Submitted:
07 August 2025
Posted:
07 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
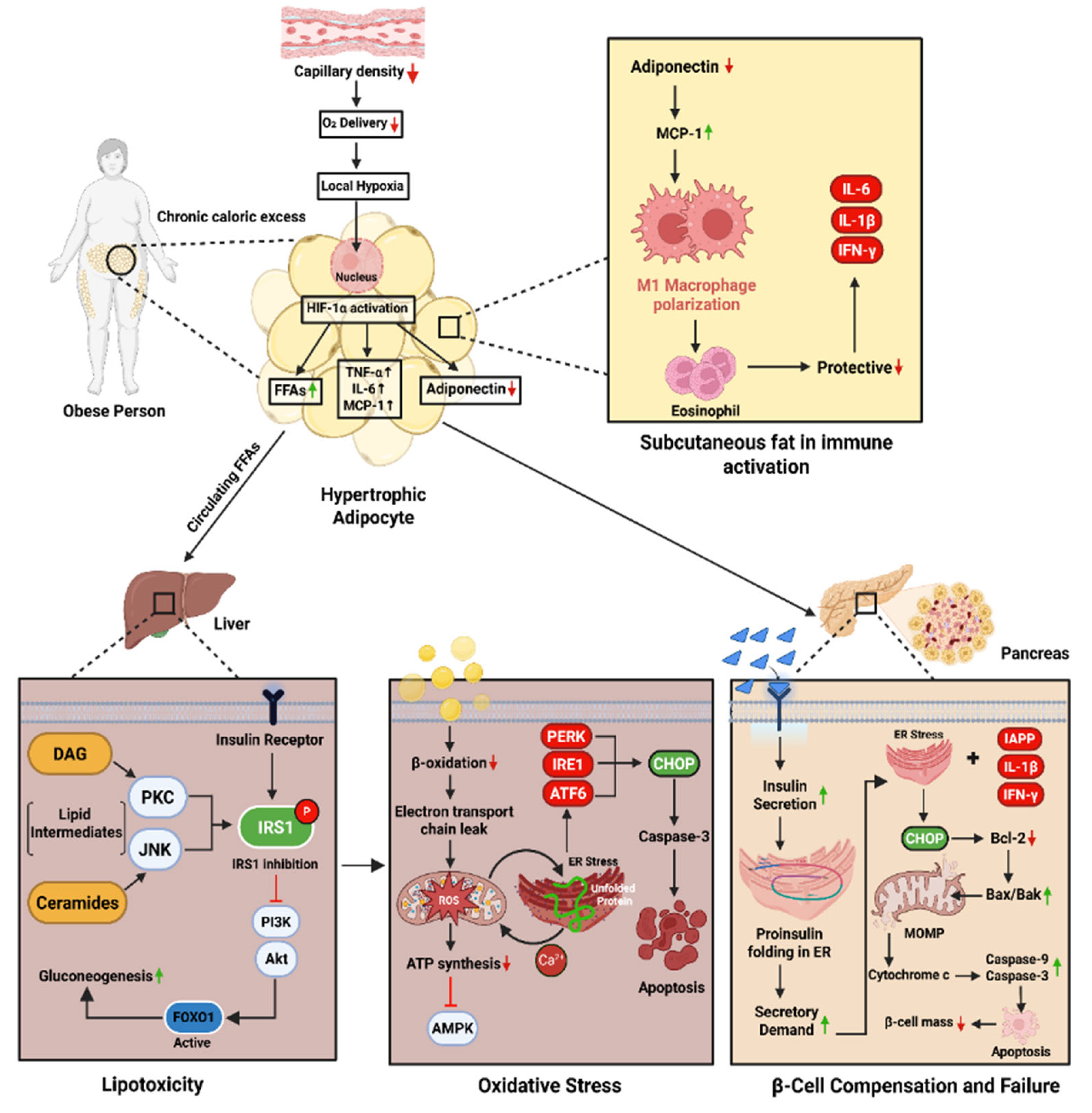
2. Pathophysiology of Obesity-Induced Diabetes
2.1. Lipotoxicity and Free Fatty Acid-Mediated Insulin Resistance
2.2. Oxidative Stress, Mitochondrial Dysfunction, and ER Disruption
2.3. β-Cell Compensation and Failure
2.4. Adipose Tissue Dysfunction and Immune Activation
3. Citrus Polyphenols in Metabolic Reprogramming
3.1. Sour Yet Sweet Salvation: How Citrus Polyphenols Rewire Diabetic Metabolism
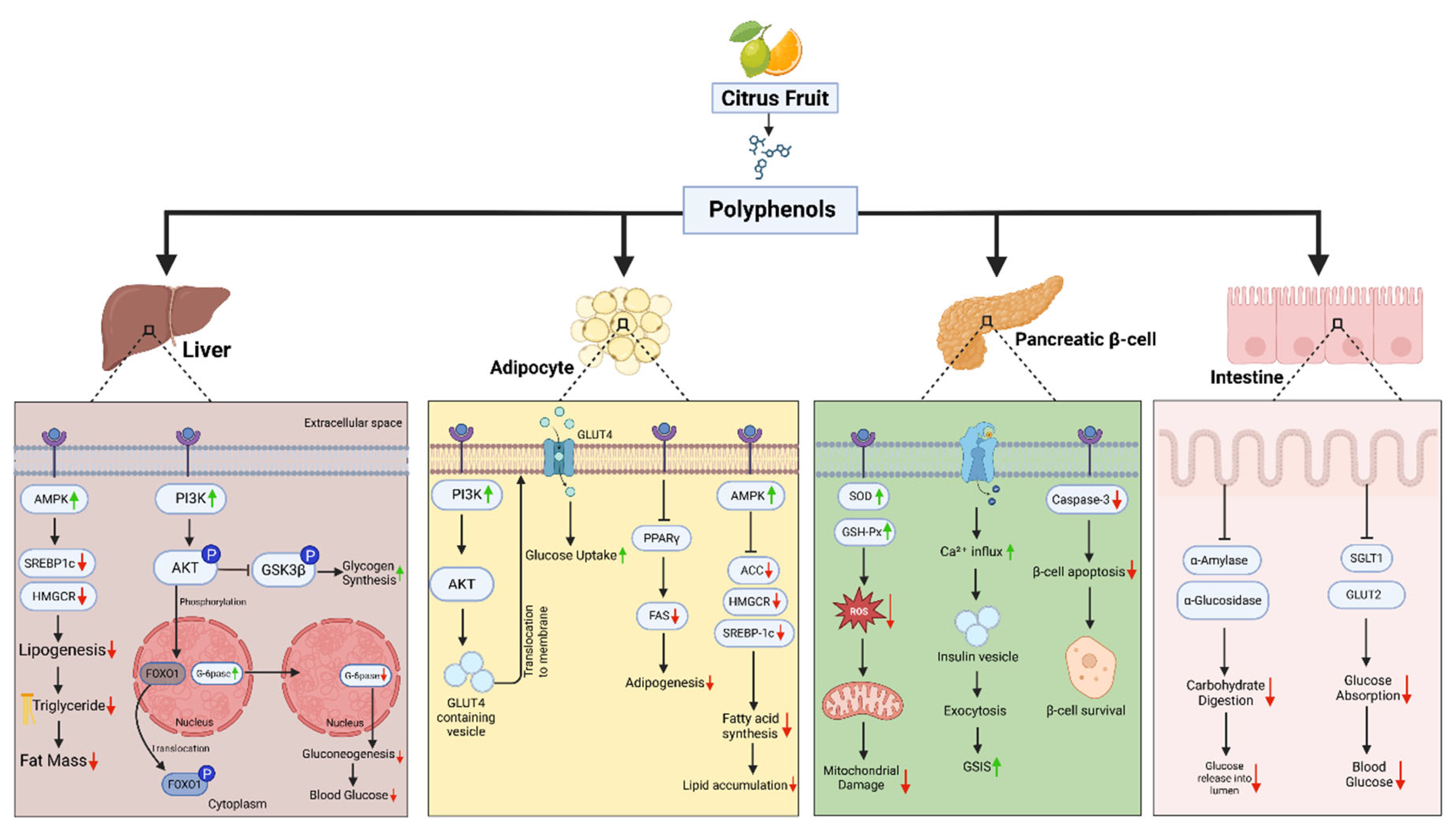
3.2. Citrus Polyphenols and Inflammatory Reprogramming in Diabesity
3.3. Role of Citrus in Mitochondrial Health and Endoplasmic Reticulum Stress: Restoring Protein Homeostasis
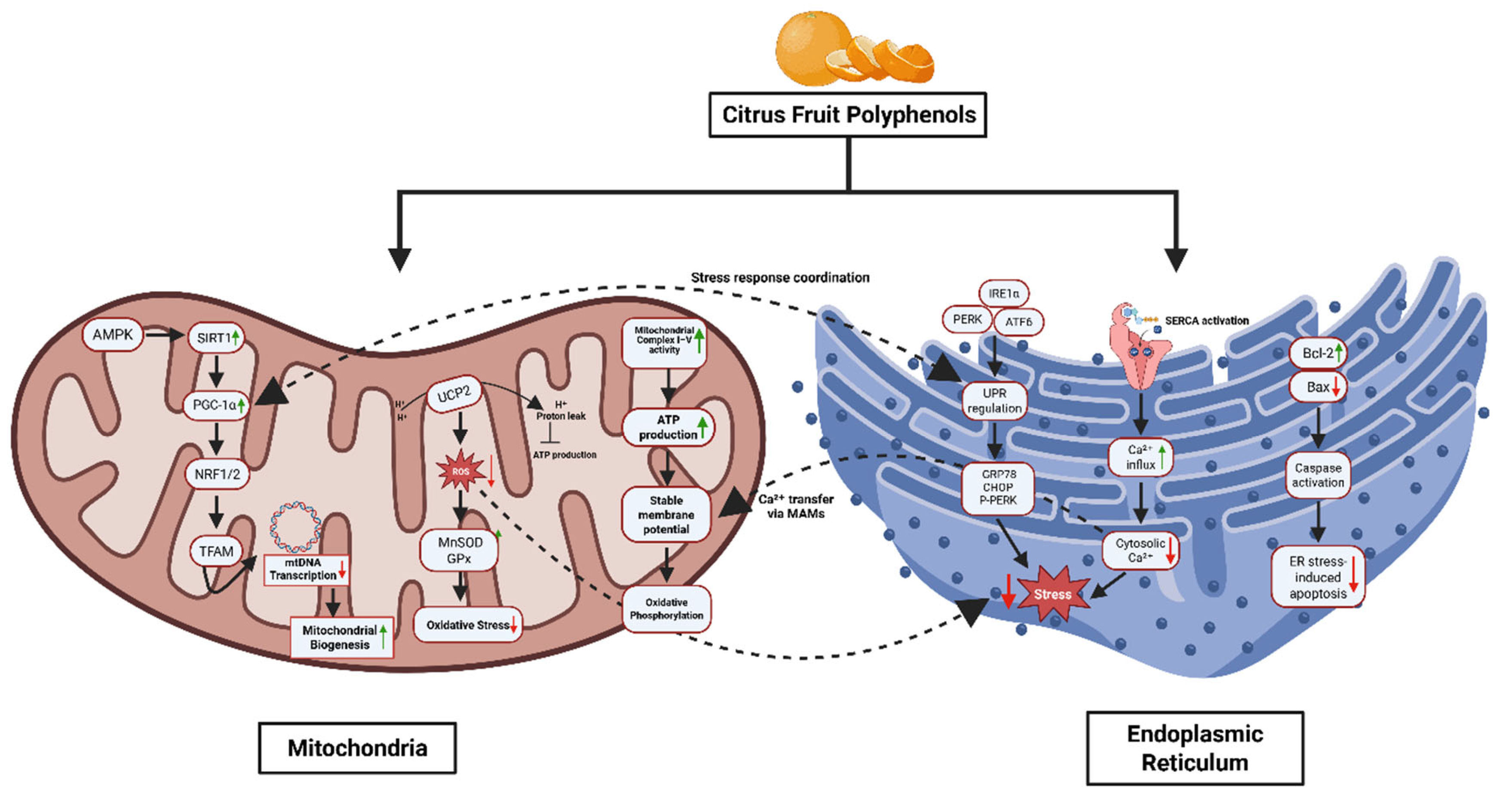
3.4. Free radicals, Oxidative Stress, and Citrus Polyphenols: A Natural Line of defense
| Compound | Model / System | Metabolic Mechanism(s) | Source |
| Neohesperidin | HFD-fed mice | ↑ AMPK–PGC-1α → mitochondrial biogenesis, steatosis reduction | [135] |
| Nobiletin | HFD-fed mice | ↑ FA oxidation, energy expenditure; AMPK-independent | [136] |
| Nobiletin | Hepatocytes | Restores Bmal1 → ↑ lipid/OXPHOS metabolism | [137] |
| Nobiletin | Insulin-resistant mice | ↓ VLDL secretion; improves lipid/glucose metabolism | [138] |
| Nobiletin | HepG2 cells | ↑ PGC1α, CPT1, UCP2 → β-oxidation | [138] |
| Nobiletin | ob/ob mice | ↑ GLUT4, ↑ Akt phosphorylation → improved insulin sensitivity | [139] |
| Naringenin | MCD or HFD mice | ↑ AMPK → autophagy, ↑ mitochondrial biogenesis | [140] |
| Naringenin | Hepatocytes/mice | ↑ AMPK, ↑ ATF3 → metabolic inflammation reduction | [141] |
| Naringin | HFD-fed mice | ↑ AMPK → ↓ SREBP-1c/FAS, ↑ redox balance | [142] |
| Naringin | Fructose-fed rats | ↑ Nrf2/HO-1 → antioxidant response; ↓ ChREBP/SREBP-1c | [132] |
| Naringin | HFD mice | ↑ TFEB → lipophagy → ↓ hepatic lipid droplets | [143] |
| Hesperidin | LO2 hepatocytes (HG) | ↑ ATP, restores ΔΨm via AKT/GSK3β | [144] |
| Hesperidin | Hyperlipidemic rats | ↑ SOD, catalase; preserves mitochondrial enzymes | [145] |
| Hesperidin | Neurons (hyperglycemia) | Improves ATP/redox; ↓ mitochondrial dysfunction | [146] |
| Hesperetin | Aging mice | ↑ Cisd2 expression → metabolic health maintenance | [147] |
| Limonene | Mice model | ↑ mitochondrial respiration, ↓ ROS | [148,149] |
| Eriocitrin | HFD rats | ↑ mitochondrial biogenesis, ↓ steatosis | [150] |
| Sudachitin | C57BL/6J, db/db mice | ↑ β-oxidation, ↑ mitochondrial biogenesis | [151] |
| Tangeretin | Diabetic rats | ↑ GLUT4, antioxidant enzymes | [152] |
| Naringenin | NAFLD mice | ↓ NLRP3/NF-κB, ↓ IL-1β → metabolic reprogramming | [153] |
| Naringenin | NAFLD mice (metabolomics) | Gut microbiota modulation → improved host metabolism | [154] |
| Naringenin | Muscle cells | ↑ p-AMPK → ↑ glucose uptake, ↑ mitochondria | [83] |
| Naringin | Hepatocytes, HFD mice | AMPK–IRS1–MAPK pathway → improved insulin signaling | [127] |
| Naringenin NP | MASLD mice | ↑ PPAR, lipid oxidation, gut microbiota shift | [155] |
| Naringenin | Mice (aerobic fitness) | ↑ oxidative fibers, ↑ aerobic metabolism | [156] |
| Naringin | KK-A(y) mice | ↑ AMPK → ↓ glucose/lipids, ↑ insulin sensitivity | [157] |
| Neohesperidin | DIO mice, HepG2 cells | ↑ FGF21, ↑ AMPK → improved lipid regulation | [158] |
| Hesperidin | MASLD mice | ↓ insulin resistance, ↓ oxidative stress | [159] |
| Nobiletin | HepG2 cells | ↑ AMPK, ↓ lipogenesis | [160] |

3.5. Translating Mechanisms to Humans: Clinical Evidence of Citrus Polyphenol-Driven Metabolic Reprogramming
| Compound(s) | Source | Reported Outcome | Mechanism of Action | Source |
| Neoeriocitrin, Naringin, Neohesperidin | Citrus bergamia (Bergamot) | ↓ Liver fat content, ↓ body weight (vs. placebo) | Enhances bile flow; antioxidant activity reduces oxidative stress | [167] |
| Hesperidin, Naringin, Neohesperidin | Various citrus fruits | ↑ Endothelial function (↑ FMD, vascular tone) | Bile secretion support; antioxidant effects improve vascular inflammation and nitric oxide availability | [168] |
| Hesperidin→Hesperetin; SCFAs | Citrus fruit extracts | Modulates gut microbiota → ↑ beneficial bacteria → systemic inflammation ↓ | Hesperidin metabolized to hesperetin → SCFA production → improved endothelial protection and anti-inflammatory response | [169] |
| Flavones, Flavanones, Oleuropein | Citrus + olive | ↓ Cardiovascular risk biomarkers; improved metabolic-inflammatory profile | Antioxidant activity; inhibits neuro-immune-inflammatory pathways (e.g., cytokines, NF-κB) | [170] |
| Hesperidin, Naringin, Oleuropein | Citrus fruits + olive leaves | ↓ LDL oxidation; ↓ pro-inflammatory cytokines (e.g., TNF-α, IL-6) | Free radical scavenging; anti-inflammatory cytokine modulation | [171] |
| Hesperidin | Orange juice (Citrus sinensis) | ↓ BMI, ↓ waist circumference, ↓ IL-1, IL-6, TNF-α | Inhibits pro-inflammatory cytokine release; protects cells (e.g., keratinocytes, endothelial) from oxidative damage | [172] |
| Hesperidin (1 g/day + lifestyle) | RCT in NAFLD patients | ↓ Liver fat, ALT, weight; improved lipids | NF-κB inhibition; ↓ TNF-α, hs-CRP | [173] |
| Hesperidin (meta-analysis) | RCTs in metabolic subjects (n=525) | ↓ TG, TC, LDL (in BMI>30) | ↓ TNF-α, ↓ IL-6 at high dose | [174] |
| Orange juice (flavonoids) | 4-week RCT in MASLD (n=62) | ↓ Liver steatosis (FibroScan) | ↓ GGT; modest inflammatory effects | [175] |
| Flavonoid-enriched orange juice | RCT in metabolic patients | ↑ antioxidant status; improved glycemic trend | ↓ CRP, ↓ endothelial inflammation | [176] |
| Hesperidin | Vascular study (metabolic syndrome) | ↑ FMD, endothelial function | ↑ NO; ↓ IL-6, TNF-α | [22] |
| Eriomin® (Eriocitrin) | Crossover RCT (n=103) | ↓ FBG, HOMA-IR; ↑ GLP-1, adiponectin | ↓ IL-6, TNF-α, hs-CRP | [177] |
| Polyphenols incl. naringenin | Meta-analysis in NAFLD | ↓ BMI, ALT, AST, TG | ↓ TNF-α (across multiple flavonoids) | [178] |
4. Conclusions & Future Direction
Acknowledgments
Conflicts of Interest
References
- Allocca, S., et al., Endocrine and Metabolic Mechanisms Linking Obesity to Type 2 Diabetes: Implications for Targeted Therapy. Healthcare (Basel), 2025. 13(12). [CrossRef]
- Kyrou, I., et al., Clinical problems caused by obesity. 2015.
- Saeedi, P., et al., Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract, 2019. 157: p. 107843. [CrossRef]
- Ong, K.L., et al., Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet, 2023. 402(10397): p. 203-234.
- Kocatepe, D., D.C. Büyükkol, and K.N. Hinislioğlu, Obesity Prevalence in World and Türkiye. Northern Journal of Health Sciences, 2025. 1(1): p. 26-32.
- Shimu, S.J., A 10-Year Retrospective Quantitative Analysis of The CDC Database: Examining the Prevalence of Depression in the Us Adult Urban Population. [CrossRef]
- Zatterale, F., et al., Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Frontiers in physiology, 2020. 10: p. 1607. [CrossRef]
- Makki, K., P. Froguel, and I. Wolowczuk, Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. International Scholarly Research Notices, 2013. 2013(1): p. 139239. [CrossRef]
- Li, X., et al., Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Frontiers in Immunology, 2023. 14: p. 1153915. [CrossRef]
- Gkrinia, E.M.M. and A. Belančić, The Mechanisms of Chronic Inflammation in Obesity and Potential Therapeutic Strategies: A Narrative Review. Current Issues in Molecular Biology, 2025. 47(5): p. 357. [CrossRef]
- Gudise, V. and B. Chowdhury, Molecular mechanisms and the vital roles of resistin, TLR 4, and NF-κB in treating type 2 diabetic complications. Beni-Suef University Journal of Basic and Applied Sciences, 2020. 9(1): p. 54.
- Kim, J.K., Endothelial nuclear factor κB in obesity and aging: is endothelial nuclear factor κB a master regulator of inflammation and insulin resistance? 2012, Lippincott Williams & Wilkins Hagerstown, MD. p. 1081-1083.
- Chen, L., et al., Mechanisms linking inflammation to insulin resistance. International journal of endocrinology, 2015. 2015(1): p. 508409. [CrossRef]
- Sergi, D., et al., Ceramides as the molecular link between impaired lipid metabolism, saturated fatty acid intake and insulin resistance: are all saturated fatty acids to be blamed for ceramide-mediated lipotoxicity? Nutrition Research Reviews, 2024: p. 1-11.
- Römer, A., T. Linn, and S.F. Petry, Lipotoxic impairment of mitochondrial function in β-cells: A review. Antioxidants, 2021. 10(2): p. 293. [CrossRef]
- Corkey, B.E., Reactive oxygen species: role in obesity and mitochondrial energy efficiency. Philosophical Transactions of the Royal Society B, 2023. 378(1885): p. 20220210. [CrossRef]
- Apostolova, N., et al., Mitochondrial dysfunction and mitophagy in type 2 diabetes: pathophysiology and therapeutic targets. Antioxidants & Redox Signaling, 2023. 39(4-6): p. 278-320. [CrossRef]
- Patel, S. and A. Majumdar, Endoplasmic Reticulum Stress and Unfolded Protein Response in Metabolic Syndrome, in Biochemical Mechanisms for Metabolic Syndrome. 2024, Springer. p. 203-222.
- Ghosh, R., K. Colon-Negron, and F.R. Papa, Endoplasmic reticulum stress, degeneration of pancreatic islet β-cells, and therapeutic modulation of the unfolded protein response in diabetes. Molecular metabolism, 2019. 27: p. S60-S68. [CrossRef]
- Aufi, S.S.A. and K. Hasnin, Diabesity An Emerging Epidemic and Advances in Treatment. Barind Medical College Journal, 2024. 10(1): p. 29-34.
- Mohib, M., et al., Beneficial role of citrus fruit polyphenols against hepatic dysfunctions: a review. Journal of dietary supplements, 2018. 15(2): p. 223-250. [CrossRef]
- Rizza, S., et al., Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. The Journal of Clinical Endocrinology & Metabolism, 2011. 96(5): p. E782-E792. [CrossRef]
- Gandhi, G.R., et al., Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients, 2020. 12(10): p. 2907. [CrossRef]
- Zygmunt, K., et al., Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochemical and biophysical research communications, 2010. 398(2): p. 178-183.
- Den Hartogh, D.J. and E. Tsiani, Antidiabetic properties of naringenin: A citrus fruit polyphenol. Biomolecules, 2019. 9(3): p. 99. [CrossRef]
- Hammerschmidt, P. and J.C. Brüning, Contribution of specific ceramides to obesity-associated metabolic diseases. Cellular and Molecular Life Sciences, 2022. 79(8): p. 395. [CrossRef]
- Sears, B. and M. Perry, The role of fatty acids in insulin resistance. Lipids in health and disease, 2015. 14(1): p. 121. [CrossRef]
- Yung, J.H.M. and A. Giacca, Role of c-Jun N-terminal kinase (JNK) in obesity and type 2 diabetes. Cells, 2020. 9(3): p. 706. [CrossRef]
- Kim, G.-T., et al., Hepatic expression of the serine palmitoyltransferase subunit Sptlc2 reduces lipid droplets in the liver by activating VLDL secretion. Journal of lipid and atherosclerosis, 2020. 9(2): p. 291. [CrossRef]
- Elkanawati, R.Y., S.A. Sumiwi, and J. Levita, Impact of lipids on insulin resistance: insights from human and animal studies. Drug design, development and therapy, 2024: p. 3337-3360. [CrossRef]
- Sokolowska, E. and A. Blachnio-Zabielska, The role of ceramides in insulin resistance. Frontiers in Endocrinology, 2019. 10: p. 577. [CrossRef]
- Schrauwen, P. and M.K. Hesselink, Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes, 2004. 53(6): p. 1412-1417.
- Dubé, J.J., et al., Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. American Journal of Physiology-Endocrinology and Metabolism, 2008. 294(5): p. E882-E888.
- Goodpaster, B.H. and L.M. Sparks, Metabolic flexibility in health and disease. Cell metabolism, 2017. 25(5): p. 1027-1036. [CrossRef]
- Mohib, M.M., et al., Eplerenone, a mineralocorticoid receptor inhibitor, reduces cirrhosis associated changes of hepatocyte glucose and lipid metabolism. Cell Communication and Signaling, 2024. 22(1): p. 614.
- Cusi, K., Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology, 2012. 142(4): p. 711-725. e6. [CrossRef]
- Qin, W. and J. Weng, Hepatocyte NLRP3 interacts with PKCε to drive hepatic insulin resistance and steatosis. Science Bulletin, 2023. 68(13): p. 1413-1429. [CrossRef]
- Mota, M., et al., Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism, 2016. 65(8): p. 1049-1061.
- Huang, W., et al., Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes, 2010. 59(2): p. 347-57. [CrossRef]
- Ziolkowska, S., et al., The Interplay between Insulin Resistance, Inflammation, Oxidative Stress, Base Excision Repair and Metabolic Syndrome in Nonalcoholic Fatty Liver Disease. Int J Mol Sci, 2021. 22(20). [CrossRef]
- Gruben, N., et al., Nonalcoholic fatty liver disease: A main driver of insulin resistance or a dangerous liaison? Biochim Biophys Acta, 2014. 1842(11): p. 2329-2343.
- Chen, Z., et al., A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis, 2017. 16(1): p. 203.
- Ly, L.D., et al., Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med, 2017. 49(2): p. e291. [CrossRef]
- Morawietz, H., et al., Cross-Talk of NADPH Oxidases and Inflammation in Obesity. Antioxidants (Basel), 2023. 12(8).
- Vilas-Boas, E.A., et al., Lipotoxicity and β-Cell Failure in Type 2 Diabetes: Oxidative Stress Linked to NADPH Oxidase and ER Stress. Cells, 2021. 10(12). [CrossRef]
- Rains, J.L. and S.K. Jain, Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med, 2011. 50(5): p. 567-75.
- Manna, P. and S.K. Jain, Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab Syndr Relat Disord, 2015. 13(10): p. 423-44.
- Bhatti, J.S., G.K. Bhatti, and P.H. Reddy, Mitochondrial dysfunction and oxidative stress in metabolic disorders - A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis, 2017. 1863(5): p. 1066-1077. [CrossRef]
- Cojocaru, K.A., et al., Mitochondrial Dysfunction, Oxidative Stress, and Therapeutic Strategies in Diabetes, Obesity, and Cardiovascular Disease. Antioxidants (Basel), 2023. 12(3).
- Argaev-Frenkel, L. and T. Rosenzweig, Redox Balance in Type 2 Diabetes: Therapeutic Potential and the Challenge of Antioxidant-Based Therapy. Antioxidants (Basel), 2023. 12(5).
- Shrestha, N., et al., Pathological β-Cell Endoplasmic Reticulum Stress in Type 2 Diabetes: Current Evidence. Front Endocrinol (Lausanne), 2021. 12: p. 650158. [CrossRef]
- Kim, G., et al., Endoplasmic Reticulum Stress and Its Impact on Adipogenesis: Molecular Mechanisms Implicated. Nutrients, 2023. 15(24).
- Bhattarai, K.R., et al., The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp Mol Med, 2021. 53(2): p. 151-167. [CrossRef]
- Veluthakal, R., et al., Mitochondrial Dysfunction, Oxidative Stress, and Inter-Organ Miscommunications in T2D Progression. Int J Mol Sci, 2024. 25(3).
- Benito-Vicente, A., et al., Molecular mechanisms of lipotoxicity-induced pancreatic β-cell dysfunction. Int Rev Cell Mol Biol, 2021. 359: p. 357-402.
- Biondi, G., et al., Adipose Tissue Secretion Pattern Influences β-Cell Wellness in the Transition from Obesity to Type 2 Diabetes. Int J Mol Sci, 2022. 23(10).
- Ježek, P., et al., Fatty Acid-Stimulated Insulin Secretion vs. Lipotoxicity. Molecules, 2018. 23(6).
- Kim, J.W. and K.H. Yoon, Glucolipotoxicity in Pancreatic β-Cells. Diabetes Metab J, 2011. 35(5): p. 444-50.
- Dalle, S., A. Abderrahmani, and E. Renard, Pharmacological inhibitors of β-cell dysfunction and death as therapeutics for diabetes. Front Endocrinol (Lausanne), 2023. 14: p. 1076343. [CrossRef]
- Brozzi, F. and D.L. Eizirik, ER stress and the decline and fall of pancreatic beta cells in type 1 diabetes. Ups J Med Sci, 2016. 121(2): p. 133-9. [CrossRef]
- Lee, J.H. and J. Lee, Endoplasmic Reticulum (ER) Stress and Its Role in Pancreatic β-Cell Dysfunction and Senescence in Type 2 Diabetes. Int J Mol Sci, 2022. 23(9).
- Yang, L., et al., Puerarin Protects Pancreatic β-Cells in Obese Diabetic Mice via Activation of GLP-1R Signaling. Mol Endocrinol, 2016. 30(3): p. 361-71. [CrossRef]
- Yusta, B., et al., GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab, 2006. 4(5): p. 391-406. [CrossRef]
- Li, Y., et al., Recent advances in pancreatic α-cell transdifferentiation for diabetes therapy. Front Immunol, 2025. 16: p. 1551372.
- Choe, S.S., et al., Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front Endocrinol (Lausanne), 2016. 7: p. 30. [CrossRef]
- Huynh, P.M., F. Wang, and Y.A. An, Hypoxia signaling in the adipose tissue. J Mol Cell Biol, 2025. 16(8). [CrossRef]
- Mirabelli, M., et al., Hypoxia in Human Obesity: New Insights from Inflammation towards Insulin Resistance-A Narrative Review. Int J Mol Sci, 2024. 25(18). [CrossRef]
- Begum, M., et al., Adiponectin: a promising target for the treatment of diabetes and its complications. Life, 2023. 13(11): p. 2213.
- Corvera, S., Cellular Heterogeneity in Adipose Tissues. Annu Rev Physiol, 2021. 83: p. 257-278.
- Massier, L., et al., An integrated single cell and spatial transcriptomic map of human white adipose tissue. Nat Commun, 2023. 14(1): p. 1438.
- Sultana, A., et al., Bangladeshi parents’ knowledge and awareness about cervical cancer and willingness to vaccinate female family members against human papilloma virus: a cross sectional study. Int J Community Med Public Health, 2023. 10(10): p. 3446-3453.
- Khin, P.P., J.H. Lee, and H.-S. Jun, Pancreatic beta-cell dysfunction in type 2 diabetes. European Journal of Inflammation, 2023. 21: p. 1721727X231154152. [CrossRef]
- Chen, B., et al., Lipotoxicity: A New Perspective in Type 2 Diabetes Mellitus. Diabetes, Metabolic Syndrome and Obesity, 2025: p. 1223-1237.
- Sandoval, V., et al., Metabolic impact of flavonoids consumption in obesity: from central to peripheral. Nutrients, 2020. 12(8): p. 2393.
- Mirzaei, A., et al., Promising influences of hesperidin and hesperetin against diabetes and its complications: a systematic review of molecular, cellular, and metabolic effects. Excli j, 2023. 22: p. 1235-1263.
- Russo, B., et al., Flavonoids and insulin-resistance: from molecular evidences to clinical trials. International journal of molecular sciences, 2019. 20(9): p. 2061. [CrossRef]
- Yi, H., et al., The therapeutic effects and mechanisms of quercetin on metabolic diseases: pharmacological data and clinical evidence. Oxidative medicine and cellular longevity, 2021. 2021(1): p. 6678662. [CrossRef]
- Dong, J., et al., Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: a mechanism including AMPKα1/SIRT1. J Lipid Res, 2014. 55(3): p. 363-74.
- Tsai, C.F., et al., Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance. Nutrients, 2021. 14(1).
- Kohjima, M., et al., SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. International journal of molecular medicine, 2008. 21(4): p. 507-511. [CrossRef]
- Lee, J., et al., GLP-1 receptor agonist and non-alcoholic fatty liver disease. Diabetes & metabolism journal, 2012. 36(4): p. 262.
- Li, S., et al., Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK. Nutrition & diabetes, 2019. 9(1): p. 28. [CrossRef]
- Zygmunt, K., et al., Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem Biophys Res Commun, 2010. 398(2): p. 178-83. [CrossRef]
- Nery, M., et al., Physiological effects of tangeretin and heptamethoxyflavone on obese C57BL/6J mice fed a high-fat diet and analyses of the metabolites originating from these two polymethoxylated flavones. Food Science & Nutrition, 2021. 9(4): p. 1997-2009.
- La Spina, M., et al., Browning effects of a chronic pterostilbene supplementation in mice fed a high-fat diet. International journal of molecular sciences, 2019. 20(21): p. 5377.
- Chen, Q., et al., Tangeretin prevents obesity by modulating systemic inflammation, fat browning, and gut microbiota in high-fat diet-induced obese C57BL/6 mice. The Journal of Nutritional Biochemistry, 2022. 101: p. 108943.
- Gao, J., et al., The combination of cinnamaldehyde and kaempferol ameliorates glucose and lipid metabolism disorders by enhancing lipid metabolism via AMPK activation. Journal of Functional Foods, 2021. 83: p. 104556. [CrossRef]
- Park, J.-e., J. Yoo, and J.-s. Han, HM-Chromanone Alleviates Hyperglycemia by Activating AMPK and PI3K/AKT Pathways in Mice Fed a High-Fat Diet. Nutrients, 2024. 16(22): p. 3972. [CrossRef]
- Chang, W.-T., et al., Rutin and gallic acid regulates mitochondrial functions via the SIRT1 pathway in C2C12 myotubes. Antioxidants, 2021. 10(2): p. 286.
- Alkhalidy, H., et al., The flavonoid kaempferol ameliorates streptozotocin-induced diabetes by suppressing hepatic glucose production. Molecules, 2018. 23(9): p. 2338. [CrossRef]
- Hotamisligil, G.S., N.S. Shargill, and B.M. Spiegelman, Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science, 1993. 259(5091): p. 87-91.
- Hotamisligil, G.S., et al., Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest, 1995. 95(5): p. 2409-15. [CrossRef]
- Shimu, S.J. and S. Islam, Gender Differences in Drug Addiction: Neurobiological, Social, and Psychological Perspectives in Women–A Systematic Review. Journal of Primeasia, 2025. 6(1): p. 1-13.
- Gao, Z., et al., Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem, 2002. 277(50): p. 48115-21.
- Kanety, H., et al., Tumor necrosis factor alpha-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J Biol Chem, 1995. 270(40): p. 23780-4.
- Martínez Báez, A., et al., Phosphorylation codes in IRS-1 and IRS-2 are associated with the activation/inhibition of insulin canonical signaling pathways. Current issues in molecular biology, 2024. 46(1): p. 634-649.
- Baker, R.G., M.S. Hayden, and S. Ghosh, NF-κB, inflammation, and metabolic disease. Cell Metab, 2011. 13(1): p. 11-22.
- Kanda, H., et al., MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of clinical investigation, 2006. 116(6): p. 1494-1505. [CrossRef]
- Morshedzadeh, N., et al., A narrative review on the role of hesperidin on metabolic parameters, liver enzymes, and inflammatory markers in nonalcoholic fatty liver disease. Food Science & Nutrition, 2023. 11(12): p. 7523-7533.
- Alam, M.A., et al., Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Advances in nutrition, 2014. 5(4): p. 404-417. [CrossRef]
- Shen, C.-Y., et al., The development of maillard reaction, and advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related diseases. Molecules, 2020. 25(23): p. 5591.
- Syed, A.A., et al., Naringin ameliorates type 2 diabetes mellitus-induced steatohepatitis by inhibiting RAGE/NF-κB mediated mitochondrial apoptosis. Life sciences, 2020. 257: p. 118118.
- Cepas, V., et al., Redox Signaling and Advanced Glycation Endproducts (AGEs) in Diet-Related Diseases. Antioxidants (Basel), 2020. 9(2).
- Al-Aubaidy, H.A., et al., Twelve-week mediterranean diet intervention increases citrus bioflavonoid levels and reduces inflammation in people with type 2 diabetes mellitus. Nutrients, 2021. 13(4): p. 1133.
- Passaro, A., et al., The complex interplay between oxinflammation, mitochondrial dysfunction and lipotoxicity: Focus on their role in the pathogenesis of skeletal muscle insulin resistance and modulation by dietary fatty acids. Advances in Redox Research, 2024. 11: p. 100100. [CrossRef]
- Cavaliere, G., et al., From obesity-induced low-grade inflammation to lipotoxicity and mitochondrial dysfunction: altered multi-crosstalk between adipose tissue and metabolically active organs. Antioxidants, 2023. 12(6): p. 1172.
- Kicinska, A. and W. Jarmuszkiewicz, Flavonoids and mitochondria: activation of cytoprotective pathways? Molecules, 2020. 25(13): p. 3060. [CrossRef]
- Armani, A., et al., Nutraceuticals in Brown Adipose Tissue Activation. Cells, 2022. 11(24).
- Zong, Y., et al., Mitochondrial dysfunction: mechanisms and advances in therapy. Signal transduction and targeted therapy, 2024. 9(1): p. 124. [CrossRef]
- Muoio, D.M. and P.D. Neufer, Lipid-induced mitochondrial stress and insulin action in muscle. Cell metabolism, 2012. 15(5): p. 595-605.
- Narendra, D.P., et al., PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS biology, 2010. 8(1): p. e1000298.
- Lemos, G.d.O., R.S. Torrinhas, and D.L. Waitzberg, Nutrients, physical activity, and mitochondrial dysfunction in the setting of metabolic syndrome. Nutrients, 2023. 15(5): p. 1217.
- Ouyang, Y.B., et al., Overexpressing GRP78 influences Ca2+ handling and function of mitochondria in astrocytes after ischemia-like stress. Mitochondrion, 2011. 11(2): p. 279-86.
- Chen, X., et al., Endoplasmic reticulum stress: molecular mechanism and therapeutic targets. Signal transduction and targeted therapy, 2023. 8(1): p. 352.
- Alotaibi, G. and A. Alkhammash, Pharmacological landscape of endoplasmic reticulum stress: Uncovering therapeutic avenues for metabolic diseases. European Journal of Pharmacology, 2025: p. 177509. [CrossRef]
- Lenghel, A., et al., What is the sweetest UPR flavor for the β-cell? That is the question. Frontiers in Endocrinology, 2021. 11: p. 614123.
- Lee, J.-H. and J. Lee, Endoplasmic reticulum (ER) stress and its role in pancreatic β-cell dysfunction and senescence in type 2 diabetes. International journal of molecular sciences, 2022. 23(9): p. 4843.
- Zhao, T., J. Du, and H. Zeng, Interplay between endoplasmic reticulum stress and non-coding RNAs in cancer. Journal of Hematology & Oncology, 2020. 13(1): p. 163.
- Amodio, G., et al., Structural and functional significance of the endoplasmic reticulum unfolded protein response transducers and chaperones at the mitochondria–ER contacts: a cancer perspective. Frontiers in Cell and Developmental Biology, 2021. 9: p. 641194. [CrossRef]
- Park, S., et al., Naringenin induces mitochondria-mediated apoptosis and endoplasmic reticulum stress by regulating MAPK and AKT signal transduction pathways in endometriosis cells. MHR: Basic science of reproductive medicine, 2017. 23(12): p. 842-854. [CrossRef]
- Li, X., et al., Effects of hesperidin on mitochondrial function, mitochondria-associated endoplasmic reticulum membranes and IP3R–MCU calcium axis in the intestine of piglets exposed to deoxynivalenol. Food & Function, 2024. 15(12): p. 6459-6474.
- Gou, F., et al., Hesperidin alleviated intestinal barrier injury, mitochondrial dysfunction, and disorder of endoplasmic reticulum mitochondria contact sites under oxidative stress. Journal of Agricultural and Food Chemistry, 2024. 72(29): p. 16276-16286. [CrossRef]
- Khoi, C.-S., T.-Y. Lin, and C.-K. Chiang, Targeting Insulin Resistance, Reactive Oxygen Species, Inflammation, Programmed Cell Death, ER Stress, and Mitochondrial Dysfunction for the Therapeutic Prevention of Free Fatty Acid-Induced Vascular Endothelial Lipotoxicity. Antioxidants, 2024. 13(12): p. 1486. [CrossRef]
- Karunakaran, U. and K.G. Park, A systematic review of oxidative stress and safety of antioxidants in diabetes: focus on islets and their defense. Diabetes Metab J, 2013. 37(2): p. 106-12.
- Sergi, D., et al., Mitochondrial (dys) function and insulin resistance: from pathophysiological molecular mechanisms to the impact of diet. Frontiers in physiology, 2019. 10: p. 449821. [CrossRef]
- González, P., et al., Hyperglycemia and oxidative stress: an integral, updated and critical overview of their metabolic interconnections. International journal of molecular sciences, 2023. 24(11): p. 9352.
- Pu, P., et al., Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch Biochem Biophys, 2012. 518(1): p. 61-70.
- Ali, A.M., et al., Antidiabetic Potency, Antioxidant Effects, and Mode of Actions of Citrus reticulata Fruit Peel Hydroethanolic Extract, Hesperidin, and Quercetin in Nicotinamide/Streptozotocin-Induced Wistar Diabetic Rats. Oxid Med Cell Longev, 2020. 2020: p. 1730492.
- Korac, B., et al., Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol, 2021. 42: p. 101887.
- Park, H.J., et al., Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose- and lipid-regulating enzymes in db/db mice. J Nutr Biochem, 2013. 24(2): p. 419-27. [CrossRef]
- Kim, B.M., B.O. Cho, and S.I. Jang, Anti-obesity effects of Diospyros lotus leaf extract in mice with high-fat diet-induced obesity. Int J Mol Med, 2019. 43(1): p. 603-613.
- Pengnet, S., et al., Naringin attenuates fructose-induced NAFLD progression in rats through reducing endogenous triglyceride synthesis and activating the Nrf2/HO-1 pathway. Front Pharmacol, 2022. 13: p. 1049818.
- T, L.S., et al., Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants (Basel), 2020. 9(10).
- Tsai, H.L., S.K. Chang, and S.J. Chang, Antioxidant content and free radical scavenging ability of fresh red pummelo [Citrus grandis (L.) Osbeck] juice and freeze-dried products. J Agric Food Chem, 2007. 55(8): p. 2867-72.
- Wang, S.W., et al., Neohesperidin enhances PGC-1α-mediated mitochondrial biogenesis and alleviates hepatic steatosis in high fat diet fed mice. Nutr Diabetes, 2020. 10(1): p. 27. [CrossRef]
- Morrow, N.M., et al., The citrus flavonoid nobiletin confers protection from metabolic dysregulation in high-fat-fed mice independent of AMPK. J Lipid Res, 2020. 61(3): p. 387-402.
- Lu, Z., et al., Liver-Specific Bmal1 Depletion Reverses the Beneficial Effects of Nobiletin on Liver Cholesterol Homeostasis in Mice Fed with High-Fat Diet. Nutrients, 2023. 15(11). [CrossRef]
- Mulvihill, E.E., et al., Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes, 2011. 60(5): p. 1446-57.
- Lee, Y.S., et al., Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem Pharmacol, 2010. 79(11): p. 1674-83. [CrossRef]
- Yang, Y., et al., Naringenin Attenuates Non-Alcoholic Fatty Liver Disease by Enhancing Energy Expenditure and Regulating Autophagy via AMPK. Front Pharmacol, 2021. 12: p. 687095.
- Liu, X., et al., The citrus flavonoid naringenin confers protection in a murine endotoxaemia model through AMPK-ATF3-dependent negative regulation of the TLR4 signalling pathway. Sci Rep, 2016. 6: p. 39735. [CrossRef]
- Sarkar, S., S. Ghosh, and M. Biswas, Naringin ameliorates high-fat diet-induced hepatotoxicity and dyslipidemia in experimental rat model via modulation of anti-oxidant enzymes, AMPK and SERBP-1c signaling pathways. Toxicol Rep, 2025. 14: p. 102062. [CrossRef]
- Guan, L., et al., Naringin Protects against Non-Alcoholic Fatty Liver Disease by Promoting Autophagic Flux and Lipophagy. Mol Nutr Food Res, 2024. 68(3): p. e2200812.
- Tian, M., et al., Hesperidin alleviates insulin resistance by improving HG-induced oxidative stress and mitochondrial dysfunction by restoring miR-149. Diabetol Metab Syndr, 2021. 13(1): p. 50.
- Estruel-Amades, S., et al., Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats. Nutrients, 2019. 11(4). [CrossRef]
- Morshedzadeh, N., et al., A narrative review on the role of hesperidin on metabolic parameters, liver enzymes, and inflammatory markers in nonalcoholic fatty liver disease. Food Sci Nutr, 2023. 11(12): p. 7523-7533.
- Yeh, C.H., et al., Hesperetin promotes longevity and delays aging via activation of Cisd2 in naturally aged mice. J Biomed Sci, 2022. 29(1): p. 53. [CrossRef]
- Durço, A.O., et al., d-Limonene Ameliorates Myocardial Infarction Injury by Reducing Reactive Oxygen Species and Cell Apoptosis in a Murine Model. J Nat Prod, 2019. 82(11): p. 3010-3019.
- Valerii, M.C., et al., Effect of a Fiber D-Limonene-Enriched Food Supplement on Intestinal Microbiota and Metabolic Parameters of Mice on a High-Fat Diet. Pharmaceutics, 2021. 13(11).
- Hiramitsu, M., et al., Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis. Sci Rep, 2014. 4: p. 3708. [CrossRef]
- Tsutsumi, R., et al., Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle. Nutr Metab (Lond), 2014. 11: p. 32.
- Sundaram, R., P. Shanthi, and P. Sachdanandam, Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine, 2014. 21(6): p. 793-9. [CrossRef]
- Wang, Q., et al., Naringenin attenuates non-alcoholic fatty liver disease by down-regulating the NLRP3/NF-κB pathway in mice. Br J Pharmacol, 2020. 177(8): p. 1806-1821.
- Wu, L., et al., Naringenin Promotes Gastrointestinal Motility in Mice by Impacting the SCF/c-Kit Pathway and Gut Microbiota. Foods, 2024. 13(16).
- Dong, L., et al., Naringenin cationic lipid-modified nanoparticles mitigate MASLD progression by modulating lipid homeostasis and gut microbiota. J Nanobiotechnology, 2025. 23(1): p. 168. [CrossRef]
- Lv, Z., et al., Naringenin improves muscle endurance via activation of the Sp1-ERRγ transcriptional axis. Cell Rep, 2023. 42(11): p. 113288.
- Jia, S., et al., Hypoglycemic and hypolipidemic effects of neohesperidin derived from Citrus aurantium L. in diabetic KK-A(y) mice. Food Funct, 2015. 6(3): p. 878-86.
- Wu, H., et al., Neohesperidin Exerts Lipid-Regulating Effects in vitro and in vivo via Fibroblast Growth Factor 21 and AMP-Activated Protein Kinase/Sirtuin Type 1/Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1α Signaling Axis. Pharmacology, 2017. 100(3-4): p. 115-126.
- Jamal, A., et al., Reduced Insulin Resistance and Oxidative Stress in a Mouse Model of Metabolic Syndrome following Twelve Weeks of Citrus Bioflavonoid Hesperidin Supplementation: A Dose-Response Study. Biomolecules, 2024. 14(6). [CrossRef]
- Yuk, T., et al., Nobiletin Inhibits Hepatic Lipogenesis via Activation of AMP-Activated Protein Kinase. Evid Based Complement Alternat Med, 2018. 2018: p. 7420265.
- Namkhah, Z., et al., Does naringenin supplementation improve lipid profile, severity of hepatic steatosis and probability of liver fibrosis in overweight/obese patients with NAFLD? A randomised, double-blind, placebo-controlled, clinical trial. Int J Clin Pract, 2021. 75(11): p. e14852. [CrossRef]
- Naeini, F., et al., Effects of naringenin supplementation in overweight/obese patients with non-alcoholic fatty liver disease: study protocol for a randomized double-blind clinical trial. Trials, 2021. 22(1): p. 801. [CrossRef]
- Goldwasser, J., et al., Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: role of PPARalpha, PPARgamma and LXRalpha. PLoS One, 2010. 5(8): p. e12399.
- Sleiman, D., M.R. Al-Badri, and S.T. Azar, Effect of mediterranean diet in diabetes control and cardiovascular risk modification: a systematic review. Front Public Health, 2015. 3: p. 69. [CrossRef]
- Carpenito, M., et al., Unveiling the Power of Bergamot: Beyond Lipid-Lowering Effects. Nutrients, 2025. 17(11). [CrossRef]
- Fogacci, F., et al., Metabolic and vascular effect of a new standardized bergamot phytocomplex: a three-arm, placebocontrolled, double-blind clinical trial. Arch Med Sci, 2023. 19(5): p. 1228-1235. [CrossRef]
- Ferro, Y., et al., Citrus Bergamia and Cynara Cardunculus Reduce Serum Uric Acid in Individuals with Non-Alcoholic Fatty Liver Disease. Medicina (Kaunas), 2022. 58(12).
- Jalili, F., et al., The effects of citrus flavonoids supplementation on endothelial function: A systematic review and dose-response meta-analysis of randomized clinical trials. Phytother Res, 2024. 38(6): p. 2847-2859.
- Sost, M.M., et al., A Citrus Fruit Extract High in Polyphenols Beneficially Modulates the Gut Microbiota of Healthy Human Volunteers in a Validated In Vitro Model of the Colon. Nutrients, 2021. 13(11). [CrossRef]
- Sánchez Macarro, M., et al., Effect of a Combination of Citrus Flavones and Flavanones and Olive Polyphenols for the Reduction of Cardiovascular Disease Risk: An Exploratory Randomized, Double-Blind, Placebo-Controlled Study in Healthy Subjects. Nutrients, 2020. 12(5).
- Victoria-Montesinos, D., et al., Effect of Dietary Supplementation with a Natural Extract of Sclerocarya birrea on Glycemic Metabolism in Subjects with Prediabetes: A Randomized Double-Blind Placebo-Controlled Study. Nutrients, 2021. 13(6). [CrossRef]
- Morand, C., et al., Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr, 2011. 93(1): p. 73-80. [CrossRef]
- Yari, Z., et al., The effect of hesperidin supplementation on metabolic profiles in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Eur J Nutr, 2020. 59(6): p. 2569-2577.
- Khorasanian, A.S., et al., The effects of hesperidin supplementation on cardiovascular risk factors in adults: a systematic review and dose-response meta-analysis. Front Nutr, 2023. 10: p. 1177708. [CrossRef]
- Notarnicola, M., et al., Daily Orange Consumption Reduces Hepatic Steatosis Prevalence in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: Exploratory Outcomes of a Randomized Clinical Trial. Nutrients, 2024. 16(18).
- Navajas-Porras, B., et al., Effects of a flavonoid-enriched orange juice on antioxidant capacity, lipid profile, and inflammation in obese patients: A randomized placebo-controlled trial. Food Res Int, 2025. 217: p. 116759. [CrossRef]
- Cesar, T.B., F.M.M. Ramos, and C.B. Ribeiro, Nutraceutical Eriocitrin (Eriomin) Reduces Hyperglycemia by Increasing Glucagon-Like Peptide 1 and Downregulates Systemic Inflammation: A Crossover-Randomized Clinical Trial. J Med Food, 2022. 25(11): p. 1050-1058. [CrossRef]
- Wang, X.C., L. Song, and X.H. Wang, Efficacy of dietary polyphenol supplement in patients with non-alcoholic fatty liver disease: a network meta-analysis. Front Nutr, 2025. 12: p. 1582861.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





