1. Introduction
Since the beginning of the 21st century, birth rates have declined and life expectancy has increased worldwide, and population aging has accelerated markedly [
1,
2]. The aging population is expected to continue to grow in the future [
3,
4], raising concerns about the increasing strain on medical resources owing to the decline in the working-age population [
1]. Furthermore, as the population continues to age, aging itself has become a major risk factor for chronic diseases, and aging-related diseases and multimorbidity have led to rising healthcare expenditure per person, placing increasing strain on healthcare financing systems [
2,
5]. Therefore, interventions targeting the aging process are increasingly being recognized as crucial for preventing or inhibiting the progression of chronic diseases [
6,
7].
In research on health and anti-aging in the elderly, clinical trials on pharmacological interventions have been conducted in the elderly [
8]. However, it is often difficult to conduct clinical trials on elderly patients because of their decline in physical function and the presence of comorbidities [
9,
10]. Additionally, there are difficulties in obtaining informed consent from patients with cognitive impairment, which may limit their participation in clinical trials [
11].
Various animal species have been used in aging research, including rodents (mice and rats), primates (non-human primates, NHPs), carnivorans (dogs and cats) [
12], birds, and fish [
13]. Among them, NHPs have been widely used as animal models in biomedical research because of their genetic and physiological similarities to humans. Although NHPs have been extensively used in aging research, their use has been limited in recent years owing to their long lifespans and high associated costs. On the other hand, mice and rats are the most commonly used animals in aging research due to their well-characterized genetic backgrounds, known physiological traits, ease of handling, shorter lifespans, and lower research costs compared to NHPs. However, validating the Human Equivalent Dose in rodents is challenging [
14,
15].
Pigs share many anatomical and physiological similarities with humans and have attracted attention as experimental animals, and translational research using swine models has been actively developed [
16,
17]. Domestic pigs may be used depending on the research purpose; however, miniature pigs bred for smaller sizes and standardized strains are mainly used [
18,
19]. One of the authors, E.K., has been conducting research and development on the microminipig (MMP), the world’s smallest experimental minipig, and has managed a colony of MMPs continuously for over 10 years in order to conduct this study.
This study evaluated the effects of the long-term administration of sweet potato petioles and leaves on super-aged MMPs. To assess these effects in a minimally invasive manner, gut microbiota analysis and evaluation of senescence-associated cells in the peripheral blood were performed. Polyphenols found in most plants are expected to be utilized as health foods because of their antioxidant effects and potential to ameliorate arteriosclerosis, diabetes, and obesity [
20,
21,
22,
23]. Sweet potato (
Ipomoea batatas L.), an edible crop belonging to the family
Convolvulaceae and order
Solanales, originated in Central and South America more than 5,000 years ago and is consumed worldwide [
24]. Although the tuberous roots of sweet potatoes are primarily consumed, the petioles and leaves, which are usually discarded, are also rich in dietary fiber, polyphenols, vitamins, and minerals, and have been reported to have antioxidant [
25,
26], anti-obesity [
27,
28], antidiabetic [
29,
30,
31,
32], anti-inflammatory [
33,
34], and anti-aging effects [
35].
In this study, we used a colony of super-aged experimental MMPs to evaluate the efficacy and safety of drugs and health foods for the elderly. A minimally invasive test of these MMPs demonstrated their potential to contribute to the development of health foods for the elderly with the goal of lifelong breeding.
2. Materials and Methods
2.1. Animals
Super-aged microminipigs (MMPs), aged 8–13 years (two males and three females), were used in this study. Before the start of this study, animals were housed for long-term in an environment maintained at 20 ± 5 °C, 55 ± 35% humidity, and a 11-hour:13-hour light-dark cycle, and were provided with 360 g of formulated feed for medium pigs (Multirack, CHUBU SHIRYO Co., Ltd., Aichi, Japan) once daily in the morning, with both feed and water available ad libitum.
Generally, the average lifespan of pigs is approximately 20 years, and their maximum expected lifespan is 27 years [
17,
36]. However, domestic pigs are typically slaughtered early due to their rapid growth, resulting in a much shorter average lifespan of approximately 1.8 years [
36]. Although there are reports of studies using aged experimental pigs [
37,
38,
39,
40,
41,
42], the animals described were generally approximately 10 years old; therefore, the MMPs used in this study are regarded as super-aged.
2.2. Experimental Protocols
In this study, we used the aboveground stems (vines) of the Japanese sweet potato cultivar Silk Sweet cultivated in Kokubunji, Tokyo, Japan. After harvesting, the vines were sun-dried and separated into petioles and stems. The petioles and leaves were pulverized using a grinder (Ikemoto Rika Kogyo Co., Ltd., Kanagawa, Japan), and the resulting powder, which was sieved through a 1-mm mesh, was used in the experiment. The effects and toxicity of the sweet potato petiole and leaf powder used in this study were verified in a previous study using small animals (gerbils) at the Nippon Veterinary and Life Science University (Permit No. 2024 K-62).
Super-aged MMPs were divided into a control group (N = 2) fed a standard diet and a potato group (N = 3) fed a diet prepared by adding 10% powdered petioles and leaves to the standard diet. Fecal and peripheral blood samples were collected before and one month after the initiation of sweet potato petiole and leaf administration. This study was approved by the Institutional Animal Care and Use Committee of Clino Corp. (Miyagi, Japan) in accordance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care International (Permit No. Clino25005). All sections of the study adhered to the ARRIVE guidelines for animal research [
43].
2.3. Gut Microbiota Analysis from Fecal Samples
A small amount of feces was homogenized in 4 M guanidinium thiocyanate buffer, and DNA was extracted. The 16S rDNA region from each fecal DNA sample was amplified using dual-index primers, and sequencing was performed using the MiSeq platform (Illumina, Inc., San Diego, CA, US) to generate FASTQ files. The FASTQ files were processed using QIIME2 version 2023.5 [
44], and taxonomic classification was performed with reference to the SILVA database version 138. From the resulting data, α-diversity (Chao1 index) and ß-diversity (principal coordinate analysis using both weighted and unweighted UniFrac distances) were calculated. Taxonomic composition at the genus level (level 7) was analyzed, and LEfSe (Linear Discriminant Analysis Effect Size) analysis [
45] was performed to identify bacterial taxa that were significantly altered following sweet potato petiole and leaf administration. In addition, metabolic pathway prediction was conducted using PICRUSt2 version 2021.2 [
46] and the ggpicrust2 package in R [
47], with functional annotation based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Statistical analyses were conducted using R version 4.3.2, and differences before and after treatment were assessed using paired t-tests or Wilcoxon signed-rank tests with a significance threshold of
P < 0.05. For multiple comparisons, a false discovery rate (FDR) < 0.2 was considered statistically significant.
2.4. Analysis of Senescence-Associated Cells in Peripheral Blood
5 mL of peripheral blood was collected from the subclavian veins of the animals. The collected blood was diluted two-fold with PBS (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan), and peripheral blood mononuclear cells were isolated by density gradient centrifugation using Ficoll-Paque™ PLUS (Cyteva, Marlborough, MA, US) at 400 × g, 24 °C for 30 minutes. The collected mononuclear cell layer was washed with 10 volumes of PBS and centrifuged again at 400 × g, 24 °C for 5 minutes. After removing the supernatant, the cells were resuspended in RPMI 1640 medium (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) containing 10% FBS (Thermo Fisher Scientific, Waltham, MA, US).
As an indicator of senescent cells, ß-galactosidase (ß-gal) staining was performed using the Cellular Senescence Detection Kit - SPiDER-ßGal (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Specifically, the cell suspension was centrifuged at 400 × g, 24 °C for 5 minutes, and the supernatant was removed. The cells were then resuspended in 1 mL of HBSS (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan), centrifuged again under the same conditions, and the supernatant was discarded. Subsequently, the cells were resuspended in 1 mL of Bafilomycin A1 working solution and incubated at 37 °C for 1 h. Next, 1 mL of SPiDER-ßGal working solution was added, and the mixture was incubated at 37 °C for 30 min. After staining, the cells were centrifuged (400 × g, 24 °C, 5 minutes), the supernatant was removed, and the cells were resuspended in 10% FBS/RPMI 1640 medium, followed by another centrifugation. Finally, the cells were resuspended in 1% BSA/PBS. APC-conjugated anti-CD3 antibody (BD Biosciences, Franklin Lakes, NJ, US) was added to the stained cells and incubated on ice for 15 min. After two washes with 1% BSA/PBS (400 × g, 24 °C, 5 min), the cells were resuspended again in 1% BSA/PBS. Flow cytometric analysis was performed using a BD FACSLyric flow cytometer (BD, Franklin Lakes, NJ, US), and data were analyzed using the FlowJo v10 software platform (FlowJo LLC., Ashland, OR, US).
Lymphocyte proliferative capacity was assessed by Ki-67 staining using the BD Pharmingen Transcription Factor Buffer Set (BD, Franklin Lakes, NJ, US). Specifically, the cell suspension was centrifuged at 400 × g, 24 °C for 5 minutes, then resuspended in 1x Fix/Purm solution and incubated at 4 °C for 40 minutes. After two washes with 1x Perm/Wash buffer (400 × g, 24 °C, 5minutes), the supernatant was removed, and cells were incubated with APC-conjugated anti-Ki-67 antibody (BD, Franklin Lakes, NJ, US) diluted in 1x Perm/Wash buffer at 4 °C for 40 minutes. After two additional washes, cells were resuspended in 1% BSA/PBS medium. Flow cytometric analysis was performed using BD FACSLyric, and the data were analyzed using FlowJo v10.
A stimulation assay with anti-CD3/CD28 antibodies was performerd on cryopreserved lymphocytes. Specifically, 96-well flat-bottom plates were coated with anti-CD3 antibody (Novus Biologicals, Centennial, CO, US) diluted in PBS (final concentration: 1 μg/mL) and incubated for 2 hours at 37 °C in a 5% CO₂ incubator. After two washes with PBS, the cell suspension was added to each well. Anti-CD28 antibody (MyBioSource, San Diego, CA, US) diluted in 10% FBS/RPMI 1640 medium (final concentration: 1 μg/mL) was then added, and the plate was centrifuged at 200 × g for 2 minutes at 24 °C to facilitate cell contact with the well bottom. Cells were cultured for 6 d at 37 °C in a 5% CO₂ incubator. After incubation, the cells were harvested, and Ki-67–positive cells were detected.
Statistical analyses of these experimental results were performed using paired t-tests, with differences considered significant at P < 0.05.
3. Results
3.1. Gut Microbiota Changes Induced by Sweet Potato Petiole and Leaf
16S rDNA V3-V4 region sequencing using fecal DNA collected before and after the administration of sweet potato petioles and leaves yielded 102,702 ± 11,321 non-chimeric reads per sample. Based on these results, statistical analyses were conducted to evaluate the changes in the gut microbiota before and after the administration of sweet potato petioles and leaves.
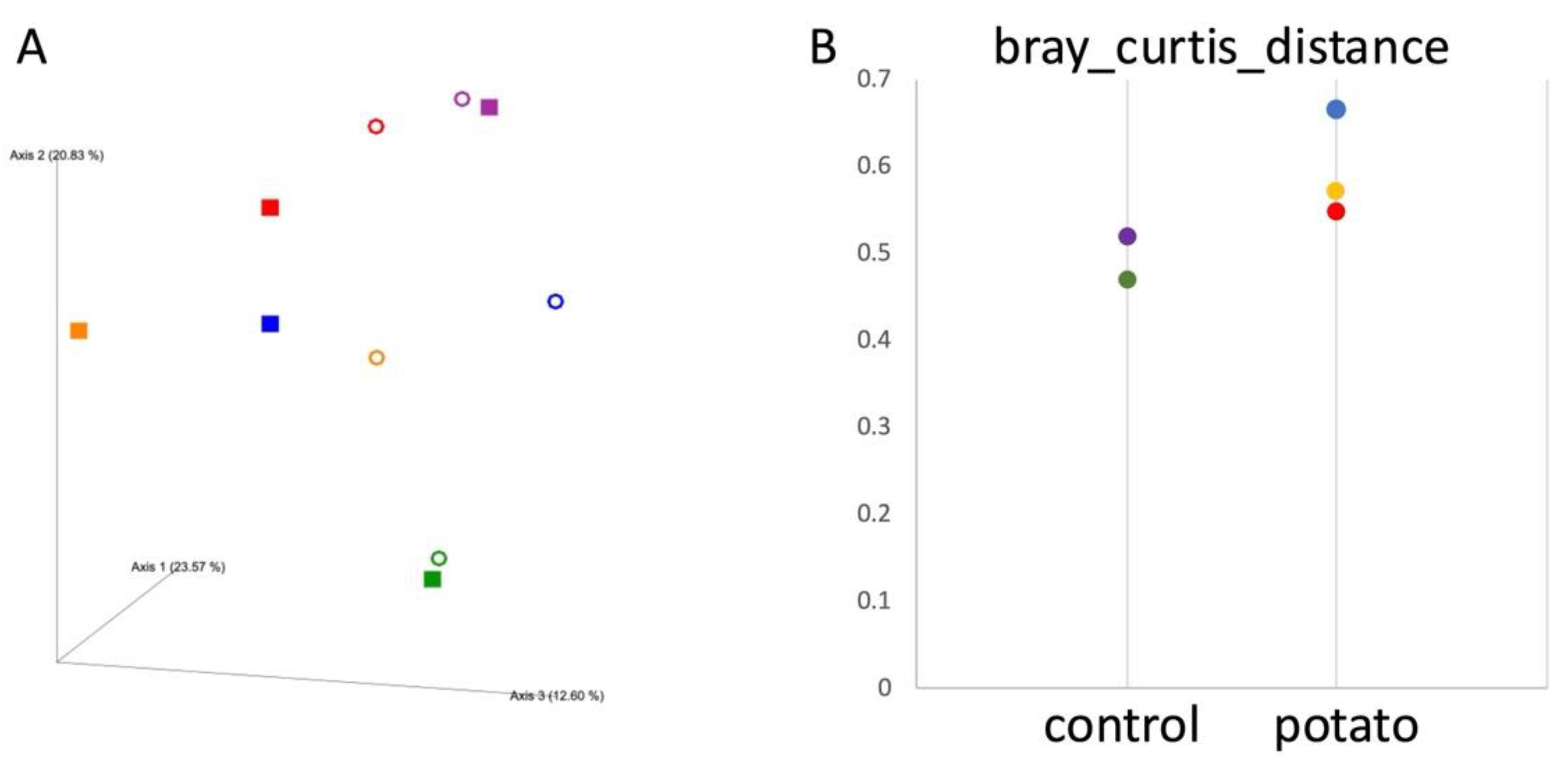
No significant changes were observed in α-diversity analysis, including observed OTUs, Chao1, Shannon index, and Faith’s phylogenetic diversity, either between the control and potato groups or between pre- and post-administration. In ß-diversity analysis, principal coordinate analysis (PCoA) based on the Bray–Curtis distance revealed that in the potato group, the distances between pre- and post-administration samples from the same individual were greater than those in the control group (
Figure 1A). Although the Bray–Curtis distances tended to be greater in the potato group than in the control group, this difference was not statistically significant (
Figure 1B).
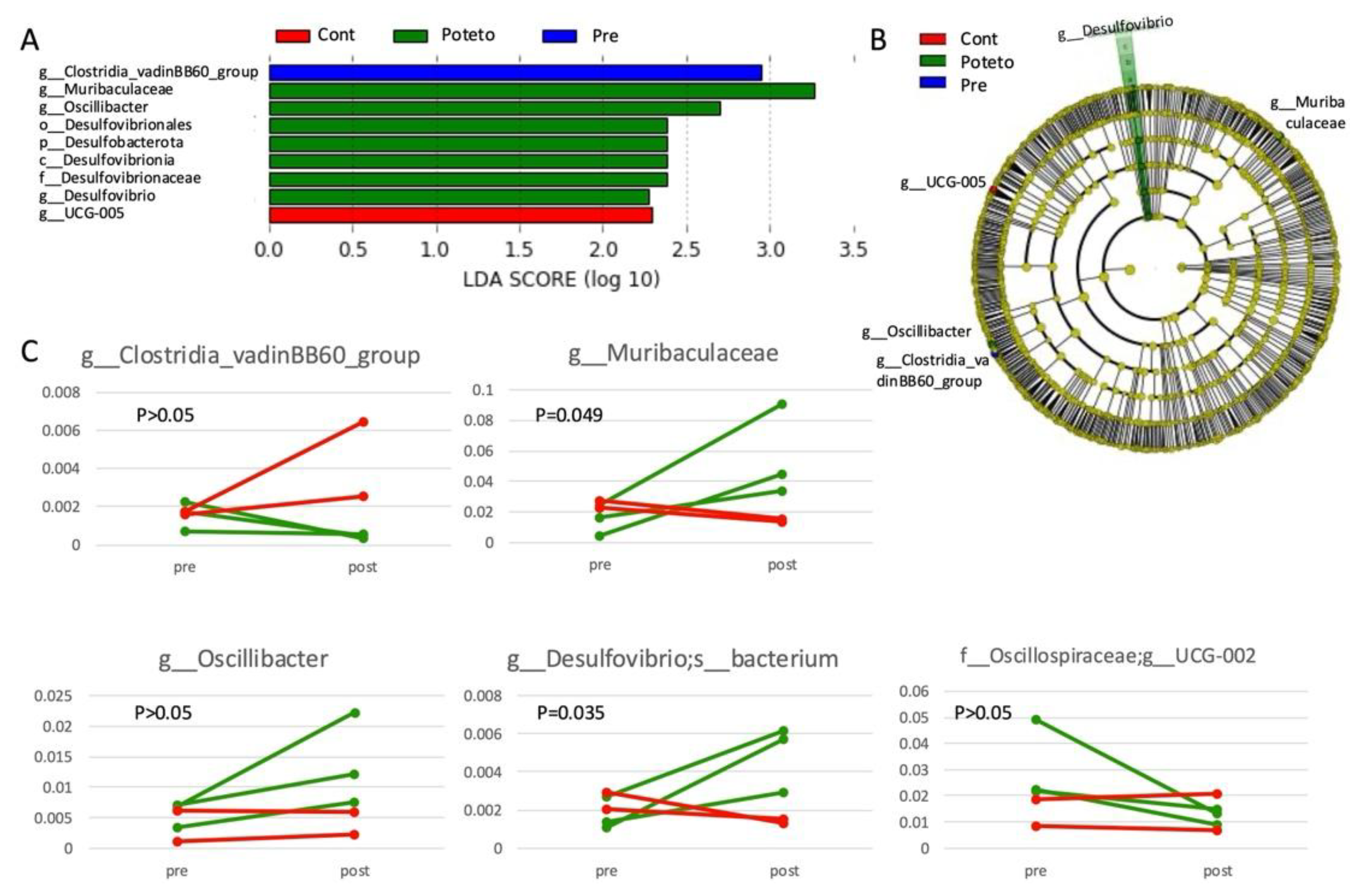
In the LEfSe analysis using the individual as a subclass variable, the administration of sweet potato petioles and leaves resulted in an increase in
Muribaculaceae (log LDA = 3.262.
P = 0.040),
Oscillibacter (log LDA = 2.699,
P = 0.048), and
Desulfovibrio (log LDA = 2.385,
P = 0.048), as well as a decrease in the genus
UCG-002 within the family
Oscillospiraceae (log LDA = 2.949,
P = 0.022), whereas the control group showed an increase in the genus
Clostridia_vadinBB60_group (log LDA = 2.293,
P = 0.022) (
Figure 2A, B). Among these, the genera
Muribaculaceae and
Desulfovibrio showed a significant increase in pre- and post-administration of sweet potato petioles and leaves, as confirmed by the paired t-test (
Figure 2C).
By predicting the activated or suppressed metabolic enzymes from the percentage of microbiota using PICRUSt, 431 enzymes were found to differ significantly (
P < 0.05) before and after sweet potato petiole and leaf administration based on a paired t-test, and 77 enzymes remained significant after multiple testing corrections. Although no significantly enriched KEGG pathways were identified in the ggpicrust2 analysis, the KEGG Mapper analysis of the 77 enzymes that remained significant after multiple testing corrections revealed their involvement in 27 pathways (
Table 1).
3.2. Senescence-Associated Cellular Changes in Peripheral Blood Induced by Sweet Potato Petiole and Leaf
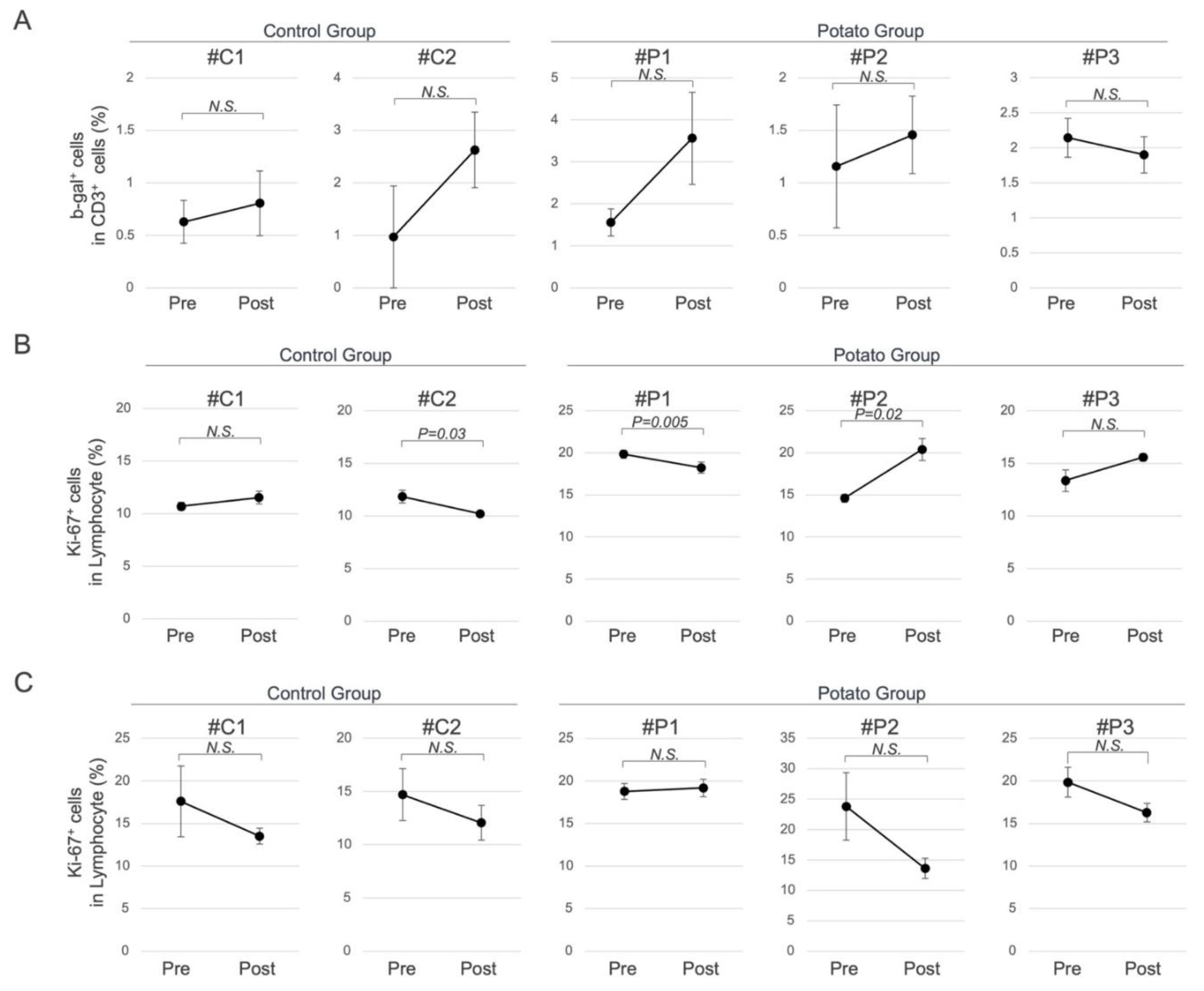
Given the use of super-aged MMPs in this study, senescent cells in peripheral blood lymphocytes were evaluated by flow cytometry in both potato and control groups. No significant differences were observed in the proportion of ß-galactosidase (ß-gal)-positive cells, a widely recognized marker of cellular senescence (
Figure 3A). In contrast, a significant increase in the proportion of cells expressing Ki-67, a marker of cellular proliferation, was observed in some individuals of the potato group (
Figure 3B). Furthermore, to assess lymphocyte responsiveness, the proportion of Ki-67-positive cells was measured following stimulation with anti-CD3 and anti-CD28 antibodies; however, no significant differences were observed between the groups (
Figure 3C).
4. Discussion
As global population aging has become an increasingly serious social issue, it is becoming increasingly important to prevent or inhibit aging because it is a major risk factor for various chronic diseases [
6,
7,
48,
49]. However, clinical trials involving elderly subjects are limited, making the use of experimental animals in aging research more common. Although previous studies using aged experimental pigs have reported that the oldest animals are approximately 10 years [
37,
38,
39,
40,
41,
42], to our knowledge, this is the first study to utilize super-aged MMPs at 13 years of age.
Sweet potatoes are divided into four parts: tuberous roots, stems, petioles, and leaves, with the tuberous roots being primarily consumed. Although parts other than the tuberous root are usually discarded, they are rich in dietary fiber, polyphenols, vitamins, and minerals and have gained attention because of their antioxidant [
25,
26], anti-obesity [
27,
28], antidiabetic [
29,
30,
31,
32], anti-inflammatory [
33,
34], and anti-aging effects [
35]. Therefore, using the discarded petioles and leaves of sweet potatoes as a health food is considered valuable from the perspective of Sustainable Development Goals.
Analysis of the gut microbiota from the feces of super-aged MMPs administered to sweet potato petioles and leaves showed a tendency toward greater divergence in Bray–Curtis distance in ß-diversity analysis compared to those in the control group. The Bray–Curtis distance is an index of ecological similarity based on the abundance of each microbial species, and the results suggest that the administration of sweet potato petioles and leaves altered the relative abundance of certain species. Consistently, LEfSe analysis revealed an increase in the genera
Muribaculaceae,
Oscillibacter, and
Desulfovibrio, and a decrease in the genus
UCG-002 (family
Oscillospiraceae) following the administration of sweet potato petioles and leaves. Among these, increases in the genera
Muribaculaceae and
Desulfovibrio were validated using a paired t-test. Bacteria of the genus
Muribaculaceae cooperate with
Bifidobacterium and
Lactobacillus to produce short-chain fatty acids from plant-derived fibers, contributing to beneficial effects in inflammatory bowel disease, obesity, and type 2 diabetes [
50]. Therefore, prebiotics that promote an increase in
Muribaculaceae bacteria have been studied, and in addition to plant-derived fibers, polyphenols have also been reported to contribute to their proliferation [
50]. The petioles and leaves of sweet potatoes, which are rich in both dietary fibers and polyphenols, may serve as ideal prebiotic materials.
Desulfovibrio species are also involved in short-chain fatty acid synthesis; however, they are known to influence immune signaling regulation by catalyzing hydrogen sulfide (H
2S) production [
51]. H
2S can exert immunosuppressive effects by negatively regulating immune signaling pathways such as NLRP3; however, it can also activate immune responses by positively modulating MAPK and ERK-NF-κB signaling pathways. Therefore, there are conflicting reports regarding its involvement in the aforementioned diseases and carcinogenesis [
51]. PICRUSt analysis did not reveal any changes in the pathways related to sulfate metabolism; however, enzymes associated with butanoate metabolism showed significant alterations. These results suggest that sweet potato petiole and leaf administration may promote short-chain fatty acid synthesis, potentially exerting anti-inflammatory effects, and improving glucose and lipid metabolism.
The measurement of senescence markers in the peripheral blood lymphocytes of super-aged MMPs administered to sweet potato petioles and leaves revealed no significant changes in senescent cells due to the administration. However, increased lymphocyte proliferation has also been observed in some individuals. In these individuals, neither a decrease in ß-gal-positive cells nor an improvement in stimulation responsiveness was observed. Therefore, although this does not directly indicate a reduction in senescent cells, the administration of sweet potato petioles and leaves may induce an increase in highly proliferative lymphocytes in peripheral blood. Furthermore, considering the enhanced short-chain fatty acid synthesis by the gut microbiota, as described above, activation of MAPK and ERK-NF-κB pathways promoting cell proliferation may underlie the observed data. Taken together, the administration of sweet potato petioles and leaves may not directly affect senescent cells in the peripheral blood, but rather induce lymphocytes influenced by short-chain fatty acids in the gut to circulate systemically, potentially affecting the overall immune function. However, these changes were observed only in some individuals, and the generalization of the results requires careful consideration.
In aging research, the use of experimental animals with rapid growth and short lifespans allows efficient study progression. However, similarities to humans differ depending on the animal species. Moreover, many major age-related diseases in humans rarely occur spontaneously in experimental animals, and animal models that develop aging-related diseases at a young age often differ substantially in background and disease progression from those observed in elderly humans [
52,
53]. Therefore, caution should be exercised when extrapolating results from experimental animals to humans [
12]. A comprehensive understanding of human aging and age-related diseases can be achieved by adopting multifaceted approaches in various organisms.
5. Conclusions
This is the first study to use super-aged MMPs. Furthermore, sweet potato petioles and leaves administered with super-aged MMPs were suggested to possess anti-inflammatory effects and improve glucose and lipid metabolism. Although sweet potato petioles and leaves are generally discarded, this study highlights their potential as effective health food ingredients.
Author Contributions
Conceptualization: E.K.; Methodology: Y.M., T.S., Y.H., T.M., S.K. S.I., and E.K.; Software: Y.M., and S.I.; Validation: K.S., T.S., Y.H., and E.K.; Formal analysis: Y.M., and S.I.; Investigation: K.S., Y.H., T.M., S.K., and E.K.; Resources: K.S., Y.H., T.M., S.K., and E.K.; Data curation: Y.M., T.S., S.I., and E.K.; Writing—original draft preparation: K.S., Y.M., S.I., and E.K.; Writing—review and editing: K.S., and E.K.; Visualization: K.S., Y.M., and S.I.; supervision: E.K.; Project administration: K.S., Y.M., S.K, S.I., and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the grant from Kobayashi Regenerative Research Institute, LLC, Wakayama, Japan.
Institutional Review Board Statement
This study was approved by the Institutional Animal Care and Use Committee of Clino Corp. (Miyagi, Japan) in accordance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care International (Permit No. Clino25005).
Data Availability Statement
The data supporting the findings of this study are available from the author upon request.
Acknowledgements
Mr. Noriaki Satake, CEO of Fuji Micra, Inc. (Shizuoka, Japan), contributed to the maintenance of the super-aged MMP colony through long-term rearing of the world’s smallest laboratory miniature pigs established in Japan. The development of preclinical protocols for super-aged MMPs using minimally invasive examinations in this colony was carried out under the guidance of Mr. Azusa Seki, DVM, and the director of Fukushima Medical Device Development Support Centre (Fukushima, Japan). Valuable insights on the physiological activity of sweet potato petioles were provided by Mr. Hisanobu Hirano (TES Holdings Inc., Tokyo, Japan).
Conflicts of Interest
Y.M. and T.S. are the employees of Sysmex Corporation, Hyogo, Japan. S.K., S.I. and E.K. served as staff members and the CEO of the Kobayashi Regenerative Research Institute, LLC. All other authors declare no conflicts of interest.
References
- Nakatani, H. Ageing and shrinking population: The looming demographic challenges of super-aged and super-low fertility society starting from Asia. Glob Health Med 2023, 5, 257–263. [Google Scholar] [CrossRef]
- Guillemot, J.R.; Zhang, X.; Warner, M.E. Population Aging and Decline Will Happen Sooner Than We Think. Social Sciences 2024, 13. [Google Scholar] [CrossRef]
- Bloom, D.E.; Luca, D.L. Chapter 1 - The Global Demography of Aging: Facts, Explanations, Future. In Handbook of the Economics of Population Aging, Piggott, J., Woodland, A., Eds.; North-Holland: 2016; Volume 1, pp. 3-56.
- Rosenberg, M.; Tomioka, S.; Barber, S.L. Research to inform health systems’ responses to rapid population ageing: a collection of studies funded by the WHO Centre for Health Development in Kobe, Japan. Health Res Policy Syst 2022, 20, 128. [Google Scholar] [CrossRef]
- Hong, C.; Sun, L.; Liu, G.; Guan, B.; Li, C.; Luo, Y. Response of Global Health Towards the Challenges Presented by Population Aging. China CDC Wkly 2023, 5, 884–887. [Google Scholar] [CrossRef]
- Sierra, F. The Emergence of Geroscience as an Interdisciplinary Approach to the Enhancement of Health Span and Life Span. Cold Spring Harb Perspect Med 2016, 6, a025163. [Google Scholar] [CrossRef]
- Polidori, M.C. Geroscience in the continuum from healthy longevity to frailty. Z Gerontol Geriatr 2024, 57, 361–364. [Google Scholar] [CrossRef]
- Petr, M.A.; Matiyevskaya, F.; Osborne, B.; Berglind, M.; Reves, S.; Zhang, B.; Ezra, M.B.; Carmona-Marin, L.M.; Syadzha, M.F.; Mediavilla, M.C.; et al. Pharmacological interventions in human aging. Ageing Res Rev 2024, 95, 102213. [Google Scholar] [CrossRef]
- Herrera, A.P.; Snipes, S.A.; King, D.W.; Torres-Vigil, I.; Goldberg, D.S.; Weinberg, A.D. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health 2010, 100 Suppl 1, S105–112. [Google Scholar] [CrossRef]
- Provencher, V.; Mortenson, W.B.; Tanguay-Garneau, L.; Belanger, K.; Dagenais, M. Challenges and strategies pertaining to recruitment and retention of frail elderly in research studies: a systematic review. Arch Gerontol Geriatr 2014, 59, 18–24. [Google Scholar] [CrossRef]
- Ridda, I.; Lindley, R.; MacIntyre, R.C. The challenges of clinical trials in the exclusion zone: the case of the frail elderly. Australas J Ageing 2008, 27, 61–66. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Scheibye-Knudsen, M.; Longo, D.L.; de Cabo, R. Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci 2015, 3, 283–303. [Google Scholar] [CrossRef]
- Holtze, S.; Gorshkova, E.; Braude, S.; Cellerino, A.; Dammann, P.; Hildebrandt, T.B.; Hoeflich, A.; Hoffmann, S.; Koch, P.; Terzibasi Tozzini, E.; et al. Alternative Animal Models of Aging Research. Front Mol Biosci 2021, 8, 660959. [Google Scholar] [CrossRef]
- Tohyama, S.; Kobayashi, E. Age-Appropriateness of Porcine Models Used for Cell Transplantation. Cell Transplant 2019, 28, 224–228. [Google Scholar] [CrossRef]
- Shirakawa, K.; Kobayashi, E.; Ichihara, G.; Kitakata, H.; Katsumata, Y.; Sugai, K.; Hakamata, Y.; Sano, M. H(2) Inhibits the Formation of Neutrophil Extracellular Traps. JACC Basic Transl Sci 2022, 7, 146–161. [Google Scholar] [CrossRef]
- Santulli, G.; Borras, C.; Bousquet, J.; Calza, L.; Cano, A.; Illario, M.; Franceschi, C.; Liotta, G.; Maggio, M.; Molloy, W.D.; et al. Models for preclinical studies in aging-related disorders: One is not for all. Transl Med UniSa 2015, 13, 4–12. [Google Scholar]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci Transl Med 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Gutierrez, K.; Dicks, N.; Glanzner, W.G.; Agellon, L.B.; Bordignon, V. Efficacy of the porcine species in biomedical research. Front Genet 2015, 6, 293. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, W.; Li, J.; Jin, Y.; Qiu, Z. Application of the transgenic pig model in biomedical research: A review. Front Cell Dev Biol 2022, 10, 1031812. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomas-Barberan, F.A. The effects of polyphenols and other bioactives on human health. Food Funct 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit Rev Food Sci Nutr 2021, 61, 690–711. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J Food Biochem 2022, 46, e14264. [Google Scholar] [CrossRef]
- Escobar-Puentes, A.A.; Palomo, I.; Rodriguez, L.; Fuentes, E.; Villegas-Ochoa, M.A.; Gonzalez-Aguilar, G.A.; Olivas-Aguirre, F.J.; Wall-Medrano, A. Sweet Potato (Ipomoea batatas L.) Phenotypes: From Agroindustry to Health Effects. Foods 2022, 11. [Google Scholar] [CrossRef]
- Chen, W.P.; Mao, T.J.; Fan, L.; Zhou, Y.H.; Yu, J.; Jin, Y.; Hou, P.C. [Effect of purple sweet potato on lipid metabolism and oxidative stress in hyperlipidemic rats]. Zhejiang Da Xue Xue Bao Yi Xue Ban 2011, 40, 360–364. [Google Scholar] [CrossRef]
- Insanu, M.; Amalia, R.; Fidrianny, I. Potential Antioxidative Activity of Waste Product of Purple Sweet Potato (Ipomoea batatas Lam.). Pak J Biol Sci 2022, 25, 681–687. [Google Scholar] [CrossRef]
- Han, K.H.; Matsumoto, A.; Shimada, K.; Sekikawa, M.; Fukushima, M. Effects of anthocyanin-rich purple potato flakes on antioxidant status in F344 rats fed a cholesterol-rich diet. Br J Nutr 2007, 98, 914–921. [Google Scholar] [CrossRef]
- Li, C.; Feng, Y.; Li, J.; Lian, R.; Qin, L.; Wang, C. Extraction, purification, structural characterization, and hepatoprotective effect of the polysaccharide from purple sweet potato. J Sci Food Agric 2023, 103, 2196–2206. [Google Scholar] [CrossRef]
- Herawati, E.R.N.; Santosa, U.; Sentana, S.; Ariani, D. Protective Effects of Anthocyanin Extract from Purple Sweet Potato (Ipomoea batatas L.) on Blood MDA Levels, Liver and Renal Activity, and Blood Pressure of Hyperglycemic Rats. Prev Nutr Food Sci 2020, 25, 375–379. [Google Scholar] [CrossRef]
- Hisamuddin, A.S.B.; Naomi, R.; Manan, K.A.B.; Bahari, H.; Othman, F.; Embong, H.; Ismail, A.; Ahmed, Q.U.; Jumidil, S.H.; Hussain, M.K.; et al. The role of lutein-rich purple sweet potato leaf extract on the amelioration of diabetic retinopathy in streptozotocin-induced Sprague-Dawley rats. Front Pharmacol 2023, 14, 1175907. [Google Scholar] [CrossRef]
- Kinoshita, A.; Nagata, T.; Furuya, F.; Nishizawa, M.; Mukai, E. White-skinned sweet potato (Ipomoea batatas L.) acutely suppresses postprandial blood glucose elevation by improving insulin sensitivity in normal rats. Heliyon 2023, 9, e14719. [Google Scholar] [CrossRef]
- Mi, W.; Hu, Z.; Zhao, S.; Wang, W.; Lian, W.; Lu, P.; Shi, T. Purple sweet potato anthocyanins normalize the blood glucose concentration and restore the gut microbiota in mice with type 2 diabetes mellitus. Heliyon 2024, 10, e31784. [Google Scholar] [CrossRef]
- Majid, M.; Nasir, B.; Zahra, S.S.; Khan, M.R.; Mirza, B.; Haq, I.U. Ipomoea batatas L. Lam. ameliorates acute and chronic inflammations by suppressing inflammatory mediators, a comprehensive exploration using in vitro and in vivo models. BMC Complement Altern Med 2018, 18, 216. [Google Scholar] [CrossRef]
- Sun, R.; Kan, J.; Cai, H.; Hong, J.; Jin, C.; Zhang, M. In vitro and in vivo ameliorative effects of polyphenols from purple potato leaves on renal injury and associated inflammation induced by hyperuricemia. J Food Biochem 2022, 46, e14049. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, J.; Zhi, Q.; Yuan, T.; Lei, X.; Zeng, K.; Ming, J. Anti-aging effects of the fermented anthocyanin extracts of purple sweet potato on Caenorhabditis elegans. Food Funct 2021, 12, 12647–12658. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Valencak, T.G. A short life on the farm: aging and longevity in agricultural, large-bodied mammals. Geroscience 2020, 42, 909–922. [Google Scholar] [CrossRef]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Torres-Rovira, L.; Gonzalez, J.; Porrini, E.; Gonzalez-Colaco, M.; Isabel, B.; Astiz, S.; Gonzalez-Bulnes, A. Characterization of Ageing- and Diet-Related Swine Models of Sarcopenia and Sarcopenic Obesity. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
- Chen, J.; Zou, Q.; Lv, D.; Raza, M.A.; Wang, X.; Chen, Y.; Xi, X.; Li, P.; Wen, A.; Zhu, L.; et al. Comprehensive transcriptional profiling of aging porcine liver. PeerJ 2019, 7, e6949. [Google Scholar] [CrossRef]
- Kangawa, A.; Nishimura, T.; Nishimura, T.; Otake, M.; Enya, S.; Yoshida, T.; Shibata, M. Spontaneous Age-Related Histopathological Changes in Microminipigs. Toxicol Pathol 2019, 47, 817–832. [Google Scholar] [CrossRef]
- Lim, M.Y.; Song, E.J.; Kang, K.S.; Nam, Y.D. Age-related compositional and functional changes in micro-pig gut microbiome. Geroscience 2019, 41, 935–944. [Google Scholar] [CrossRef]
- Schachtschneider, K.M.; Schook, L.B.; Meudt, J.J.; Shanmuganayagam, D.; Zoller, J.A.; Haghani, A.; Li, C.Z.; Zhang, J.; Yang, A.; Raj, K.; et al. Epigenetic clock and DNA methylation analysis of porcine models of aging and obesity. Geroscience 2021, 43, 2467–2483. [Google Scholar] [CrossRef]
- Qiao, C.; Liu, C.; Ding, R.; Wang, S.; He, M. Unveiling the Metabolic Trajectory of Pig Feces Across Different Ages and Senescence. Metabolites 2024, 14. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. BMJ Open Sci 2020, 4, e100115. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol 2011, 12, R60. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Yang, C.; Mai, J.; Cao, X.; Burberry, A.; Cominelli, F.; Zhang, L. ggpicrust2: an R package for PICRUSt2 predicted functional profile analysis and visualization. Bioinformatics 2023, 39. [Google Scholar] [CrossRef]
- Li, J.; Han, X.; Zhang, X.; Wang, S. Spatiotemporal evolution of global population ageing from 1960 to 2017. BMC Public Health 2019, 19, 127. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, Y.; Schwebel, D.C.; Liu, Z.; Li, L.; Cheng, P.; Ning, P.; Hu, G. Population ageing and mortality during 1990-2017: A global decomposition analysis. PLoS Med 2020, 17, e1003138. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Zhou, H.; Huang, D.; Sun, Z.; Chen, X. Effects of intestinal Desulfovibrio bacteria on host health and its potential regulatory strategies: A review. Microbiol Res 2024, 284, 127725. [Google Scholar] [CrossRef]
- Huffman, D.M.; Justice, J.N.; Stout, M.B.; Kirkland, J.L.; Barzilai, N.; Austad, S.N. Evaluating Health Span in Preclinical Models of Aging and Disease: Guidelines, Challenges, and Opportunities for Geroscience. J Gerontol A Biol Sci Med Sci 2016, 71, 1395–1406. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, B.T.; Lei, P. Animal models of Alzheimer’s disease: Current strategies and new directions. Zool Res 2024, 45, 1385–1407. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).