Submitted:
12 August 2025
Posted:
13 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Baseline Participant Physical, Performance, and Training Characteristics
2.2. Overall Trial Characteristics and Outcomes
2.2.1. Trial Characteristics
2.2.2. Trial Average Power (Laps 2 – 4)
2.2.3. Trial Average HR and RPE (Laps 2 – 4)
2.3. Hill Climb Segment Charactersitics and Outcomes
2.3.1. Hill Climb Segment Average Power (Laps 2 – 4)
2.4. Sprint Segment Charactersitics and Outcomes
2.4.1. Sprint Segment Average Power (Laps 2 – 4)
2.5. Time Trial Charactersitics and Outcomes
2.5.1. Time Trial Average Power (Lap 5)
2.5.2. Time Trial Average HR and RPE (Lap 5)
2.9. Placebo Effects
3. Discussion
3.1. Trial Data
3.1.1. Laps 2 – 4 Repeated Efforts and Endurance
3.1.2. Durability of Time Trial Performance
3.2. Performance Replication and Perceived Effort
3.3. Placebo Effects and Menthol
3.4. Does Transdermal Delivery Make Sense for Buffers?
3.5. Assimilation of Present Finding with Prior Transdermal Research
3.6. Novelty and Limitations
3.7. Applications and Implications
4. Materials and Methods
4.1. Ethics Approval and Participant Characteristics
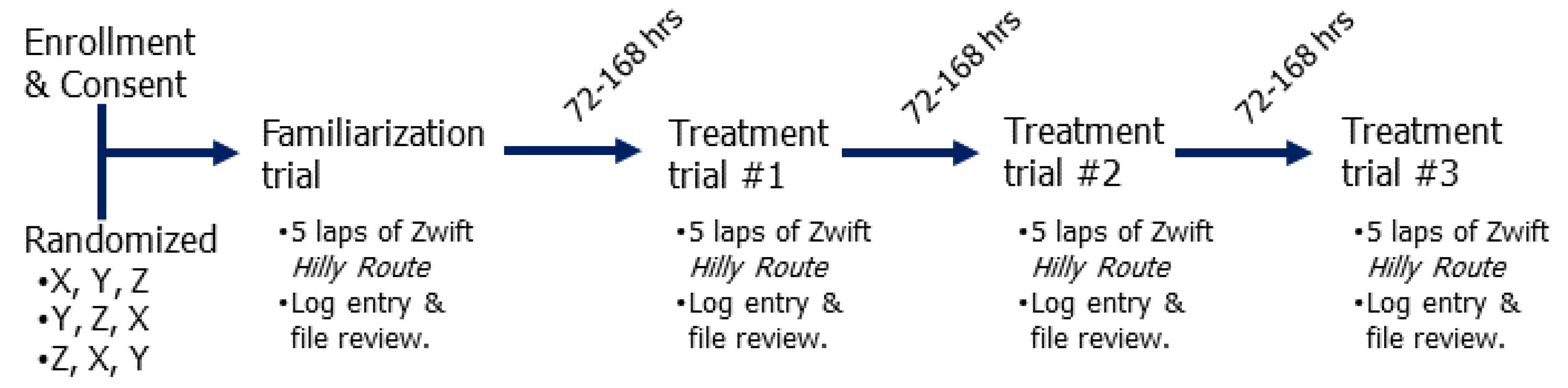
4.2. Study Design
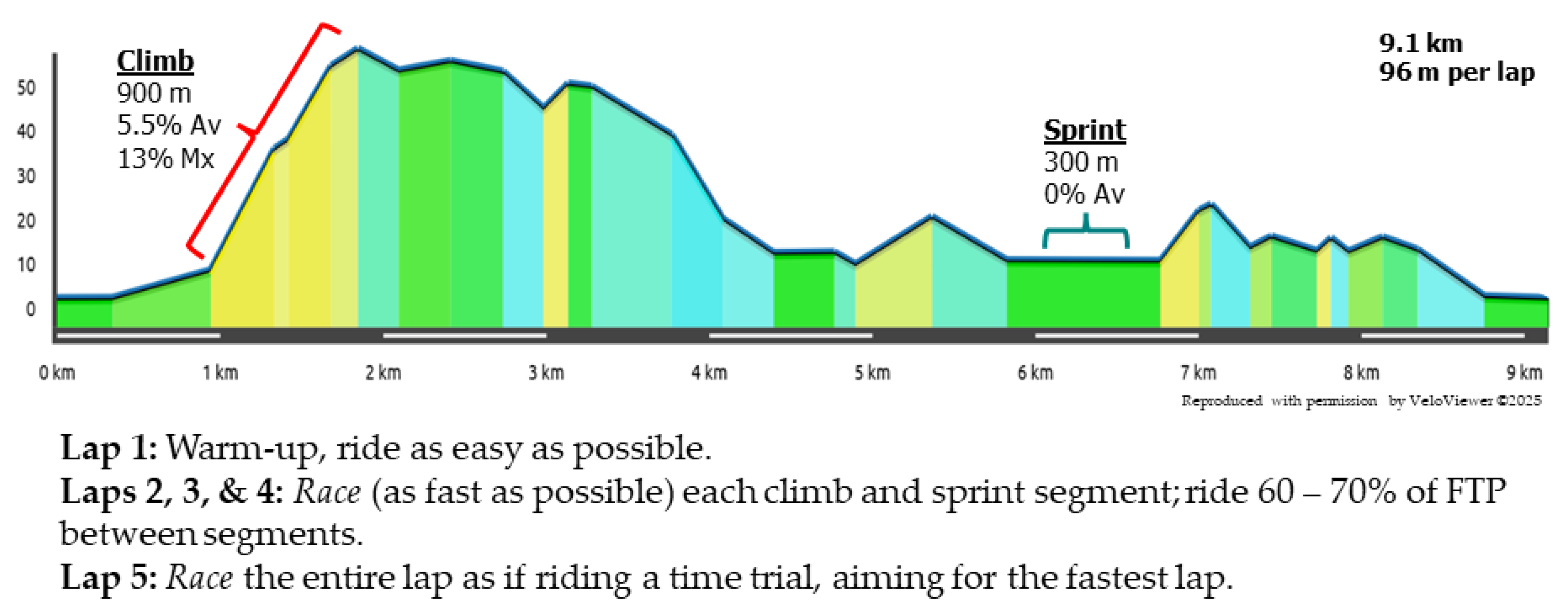
4.3. Trial Details
4.4. Trial Training Logs
- Supplement application and Trial start times.
- Trial designation (FAM, X, Y, or Z).
- Supplement rating and comments, and belief it was a supplement or PLAC.
- Environmental temperature as well as pre- and post-trial body weight.
- The Zwift virtual bike and wheels used (kept unchanged across trials as they affect game speeds).
- RPE (1 – 10) for each lap and overall trial RPE [57].
- The volume of fluids consumed and details on carbohydrate intake.
4.5. Supplmenent and Placebo Lotions
4.6. Performance Measures
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BA | Beta-alanine |
| BC | Bicarbonate |
| END | Endurance trials ≥ 90-min |
| FAM | Familiarization trial |
| FTP | Functional threshold power equal 60-min maximal power |
| HI | High-intensity intervals |
| HR | Heart Rate |
| MMP | Mean maximal power |
| MMP20 | Mean maximal power for 20-min |
| MMP30s | Mean maximal power for 30-sec |
| MMP60 | Mean maximal power for 60-min |
| MR | Mixed reality |
| OBC | Oral bicarbonate |
| PLAC | Placebo |
| RCT | Randomized controlled trial |
| RPE | Rating of perceived exertion |
| RS | Repeated sprint |
| S | Sprint |
| TBC | Transdermal bicarbonate |
| TC | Transdermal carnosine |
Appendix A
Appendix A.1. Participant Intake Form
Appendix A.2. Example Trial Training Log
Appendix B
Appendix B.1
References
- Grand View Research Sports Nutrition Market Size, Share & Trends Analysis Report By Product Type (Sports Supplements, Sports Drinks), By Formulation, By Consumer Group, By Sales Channel, By Region, And Segment Forecasts, 2024 - 2030; Grand View Research, 2023; p. 140.
- Loraine, K. Supplement Regulation for Sports Nutrition Supplements. Journal of Legal Medicine 2018, 38, 271–285. [CrossRef]
- Paton, C.D.; Hopkins, W.G. Variation in Performance of Elite Cyclists from Race to Race. European Journal of Sport Science 2006, 6, 25–31. [CrossRef]
- Chase, J.G.; Moeller, K.; Shaw, G.M.; Schranz, C.; Chiew, Y.S.; Desaive, T. When the Value of Gold Is Zero. BMC Res Notes 2014, 7, 404. [CrossRef]
- Ferguson, H.A.; Harnish, C.; Chase, J.G. Using Field Based Data to Model Sprint Track Cycling Performance. Sports Med - Open 2021, 7, 20. [CrossRef]
- Morais, J.E.; Bragada, J.A.; Magalhães, P.M.; Marinho, D.A. The Accuracy and Reliability of the Power Measurements of the TACX Neo 2T Smart Trainer and Its Agreement against the Garmin Vector 3 Pedals. Journal of Functional Morphology and Kinesiology 2024, 9, 138.
- Zadow, E.K.; Fell, J.W.; Kitic, C.M. The Reliability of a Laboratory-Based 4 Km Cycle Time Trial on a Wahoo KICKR Power Trainer. Journal of Science and Cycling 2016, 5, 23–27.
- Hoon, M.W.; Michael, S.W.; Patton, R.L.; Chapman, P.G.; Areta, J.L. A Comparison of the Accuracy and Reliability of the Wahoo KICKR and SRM Power Meter. Journal of Science and Cycling 2016, 5, 11–15.
- Reed, J.; Dunn, C.; Beames, S.; Stonehouse, P. E ‘Ride on!’: The Zwift Platform as a Space for Virtual Leisure. Leisure Studies 2023, 42, 188–202.
- Devine, A.; Devine, F.; Burns, A. An Examination of the Virtual Event Experience of Cyclists Competing on Zwift. Event Management 2024, 28, 151–167.
- Westmattelmann, D.; Stoffers, B.; Sprenger, M.; Grotenhermen, J.-G.; Schewe, G. The Performance-Result Gap in Mixed-Reality Cycling–Evidence from the Virtual Tour de France 2020 on Zwift. Frontiers in Physiology 2022, 13, 868902.
- McIlroy, B.; Passfield, L.; Holmberg, H.-C.; Sperlich, B. Virtual Training of Endurance Cycling–a Summary of Strengths, Weaknesses, Opportunities and Threats. Frontiers in sports and active living 2021, 3, 631101.
- Joyner, M.J.; Coyle, E.F. Endurance Exercise Performance: The Physiology of Champions: Factors That Make Champions. The Journal of Physiology 2008, 586, 35–44. [CrossRef]
- Stellingwerff, T.; Bovim, I.M.; Whitfield, J. Contemporary Nutrition Interventions to Optimize Performance in Middle-Distance Runners. International Journal of Sport Nutrition and Exercise Metabolism 2019, 29, 106–116. [CrossRef]
- Barsumyan, A.; Soost, C.; Burchard, R. Enhanced Durability Predicts Success in Amateur Road Cycling: Evidence of Power Output Declines. Front. Sports Act. Living 2025, 7, 1530162. [CrossRef]
- Jones, A.M. The Fourth Dimension: Physiological Resilience as an Independent Determinant of Endurance Exercise Performance. The Journal of Physiology 2024, 602, 4113–4128. [CrossRef]
- Maunder, E.; Seiler, S.; Mildenhall, M.J.; Kilding, A.E.; Plews, D.J. The Importance of ‘Durability’ in the Physiological Profiling of Endurance Athletes. Sports Med 2021, 51, 1619–1628. [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jäger, R.; Earnest, C.P.; Bannock, L. International Society of Sports Nutrition Position Stand: Beta-Alanine. Journal of the International Society of Sports Nutrition 2015, 12, 30.
- Harris, R.C.; Sale, C. Beta-Alanine Supplementation in High-Intensity Exercise. Acute topics in sport nutrition 2012, 59, 1–17.
- Carr, A.J.; Hopkins, W.G.; Gore, C.J. Effects of Acute Alkalosis and Acidosis on Performance: A Meta-Analysis. Sports medicine 2011, 41, 801–814.
- Peart, D.J.; Siegler, J.C.; Vince, R.V. Practical Recommendations for Coaches and Athletes: A Meta-Analysis of Sodium Bicarbonate Use for Athletic Performance. The Journal of Strength & Conditioning Research 2012, 26, 1975–1983.
- Saunders, B.; Sunderland, C.; Harris, R.C.; Sale, C. β-Alanine Supplementation Improves YoYo Intermittent Recovery Test Performance. Journal of the International Society of Sports Nutrition 2012, 9, 39. [CrossRef]
- De Oliveira, L.F.; Dolan, E.; Swinton, P.A.; Durkalec-Michalski, K.; Artioli, G.G.; McNaughton, L.R.; Saunders, B. Extracellular Buffering Supplements to Improve Exercise Capacity and Performance: A Comprehensive Systematic Review and Meta-Analysis. Sports Med 2022, 52, 505–526. [CrossRef]
- Saunders, B.; De Oliveira, L.F.; Dolan, E.; Durkalec-Michalski, K.; McNaughton, L.; Artioli, G.G.; Swinton, P.A. Sodium Bicarbonate Supplementation and the Female Athlete: A Brief Commentary with Small Scale Systematic Review and Meta-analysis. European Journal of Sport Science 2022, 22, 745–754. [CrossRef]
- Grgic, J.; Pedisic, Z.; Saunders, B.; Artioli, G.G.; Schoenfeld, B.J.; McKenna, M.J.; Bishop, D.J.; Kreider, R.B.; Stout, J.R.; Kalman, D.S.; et al. International Society of Sports Nutrition Position Stand: Sodium Bicarbonate and Exercise Performance. Journal of the International Society of Sports Nutrition 2021, 18, 61. [CrossRef]
- Gibson, B.M.; Needham, K.W.; Kaiser, B.W.; Wilkins, B.W.; Minson, C.T.; Halliwill, J.R. Transcutaneous Delivery of Sodium Bicarbonate Increases Intramuscular pH. Front. Physiol. 2023, 14, 1142567. [CrossRef]
- Dieter, B.P.; Macias, C.J.; Sharpe, T.J.; Roberts, B.; Wille, M.; Young, A.; Reisenauer, C.; Cantrell, B.; Bayly, W.M. Transdermal Delivery of Carnosine into Equine Skeletal Muscle. Comparative Exercise Physiology 2021, 17, 429–434. [CrossRef]
- Gurton, W.H.; Greally, J.; Chudzikiewicz, K.; Gough, L.A.; Lynn, A.; Ranchordas, M.K. Beneficial Effects of Oral and Topical Sodium Bicarbonate during a Battery of Team Sport-Specific Exercise Tests in Recreationally Trained Male Athletes. Journal of the International Society of Sports Nutrition 2023, 20, 2216678. [CrossRef]
- Gurton, W.H.; Gough, L.A.; Siegler, J.C.; Lynn, A.; Ranchordas, M.K. Oral but Not Topical Sodium Bicarbonate Improves Repeated Sprint Performance During Simulated Soccer Match Play Exercise in Collegiate Athletes. International Journal of Sport Nutrition and Exercise Metabolism 2024, 34, 362–371. [CrossRef]
- Seah, J.Z.H. Effect of Sodium Bicarbonate in a Transdermal Delivery System on Physiological Par: A Double Blind, Placebo-Controlled, Randomised, Crossover Study. 2019.
- McKay, A.K.A.; Peeling, P.; Binnie, M.J.; Goods, P.S.R.; Sim, M.; Cross, R.; Siegler, J. Topical Sodium Bicarbonate: No Improvement in Blood Buffering Capacity or Exercise Performance. International Journal of Sports Physiology and Performance 2020, 15, 1005–1011. [CrossRef]
- Kern, M.; Misell, L.M.; Ordille, A.; Alm, M.; Salewske, B. Double-Blind, Placebo Controlled, Randomized Crossover Pilot Study Evaluating The Impacts Of Sodium Bicarbonate in a Transdermal Delivery System on Physiological Parameters and Exercise Performance: 2402 Board #238 June 1 11 00 AM - 12 30 PM. Medicine & Science in Sports & Exercise 2018, 50, 595. [CrossRef]
- Sharpe, T.M.; Macias, C.J. Evaluation of the Efficacy of LactigoTM Topical Gel as an Ergogenic Aid. J. Exerc. Physiol. Online 2016, 19, 15–23.
- Harnish, C.R.; Miller, B. Transdermal Carnosine Gel Fails to Improve Repeated Wingate Performance in Trained Male Cyclists: A Randomized Controlled Cross-over Trial. J. Sports Sci. Nutr. 2023, 4, 106–111. [CrossRef]
- Brockelbank, N. The Application of a Topical Carnosine Gel and Its Effects on Intermittent High-Intensity Exercise Performance in Olympic-Level Rugby Sevens Players, The University of Waikato, 2024.
- Ravindrakumar, A.; Bommasamudram, T.; Tod, D.; Edwards, B.J.; Chtourou, H.; Pullinger, S.A. Daily Variation in Performance Measures Related to Anaerobic Power and Capacity: A Systematic Review. Chronobiology International 2022, 39, 421–455. [CrossRef]
- Hurst, P.; Schipof-Godart, L.; Szabo, A.; Raglin, J.; Hettinga, F.; Roelands, B.; Lane, A.; Foad, A.; Coleman, D.; Beedie, C. The Placebo and Nocebo Effect on Sports Performance: A Systematic Review. European Journal of Sport Science 2020, 20, 279–292. [CrossRef]
- Halson, S.L.; Martin, D.T. Lying to Win—Placebos and Sport Science. International journal of sports physiology and performance 2013, 8, 597–599.
- Garcia Matta, G. Placebo Effects on Cycling Performance in Virtual-Reality and Laboratory Environments, Canterbury Christ Church University, 2023.
- Barwood, M.J.; Gibson, O.R.; Gillis, D.J.; Jeffries, O.; Morris, N.B.; Pearce, J.; Ross, M.L.; Stevens, C.; Rinaldi, K.; Kounalakis, S.N. Menthol as an Ergogenic Aid for the Tokyo 2021 Olympic Games: An Expert-Led Consensus Statement Using the Modified Delphi Method. Sports Medicine 2020, 50, 1709–1727.
- Jeffries, O.; Waldron, M. The Effects of Menthol on Exercise Performance and Thermal Sensation: A Meta-Analysis. Journal of science and medicine in sport 2019, 22, 707–715.
- Stevens, C.J.; Best, R. Menthol: A Fresh Ergogenic Aid for Athletic Performance. Sports medicine 2017, 47, 1035–1042.
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat Biotechnol 2008, 26, 1261–1268. [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. and Transl. Res. 2022, 12, 758–791. [CrossRef]
- Velocity Animal Sciences Inc. Equine Velocity Recovery Topical Emulgel: Equestrian Horse Wellness Available online: https://www.equinevelocity.com/ (accessed on 17 July 2025).
- Downs, S.H.; Black, N. The Feasibility of Creating a Checklist for the Assessment of the Methodological Quality Both of Randomised and Non-Randomised Studies of Health Care Interventions. Journal of Epidemiology & Community Health 1998, 52, 377–384. [CrossRef]
- Pinot, J.; Grappe, F. The Record Power Profile to Assess Performance in Elite Cyclists. Int J Sports Med 2011, 32, 839–844. [CrossRef]
- Borszcz, F.; Tramontin, A.; Bossi, A.; Carminatti, L.; Costa, V. Functional Threshold Power in Cyclists: Validity of the Concept and Physiological Responses. Int J Sports Med 2018, 39, 737–742. [CrossRef]
- Reilly, T.; Atkinson, G.; Waterhouse, J. Chronobiology and Physical Performance. Exercise and sport science 2000, 24, 351–372.
- Thomas, K.; Stone, M.R.; Thompson, K.G.; St Clair Gibson, A.; Ansley, L. Reproducibility of Pacing Strategy during Simulated 20-Km Cycling Time Trials in Well-Trained Cyclists. European journal of applied physiology 2012, 112, 223–229.
- Foster, C.; Hendrickson, K.J.; Peyer, K.; Reiner, B.; deKoning, J.J.; Lucia, A.; Battista, R.A.; Hettinga, F.J.; Porcari, J.P.; Wright, G. Pattern of Developing the Performance Template. British journal of sports medicine 2009, 43, 765–769.
- Hibbert, A.W.; Billaut, F.; Varley, M.C.; Polman, R.C.J. Familiarization Protocol Influences Reproducibility of 20-Km Cycling Time-Trial Performance in Novice Participants. Front. Physiol. 2017, 8, 488. [CrossRef]
- Hopkins, W.G. Measures of Reliability in Sports Medicine and Science. Sports medicine 2000, 30, 1–15.
- Gotti, D.; Codella, R.; Vergallito, L.; Meloni, A.; Arrighi, T.; La Torre, A.; Filipas, L. From Amateur to Professional Cycling: A Case Study on the Training Characteristics of a Zwift Academy Winner. Sports 2025, 13, 234.
- Currell, K.; Jeukendrup, A.E. Validity, Reliability and Sensitivity of Measures of Sporting Performance. Sports Medicine 2008, 38, 297–316. [CrossRef]
- Clark, B.; Paton, C.D.; O’Brien, B.J. The Reliability of Performance during Computer-Simulated Varying Gradient Cycling Time Trials. J Sci Cycling 2014, 3, 29–33.
- Foster, C.; Florhaug, J.A.; Franklin, J.; Gottschall, L.; Hrovatin, L.A.; Parker, S.; Doleshal, P.; Dodge, C. A New Approach to Monitoring Exercise Training. J Strength Cond Res 2001, 15, 109–115.
- Maxwell, S.E.; Delaney, H.D.; Kelley, K. Designing Experiments and Analyzing Data: A Model Comparison Perspective; Routledge, 2017; ISBN 1-315-64295-6.
| Overall | Zwift A | Zwift B | Zwift C | Zwift D | |
|---|---|---|---|---|---|
| Physical Characteristics | |||||
| n | 15 | 4 | 3 | 4 | 4 |
| Age (years) | 45.1 ± 12.1 | 42.0 (28.0, 50.0) | 54.0 (47, 61) | 46.0 (30.0, 51.0) | 53.5 (18, 55) |
| Height (cm) | 175.6 ± 6.9 | 176.7 (175.0, 180.3) | 181.6 (177.8, 183) | 170.2 (159, 182.9) | 176.1 (167.6, 180.3) |
| Weight (kg) | 74.4 ± 11.6 | 70.5 (65.4, 77.2) | 79.8 (69.5, 88.6) | 75.4 (60.5, 94.0) | 76.1 (55.5, 82.0) |
| RHR (bpm) | 51.3 ± 7.8 | 47.5 (45.0, 51.0) | 45.0 (39.0, 56.0) | 53.5 (48.0, 58.0) | 58.5 (43.0,66.0) |
| MHR (bpm) | 177.1 ± 11.0 | 185.0 (185, 190.0) | 172.0 (168.0, 180.0) | 183.0 (161.0, 194.0) | 164.5 (161.0,181.0) |
| Performance Characteristics | |||||
| MMP30s (W/kg) | 7.1 ± 2.3 | 9.8 (9.2, 10.4)#D | 6.8 (6.7, 6.9) | 6.7 (3.6, 9.3) | 5.8 (2.7, 7.4) |
| MMP20 (W/kg) | 3.2 ± 1.0 | 4.4 (4.0, 4.6)*D | 3.5 (3.5, 3.7) | 2.8 (2.6, 2.9) | 2.2 (1.5, 2.5) |
| MMP60 (W/kg) | 2.9 ± 0.9 | 4.0 (3.6, 4.1)*D | 3.3 (3.2, 3.4) | 2.6 (2.3, 3.1) | 1.9 (1.3, 2.2) |
| Training Characteristics | |||||
| Years training (years) | 13.4 ± 11.8 | 18.5 (3.0, 36.0) | 17.0 (12.0, 31.0) | 6.0 (1.0, 10.0) | 7.0 (2.0, 23.0) |
| 6-wk training Volume (min) | 454.0 ± 209.7 | 630.0 (180.0, 300.0) | 480.0 (480.0, 810.0) | 315.0 (180.0, 480.0) | 330.0 (90.0, 600.0) |
| Typical training ride (min) | 123.0 ± 69.5 | 135.0 (90.0, 240.0) | 135.0 (120.0, 240.0) | 90.0 (60.0, 150.0) | 75.0 (60.0, 90.0) |
| Typical longest ride (min) | 187.3 ± 68.6 | 240.0 (180.0, 300.0)#D | 240.0 (120.0, 300.0) | 150.0 (120.0, 270.0) | 150.0 (60.0, 180.0) |
| Typical Race Duration (min) | 140 ± 86.8 | 60 - 600 | 60 - 300 | 60 – 120 | 60 - 180 |
| Lap | Trial Condition |
Entire Lap Average Power (W) |
Average HR (bpm) |
RPE (1 – 10) |
Hill Climb Segment Average Power (W) |
Sprint Segment Average Power (W) |
|---|---|---|---|---|---|---|
| FAM | 140.1 ± 38.9 | 114.4 ± 10.8 | 3.3 ± 1.7 | 159.3 ± 29.3 | 151.2 ± 55.7 | |
| 1 | TBC | 134.8 ± 41.3 | 115.6 ± 15.1 | 2.8 ± 1.6 | 151.7 ± 34.7 | 138.4 ± 56.8 |
| TC | 136.3 ± 40.0 | 116.1 ± 15.4 | 3.1 ± 2.0 | 156.5 ± 34.0 | 121.3 ± 52.0 | |
| PLAC | 136.2 ± 39.2 | 114.5 ± 11.9 | 3.2 ± 1.5 | 153.3 ± 35.1 | 141.3 ± 49.6 | |
| FAM | 180.5 ± 56.3 | 138.4 ± 13.9 | 5.3 ± 1.4 | 267.2 ± 97.5* | 431.0 ± 211.2 | |
| AVG 2-4 | TBC | 180.8 ± 56.4 | 140.8 ± 16.1 | 5.3 ± 1.5 | 300.6 ± 101.6 | 483.0 ± 211.5# |
| TC | 185.1 ± 56.2 | 141.5 ± 16.9 | 5.5 ± 1.6 | 292.9 ± 103.0 | 464.9 ± 207.0 | |
| PLAC | 192.8 ± 52.2 | 143.4 ± 15.6 | 5.3 ± 1.5 | 298.6 ± 101.2 | 488.5 ± 221.8# | |
| FAM | 181.0 ± 58.1 | 133.5 ± 14.6 | 5.6 ± 1.3 | 274.8 ± 100.8 | 437.4 ± 215.7 | |
| 2 | TBC | 185.3 ± 57.2 | 137.8 ± 18.0 | 5.7 ± 1.6 | 309.4 ± 103.5 | 502.8 ± 222.6 |
| TC | 187.3 ± 55.6 | 136.7 ± 17.3 | 5.9 ± 1.6 | 298.8 ± 111.6 | 468.2 ± 235.6 | |
| PLAC | 196.6 ± 52.4 | 138.0 ± 16.8 | 5.6 ± 1.6 | 304.6 ± 102.8 | 494.9 ± 214.8 | |
| FAM | 182.8 ± 55.2 | 136.6 ± 14.4 | 5.6 ± 1.5 | 256.0 ± 101.1 | 414.6 ± 223.0 | |
| 3 | TBC | 181.3 ± 55.2 | 139.8 ± 17.8 | 6.0 ± 1.6 | 292.2 ± 110.0 | 464.4 ± 230.0 |
| TC | 185.3 ± 54.6 | 140.1 ± 18.7 | 5.9 ± 1.6 | 273.0 ± 103.4 | 458.7 ± 225.2 | |
| PLAC | 191.6 ± 52.5 | 141.2 ± 16.6 | 5.8 ± 1.8 | 291.5 ± 107.8 | 475.5 ± 251.6 | |
| FAM | 177.7 ± 57.6 | 138.5 ± 12.7 | 6.6 ± 1.1 | 270.6 ± 104.2 | 440.9 ± 216.9 | |
| 4 | TBC | 175.9 ± 58.8 | 139.5 ± 16.3 | 6.7 ± 1.3 | 300.2 ± 101.8 | 481.8 ± 203.9 |
| TC | 182.8 ± 60.1 | 141.4 ± 15.8 | 6.9 ± 1.0 | 297.0 ± 102.1 | 467.7 ± 188.7 | |
| PLAC | 190.3 ± 52.9 | 143.6 ± 15.0 | 6.5 ± 1.3 | 299.8 ± 102.5 | 495.1 ± 210.8 | |
| FAM | 220.8 ± 76.8* | 152.1 ± 12.2 | 8.2 ± 0.9 | 235.2 ± 66.9 | 247.0 ± 74.4 | |
| 5 | TBC | 232.9 ± 69.5 | 155.7 ± 13.6 | 8.5 ± 1.2 | 246.3 ± 69.9 | 240.7 ± 56.9 |
| TC | 229.5 ± 67.3 | 155.4 ± 15.4 | 8.6 ± 1.2 | 243.4 ± 65.2 | 244.7 ± 63.6 | |
| PLAC | 233.0 ± 73.1 | 156.8 ± 14.0 | 8.3 ± 1.1 | 245.3 ± 72.9 | 236.3 ± 69.8 |
| Author | Condition/Treatment | Sport | n | RCT | PLAC | Matched PLAC | Measures | Improvement | DB Score (30) |
|---|---|---|---|---|---|---|---|---|---|
| Harnish et al. 2025 | TBC/TC | Cycling | 15 | Yes | Yes | No | RS, HI, TT, END | No improvement across all measures. | 25 |
| Gurton et. al. 2024 | OBC/TBC | Soccer | 10 | Yes | Yes | No | RS | No change in acid-base balance and no improvement across all measures for TBC. | 24 |
| Gurton et. al. 2023 | OBC/TBC | Team Field Sport | 14 | Yes | Yes | No | RS | No change in acid-base balance, similar < 2% improvement for RS compared to OBC. | 24 |
| McKay et al. 2020 | OBC/TBC | Cycling | 10 | Yes | Yes | No | RS | No change in acid-base balance and no improvement across all measures for TBC. | 23 |
| Kern et al. 2019* | TBC | Cycling | 20 | Yes | Yes | No | S, TT | No change in acid-base balance and no improvement across all measures for TBC. | 22 |
| Seah et al. 2019^ | TBC | Team Field Sport | 10 | Yes | Yes | ? | RS | No improvement across all measures. | 6 |
| Brockelbank 2024# | TC | Rugby | 12 | Yes | Yes | Yes | RS | No improvement in average peak or mean sprint power. | 24 |
| Harnish & Miller 2023 | TC | Cycling | 15 | Yes | Yes | No | RS | No improvement across all measures. | 28 |
| Sharpe & Macias 2016 | TC | Soccer | 11 | No | No | No | RS, TT | Yes. Final TC trials showed small TT improvement. | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





