Submitted:
12 August 2025
Posted:
14 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
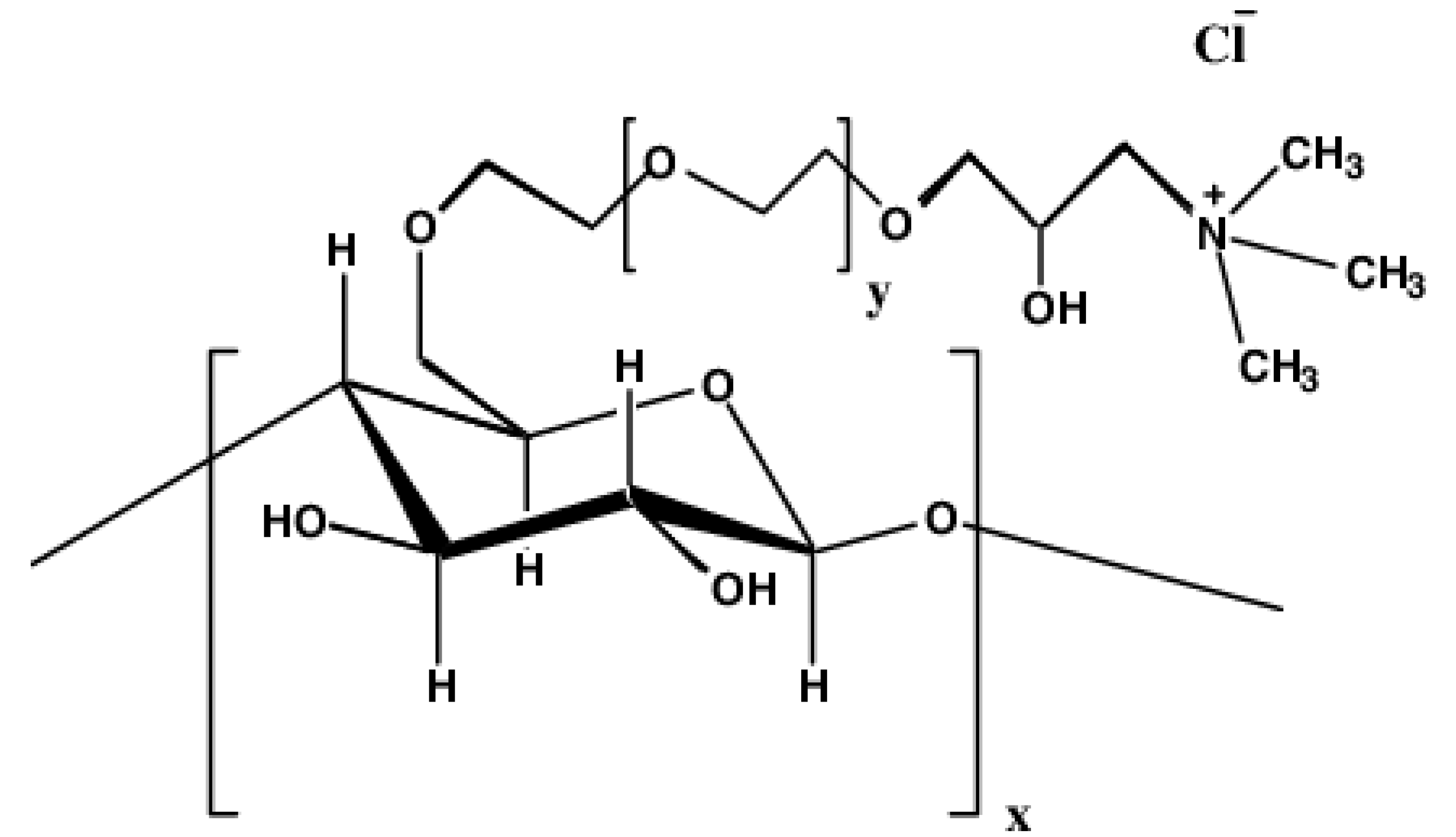
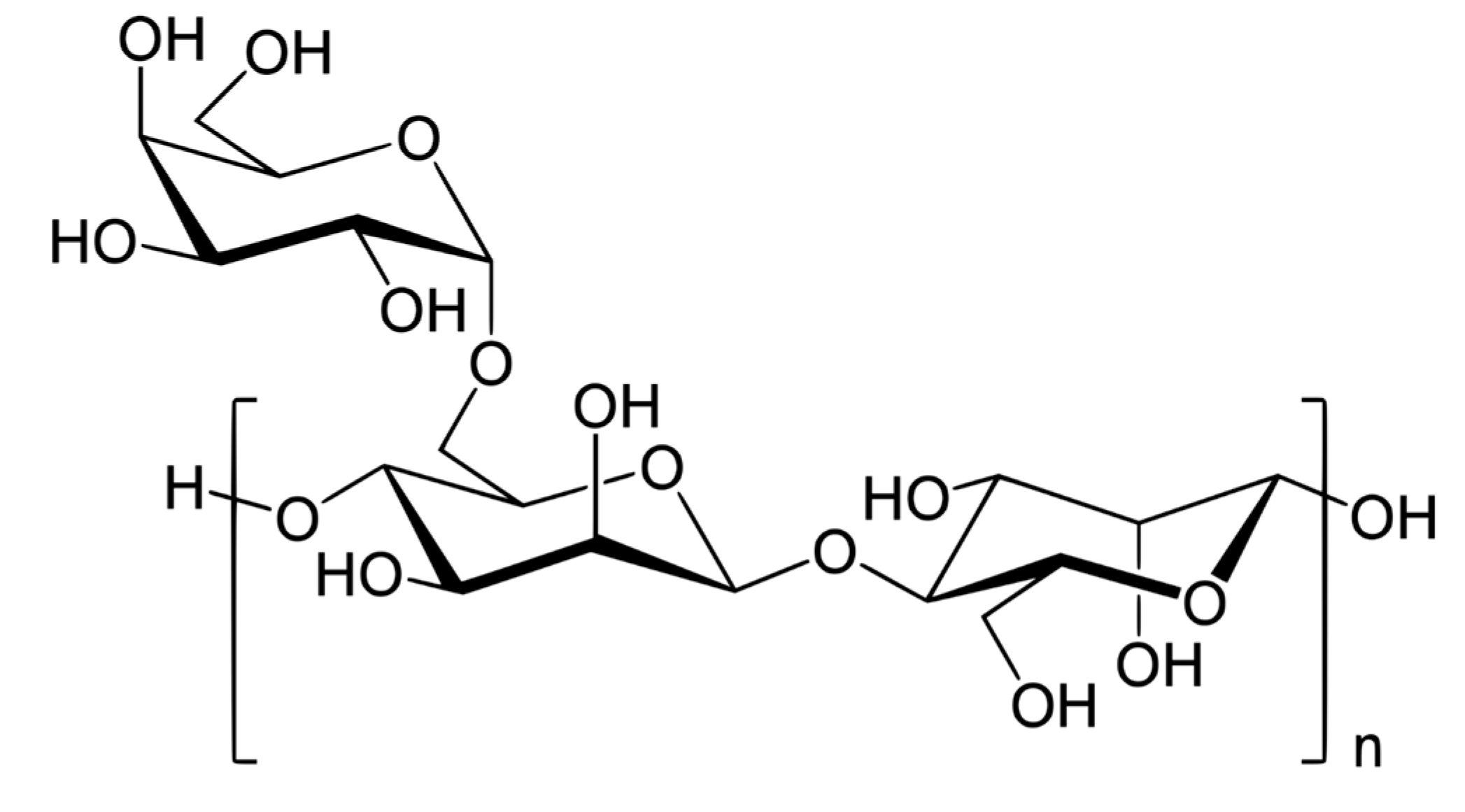
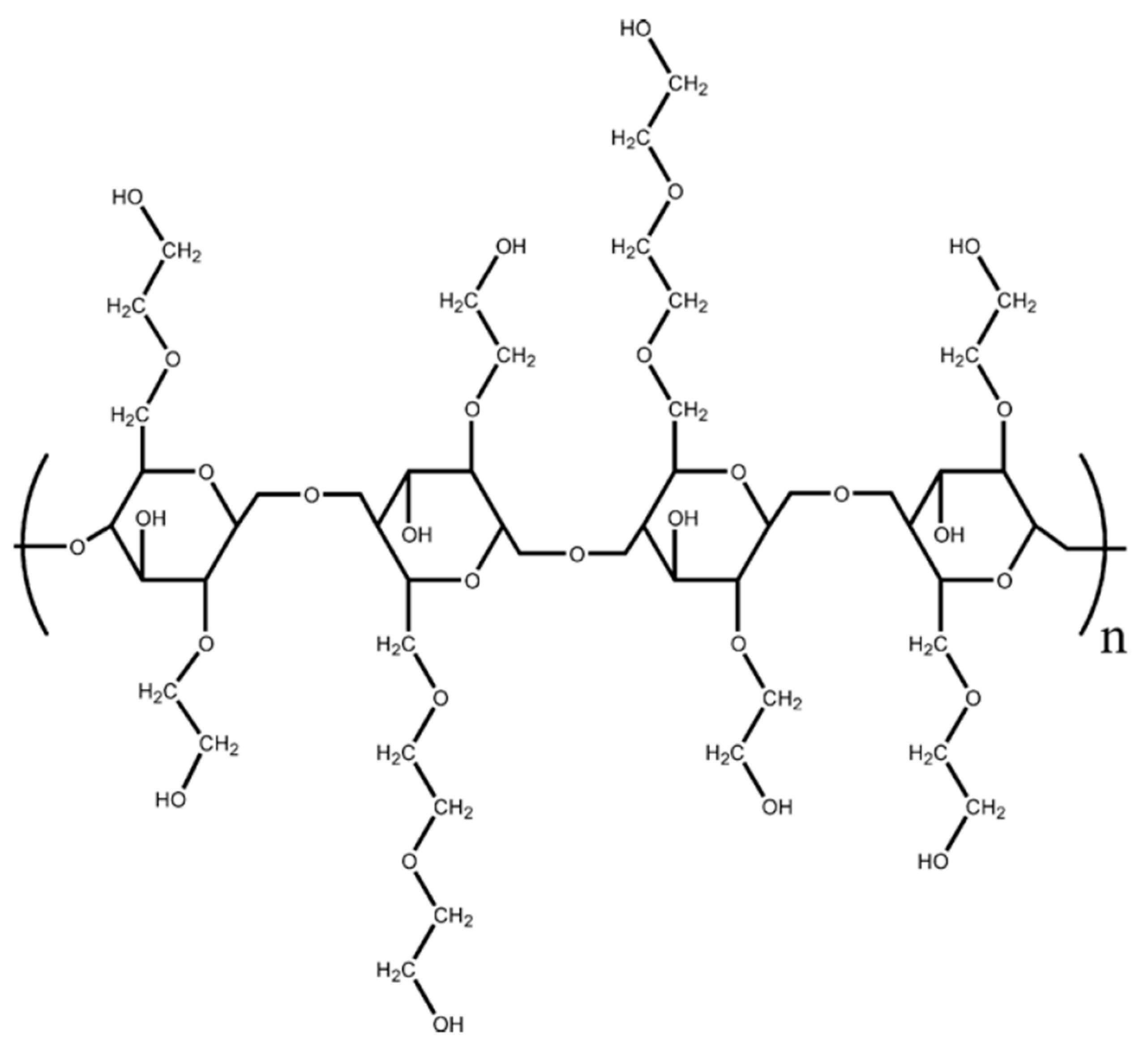
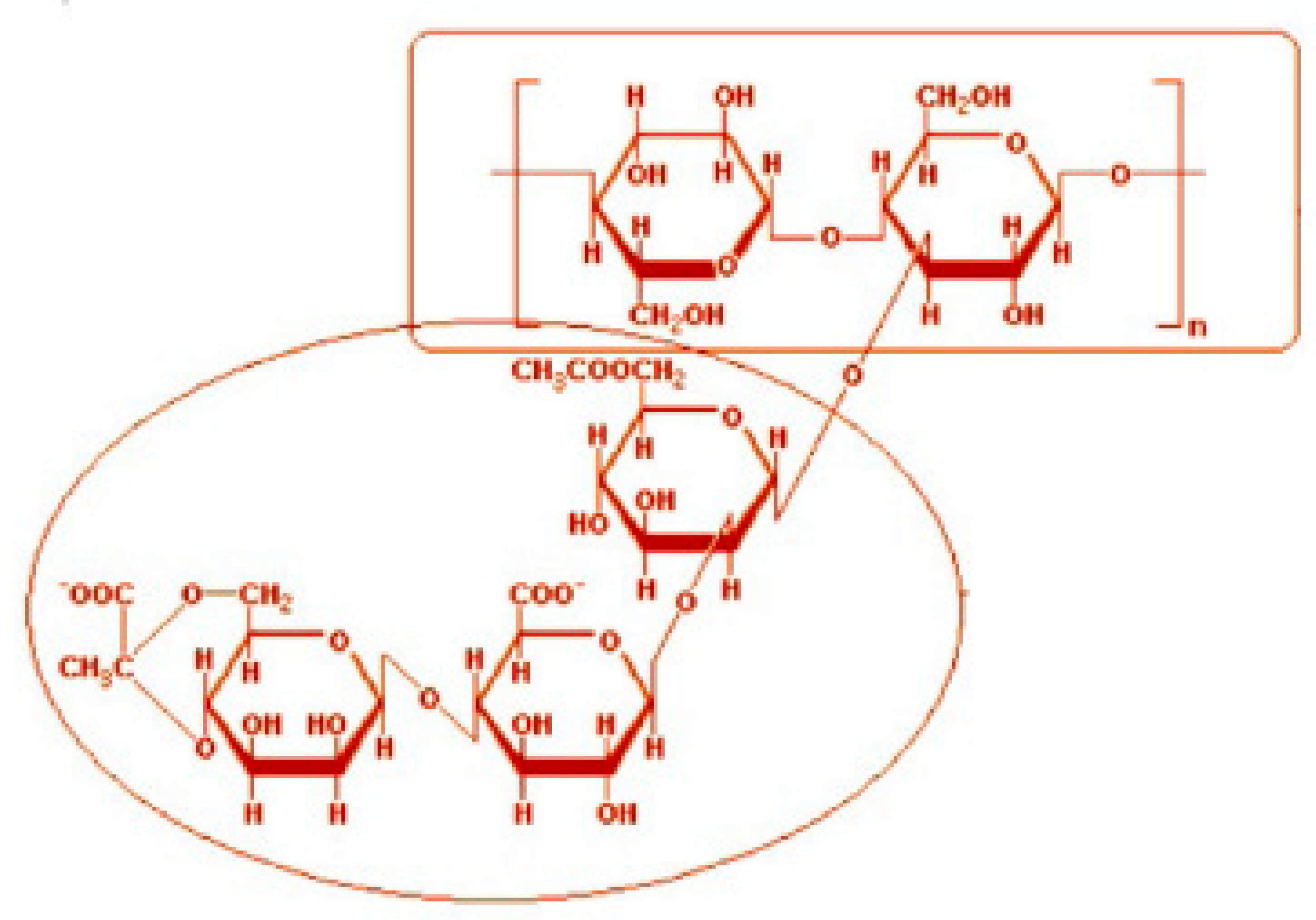
2.1. Materials
2.2. Preparation of Polymer and Surfactant-Polymer Solutions
2.3. Measurement of Rheology
2.4. Measurement of Surface Tension
2.5. Measurement of Electrical Conductivity
3. Results and Discussion
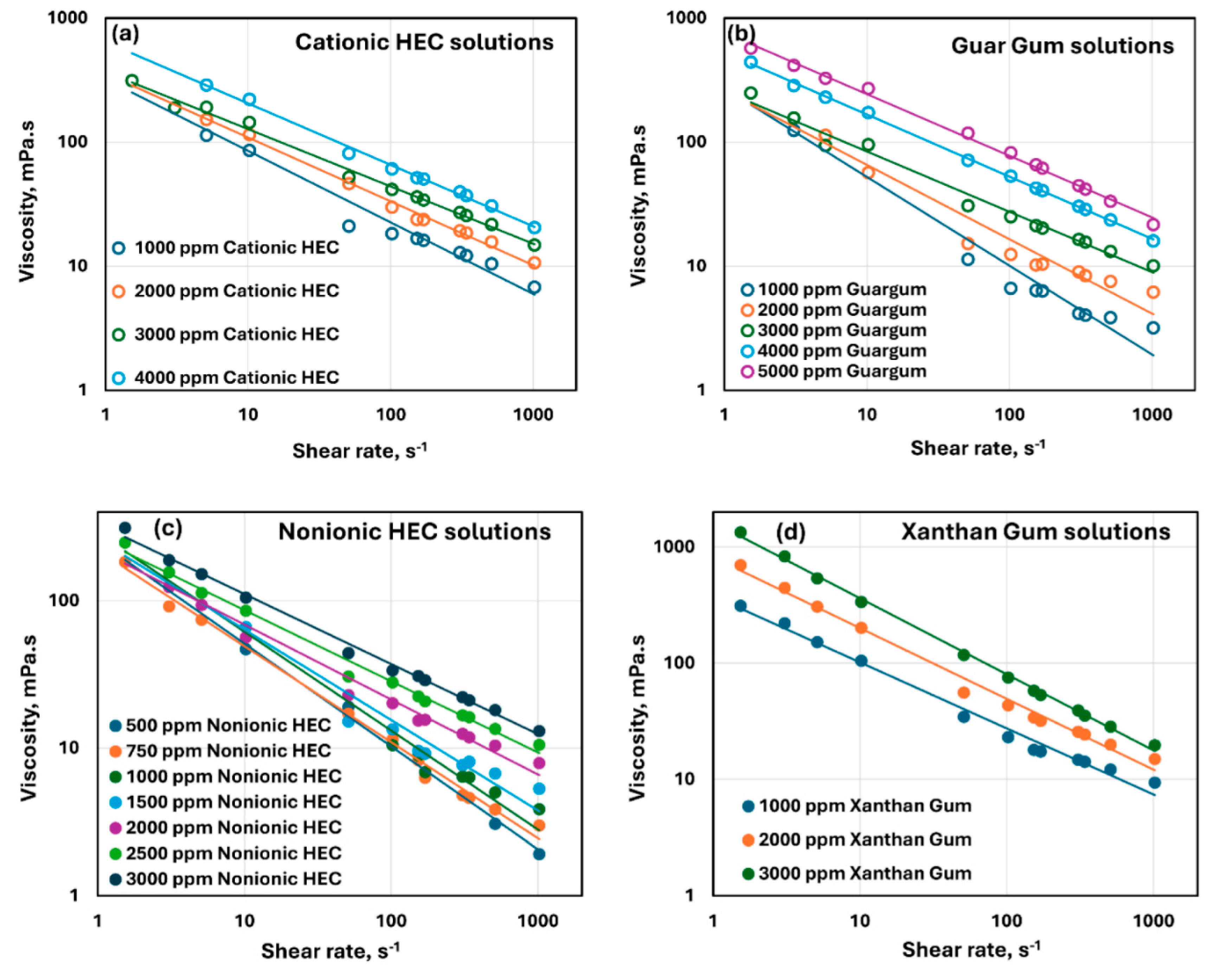
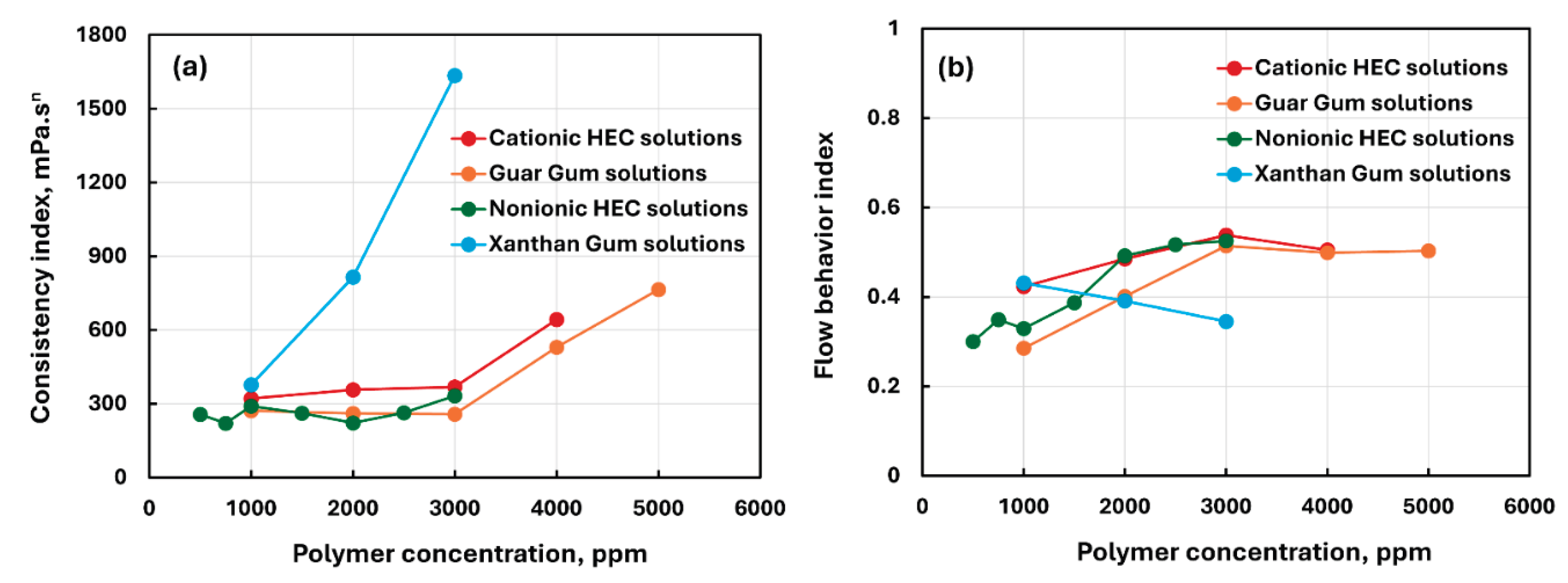
3.1. Rheology of Pure Polymer Solutions
3.2. Rheology and Surface Activity of Polymer-Surfactant Solutions
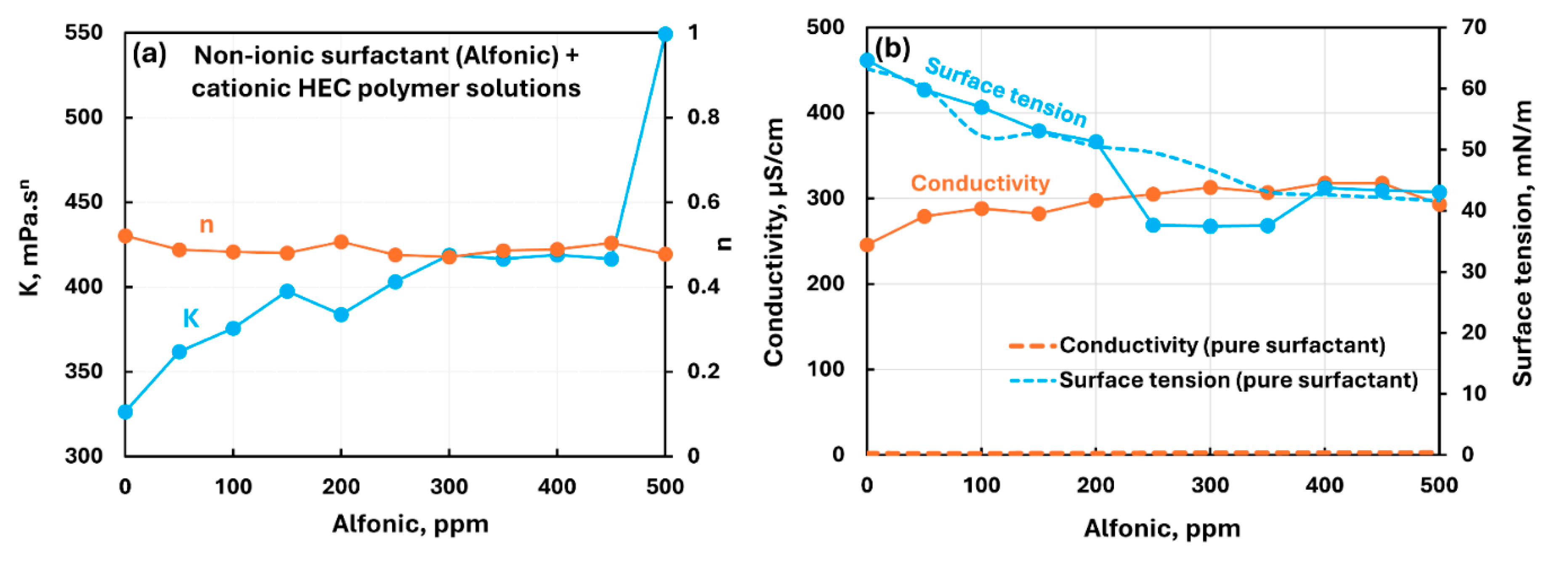
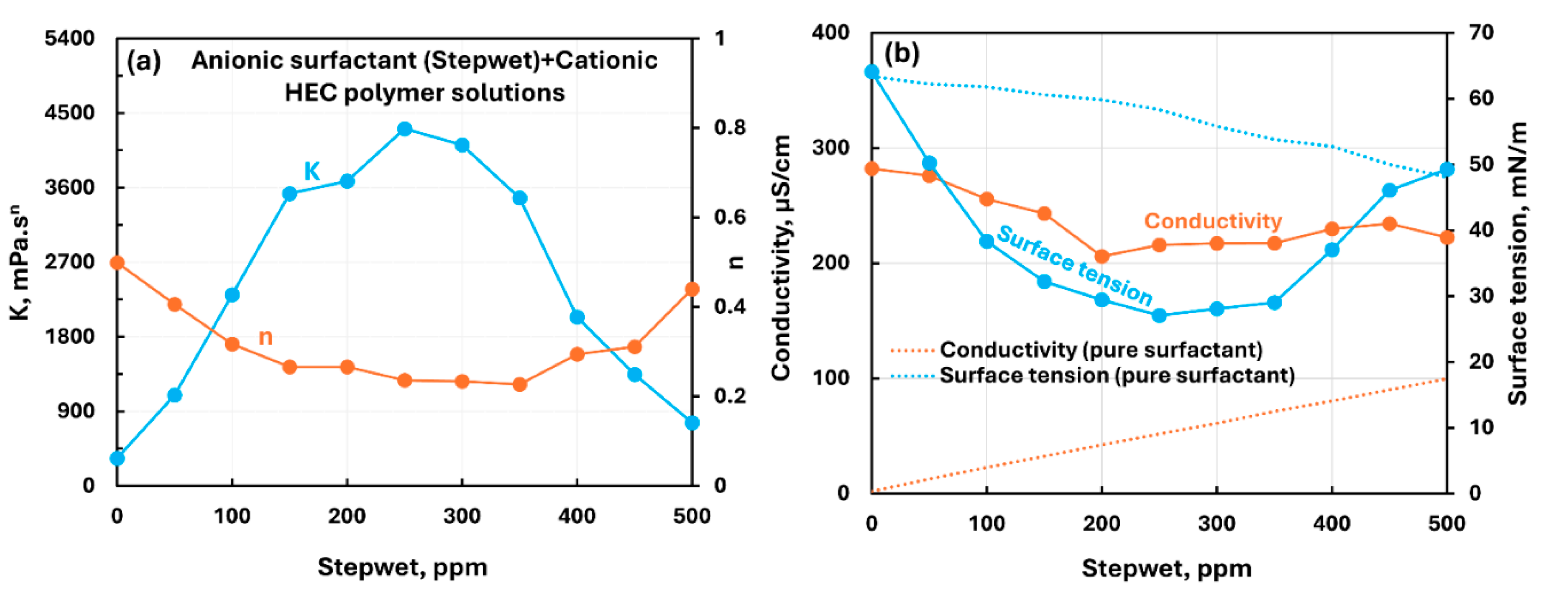
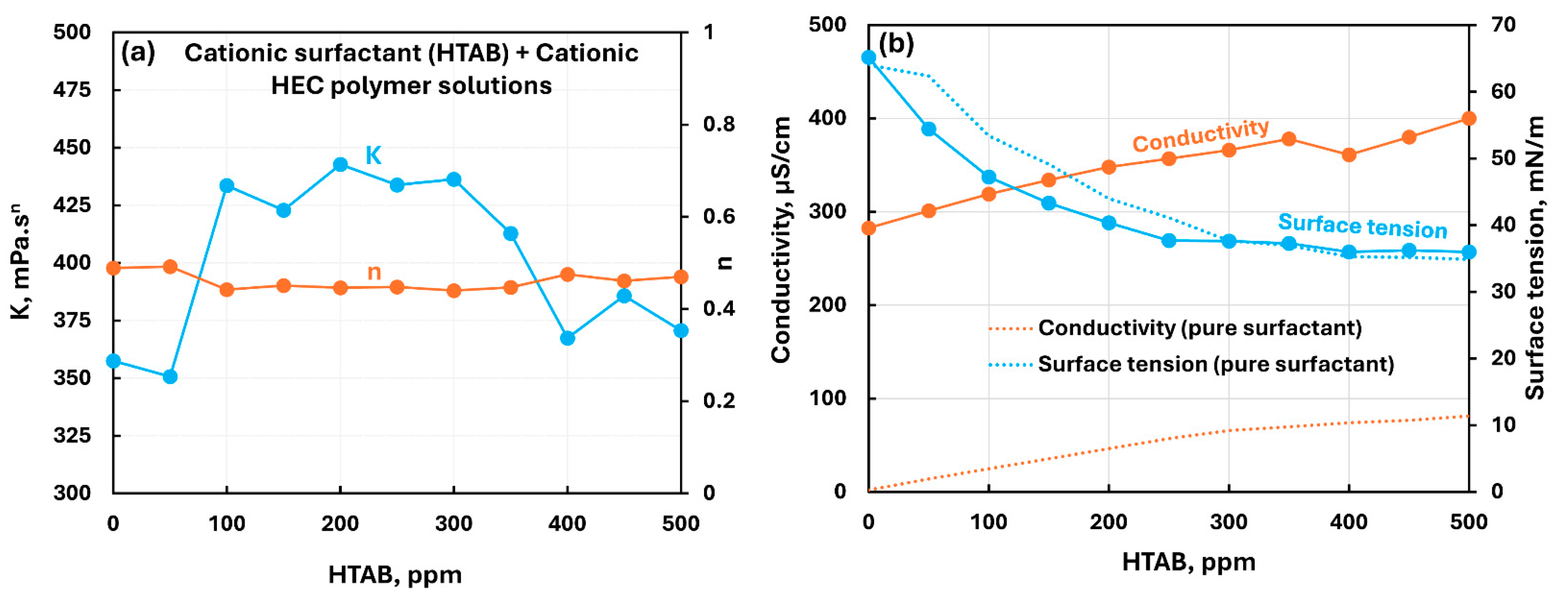
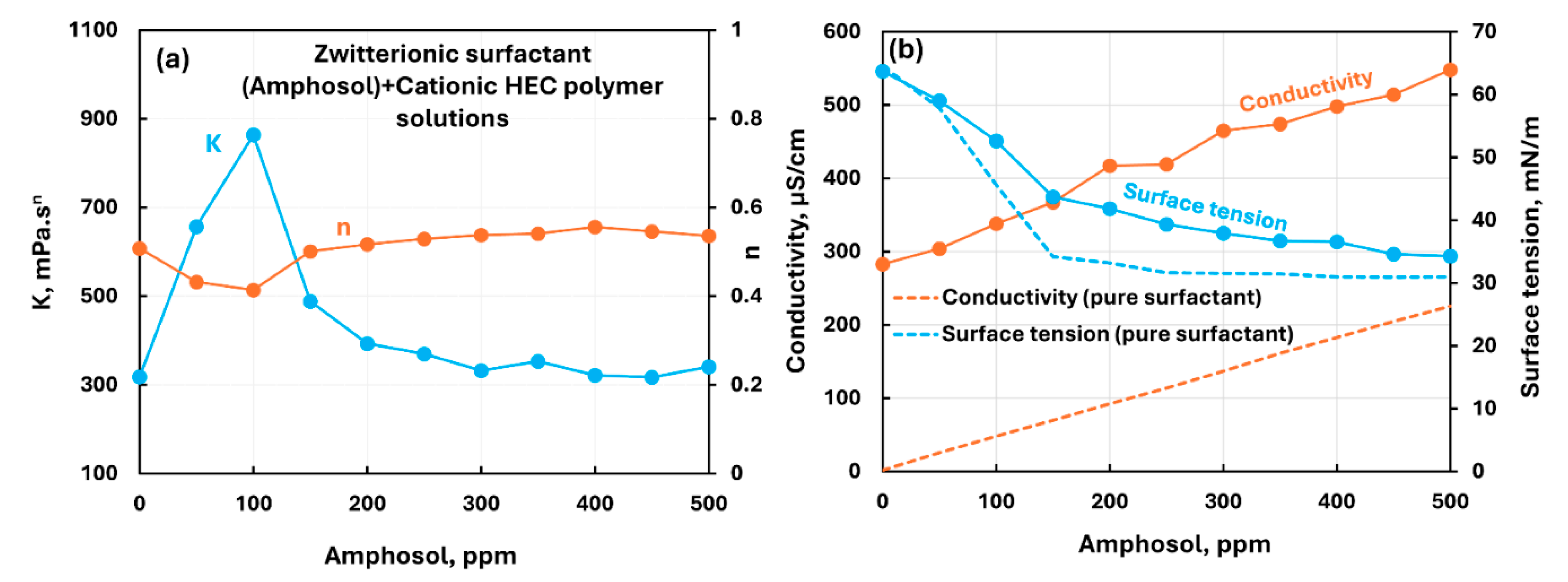
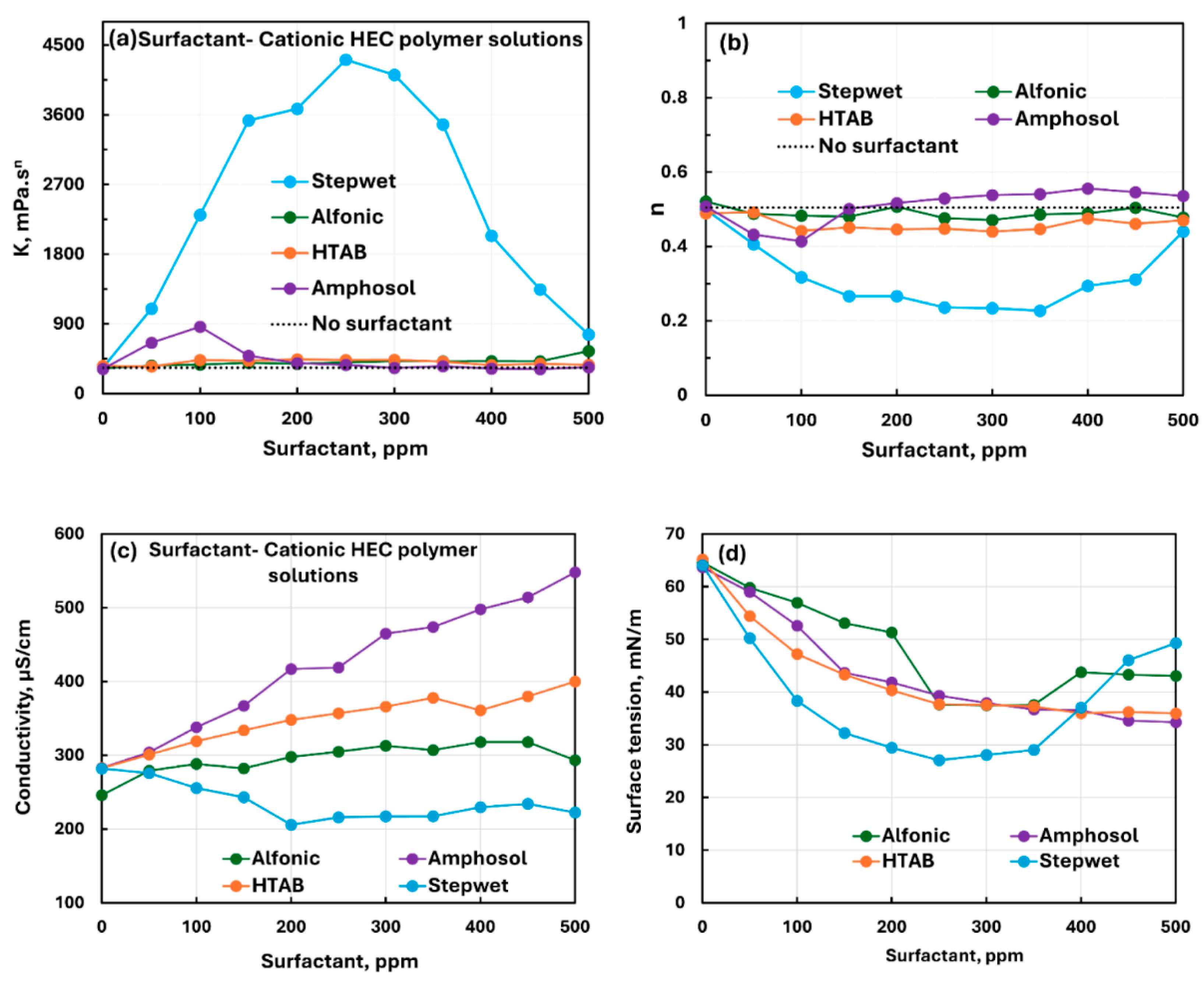
3.2.1. Cationic Polymer (CHEC) + Surfactant Solutions
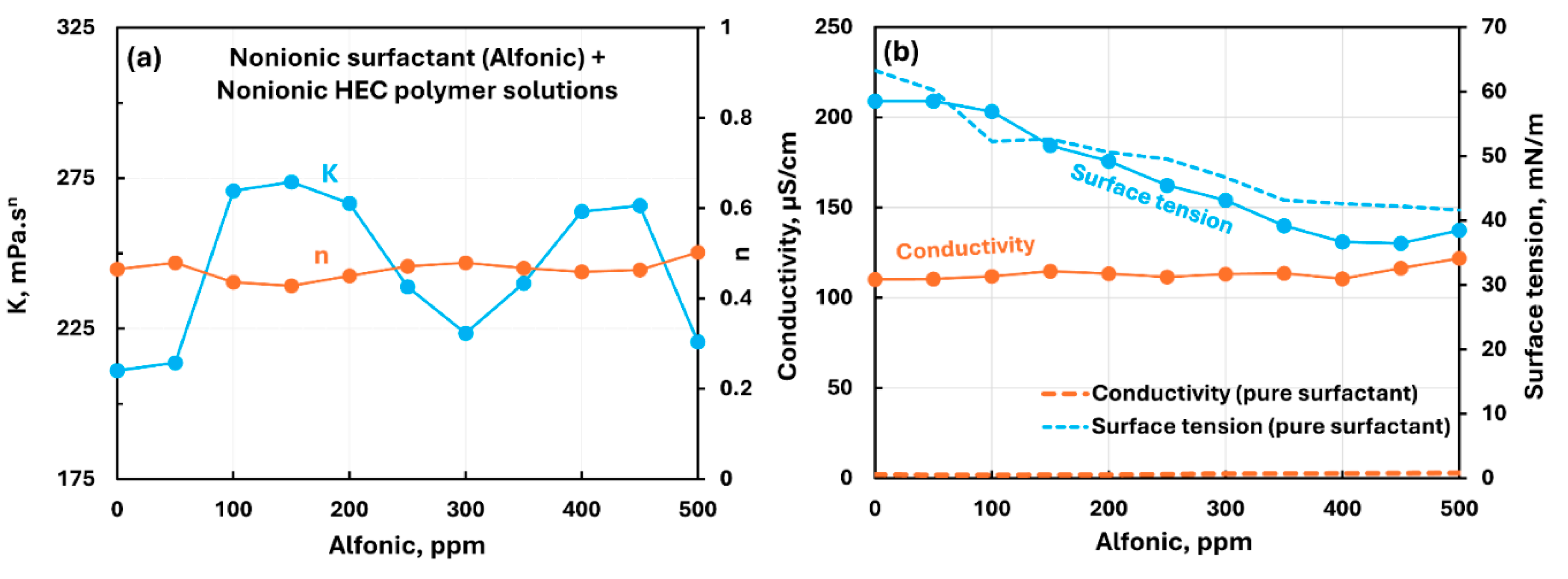
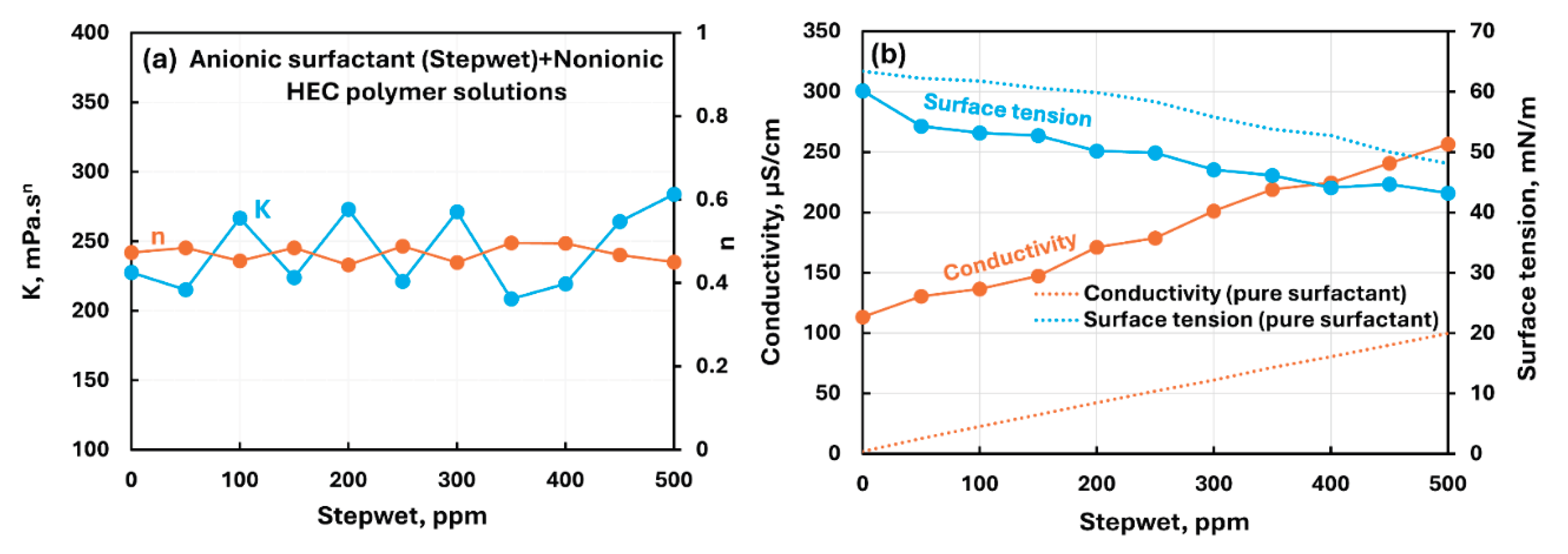
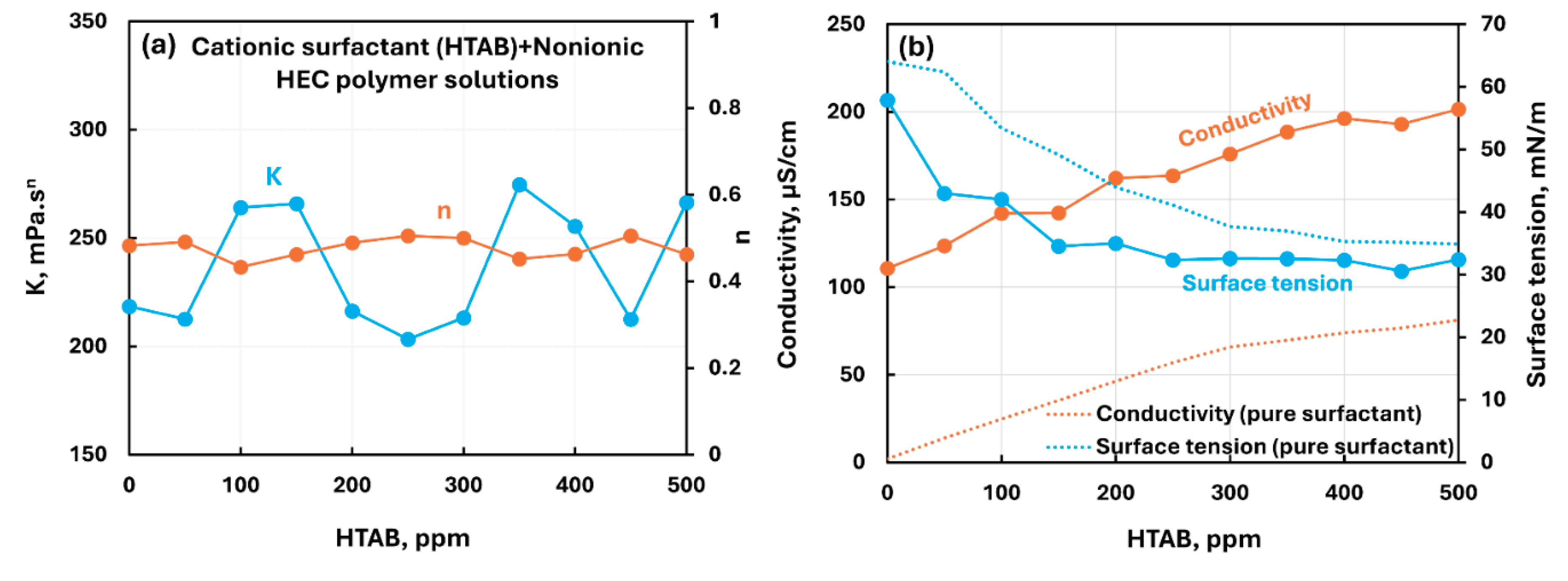
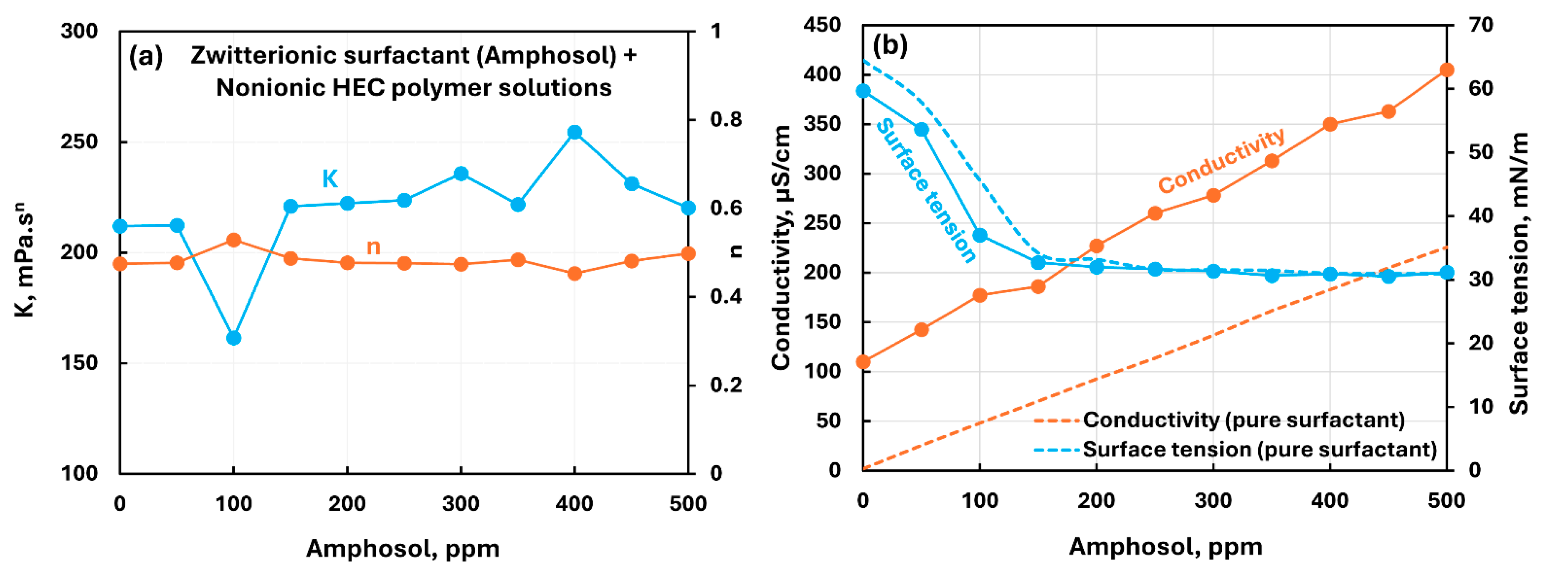
3.2.2. Nonionic Polymer (NHEC) + Surfactant Solutions
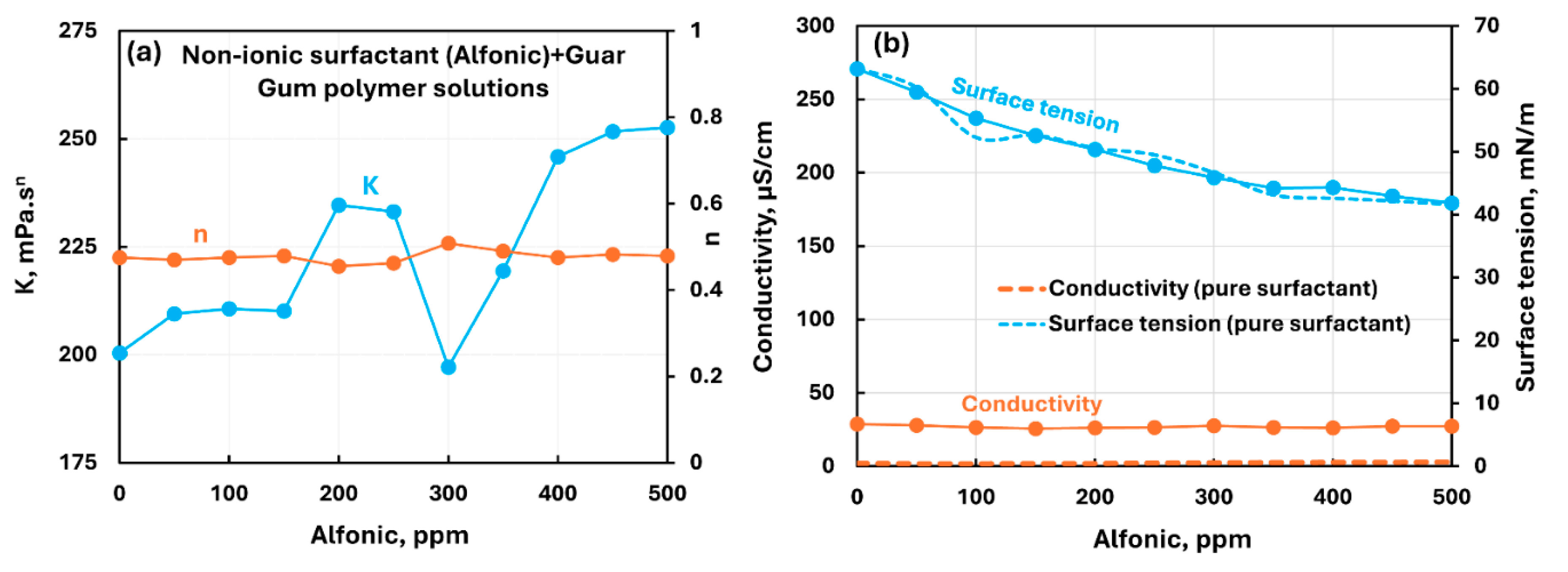
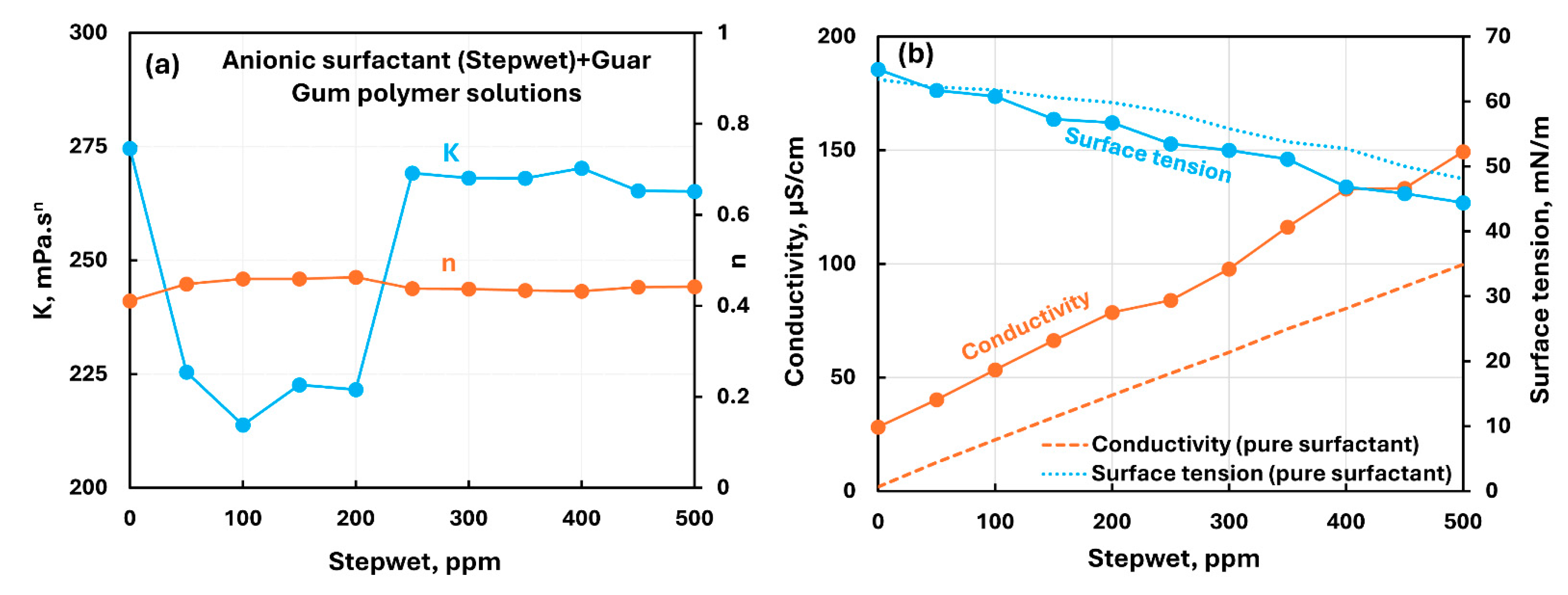
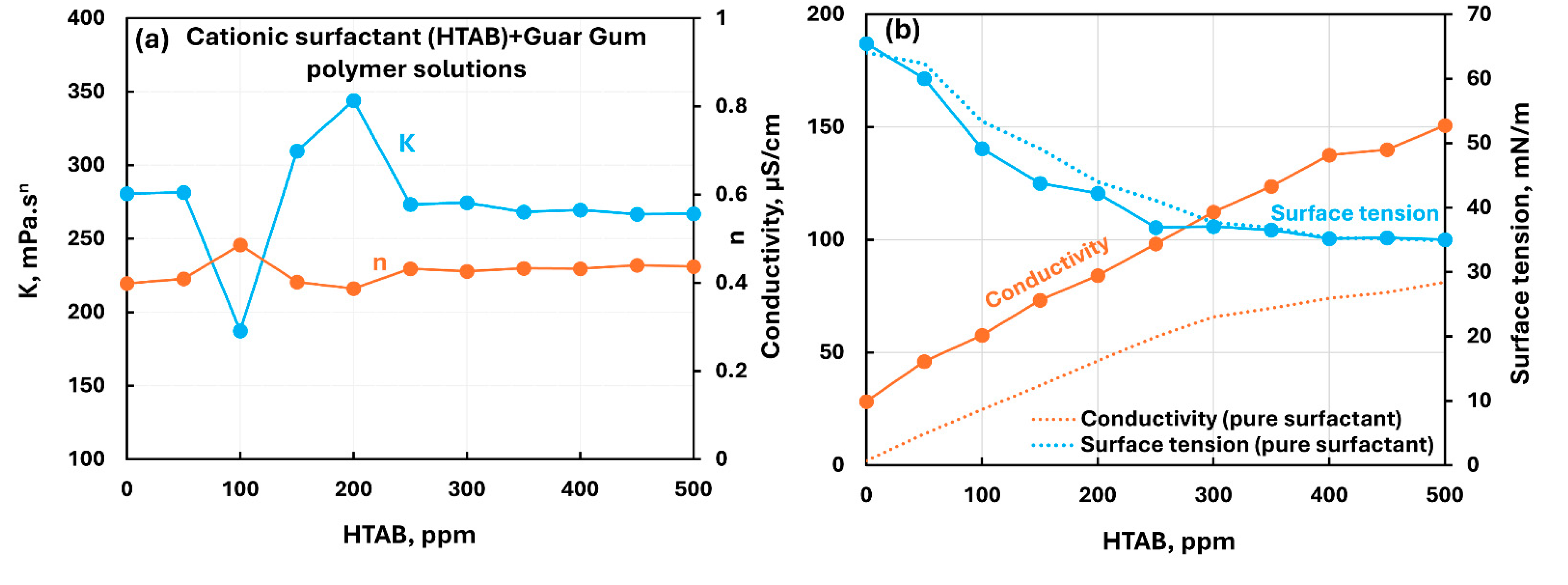
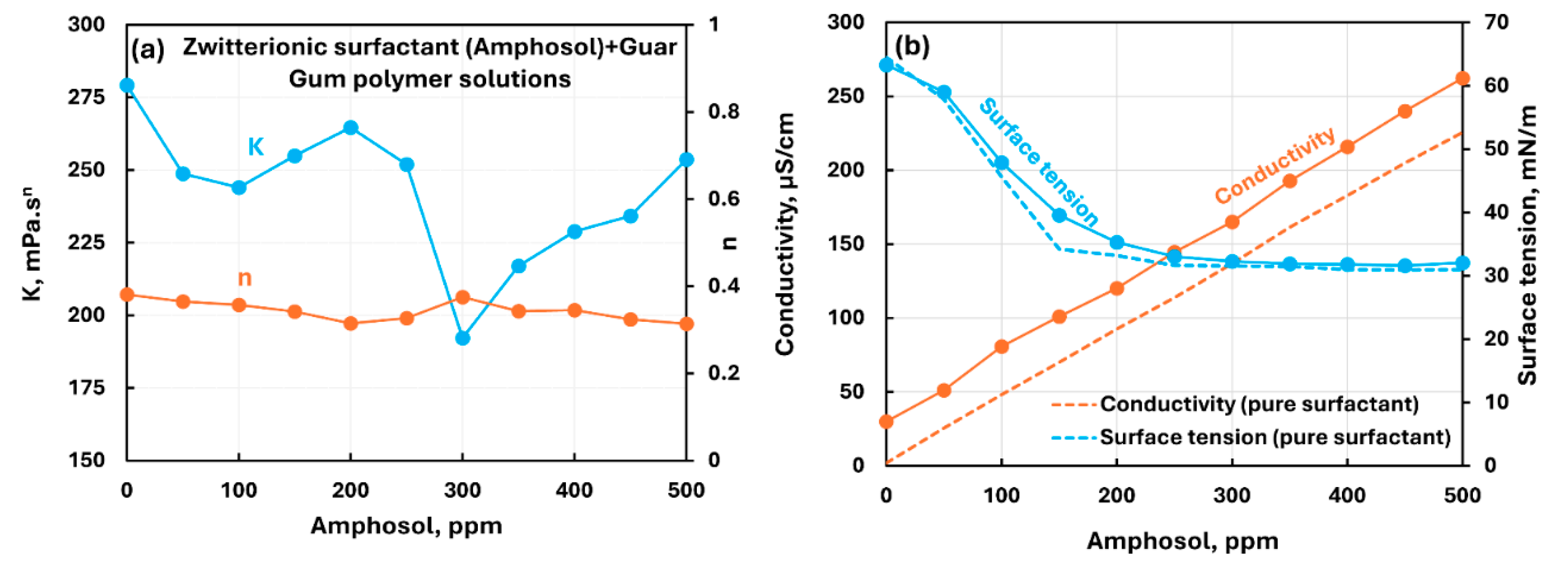
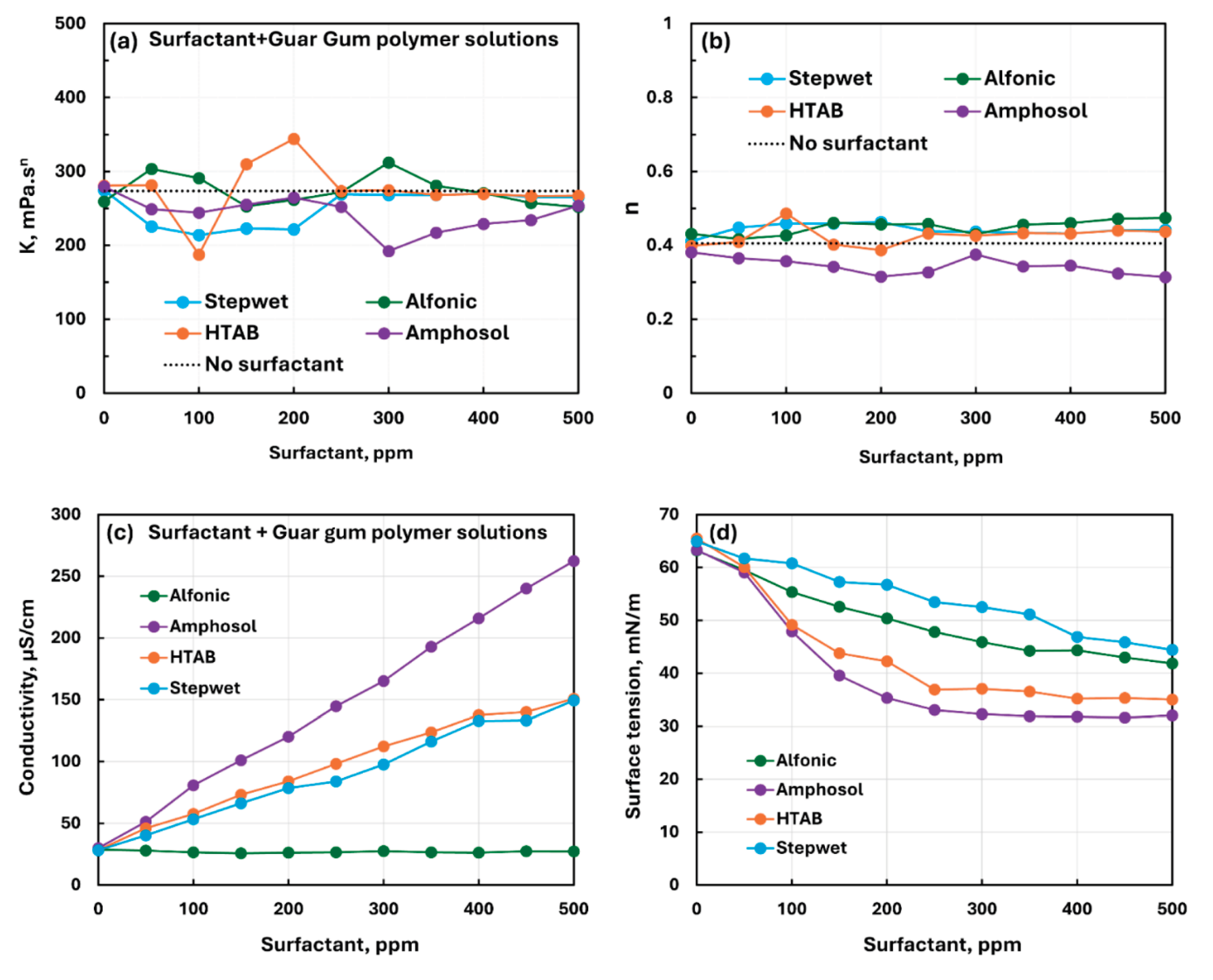
3.2.3. Nonionic Polymer (Guar Gum) + Surfactant Solutions
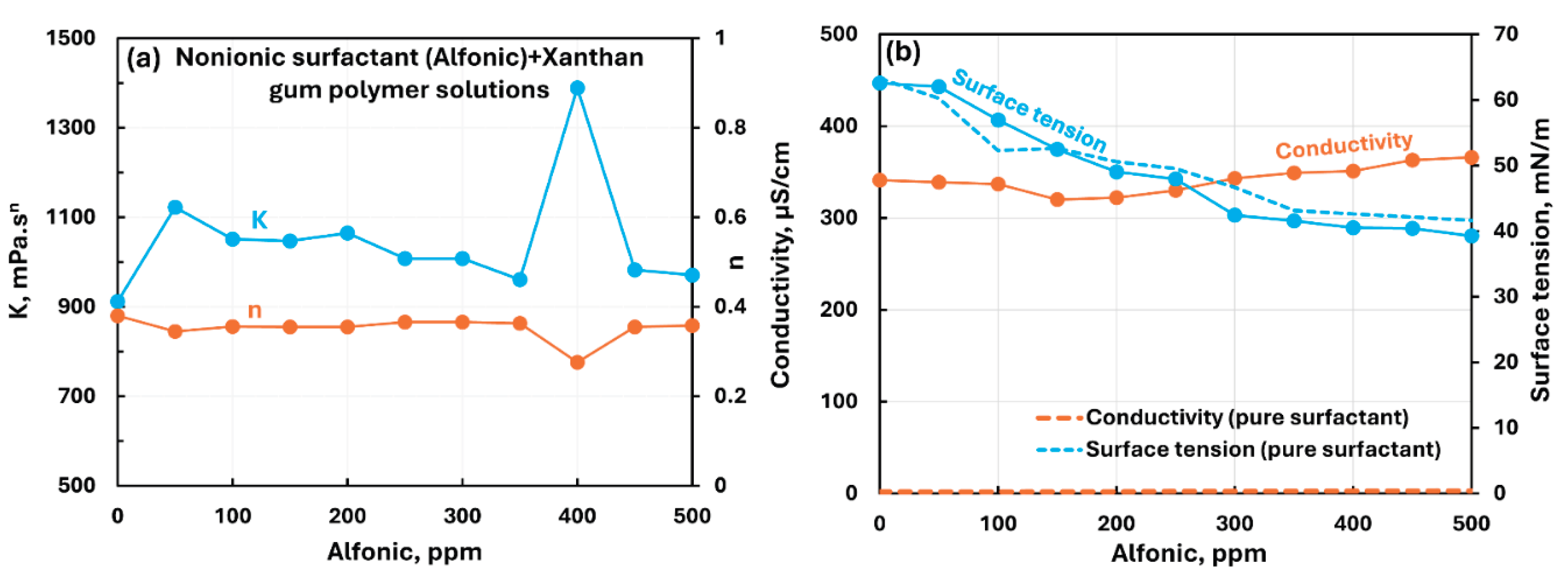
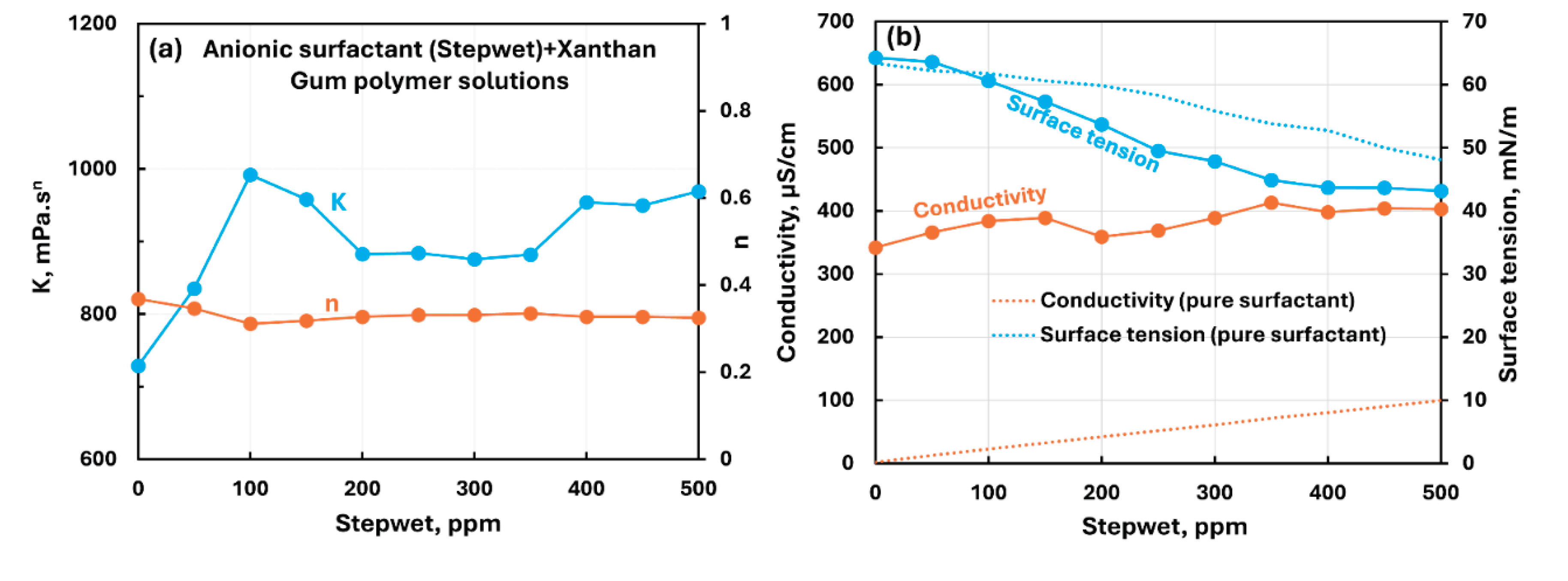
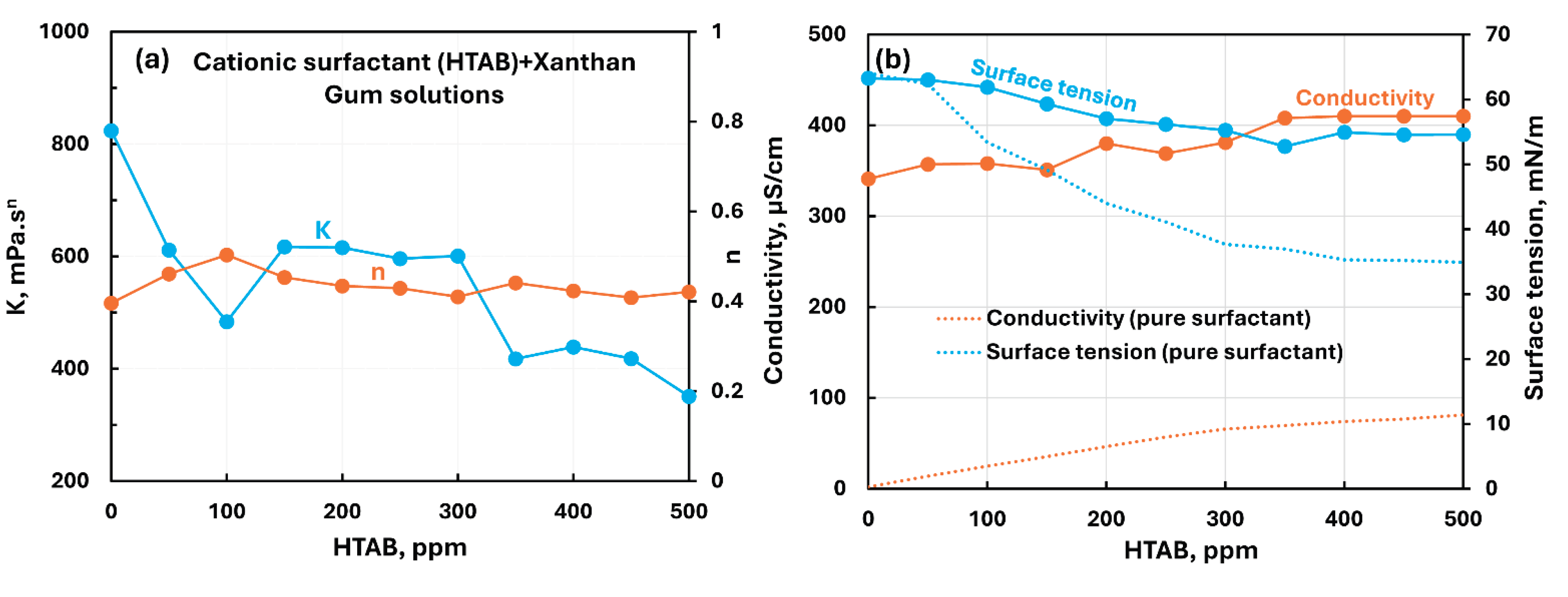
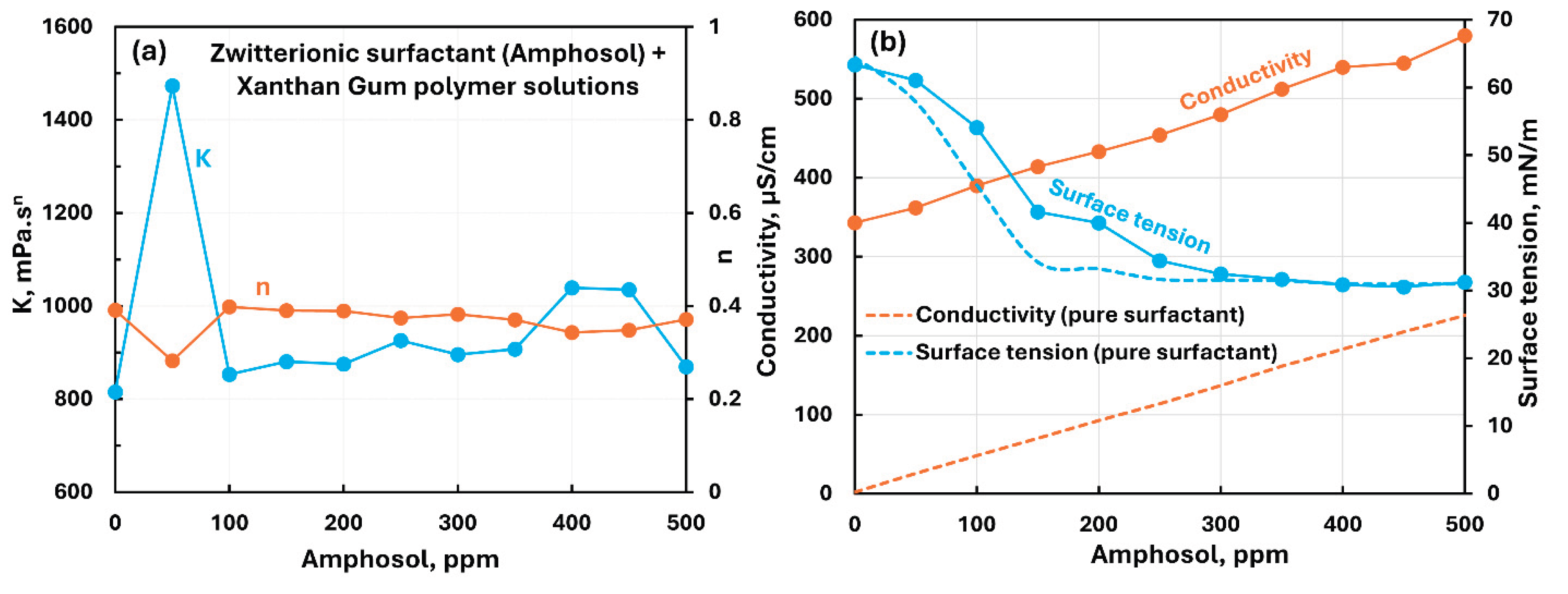
3.2.4. Anionic Polymer (Xanthan Gum) + Surfactant Solutions
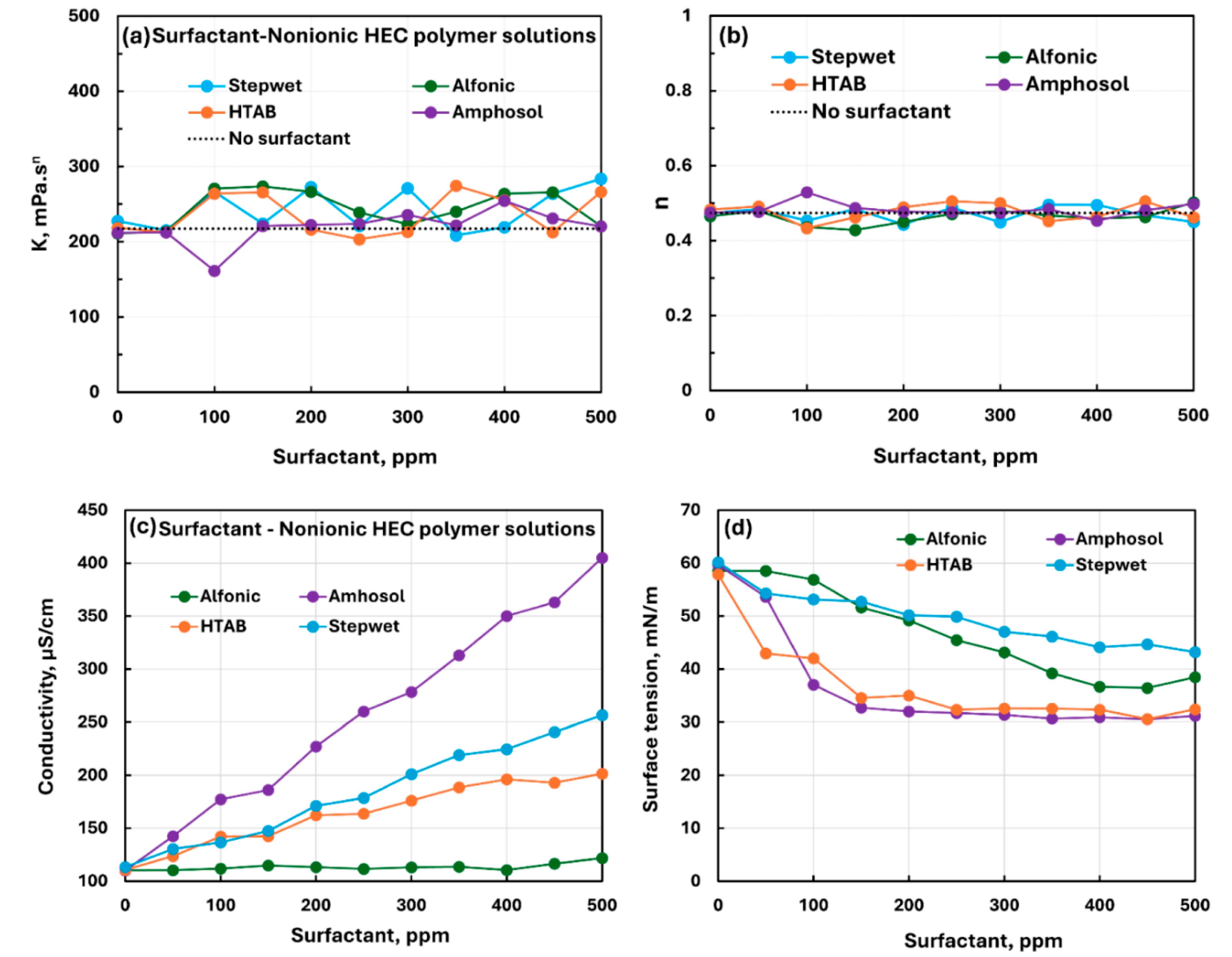
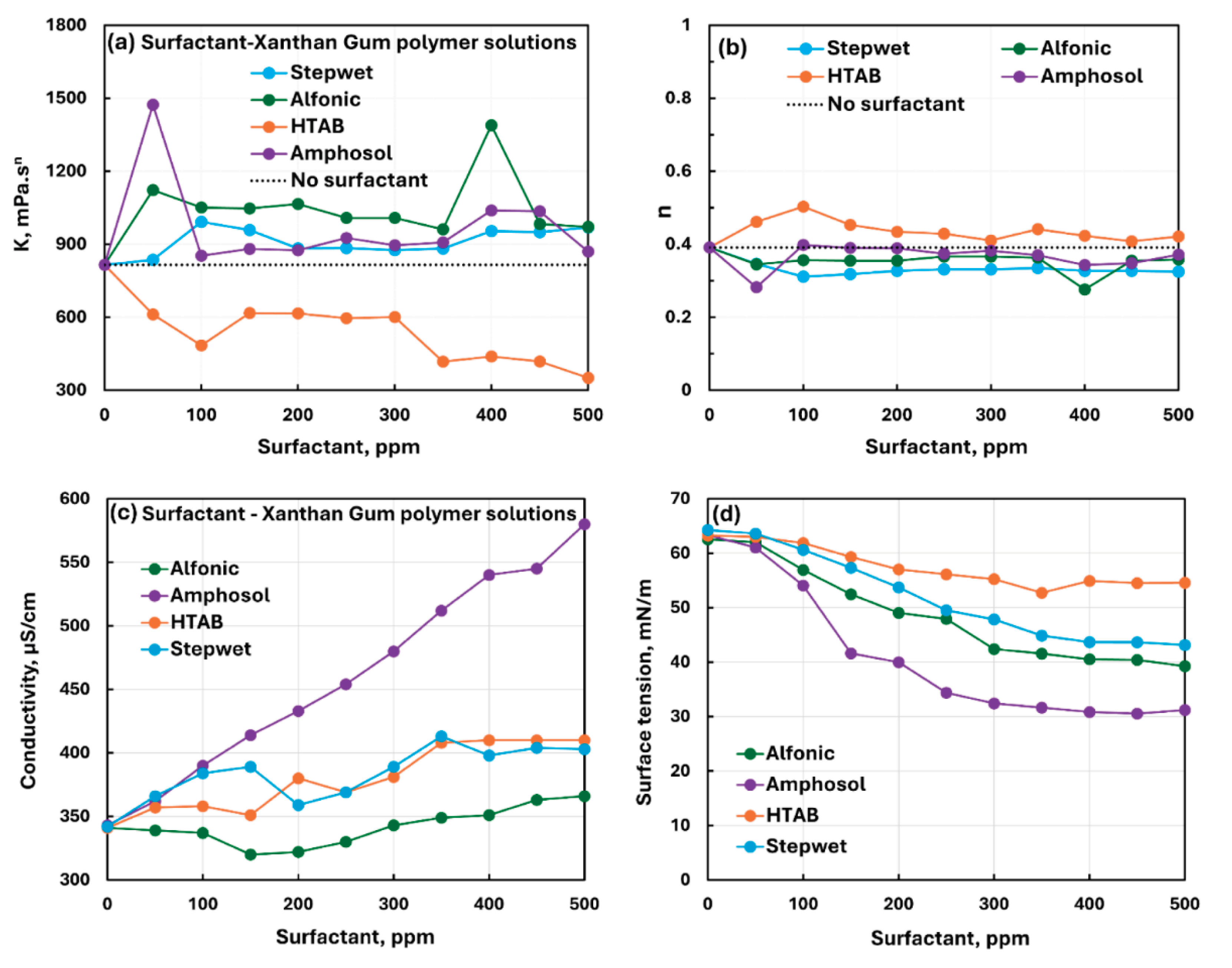
3.3. Summary of Interactions between Different Surfactants and Polymers
4. Conclusions
- The cationic hydroxyethyl cellulose (CHEC) polymer exhibits extraordinarily strong interaction with anionic surfactant (Stepwet). Dramatic changes occur in the rheological and surface-active properties upon addition of surfactant to polymer solution.
- The interactions between CHEC and three other surfactants (non-ionic Alfonic, cationic HTAB, zwitterionic Amphosol) are moderate. The consistency generally increases with the addition of surfactants. Except for zwitterionic Amphosol, the surfactant-polymer complexes formed are more surface-active than pure surfactant. Migration of surfactant from solution to polymer occurs resulting in decrease in surface-activity of solution when zwitterionic Amphosol is added to CHEC.
- The non-ionic hydroxyethyl cellulose (NHEC) polymer exhibits weak to mild interactions with the surfactants investigated. The consistency index either varies to a small extent and/or fluctuates with the increase in surfactant concentration. Generally, the surface-activity of solutions is higher than that of pure surfactants as the polymer NHEC itself is surface-active.
- The non-ionic guar gum exhibits weak to mild interactions with surfactants investigated. The consistency varies mildly upon addition of surfactant. The surface-activity of surfactant-polymer solution is enhanced compared with pure surfactant solutions in the case of anionic (Stepwet) and cationic (HTAB) surfactants. With non-ionic (Alfonic) and zwitterionic (Amphosol) surfactants, the surface-activity of surfactant-polymer solutions is unaltered from pure surfactant solutions.
- The anionic xanthan gum exhibits strong interaction with cationic surfactant (HTAB). The consistency index decreases substantially with the addition of surfactant. The other three surfactants (non-ionic, anionic, and zwitterionic) show mild to moderate interactions resulting in some increase in consistency. In the case of non-ionic Alfonic and anionic stepwet surfactants, the surfactant interacts with polymer to forms complexes which are more surface active than pure surfactant. Upon addition of cationic (HTAB) and zwitterionic (Amphosol) surfactants, the surfactant-polymer solutions become less surface active compared with pure surfactant solutions due to migration of surfactant from solution to polymer.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Q.; Pal, R. Steady shear rheology and surface activity of polymer-surfactant mixtures. Polymers 2025, 17, 364. [Google Scholar] [CrossRef]
- Gradzielski, M. Polymer–surfactant interaction for controlling the rheological properties of aqueous surfactant solutions. Current Opinion in Colloid & Interface Science 2023, 63, 101662. [Google Scholar]
- Davoodi, S.; Al-Shargabi, M.; Wood, D. A.; Rukavishnikov, V. S. A comprehensive review of beneficial applications of viscoelastic surfactants in wellbore hydraulic fracturing fluids. Fuel 2023, 338, 127228. [Google Scholar] [CrossRef]
- Machale, J.; Majumder, S. K.; Ghosh, P.; Sen, T. K. Role of chemical additives and their rheological properties in enhanced oil recovery. Reviews in Chemical Engineering 2020, 36, 789–830. [Google Scholar] [CrossRef]
- Raffa, P.; Broekhuis, A. A.; Picchioni, F. Polymeric surfactants for enhanced oil recovery: A review. Journal of Petroleum Science and Engineering 2016, 145, 723–733. [Google Scholar] [CrossRef]
- Liu, J.; Liu, P.; Du, J.; Wang, Q.; Chen, X.; Zhao, L. Review on High-Temperature-Resistant Viscoelastic Surfactant Fracturing Fluids: State-of-the-Art and Perspectives. Energy & Fuels 2023, 37, 9790–9821. [Google Scholar]
- Goddard, E. D.; Ananthapadmanabhan, K. P. Interactions of surfactants with polymers and proteins. CRC Press, Boca Raton, 1993.
- Somasundaran, P.; Krishnakumar, S. Adsorption of surfactants and polymers at the solid-liquid interface. Colloids and Surfaces A: Physicochemical and Engineering Aspects 1997, 123-124, 491–513. [Google Scholar] [CrossRef]
- Goddard, E. D.; Gruber, J. V. Principles of Polymer Science and Technology in Cosmetics and Personal Care, Taylor & Francis, Boca Raton,1999.
- Taylor, D. J. F.; Thomas, R. K.; Penfold, J. Polymer/surfactant interactions at the air/water interface. Advances in Colloid and Interface Science 2007, 132, 69–110. [Google Scholar] [CrossRef]
- Talwar, S.; Scanu, L.F.; Khan, S.A. Hydrophobic interactions in associative polymer/nonionic surfactant systems: Effects of surfactant architecture and system parameters. J. Rheol 2006, 50, 831–847. [Google Scholar] [CrossRef]
- Petkova, R.; Tcholakova, S.; Denkov, N. D. Foaming and Foam Stability for Mixed Polymer–Surfactant Solutions: Effects of Surfactant Type and Polymer Charge. Langmuir 2012, 28, 4996–5009. [Google Scholar] [CrossRef]
- Kirtil, E.; Oztop, M. H. Mechanism of adsorption for design of role-specific polymeric surfactants. Chemical Papers 2023, 77, 2343–2361. [Google Scholar] [CrossRef]
- Lazaridis, N.; Alexopoulos, A. H.; Chatzi, E. G.; Kiparissides, C. Steric stabilization in emulsion polymerization using oligomeric nonionic surfactants. Chemical Engineering Science 1999, 54, 3251–3261. [Google Scholar] [CrossRef]
- Duro, R.; Souto, C.; Gómez-Amoza, J. L.; Martínez-Pacheco, R.; Concheiro, A. Interfacial Adsorption of Polymers and Surfactants: Implications for the Properties of Disperse Systems of Pharmaceutical Interest. Drug Development and Industrial Pharmacy 1999, 25, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Cazeneuve, C.; Baghdadli, N.; Ringeissen, S.; Leermakers, F.A.M.; Luengo, G.S. Surfactant–polymer interactions: molecular architecture does matter. Soft Matter 2015, 11, 2504–2511. [Google Scholar] [CrossRef]
- Rapp, M. V.; Donaldson Jr, S.H.; Gebbie, M.A.; Gizaw, Y.; Koenig, P.; Roiter, Y.; Israelachvili, J.N. Effects of Surfactants and Polyelectrolytes on the Interaction between a Negatively Charged Surface and a Hydrophobic Polymer Surface. Langmuir 2015, 31, 8013–8021. [Google Scholar] [CrossRef]
- Raffa, P.; Wever, D. A. Z.; Picchioni, F.; Broekhuis, A. A. Polymeric Surfactants: Synthesis, Properties, and Links to Applications. Chemical Reviews 2015, 115, 8504–8563. [Google Scholar] [CrossRef]
- Tam, K. C.; Wyn-Jones, E. Insights on polymer surfactant complex structures during the binding of surfactants to polymers as measured by equilibrium and structural techniques. Chemical Society Reviews 2006, 35, 693–709. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, C.; Wang, C.; Wang, J.; Golas, P.; Batteas, J.; Kreeger, L. Phase Behavior of Cationic Hydroxyethyl Cellulose−Sodium Dodecyl Sulfate Mixtures: Effects of Molecular Weight and Ethylene Oxide Side Chain Length of Polymers. Langmuir 2004, 20, 8482–8489. [Google Scholar] [CrossRef]
- Mohsenipour, A.A.; Pal, R. A review of polymer-surfactant interactions, in Birdi, K.S. (Ed.), Handbook of Surface and Colloid Chemistry, fourth ed., CRC Press, Boca Raton, 2015, pp. 639–684.
- Diamant, H.; Andelman, D. Onset of self-assembly in polymer-surfactant systems. EPL (Europhysics Letters), 1999; 48, 170. [Google Scholar]
- Hansson, P.; Lindman, B. Surfactant-polymer interactions. Current Opinion in Colloid & Interface Science 1996, 1, 604–613. [Google Scholar] [CrossRef]
- Yang, J.; Pal, R. Investigation of surfacatant-polymer interactions using rheology ans surface tension measurements. Polymers 2020, 12, 2302. [Google Scholar] [CrossRef]
- Cao, Z.; Yan, J.; Miao, J.; Wang, J.; Wang, Z.; Zhang, L. Synthesis and evaluation of Gemini surfactant-polymer copolymers as viscosity reducer for enhancing heavy oil recovery. Chem. Eng. J. 2025, 521, 166868. [Google Scholar] [CrossRef]
- Zeariya, M.; Almarshadi, F.; Elhassan, N.E.; Humaida, M.; Essa, M.; Yousif, A.; El-Shennawy, S.; Abdel-Hameed, R.; Reda, L.M.; Metwally, A.M.; Tantawy, A.H.; ElKhawaga, H.A. Creating novel green synthesized cationic polymeric surfactants for removing the petroleum films from water surface: Surface-active and biological assessments. J. Molecular Liquids 2025, 435, 128164. [Google Scholar] [CrossRef]
- Chen, X.; Hou, Q.; Liu, Y.; Liu, G.; Zhang, H.; Sun, H.; Zhu, Z.; Liu, W. Experimental Study on Surfactant–Polymer Flooding After Viscosity Reduction for Heavy Oil in Matured Reservoir. Energies 2025, 18, 756. [Google Scholar] [CrossRef]
- Mohsenipour, A.A.; Pal, R. Drag reduction in turbulent pipeline flow of mixed nonionic polymer and cationic surfactant systems. Can. J. Chem. Eng. 2013, 91, 190–201. [Google Scholar] [CrossRef]
- Mohsenipour, A.A.; Pal, R. The role of surfactants in mechanical degradation of drag-reducing polymers. Ind. Eng. Chem. Res. 2013, 52, 1291–1302. [Google Scholar] [CrossRef]
- Chen, H.; Muros-Cobos, J.L.; Holgado-Terriza, J.A.; Amirfazli, A. Surface tension measurement with a smartphone using a pendant drop. Colloids Surfaces A 2017, 533, 213–217. [Google Scholar] [CrossRef]
- Pal, R. Rheology of high internal phase ratio emulsions and foams. Adv. Colloid Interface Sci. 2025, 339, 103426. [Google Scholar] [CrossRef]
| Viscometer | Length of inner cylinder | Gap-width | ||
|---|---|---|---|---|
| Fann 35A/SR-12 | 1.72 cm | 1.84 cm | 3.8 cm | 0.12 cm |
| Haake Roto- visco RV 12 with MV I | 2.00 cm | 2.1 cm | 6.0 cm | 0.10 cm |
| Polymer | Surfactant | Surfactant -Polymer Combination | Comments |
|---|---|---|---|
| Cationic hydroxyethyl cellulose (CHEC) | Non-ionic (Alfonic) | S0 P+ | Moderate interaction between surfactant and polymer; consistency increases; solution surface tension lower than pure surfactant; surfactant-polymer complexes formed are more surface active than pure surfactant. |
| Cationic hydroxyethyl cellulose (CHEC) | Anionic (Stepwet)razmak | S− P+ | Extraordinarily strong interaction between surfactant and polymer; consistency increases sharply and goes through a maximum; solution becomes very shear-thinning; surface tension falls by a large amount; surfactant-polymer complexes formed are much more surface active than pure surfactant. |
| Cationic hydroxyethyl cellulose (CHEC) | Cationic (HTAB)razmak | S+ P+ | Moderate interaction between surfactant and polymer; consistency increases; solution surface tension lower than pure surfactant; surfactant-polymer complexes formed are more surface active than pure surfactant. |
| Cationic hydroxyethyl cellulose (CHEC) | Zwitterionic (Amphosol)razmak | S+- P+ | Moderate interaction between surfactant and polymer; consistency increases; migration of surfactant from solution to polymer increases the surface tension of solution. |
| Non-ionic hydroxyethyl cellulose (NHEC) | Non-ionic (Alfonic) | S0 P0 | Mild interaction between surfactant and polymer; consistency fluctuates; solution surface tension lower than pure surfactant; surfactant-polymer complexes formed are more surface active than pure surfactant. |
| Non-ionic hydroxyethyl cellulose (NHEC) | Anionic (Stepwet)razmak | S− P0 | Weak interaction between surfactant and polymer; negligible change in consistency; solution surface tension lower than pure surfactant due to surface activity of polymer itself; no unambiguous evidence of formation of surfactant-polymer complexes. |
| Non-ionic hydroxyethyl cellulose (NHEC) | Cationic (HTAB)razmak | S+ P0 | Mild interaction between surfactant and polymer; minor changes in consistency; solution surface tension lower than pure surfactant due to surface activity of polymer itself; no unambiguous evidence of formation of surfactant-polymer complexes. |
| Non-ionic hydroxyethyl cellulose (NHEC) | Zwitterionic (Amphosol)razmak | S+- P0 | Weak interaction between surfactant and polymer; consistency fluctuates; no unambiguous evidence of formation of surfactant-polymer complexes. |
| Non-ionic Guar Gum | Non-ionic (Alfonic) | S0 P0 | Mild interaction between surfactant and polymer; consistency increases mildly; no unambiguous evidence of formation of surfactant-polymer complexes. |
| Non-ionic Guar Gum | Anionic (Stepwet) | S− P0 | Moderate interaction between surfactant and polymer; consistency decreases; solution surface tension lower than pure surfactant; surfactant-polymer complexes formed are more surface active than pure surfactant. |
| Non-ionic Guar Gum | Cationic (HTAB) | S+ P0 | Weak interaction between surfactant and polymer; consistency fluctuates; enhanced surface-activity due to formation of surface active factant-polymer compexes. |
| Non-ionic Guar Gum | Zwitterionic (Amphosol) | S+- P0 | Mild interactions between surfactant and polymer; consistency changes small; surface activity of surfactant-polymer solution is nearly the same as that of pure surfactant solution. No evidence of formation of surfactant-polymer complexes.razmak |
| Anionic Xanthan Gum | Non-ionic (Alfonic) | S0 P- | Moderate interaction between surfactant and polymer; consistency index generally increases; Surfactant interacts with polymer to forms complexes which are more surface active than pure surfactant. |
| Anionic Xanthan Gum | Anionic (Stepwet) | S− P- | Moderate interaction between surfactant and polymer; consistency index increases significantly; Surfactant interacts with polymer to forms complexes which are more surface active than pure surfactant. |
| Anionic Xanthan Gum | Cationic (HTAB) | S+ P- | Strong interaction between surfactant and polymer; consistency index decreases substantially; polymer-surfactant solution is less surface active than pure surfactant due to migration of surfactant from solution to polymer |
| Anionic Xanthan Gum | Zwitterionic (Amphosol) | S+- P- | Mild interaction between surfactant and polymer; consistency fluctuates with small overall increase; migration of surfactant from solution to polymer increases the surface tension. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





