Submitted:
13 August 2025
Posted:
13 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
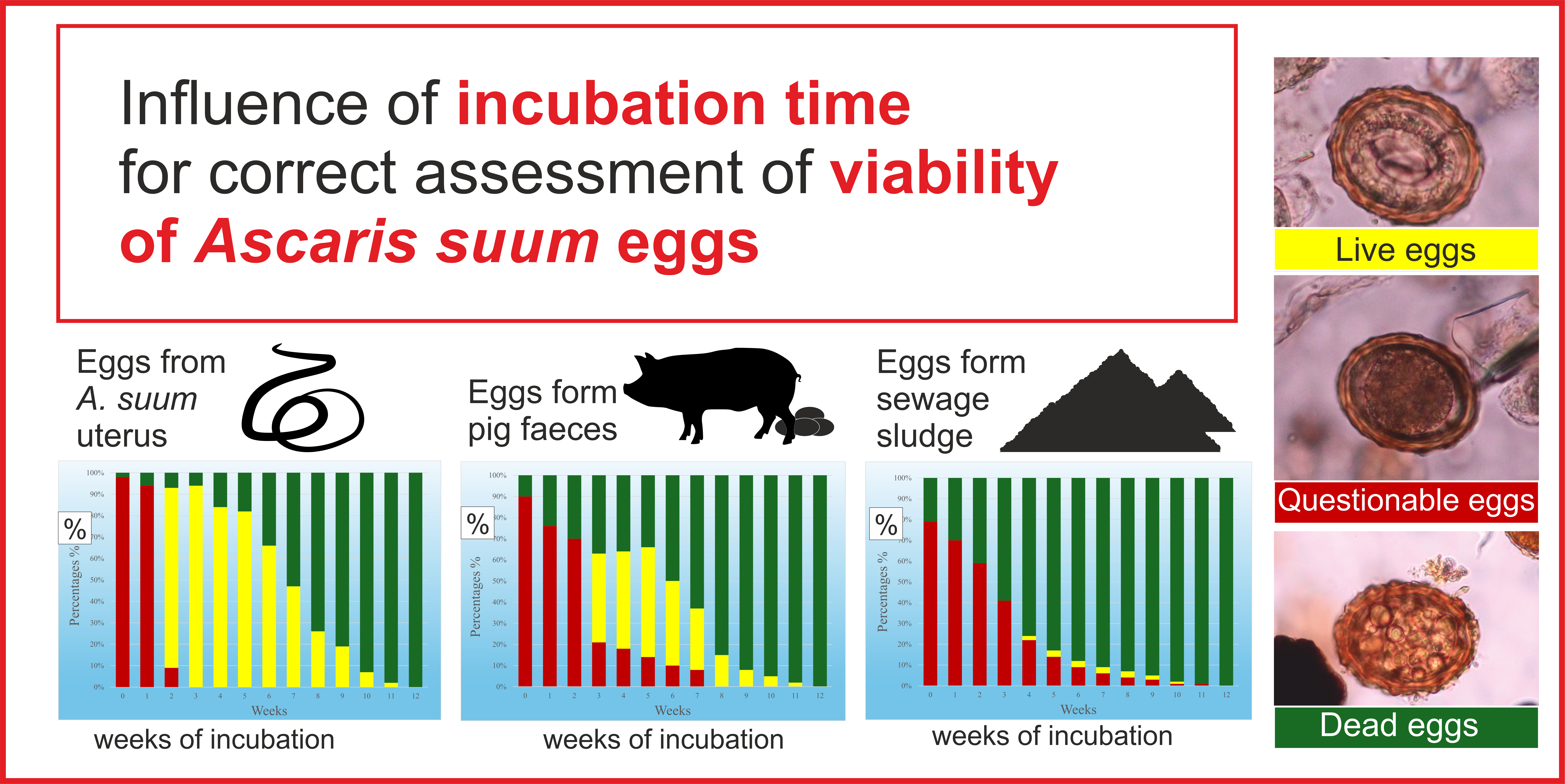
2.1. Parasite Eggs
2.2. The Process of Obtaining Eggs from the Uterus of Mature Female Nematodes (U)
2.3. The Process of Isolating Eggs from Pig Faeces (F)
2.4. The Process of Isolating Eggs from Dewatered Sewage Sludge Obtained from a Wastewater Treatment Plant (S)
2.5. Experiment: Estimation of Egg Viability
- 12 plates with eggs isolated from the uterus of adult female Ascaris suum (group U),
- 12 plates with eggs isolated from pig faeces (group F),
- 12 plates with eggs isolated from sewage sludge (group S).
- eggs with clear deformations (such as granular appearance, deformed cytoplasm, damaged shell or empty shell) were classified as dead eggs (DE),
- eggs in which motile larvae had developed were classified as live eggs (LE),
- eggs that retained correct structural features but did not show signs of embryo development (such as cleavage or larval development) were classified as eggs of questionable viability (QE).
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Regulation of the Polish Minister of Environment on the municipal sewage sludge. 2015, Gazette No. 257.
- Regulation of the Minister of Agriculture and Rural Development on the implementation of certain provisions of the Act on Fertilizers and Fertilization. 2024, Gazette No. 1261.
- European Commission. Environmental, economic and social impacts of the use of sewage sludge on land. Consultation Report on Options and Impacts, Report by RPA, Milieu Ltd. and WRc for the European Commission, DG Environment, European Commission. 2009.
- World Health Organization. Guidelines for the Safe Use of Wastewater Excreta and Greywater; World Health Organization: Geneva, Switzerland, 2006.
- Zdybel, J., Cencek, T., Karamon, J., Kłapeć, T. Effectiveness of selected stages of wastewater treatment in elimination of eggs of intestinal parasites. Bulletin of the Veterinary Institute in Pulawy. 2015, 59, 51-57. [CrossRef]
- Khurana, S., Singh, S., Mewara, A. Diagnostic Techniques for Soil-Transmitted Helminths - Recent Advances. Research and reports in tropical medicine. 2021, 12, 181–196. [CrossRef]
- Dąbrowska, J., Zdybel, J., Karamon, J., Kochanowski, M., Stojecki, K., Cencek, T., Kłapeć, T., Assessment of viability of the nematode eggs (Ascaris, Toxocara, Trichuris) in sewage sludge with the use of LIVE/DEAD Bacterial Viability Kit. Ann. Agric. Environ. Med. 2014, 21(1), 35-41.
- Karkashan A., Khallaf B., Morris J., Thurbon N., Rouch D., Smith S.R., Deighton M. Comparison of methodologies for enumerating and detecting the viability of Ascaris eggs in sewage sludge by standard incubation-microscopy, the BacLight Live/Dead viability assay and other vital dyes. Water Research. 2015, 68, 533-544. [CrossRef]
- Włodarczyk M., Zdybel J., Próchniak M., Osiński Z., Karamon J., Kłapeć T., Cencek T. Viability assessment of Ascaris suum eggs stained with fluorescent dyes using digital colorimetric analysis. Experimental parasitology. 2017, 178, 7–13. [CrossRef]
- US. Environmental Protetcion Agency. Control of Pathogenes and Vector Attraction in Sewage Sludge. EPA/625/R-92-013 (Appendix I. Washington D.C). 1992.
- ANFOR XP X 33-017. Characterisation of Sludges e Enumeration and Viability of Parasite Helminth Eggs - Triple Flotation. Ed. Diffuse Par I’. Association Francaise de Normalisation (ANFOR). 2004.
- Raynaud J.P. Etude de l’efficacited’une technique de coproscopie quantitative pour le diagnostic de routine et le controle des infestations parasitaires des bovins, ovins, equines et porcins. Annales de Parasitologie (Paris). 1970, 45, 321–342.
- Cringoli G., Rinaldi L., Maurelli M.P., Utzinger J. FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010, 5, 503–515.
- Vasilkova ZG, Gefter VA. Methods for studying soil for helminth eggs. Med Parasitol Parasitic Dis. 1948, 2, 139–43.
- Dada B.J. A new technique for the recovery of Toxocara eggs from soil. Journal of helminthology. 1979, 53(2), 141–144. [CrossRef]
- Quinn, R., Smith, H.V., Bruce, R.G., Girdwood, R.W. Studies on the incidence of Toxocara and Toxascaris spp. ova in the environment. 1. A comparison of flotation procedures for recovering Toxocara spp. ova from soil. The Journal of hygiene. 1980, 84(1), 83–89. [CrossRef]
- Gundłach J.L., Sadzikowski A.B., Tomczuk K. Contamination by Toxocara spp. eggs of selected urban and rural environments. Medycyna Weterynaryjna. 1996, 52, 395–396.
- Cruz, L. M., Allanson, M., Kwa, B., Azizan, A., Izurieta, R. Morphological changes of Ascaris spp. eggs during their development outside the host. The Journal of parasitology. 2012, 98(1), 63–68. [CrossRef]
- Polish Standardisation Committee PN-Z-19005:2018-10. Soil Quality- Evaluation of the Sanitary Condition of Materials Introduced into the Soil-Detection and Quantification of Intestinal Parasitic Eggs from the Genera Ascaris, Trichuris and Toxocara in Dehydrated Sewage Sludge, Intended for Introduction into the Soil.
- Polish Standardisation Committee PN-Z-19006:2023-04. Soil Quality- Evaluation of the Sanitary Condition of Materials Introduced into the Soil-Detection of Intestinal Parasitic Eggs from the Genera Ascaris, Trichuris and Toxocara in organic fertilisers.
- Zdybel J., Karamon J., Różycki M., Bilska-Zając E., Kłapeć T., Cencek T. Characterisation of a new, highly effective method for detecting nematode eggs (Ascaris spp., Toxocara spp., Trichuris spp.) in sewage sludge containing flocculants. Experimental Parasitology. 2016, 170, (198-206). [CrossRef]
- Maya C., Ortiz M., Jiménez B. Viability of Ascaris and other helminth genera non larval eggs in different conditions of temperature, lime (pH) and humidity. Water Sci Technol. 2010, 62 (11): 2616–2624. [CrossRef]
- Jimenez B. Helminth Ova Control in Wastewater and Sludge for Agricultural Reuse. In: W. O. K. Grabow, Ed., Water Reuse New Paradigm towards Integrated Water Resources Management in Encyclopedia of Biological, Physiological and Health Sciences. Water and Health. II. Life Support System, EOLSS Publishers Co Ltd., UNESCO, 2008, pp. 429-449.
- World Health Organization; Health Guidelines for the Use of Wastewater in Agriculture and Aquaculture. Technical Report. Series No 778. World Health Organization, Geneva, Switzerland, 1989.
- Arene FOI. Ascaris suum: Influence of embryonation temperature on the viability of the infective larva. J Thermal Biol. 1986, 11(1):9-15.
- Strauch D. Survival of pathogenic micro–organisms and parasites in excreta, manure and sewage sludge. Part I. Medycyna Weterynaryjna. 1993, 49, 59–65.
- Strauch D. Survival of pathogenic micro–organisms and parasites in excreta, manure and sewage sludge. Part II. Medycyna Weterynaryjna. 1993, 49, 117–121.
- Figura, A., Cencek, T., Żbikowska, E. Parasitic threat in commercial organic fertilizers. Parasitology research. 2022, 121(3), 945–949. [CrossRef]
- Zdybel J. ,Karamon J., Dąbrowska J., Różycki M., Bilska-Zając E., Kłapeć T, Cencek T. Parasitological contamination with eggs Ascaris spp., Trichuris spp. and Toxocara spp. of dehydrated municipal sewage sludge in Poland. Environmental Pollution. 2019, 248, 621-626. [CrossRef]
- Rocha, M.C.V.D., Barés, M.E., Braga, M.C.B. Quantification of viable helminth eggs in samples of sewage sludge. Water research. 2016, 103, 245–255. [CrossRef]
- Zhou C., Li M., Yuan K., Deng S., Peng W., , Pig Ascaris: an important source of human ascariasis in China. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2012, 12(6), 1172-1177. [CrossRef]
- Eijck, I. A., Borgsteede, F. H. A survey of gastrointestinal pig parasites on free-range, organic and conventional pig farms in The Netherlands. Veterinary research communications. 2005, 29(5), 407–414. [CrossRef]
- Szostak, B., Bekier-Jaworska, E. Microbiological and parasitological pollution of soil in the vicinity of swine farms. Medycyna Weterynaryjna. 2003, 59 (3), 251-254.
- Nejsum, P., Thamsborg, S. M., Petersen, H. H., Kringel, H., Fredholm, M., Roepstorff, A. Population dynamics of Trichuris suis in trickle-infected pigs. Parasitology. 2009, 136(6), 691–697. [CrossRef]
- Horák P. Helminth eggs in the sludge from three sewage treatment plants in Czechoslovakia. Folia parasitological. 1992, 39(2), 153–157.
- Water Research Commission , Priya Moodley, Colleen Archer and David Hawksworth in association with Lizette Leibach Standards methods for the recovery and enumeration od helminth ova in wastewater, sludge, compost and urine-diversion waste in South Africa, WRC Report No. TT322/08. Republic of South Africa, 2008.
- Jeska, E. L., Caruso, J. P., Donahue, M. J. Collection of fertile Ascaris suum eggs. The Journal of parasitology. 1986, 72(6), 964–965.
- Oksanen, A., Eriksen, L., Roepstorff, A., Ilsøe, B., Nansen, P., Lind, P. Embryonation and infectivity of Ascaris suum eggs. A comparison of eggs collected from worm uteri with eggs isolated from pig faeces. Acta veterinaria Scandinavica. 1990, 31(4), 393–398. [CrossRef]
- Popat, S.C., Yates, M.V., Deshusses, M.A. Kinetics of inactivation of indicator pathogens during thermophilic anaerobic digestion. Water research. 2010, 44 (20), 5965–5972. [CrossRef]
- Gaspard P.G., Wiart J., Schwartzbrod J. Urban sludge reuse in agriculture: waste treatment and parasitological risk. Bioresource Technology. 1995, 52(1), 37–40. [CrossRef]
- Pecson, B.M., Barrios, J.A., Jiménez, B.E., Nelson, K.L. The effects of temperature, pH, and ammonia concentration on the inactivation of Ascaris eggs in sewage sludge. Water Research. 2007, 41 (13), 2893–2902. [CrossRef]
- Senecal, J., Nordin, A., Vinnerås, B., Fate of Ascaris at various pH, temperature and moisture levels. Journal of water and health. 2020, 18(3), 375–382. [CrossRef]
- Enigk K. Dey-Hazra A., Zur Oberflachenstruktur und Funktion der ausseren Proteinhiille der Eies von Ascaris suum (Nematoda). (The surface and function of the protein layer of Ascaris suum eggs). Berliner und Munchener tierarztliche Wochenschrift. 1976, 89(14), 276–281.
- Geenen, P. L., Bresciani, J., Boes, J., Pedersen, A., Eriksen, L., Fagerholm, H. P., Nansen, P., The morphogenesis of Ascaris suum to the infective third-stage larvae within the egg. The Journal of parasitology. 1999, 85(4), 616–622.
- Maya, C., Pérez, M., Velásquez, G., Barrios, J. A., Román, A., Jiménez, B. Quick incubation process to determine inactivation of Ascaris and Toxocara eggs. Water science and technology: a journal of the International Association on Water Pollution Research. 2019, 80(12), 2328–2337. [CrossRef]
- Katakam, K. K., Mejer, H., Dalsgaard, A., Kyvsgaard, N. C., Thamsborg, S. M. Survival of Ascaris suum and Ascaridia galli eggs in liquid manure at different ammonia concentrations and temperatures. Veterinary parasitology. 2014, 204(3-4), 249–257. [CrossRef]
- Eriksen, L., Andeeasen, P., Ilsoe, B. Inactivation od Ascaris suum eggs during storage in lime treated sewage sludge. Water. Research. 1996, 30(4,), 1026-1029. [CrossRef]
- Husna, M.S., Barti, S.M., Mayrina, F. The Efficiency of Ascaris Spp. Eggs Inactivation in Sewage Sludge by Lime Dosage, Ammonia Concentration, and Temperature Variation. Journal of Environmental Health. 2023, 15(4), 267-274. [CrossRef]
- Rudolfs W., Falk L.L., Ragotzkie R.A. Contamination of Vegetables Grown in Polluted Soil: III. Field Studies on Ascaris Eggs. Sewage and Industrial Wastes. 1951, 23(5), 656–660. https://www.jstor.org/stable/25031661.
- Kowalczyk K., Kłapeć T. Contamination of soil with eggs of geohelminths Ascaris spp., Trichuris spp., Toxocara spp. in Poland – potential source of health risk in farmers. Annals of parasitology. 2020, 66(4), 433–440. [CrossRef]
|
Eggs category |
Statistical parameters |
Incubation time [week] | ||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
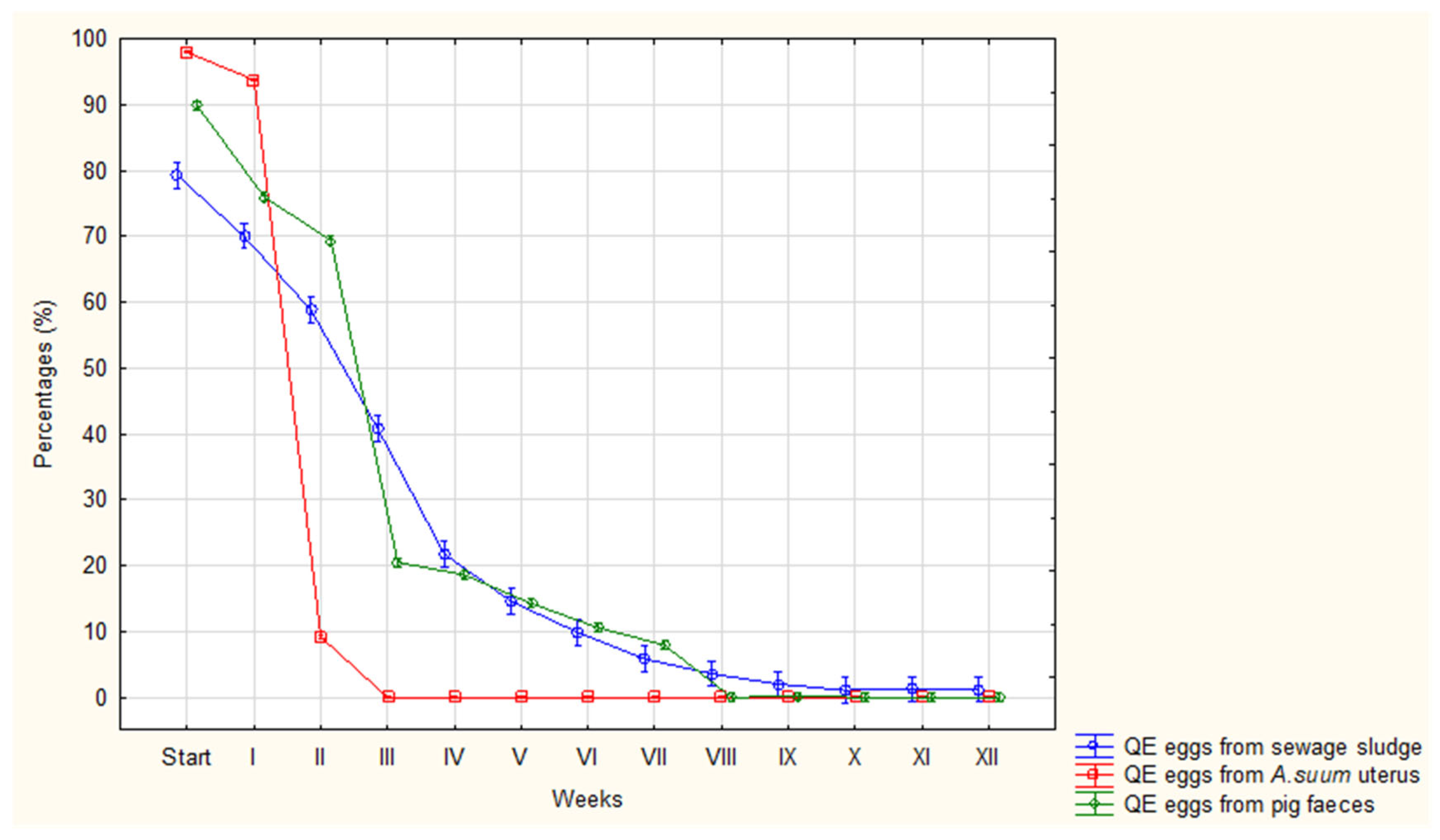
| QE | Avg (%) | 98 | 94 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Range | 96-99 | 91-95 | 8-10 | 0-0 | 0-0 | 0-0 | 0-0 | 0-0 | 0-0 | 0-0 | 0-0 | 0-0 | 0-0 | |
| SD | 1.00 | 1.42 | 0.58 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Standardized scores | 82.47 | 78.30 | -6.28 | -15.45 | -15.45 | -15.45 | -15.45 | -15.45 | -15.45 | -15.45 | -15.45 | -15.45 | ||
| t-value | 585.9 | 556.3 | -44.6 | -109.8 | -109.8 | -109.8 | -109.8 | -109.8 | -109.8 | -109.8 | -109.8 | -109.8 | ||
| p-value | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
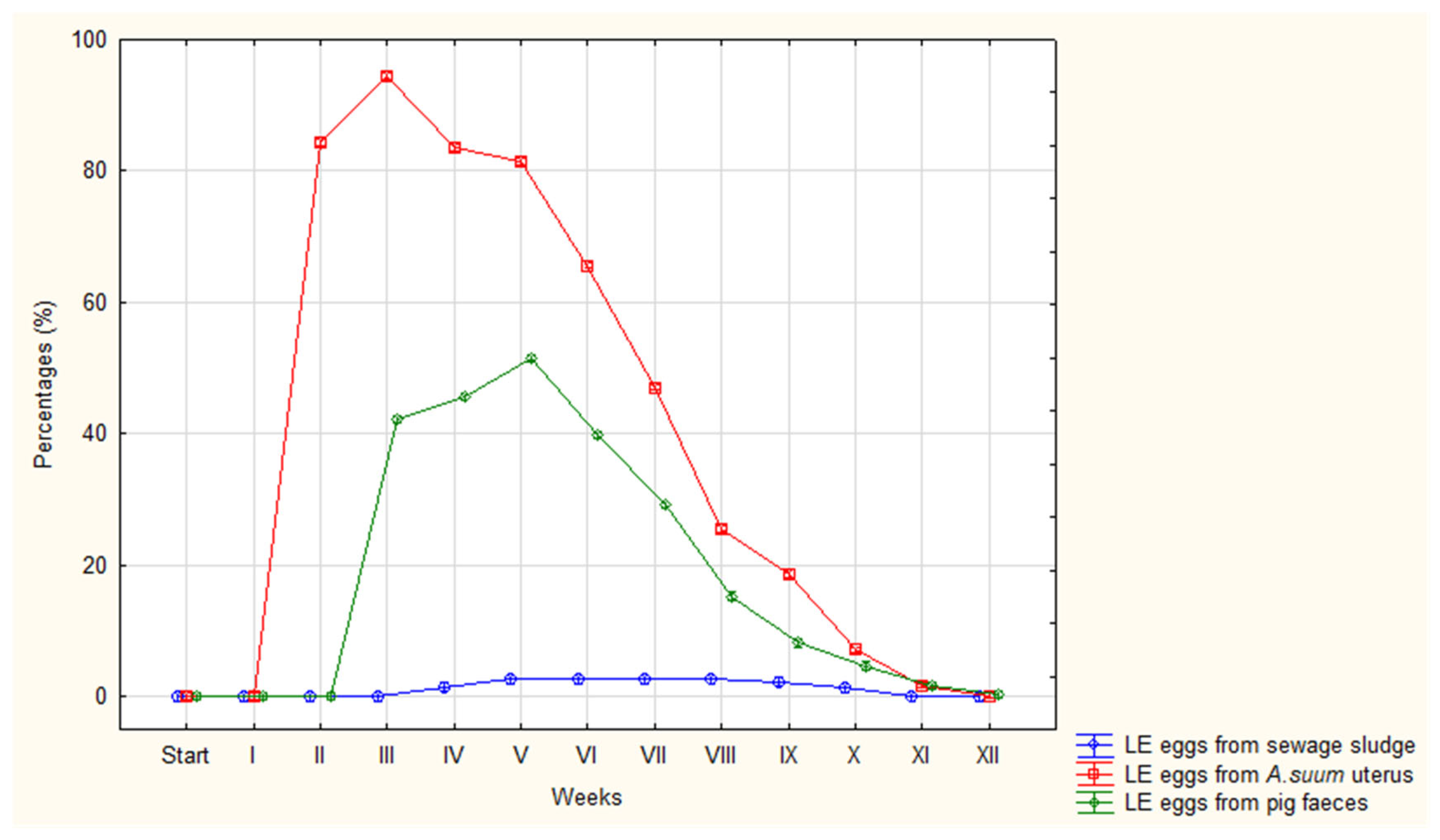
| LE | Avg (%) | 0 | 0 | 84 | 94 | 84 | 82 | 66 | 47 | 26 | 19 | 7 | 2 | 0 |
| Range | 0-0 | 0-0 | 82-87 | 92-96 | 78-87 | 79-85 | 63-70 | 41-50 | 23-28 | 17-20 | 4-10 | 0-5 | 0-1 | |
| SD | 0 | 0 | 1.37 | 1.31 | 2.94 | 2.15 | 2.31 | 2,71 | 1.38 | 0.98 | 1.83 | 1.42 | 0.39 | |
| Standardized scores | -39.22 | -39.22 | 45.12 | 55.20 | 44.37 | 42.28 | 26.45 | 7.87 | -13.63 | -20.55 | -32.13 | -37.47 | ||
| t-value | -82.76 | -82.76 | 95.20 | 116.48 | 93.62 | 89.22 | 55.81 | 16.60 | -28.77 | -43.37 | -67.81 | -79.06 | ||
| p-value | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
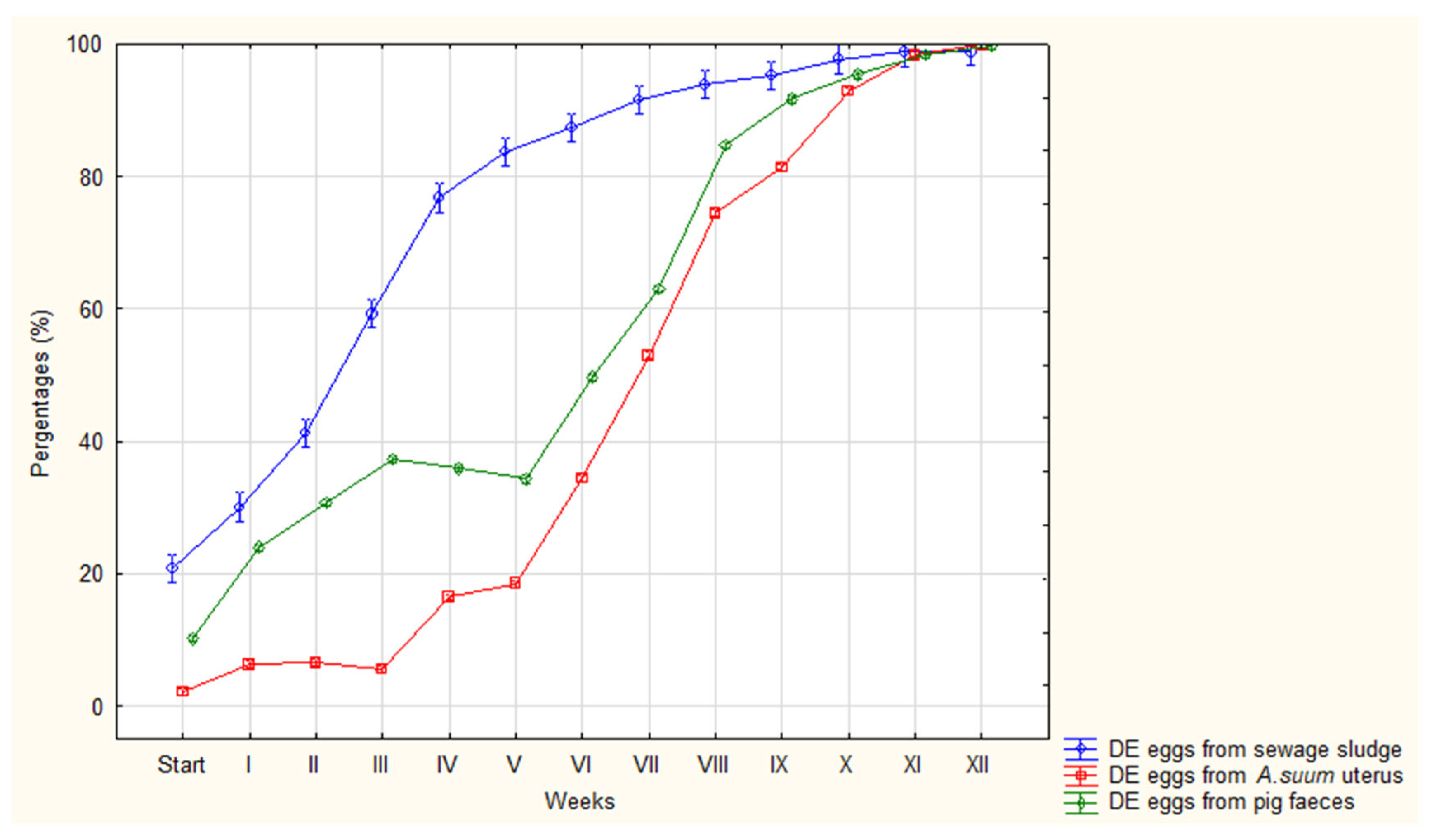
| DE | Avg (%) | 2 | 6 | 7 | 6 | 16 | 18 | 34 | 53 | 74 | 81 | 93 | 98 | 100 |
| Range | 1-4 | 5-9 | 3-9 | 4-8 | 13-22 | 15-21 | 30-37 | 50-59 | 72-77 | 80-83 | 90-96 | 95-100 | 99-100 | |
| SD | 1.00 | 1.42 | 1.68 | 1.31 | 2.94 | 2.15 | 2.31 | 2.71 | 1.38 | 0.98 | 1.83 | 1.42 | 0.39 | |
| Standardized scores | -43.25 | -39.08 | -38.83 | -39.75 | -28.92 | -26.83 | -11.00 | 7.58 | 29.08 | 36.00 | 47.58 | 52.92 | ||
| t-value | -86.85 | -78.48 | -77.98 | -79.82 | -58.07 | -53.88 | -22.09 | 15.23 | 58.40 | 72.29 | 95.55 | 106.26 | ||
| p-value | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
|
Eggs category |
Statistical parameters |
Incubation time [week] | ||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| QE | Avg (%) | 90 | 76 | 70 | 21 | 18 | 14 | 10 | 8 | 0 | 0 | 0 | 0 | 0 |
| Range | 87-97 | 72-80 | 66-73 | 14-25 | 14-24 | 10-18 | 8-13 | 6-10 | 0-0 | 0-2 | 0-0 | 0-0 | 0-0 | |
| SD | 2.82 | 2.66 | 2.02 | 3.18 | 4.52 | 2.38 | 1.93 | 1.68 | 0 | 0.58 | 0 | 0 | 0 | |
| Standardized scores | 66.21 | 52.37 | 45.71 | -3.13 | -5.04 | -9.38 | -13.04 | -15.71 | -23.63 | -23.46 | -23.63 | -23.63 | ||
| t-value | 109.47 | 86.59 | 75.57 | -5.17 | -8.34 | -15.51 | -21.57 | -25.98 | -39.07 | -38.79 | -39.07 | -39.07 | ||
| p-value | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| LE | Avg (%) | 0 | 0 | 0 | 42 | 46 | 52 | 40 | 29 | 15 | 8 | 5 | 2 | 0 |
| Range | 0-0 | 0-0 | 0-0 | 39-49 | 40-51 | 49-55 | 37-43 | 25-34 | 12-20 | 6-11 | 3-7 | 1-3 | 0-2 | |
| SD | 0 | 0 | 0 | 3.36 | 4.38 | 1.78 | 1.91 | 2.21 | 2.09 | 1.70 | 1.30 | 0.78 | 0.65 | |
| Standardized scores | -18.33 | -18.33 | -18.33 | 23.92 | 27.25 | 33.17 | 21.42 | 10.83 | -3.08 | -10.17 | -13.67 | -16.67 | ||
| t-value | -32.99 | -32.99 | -32.99 | 43.04 | 49.04 | 59.69 | 38.54 | 19.50 | -5.55 | -18.30 | -24.59 | -29.99 | ||
| p-value | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| DE | Avg (%) | 10 | 24 | 30 | 37 | 36 | 34 | 50 | 63 | 85 | 92 | 95 | 98 | 100 |
| Range | 3-13 | 20-28 | 27-34 | 35-40 | 35-38 | 31-39 | 47-53 | 59-66 | 80-88 | 89-94 | 93-97 | 97-99 | 98-100 | |
| SD | 2.82 | 2.66 | 2.02 | 1.64 | 0.94 | 1.91 | 1.97 | 2.27 | 2.09 | 1.56 | 1.30 | 0.78 | 0.65 | |
| Standardized scores | -47.87 | -34.04 | -27.37 | -20.79 | -22.21 | -23.79 | -8.37 | 4.88 | 26.71 | 33.63 | 37.29 | 40.29 | ||
| t-value | -92.59 | -65.83 | -52.94 | -40.21 | -42.95 | -46.01 | -16.19 | 9.43 | 51.66 | 65.04 | 72.13 | 77.93 | ||
| p-value | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
|
Eggs category |
Statistical parameters |
Incubation time [week] | ||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| QE | Avg (%) | 79 | 70 | 59 | 41 | 22 | 14 | 9 | 6 | 4 | 3 | 1 | 1 | 0 |
| Range | 66-92 | 54-76 | 35-68 | 28-55 | 13-37 | 4-21 | 4-16 | 2-11 | 0-8 | 0-7 | 0-3 | 0-4 | 0-0 | |
| SD | 8.98 | 8.21 | 12.89 | 8.68 | 9.01 | 5.85 | 4.12 | 3.51 | 2.71 | 2.57 | 1.16 | 1.42 | 0 | |
| Standardized scores | 55.40 | 46.15 | 34.99 | 16.99 | -2.10 | -9.26 | -14.01 | -18.01 | -20.26 | -21.85 | -22.76 | -22.60 | ||
| t-value | 30.34 | 25.27 | 19.16 | 9.30 | -1.15 | -5.07 | -7.67 | -9.86 | -11.10 | -11.96 | -12.46 | -12.37 | ||
| p-value | 0 | 0 | 0 | 0 | 0.25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| LE | Avg (%) | 0 | 0 | 0 | 0 | 2 | 3 | 3 | 3 | 3 | 2 | 1 | 0 | 0 |
| Range | 0-0 | 0-0 | 0-0 | 0-0 | 0-5 | 0-10 | 0-10 | 0-10 | 0-10 | 0-10 | 0-5 | 0-0 | 0-2 | |
| SD | 0 | 0 | 0 | 0 | 1.88 | 3.78 | 3.78 | 3.78 | 3.78 | 3.74 | 1.91 | 0 | 0.65 | |
| Standardized scores | -1.179 | -1.179 | -1.179 | -1.179 | 0.321 | 1.404 | 1.404 | 1.404 | 1.404 | 1.071 | 0.071 | -1.179 | ||
| t-value | -1.733 | -1.733 | -1.733 | -1.733 | 0.471 | 2.063 | 2.063 | 2.063 | 2.063 | 1.573 | 0.104 | -1.733 | ||
| p-value | 0.09 | 0.09 | 0.09 | 0.09 | 0.64 | 0.04 | 0.04 | 0.04 | 0.04 | 0.12 | 0.92 | 0.09 | ||
| DE | Avg (%) | 21 | 30 | 41 | 59 | 76 | 83 | 88 | 91 | 93 | 95 | 98 | 99 | 99 |
| Range | 8-34 | 24-46 | 32-65 | 45-72 | 58-87 | 72-96 | 78-96 | 81-98 | 86-100 | 90-99 | 95-100 | 96-100 | 97-100 | |
| SD | 8.98 | 8.21 | 12.89 | 8.67 | 10.85 | 8.58 | 6.88 | 6.53 | 5.41 | 3.41 | 1.72 | 1.42 | 1.03 | |
| Standardized scores | -54.23 | -44.98 | -33.81 | -15.73 | 1.77 | 8.69 | 12.35 | 16.60 | 18.85 | 20.19 | 22.69 | 23.77 | ||
| t-value | -26.24 | -21.76 | -16.36 | -7.61 | 0.86 | 4.20 | 5.98 | 8.03 | 9.12 | 9.77 | 10.98 | 11.50 | ||
| p-value | 0 | 0 | 0 | 0 | 0.39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





