Submitted:
16 September 2025
Posted:
17 September 2025
Read the latest preprint version here
Abstract
Medetomidine, a potent veterinary α2 agonist, has emerged as a fentanyl adulterant in the non-medical opioid supply. Its use has been linked to a novel withdrawal syndrome that is often resistant to conventional treatment protocols. Four cases are presented demonstrating extreme forms of this withdrawal syndrome. A literature review is provided showing both the paucity of available literature as well as potential avenues for treatment and future research. As adulterants continue to proliferate in the illicit drug supply, clinicians should anticipate atypical withdrawal phenotypes and consider early intervention.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Case Series Review
2.3. Medication Usage
2.4. Narrative Literature Review
3. Results
3.1. Case One
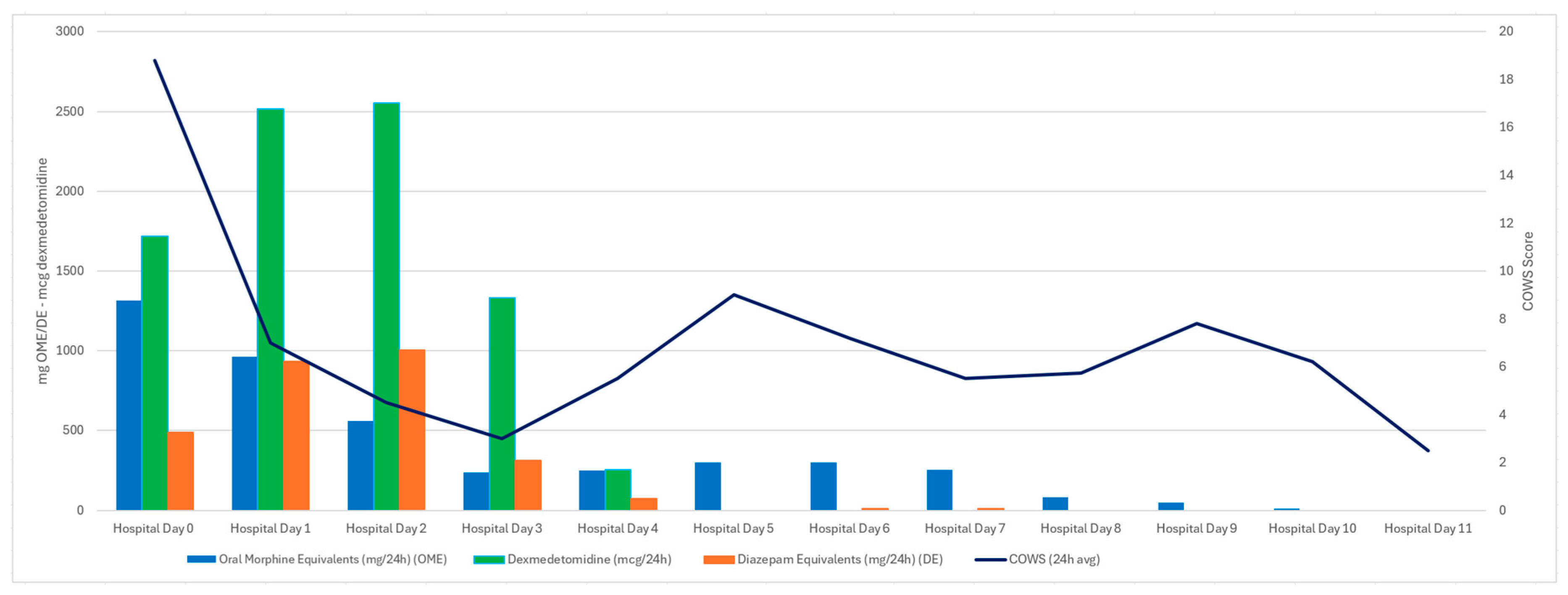
3.2. Case Two
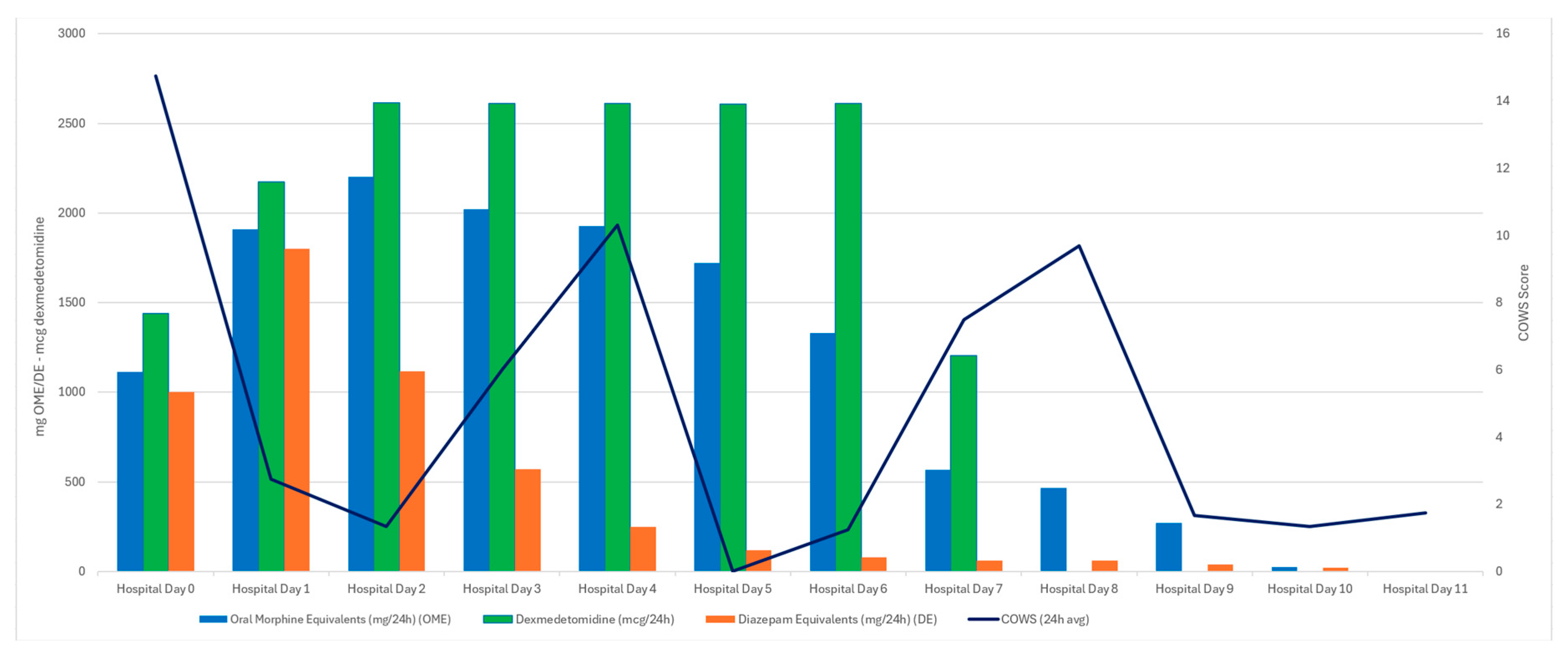
3.3. Case Three
3.4. Case Four
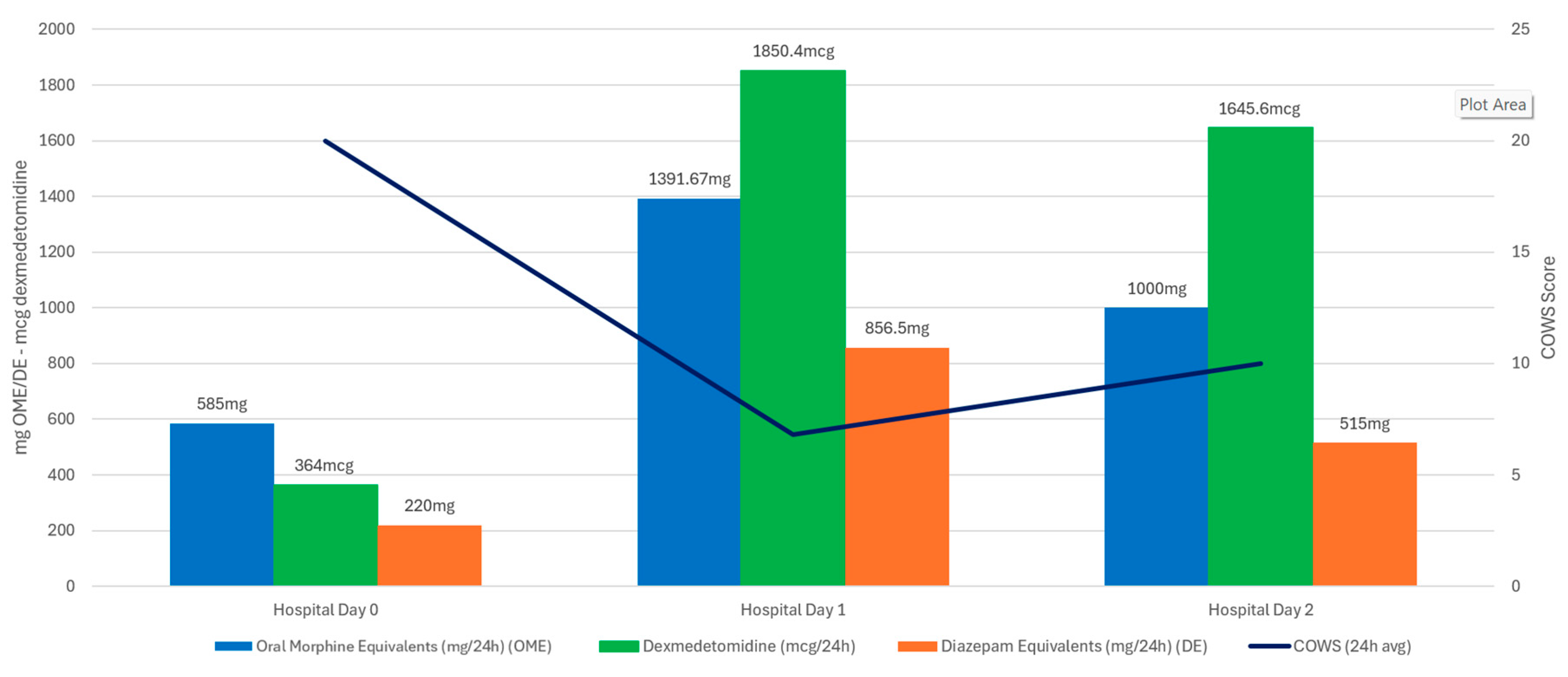
4. Discussion
4.1. Summary
4.2. Introduction to Narrative Review
4.3. Pharmacology and Comparison to Xylazine
4.4. Dexmedetomidine Withdrawal Syndrome
4.5. Timeline and Epidemiology
4.6. Concepts in Clinical Management
4.7. Combination α2 Agonist Therapy
4.8. Low Dose Dexmedetomidine Infusion Outside the ICU
4.9. High Dose Dexmedetomidine
4.10. Transdermal Clonidine
4.11. Public Health Implications and Future Directions
4.12. Limitations
6. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| MICU | Medical Intensive Care Unit |
| ED | Emergency Department |
| α2 | Alpha-2 (adrenergic receptor) |
| ECG | Electrocardiogram |
| HR | Heart Rate |
| BP | Blood Pressure |
| COWS | Clinical Opiate Withdrawal Scale |
| PHA | Public Health Alert |
| CNS | Central Nervous System |
| PO | Per Os (by mouth/oral) |
| IVF | Intravenous Fluid |
| ODT | Oral Dissolving Table |
| IVP | Intravenous Push |
| QTc | Corrected QT Interval |
| SANRA | Scale for the Assessment of Narrative Review Articles |
| CARE | CAse REport guidelines |
| GABA | Gamma-Aminobutyric Acid |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| AV | Atrioventricular |
References
- Quijano T, Crowell J, Eggert K, Clark K, Alexander M, Grau L, Heimer R. Xylazine in the drug supply: emerging threats and lessons learned in areas with high levels of adulteration. Int. J. Drug Policy 2023, 120, 104154. [CrossRef]
- Reed MK, Esteves Camacho T, Olson R, Grover Z, Rapoza T, Larson MJ. Xylazine’s impacts on the community in Philadelphia: perspectives of people who use opioids and harm reduction workers. Subst. Use Misuse 2024, 60(1), 100–107. [CrossRef]
- Alexander R, Agwuncha C, Wilson C, Schrecker J, Holt A, Heltsley R. Withdrawal signs and symptoms among patients positive for fentanyl with and without xylazine. J. Addict. Med. 2025, 19, 202–207. [CrossRef]
- London K, Li Y, Kahoud JL, Cho D, Mulholland J, Roque S, Slovis B. Tranq dope: characterization of an ED cohort treated with a novel opioid withdrawal protocol in the era of fentanyl/xylazine. Am. J. Emerg. Med. 2024, 85, 130–139. [CrossRef]
- Huo S, London K, Murphy L, et al. Notes from the Field: Suspected Medetomidine Withdrawal Syndrome among Fentanyl-Exposed Patients—Philadelphia, Pennsylvania, September 2024–January 2025. MMWR Morb. Mortal. Wkly. Rep. 2025, 74(15), 266–268. [CrossRef]
- Philadelphia Department of Public Health. Health Alert: Medetomidine as an emerging adulterant in the illicit drug supply—December 10, 2024. Available online: https://hip.phila.gov/document/4874/PDPH-HAN-00444A-12-10-2024.pdf (accessed on 30 July 2025).
- Bryant CE, England GCW, Clarke KW. A comparison of the sedative effects of medetomidine and xylazine in the horse. J. Vet. Anaesth. 1991, 18, 55–57. [CrossRef]
- Tyner CL, Woody BJ, Reid JS, Chafetz EP, Lederer HA, Norton JF, Jöchle W. Multicenter clinical comparison of sedative and analgesic effects of medetomidine and xylazine in dogs. J. Am. Vet. Med. Assoc. 1997, 211, 1413–1417. [CrossRef]
- London KS, Durney P, Warrick-Stone T, Alexander K, Kahoud JL. Decreased effectiveness of a novel opioid withdrawal protocol following the emergence of medetomidine as a fentanyl adulterant. Biomedicines 2025, 5(2), 13. [CrossRef]
- Wesson, D.R.; Ling, W. The clinical opiate withdrawal scale (COWS). J. Psychoact. Drugs 2003, 35, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ashton, C.H. Benzodiazepines: How They Work and How to Withdraw (The Ashton Manual); Institute of Neuroscience, Newcastle University: Newcastle upon Tyne, UK, 2002.
- McPherson, M.L.; American Society of Health-System Pharmacists, Eds. Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing, 2nd ed.; American Society of Health-System Pharmacists: Bethesda, MD, USA, 2018.
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. J. Clin. Epidemiol. 2014, 67(1), 46–51. [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Dexmedetomidine: a review of its use for sedation in the intensive care setting. Drugs 2015, 75, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Shukry, M.; Miller, J.A. Update on dexmedetomidine: use in nonintubated patients requiring sedation for surgical procedures. Ther. Clin. Risk Manag. 2010, 6, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Maze M, Tranquilli W. Alpha-2 adrenoceptor agonists: defining the role in clinical anesthesia. Anesthesiology 1991, 74(3), 581–605. [CrossRef]
- Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc. (Baylor Univ. Med. Cent.) 2001, 14(1), 13–21. [CrossRef]
- Karol, M.D.; Maze, M. Pharmacokinetics and interaction pharmacodynamics of dexmedetomidine in humans. Best Pract. Res. Clin. Anaesthesiol. 2000, 14, 261–269. [Google Scholar] [CrossRef]
- Ingersoll-Weng E, Manecke Jr GR, Thistlethwaite PA. Dexmedetomidine and cardiac arrest. Anesthesiology 2004, 100, 738–739. [CrossRef]
- Scheinin H, Karhuvaara S, Olkkola KT, Kallio A, Anttila M, Vuorilehto L, Scheinin M. Pharmacodynamics and pharmacokinetics of intramuscular dexmedetomidine. Clin. Pharmacol. Ther. 1992, 52, 537–546. [CrossRef]
- Kallio A, Salonen M, Forssell H, Scheinin H, Scheinin M, Tuominen J. Medetomidine premedication in dental surgery: a double-blind cross-over study with a new alpha 2-adrenoceptor agonist. Acta Anaesthesiol. Scand. 1990, 34(3), 171–175. [CrossRef]
- Li A, Yuen VM, Goulay-Dufay S, Kwok PC. Pharmacokinetics and pharmacodynamics of dexmedetomidine. Drug Dev. Ind. Pharm. 2016, 42, 1917–1927. [CrossRef] [PubMed]
- Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an α2-adrenoceptor agonist. Eur. J. Pharmacol. 1988, 150, 9–14. [CrossRef] [PubMed]
- Yaygıngül R, Belge A. The comparison of clinical and cardiopulmonary effects of xylazine, medetomidine and detomidine in dogs. Ankara Univ. Vet. Fak. Derg. 2018, 65, 313–322. [CrossRef]
- Maurer PM, Bartkowski RR. Drug interactions of clinical significance with opioid analgesics. Drug Saf. 1993, 8, 30–48. [CrossRef] [PubMed]
- Srivastava AB, Mariani JJ, Levin FR. New directions in the treatment of opioid withdrawal. Lancet 2020, 395, 1938–1948. [CrossRef] [PubMed]
- Rudolf G, Walsh J, Plawman A, Gianutsos P, Alto W, Mancl L, Rudolf V. A novel non-opioid protocol for medically supervised opioid withdrawal and transition to antagonist treatment. Am. J. Drug Alcohol Abuse 2018, 44(3), 302–309. [CrossRef]
- Ehrman-Dupre R, Kaigh C, Salzman M, Haroz R, Peterson LK, Schmidt R. Management of xylazine withdrawal in a hospitalized patient: a case report. J. Addict. Med. 2022, 16(6), 595–598. [CrossRef]
- Ayub S, Parnia S, Poddar K, Bachu AK, Sullivan A, Khan AM, Ahmed S, Jain L. Xylazine in the opioid epidemic: a systematic review of case reports and clinical implications. Cureus 2023, 15(3), e36864. [CrossRef]
- Kukoyi AT, Coker SA, Lewis LD, Nierenberg DW. Two cases of acute dexmedetomidine withdrawal syndrome following prolonged infusion in the intensive care unit: report of cases and review of the literature. Hum. Exp. Toxicol. 2013, 32(1), 107–110. [CrossRef]
- Pathan S, Kaplan JB, Adamczyk K, et al. Evaluation of dexmedetomidine withdrawal in critically ill adults. J. Crit. Care 2021, 62, 19–24. [CrossRef] [PubMed]
- Bouajram RH, Bhatt K, Croci R, Baumgartner L, Puntillo K, Ramsay J, Thompson A. Incidence of dexmedetomidine withdrawal in adult critically ill patients: a pilot study. Crit. Care Explor. 2019, 1(1), e0035. [CrossRef]
- Flieller LA, Alaniz C, Pleva MR, Miller JT. Incidence of rebound hypertension after discontinuation of dexmedetomidine. Pharmacotherapy 2019, 39(10), 970–974. [CrossRef]
- Ferguson, L.; Hooper, S. Dexmedetomidine withdrawal syndrome and opioid sensitivity. BMJ Support. Palliat. Care 2023, 13 (Suppl 1), e105–e107. [Google Scholar] [CrossRef]
- Bhatt K, Quan AT, Baumgartner L, et al. Effects of a clonidine taper on dexmedetomidine use and withdrawal in adult critically ill patients—a pilot study. Crit. Care Explor. 2020, 2(11), e0245. [CrossRef]
- Kim CS, McLaughlin KC, Romero N, Crowley KE. Evaluation of dexmedetomidine withdrawal and management after prolonged infusion. Clin. Ther. 2024, 46(12), 1034–1040. [CrossRef]
- Philadelphia Department of Public Health, Division of Substance Use Prevention and Harm Reduction. Health Alert: In Philadelphia, medetomidine, a potent non-opioid veterinary sedative, has been detected in the illicit drug supply [Health Alert PDPH-HAN-0441A]. Philadelphia Department of Public Health; 13 May 2024. Available online: https://hip.phila.gov/document/4421/PDPH-HAN-0441A-05-13-24.pdf (accessed on 30 July 2025).
- Murphy, L.; Krotulski, A.; Hart, B.; Wong, M.; Overton, R.; McKeever, R. Clinical characteristics of patients exposed to medetomidine in the illicit opioid drug supply in Philadelphia: a case series. Clin. Toxicol. 2025, 63, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Nham A, Le JN, Thomas SA, Gressick K, Ussery EN, Ko JY, Gladden RM, Mikosz CA, Schier JG, Vivolo-Kantor A, et al. Overdoses involving medetomidine mixed with opioids—Chicago, Illinois, May 2024. MMWR Morb. Mortal. Wkly. Rep. 2025, 74(15), 258–265. [CrossRef]
- Ostrowski SJ, Tamama K, Trautman WJ, Stratton DL, Lynch MJ. Notes from the Field: Severe Medetomidine Withdrawal Syndrome in Patients Using Illegally Manufactured Opioids — Pittsburgh, Pennsylvania, October 2024–March 2025. MMWR Morb. Mortal. Wkly. Rep. 2025, 74(15), 269–271. [CrossRef]
- Durney P, Kahoud JL, Warrick-Stone T, Montesi M, Carter M, Butt S, Mencia AM, Omoregie L, Shah M, Bloomfield M, et al. Biochemical identification and clinical description of medetomidine exposure in people who use fentanyl in Philadelphia, PA. Int. J. Mol. Sci. 2025, 26(14), 6715. [CrossRef]
- Johnson RJ, Casey ER, Zwiebel SJ. Diagnosis and management of medetomidine withdrawal: clinical implications of the shifting illicit opioid landscape. J. Acad. Consult. Liaison Psychiatry 2025, published online. [CrossRef]
- London KS, Huo S, Murphy L, Warrick-Stone T, Goodstein D, Montesi M, Carter M, Butt S, Alexander K, Satz W, Tasillo A, Xu L, Arora M, Casey E, McKeever R, Lowenstein M, Durney P, Hart B, Perrone J. Severe fentanyl withdrawal associated with medetomidine adulteration: a multicenter study from Philadelphia, PA. J. Addict. Med. 2025, 00(00), 1–7. [CrossRef]
- Pennsylvania Coordinated Medication-Assisted Treatment Program (PENNCAMP). Medetomidine. Published 2024. Available online: https://penncamp.org/medetomidine/ (accessed on 29 July 2025).
- Fairbanks CA, Kitto KF, Nguyen HO, Stone LS, Wilcox GL. Clonidine and dexmedetomidine produce antinociceptive synergy in mouse spinal cord. Anesthesiology 2009, 110, 638–647. [CrossRef]
- Stone LS, Fairbanks CA, Wilcox GL. α₂ receptors and agonists in pain management. Curr. Opin. Anaesthesiol. 2001, 14(5), 527–533. [CrossRef]
- Chan AKM, Cheung CW, Chong YK. Alpha-2 agonists in acute pain management. Expert Opin. Pharmacother. 2010, 11(17), 2849–2868. [CrossRef]
- Ono H, Mishima A, Ono S, Fukuda H, Vasko MR. Inhibitory effects of clonidine and tizanidine on release of substance P from slices of rat spinal cord and antagonism by α adrenergic receptor antagonists. Neuropharmacology 1991, 30(6), 585–589. [CrossRef]
- Wong A, Smithburger PL, Kane-Gill SL. Review of adjunctive dexmedetomidine in the management of severe acute alcohol withdrawal syndrome. Am. J. Drug Alcohol Abuse 2015, 41(5), 382–391. [CrossRef]
- American Association of Critical Care Nurses (AACN). Dexmedetomidine (Precedex) in the Progressive Care Unit. Available online: https://www.aacn.org/education/ce-activities/nti23330/dexmedetomidine-precedex-in-the-progressive-care-unit (accessed on 29 July 2025).
- Patch RK, Eldrige JS, Moeschler SM, Pingree MJ. Dexmedetomidine as part of a multimodal analgesic treatment regimen for opioid induced hyperalgesia in a patient with significant opioid tolerance. Case Rep. Anesthesiol. 2017, 2017, 9876306. [CrossRef]
- Durney P, Carter M, London K, Goodstein DC, Montesi M, Butt S, Warrick-Stone T, Fiore J. Low-Dose Dexmedetomidine: Repurposing an Intravenous Agent for Opioid & Alpha-2 Withdrawal. Abstract presented at: American Society of Addiction Medicine (ASAM) Annual Conference; April 24–27, 2025; Denver, CO.
- Dexmedetomidine Hydrochloride Injection [package insert]. Hospira, Inc; revised 2021 (21 038/S 017). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/206628s017lbl.pdf (accessed on 29 July 2025).
- Kobayashi K, et al. High versus standard dose dexmedetomidine in critically ill patients: a randomized controlled trial. Crit. Care Med. 2015, 43(12 Suppl 1), 850. [CrossRef]
- Tobias, J.D. Dexmedetomidine: applications in pediatric critical care and pediatric anesthesiology. Open Anesth. J. 2011, 5, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Shehabi Y, et al. High-dose dexmedetomidine—a new option for intensive care sedation? Crit. Care 2010, 14, R194. [CrossRef]
- Iirola T, Aantaa R, Laitio R, Kentala E, Lahtinen M, Wighton A, Garratt C, Ahtola-Sätilä T, Olkkola KT. Pharmacokinetics of prolonged infusion of high-dose dexmedetomidine in critically ill patients. Crit. Care 2011, 15(5), R257. [CrossRef]
- Voscopoulos C, Kirk FL, Lovrincevic M, Lema M. The use of “high dose” dexmedetomidine in a patient with critical tracheal stenosis and anterior mediastinal mass. Open Anesth. J. 2011, 5(1), 42. [CrossRef]
- Selvam RP, Singh AK, Sivakumar T. Transdermal drug delivery systems for antihypertensive drugs—a review. Int. J. Pharm. Biomed. Res. 2010, 1(1), 1–8. [CrossRef]
- Glaess SS, Attridge RL, Gutierrez GC. Clonidine as a strategy for discontinuing dexmedetomidine sedation in critically ill patients: a narrative review. Am. J. Health Syst. Pharm. 2020, 77(7), 515–522. [CrossRef]
- Lardieri AB, Fusco NM, Simone S, et al. Effects of clonidine on withdrawal from long-term dexmedetomidine in the pediatric patient. J. Pediatr. Pharmacol. Ther. 2015, 20(1), 45–53. [CrossRef]
- Spencer, L.; Gregory, M. Clonidine transdermal patches for use in outpatient opiate withdrawal. J. Subst. Abuse Treat. 1989, 6, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Sica DA, Grubbs R. Transdermal clonidine: therapeutic considerations. J. Clin. Hypertens. 2005, 7(9), 558–562. [CrossRef]
- Deutsch AB, Hartman CF, Flaherty CP, et al. Novel use of clonidine patch to treat tizanidine withdrawal. Cureus 2024, 16(2), e54831. [CrossRef]
- Sood N. Rise of illicit medetomidine use: a worrisome trend. Am. J. Addict. 2025, [online ahead of print]. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




