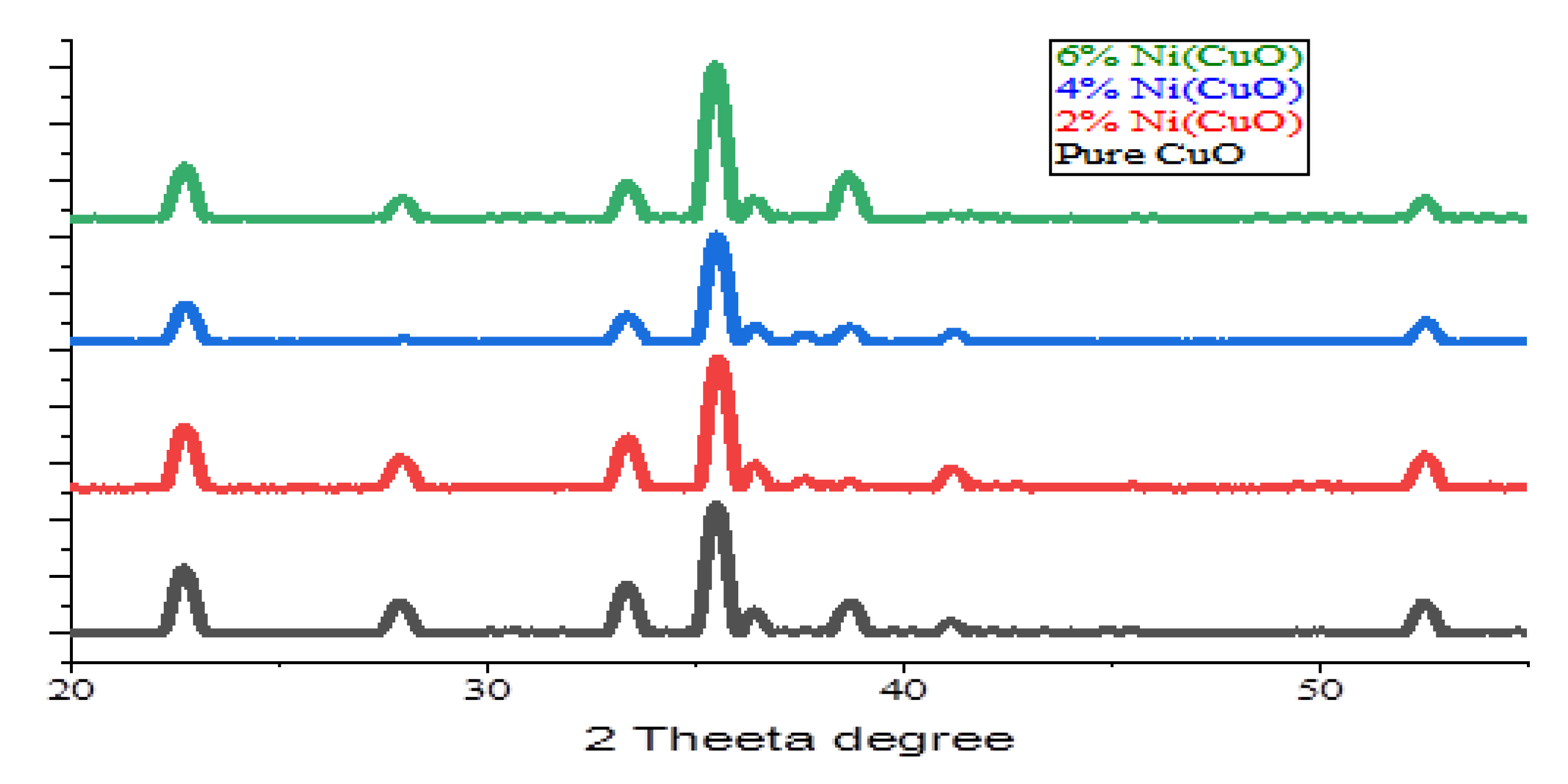
Figure (4) depicts that X-ray diffraction pattern of CuO-NPs. A red color pattern shows the data of the cooper oxide standard XRD. In XRD pattern, each of the peaks was indexed using JCPDS card number 36-1451. As seen in the picture, it was clear that all the peaks of the XRD pattern that was characteristic of the CuO NPs were in perfect conformity with the standard CuO x-ray powder diffraction data. The absence of any other peak signifies (CuO) NPs syntheses using hydrothermal method that the formation of polycrystalline copper oxide nanoparticle is taking place in one phase. By broadening of ZRD signal, the size of components of CuO powder is on nano regime and smaller. The average grain size or crucial crystallite size of (CuO) NPs was calculated with the help of the Scherer equation below.
In this example, the size of synthesized nanoparticles described by (D), The Debye- Scherer constant (9.8 in case of a spherical particle(s)), the wavelength of the XRD source (0.15406 nanometer), FWHM and 0 (peak location in radians). Origin software, therefore, measured the positions of the peak and FWHM of every crystalline peak. Therefore, the usage of equation [
15]. It was noted that the average primary crystallite size of the carbon nano particle was 8.0 nanometre.
3.2. TEM Analysis
Transmission electron microscopy (TEM) was used to assess both the form and the size of the (CuO) NPs. The TEM images of CuO nanoparticle at the higher and lower magnification scales are elaborated in
Figure 4 (A) and 4(B) respectively. The low-magnification image of the nanoparticle reveals that (CuO) nanoparticle has the shape of a sphere and size is regular. Consistent with the implications of the XRD study, an experimental average particle size of the range; 9.5nm is evidently obtained in
Figure 4(b)[
10].
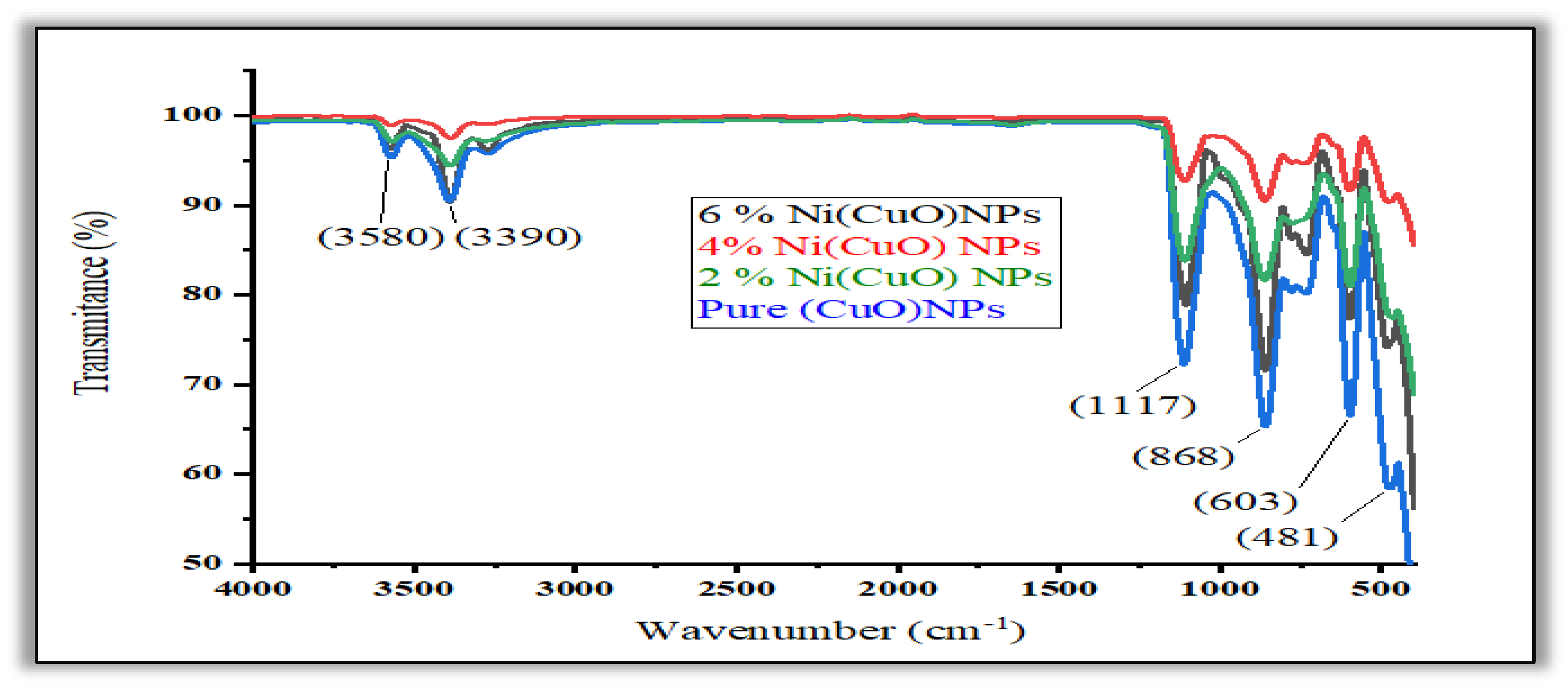
3.3. FTIR Analysis
The presence of (F.G) functional groups in the synthesized CuO and the doped CuO NPs was identified by using Fourier Transform Infrared Spectroscopy (FTIR) that is illustrated in
Figure 4(a). Conversely, a peak showed up at 603 cm-1 which implied the existence of typical absorption band of CuO metal oxide. The synthesis of CuO nanopreciptates was confirmed by its maximum appearing in 481 cm-1. The stretch mode absorptions of the O-H group at the nickel-CuO region between 3390 and 3580cm-1 can be attributed to the peak. The bending of long polymeric chains would be one of the possible explanations to the peaks between 868 and 1117 cm-1.[
16].
Figure 5.
FTIR spectra of (CuO) and Ni(CuO) Nanoparticle.
Figure 5.
FTIR spectra of (CuO) and Ni(CuO) Nanoparticle.
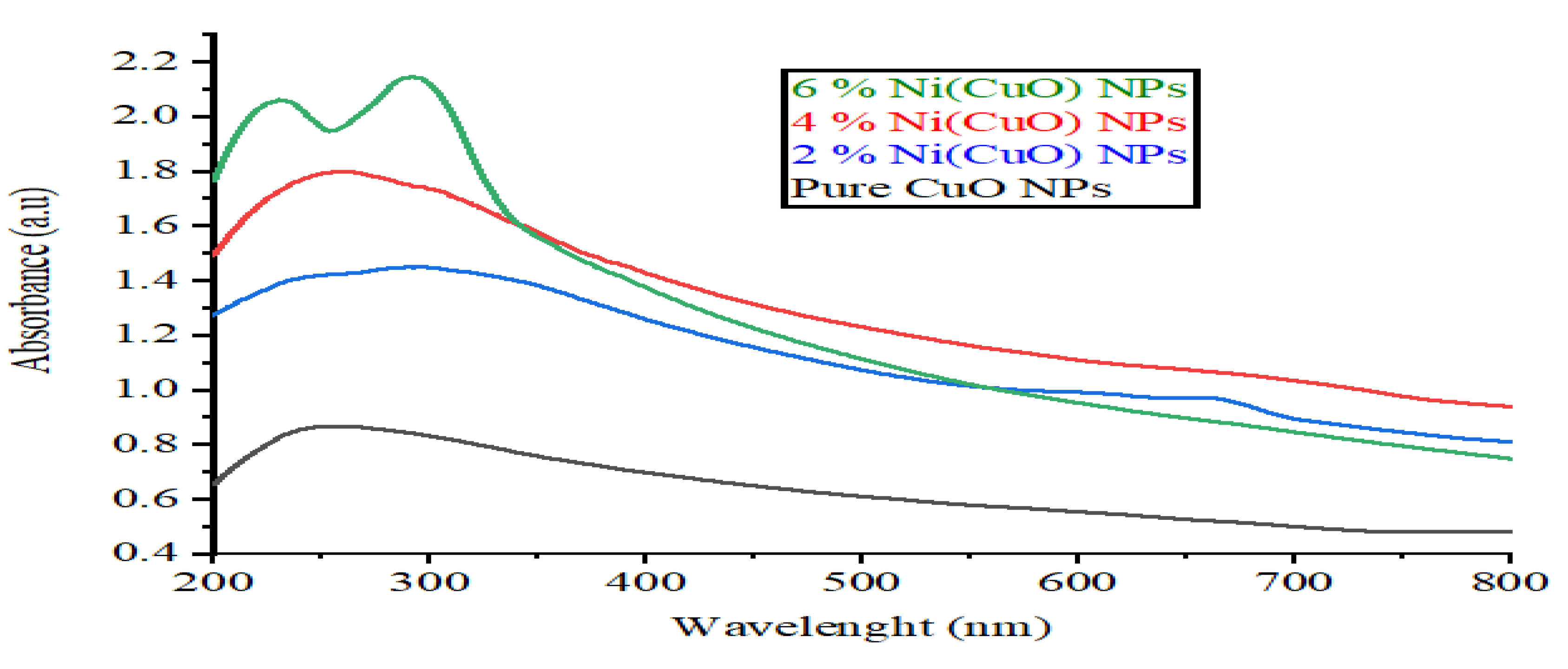
3.4. UV–Vis Spectroscopy Analysis
Absorption spectra of doped and non-doped (CuO)NPs were represented in
Figure 4(b). In the two spectra, wavelength of 256 nanometer implies maximum absorption in the range of UV-visible. Compared to the doped CuO-NPs, CuO-NPs have shown greater absorption capacity in binding the Ni molecules which have been effectively deposited on the surface of the doped CuO nanoparticle as seen on the reduction on the photon absorption in the UV- visible region of those particles. There is a probability of red shifting in the wavelength absorption because Nickel polymers that are deposited on the (CuO) side can minimize the degree of the strength of the copper oxide CuO NPs absorption. The UV-vis incorporation spectra of CuO particles with and without doping is displayed in the
Figure 4(b). As can be observed in the figure of CuO nanoparticles, you will find out that there is a broad absorption peak at 292nm and this absorption peak is able to relate to the band gap energy of 1.2 and 2.0 eV. On the other hand, the absorption of the doped-CuO NPs carries the band gap energy of approximately (1-1. 5eV) and located at nearly 292 nm[
17].
Figure 6.
UV spectra of (CuO) and Ni(CuO) Nanoparticle.
Figure 6.
UV spectra of (CuO) and Ni(CuO) Nanoparticle.
3.5. Antibacterial Activity Results
To establish the antibacterial efficacy of the designed NPs, well diffusion method was done for all the three MDR bacterial types, namely
Escherichia coli, Klebsiella, B.Subtillis. MacConkey Agar was inoculated with bacterial strains’ broth cultures which were spread evenly. The nanoparticle sample solution was dispensed into the wells, and the plates were exposed to sunlight for a few minutes to initiate the activity of NPs. The next day, the zone of inhibition for each strain was assessed[
18]. k.p.4% coli, 4% k.p, 4% B, 6% coli, 6% k.p., and 6% B.subilites, respectively. Zone inhibition was assessed on the third day, one day (24 hours)[
14].
3.6. Hemolysis Assay Results
The process of dispersing sea salt in double distilled water yielded brine shrimp hatching media. The prepared medium was used to raise brine shrimp for 48 hours.After that, six (6) petri dishes containing twenty (20) live larvae each were taken out of the medium. Different petri plates were filled with NPs solutions at different concentrations (100, 1000, and 1500μg/ml), and incubated at 25 °C for 24 hours under controlled lighting and suitable aeration[
19].
3.7. Brine Shrimp Cytotoxicity Results
With sea salt being dissolved in double-distilled water, a brine shrimp hatching medium is produced. Brine shrimp were grown in the prepared medium during 48 hours. Six (6) petri dishes with a concentration of twenty (20) live larvae each was thereafter removed out of a medium of communication. Best petri plates were introduced with NPs solutions at various concentrations (100,1000 and 1500ug/ml) and incubated at 25 C were maintained under adequate ventilation and controlled light for 24hours[
20].
4. Discussion
The need of our study carried out with the goal of making pure and Ni(CuO) NPs of desired size to carry a bio- application . Hence, it was not only necessary to determine the efficacy of the two different types of NPs in fighting against three different strains of bacteria that are well known to be drug resistant[
4]. Findings that seemed promising about the study were that the following had been identified: As research works have indicated, CuO nanoparticles with other linked properties of signal transduction, material transport, energy metabolism, and other biochemical processes have proven to be anti-E.c oli properties out of all CuO NPs that had been doped, the non-doping method exhibited superior antibacterial activity with reference to all the types of bacteria over non-doped CuO NPs
Figure 4(c) Antibacterial activities of Pure (CuO) nanoparticle. [
21]. It seems that Ag-dop-CuO NP, and the other modification, has been found to be more effective against Streptococcus than Ag-dop-CuO NP due to the fact that particle size of CuO NPs that we synthesized was small enough and thus has shown elevated antibacterial properties due to the ability to generate ROS in bacterial cells; ROS production in prokaryotic cells is strongly connected with the decrease in the particle size[
4]. Increases in populations of Zn+ 2 in the living cells cause crushing of the bacterial cell as mentioned by other articles published. The nickel doping should enhance the number of time our nanoparticles remain in the bacterial cell which results to better lysis of the cell and better antibacterial capabilities. Another rather interesting discovery was the fact that the ROS produced by the nanoparticles (NPs) were bacteriocidal even though they were less toxic to the human blood cells. Tam et al has carried out a study with an aim of determining how well CuO nanorod can combat gram positive B bacteria (atrophies) & gram negative E.coli. The reason according to them is the destruction of the membrane. The reaction occurred between the CuO nanorods and the hydrogen peroxide to create the radicals that exposed the bacterial cell walls and left the bacteria dead; this was attested by the CL test. The other causative agent that is yielding positive results with CuO NPs as an antibacterial agent is anti-capsulation of multidrug-resistant (MDR) pathogen Acinetobacter baumannii (A. Baumannii) which inhibited both biofilms and capsules triggered by A. Gentamicin and NPs of CuO that had a synergistic perspective that boosted the effects of the antibiotic. Lately, the genes involved in capsules biosynthesis (mexX, espA and Ptk) have been found reduced by CuO NPs. The application of CuO NPs served as a potent effective agent against A. baumannii since the generation of dangerous ROS caused the cell membrane to be distracted and led to the loss of the cell constituents including the reducing sugars, proteins, and DNA[
22]. The research carried out by Vishvanath Tiwari and his team embraced the application of CuO NPs on the MDR pathogen which is A. baumannii. Reduced amount of sugars, proteins and DNA was observed to be Leaked out of the bacterial membrane when CuO NPs were added and this was the reason why they could be destructive against this deadly bacterium. As evidenced in the discussion above, it was clarified that the leakage of this membrane was due to the generation of ROS. The curiosity of the non-hemolytic 35 CuO NPs at various concentration was satisfied by Dhaneswarwewr Das and colleagues. They speculated that the CuO NPs portrayed lesser percentage of their TOX at 2 percent relative to other researches conducted by other scientists previously. Some acts that would require the utilization of rTM are 5 percent hemolysis of 2 in concentration. Therefore, the response of two enzymes vis-a-vis Horse RBCs demonstrates that the hemolytic activity of both enzymes is almost insignificant that is 5 mg/ml. As it has been assumed, the hemolysis test is quite trustworthy in case of an assessment of a type of nanoparticle biocompatibility. The compound material also displayed small swelling at equilibrium but the finding was much less than the NICKEL-doped sample which just increased by about 1%. The maximum tolerated dose seemed to be at 5 percent hemolysis and 70 μ g /ml and therefore MTD of our samples was at most of the doses. Inasmuch as the 5 percent is a legal level, to that end then, by this token we can say that the samples mentioned above were biocompatible. According to our findings indicating weak hemolytic activity of (CuO) NPs, the studies were performed in the past. The last and most evidently, in NICKEL-doped samples, the hemolysis rate was even lower, which constitutes to the fact that our manufactured nanoparticles are highly biocompatible in the permitted portion of 5 per cent. The work published and which showed the Cytotoxicity of the phytogenic CuO NPs was the one done by Krishnasamy and associates. Once again, relying on the evidence of the copper ion generation, that caused the death of the cells, they were able to prove that 51. The cytotoxicity of A549 cells with significance was determined at 25 mu g/mL. Additional studies have also clarified that CuO NPs interfere with the rotation of the cells during G1 phase hence placing it on hold. Therefore, the results that were attained in this research work correspond to the earlier studies concerning the effect of CuO NPs on cytotoxicity. Jasim and other researchers were also interested in the cytotoxic effects of CuO NPs on colon cancer cell lines. According to what they have found, CuO NPs has a potential to be internalized into the tumor cells due to its small size and the properties of the surface area. Applying MTT test in finding out the growth of the tumor, it was established that the maximum level of the anticancer activity line against cancer cells was achieved using dilution values of 15 µg/mL and 8 µg/mL though it was highly toxic. Considering these results, there is a possibility that CuO NPs may be used as anticancerous drugs in the management of cancer in the future.
Figure 7.
Figure and table shows the results of antibacterial assay for (CuO) NPs.
Figure 7.
Figure and table shows the results of antibacterial assay for (CuO) NPs.
5. Conclusion
Our finding in turn, shows that CuO NPs are perspective to produce anticancer medications since we have observed very similar perspectives about the fatal dose at the approximate dose. CuO-folic acid and treatment of the glioblastoma cells using the complex have demonstrated a very high degree of cytotoxicity with cell death time dependent and dose dependent manner. Medical applications of CuO through biological applications have been achieved as indicated in the following content: CuO is in this way proposed to non-o-medicine among which it is one of the best competitors. The final observation, as suggested in the present paper, is that the prime goal was to obtain the resultant minimum quantity of CuO NPs through the application of the modified hydrothermal chemical method. The doped constituent in this process was nickel sulphate otherwise called Ni (SO4). These NPs whose overall average size was nearly 9.8 nanometre and were clustered were produced and their size and shape were verified and assured by the use of state of art mechanisms.
The antiseptic inquiry against both pure and nickel loaded NPs was tested on multi-drug resistant bacteria like Escherichia coli, Klebsiella, Subtills, and so on. An astronomical concentration of 500, 1000 and 1500 8g/ml was utilized in the test and the results derived were significant; both the forms of NPs showed antibacterial activity in the test pathogens. What is more important, they discovered that NPs doped with nickel were much more efficient than others. Besides, the Nps through preparing by blend process were also evaluated in terms of their cytotoxicity effects on human red blood cells and the percentage of hemolysis was noted to be low. Composition of the doped NPs with nickel enhanced the biocompatibility of the NPs effectively. This paper has also explained the ability to use these NPs in the PDT process as cancer treating photosensitizers.
When the NPs were treated it was discovered that the tumor cell produced ROS and underwent apoptosis and death. This flattering property confirms them in PDT towards treatment of cancer. The information retrieved was interpreted with focus on antibacterial activity CuO NPs, low cytotoxicity of CuO NPs on human blood cells and mechanic of action of CuO NPs. The study also indicated that the tests of hemolysis are an important aspect of the puzzle in interpreting the biocompatibility and the developed NPs and especially nickel-doped are non-toxic.
It was also explored the way these NPs impacted on the other cell lines which included A549 and the colon cancer cells in a cytotoxic way. The results indicated their possible cytotoxicity and potential applications in anticancer. This study went further to look at the effects of such NPs on glioblastoma cells, which show evidence of their cytotoxicity effects as time goes by and at the various amounts present. This detailed research review brings into sharp focus the utilization of CuO NPs in the oncological direction, as well as biocompatibility and safety across various living systems, in addition to its ability applications in anti-skin cancer and anti-bacterial activity. The observations, in turn, indicate that these NPs have a chance to enhance human health together with quality of life and facilitate the exploration of new and varied applications in biological settings.
So, in conclusion, a careful examination of this research topic allows realizing the numerous applications of CuO nanoparticles that involves them in fighting germs, in biocompatibility or even in anti-cancer activity. The mechanism of action helps us understand the therapeutic potential of CuO NPs better, which is supported by studies of comparison and cytotoxicity analysis. A greater benefit of our manufactured nanoparticles may occur through even more research into targeted treatment.
Conflicts of Interest
There is no conflict of interest on the behalf of all authors
References
- Malik, S. , Muhammad, K., & Waheed, YNanotechnology: A revolution in modern industry. Molecules, 2023, 28, 661. [Google Scholar]
- Chinemerem Nwobodo, D. , Ugwu, M. C., Oliseloke Anie, C., Al-Ouqaili, M. T., Chinedu Ikem, J., Victor Chigozie, U., & Saki, M, Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. Journal of clinical laboratory analysis, 2022, 36, e24655. [Google Scholar] [PubMed]
- Morrison, L. , & Zembower, T. R. Antimicrobial resistance. Gastrointestinal Endoscopy Clinics 2020, 30, 619–635. [Google Scholar]
- Naz S, Gul A, Zia M, Javed R. Synthesis, biomedical applications, and toxicity of CuO nanoparticles. Applied Microbiology and Biotechnology. 2023, 107, 1039–61. [Google Scholar] [CrossRef] [PubMed]
- Sharma S, Sudhakara P, Singh J, Ilyas RA, Asyraf MR, Razman MR. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers. 2021, 13, 2623. [Google Scholar] [CrossRef]
- Zarenezhad E, Abdulabbas HT, Marzi M, Ghazy E, Ekrahi M, Pezeshki B, Ghasemian A, Moawad AA. Nickel Nanoparticles: Applications and antimicrobial role against Methicillin-Resistant Staphylococcus aureus infections. Antibiotics. 2022, 11, 1208. [Google Scholar]
- Malik, S., K. Muhammad, and Y. Waheed, Nanotechnology: A revolution in modern industry. Molecules 2023, 28, 661. [CrossRef] [PubMed]
- Chinemerem Nwobodo, D. , et al., Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. Journal of clinical laboratory analysis, 2022, 36, e24655.
- Morrison, L. and T.R. Zembower, Antimicrobial resistance. Gastrointestinal Endoscopy Clinics, 2020, 30, 619-635.
- Naz, S. , et al., Synthesis, biomedical applications, and toxicity of CuO nanoparticles. Applied Microbiology and Biotechnology, 2023, 107, 1039-1061.
- Sharma, S. , et al., Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers, 2021, 13, 2623.
- Zarenezhad, E. , et al., Nickel Nanoparticles: Applications and antimicrobial role against Methicillin-Resistant Staphylococcus aureus infections. Antibiotics, 2022, 11, 1208.
- Arun, K. , et al., Surfactant free hydrothermal synthesis of copper oxide nanoparticles. Am. J. Mater. Sci, 2015, 5, 36-38.
- Al-Amri, S. , et al., Ni doped CuO nanoparticles: structural and optical characterizations. Current Nanoscience, 2015, 11, 191-197.
- Pike, J. , et al., Formation of stable Cu2O from reduction of CuO nanoparticles. Applied Catalysis A: General, 2006, 303, 273-277.
- Mobarak, M.B. , et al., Synthesis and characterization of CuO nanoparticles utilizing waste fish scale and exploitation of XRD peak profile analysis for approximating the structural parameters. Arabian Journal of Chemistry, 2022, 15, 104117.
- Rifani, N.D. , et al., Synthesis, characterization, and antimicrobial properties of copper oxide nanoparticles produced by pulse laser ablation method in chitosan solution. Journal of applied research and technology, 2023, 21, 196-204.
- Ramyadevi, J. , et al., Copper nanoparticles synthesized by polyol process used to control hematophagous parasites. Parasitology research, 2011, 109, 1403-1415.
- Amiri, M. , et al., Antimicrobial effect of copper oxide nanoparticles on some oral bacteria and candida species. Journal of dental biomaterials, 2017, 4, 347.
- Naatz, H. , et al., Safe-by-design CuO nanoparticles via Fe-doping, Cu–O bond length variation, and biological assessment in cells and zebrafish embryos. ACS nano, 2017, 11, 501-515.
- Lanje, A.S. , et al., Synthesis and optical characterization of copper oxide nanoparticles. Adv Appl Sci Res, 2010, 1, 36-40.
- Zayyoun, N. , et al., The effect of pH on the synthesis of stable Cu 2 O/CuO nanoparticles by sol–gel method in a glycolic medium. Applied Physics A, 2016, 122, 1-6.
- Vinothkumar, P., et al., Effect of reaction time on structural, morphological, optical and photocatalytic properties of copper oxide (CuO) nanostructures. Journal of Materials Science: Materials in Electronics 2019, 30, 6249–6262.
- Mishra, A., et al., Metal nanoparticles against multi-drug-resistance bacteria. Journal of inorganic biochemistry 2022, 237, 111938. [CrossRef] [PubMed]
- Walstad, D. , HATCHING and GROWING BRINE SHRIMP (Artemia).
- Gandhi, A.D. , et al., Panchagavya mediated copper nanoparticles synthesis, characterization and evaluating cytotoxicity in brine shrimp. 2019.
- Ivanova, I.A., et al., Copper and Copper Nanoparticles Applications and Their Role against Infections: A Minireview. Processes 2024, 12, 352. [CrossRef]
- Al-Kadmy, I.M., et al., Anti-capsular activity of CuO nanoparticles against Acinetobacter baumannii produce efflux pump. Microbial Pathogenesis 2023, 181, 106184. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).