1. Introduction
Prolonged and intensive endurance exercise causes post-exercise inflammation, oxidative stress, immune suppression, and extensive perturbations in hundreds of circulating proteins and metabolites. Nutrition-based interventions are being investigated for their potential to improve long distance running and cycling performance, mitigate physiological stress, and augment metabolic recovery [
1,
2,
3,
4,
5].
Betaine (trimethylglycine) is a non-essential amino acid and is naturally present in beets, spinach, wheat germ and bran, and shellfish. Dietary intake ranges from 0.5 to 2.5 g/d depending on diet composition [
6]. Betaine is also produced in vivo by means of choline metabolism. Betaine is one of several key methyl donors in the one-carbon metabolism pathway and participates in the methionine cycle by transferring a methyl group to homocysteine and contributing to the formation of S-adenosylmethionine (SAM) [
7]. SAM is the universal methyl donor that supports histone and DNA methylation, two epigenetic mechanisms that regulate gene expression [
8]. Betaine also functions as an organic osmolyte to maintain and regulate fluid balance, and intracellular fluid concentrations and cell volume [
9,
10]. Cell culture and animal studies suggest that betaine can reduce oxidative damage, improve enterocyte health, and counter negative changes in intestinal permeability [
7,
11]. Animal data indicate that betaine helps regulate intestinal epithelial barrier function by improving the intestinal barrier structure and the expression of tight junction proteins including occludin and claudin-1 [
12,
13,
14]. Plasma betaine concentrations are inversely associated with cardiovascular events [
15] and higher levels have been linked to a pattern of favorable lifestyle habits and leanness [
16].
Limited and conflicting data indicate a potential effect of betaine supplementation on human exercise performance and recovery [
17,
18]. The underlying mechanisms are poorly understood but may involve beneficial influences on fat oxidation, regulation of gene expression, growth hormone and insulin-like growth factor-1 (IGF-1) secretion, creatine and protein synthesis, regulation of inflammation and oxidative stress, cortisol production, regulation of organic osmolytes and cell water retention, and sensations of fatigue [
17,
18]. These betaine-related physiological effects suggest a potential influence on performance and metabolic recovery from both resistance exercise and long-distance cycling and running. However, most betaine supplementation studies have focused on strength-related outcomes or short bursts of intense exercise [
18,
19,
20,
21]. A recent review of 17 studies concluded that betaine supplementation for at least 7 days may improve various measures of muscular strength [
18]. In this systematic review and meta-analysis, a significant effect size of 0.47 was reported for maximal strength in the lower body but not for upper body strength, cycling sprint power, or muscular endurance [
18]. Few studies have investigated betaine’s influence on long distance cycling and running performance and metabolic recovery. One study reported no effects of 2-weeks intake of betaine supplements (2.5 g/d) on intensive running time to exhaustion (~33 minutes) and post-exercise oxidative stress, with a modest effect in lowering lymphocyte apoptosis [
2]. Another study showed no performance effect of acutely ingesting 5g betaine in one liter of rehydration beverages prior to 75 minutes of treadmill running followed by a performance sprint to exhaustion (3-4 minutes at 84% VO2max) [
23].
The impact of betaine supplementation on long-distance cycling performance, gut permeability, and metabolic recovery has not been investigated. We hypothesized that ingestion of 3 g/d betaine versus placebo for two weeks in trained cyclists prior to a 60-km cycling time trial would improve performance, moderate exercise-induced changes in gut permeability, and improve metabolic recovery using a randomized crossover trial design. Outcome measurements also included the Profile of Mood States (POMS) to determine betaine’s potential influence on fatigue, vigor, and other mood states. A moderate dose of betaine (3g/d) for a 2-week period was chosen for this investigation and represents the upper level of dietary intake achievable by individuals with diets high in whole grains, spinach, beets, and seafood [
6]. The ergogenic and clinical effects of betaine have been investigated with doses ranging from 0.5 to 20 g/d, with 1.5–6 g/d found to be sufficient to increase plasma betaine and methionine concentrations and lower homocysteine within 2 weeks [
6].
2. Materials and Methods
Study Participants
Healthy male and female cyclists were invited to take part in this study if they met the inclusion criteria including 18 to 65 years of age, capable of cycling 60 km in a laboratory setting, and a willingness during the 6-week study period to train normally, maintain body weight, restrict intake of betaine-rich foods such as beets, spinach, wheat bran, and wheat germ, avoid betaine supplements and other nutrient supplements and herbs, and abstain from use of medications with a potential to influence post-exercise inflammation (in particular, nonsteroidal anti-inflammatory drugs or NSAIDs). To be included in the study, study participants had to report that they did not have a gastrointestinal disease (irritable bowel syndrome, chronic nausea, vomiting, and diarrhea, Crohn’s disease, Celiac disease, diverticulosis) or chronic disease. Participants agreed to taper exercise training and ingest a moderate-carbohydrate diet using a food list restricting high fat foods and visible fats.
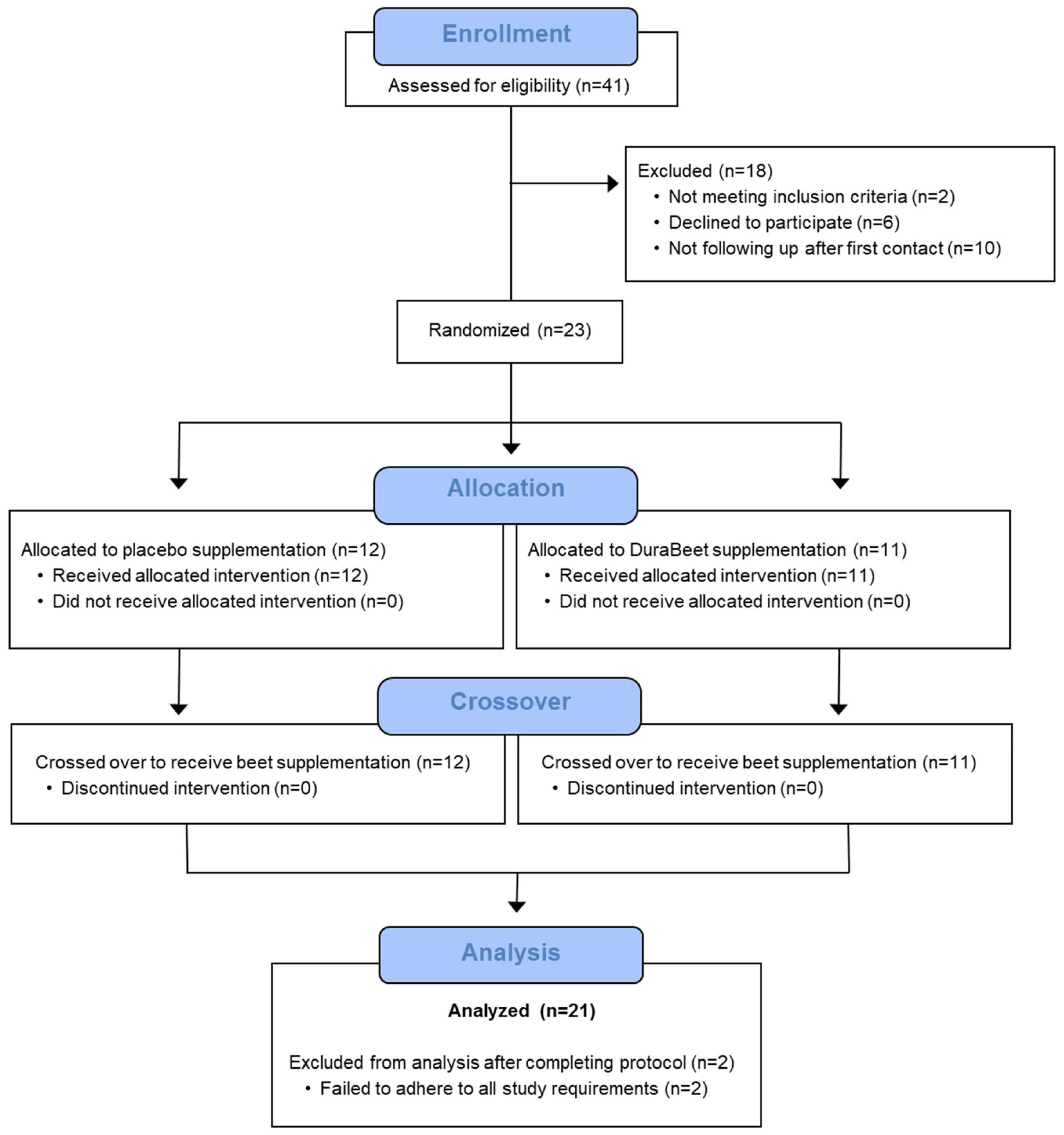
A total of 41 participants were assessed for eligibility and 23 were entered into the study, with 21 completing all aspects of the protocol (
Figure 1). A post-hoc analysis using repeated measures for between trials and timepoints showed a statistical power of 0.93 for plasma betaine and dimethylglycine (two metabolites of interest in this study) at an alpha level of 0.05 with 21 subjects (WebPower, R package v. 0.9.4). Participants voluntarily signed the informed consent, and procedures were approved by the university’s Institutional Review Board. Trial Registration: ClinicalTrials.gov, U.S. National Institutes of Health, identifier: NCT06392360.
Study Design
This study employed a randomized, placebo controlled, double-blind, crossover design with two 2-week supplementation periods (betaine and placebo capsules) and a 2-week washout period. The 2-week supplementation period was utilized because this duration was similar to what was used in 13 of 17 studies in a recent review. The supplement capsules were supplied in coded bottles by the sponsor, with the double-blind code held until after all study samples had been analyzed. Study procedures were conducted at the Appalachian State University Human Performance Laboratory (HPL), North Carolina Research Campus, Kannapolis, NC.
Subjects came to the lab for orientation/baseline testing, pre-and post-2-weeks supplementation blood sample collections, and two 60-km cycling sessions. During the first lab visit, study participants reported to the lab in an overnight fasted state, voluntarily signed the consent form, and completed questionnaires including a 1-10 rating of delayed onset of muscle soreness (DOMS) [
24], profile of moods state (POMS) [
25], and demographic, health, and training histories. An abbreviated 40-item version of POMS was used, and participants rated moods using the right-now approach [
25]. Responses were based on a five-point scale anchored by not at all (score of 0) and extremely (score of 4). Scores for the seven subscales were calculated by summing the numerical ratings for items that contributed to each subscale, with the total mood disturbance (TMD) calculated by summing the totals for the negative subscales (tension, depression, anger, fatigue, confusion) and then subtracting the total for the positive subscales (vigor, esteem-related affect), and adding 100 to eliminate negative scores. A 35 ml blood sample was collected. Height and body weight were assessed, with body composition measured using the BodPod system (Cosmed, Rome, Italy). The seca medical Body Composition Analyzer (mBCA) (Chino, CA) with 8-point bioelectrical impedance (BIA) was used to assess body composition, total body water, and extracellular water. Participants next ingested a nonabsorbable sugar solution (SS) containing 5 g lactulose (Sigma Aldrich, St. Louis, MO), 100 mg
13C mannitol (Cambridge Isotope Laboratories, Tewksbury, MA), and 1.9 g
12C mannitol (Sigma Aldrich) in 450 ml water. All urine excreted from 0-5 h post SS ingestion was collected in a urine collection container. Participants were urged to drink water after the first hour of SS ingestion to ensure adequate urine output. An increase in the post-exercise urine lactulose/
13C mannitol ratio (L:
13CM) was used as the primary indicator of increased gut permeability [
15]. The Boost beverage was next consumed at 7 kcal/kg 1.5 h post SS ingestion (to simulate what occurred in this study post-exercise). The urine collection container was placed in the refrigerator until participants returned to the lab the next day. The total urine volume was measured, and four 50 ml aliquots were frozen in a minus 80°C freezer until analysis.
During the second lab visit the next day, participants returned the urine collection container. Study participants were tested for maximal aerobic capacity (VO2max) during a graded, cycling test (25 watt increase every two minutes starting at 50 watts for females and 100 watts for males) with the Lode cycle ergometer (Lode B.V., Groningen, Netherlands) and the Cosmed CPET metabolic cart (Cosmed, Rome, Italy). A 2-week supply of the coded supplement capsules (either betaine or placebo) was given to the participants with instructions to ingest 6 capsules per day (3 g/d, 500 mg/capsule) with 3 capsules ingested daily with the first meal in the morning and 3 more with the last meal of the day. The betaine and placebo capsules were prepared by the study sponsor (AGRANA Beteiligungs-AG, Vienna, Austria). The white DuraBeet® crystalline betaine was produced from beet molasses at the betaine crystallization plant in Austria according to European standards. The placebo capsules each contained 355 mg microcrystalline cellulose, 3 mg magnesium stearate, and 2 mg precipitated silica in identical looking capsules. Participants were instructed to swallow the capsules whole with a beverage and not bite down on them. The coded supplement bottles were brought back to the lab after 2 weeks to help verify compliance with the supplementation regimen.
During the 3-day period prior to the 60-km cycling bouts, participants tapered exercise training and ingested a moderate-carbohydrate diet using a food list restricting high fat foods, visible fats, and caffeine. Participants recorded all food and beverage intake in 3-day food records with macro- and micro-nutrient intake calculated using the Food Processor dietary analysis software system (Version 11.11, ESHA Research, Salem, OR, USA).
After the 2-week supplementation period, study participants reported to the Human Performance Lab. The testing protocol during the lab sessions with the 60-km cycling time trial sessions were organized as follows:
7:00 am: Participants turned in the 3-d food record. A 35 ml blood sample was collected. Participants provided DOMS and POMS ratings and completed a 2-week retrospective symptom survey (with ratings of gastrointestinal symptoms, mental health, respiratory illness, sleep quality, pain symptoms, and overall wellbeing).
7:10 am: Participants ingested 3 betaine or placebo supplement capsules with 500 ml water.
7:30 am: After a warm-up, participants cycled for 60-km at race pace intensity on their own bicycles fitted to Saris H3 direct drive smart trainers (Madison, WI, USA) with monitoring by the Zwift online training platform (Long Beach, CA, USA) and the Cosmed CPET metabolic cart (Rome, Italy). Heart rate, cycling speed, cadence, distance, and power were measured and recorded continuously during the 60-km bout. Metabolic parameters such as breathing rate, ventilation, and oxygen consumption were measured after 15 min and then every 30 min during the cycling session. Participants consumed 3 ml/kg water every 15 min. No other beverage or food containing energy or nutrients were allowed during the cycling sessions.
Exercise-recovery period: Blood samples were collected immediately after completing the cycling session, and then 1.5h-, 3.0h-, and 24h-post-exercise. Participants ingested the 450 mL SS within the first minute of getting off the bicycle, and urine was collected for the next five hours. Participants were allowed to shower and change their clothes. The DOMS and POMS questionnaires were administered each time blood samples were collected. Body composition, total body water, and extracellular water were measured using the seca mBCA each time blood samples were collected. No food or beverage other than water (7 ml/kg) was ingested during the first 1.5h post-exercise. After the 1.5h post-exercise blood draw, participants ingested 7 kcal/kg of a fortified nutrient meal replacement beverage (480 kcal/474 ml) (Boost, Nestlé S.A., Vevey, Switzerland). Another blood sample was collected 3-h post-exercise prior to leaving the lab. Participants ingested 3 betaine or placebo supplement capsules with the last meal of the day. Participants returned the next morning at approximately 7:00 am (24h) to turn in the 5h urine container, complete the DOMS and POMS questionnaires, and provide a blood sample. Body composition, total body water, and extracellular water were measured using the seca mBCA.
After the first cycling session, participants completed a 2-week washout period without the supplements, crossed over to the opposite treatment arm, and then repeated all procedures. Participants maintained their normal diets and exercise routines during the 2-week washout period and the entire 6-week study.
Sample Analysis
Plasma aliquots were prepared from EDTA blood collection tubes and stored in a -80°C freezer for metabolomics and intestinal fatty acid binding protein (I-FABP) analysis. The 5h urine samples were weighed with sample aliquots prepared and stored in a -80°C freezer for sugar analysis. Serum creatine kinase, myoglobin, and cortisol, and complete blood counts (CBCs) with a white blood cell differential count (EDTA tubes) were analyzed using Labcorp services (Burlington, NC).
Urine Sugar and Plasma I-FABP Analysis:
The urine samples were analyzed using a high-performance liquid chromatography (HPLC) method for
12C- and
13C-mannitol, and lactulose at the Mayo Clinic’s Immunochemical Core Lab (Rochester, MN) [
26,
27]. The HPLC-MS/MS System included an API 5000 triple-quadruple mass spectrometer (Applied Biosystems/MDS SCIEX, Foster City, CA/Concord, Ontario, Canada) coupled with an electrospray ionization source that was operated at 700°C in the negative ion mode. Urine samples (25 µL) were added to a 96 deep-well plate. Samples, quality controls, and calibrators were diluted 11x by the addition of 250 µL of an internal standard consisting of a mixture of 13C-mannitol and lactulose. The analytes were separated by normal phase HPLC and detected on a tandem mass spectrometer (LC-MS/MS) utilizing electrospray ionization, operating in the multiple-reaction monitoring negative mode. The calibration utilized two different six-point standard curves over a concentration range of 0.5–500 µg/mL for mannitol and 0.125–125 µg/mL for lactulose. The 13C-mannitol internal standard was used to normalize the mannitol values, and the lactulose internal standard normalized the lactulose values. Sugar peaks were identified and measured using Analyst 1.6 software package (MDS SCIEX, Concord, Ontario, Canada). The limit of detection, the lowest analyte concentration likely to be reliably distinguished from the limit of blank, was 0.3 mg/mL for 12C-mannitol, 0.5 mg/mL for 13C-mannitol, and 0.3 mg/mL for lactulose. Plasma I-FABP concentrations were analyzed using EDTA plasma via enzyme-linked immunosorbent assay kits (HK406, Hycult Biotech Inc., Pennsylvania, USA).
Plasma Untargeted Metabolomics Analysis:
Plasma samples were analyzed using untargeted metabolomics procedures at Metabolon (Morrisville, NC) [
28]. Samples were prepared using the automated MicroLab STAR
® system from Hamilton Company (Reno, NV). Several recovery standards were added prior to the first step in the extraction process for quality control (QC) purposes. Proteins were precipitated with methanol under vigorous shaking (Glen Mills GenoGrinder 2000) followed by centrifugation. The resulting extract was divided into multiple fractions: two for analysis by two separate reverse phase (RP)/UPLC-MS/MS methods with positive ion mode electrospray ionization (ESI), one for analysis by RP/UPLC-MS/MS with negative ion mode ESI, one for analysis by HILIC/UPLC-MS/MS with negative ion mode ESI, while the remaining fractions were reserved for backup. Samples were placed briefly on a TurboVap
® (Zymark) to remove the organic solvent. Several types of QC controls were analyzed in concert with the experimental samples: a pooled matrix sample, extracted water samples, and a cocktail of QC standards. Instrument variability was determined by calculating the median relative standard deviation (RSD) for the standards that were added to each sample prior to injection into the mass spectrometers. Overall process variability was determined by calculating the median RSD for all endogenous metabolites present in 100% of the pooled matrix samples. Experimental samples were randomized across the platform run with QC samples spaced evenly among the injections. All methods utilized a Waters ACQUITY ultra-performance liquid chromatography (UPLC) and a Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. Raw data was extracted, peak-identified and QC processed using a combination of Metabolon developed software services. Metabolon maintains a library based on authenticated standards that contains the retention time/index (RI), mass to charge ratio (m/z), and fragmentation data on all molecules present in the library. Peaks were quantified using area-under-the-curve. Data was normalized by the medians equal to one method and normalizing each data point proportionately.
Statistical Procedures
Data are expressed as mean±SE. Except where described, data sets were analyzed using the generalized linear model (GLM), repeated measures ANOVA module in SPSS (IBM SPSS Statistics, Version 28.0, IBM Corp, Armonk, NY, USA). The statistical model utilized the within-subjects approach: 2 (supplement) (betaine or placebo) x 6 (time points) (pre-and post-2 weeks supplementation, and 0h, 1.5h, 3h, and 24h post-exercise) repeated measures ANOVA and provided time (i.e., the collective effect of the cycling exercise bout), supplement (i.e., the collective supplement effect), and interaction effects (i.e., whether the data pattern over time differed between supplement trials). If the interaction effect was significant (p≤0.05), then post-hoc analyses were conducted using paired t-tests comparing time point contrasts between supplement trials. An alpha level of p<0.05 was used for statistical significance testing for paired t-tests (non-repeated supplement trial comparisons). A p-value of ≤0.0125 was used after Bonferroni correction for 4 multiple tests of changes from pre-study trial comparisons within repeated measures ANOVA analyses.
The metabolomics data was analyzed statistically using the ArrayStudio/Jupyter Notebook on log transformed data. Statistical analyses included principal component analysis (PCA) and two treatment two period crossover ANOVA. P-values and q-values were used for statistical significance testing. Partial Least Squares Discriminant Analysis (PLS-DA) was used [
1] to identify metabolites that were discriminatory between the betaine and placebo trials, and [
2] for differentiation between all six timepoints (ropls R package v.1.38.0). Metabolites with Variable Importance in the Projection scores (VIPs) were considered to be significant if ≥2.5. VIPs were cross-validated using features produced from Lasso regression with an expected binary outcome (alpha = 0.7) (caret R package v.7.0-1). Model performance was evaluated using cross-validation inherent within the function for 7 iterations. The resultant cumulative Q2 and proportion of the variance in response to the treatment or timepoint (R2Y) values were used to assess model predictability and quality. The effect of sex on metabolite changes was determined using student’s t-test or a non-parametric equivalent between the sexes with all time-point measurements. A two-way repeated-measures ANOVA was also utilized to check for interactions between sex and treatment. The steps for t-test analysis included assessing statistical power using Cohen’s D effect size, checking the assumptions of normality (Shapiro-Wilks) and equal variances (F-test), and then applying the appropriate test (Student’s T-test or Wilcox). ANOVA procedures included 0-3h post-exercise time-points and all time-points. Statistical power was assessed for two-way ANOVA using Cohen’s F effect size for each group and checked using assumptions of normality including the Mauchly’s test. Greenhouse-Geisser p-value corrections were made if the assumption of sphericity was not met. FDR corrections for p-values for the main tests were made using Benjamini-Hochberg.
3. Results
A total of 21 study participants (n=15 males, n=8 females) completed all study procedures (
Table 1). Age, BMI, percent body fat, and maximal heart rates were comparable between sex groups, with higher body mass, cardiorespiratory capacity (VO
2max), maximal cycling power, and ventilation capacity for the male versus female cyclists (p<0.05). The pattern of change over time did not differ between the male and female cyclists for urine lactulose/
13C mannitol ratio (L:
13CM) (supplement x time x sex interaction effect, p-value =0.634, plasma I-FABP, interaction p-value=0.461), or plasma betaine (interaction p-value=0.852). No sex differences were found for 60-km cycling performance in terms of average percent maximal heart rate, power output in watts, and oxygen consumption rates (all p>0.13). In both types of ANOVA analyses, there were no statistically significant differences found between supplement trials examining all metabolites and no interactions between supplement and gender were found. Thus, outcome measures are presented for all study participants combined.
Performance data for the 60-km cycling time trials after 2-weeks supplementation with betaine and placebo are summarized in
Table 2. The time to complete the 60-km cycling time trials was faster with betaine compared to placebo (-1.41±0.7 minutes) (effect size=0.457, p=0.049). No supplement trial differences were found for average power output, speed, oxygen consumption rates, respiratory exchange ratio (RER), rating of perceived exertion (RPE), and heart rates.
Three-day food records were collected at the end of each 2-week supplementation period. No differences in macro- and micro-nutrient intake were found between trials. Intake during the betaine and placebo trials did not differ for folate (355±53.9, 358±56.4 µg/d, p=0.956) and choline (344±33.3, 315±28.2 mg/d, p=0.342), respectively. Nutrient data from the two 3-day food records were averaged for the 21 cyclists, and energy intake averaged 2117±122 kcal/d (8.86±0.51 MJ/d), and carbohydrate, protein, fat, and alcohol represented 42.0±1.7, 20.6±1.3, 36.8±1.4, and 1.4±0.5%, respectively, of total energy. Symptom logs revealed no significant trial differences (data not shown).
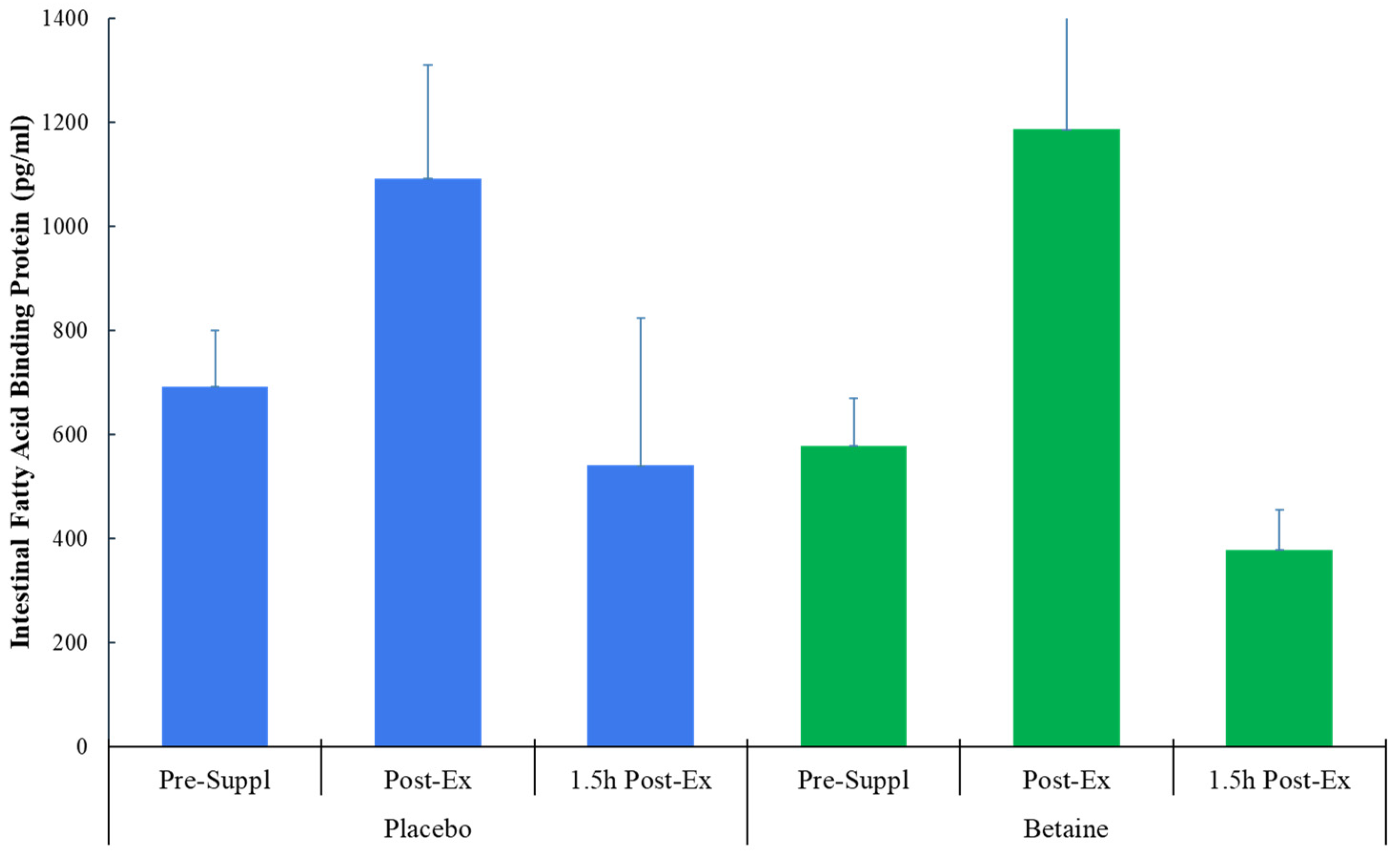
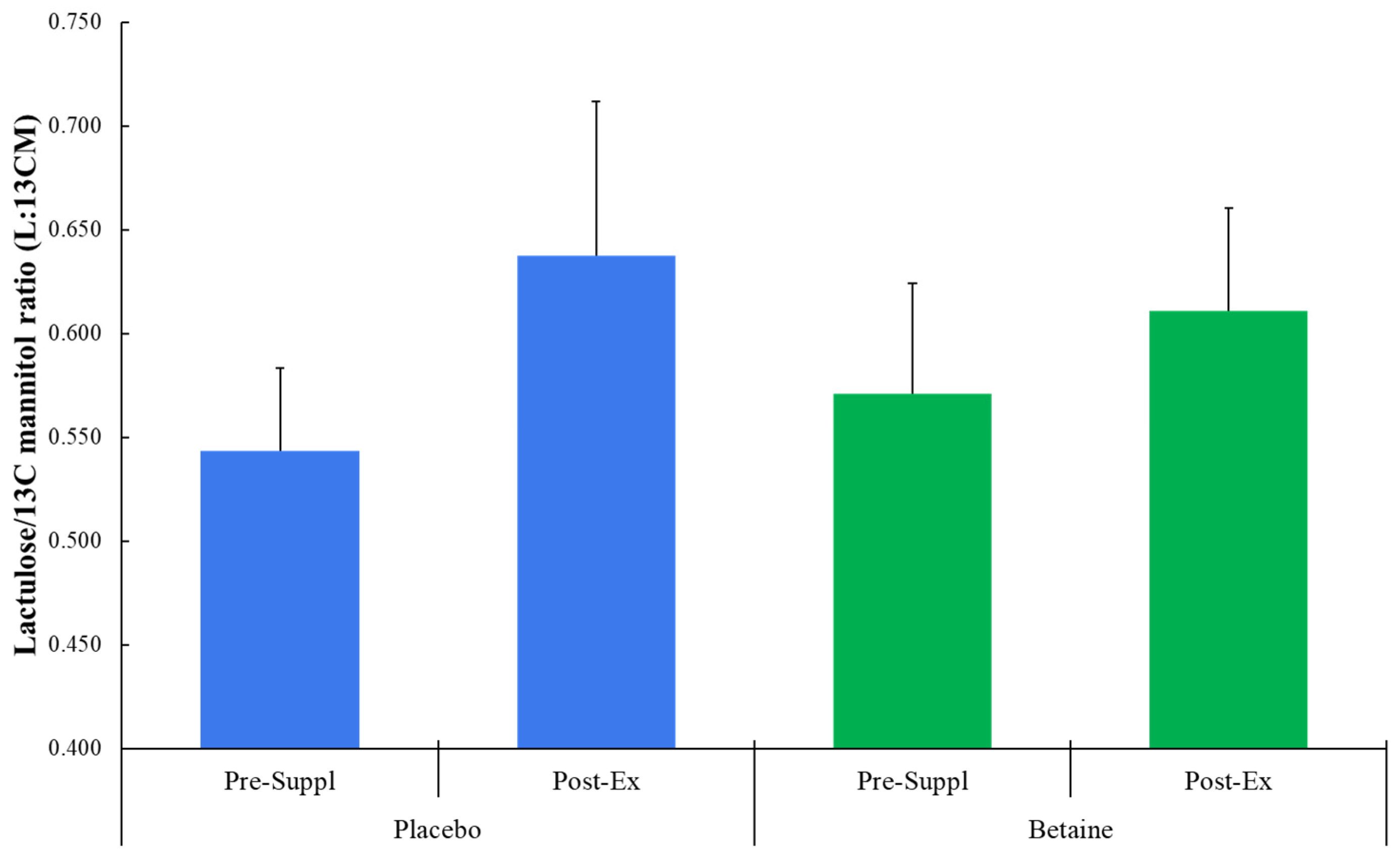
No trial differences were found for plasma I-FABP (time effect, p=0.013, interaction effect, p=0.076) (
Figure 2), L:
13CM (time p=0.237, interaction p=0.559) (
Figure 3), or L:
12CM (time p=0.071, interaction p=0.695, data not shown). Plasma volume shifts did not differ between the placebo and betaine trials immediately post-exercise (-7.7±1.2% and -7.3±0.8%, respectively, p=0.800) and 1.5h-post-exercise (1.6±0.9% and 2.3±1.2%, respectively, p=0.637).
The seca BIA data showed that the pattern of change over time differed for total body intracellular water between the betaine and placebo trials (interaction effect, p=0.010), with changes from pre-supplementation levels decreasing in the betaine group compared to placebo (
Table 3). No trial differences were found for total body extracellular water (interaction effect, p=0.640).
The plasma metabolomics dataset after sample analysis included 1,483 biochemicals with 1,214 compounds of known identity and 269 compounds of unknown structural identity. Of the 1,483 detected biochemicals, 1,278 exhibited significant time-dependent effects. Thus, the 60-km cycling time trial caused an extensive perturbation in circulating metabolites. Statistical analysis showed that 214 metabolites exhibited significant trial treatment effects and 130 metabolites had significant trial x time interaction effects. Supplement Data Sheets S1 and S2 include the raw and batch normalized values for each sample and the statistical analysis results in the form of a heatmap.
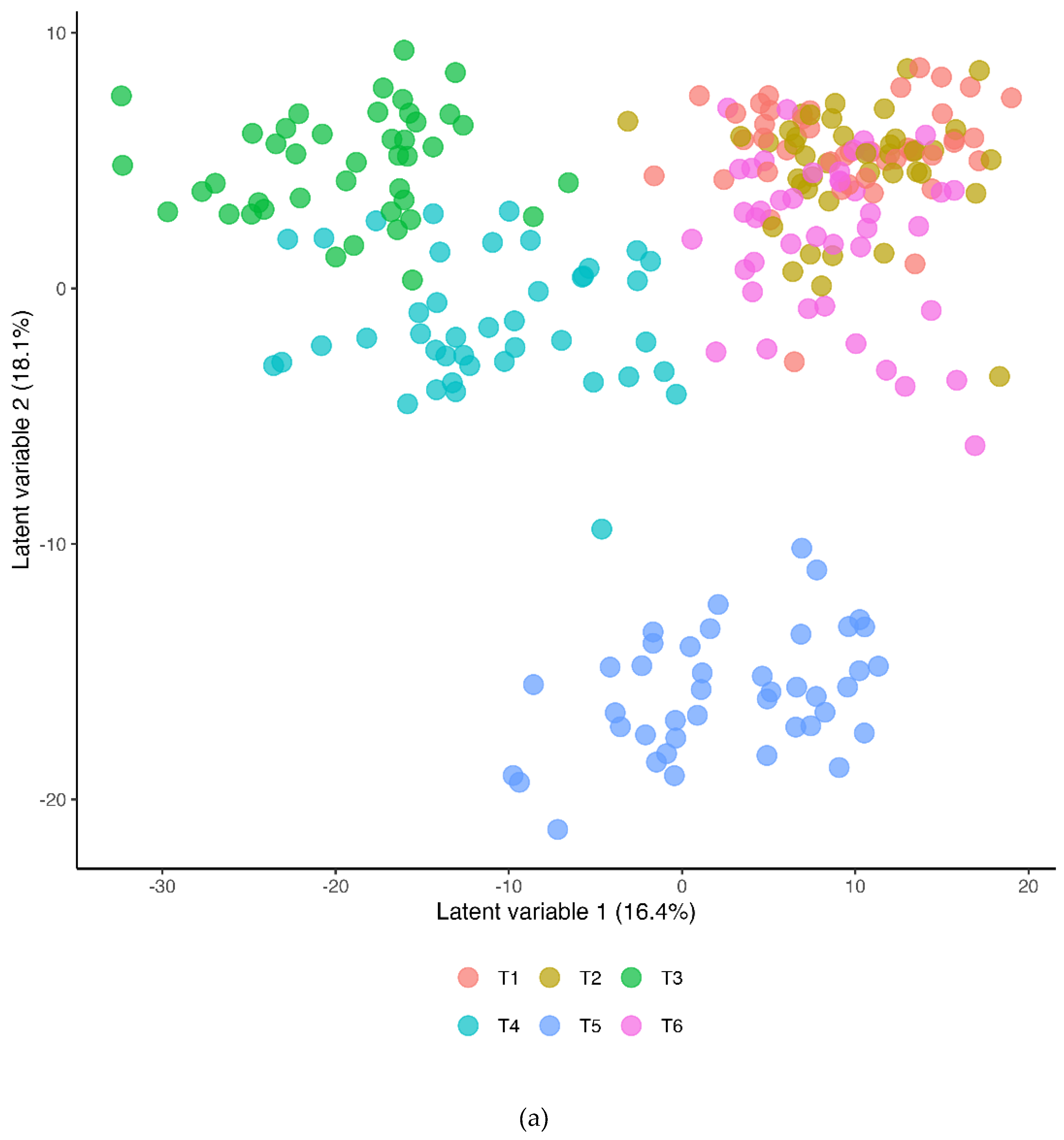
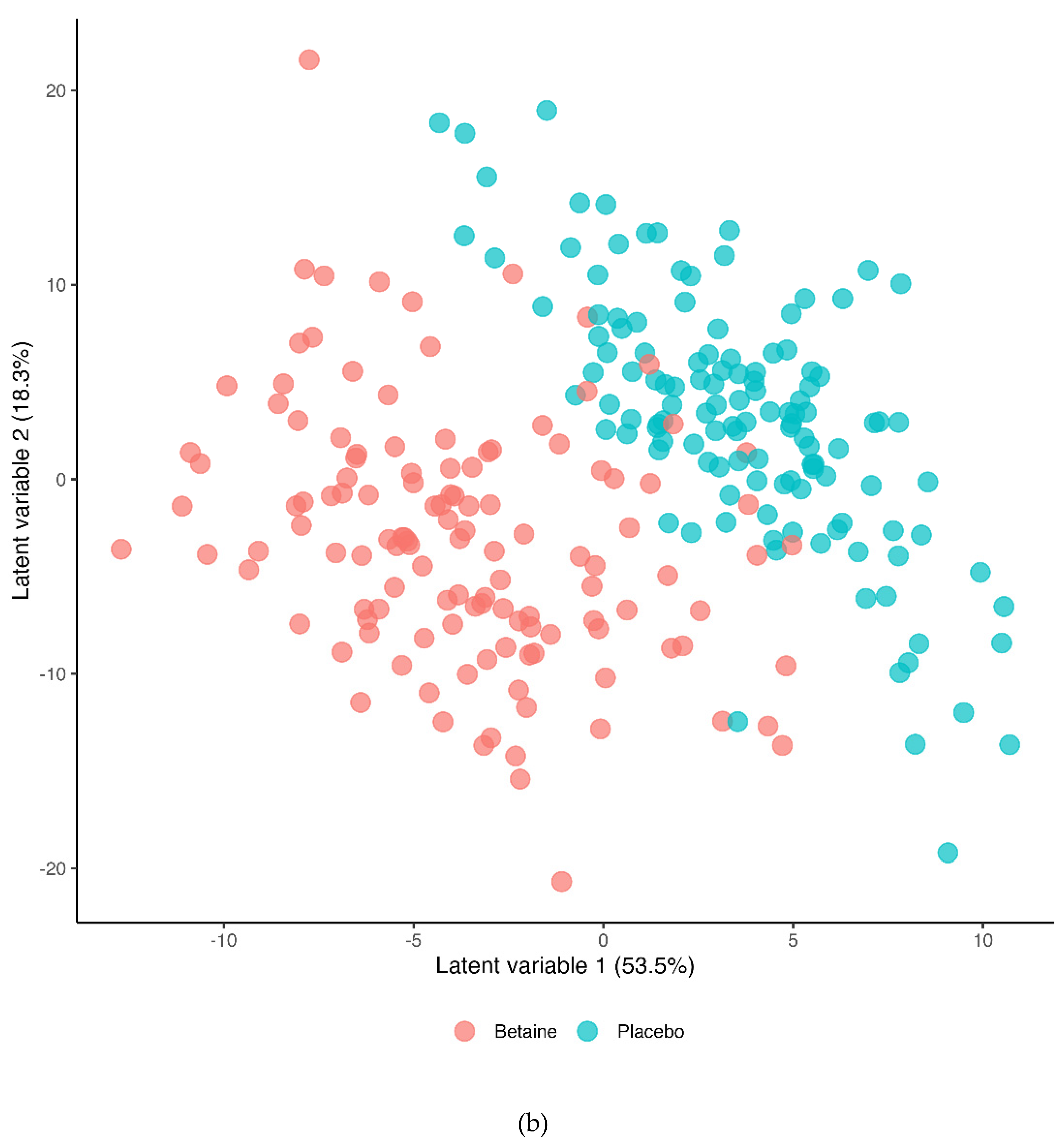
The Partial Least Squares Discriminant Analysis (PLS-DA) for the betaine and placebo trials across all six timepoints (R2Y=0.495, Q2=0.451) is shown in
Figure 4a (T1=presupplementation, T2=post-2-weeks supplementation, T3=immediately post-exercise, T4=1.5 hours post-exercise, T5=3 hours post-exercise, T6=24 hours post-exercise). The 60-km cycling time trial had a significant effect on metabolite shifts especially for the first three hours of recovery.
Figure 4b shows the PLS-DA contrast for the betaine and placebo trials (all 6 time points). A distinct difference was shown between the betaine and placebo trials (R2Y=0.769, Q2=0.574). Lasso regression at a level of 0.7 and Variable Importance in Projection (VIP) scores of 2.5 and higher identified nine metabolites that were most influential in distinguishing the betaine and placebo trials including plasma betaine, dimethylglycine (DMG), sarcosine, carnitine, deoxycarnitine, 5’methylthioadensone (MTA), acetylcarnitine (C2), alpha-ketoglutaramate, and N-acetylserine.
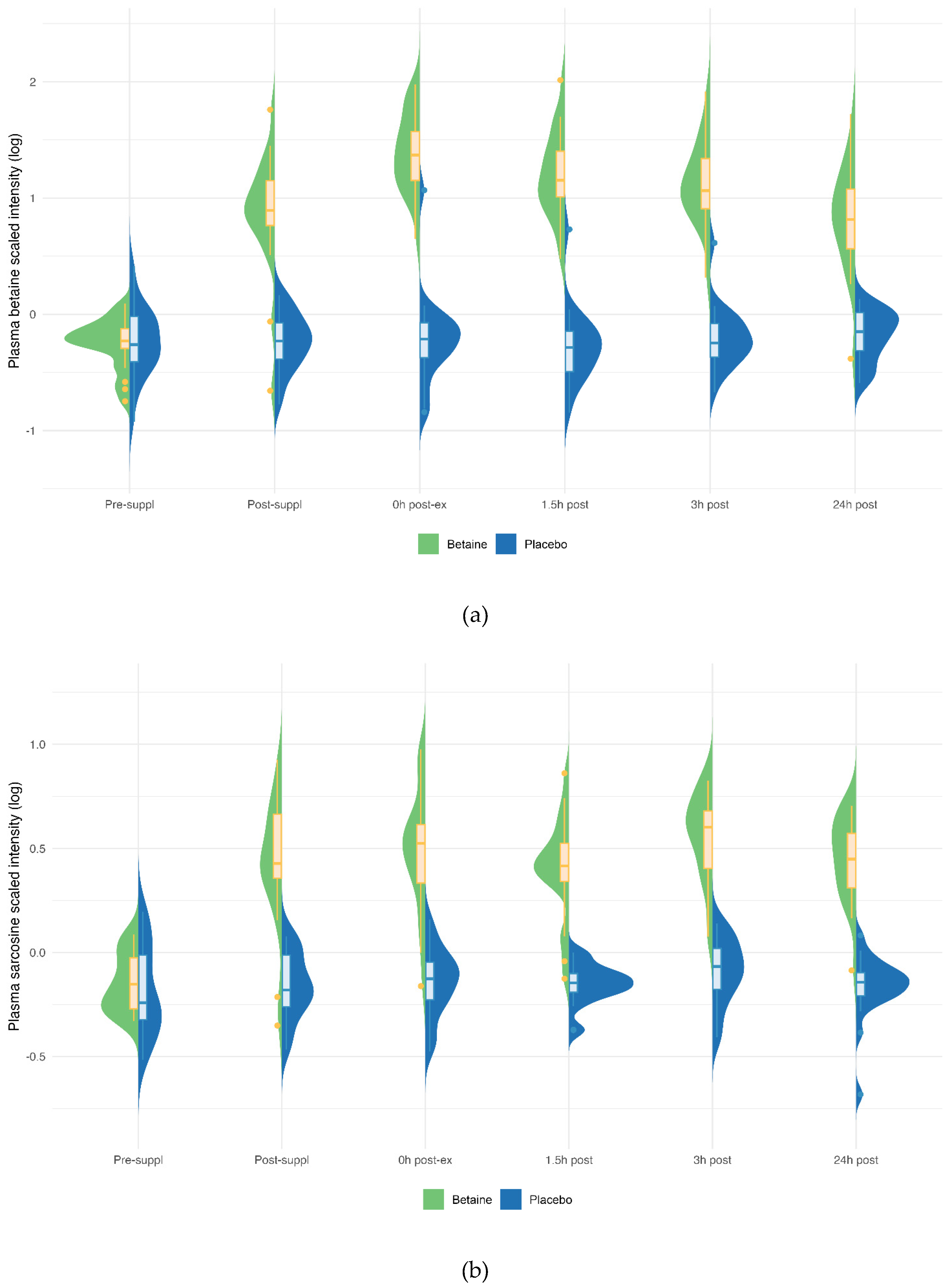
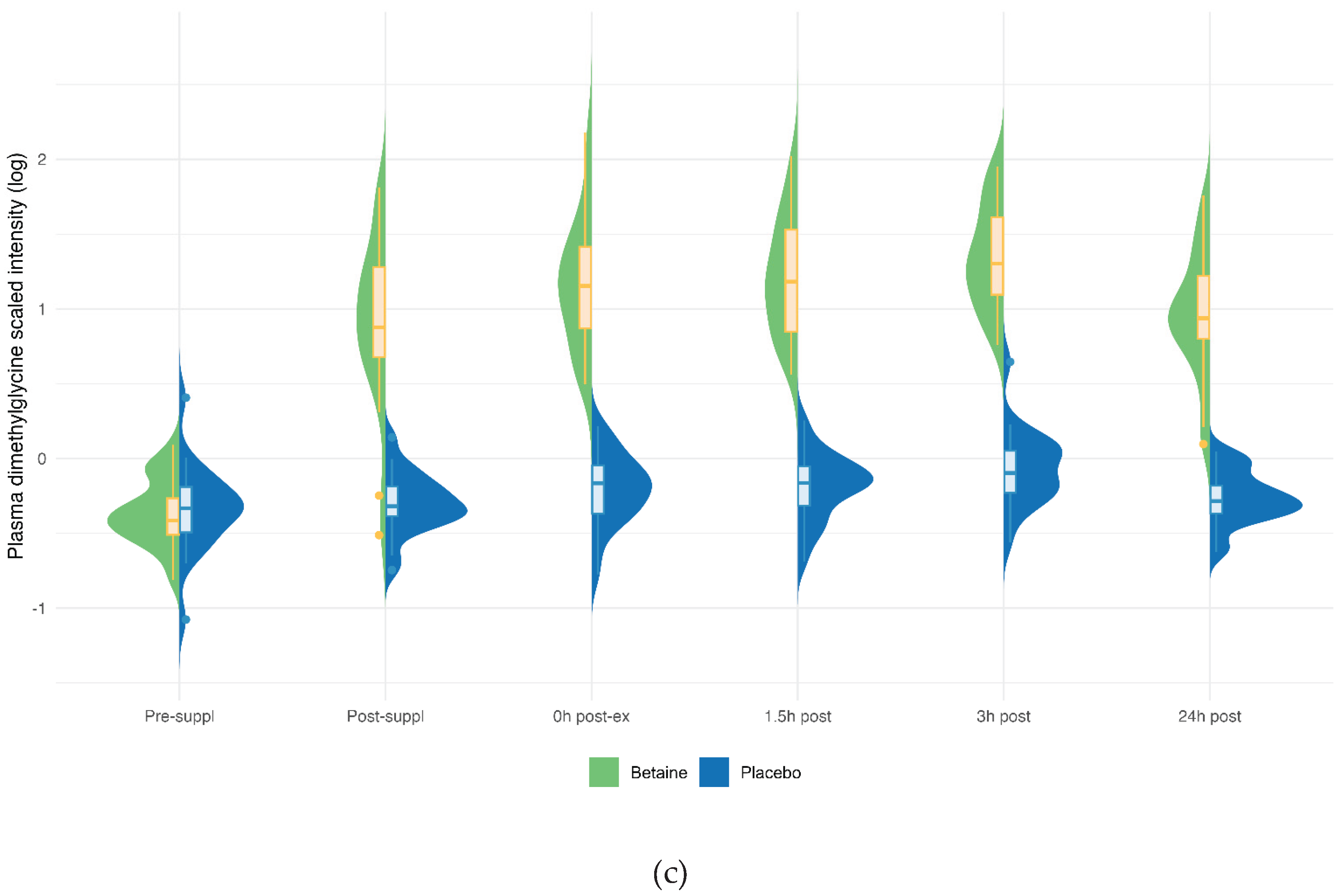
Betaine versus placebo supplementation was linked to significant increases in plasma betaine (treatment and timepoint interaction effect, q<0.001) and the betaine metabolites dimethylglycine (DMG) (q<0.001), and sarcosine (q<0.001) (
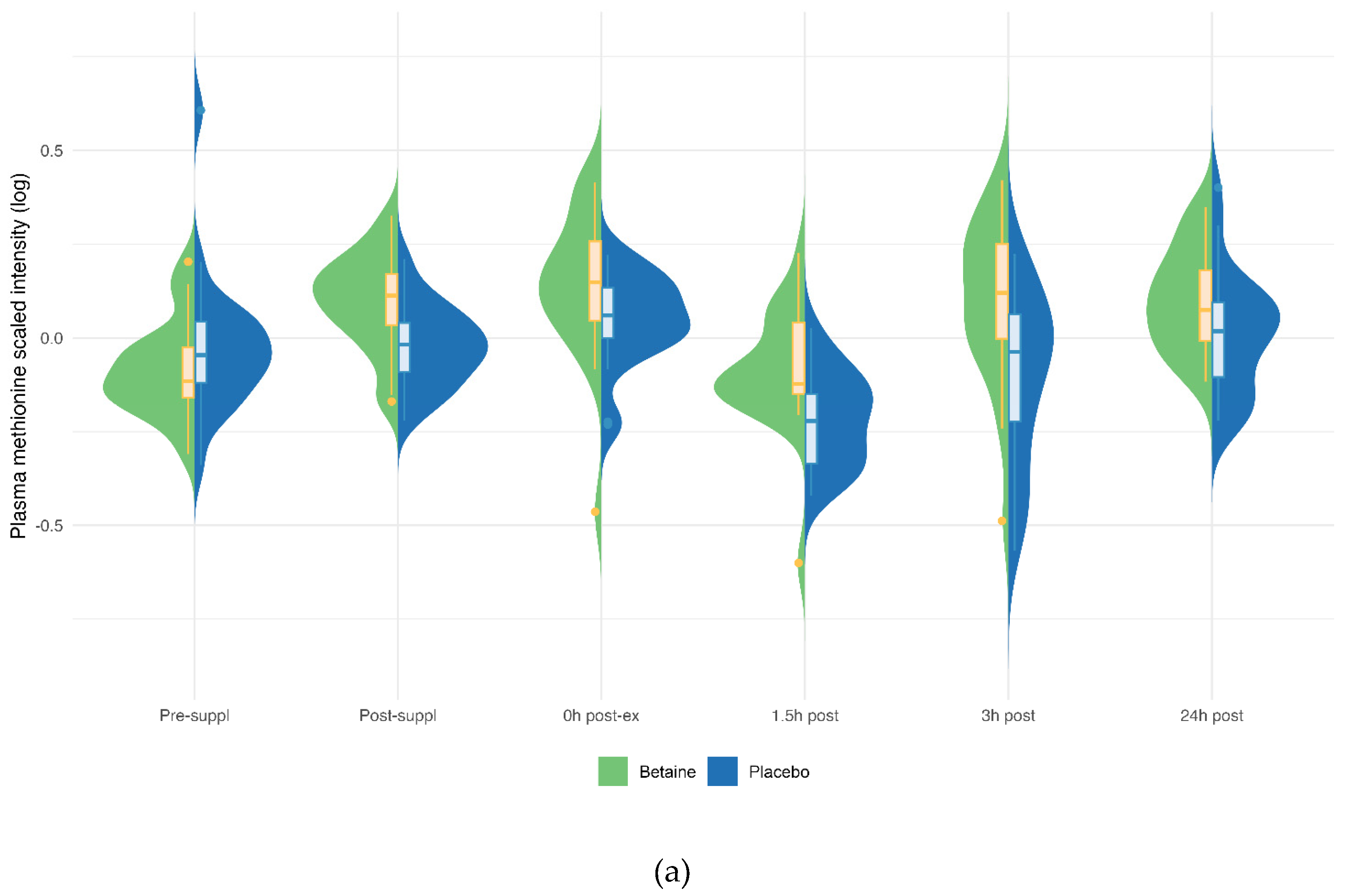
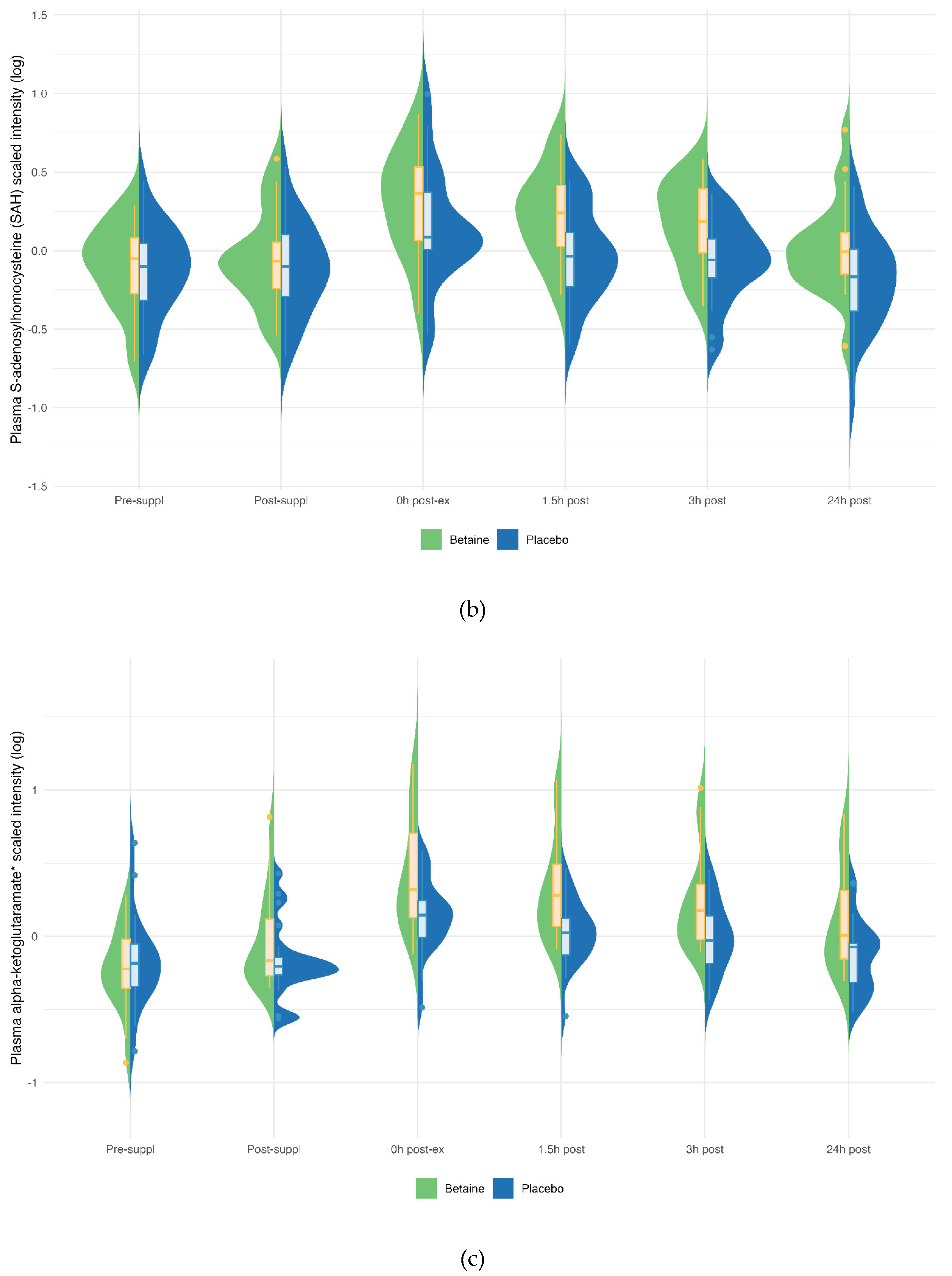
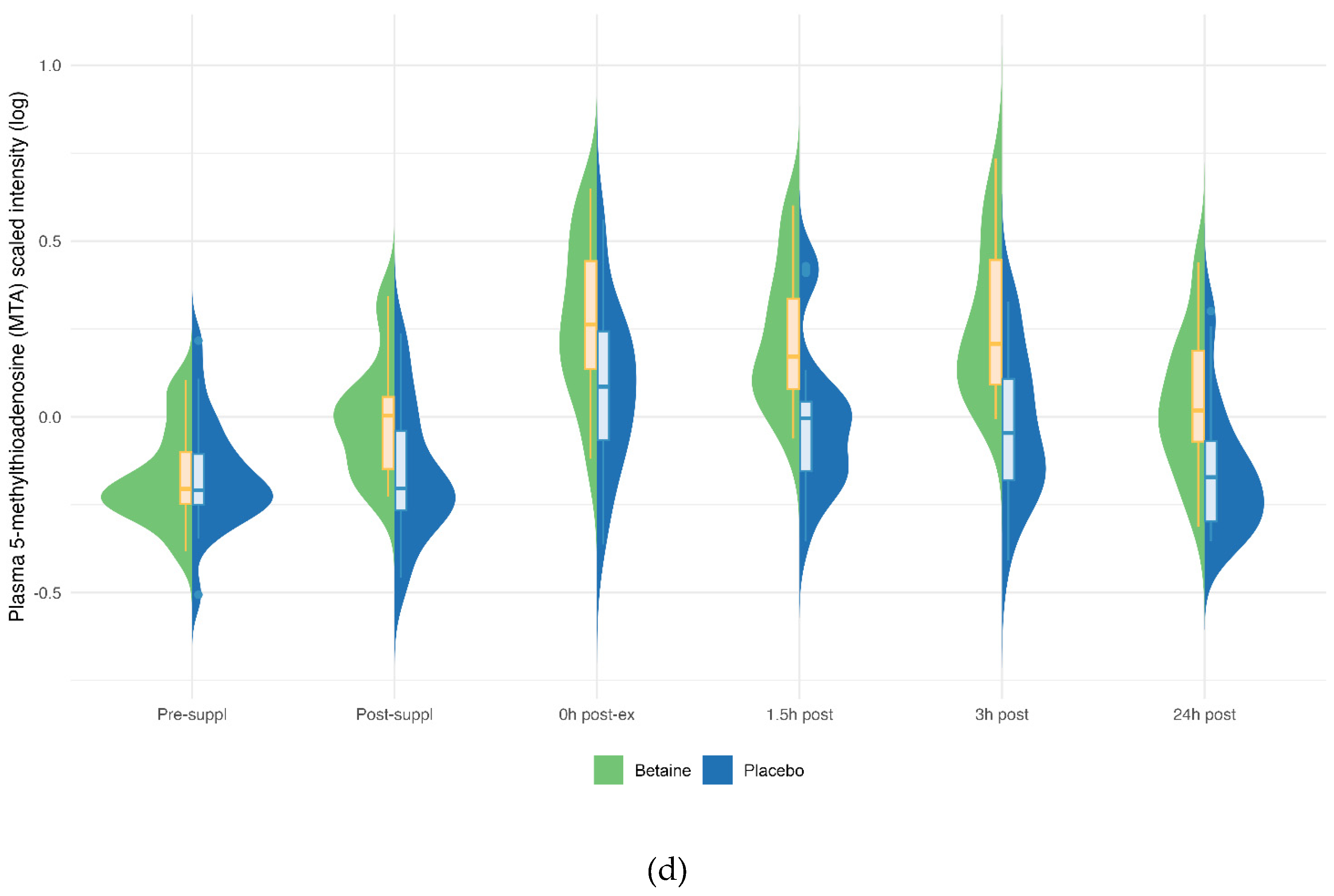
Figure 5a–c). Betaine supplementation was also associated with significant increases in methionine (q=0.036) and related metabolites S-adenosylhomocysteine (SAH) (q=0.025), alpha-ketoglutaramate (q<0.001), and 5’methylthioadensone (MTA) (q<0.001) (
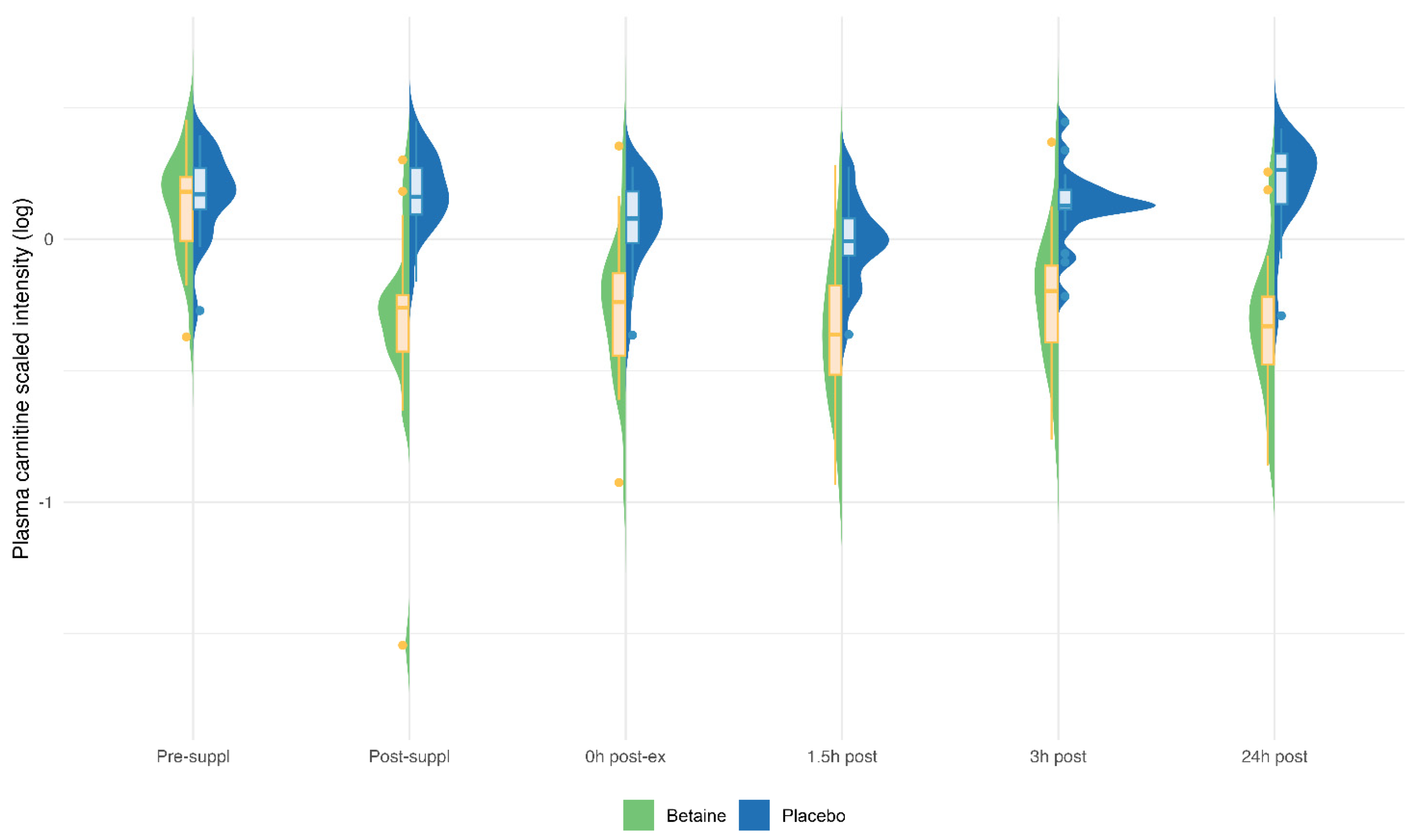
Figure 6a–d). Betaine supplementation was also related to a decrease in plasma carnitine (q<0.001) (
Figure 7) and numerous acylcarnitines (Supplement Data Sheet S2).
Significant post-exercise increases (all p<0.001) were measured for plasma inflammation-related metabolites including 13-HODE + 9-HODE, 9,10- and 12,13-DiHOMEs, and 12-HETE. The pattern of increase over time for each of these metabolites, however, did not differ between the betaine and placebo trials (all p>0.05) (Supplement Data Sheets S1 and S2).
4. Discussion
This study showed that the intake of a modest 3 g/d dose of betaine for a relatively short 2-week supplementation period improved 60-km cycling time trial performance. Betaine supplementation was also linked to significant increases in pre- and post-exercise plasma levels of betaine and related metabolites in the one carbon metabolism pathway, with reduced levels of carnitine and acylcarnitines. No effect of betaine supplementation was found for gut permeability, I-FABP, muscle soreness or muscle damage biomarkers, serum cortisol, exercise-induced mood disturbance, or inflammation biomarkers. Contrary to expectations, betaine supplementation was linked to a small but significant decrease in intracellular water.
This is the first study to show that betaine supplementation improves prolonged and intensive cycling performance. Most previous studies in this area focused on strength and power performance outcomes, and systematic reviews from 2017 and 2024 indicated moderate to high study heterogeneity and an inconsistent or modest betaine supplementation effect on lower body strength [
18,
20]. Proposed mechanisms include betaine-related effects on increased plasma levels of anabolic hormones, decreased cortisol responses, increased creatine and protein synthesis, improved cell water retention, and decreased feelings of fatigue (18,20,29). However, data to support these suppositions are inconsistent or lacking in randomized human trials. Our data showed a modest but significant effect of betaine supplementation on 60-km cycling time trial performance using a randomized, crossover design and double-blinded, placebo-controlled supplementation methods. No trial differences were found for exercise-induced muscle soreness or damage, inflammation, changes in serum cortisol, total mood disturbance (an index that includes fatigue), or shifts in plasma volume. Contrary to what has been proposed, BIA data supported a small but significant decrease in post-exercise intracellular water with betaine supplementation.
Enhanced cycling performance with betaine supplementation may in part be related to increases in the methyl donor pool that lead to favorable epigenetic influences on gene expression within muscle tissue [
18]. The elevated pre- and post-exercise plasma levels of betaine, methionine, dimethylglycine, and sarcosine with betaine supplementation confirms its efficacy in influencing the one-carbon metabolism pathway. One-carbon metabolism is significantly involved in epigenetic modifications by providing methyl groups for DNA methylation, impacting gene expression [
8]. Methionine plays a pivotal role in multiple biological functions and serves as a protein building block as well as functioning as a substrate/precursor for several important downstream metabolites, including the universal methyl donor S-adenosylmethionine (SAM) and the antioxidant glutathione. Although SAM was not detected in this study using untargeted metabolomics, its post-methyl-donating product, S-adenosylhomocysteine (SAH), was significantly and robustly elevated following betaine supplementation. This suggests that betaine supplementation promotes one-carbon metabolism by efficiently re-methylating homocysteine to methionine, which can then be used to generate additional methyl-donating SAM [
30]. Animal studies support that elevations in SAM via betaine supplementation promote muscle protein synthesis and improved grip strength through a regulatory effect on the mammalian target of rapamycin complex1 protein kinase (mTORC1) [
31].
Prolonged and intensive endurance exercise evokes extensive physiological adaptive responses through transcriptional reactions that are associated with epigenetic DNA methylation and histone modifications [
32,
33]. There is increasing evidence that physical activity and nutrition work together as major environmental factors to shape human genotypes phenotypes [
34]. Whether or not an increase in the DNA methyl donor pool through betaine supplementation amplifies and facilitates the epigenetic regulation of gene transcription for exercise-induced adaptations has not yet been investigated in humans. A study with young and old mice showed that betaine supplementation transcriptionally improved age-related mitochondrial respiration in skeletal muscle and improved running distance to exhaustion [
35]. A cell culture study with murine myoblasts showed that betaine stimulated muscle fiber differentiation by promoting the expression profile of genes encoding proteins involved in the IGF-1 pathway [
36].
Animal studies support decreases in fat mass and reduced intramyocellular lipid accumulation with betaine supplementation [
37,
38]. A systematic review of human studies showed a modest fat-lowering effect with higher intakes of betaine [
39]. The downward shift observed in circulating carnitine concentrations with betaine supplementation may represent a translocation of circulating carnitine into organ-bound liver and muscle carnitine. Our results are consistent with a recent metabolomics-based investigation of 32 dogs that showed a decrease in many plasma carnitine-containing metabolites with betaine supplementation [
40]. Betaine acts as a methyl donor in the biosynthesis of carnitine suggesting a positive impact on fat metabolism by enhancing fatty acid transport into the mitochondria through increased carnitine availability. However, our RER data did not support this potential glycogen-sparing effect within an exercise context. Additionally, plasma levels of major lipid oxidation intermediates formed during exercise (see Supplement Data Sheets S1 and S2) were similar across the betaine and placebo arms, suggesting that lipid oxidation rates during exercise were not significantly impacted by betaine supplementation. The performance and physiological effects of betaine’s influence in lowering plasma levels of carnitines and acylcarnitines remain to be determined.
Betaine is an osmolyte that helps maintain fluid balance and intracellular fluid volumes [
9,
10]. However, the data from our study showed a small but significant decrease in intracellular water with betaine supplementation. The 8-point BIA method used in our study is considered an accurate and reliable method for estimating total body, extracellular, and intracellular water [
41]. Other exercise-related studies using similar methods reported no effect of betaine supplementation on total body water [
42]. Body water responses to betaine supplementation may depend on the dosing regimen and the degree of physiological stress imposed during the exercise intervention.
Betaine supplementation had no significant effect on the modest exercise-induced changes in I-FABP and gut permeability. Animal data indicates that betaine may influence gut permeability by regulating intestinal epithelial barrier function and structure (11-14, 43). The underlying mechanisms may include betaine’s protective effect on epithelial cell health and the upregulation of gene expression for tight junction proteins like occludin and zonula occludens-1 especially under conditions of heat or oxidative stress [
43]. A limitation in this study was that changes in gut permeability were modest following the 60-km cycling bout. Additional studies should focus on similar exercise bouts combined with environmental heat stress [
11]. Other limitations in this study included the lack of epigenetic and cytokine measurements to determine the potential influence of betaine supplementation on gene expression within muscle tissue and post-exercise inflammation. The betaine dosing regimen used in this study was similar to that used in most other related studies [
18]. The potential influence of a larger betaine dose for a greater time period remains to be determined. This study utilized an acute 60-km cycling trial as a performance measurement and the favorable results may not apply to other indices including exercise time to exhaustion or chronic performance. The finding that betaine supplementation was linked to a small decrease in intracellular water was unexpected. This result may vary depending on the type of method used to assess fluid balance and intracellular fluid volumes.
5. Conclusions
Betaine supplementation was linked in this study to a faster 60-km cycling performance time and significant increases in circulating metabolites that are part of the methyl donor pool involved in various biological reactions within cells. These are novel data, and while promising, research on betaine’s potential role in exercise epigenetics is still in its early stages. The data from this investigation can form the basis for additional studies to determine if betaine supplementation contributes to improved exercise performance, recovery, and adaptation by modulating epigenetics through methylation.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Supplement Data Sheet S1,Metabolite raw and normalized data; Supplement Data Sheet S2, Heat map of statistically significant biochemicals profiled in this study.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, methodology, investigation, and resources, DCN, CAS, JCW; formal analysis and data curation, DCN, CAS, JCW, JL, KCL; writing—original draft preparation, DCN; writing—review and editing, DCN, CAS, JCW, JL, KCL; supervision and project administration, DCN, CAS; funding acquisition, DCN. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Appalachian State University (protocol code HS-24-248, date of approval 23 April 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. The metabolomics data has been deposited to the MetaboLights repository (
https://www.ebi.ac.uk/metabolights/MTBLS976) with the dataset identifier MTBLS976.
Acknowledgments
The authors thank AGRANA for providing supplements for this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Nieman DC, Lila MA, Gillitt ND. Immunometabolism: A multi-omics approach to interpreting the influence of exercise and diet on the immune system. Annu Rev Food Sci Technol. (2019) 10:341-363. [CrossRef]
- Nieman DC. Multiomics approach to precision sports nutrition: limits, challenges, and possibilities. Front Nutr. (2021) 8:796360. [CrossRef]
- Nieman DC, Woo J, Sakaguchi CA, Omar AM, Tang Y, Davis K, et al. Astaxanthin supplementation counters exercise-induced decreases in immune-related plasma proteins. Front Nutr. (2023) 10:1143385. [CrossRef]
- Nieman DC, Gillitt ND, Chen GY, Zhang Q, Sha W, Kay CD, et al. Blueberry and/or banana consumption mitigate arachidonic, cytochrome p450 oxylipin generation during recovery from 75-km cycling: a randomized trial. Front Nutr. (2020) 7:121. [CrossRef]
- Nieman DC, Sakaguchi CA, Williams JC, Mulani FA, Suresh PS, Omar AM, Zhang Q. Beet supplementation mitigates post-exercise inflammation. Front Nutr. (2024) 11:1408804.
- Olthof MR, van Vliet T, Boelsma E, Verhoef P. Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J Nutr. 2003 Dec;133(12):4135-8. [CrossRef]
- Arumugam MK, Paal MC, Donohue TM Jr, Ganesan M, Osna NA, Kharbanda KK. Beneficial effects of betaine: a comprehensive review. Biology (Basel). 2021 May 22;10(6):456. [CrossRef]
- Bekdash RA. Methyl donors, epigenetic alterations, and brain health: understanding the connection. Int J Mol Sci. 2023 Jan 25;24(3):2346. [CrossRef]
- Dobrijević D, Pastor K, Nastić N, Özogul F, Krulj J, Kokić B, Bartkiene E, Rocha JM, Kojić J. Betaine as a functional ingredient: metabolism, health-promoting attributes, food sources, applications and analysis methods. Molecules. 2023 Jun 17;28(12):4824. [CrossRef]
- Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004 Sep;80(3):539-49. [CrossRef]
- Willingham BD, Ragland TJ, Ormsbee MJ. Betaine supplementation may improve heat tolerance: potential mechanisms in humans. Nutrients. 2020 Sep 25;12(10):2939. [CrossRef]
- Wu J, He C, Bu J, Luo Y, Yang S, Ye C, Yu S, He B, Yin Y, Yang X. Betaine attenuates LPS-induced downregulation of Occludin and Claudin-1 and restores intestinal barrier function. BMC Vet Res. 2020 Mar 4;16(1):75. [CrossRef]
- Li Z, Pu J, Zeng T, Cai J, Jia G, Zhao H, Liu G, Zeng Q, Luo Y, Tian G. Effects of betaine on growth performance and intestinal health of rabbits fed different digestible energy diets. J Anim Sci. 2024 Jan 3;102:skae029.
- Chen Q, Wang Y, Jiao F, Shi C, Pei M, Wang L, Gong Z. Betaine inhibits Toll-like receptor 4 responses and restores intestinal microbiota in acute liver failure mice. Sci Rep. 2020 Dec 14;10(1):21850. [CrossRef]
- Zhong C, Miao M, Che B, Du J, Wang A, Peng H, Bu X, Zhang J, Ju Z, Xu T, He J, Zhang Y. Plasma choline and betaine and risks of cardiovascular events and recurrent stroke after ischemic stroke. Am J Clin Nutr. 2021 Oct 4;114(4):1351-1359. [CrossRef]
- Pathmasiri W, Rushing BR, McRitchie S, Choudhari M, Du X, Smirnov A, et al. Untargeted metabolomics reveal signatures of a healthy lifestyle. Sci Rep. (2024) 14:13630. [CrossRef]
- Cholewa JM, Guimarães-Ferreira L, Zanchi NE. Effects of betaine on performance and body composition: a review of recent findings and potential mechanisms. Amino Acids. 2014 Aug;46(8):1785-93. [CrossRef]
- Zawieja E, Machek S, Zanchi NE, Cholewa J, Woźniewicz M. Effects of chronic betaine supplementation on exercise performance: Systematic review and meta-analysis. J Sports Sci. 2024 Nov;42(22):2131-2144. [CrossRef]
- Waldman HS, Bryant AR, McAllister MJ. Effects of betaine supplementation on markers of metabolic flexibility, body composition, and anaerobic performance in active college-age females. J Diet Suppl. 2023;20(1):89-105. [CrossRef]
- Ismaeel A. Effects of betaine supplementation on muscle strength and power: a systematic review. J Strength Cond Res. 2017 Aug;31(8):2338-2346. [CrossRef]
- Trepanowski JF, Farney TM, McCarthy CG, Schilling BK, Craig SA, Bloomer RJ. The effects of chronic betaine supplementation on exercise performance, skeletal muscle oxygen saturation and associated biochemical parameters in resistance trained men. J Strength Cond Res. 2011 Dec;25(12):3461-71. [CrossRef]
- Yang MT, Lee XX, Huang BH, Chien LH, Wang CC, Chan KH. Effects of two-week betaine supplementation on apoptosis, oxidative stress, and aerobic capacity after exhaustive endurance exercise. Antioxidants (Basel). 2020 Nov 27;9(12):1189. [CrossRef]
- Armstrong LE, Casa DJ, Roti MW, Lee EC, Craig SA, Sutherland JW, Fiala KA, Maresh CM. Influence of betaine consumption on strenuous running and sprinting in a hot environment. J Strength Cond Res. 2008 May;22(3):851-60. [CrossRef]
- Smith LL, Brunetz MH, Chenier TC, McCammon MR, Houmard JA, Franklin ME, et al. The effects of static and ballistic stretching on delayed onset muscle soreness and creatine kinase. Res Q Exerc Sport. 1993;64:103–107. [CrossRef]
- Curran SL, Andrykowski MA, Studts J L. Short form of the Profile of Mood States (POMS-SF): Psychometric information. Psyc Assess. 1995;7:80–83. doi.org/10.1037/1040-3590.7.1.80.
- Khoshbin K, Khanna L, Maselli D, Atieh J, Breen-Lyles M, Arndt K, et al. Development and validation of test for “leaky gut” small intestinal and colonic permeability using sugars in healthy adults. Gastroenterology. 2021;161:463-475.e13. [CrossRef]
- Larkey NE, Fatica EM, Singh RJ. Detection of 13C-mannitol and other saccharides using tandem mass spectrometry for evaluation of intestinal permeability or leaky gut. Methods Mol Biol. 2022;2546:285-294. [CrossRef]
- Ford L, Kennedy AD, Goodman KD, Pappan KL, Evans AM, Miller LAD, Wulff JE, Wiggs BR, Lennon JJ, Elsea S, Toal DR. Precision of a clinical metabolomics profiling platform for use in the identification of inborn errors of metabolism. J Appl Lab Med. 2020 Mar 1;5(2):342-356. [CrossRef]
- Zawieja E, Durkalec-Michalski K, Sadowski M, Główka N, Chmurzynska A. Betaine supplementation improves CrossFit performance and increases testosterone levels, but has no influence on Wingate power: randomized crossover trial. J Int Soc Sports Nutr. 2023 Dec;20(1):2231411. [CrossRef]
- Mahmoud AM, Ali MM. Methyl donor micronutrients that modify DNA methylation and cancer outcome. Nutrients. 2019 Mar 13;11(3):608. [CrossRef]
- Chen S, Lu XT, He TT, Yishake D, Tan XY, Hou MJ, Luo Y, Long JA, Tang ZH, Zhong RH, Fang AP, Zhu HL. Betaine delayed muscle loss by attenuating samtor complex inhibition for mTORC1 signaling via increasing SAM level. Mol Nutr Food Res. 2021 Aug;65(15):e2100157. [CrossRef]
- Widmann M, Nieß AM, Munz B. Physical exercise and epigenetic modifications in skeletal muscle. Sports Med. 2019 Apr;49(4):509-523. [CrossRef]
- Mallett G. The effect of exercise and physical activity on skeletal muscle epigenetics and metabolic adaptations. Eur J Appl Physiol. 2025 Jan 8. [CrossRef]
- Petracci I, Gabbianelli R, Bordoni L. The Role of nutri(epi)genomics in achieving the body’s full potential in physical activity. Antioxidants (Basel). 2020 Jun 7;9(6):498. [CrossRef] [PubMed] [PubMed Central]
- Chen S, He T, Chen J, Wen D, Wang C, Huang W, Yang Z, Yang M, Li M, Huang S, Huang Z, Zhu H. Betaine delays age-related muscle loss by mitigating Mss51-induced impairment in mitochondrial respiration via Yin Yang1. J Cachexia Sarcopenia Muscle. 2024 Oct;15(5):2104-2117. [CrossRef]
- Senesi P, Luzi L, Montesano A, Mazzocchi N, Terruzzi I. Betaine supplement enhances skeletal muscle differentiation in murine myoblasts via IGF-1 signaling activation. J Transl Med. 2013 Jul 19;11:174. [CrossRef]
- Eklund M, Bauer E, Wamatu J, Mosenthin R. Potential nutritional and physiological functions of betaine in livestock. Nutr Res Rev. 2005 Jun;18(1):31-48. [CrossRef]
- Du J, Shen L, Tan Z, Zhang P, Zhao X, Xu Y, Gan M, Yang Q, Ma J, Jiang A, Tang G, Jiang Y, Jin L, Li M, Bai L, Li X, Wang J, Zhang S, Zhu L. Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients. 2018 Jan 26;10(2):131. [CrossRef]
- Gao X, Zhang H, Guo XF, Li K, Li S, Li D. Effect of betaine on reducing body fat-a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2019 Oct 16;11(10):2480. [CrossRef]
- Jewell DE, Tavener SK, Creech R, Panickar KS. Betaine and L-carnitine synergistically influence the metabolome and immune response in dogs. Animals (Basel). 2024 Jan 23;14(3):357. [CrossRef]
- Bedogni G, Malavolti M, Severi S, Poli M, Mussi C, Fantuzzi AL, Battistini N. Accuracy of an eight-point tactile-electrode impedance method in the assessment of total body water. Eur J Clin Nutr. 2002 Nov;56(11):1143-8. [CrossRef]
- Moro T, Badiali F, Fabbri I, Paoli A. Betaine Supplementation Does Not Improve Muscle Hypertrophy or Strength Following 6 Weeks of Cross-Fit Training. Nutrients. 2020 Jun 5;12(6):1688. [CrossRef]
- Alhotan RA, Al Sulaiman AR, Alharthi AS, Abudabos AM. Protective influence of betaine on intestinal health by regulating inflammation and improving barrier function in broilers under heat stress. Poult Sci. 2021 Sep;100(9):101337. [CrossRef]
Figure 1.
Study participant flow diagram.
Figure 1.
Study participant flow diagram.
Figure 2.
Betaine and placebo trial changes in intestinal fatty acid binding protein (I-FABP) across three timepoints (pre-supplementation, immediately- and 1.5 hours post-exercise (60-km cycling time trial).
Figure 2.
Betaine and placebo trial changes in intestinal fatty acid binding protein (I-FABP) across three timepoints (pre-supplementation, immediately- and 1.5 hours post-exercise (60-km cycling time trial).
Figure 3.
Betaine and placebo trial changes in lactulose to 13C mannitol ratios from 5-hour urine samples collected pre-supplementation and post-exercise (60-km cycling time trial).
Figure 3.
Betaine and placebo trial changes in lactulose to 13C mannitol ratios from 5-hour urine samples collected pre-supplementation and post-exercise (60-km cycling time trial).
Figure 4.
a. Partial Least Squares Discriminant Analysis (OPLS-DA) for both trials across all six timepoints (T1=presupplementation, T2=post-2-weeks supplementation, T3=immediately post-exercise, T4=1.5 hours post-exercise, T5=3 hours post-exercise, T6=24 hours post-exercise). The 60-km cycling time trial had a significant effect on metabolite shifts (R2Y=0.495, Q2=0.451). b. OPLS-DA contrasting the betaine and placebo trials (all 6 time points). A distinct difference was shown between the betaine and placebo trials (R2Y=0.769, Q2=0.574). R2Y values attributed to the contributory latent variables are listed for each axis in the parentheses.
Figure 4.
a. Partial Least Squares Discriminant Analysis (OPLS-DA) for both trials across all six timepoints (T1=presupplementation, T2=post-2-weeks supplementation, T3=immediately post-exercise, T4=1.5 hours post-exercise, T5=3 hours post-exercise, T6=24 hours post-exercise). The 60-km cycling time trial had a significant effect on metabolite shifts (R2Y=0.495, Q2=0.451). b. OPLS-DA contrasting the betaine and placebo trials (all 6 time points). A distinct difference was shown between the betaine and placebo trials (R2Y=0.769, Q2=0.574). R2Y values attributed to the contributory latent variables are listed for each axis in the parentheses.
Figure 5.
Betaine and placebo trial changes across six time points for plasma betaine (a), sarcosine (b), dimethylglycine (DMG) (c). The treatment and timepoint interactions effects were each q<0.001. The split violin graphs reveal the differences between plasma metabolite values for both trials across all six timepoints. Overlayed box-and-whisker plots further describe the minimum, maximum, and median values, the interquartile range, and detected outliers.
Figure 5.
Betaine and placebo trial changes across six time points for plasma betaine (a), sarcosine (b), dimethylglycine (DMG) (c). The treatment and timepoint interactions effects were each q<0.001. The split violin graphs reveal the differences between plasma metabolite values for both trials across all six timepoints. Overlayed box-and-whisker plots further describe the minimum, maximum, and median values, the interquartile range, and detected outliers.
Figure 6.
Betaine and placebo trial changes across six time points for plasma methionine (a), S-adenosylhomocysteine (SAH) (b), alpha-ketoglutaramate (c), and 5-methylthioadenosine (MTA) (d). The treatment and timepoint interactions effects were q=0.036, 0.025, q<0.001, and q<0.001, respectively.
Figure 6.
Betaine and placebo trial changes across six time points for plasma methionine (a), S-adenosylhomocysteine (SAH) (b), alpha-ketoglutaramate (c), and 5-methylthioadenosine (MTA) (d). The treatment and timepoint interactions effects were q=0.036, 0.025, q<0.001, and q<0.001, respectively.
Figure 7.
Betaine and placebo trial changes across six time points for plasma carnosine (q<0.001).
Figure 7.
Betaine and placebo trial changes across six time points for plasma carnosine (q<0.001).
Table 1.
Study participant characteristics for male and female cyclists (n=21). *p<0.05.
Table 1.
Study participant characteristics for male and female cyclists (n=21). *p<0.05.
| Variable |
Males (n=15) |
Females (n=6) |
| Age (years)
|
45.5±2.5 |
44.2±4.8 |
| Weight (kilograms)
|
79.5±2.4* |
62.5±2.8 |
| Height (centimeters)
|
180±1.6* |
167±3.6 |
| Body mass index (BMI) |
24.5±0.5 |
22.6±1.4 |
| Body fat % |
21.3±1.7 |
23.5±1.9 |
| V02max (ml.kg.-1min-1)
|
43.6±1.6* |
36.7±1.7 |
| Maximal cycling power (watts)
|
268±11.6* |
179±15.0 |
| Maximal heart rate (beats/min)
|
167±3.0 |
162±3.7 |
| Maximal ventilation rate (liters/min)
|
126±3.9* |
89.4±9.9 |
Table 2.
60-km cycling performance data for n=21 cyclists in the placebo and betaine trials (mean±SE).
Table 2.
60-km cycling performance data for n=21 cyclists in the placebo and betaine trials (mean±SE).
| Performance Variable |
Supplement |
Mean±SE
|
T-test
p-value
|
| Time to complete 60 km cycling trial (min) |
Placebo
Betaine |
114.2±2.6
112.8±2.3 |
0.049
|
| Average speed (km/h) |
Placebo
Betaine |
31.8±0.7
32.1±0.6 |
0.132
|
Average watts and
%maximal |
Placebo
Betaine |
148±9.1; 61.0±1.9
147±9.3; 60.5±1.9 |
0.692;
0.674 |
| Average VO2 (ml.kg-1.min-1) and %maximal |
Placebo
Betaine |
30.6±6.4; 74.6±8.1
30.9±6.4; 75.3±8.5 |
0.327;
0.291 |
Average respiratory
exchange ratio (RER) (VCO2/VO2) |
Placebo
Betaine |
0.840±0.007
0.838±0.007 |
0.796 |
| Average heart rate (bpm) and %maximal |
Placebo
Betaine |
137±3.9; 82.3±1.7
138±3.6; 82.9±1.6 |
0.640;
0.608 |
Average rating of perceived
exertion (RPE) |
Placebo
Betaine |
13.7±0.3
13.5±0.3 |
0.412 |
Table 3.
Trial comparisons for n=21 participants across all time points for intracellular water, total mood disturbance (TMD) from the Profile of Moods States (POMS) questionnaire, and physiological outcomes. P-values represent time (first value) and trial x time interaction effects.
Table 3.
Trial comparisons for n=21 participants across all time points for intracellular water, total mood disturbance (TMD) from the Profile of Moods States (POMS) questionnaire, and physiological outcomes. P-values represent time (first value) and trial x time interaction effects.
| Variable |
Trial |
Pre-
Study |
2-Wks Suppl. |
0h
Post-Ex |
1.5h Post-Ex |
3h
Post-Ex |
24h Post-Ex |
P-
value |
| Intracellular water (liters) |
Placebo |
25.1±1.0 |
25.2±1.0 |
25.6±1.0 |
25.0±1.0 |
25.2±1.0 |
25.1±1.0 |
0.004;
0.010 |
| Betaine |
25.6±1.0 |
25.0±1.0* |
25.2±1.0* |
25.1±1.0* |
25.0±1.0* |
25.2±1.0 |
| Total mood disturbance |
Placebo |
91.2±2.2 |
94.3±2.1 |
101±2.2 |
98.5±2.3 |
96.8±2.0 |
91.8±2.0 |
<0.001;
0.304 |
| Betaine |
92.2±1.7 |
90.4±2.1 |
98.0±2.3 |
96.0±2.3 |
93.9±1.9 |
90.7±1.8 |
DOMS
(1-10 scale) |
Placebo |
1.9±0.2 |
1.8±0.2 |
4.5±0.4 |
4.3±0.5 |
3.9±0.4 |
2.8±0.4 |
<0.001;
0.465 |
| Betaine |
1.7±0.2 |
1.8±0.2 |
4.1±0.5 |
3.7±0.4 |
3.0±0.3 |
2.4±0.4 |
Creatine
kinase (U/L) |
Placebo |
253±61.7 |
192±35.2 |
224±37.9 |
215±34.9 |
235±38.2 |
254±65.7 |
<0.001;
0.790 |
| Betaine |
175±22.3 |
152±21.4 |
182±24.2 |
178±23.3 |
208±27.1 |
233±37.6 |
| Myoglobin (ng/ml) |
Placebo |
57.1±17.3 |
43.8±10.0 |
56.9±5.2 |
107±26.2 |
105±39.3 |
36.9±2.8 |
<0.001;
0.849 |
| Betaine |
39.5±2.8 |
33.9±2.3 |
55.3±5.1 |
96.7±18.0 |
94.8±22.2 |
38.2±2.9 |
| Serum glucose (mg/dl) |
Placebo |
96.2±2.1 |
95.0±1.6 |
92.7±3.3 |
84.4±2.7 |
116±4.8 |
93.0±3.0 |
<0.001;
0.391 |
| Betaine |
95.7±2.0 |
92.6±1.9 |
96.3±3.0 |
85.6±2.5 |
113±5.0 |
94.4±1.9 |
| Neutrophil/ lymphocyte |
Placebo |
1.9±0.1 |
1.8±0.2 |
4.3±0.3 |
6.7±0.7 |
6.3±0.5 |
2.0±0.2 |
<0.001;
0.107 |
| Betaine |
2.0±0.2 |
1.7±0.1 |
4.5±0.4 |
7.2±0.7 |
6.6±0.8 |
2.2±0.3 |
| Cortisol (µg/dl) |
Placebo |
17.3±1.2 |
18.4±1.2 |
21.5±1.5 |
14.9±1.3 |
11.4±1.3 |
16.2±1.5 |
<0.001;
0.955 |
| Betaine |
17.3±1.1 |
18.0±1.1 |
21.8±1.7 |
15.1±1.4 |
11.0±0.9 |
15.8±1.1 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).