1. Introduction
Mitochondria are double membrane-bound organelles in most eukaryotic cells that are primarily responsible for energy production through the oxidative phosphorylation (OXPHOS) system. In addition to their role in cellular bioenergetics, mitochondria are involved in a wide range of important biological processes, including the regulation of intermediary metabolism, calcium homeostasis, ferroptosis, apoptosis and aging [1-3]. Mitochondria possess their own genome (mtDNA), which encodes several essential proteins required for the assembly and function of the electron transport chain (ETC). In the fission yeast Schizosaccharomyces pombe (S. pombe), the mtDNA is ~19 kb in size and encodes seven key subunits of the ETC (cytb of complex III; cox1, cox2, and cox3 of complex IV; atp6, atp8, and atp9 of ATP synthase), two rRNAs (rns and rnl), one small subunit of ribosomal protein (rps3), and a full set of tRNAs [4].
The expression of mtDNA-encoded proteins relies on a dedicated mitochondrial translation machinery, which is more closely related to the bacterial counterpart than to the cytosolic one, reflecting the endosymbiotic origin of mitochondria [5,6]. Unlike bacterial translation initiation, which requires three essential initiation factors (IF1, IF2 and IF3), mitochondrial translation initiation typically involves only two initiation factors, mtIF2 and mtIF3 [7,8]. Mammalian mtIF2 proteins contain a unique 37-amino-acid insertion domain that exhibits functional similarities to bacterial IF1 and is required for efficient mitochondrial translation in vitro [9]. S. pombe contains two mitochondrial translation initiation factors, Mti2 (mtIF2) and Mti3 (mtIF3), with Mti2 playing a more critical role. Deletion of mti2 disrupts mitochondrial protein synthesis and impairs mitoribosome assembly [10]. A recent study has further demonstrated that the insertion domain of Mti2 is critical for its proper folding and for maintaining mitochondrial translation efficiency in S. pombe [11]. Another distinct feature of mitochondrial translation is the requirement for translational activators, which function in conjunction with the initiation factors. In the budding yeast Saccharomyces cerevisiae, activators such as Sov1, Cbp1, Pet309, and Mss51 regulate the translation of specific mitochondrial mRNAs [6]. Similarly, Ppr10 and Mpa1 are key activators required for efficient translation in S. pombe [12,13]. Due to the essential roles of mitochondria in cellular homeostasis, mitochondrial dysfunction is associated with various human diseases, including neurodegenerative disorders such as Parkinson’s and Alzheimer’s [14,15].
Mitochondrial dysfunction can impact nuclear transcription through retrograde (mitonuclear) signaling pathways, enabling mitochondria to communicate with the nucleus and trigger adaptive gene expression programs, a process known as the retrograde response [16-18]. The retrograde response enables cells to maintain cellular homeostasis by compensating for mitochondrial problems, such as impaired oxidative phosphorylation or loss of mitochondrial membrane potential. The retrograde response is conserved across yeasts, mammals, and other eukaryotes [19-21]. For example, in S. cerevisiae, chemical inhibition of ETC complexes induces a retrograde response [22], and in S. pombe, mutations in respiratory genes lead to similar coordinated changes in nuclear transcription [17,23].
Cells can also adapt their carbon metabolism in response to available sugars in the environment using nutrient-sensing regulatory networks. Glucose is the preferred carbon source for most cells, and many microorganisms have evolved regulatory mechanisms to suppress the utilization of alternative carbon sources when glucose is available. This process is referred to as carbon catabolite repression (CCR), enabling cells to respond to changing environmental carbon sources by adjusting the expression of genes involved in sugar transport, carbon metabolism, and cell growth [24-26]. In S. pombe, the CCR is mainly mediated by two conserved C2H2 zinc finger transcription factors, Scr1 and Rst2 [27-29]. Scr1 functions primarily as a transcriptional repressor under glucose-sufficient conditions, and its activity requires the corepressors Tup11 and Tup12 to establish full repression. Scr1 represses the expression of fbp1 in glucose-sufficient conditions. Upon glucose depletion, Scr1 is hyper-phosphorylated by the AMP-activated protein kinase (AMPK) Ssp2, leading to its export from the nucleus. This enables the transcriptional activator Rst2 to bind to the promoter of fbp1, thereby activating its expression [27-29]. ChIP-seq analysis of Scr1,Rst2, Tup11 and Tup12 suggests a model by which the transcriptional network governing carbon metabolism involves a dynamic balance between Scr1-mediated repression and Rst2-mediated activation at shared target genes [26].
Here, we investigate whether and how the inhibition of mitochondrial translation affects the nuclear transcriptome of S. pombe in different carbon sources and growth phases. Proliferating S. pombe cells will grow mainly by fermentation in standard glucose medium, and once glucose becomes limiting, they will enter a non-dividing state (stationary phase), when mitochondrial respiration increases. Stationary phase and other non-dividing cell states have been much less studied than cell proliferation. We find that only during stationary phase in glucose medium, inhibition of mitochondrial translation triggers an extensive transcriptional reprogramming of carbon metabolism, along with a general stress-like transcriptional response. We show that Scr1 and Rst2 contribute to this adaptive reprogramming process by regulating the expression of a shared set of genes, many of which are involved in carbon metabolism. In stationary-phase cells, Rst2 functions as a primary transcriptional activator and remains in the nucleus only when mitochondrial translation is inhibited. The retrograde response and CCR have been studied in isolation in S. pombe. Our findings reveal new aspects of the mitochondrial retrograde response and CCR regulators that coordinate gene-expression reprogramming in response to inhibition of mitochondrial translation in stationary-phase cells.
2. Materials and Methods
2.1. Fission Yeast Strains and Growth Media
S. pombe strains used in this study are listed in
Supplementary Table S1. The deletion strains Δ
rst2 and Δ
scr1 were generated by homologous recombination using pFA6a-kanMX6 [30] in the wild-type strain
972 h- background. The
S. pombe Bioneer haploid deletion library v5.0, containing 3420 deletion mutants, was obtained from the Bähler lab stock. The query strains (
h+ rst2::
natMX6) for SGA with a nourseothricin cassette were constructed in a
975 h+ background by overlapping PCR [31]. As a control query strain for SGA, we used a wild-type strain (
h⁺ natMX6@(NC_003421.2:1959559_1959560ins)), with genotype named according to [32], in which a nourseothricin cassette was inserted, hereafter referred to as Natᴿ wild-type. This insertion does not affect the fitness of the wild-type strain (
Supplementary Figure S5). All deletion strains were verified by colony PCR using check primers, with all primers used in this study listed in
Supplementary Table S2. Strains expressing Rst2 C-terminal tagged GFP from its endogenous promoter were generated by overlapping PCR as described [33]. The GFP-tagged strain does not affect cell growth and mitochondrial function (
Supplementary Figure S6).
S. pombe cells were cultured in rich Yeast Extract and Supplements medium (YES: 0.5% yeast extract, 225 mg/L adenine, histidine, leucine, uracil and lysine hydrochloride) supplemented with 3% glucose for fermentative growth or 3% glycerol and 0.1% glucose for respiratory growth. Malt Extract Agar (MEA) media was obtained from Formedium (PCM 0810).
2.2. RNA Sequencing Experiments
The schematic overview of samples for total RNA extraction following CAP treatment is shown in
Figure 1a. Briefly, cells were precultured overnight in YES medium containing 3% glucose. The cultures were subsequently harvested, washed with ddH
2O and diluted with fresh YES containing 3% glucose (Glucose medium, fermentative condition) or 3% glycerol (Glycerol medium, respiratory condition) and 0.1% glucose to an initial OD
600 of 0.2. Chloramphenicol (CAP) was then added to a final concentration of 2 mg/mL, where indicated. To control for any solvent effects, 2% ethanol (EtOH) was added to untreated samples, as CAP was dissolved in EtOH. Cells were then collected for RNA extraction in both exponential (6 hours) and stationary (30 hours) phases. The schematic overview of the Δ
rst2 and wild-type (wt) samples preparation is shown in Figure 5a. Cells were precultured overnight in YES medium, diluted to an initial OD
600 of 0.2, and then harvested for RNA extraction in both exponential (6 hours) and stationary (30 hours) phases. Three independent biological repeats were prepared for each condition.
Total RNA was extracted using the PureLink RNA Mini Kit (Thermo Fisher Scientific) following the manufacturer’s instructions. Cells were homogenized with 0.5 mm acid-washed beads (Sigma-Aldrich) using a FastPrep instrument (MP Biomedicals). RNA was purified with RNA Mini Columns (Qiagen) and digested with DNase I for 15 min at room temperature to remove genomic contamination. RNA quality was assessed using the High Sensitivity D5000 ScreenTape Assay on the TapeStation System (Agilent Technologies). Strand-specific RNA-seq libraries were prepared using poly(A) mRNA enrichment from total RNA samples and sequenced on an Illumina platform with 150 bp paired-end reads in two lanes. Raw FASTQ files were provided for downstream analysis. Sequencing quality was assessed by FastQC (version 0.12.1,
https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Transcript abundance was estimated by quasi-mapping to
S. pombe reference genome sequences and quantified with
Salmon (
https://combine-lab.github.io/salmon/) [34]. Differential expression analysis was performed by DESeq2 (version 1.44.0). A linear model was constructed incorporating different carbon sources (Glucose vs. Glycerol), mitochondrial translation status (Uninhibited vs. Inhibited) and growth phase (Exponential vs. Stationary) as independent factors. The designed model formula used for DESeq2 was specified as “~ Carbon source * Mitochondrial translation status * Growth phase”. The
S. pombe genome sequence and gene annotation files were obtained from PomBase [35]. Gene Ontology (GO) term and KEGG pathway enrichment analysis were performed by DAVID [36,37] with default parameters and visualized by
R (version 4.4.1) [38]. Overlap analysis used in this study was performed using two approaches. Pairwise overlap between two gene sets were assessed by the
GeneOverlap (version 1.40.0) [39] R package. For overlaps among three gene sets, we used
SuperExactTest (version 1.1.0) [40] R package.
2.3. Quantitative Cell Growth Assays in Liquid Culture
Quantitative growth assays were performed using 48-well FlowerPlates in a BioLector microbioreactor (m2p-labs) as previously described [41]. Briefly, wild-type, Δrst2 and Δscr1 strains were precultured overnight in YES media and subsequently diluted to an initial OD600 of 0.2 in fresh YES. After ~4 hours of cultivation to mid-exponential phase, the cultures were further diluted with 1.5 mL of fresh YES media supplemented with either 3% glucose or 3% glycerol and 0.1% glucose to an initial OD600 of 0.02. Mitochondrial translation was inhibited with the treatment of CAP at a final concentration of 2 mg/mL. Cultures were incubated in three replicates at 32℃ with shaking at 1000 rpm and 85% humidity. Growth was monitored in real time, and growth data were normalized to the initial time point. The mean growth curves were fitted, and growth rates and lag period were calculated by grofit [42]. All pairwise comparisons were analysed using one-way ANOVA, followed by Tukey’s Honest Significant Difference test. Data visualization was performed in R (version 4.4.1) [38].
2.4. Spot Assays
The wild-type, Δrst2 and Δscr1 strains were precultured overnight in YES medium at 32℃, and cultures were subsequently diluted to an initial OD600 of 0.2. CAP (dissolved in EtOH) was added to a final concentration of 2 mg/mL to inhibit mitochondrial translation, and 2% EtOH was added as a control for solvent effects. A total of 3 OD600 of cells were collected at 6, 12, 24, 36, 48 h and 10-fold serial dilutions were prepared and spotted onto YES plates. Plates were incubated at 32℃ for 3 days before being photographed.
The spot assays for wild-type strains in the SGA analysis were performed as follows. The wild-type, Δ
ade6 (see Methods 2.6), and Natᴿ wild-type strains were precultured overnight in YES medium at 32℃. Cultures were diluted to an initial OD₆₀₀ of 0.1 and grown for 4 h to ensure collection during the logarithmic growth phase. The OD₆₀₀ was then adjusted to 0.2, and serial dilutions were prepared and spotted onto YES plates, either without additives (control) or supplemented with the indicated stress agents: oxidative stress (0.075% MMS), heat stress (38℃), low glucose (0.1%), non-fermentable carbon source (3% glycerol + 0.05% glucose), ER stress (75 fg/mL tunicamycin), TOR inhibition (5 µM Torin), and rapamycin + caffeine (100 µg/mL + 10 mM). Plates were incubated at 32℃ for 3 days before scanning (Supplemental
Figure S5).
2.5. Chronological Lifespan (CLS) Assays
CLS assays assess the viability of non-dividing cells during ageing by measuring colony-forming units (CFUs) at different time points. CLS assays shown in
Figure 3d were performed as previously described [43]. Briefly, cells were precultured overnight in YES medium at 32℃, diluted to an initial OD
600 of 0.02 and cultured to stationary phase (Day 0). CFUs were measured daily and normalized to the CFUs at Day 0 (100% cell viability). At each time point, aliquots of ageing cultures were collected, 3-fold serially diluted, and spotted onto YES plates using a RoToR HDA pinning robot (Singer Instruments). Three independent biological replicates were prepared for each condition. Plates were incubated at 32℃ for 3 days and subsequently imaged. The scanned images were analyzed using the R package,
DeadOrAlive (
https://github.com/JohnTownsend92/DeadOrAlive) [43].
2.6. Synthetic Genetic Array (SGA) Analysis
A genome-wide genetic interaction analysis was performed to identify the genes that genetically interact with
rst2 and
scr1. The SGAs were carried out as previously described [44]. The deletion strains Δ
rst2 (
h+ rst2::
natMX6) and Δ
scr1 (
h+ scr1::
natMX6) were used as query strains to mate with the
S. pombe Bioneer haploid deletion library v5.0, consisting of 3420 deletion mutants marked with
kanMX6. Traditionally, an
ade6- strain was used as a control strain, but we observed that this strain features some traits different from wild-type, like altered growth and chronological lifespan (Supplemental
Figure S5a,b). We therefore generated a new prototrophic control strain, Natᴿ wild-type (as described in Methods 2.1), which shows the same fitness as the regular wild-type strain (Supplementary
Figure S5c,d). The query strains were mated with the library mutant strains to create double mutant strains on MEA plates. After incubation for 3 days at 25℃ for mating and sporulation and 3 days at 42℃ to eliminate the parental cells, the resulting colonies were selected on YES agar plates containing G418 and nourseothricin. Once colonies were sufficiently grown, they were printed onto either YES or YES containing 2 mg/mL CAP agar plates, followed by incubation at 32℃ for 3 days. The plates were imaged by an EPSON V800 scanner. Each condition was performed in three biological repeats.
The SGA image analysis was conducted in R (version 4.4.1) [38]. The colony sizes were quantified by the R package gitter [45]. Systematic gene IDs were assigned to colony data according to the Bioneer library plate number and row-column position. Small (<50 pixels) and absent colonies were excluded to minimize false positives potentially caused by technical issues such as incomplete library or poorly pinned mutants. The colony sizes were subsequently normalized to reduce spatial and plate-to-plate variation effects by median smoothing and row-column median normalization [46]. Genes located within 250 kb of the query loci were excluded to avoid effects from linked loci. Genetic interaction scores (GIS) were calculated as the log2-transformed ratio between the normalized colony sizes of the mutant strain and the wild-type control. Genetic interaction hits were defined as genes with a GIS beyond the threshold of ±0.15 and a p-value less than 0.1.
2.7. Fluorescence Microscopy
Overnight cultures of cells expressing Rst2-GFP were diluted to an initial OD600 of 0.2 and grown for the indicated hours (6, 12, 18, 24, 30, 36 h) before analysing by fluorescence microscopy. The green fluorescence signals were captured using a Zeiss Axio Imager 2 microscope (Carl Zeiss, Jena, Germany) with excitation wavelengths of 488 nm.
3. Results
3.1. Mitochondrial Translation Inhibition Affects Genome Regulation Mainly During Stationary Phase
To investigate the effect of mitochondrial translation inhibition on the transcriptome in
S. pombe, we performed RNA sequencing before and after treatment inhibiting mitochondrial translation with chloramphenicol (CAP) [47,48]. We sequenced the transcriptomes of cells grown in glucose and glycerol media, both in the exponential and stationary phases. Exponentially growing cells require low mitochondrial respiratory activity in glucose media (fermentative condition), whereas they require high mitochondrial activity in glycerol media (respiratory condition). Stationary-phase cells have stopped proliferating due to carbon source depletion and require higher mitochondrial respiratory activity than exponentially growing cells in glucose media. The experimental design for the eight RNA-seq conditions is illustrated in
Figure 1a. We carried out three independent biological repeats for each condition. The numbers of differentially expressed genes following CAP treatment under each condition are summarized in
Table 1, and the annotated gene lists and transcriptome-wide expression data are provided in Supplementary Dataset S1. Principal component analysis (PCA) based on regularized log (rlog)-transformed normalized expression values of the transcriptome signatures in all conditions revealed that the largest variance in the data is attributed to differences between exponentially growing and stationary-phase cells, irrespective of CAP treatment, while the second-largest variance is based on differences between CAP-treated and untreated cells in stationary-phase cells, particularly in glucose medium (
Figure 1b;
Table 1). In exponentially growing cells, on the other hand, CAP treatment had only a subtle effect on the transcriptome, especially in glycerol medium (
Figure 1b;
Table 1). These results are consistent with the much higher requirement for mitochondrial respiration in stationary-phase cells, particularly in glucose medium. However, with glycerol as the carbon source, high mitochondrial respiration is required even in exponentially growing cells to sustain energy production [49]. Thus, the altered expression of only 23 genes in response to CAP treatment in this condition is surprising. Nevertheless, cell growth was almost completely abolished in CAP-treated cells in this condition (
Supplementary Figure S1), indicating that mitochondrial translation is essential for proliferation in glycerol medium, as expected.
Unlike in exponentially growing cells in glycerol medium, CAP treatment triggered a strong response in stationary-phase cells, with 391 genes showing altered expression (
Table 1). Gene Ontology (GO) and KEGG pathway analysis revealed that the induced genes were enriched in glycolysis/gluconeogenesis as well as genome-maintenance processes, including DNA integration, recombination and polymerase activity, while the repressed genes were enriched in nucleotide metabolism-related pathways and chromatin functions (
Supplementary Figure S2). These results suggest a potential trade-off between energy metabolism and genome maintenance under mitochondrial translation stress.
CAP-treated stationary-phase cells grown in glucose medium showed the strongest transcriptome response, with 888 genes showing altered expression levels (
Table 1). Therefore, the remainder of the results focuses on this condition.
3.2. Mitochondrial Translation Inhibition During Stationary Phase Induces Genes Involved in the General Stress and Retrograde Responses
We compared the 432 genes that were induced in CAP-treated stationary-phase cells grown in glucose medium with the following gene sets induced under other stressful conditions: 1) response to treatment with antimycin A (ANT), an inhibitor of the mitochondrial ETC complex III [17]; 2) mito-nuclear retrograde (MNR) response, comprising genes altered in response to ANT as well as in two respiratory-deficient mutants [17]; 3) the core environmental stress response (CESR), which includes genes responsive to oxidative stress, heavy metal stress, heat shock, osmotic stress, and DNA damage [50]; and 4) the core oxidative stress genes (COSG), which are induced in three different oxidants [51]. We observed significant overlaps between our set of induced 432 genes and all four stress-related gene sets induced in different conditions (
Figure 1c). Various numbers of CAP-induced genes overlapped with different combinations of the other stress-related lists (
Supplementary Figure S3). In contrast, the genes induced in the other CAP-treated conditions did not show significant overlaps with genes induced in any of the four stress conditions, and no significant overlaps were observed among the genes repressed in the CAP-treated conditions.
To further elucidate the biological processes associated with the genes induced in CAP-treated stationary-phase cells in glucose medium, we performed Gene Ontology (GO) and KEGG pathway enrichment analyses. The 432 genes were significantly enriched in terms related to stress response and protein homeostasis processes (
Figure 1d). These enrichments are consistent with the significant overlaps with the responses to other stressful conditions (
Figure 1c). Together, these findings indicate that mitochondrial translation inhibition triggers a transcriptional response similar to that observed in other stressful conditions, but only in stationary-phase cells grown in glucose medium.
3.3. Mitochondrial Translation Inhibition During Stationary Phase Induces Carbon-Metabolism Genes Functioning in Non-Mitochondrial Pathways
When glucose is used as a carbon source (fermentative condition), exponentially growing cells primarily rely on cytosolic glycolysis to convert glucose to pyruvate, which is then fermented to ethanol [52]. During stationary phase, however, cells shift to mitochondrial respiration whereby pyruvate is oxidized through the Tricarboxylic Acid (TCA) cycle and oxidative phosphorylation to generate ATP [53].
Figure 1d shows that the genes induced in CAP-treated stationary-phase cells grown in glucose medium were also significantly enriched in several pathways related to carbon metabolism, including glycolysis, the pentose phosphate pathway, and pyruvate metabolism (
Figure 1d). In contrast, no significant enrichments were detected among the genes repressed in response to CAP treatment. To further characterize this response, we examined all genes associated with major carbon metabolic pathways in PomBase [54], including glycolysis, the TCA cycle, the pentose phosphate pathway, fermentation, and glycerol metabolism.
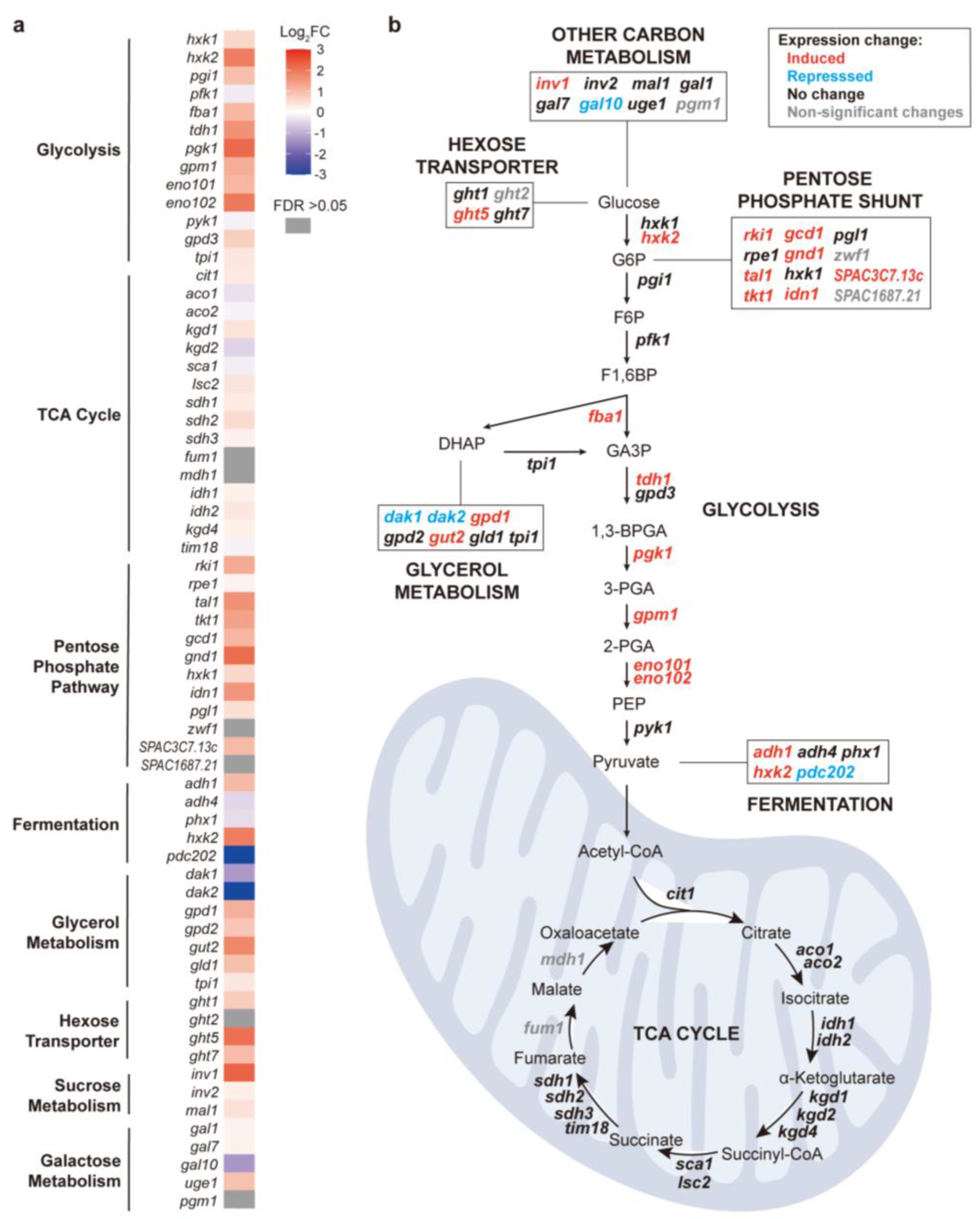
Figure 2a shows the expression changes of all these genes in CAP-treated stationary-phase cells grown in glucose medium, grouped by the different metabolic pathways. Notably, the pathways that include most of the induced genes all function in the cytoplasm, outside of mitochondria and upstream of the TCA cycle, most notably genes functioning in glycolysis and the pentose phosphate shunt (
Figure 2b). Together, these findings indicate that the inhibition of mitochondrial translation activates a substantial metabolic reprogramming that enhances the cytosolic carbon flux, likely as a compensatory mechanism shifting energy metabolism from respiration to fermentation to maintain cellular homeostasis when mitochondrial oxidative phosphorylation is impaired.
Figure 2.
Expression changes of carbon-metabolism genes in response to CAP treatment of stationary-phase cells in glucose medium. (a) Heatmap of expression fold-changes (FC) for genes involved in major carbon-metabolism pathways. The log2FC values are color-coded as indicated, with grey boxes indicating non-significant expression changes (FDR >0.05). (b) Carbon metabolism network scheme to map gene-expression changes in response to CAP treatment in stationary-phase cells grown in glucose medium. Genes are colored according to their log2FC shown in (a): red indicates significantly induced genes (log2FC >1), blue indicates significantly repressed genes (log2FC <-1), black indicates small fold-changes (-1< log2FC <1), and grey indicates non-significant changes (FDR >0.05).
Figure 2.
Expression changes of carbon-metabolism genes in response to CAP treatment of stationary-phase cells in glucose medium. (a) Heatmap of expression fold-changes (FC) for genes involved in major carbon-metabolism pathways. The log2FC values are color-coded as indicated, with grey boxes indicating non-significant expression changes (FDR >0.05). (b) Carbon metabolism network scheme to map gene-expression changes in response to CAP treatment in stationary-phase cells grown in glucose medium. Genes are colored according to their log2FC shown in (a): red indicates significantly induced genes (log2FC >1), blue indicates significantly repressed genes (log2FC <-1), black indicates small fold-changes (-1< log2FC <1), and grey indicates non-significant changes (FDR >0.05).
3.4. The Scr1 and Rst2 Transcription Factors Regulate Common Metabolic Genes Upon Inhibition of Mitochondrial Translation in Stationary-Phase Cells
The CAP-induced gene-expression response appears to be a complex program that likely involves multiple regulatory factors. The Scr1 and Rst2 transcription factors are key regulators of the CCR (see Introduction). ChIP-seq experiments have identified 140 Scr1 and 413 Rst2 target genes in glucose and glycerol media, respectively, where these factors are most active [26]. As expected, these two gene sets share many common target genes (
Figure 3a). To investigate the involvement of Scr1 and Rst2 in the CAP-induced gene-expression response, we performed an overlap analysis between their published target genes and our 432 genes induced in CAP-treated stationary-phase cells grown in glucose medium. These genes showed significant overlaps with both the Scr1 and Rst2 target genes (
Figure 3b). Moreover, many of the carbon-metabolism genes analysed above (
Figure 2) were included among the Scr1 and Rst2 target genes (
Figure 3c). In contrast, the 456 repressed genes in CAP-treated stationary-phase cells showed no significant overlaps with Scr1 or Rst2 target genes (
Figure 3d).
Figure 3.
Scr1 and Rst2 regulate shared metabolic genes in response to CAP treatment. (
a) Venn diagram to show the overlap between published Scr1 and Rst2 target genes [26]. Statistical significance of overlaps was calculated using Fisher’s exact test with
GeneOverlap R package [39], with the resulting
p-values of the overlaps indicated. The background gene set used for the analysis corresponds to all genes detected in the RNA-seq dataset in this study. (
b-d) Overlap analysis of CAP-induced genes (b), carbon metabolism genes in
Figure 2 (c), and CAP-repressed genes (d) with Scr1 and Rst2 target genes, with the resulting
p-values of the overlaps indicated. Statistical significance of overlaps was calculated using Fisher’s exact test with
GeneOverlap R package [39], with the resulting
p-values of the overlaps indicated. The background gene set used for the analysis encompasses all genes detected in the RNA-seq dataset in this study. (
e) Transcriptional response of Scr1 and Rst2 target genes to CAP treatment. Box plots show log
2 fold changes in gene expression of Scr1 and Rst2 targets in CAP-treated versus untreated cells during exponential and stationary phases, as indicated. Substantial changes in gene expression (mainly induction) of Scr1 and Rst2 targets were observed predominantly during stationary phase. Red horizontal lines mark log
2 fold change = 0 (no change); dashed lines mark log
2 fold-change thresholds of ±1. P-values were calculated using the Wilcoxon signed-rank test [55]. (
f) Overlap analysis of Scr1 and Rst2 targets that were induced (top) or repressed (bottom) based on the RNA-seq data shown in (e), with the
p-values of the overlap indicated. (
g) KEGG pathway enrichment analysis of Scr1 and Rst2 target genes that are induced in our data. The most enriched pathways are related to carbon metabolism. Dot size represents gene count per pathway, and dot color indicates FDR values, as shown at right. The analysis was performed using DAVID [36,37] with default parameters and visualized by
R (version 4.4.1, R Foundation for Statistical Computing, Vienna) [38].
Figure 3.
Scr1 and Rst2 regulate shared metabolic genes in response to CAP treatment. (
a) Venn diagram to show the overlap between published Scr1 and Rst2 target genes [26]. Statistical significance of overlaps was calculated using Fisher’s exact test with
GeneOverlap R package [39], with the resulting
p-values of the overlaps indicated. The background gene set used for the analysis corresponds to all genes detected in the RNA-seq dataset in this study. (
b-d) Overlap analysis of CAP-induced genes (b), carbon metabolism genes in
Figure 2 (c), and CAP-repressed genes (d) with Scr1 and Rst2 target genes, with the resulting
p-values of the overlaps indicated. Statistical significance of overlaps was calculated using Fisher’s exact test with
GeneOverlap R package [39], with the resulting
p-values of the overlaps indicated. The background gene set used for the analysis encompasses all genes detected in the RNA-seq dataset in this study. (
e) Transcriptional response of Scr1 and Rst2 target genes to CAP treatment. Box plots show log
2 fold changes in gene expression of Scr1 and Rst2 targets in CAP-treated versus untreated cells during exponential and stationary phases, as indicated. Substantial changes in gene expression (mainly induction) of Scr1 and Rst2 targets were observed predominantly during stationary phase. Red horizontal lines mark log
2 fold change = 0 (no change); dashed lines mark log
2 fold-change thresholds of ±1. P-values were calculated using the Wilcoxon signed-rank test [55]. (
f) Overlap analysis of Scr1 and Rst2 targets that were induced (top) or repressed (bottom) based on the RNA-seq data shown in (e), with the
p-values of the overlap indicated. (
g) KEGG pathway enrichment analysis of Scr1 and Rst2 target genes that are induced in our data. The most enriched pathways are related to carbon metabolism. Dot size represents gene count per pathway, and dot color indicates FDR values, as shown at right. The analysis was performed using DAVID [36,37] with default parameters and visualized by
R (version 4.4.1, R Foundation for Statistical Computing, Vienna) [38].
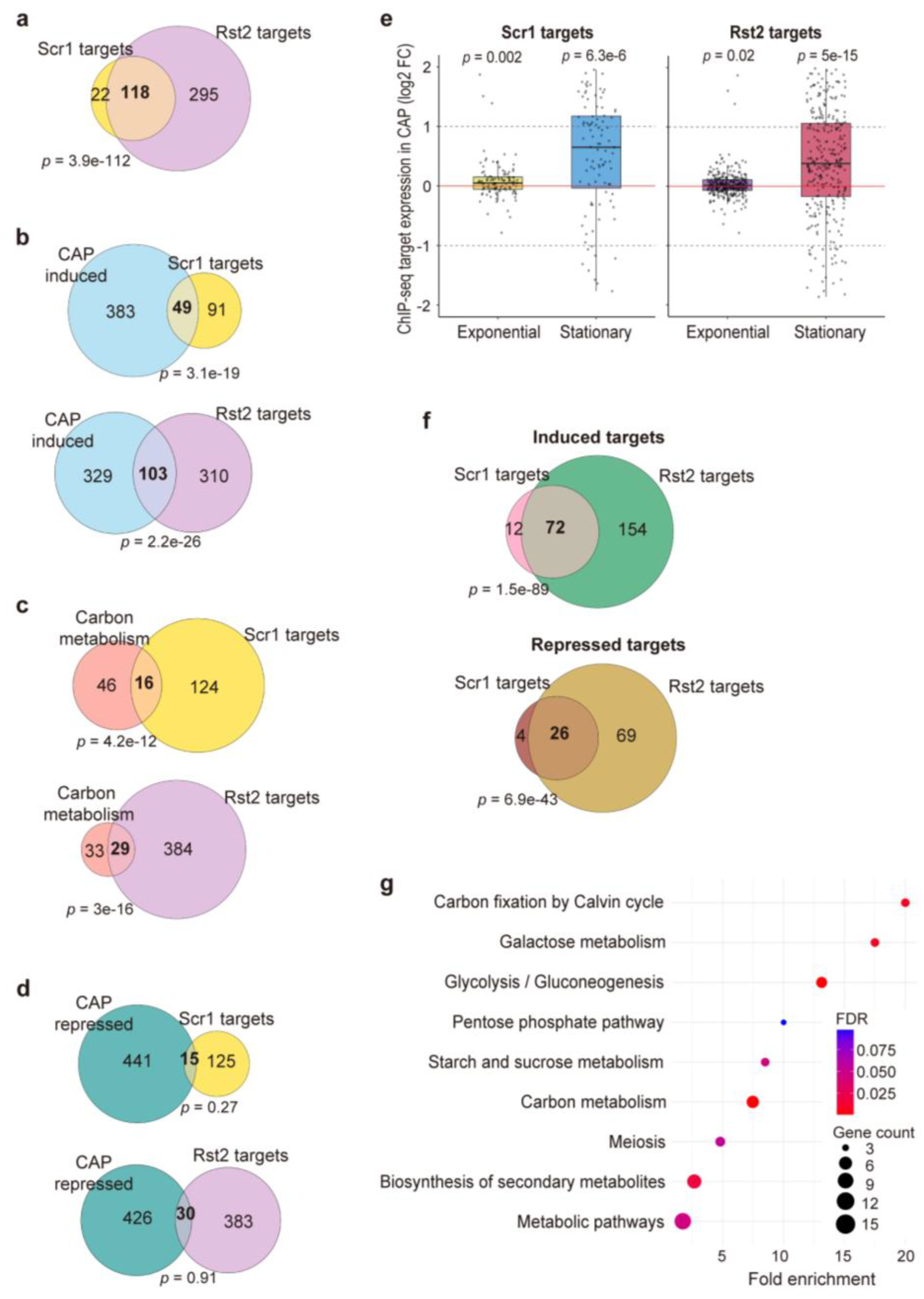
We then tested whether the Scr1 and Rst2 target genes showed altered expression in response to CAP treatment in exponentially growing or stationary-phase cells in glucose medium. On average, both the Scr1 and Rst2 target genes were significantly induced in stationary-phase cells, while they showed only minor expression differences in exponentially growing cells (
Figure 3e). These findings further support the conclusion that Scr1 and Rst2 play an important role in regulating the strong transcriptional response to mitochondrial translation inhibition in stationary-phase cells.
To dissect the regulatory relationship between Scr1 and Rst2, we looked for their target genes that were significantly induced or repressed in our RNA-seq data of CAP-treated stationary-phase cells. Notably, the target genes common to Scr1 and Rst2 included significant numbers of both induced and repressed genes, although fewer of the latter (
Figure 3f). In contrast, no overlap was observed between induced Scr1 target genes and repressed Rst2 target genes, or
vice versa. These results indicate that Scr1 and Rst2 cooperatively regulate a set of common genes, and most of these genes are induced in response to CAP treatment. KEGG pathway analysis of the genes that are positively regulated by both Scr1 and Rst2 revealed significant enrichments in several processes related to carbon metabolism (
Figure 3g). Collectively, these findings support a model of functional convergence, in which Scr1 and Rst2 jointly promote the reprogramming of carbon metabolism in response to mitochondrial translation inhibition.
3.5. Scr1 and Rst2 Antagonistically Affect Cellular Growth and Viability Upon Inhibition of Mitochondrial Translation
To further investigate the roles of Rst2 and Scr1 in CAP-treated cells, we constructed Δ
rst2 and Δ
scr1 deletion strains. We then assessed various traits of the cells lacking Rst2 or Scr1 as a function of CAP treatment. Without CAP treatment, Δ
rst2 cells showed a slightly faster growth rate, shorter lag phase, and higher final cell mass compared to wild-type cells, while Δ
scr1 cells showed a slower growth rate, prolonged lag phase, and lower final cell mass (
Figure 4a). With CAP treatment, all three strains exhibited reduced overall growth patterns, but with the same relative differences of increased growth patterns in Δ
rst2 and decreased growth patterns in Δ
scr1 compared to wild-type cells (
Figure 4a). To assess whether CAP affects cell viability over time, we performed serial-dilution spot assays at multiple time points following CAP treatment. In untreated cells, deletion of either
rst2 or
scr1 did not significantly affect cell viability at any time points compared to wild-type (
Figure 4b). CAP-treated cells of all three strains, on the other hand, showed markedly reduced viability at later times of culturing (36 h and 48 h), with ∆
rst2 showing slightly higher viability and ∆
scr1 slightly lower viability compared to wild type cells (
Figure 4b). This result suggests differences in the chronological lifespan (CLS) of stationary-phase cells between the three strains. To test this prediction, we conducted CLS assays to measure the longer-term viability of stationary-phase cells. Without CAP treatment, all strains showed similar CLS profiles over time. With CAP treatment, however, ∆
rst2 cells showed a substantially extended CLS compared to wild-type cells, maintaining some viability up to Day 9, whereas ∆
scr1 cells showed a dramatically shortened CLS, with a sharp decrease of viability already by Day 1 (
Figure 4c and
Supplementary Figure S4).
To explore whether the opposing effects of Scr1 and Rst2 on the CLS could be attributed to distinct target genes, we compared the transcriptional profiles of ∆
scr1 [26] and ∆
rst2 (this study;
Figure 5a) with previously identified long-and short-lived mutants, in which gene deletions extend or shorten the CLS, respectively [43]. Among the genes repressed in ∆
scr1 but not in ∆
rst2 cells, two deletion mutants shorten the CLS (
abp2,
cnp3). Notably,
abp2 showed a 2.7-fold reduction after CAP treatment. Conversely, among the genes repressed in ∆
rst2 cells but not repressed (or not detected) in ∆
scr1 cells, three deletion mutants extend the CLS (
cyp4,
mug27,
gal1). These distinct gene expression patterns might explain the opposite effects of Scr1 and Rst2 on the CLS under conditions of mitochondrial translation inhibition. Collectively, these findings indicate that Scr1 and Rst2 play antagonistic roles in the cellular adaptation to mitochondrial translation inhibition during stationary phase.
3.6. Rst2 Activates Carbon-Metabolism Genes in Stationary-Phase Cells upon Inhibition of Mitochondrial Translation
The gene-expression changes in response to CAP treatment are most pronounced during the stationary phase, a state triggered by glucose limitation. Thus, Rst2 is expected to play a dominant regulatory role in this condition based on its known function in the absence of glucose [26]. We therefore analysed the specific contribution of Rst2 to this adaptive response. To this end, we performed RNA-seq of Δ
rst2 and wild-type strains for both exponential and stationary phases (
Figure 5a). PCA based on rlog-transformed normalized expression values of the transcriptome signatures in these conditions revealed that the biggest transcriptional differences were caused by differences in the growth phases (
Figure 5b). In addition, deletion of
rst2 had a major impact on the transcriptome during the stationary phase, but not during the exponential phase (
Figure 5b). This phase-specific transcriptome response resembles the pattern observed for CAP treatment (
Figure 1b). A total of 137 genes were differentially expressed in Δ
rst2 compared to wild-type cells during stationary phase, with 30 genes induced and 107 genes repressed. These results indicate that Rst2 primarily functions as a transcriptional activator, consistent with the analyses of Rst2 target genes described above (
Figure 3 e,f) and the known function of Rst2 in the CCR response [26].
We then checked whether the genes whose expression is lowered in stationary-phase Δ
rst2 cells are part of the CAP-induced response. To this end, we compared the repressed genes in Δ
rst2 stationary-phase cells with both the published Rst2 target genes and the genes induced in CAP-treated stationary-phase cells grown in glucose medium. All three lists shared substantial numbers of genes (
Figure 5c). These results indicate that Rst2 is required for the regulation of a substantial proportion of the genes induced in CAP-treated stationary-phase cells. Accordingly, KEGG pathway analysis of the repressed genes in Δ
rst2 stationary-phase cells revealed significant enrichments of carbon-metabolism pathways (
Figure 5d). Genes involved in meiosis were also significantly enriched (see also
Figure 3g), consistent with the established role of Rst2 in activating
ste11 expression, which encodes a key transcription factor regulating sexual differentiation [56,57].
To assess whether CAP treatment affects the subcellular localization of Rst2, we tagged the genomic
rst2 gene with GFP to express a Rst2-GFP fusion protein. We then monitored the dynamics of Rst2-GFP in cells grown in glucose medium with or without CAP (
Figure 5e). In untreated cells, Rst2 was predominantly localized to the nucleus during the exponential phase but gradually shifted to the cytoplasm during the stationary phase. In contrast, in CAP-treated cells, Rst2 remained localized in the nucleus during stationary phase (
Figure 5e). This nuclear retention further points to Rst2 contributing to the transcriptional response to CAP treatment on stationary-phase cells. Together, these findings establish Rst2 as an important regulator for the transcriptional reprogramming of carbon metabolism in stationary-phase cells adapting to the inhibition of mitochondrial translation.
3.7. Rst2 Genetically Interacts with Genes Involved in Stress Protection and Nutrient Response
To systematically uncover functional relationships of Rst2 with diverse biological processes, we generated a new Δ
rst2 deletion strain (
rst2::natMX6), containing a marker suitable for a query mutant to assay genetic interactions with all 3420 non-essential prototrophic deletion mutants using the SGA method [58,59]. Genetic interactions were measured in glucose medium, both with and without CAP, based on colony size as a proxy for double-mutant fitness compared to the control mutant (see Methods). We observed 246 negative and 145 positive genetic interactions for untreated double mutants, and 247 negative and 156 positive genetic interactions for CAP-treated double mutants (Supplementary Dataset 3). Significant overlaps of 74 positive (
p = 6.8e-59) and 171 negative (
p = 4.3e-146) interactions were identified between the untreated and CAP-treated conditions. Functional enrichment analysis using AnGeLi [60] revealed that these interacting genes in both untreated and CAP-treated conditions were significantly enriched for stress-related phenotypes, including the fission yeast phenotype ontology (FYPO) terms [43,61,62] ‘increased sensitivity to chemical’ (
p = 5.5e-15 and
p = 1.0e-13 for untreated and CAP-treated, respectively), ‘sensitive to DNA damaging agents’ (
p = 4.7e-7 and
p = 9.5e-9) and ‘sensitive to hydrogen peroxide’ (
p = 7.9e-4 and
p = 4.6e-5). The term ‘decreased mating efficiency’ (
p = 2.7e-4 and
p = 9.5e-5) was also enriched, consistent with the enriched KEGG pathway ‘meiosis’ observed in
Figure 3g and
Figure 5d. Moreover, in untreated cells, the interacting genes additionally showed enrichment for several GO terms [63] related to ‘response to nutrient levels’ (
p = 2.1e-3) and ‘response to starvation’ (
p = 3.7e-3).
Among the 171 negative genetic interactions shared between untreated and CAP-treated conditions, four genes (
gal1,
ght8,
SPAC212.04c,
SPBC1861.05) were also repressed genes in Δ
rst2 stationary-phase cells;
gal1and
ght8 are involved in sugar metabolism and transport, supporting a role of Rst2 in maintaining glucose-responsive gene expression during mitochondrial stress. In addition, 14 genes overlapped with genes that are induced in CAP-treated stationary-phase cells, including
ppr5, encoding a mitochondrial pentatricopeptide repeat protein that negatively regulates mitochondrial translation [64], and
rec24, encoding a meiotic recombination protein [65,66], consistent with the enriched KEGG pathways related to meiosis (
Figure 3g;
Figure 5d). Collectively, our genetic interaction screen complements the transcriptomic data and highlights candidate genes that may work in parallel or together with Rst2 to promote the adaptation of stationary-phase cells to mitochondrial dysfunction.
4. Discussion
Mitochondrial translation is essential for cellular energy production and other mitochondrial functions [4]. Dysfunction of mitochondria is communicated to the nucleus through retrograde signaling, triggering adaptive gene expression programs [16,17]. Here, we investigated the transcriptome changes in response to impaired mitochondrial translation, induced by CAP treatment, in different metabolic and growth conditions. Our RNA-seq results indicate that CAP treatment primarily induces transcriptome changes in stationary-phase cells, whereas exponentially growing cells exhibit only a minor response (
Figure 1b).
Surprisingly, in cells growing under a respiratory condition that requires high mitochondrial activity (glycerol medium), only 23 genes were differentially expressed upon inhibition of mitochondrial translation (Table 1). This limited response could indicate that the 6-hour CAP treatment during exponential growth was insufficient to elicit a stronger transcriptional response. Such short-term CAP treatment is known to trigger only a modest reduction in the protein levels of mtDNA-encoded ETC subunits in exponential phase, whereas in the stationary phase, after longer CAP treatment, the levels of these proteins are strongly repressed [48]. Nevertheless, CAP treatment in glycerol almost completely abolished cell growth (Figure S1). Thus, it is also possible that these cells are too much impaired to launch a robust transcriptional response.
GO enrichment analysis of the differentially regulated genes in CAP-treated cells in the different conditions revealed that only the 432 genes induced in stationary-phase cells grown in glucose medium significantly overlapped with genes that are differentially expressed in response to various other stress conditions (
Figure 1c). These findings suggest that mitochondrial translation inhibition in these stationary-phase cells triggers a response similar to other stresses, including the retrograde response. Furthermore, only these 432 genes were significantly enriched in processes and pathways related to carbon metabolism, particularly cytoplasmic pathways that provide alternatives to mitochondrial respiration (
Figure 1d;
Figure 2). These transcriptome changes are consistent with a reprogramming of energy metabolism from respiration to fermentation to cope with the mitochondrial dysfunction.
Our results indicate that the CAP-induced transcriptional reprogramming of carbon metabolism involves Scr1- and Rst2-mediated regulation (
Figure 3). Scr1 primarily acts as a transcriptional repressor under glucose-sufficient conditions, while Rst2 functions as a transcriptional activator when glucose is limited [26]. Both Δ
scr1 and Δ
rst2 mutants displayed normal cell viability and lifespans under control conditions (
Figure 4), consistent with published results [26,57]. However, CAP-treated Δ
scr1 cells showed a dramatically shortened CLS, while Δ
rst2 cells featured an extended CLS compared to wild-type cells (
Figure 4c). These results suggest that Scr1 and Rst2 exert opposing regulatory roles in response to inhibition of mitochondrial translation. Scr1 and Rst2 regulate a common set of target genes, while also each controlling distinct sets of genes (
Figure 3a). The Scr1 and Rst2 target genes were, on average, significantly induced during stationary phase in CAP-treated cells (
Figure 3e), and they were enriched in carbon metabolism processes (
Figure 3g). Together with published results, these findings support a model of functional convergence, where Scr1 and Rst2 may act as a transcriptional ‘brake’ and ‘accelerator’, respectively, to tune the expression of carbon metabolism genes in response to mitochondrial defects. Similar regulators exist in
S. cerevisiae, where Mig1 is the ortholog of Scr1 that controls carbon source utilization [67,68], while Cat8 is the ortholog of Rst2 that activates metabolic genes under glucose-limited conditions [69]. These parallels suggest that coordination between glucose repression and metabolic adaptation via CCR regulators represents a conserved strategy across divergent yeast species.
Our findings further suggest that Rst2 primarily functions as a transcriptional activator for the induction of CAP-responsive metabolic genes in stationary-phase cells, based on RNA-seq (
Figure 5a-d) and genetic-interaction data. Rst2 was predominantly localized to the nucleus during exponential phase and gradually moved to the cytoplasm during late stationary phase (
Figure 5e). In CAP-treated cells, however, Rst2 was retained in the nucleus during stationary phase. This result supports the conclusion that Rst2 plays an important role in the transcriptional response of stationary-phase cells to mitochondrial dysfunction. The Rst2 localization pattern we observed in control cells seems to contradict published findings, where Rst2 is typically cytoplasmic under glucose-rich conditions and translocates to the nucleus only upon glucose depletion, regulated via AMPK-mediated phosphorylation [70]. However, a cytoplasmic localization of Rst2 has been observed in overnight cultures [71], which correspond to our stationary-phase condition. Moreover, Rst2 localization may not be exclusively regulated by glucose levels as illustrated by the following examples. Mutations in the Pka1 kinase that phosphorylates Rst2 in glucose-sufficient conditions, lead to nuclear accumulation of Rst2 [72], and Rst2 nucleocytoplasmic shuttling is impaired by deletion of ETC complex subunits [71]. Furthermore, these studies typically examined acute changes (minutes to hours) of Rst2 dynamics in response to glucose levels, whereas we captured Rst2 localization over longer times in steady-state conditions. Another difference is that we used rich medium for consistency with the RNA-seq conditions, while the other studies used minimal medium. Together these results highlight the influence of broader metabolic and signaling contexts, besides glucose levels, that affect Rst2 function and localization. A recent study identified high-occupancy target (HOT) regions in the
S. pombe genome that are bound by multiple transcription factors, and Rst2 was enriched at these HOT regions [73]. This observation might reflect a broader involvement of Rst2 in global gene regulation.
5. Conclusions
We investigated whether and how the inhibition of mitochondrial translation affects genome regulation in different metabolic conditions and growth phases. During stationary phase in glucose medium, CAP treatment triggered a transcriptional reprogramming of carbon metabolism from respiration to fermentation, along with changes similar to general stress and retrograde responses. The CCR regulators Scr1 and Rst2 control a shared set of metabolic genes in response to CAP treatment. Scr1 and Rst2 have opposing effects on the viability of CAP-treated stationary-phase cells. In these cells, which are in a glucose-limited state, Rst2 remains localized in the nucleus and functions as a transcriptional activator of carbon-metabolism genes. Genetic interactions point to functional relationships of Rst2 with stress and starvation responses. Our findings reveal a crosstalk between the stress/retrograde responses and the CCR regulators Scr1 and Rst2, and highlight a coordinated regulatory strategy that reprograms carbon metabolism in response to mitochondrial dysfunction. These findings deepen our understanding of mitochondrial-nuclear communication and the resulting transcriptome regulation in fission yeast and may also inform how other eukaryotic cells adapt their metabolism under mitochondrial stress.
Supplementary Materials
Table S1: List of S. pombe strains used in this study; Table S2: Primers used in this study; Figure S1: Growth measurement with or without CAP treatment in glucose or glycerol media; Figure S2: GO term and KEGG pathway enrichment analysis of 141 induced (a) and 250 repressed (b) genes following CAP treatment during stationary phase in glycerol medium.; Figure S3: Overlaps of CAP-induced genes with multiple stress-responsive gene sets; Figure S4: Chronological lifespan assays; Figure S5: The Natᴿ wild-type strain, unlike the Δade6 strain, shows no phenotypic differences compared to the original wild-type; Figure S6: Growth assessment of the Rst2-GFP strain; Dataset S1: Transcriptomic changes after the inhibition of mitochondrial translation under different carbon sources and growth phases; Dataset S2: Transcriptomic changes in Δrst2 compared to wild type cells. Dataset S3: SGA assays data; File S1: Original microscopy images.
Author Contributions
Conceptualization, Y.L. and J.B.; methodology, Y.L., S.H. and S.R.; formal analysis, Y.L.; investigation, Y.L.; writing—original draft preparation, Y.L.; writing—review and editing, Y.L., S.H. and J.B.; visualization, Y.L., S.H. and J.B.; supervision, J.B.; funding acquisition, Y.L. and J.B.; All authors have read and agreed to the final version of the manuscript.
Funding
This work was funded by the Wellcome Discovery Award (No. 302608/Z/23/Z to J.B.) and the China Scholarship Council (No. 202306860040 to Y.L.).
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
Acknowledgments
We thank Benjamin Murray Heineike and Manuel Lera-Ramírez for the helpful discussions, and Sam Yee Low, Qinglong Yang and Xiao Yuan for technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| mtDNA |
Mitochondrial DNA |
| ETC |
Electron transport chain |
| CCR |
Carbon catabolite repression |
| AMPK |
AMP-activated protein kinase |
| GO |
Gene ontology |
| ANT |
Antimycin A treatment |
| CESR |
Core environmental stress response |
| COSG |
Core oxidative stress genes |
| MNR |
Mito-nuclear retrograde |
| TCA |
Tricarboxylic Acid |
| CLS |
Chronological lifespan |
| FYPO |
Fission yeast phenotype ontology |
References
- Yan, C.; Duanmu, X.; Zeng, L.; Liu, B.; Song, Z. Mitochondrial DNA: Distribution, Mutations, and Elimination. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363.e353. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Chan, D.C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 2016, 212, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C.J.; Labarre-Mariotte, S.; Cornu, D.; Sophie, C.; Panozzo, C.; Michel, T.; Dujardin, G.; Bonnefoy, N. Translational activators and mitoribosomal isoforms cooperate to mediate mRNA-specific translation in Schizosaccharomyces pombe mitochondria. Nucleic Acids Res. 2021, 49, 11145–11166. [Google Scholar] [CrossRef]
- Lightowlers, R.N.; Rozanska, A.; Chrzanowska-Lightowlers, Z.M. Mitochondrial protein synthesis: figuring the fundamentals, complexities and complications, of mammalian mitochondrial translation. FEBS Lett. 2014, 588, 2496–2503. [Google Scholar] [CrossRef]
- Kuzmenko, A.; Atkinson, G.C.; Levitskii, S.; Zenkin, N.; Tenson, T.; Hauryliuk, V.; Kamenski, P. Mitochondrial translation initiation machinery: conservation and diversification. Biochimie 2014, 100, 132–140. [Google Scholar] [CrossRef]
- Agirrezabala, X.; Frank, J. Elongation in translation as a dynamic interaction among the ribosome, tRNA, and elongation factors EF-G and EF-Tu. Q. Rev. Biophys. 2009, 42, 159–200. [Google Scholar] [CrossRef]
- Kummer, E.; Ban, N. Mechanisms and regulation of protein synthesis in mitochondria. Nat Rev Mol Cell Biol 2021, 22, 307–325. [Google Scholar] [CrossRef]
- Kummer, E.; Leibundgut, M.; Rackham, O.; Lee, R.G.; Boehringer, D.; Filipovska, A.; Ban, N. Unique features of mammalian mitochondrial translation initiation revealed by cryo-EM. Nature 2018, 560, 263–267. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R.; Wang, Y.; Xie, W.; Liu, Z.; Huang, Y. Schizosaccharomyces pombe Mti2 and Mti3 act in conjunction during mitochondrial translation initiation. FEBS J. 2019, 286, 4542–4553. [Google Scholar] [CrossRef]
- Luo, Y.; Bähler, J.; Huang, Y. The Insertion Domain of Mti2 Facilitates the Association of Mitochondrial Initiation Factors with Mitoribosomes in Schizosaccharomyces pombe. Biomolecules 2025, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, J.; Zhang, Q.; Ma, X.; Zhang, J.; Su, M.; Wang, X.; Huang, Y. The Schizosaccharomyces pombe PPR protein Ppr10 associates with a novel protein Mpa1 and acts as a mitochondrial translational activator. Nucleic Acids Res. 2017, 45, 3323–3340. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Y.; Huang, Y. Schizosaccharomyces pombe Ppr10 and Mpa1 together mediate mitochondrial translational initiation. J. Biol. Chem. 2021, 297, 100869. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, T.; Chuang, C.-C. The Consequences of Damaged Mitochondrial DNA. In Mitochondrial Mechanisms of Degeneration and Repair in Parkinson's Disease, Buhlman, L.M., Ed. Springer International Publishing: Cham, 2016; 10.1007/978-3-319-42139-1_3pp. 49-61.
- Yan, C.; Duanmu, X.; Zeng, L.; Liu, B.; Song, Z. Mitochondrial DNA: Distribution, Mutations, and Elimination. Cells 2019, 8, 379. [Google Scholar] [CrossRef]
- Kotiadis, V.N.; Duchen, M.R.; Osellame, L.D. Mitochondrial quality control and communications with the nucleus are important in maintaining mitochondrial function and cell health. Biochim. Biophys. Acta 2014, 1840, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Malecki, M.; Bitton, D.A.; Rodríguez-López, M.; Rallis, C.; Calavia, N.G.; Smith, G.C.; Bähler, J. Functional and regulatory profiling of energy metabolism in fission yeast. Genome Biol. 2016, 17, 240. [Google Scholar] [CrossRef]
- Desai, R.; East, D.A.; Hardy, L.; Faccenda, D.; Rigon, M.; Crosby, J.; Alvarez, M.S.; Singh, A.; Mainenti, M.; Hussey, L.K.; et al. Mitochondria form contact sites with the nucleus to couple prosurvival retrograde response. Sci Adv 2020, 6. [Google Scholar] [CrossRef]
- Trendeleva, T.A.; Zvyagilskaya, R.A. Retrograde Signaling as a Mechanism of Yeast Adaptation to Unfavorable Factors. Biochemistry (Moscow) 2018, 83, 98–106. [Google Scholar] [CrossRef]
- Hunt, R.J.; Bateman, J.M. Mitochondrial retrograde signaling in the nervous system. FEBS Lett. 2018, 592, 663–678. [Google Scholar] [CrossRef]
- Richter, A.S.; Nägele, T.; Grimm, B.; Kaufmann, K.; Schroda, M.; Leister, D.; Kleine, T. Retrograde signaling in plants: A critical review focusing on the GUN pathway and beyond. Plant Communications 2023, 4. [Google Scholar] [CrossRef] [PubMed]
- Epstein, C.B.; Waddle, J.A.; Hale, W.t.; Davé, V.; Thornton, J.; Macatee, T.L.; Garner, H.R.; Butow, R.A. Genome-wide responses to mitochondrial dysfunction. Mol. Biol. Cell 2001, 12, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; López-Maury, L.; Shaw, M.; Bähler, J.; Norbury, C.J.; Agashe, V.R. Transcriptional and cellular responses to defective mitochondrial proteolysis in fission yeast. J. Mol. Biol. 2011, 408, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Broach, J.R. Nutritional control of growth and development in yeast. Genetics 2012, 192, 73–105. [Google Scholar] [CrossRef]
- Bahn, Y.S.; Xue, C.; Idnurm, A.; Rutherford, J.C.; Heitman, J.; Cardenas, M.E. Sensing the environment: lessons from fungi. Nat Rev Microbiol 2007, 5, 57–69. [Google Scholar] [CrossRef]
- Vassiliadis, D.; Wong, K.H.; Andrianopoulos, A.; Monahan, B.J. A genome-wide analysis of carbon catabolite repression in Schizosaccharomyces pombe. BMC Genomics 2019, 20, 251. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, T.; Fujita, Y.; Tohda, H.; Takegawa, K. Snf1-like protein kinase Ssp2 regulates glucose derepression in Schizosaccharomyces pombe. Eukaryot. Cell 2012, 11, 159–167. [Google Scholar] [CrossRef]
- Hirota, K.; Hoffman, C.S.; Ohta, K. Reciprocal nuclear shuttling of two antagonizing Zn finger proteins modulates Tup family corepressor function to repress chromatin remodeling. Eukaryot. Cell 2006, 5, 1980–1989. [Google Scholar] [CrossRef]
- Mukai, Y.; Matsuo, E.; Roth, S.Y.; Harashima, S. Conservation of histone binding and transcriptional repressor functions in a Schizosaccharomyces pombe Tup1p homolog. Mol. Cell. Biol. 1999, 19, 8461–8468. [Google Scholar] [CrossRef]
- Bähler, J.; Wu, J.Q.; Longtine, M.S.; Shah, N.G.; McKenzie, A., 3rd; Steever, A.B.; Wach, A.; Philippsen, P.; Pringle, J.R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 1998, 14, 943–951. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Y.; Huang, Y. Schizosaccharomyces pombe Ppr10 and Mpa1 together mediate mitochondrial translational initiation. J. Biol. Chem. 2021, 297, 100869. [Google Scholar] [CrossRef]
- Lera-Ramírez, M.; Bähler, J.; Mata, J.; Rutherford, K.; Hoffman, C.S.; Lambert, S.; Oliferenko, S.; Martin, S.G.; Gould, K.L.; Du, L.L.; et al. Revised fission yeast gene and allele nomenclature guidelines for machine readability. Genetics 2023, 225. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xu, Y.; Ahmad, F.; Feng, G.; Huang, Y. Characterization of Shy1, the Schizosaccharomyces pombe homolog of human SURF1. Sci Rep 2024, 14, 21678. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Lock, A.; Rutherford, K.; Harris, M.A.; Hayles, J.; Oliver, S.G.; Bähler, J.; Wood, V. PomBase 2018: user-driven reimplementation of the fission yeast database provides rapid and intuitive access to diverse, interconnected information. Nucleic Acids Res. 2019, 47, D821–d827. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–w221. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing. 2024.
- Shen, L. GeneOverlap: Test and visualize gene overlaps, 1.30.0; Bioconductor: 2022.
- Wang, M.; Zhao, Y.; Zhang, B. Efficient Test and Visualization of Multi-Set Intersections. Sci Rep 2015, 5, 16923. [Google Scholar] [CrossRef] [PubMed]
- Anver, S.; Sumit, A.F.; Sun, X.M.; Hatimy, A.; Thalassinos, K.; Marguerat, S.; Alic, N.; Bähler, J. Ageing-associated long non-coding RNA extends lifespan and reduces translation in non-dividing cells. EMBO Rep. 2024, 25, 4921–4949. [Google Scholar] [CrossRef] [PubMed]
- Kahm, M.; Hasenbrink, G.; Lichtenberg-Fraté, H.; Ludwig, J.; Kschischo, M. grofit: Fitting Biological Growth Curves with R. Journal of Statistical Software 2010, 33, 1–21. [Google Scholar] [CrossRef]
- Romila, C.A.; Townsend, S.; Malecki, M.; Kamrad, S.; Rodríguez-López, M.; Hillson, O.; Cotobal, C.; Ralser, M.; Bähler, J. Barcode sequencing and a high-throughput assay for chronological lifespan uncover ageing-associated genes in fission yeast. Microb Cell 2021, 8, 146–160. [Google Scholar] [CrossRef]
- Rallis, C.; Townsend, S.; Bähler, J. Genetic interactions and functional analyses of the fission yeast gsk3 and amk2 single and double mutants defective in TORC1-dependent processes. Sci Rep 2017, 7, 44257. [Google Scholar] [CrossRef]
- Wagih, O.; Parts, L. gitter: a robust and accurate method for quantification of colony sizes from plate images. G3 (Bethesda) 2014, 4, 547–552. [Google Scholar] [CrossRef]
- Wagih, O.; Usaj, M.; Baryshnikova, A.; VanderSluis, B.; Kuzmin, E.; Costanzo, M.; Myers, C.L.; Andrews, B.J.; Boone, C.M.; Parts, L. SGAtools: one-stop analysis and visualization of array-based genetic interaction screens. Nucleic Acids Res. 2013, 41, W591–596. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, K.; Kramhft, B. The Effect of Chloramphenicol on Respiration, Fermentation and Growth in Schizosaccharomyces pombe. Microbiology 1980, 120, 279–282. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, Y.; Yin, D.; Zhao, L.; Wang, Y.; Rui, Y.; Zhang, P.; Wu, X.; Li, M.; Hidalgo, E.; et al. Schizosaccharomyces pombe MAP kinase Sty1 promotes survival of Δppr10 cells with defective mitochondrial protein synthesis. Int J Biochem Cell Biol, 1016. [Google Scholar] [CrossRef]
- Vustin, M.M. The Biological Role of Glycerol in Yeast Cells. Yeast as Glycerol Producers. Applied Biochemistry and Microbiology 2021, 57, 907–916. [Google Scholar] [CrossRef]
- Chen, D.; Toone, W.M.; Mata, J.; Lyne, R.; Burns, G.; Kivinen, K.; Brazma, A.; Jones, N.; Bähler, J. Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell 2003, 14, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wilkinson, C.R.; Watt, S.; Penkett, C.J.; Toone, W.M.; Jones, N.; Bähler, J. Multiple pathways differentially regulate global oxidative stress responses in fission yeast. Mol. Biol. Cell 2008, 19, 308–317. [Google Scholar] [CrossRef]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic Glycolysis: Meeting the Metabolic Requirements of Cell Proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef]
- Kamrad, S.; Grossbach, J.; Rodríguez-López, M.; Mülleder, M.; Townsend, S.; Cappelletti, V.; Stojanovski, G.; Correia-Melo, C.; Picotti, P.; Beyer, A.; et al. Pyruvate kinase variant of fission yeast tunes carbon metabolism, cell regulation, growth and stress resistance. Mol. Syst. Biol. 2020, 16, e9270. [Google Scholar] [CrossRef]
- Rutherford, K.M.; Lera-Ramírez, M.; Wood, V. PomBase: a Global Core Biodata Resource-growth, collaboration, and sustainability. Genetics 2024, 227. [Google Scholar] [CrossRef]
- Woolson, R.F. Wilcoxon Signed-Rank Test. In Wiley Encyclopedia of Clinical Trials. pp. 1-3. [CrossRef]
- Takenaka, K.; Tanabe, T.; Kawamukai, M.; Matsuo, Y. Overexpression of the transcription factor Rst2 in Schizosaccharomyces pombe indicates growth defect, mitotic defects, and microtubule disorder. Biosci Biotechnol Biochem 2018, 82, 247–257. [Google Scholar] [CrossRef]
- Kunitomo, H.; Higuchi, T.; Iino, Y.; Yamamoto, M. A zinc-finger protein, Rst2p, regulates transcription of the fission yeast ste11(+) gene, which encodes a pivotal transcription factor for sexual development. Mol. Biol. Cell 2000, 11, 3205–3217. [Google Scholar] [CrossRef]
- Baryshnikova, A.; Costanzo, M.; Dixon, S.; Vizeacoumar, F.J.; Myers, C.L.; Andrews, B.; Boone, C. Synthetic genetic array (SGA) analysis in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Methods Enzymol. 2010, 470, 145–179. [Google Scholar] [CrossRef]
- Costanzo, M.; Kuzmin, E.; van Leeuwen, J.; Mair, B.; Moffat, J.; Boone, C.; Andrews, B. Global Genetic Networks and the Genotype-to-Phenotype Relationship. Cell 2019, 177, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Bitton, D.A.; Schubert, F.; Dey, S.; Okoniewski, M.; Smith, G.C.; Khadayate, S.; Pancaldi, V.; Wood, V.; Bähler, J. AnGeLi: A Tool for the Analysis of Gene Lists from Fission Yeast. Front Genet 2015, 6, 330. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Lock, A.; Bähler, J.; Oliver, S.G.; Wood, V. FYPO: the fission yeast phenotype ontology. Bioinformatics 2013, 29, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Sideri, T.; Rallis, C.; Bitton, D.A.; Lages, B.M.; Suo, F.; Rodríguez-López, M.; Du, L.L.; Bähler, J. Parallel profiling of fission yeast deletion mutants for proliferation and for lifespan during long-term quiescence. G3 (Bethesda) 2014, 5, 145–155. [Google Scholar] [CrossRef]
- The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–d334. [CrossRef]
- Kühl, I.; Dujeancourt, L.; Gaisne, M.; Herbert, C.J.; Bonnefoy, N. A genome wide study in fission yeast reveals nine PPR proteins that regulate mitochondrial gene expression. Nucleic Acids Res. 2011, 39, 8029–8041. [Google Scholar] [CrossRef]
- Bonfils, S.; Rozalén, A.E.; Smith, G.R.; Moreno, S.; Martín-Castellanos, C. Functional interactions of Rec24, the fission yeast ortholog of mouse Mei4, with the meiotic recombination-initiation complex. J. Cell Sci. 2011, 124, 1328–1338. [Google Scholar] [CrossRef]
- Steiner, S.; Kohli, J.; Ludin, K. Functional interactions among members of the meiotic initiation complex in fission yeast. Curr. Genet. 2010, 56, 237–249. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, C.Y.; Bai, X.W.; Song, H.Y.; Xiao, D.G. Effects of MIG1, TUP1 and SSN6 deletion on maltose metabolism and leavening ability of baker's yeast in lean dough. Microb. Cell Fact. 2014, 13, 93. [Google Scholar] [CrossRef]
- Alipourfard, I.; Bakhtiyari, S.; Datukishvili, N.; Haghani, K.; Di Renzo, L.; De Miranda, R.C.; Cioccoloni, G.; Basiratyan Yazdi, P.; Mikeladze, D. Saccharomyces cerevisiae, key role of MIG1 gene in metabolic switching: putative fermentation/oxidation. J. Biol. Regul. Homeost. Agents 2018, 32, 649–654. [Google Scholar] [PubMed]
- Laera, L.; Guaragnella, N.; Ždralević, M.; Marzulli, D.; Liu, Z.; Giannattasio, S. The transcription factors ADR1 or CAT8 are required for RTG pathway activation and evasion from yeast acetic acid-induced programmed cell death in raffinose. Microb Cell 2016, 3, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Watanabe, Y.; Yamamoto, M. Protein kinase A regulates sexual development and gluconeogenesis through phosphorylation of the Zn finger transcriptional activator Rst2p in fission yeast. Mol. Cell. Biol. 2002, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Liu, Q.; Kato, T.; Miao, H.; Gao, X.; Liu, K.; Chen, S.; Sakamoto, N.; Kuno, T.; Fang, Y. Role of mitochondrial complex III/IV in the activation of transcription factor Rst2 in Schizosaccharomyces pombe. Mol. Microbiol. 2021, 115, 1323–1338. [Google Scholar] [CrossRef]
- Gupta, D.R.; Paul, S.K.; Oowatari, Y.; Matsuo, Y.; Kawamukai, M. Multistep regulation of protein kinase A in its localization, phosphorylation and binding with a regulatory subunit in fission yeast. Curr. Genet. 2011, 57, 353–365. [Google Scholar] [CrossRef]
- Skribbe, M.; Soneson, C.; Stadler, M.B.; Schwaiger, M.; Suma Sreechakram, V.N.; Iesmantavicius, V.; Hess, D.; Moreno, E.P.F.; Braun, S.; Seebacher, J.; et al. A comprehensive Schizosaccharomyces pombe atlas of physical transcription factor interactions with proteins and chromatin. Mol. Cell 2025, 85, 1426–1444.e1428. [Google Scholar] [CrossRef]
Figure 1.
Mitochondrial translation inhibition primarily affects the transcriptome during stationary phase. (a) Experimental design for RNA-seq samples used in this study. For experimental details, see Materials and Methods. Three independent biological replicates were prepared for each condition. (b) Principal component analysis (PCA) based on rlog-transformed normalized expression values of transcriptome responses to CAP treatment under different carbon sources and growth phases. Each dot represents one biological replicate. Dot shapes indicate the growth phases (circle: exponential; triangle: stationary), and dot colors indicate CAP treatment (turquoise: untreated; pink: treated). The separate panels for glucose and glycerol conditions are derived from the same PCA analysis using all samples and thus share the same coordinate axes. PC1 and PC2 account for 60.3% and 14.6% of the total variance, respectively. (c) Overlap analysis of CAP-induced genes with gene sets induced in response to other stress conditions. Statistical significance of the overlaps was calculated using Fisher’s exact test with the GeneOverlap R package [39], with the resulting p-values of the overlaps indicated. The background gene set used for the analysis corresponds to all genes detected in the RNA-seq dataset in this study. (d) GO term and KEGG pathway enrichment analysis of 432 induced genes following CAP treatment during stationary phase in glucose medium. GO terms are grouped by category: BP (Biological Process) and MF (Molecular Function), and were selected for non-redundancy, specificity, and significance.
Figure 1.
Mitochondrial translation inhibition primarily affects the transcriptome during stationary phase. (a) Experimental design for RNA-seq samples used in this study. For experimental details, see Materials and Methods. Three independent biological replicates were prepared for each condition. (b) Principal component analysis (PCA) based on rlog-transformed normalized expression values of transcriptome responses to CAP treatment under different carbon sources and growth phases. Each dot represents one biological replicate. Dot shapes indicate the growth phases (circle: exponential; triangle: stationary), and dot colors indicate CAP treatment (turquoise: untreated; pink: treated). The separate panels for glucose and glycerol conditions are derived from the same PCA analysis using all samples and thus share the same coordinate axes. PC1 and PC2 account for 60.3% and 14.6% of the total variance, respectively. (c) Overlap analysis of CAP-induced genes with gene sets induced in response to other stress conditions. Statistical significance of the overlaps was calculated using Fisher’s exact test with the GeneOverlap R package [39], with the resulting p-values of the overlaps indicated. The background gene set used for the analysis corresponds to all genes detected in the RNA-seq dataset in this study. (d) GO term and KEGG pathway enrichment analysis of 432 induced genes following CAP treatment during stationary phase in glucose medium. GO terms are grouped by category: BP (Biological Process) and MF (Molecular Function), and were selected for non-redundancy, specificity, and significance.
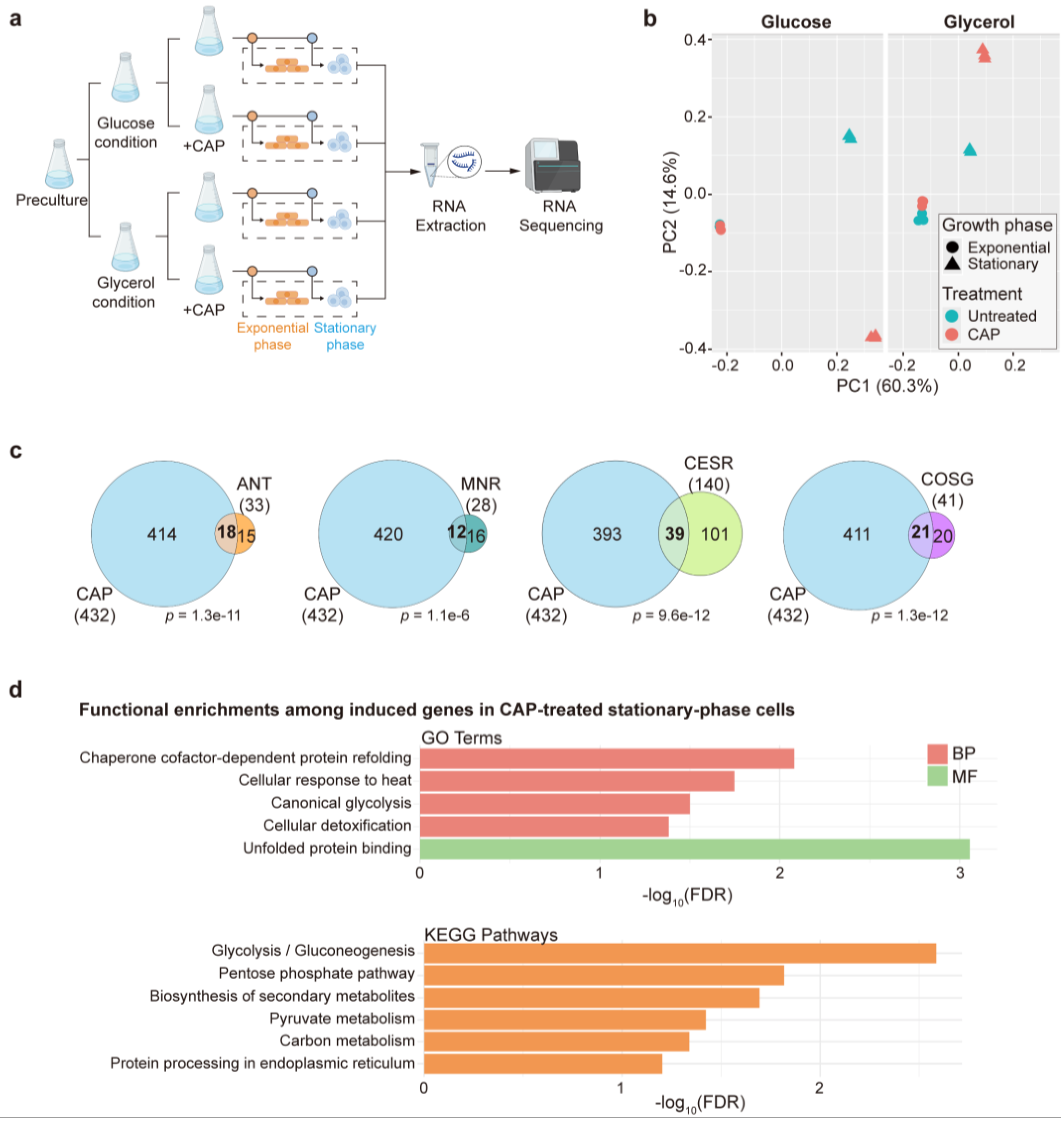
Figure 4.
Scr1 and Rst2 affect growth and viability of CAP-treated cells. (a) Growth measurement of wild-type (wt), ∆rst2 and ∆scr1 strains cultured in YES medium with or without CAP treatment. Left graph: growth curves were measured using a microbioreactor as described [41]. Mean growth curves (biomass accumulation) are shown, along with the standard deviation (shaded regions) for three independent replicates. Growth rates and lag phases (middle and right panels) were quantified from the left graph using the R package grofit [42]. The statistical analysis was conducted by one-way ANOVA, followed by Tukey’s honest significance test (ns, not significant; **, p <0.01; ***, p <0.001; and ****, p <0.0001). (b) Viability assays of wild-type, ∆rst2 and ∆scr1 strains with and without CAP treatment, with 2% EtOH serving as a solvent control. Cells grown to an initial OD600 in the media indicated at left were collected after the indicated time points, and 10-fold serial dilutions were spotted onto YES solid plates. The plates were photographed after 3 days. (c) Chronological lifespan assays of wt, ∆rst2 and ∆scr1 strains without (left graph) or with (right graph) CAP treatment. Cells were grown in YES medium to stationary phase (Day 0). A proxy for cell viability was determined over time by a robotics-based colony-forming unit (CFU) assay [43], with values normalized to Day 0 (100% viability). CFUs were measured daily from Day 0 to Day 7 and at Day 9. At each time point, aliquots of ageing cultures were collected, 3-fold serially diluted, and spotted onto YES plates, which were imaged after three days. Three independent biological replicates were prepared for each condition; error bars represent standard deviation (SD) and are shown only in the upward direction for clarity on a log-scale Y axis.
Figure 4.
Scr1 and Rst2 affect growth and viability of CAP-treated cells. (a) Growth measurement of wild-type (wt), ∆rst2 and ∆scr1 strains cultured in YES medium with or without CAP treatment. Left graph: growth curves were measured using a microbioreactor as described [41]. Mean growth curves (biomass accumulation) are shown, along with the standard deviation (shaded regions) for three independent replicates. Growth rates and lag phases (middle and right panels) were quantified from the left graph using the R package grofit [42]. The statistical analysis was conducted by one-way ANOVA, followed by Tukey’s honest significance test (ns, not significant; **, p <0.01; ***, p <0.001; and ****, p <0.0001). (b) Viability assays of wild-type, ∆rst2 and ∆scr1 strains with and without CAP treatment, with 2% EtOH serving as a solvent control. Cells grown to an initial OD600 in the media indicated at left were collected after the indicated time points, and 10-fold serial dilutions were spotted onto YES solid plates. The plates were photographed after 3 days. (c) Chronological lifespan assays of wt, ∆rst2 and ∆scr1 strains without (left graph) or with (right graph) CAP treatment. Cells were grown in YES medium to stationary phase (Day 0). A proxy for cell viability was determined over time by a robotics-based colony-forming unit (CFU) assay [43], with values normalized to Day 0 (100% viability). CFUs were measured daily from Day 0 to Day 7 and at Day 9. At each time point, aliquots of ageing cultures were collected, 3-fold serially diluted, and spotted onto YES plates, which were imaged after three days. Three independent biological replicates were prepared for each condition; error bars represent standard deviation (SD) and are shown only in the upward direction for clarity on a log-scale Y axis.
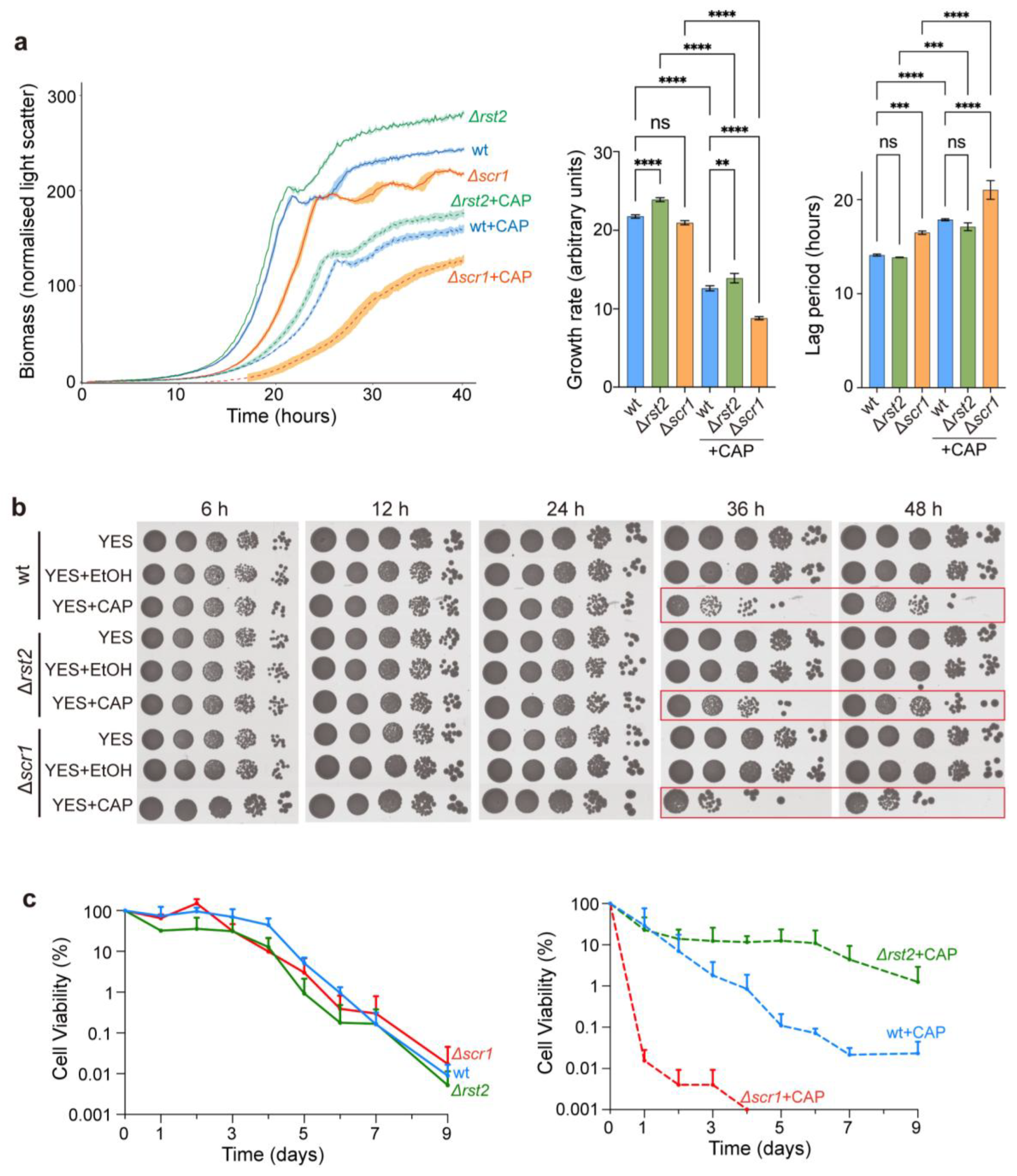
Figure 5.
Rst2 contribution to gene regulation in response to mitochondrial translation inhibition during stationary phase. (a) Experimental design of wild-type (wt) and Δrst2 strains used for RNA-seq analysis. For experimental details, see Materials and Methods. Three independent biological replicates were prepared for each condition. (b) PCA based on rlog-transformed normalized expression values of transcriptome responses from ∆rst2 and wild-type (wt) cells collected during exponential and stationary phases. Each dot represents one biological replicate. Dot shapes indicate the growth phases (circle: exponential; triangle: stationary), and dot colors indicate the genotype (turquoise: wt; pink: ∆rst2). PC1 and PC2 account for 91.2% and 5.6% of the total variance, respectively. (c) Overlap analysis of genes repressed in Δrst2 cells during stationary phase compared to CAP-induced genes during stationary phase and to Rst2 target genes [26]. The p-value represents the significance of the overlap among the three gene sets. (d) KEGG pathway enrichment analysis of the genes repressed in Δrst2 cells during stationary phase. The most enriched pathways are related to carbon metabolism and meiosis. Dot size represents gene count per pathway, and color indicates FDR values as shown at right. The analysis was performed using DAVID [36,37] with default parameters and visualized by R (version 4.4.1, R Foundation for Statistical Computing, Vienna) [38]. (e) Subcellular localization of Rst2 in untreated and CAP-treated cells. Wild-type cells expressing Rst2-GFP under its endogenous promoter were grown in YES medium to an initial OD600 of 0.2 (0 h). Cells were then treated with CAP and collected at the indicated time points for fluorescence microscopy. Scale bar, 10 μm.
Figure 5.
Rst2 contribution to gene regulation in response to mitochondrial translation inhibition during stationary phase. (a) Experimental design of wild-type (wt) and Δrst2 strains used for RNA-seq analysis. For experimental details, see Materials and Methods. Three independent biological replicates were prepared for each condition. (b) PCA based on rlog-transformed normalized expression values of transcriptome responses from ∆rst2 and wild-type (wt) cells collected during exponential and stationary phases. Each dot represents one biological replicate. Dot shapes indicate the growth phases (circle: exponential; triangle: stationary), and dot colors indicate the genotype (turquoise: wt; pink: ∆rst2). PC1 and PC2 account for 91.2% and 5.6% of the total variance, respectively. (c) Overlap analysis of genes repressed in Δrst2 cells during stationary phase compared to CAP-induced genes during stationary phase and to Rst2 target genes [26]. The p-value represents the significance of the overlap among the three gene sets. (d) KEGG pathway enrichment analysis of the genes repressed in Δrst2 cells during stationary phase. The most enriched pathways are related to carbon metabolism and meiosis. Dot size represents gene count per pathway, and color indicates FDR values as shown at right. The analysis was performed using DAVID [36,37] with default parameters and visualized by R (version 4.4.1, R Foundation for Statistical Computing, Vienna) [38]. (e) Subcellular localization of Rst2 in untreated and CAP-treated cells. Wild-type cells expressing Rst2-GFP under its endogenous promoter were grown in YES medium to an initial OD600 of 0.2 (0 h). Cells were then treated with CAP and collected at the indicated time points for fluorescence microscopy. Scale bar, 10 μm.
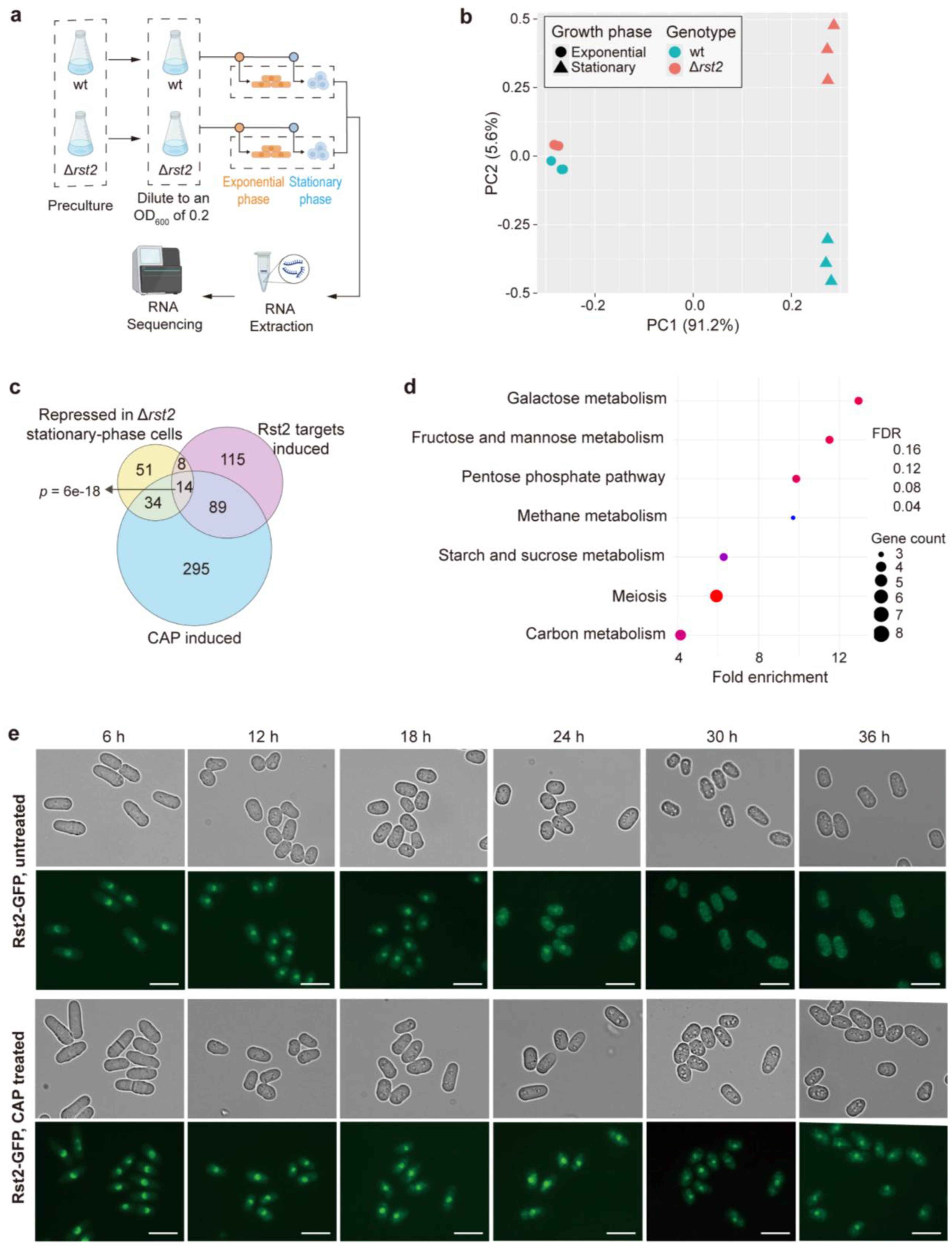
Table 1.
Numbers of differentially expressed protein-coding transcripts after CAP treatment in the different experimental conditions. Induced and repressed genes are defined as transcripts that show expression changes in CAP-treated cells relative to untreated control cells in the same condition, with a significance threshold of p <0.05 and |log2(Fold Change)| >1. A detailed list of these annotated genes is provided in Supplementary Dataset S1.
Table 1.
Numbers of differentially expressed protein-coding transcripts after CAP treatment in the different experimental conditions. Induced and repressed genes are defined as transcripts that show expression changes in CAP-treated cells relative to untreated control cells in the same condition, with a significance threshold of p <0.05 and |log2(Fold Change)| >1. A detailed list of these annotated genes is provided in Supplementary Dataset S1.
| Condition |
Induced |
Repressed |
| Glucose_Exponential |
9 |
2 |
| Glucose_Stationary |
432 |
456 |
| Glycerol_Exponential |
15 |
8 |
| Glycerol_Stationary |
141 |
250 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).