Introduction
Vascular anomalies are divided into two main categories according to the latest classification developed by the International Society for the Study of Vascular Anomalies (ISSVA): vascular tumors (benign, local aggressive/borderline, and malignant) and vascular malformations (simple, combined, of major named vessels, and associated with other anomalies). In particular, simple vascular malformations are subdivided into low-flow (capillary, lymphatic, and venous) and fast-flow (arteriovenous malformations and fistulae) lesions [
1]
Vascular malformations are relatively common in children. In particular, low-flow venous malformations are encountered in approximately 1 per 1000 births. Clinical and sonographic evaluations are considered the gold standard approaches for diagnosing such anomalies; however, magnetic resonance imaging (MRI) studies are crucial for characterizing these anomalies to optimize therapy planning [
2,
3].
Current therapeutic strategies include observation, medical therapy, sclerotherapy, laser therapy, embolization, and surgery, depending on the type and anatomical location of the malformation [
4]. Interestingly, in a recent retrospective multicenter cohort study, bleomycin electrosclerotherapy (BEST) was confirmed to be a valuable option, yielding encouraging outcomes for treating low-flow venous and lymphatic malformations in children [
5].
Herein, we describe our preliminary experience with BEST in treating peripheral low-flow vascular malformations in the pediatric population.
Materials and Methods
We prospectively collected and analyzed data from patients who underwent BEST for peripheral low-flow venous and lymphatic malformations and were treated at our institution from May 2022 onward.
Preoperative diagnostics were performed with Doppler ultrasonography for superficial and small malformations. According to our radiologists’ indications, an MRI study with contrast agent was also performed for patients with large and deep malformations. We examined the following parameters: demographic data, vascular malformation characteristics, surgical details, number of procedures, outcomes, and complications.
Owing to the small number of patients, both normally and nonnormally distributed continuous variables are expressed as medians (interquartile ranges [IQRs]). Moreover, categorical variables are expressed as numbers and percentages.
Surgical Technique
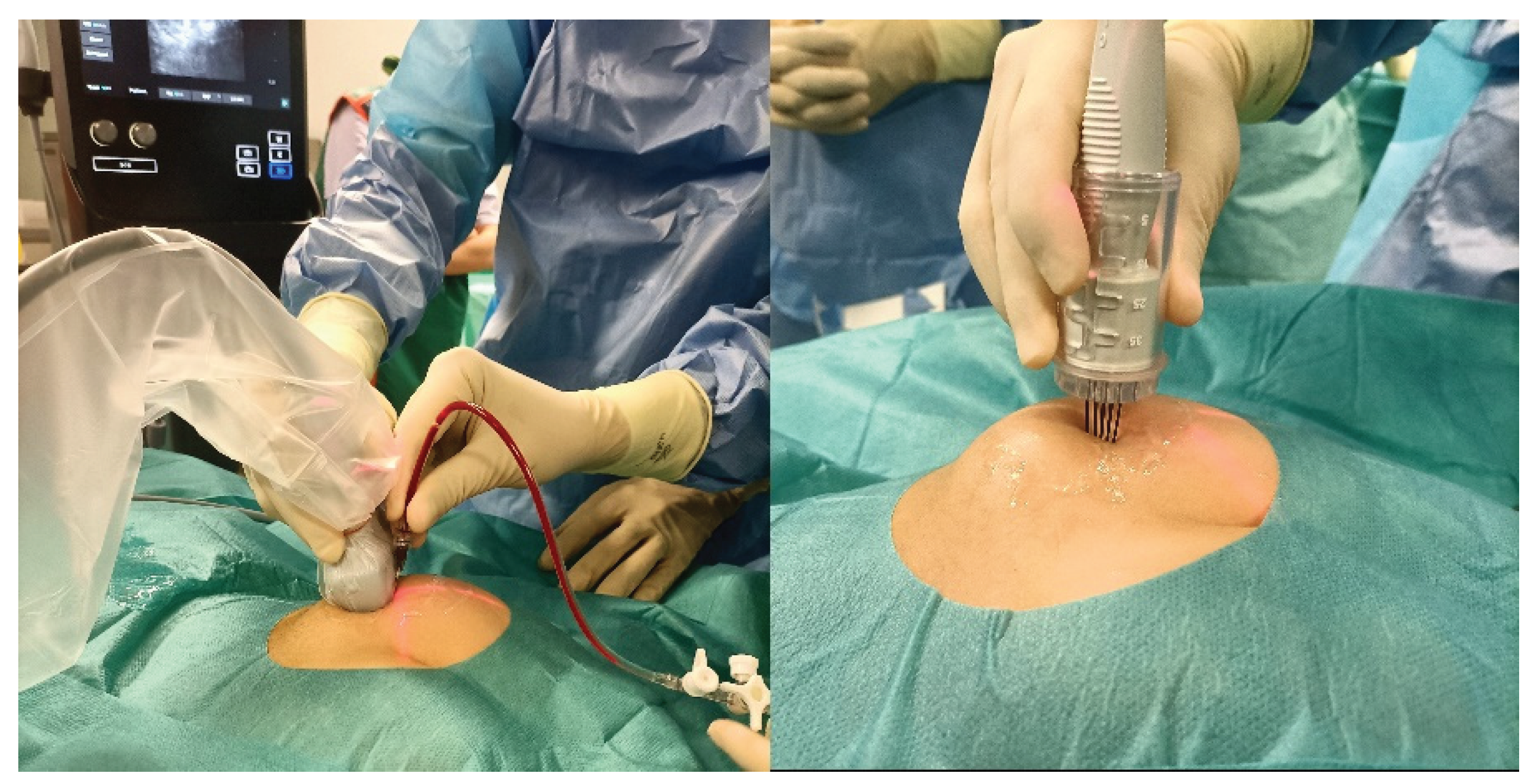
The treatment was performed in a sterile operating environment under sedation or general anesthesia, depending on the patient’s age and vascular malformation site. In the case of macrocystic lesions, intralesional injection of a solution of bleomycin (with a bleomycin concentration of 1 mg/1 ml) and contrast agent (opamidol) (with 1 ml of bleomycin diluted with 4 ml of iopamidol for a total of 20 ml of solution including a total of 5 ml of bleomycin) into the venous or lymphatic malformation was executed under ultrasonography/fluoroscopy guidance to verify the correct needle position and to study the anatomy of the vascular malformation. Bleomycin was injected at a dose of 0.5 mg/kg bleomycin, followed by the application of one or more electrical pulses generated by an electroporation device (IGEA S.p.A.) through electrodes with a specific design and adjustable length according to the lesion location and size (inserted into the lesion at a maximum depth of 4 cm) (
Figure 1). In the case of microcystic lesions, endovenous infusion of pure bleomycin at a dose of 0.3 mg/kg bleomycin (with a bleomycin concentration of 1 mg/ml) was performed. Ten minutes after the endovenous infusion, one or more electrical pulses were applied.
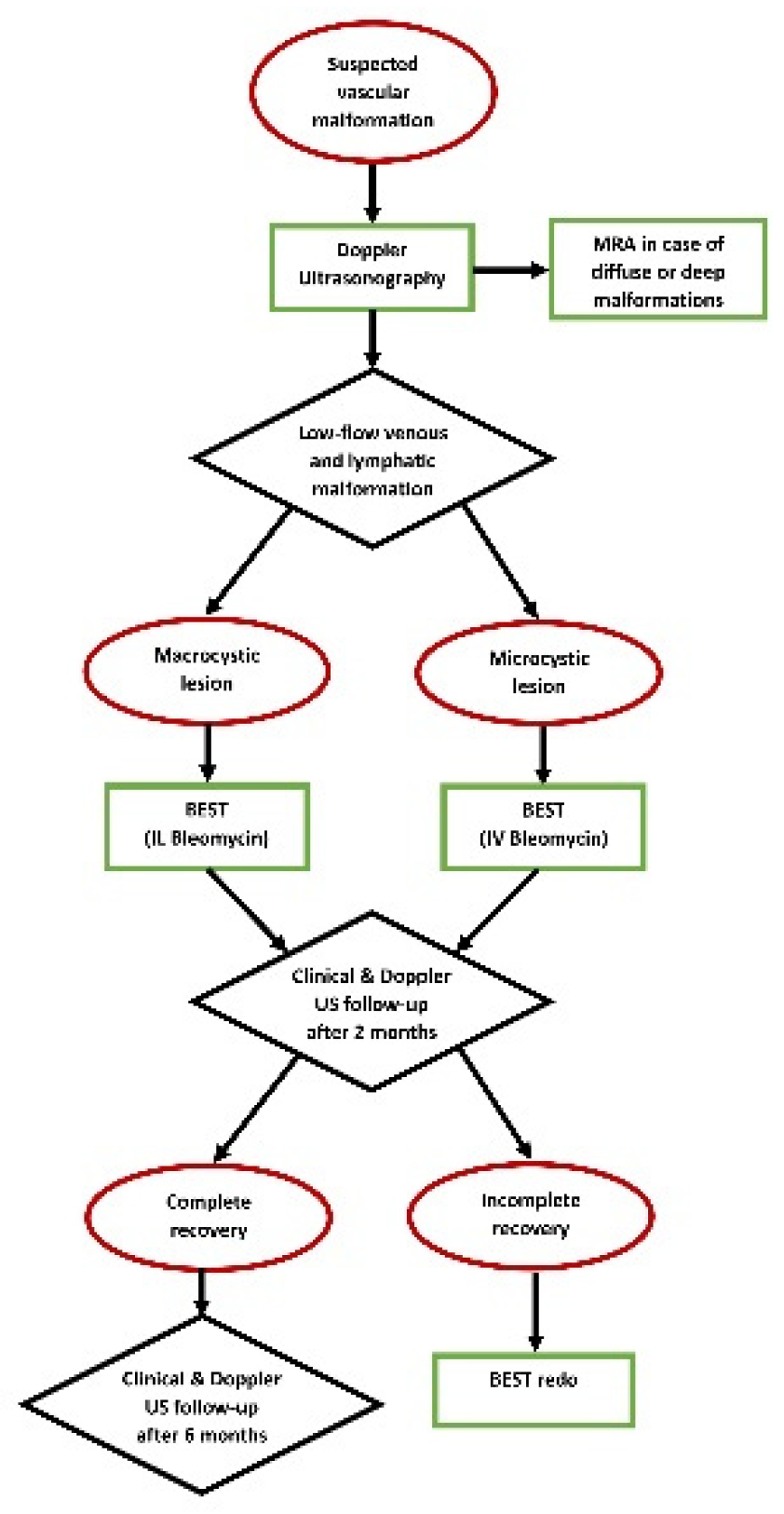
Figure 2 shows the diagnostic and therapeutic (with BEST) algorithm for low-flow vascular malformations in our tertiary-level pediatric surgical department.
Results
Twelve patients (4 boys and 8 girls) with peripheral low-flow vascular malformations were prospectively enrolled in this preliminary study. The median patient age at the first procedure was 81 months (IQR = 46–128). Notably, the youngest patient was under 1 year of age, whereas the oldest was aged 15 years.
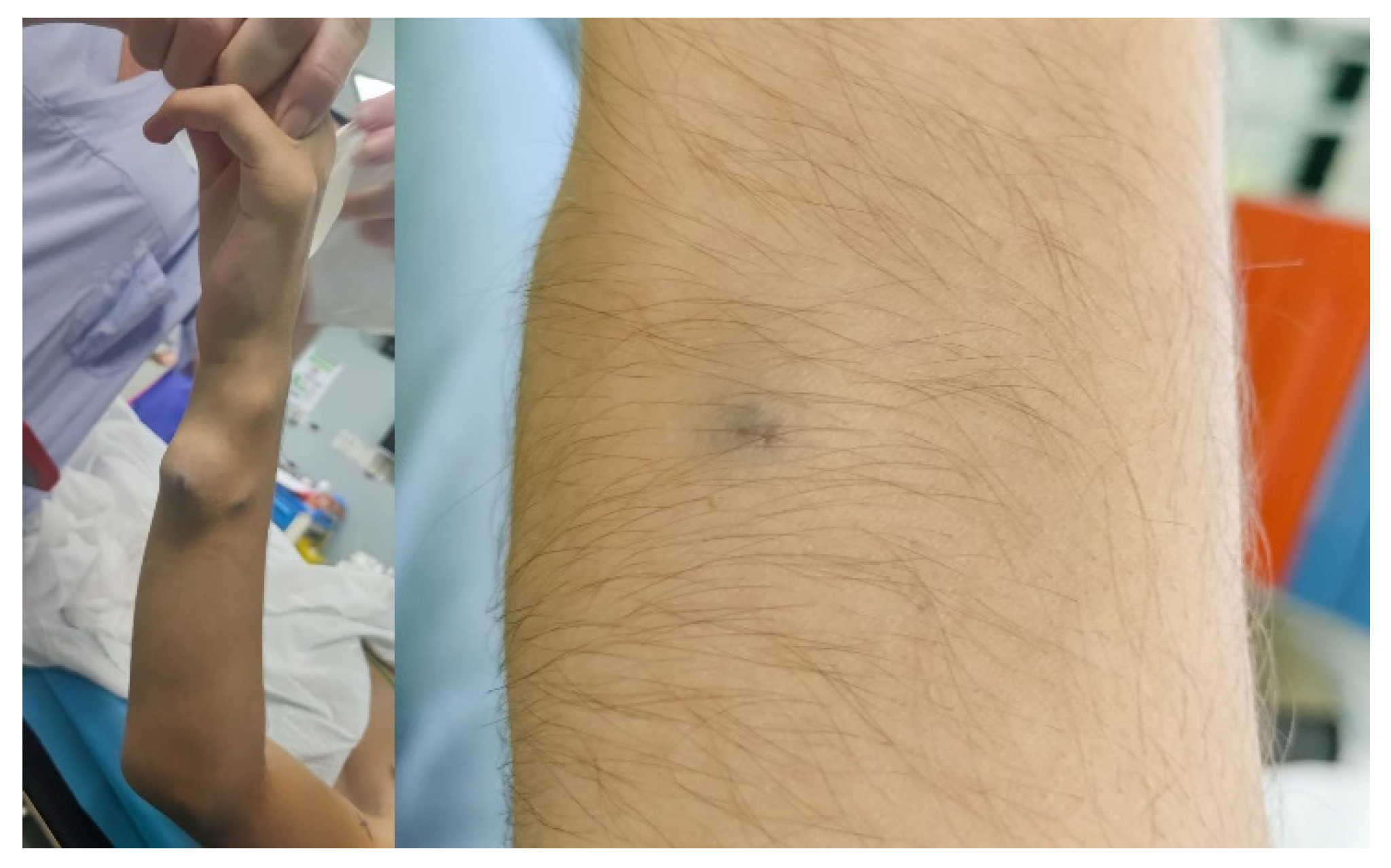
The most frequent anomaly was peripheral low-flow venous malformation (LFVM), presenting in 9 patients (75%). While, two patients (17%) presented with a lymphatic malformation and one patient (8%) with a mixed malformation. The anomalies were localized in the neck in 3 patients (25%) with one of them presenting also an involvement of the tongue, in the upper limbs in 2 (17%) patients (
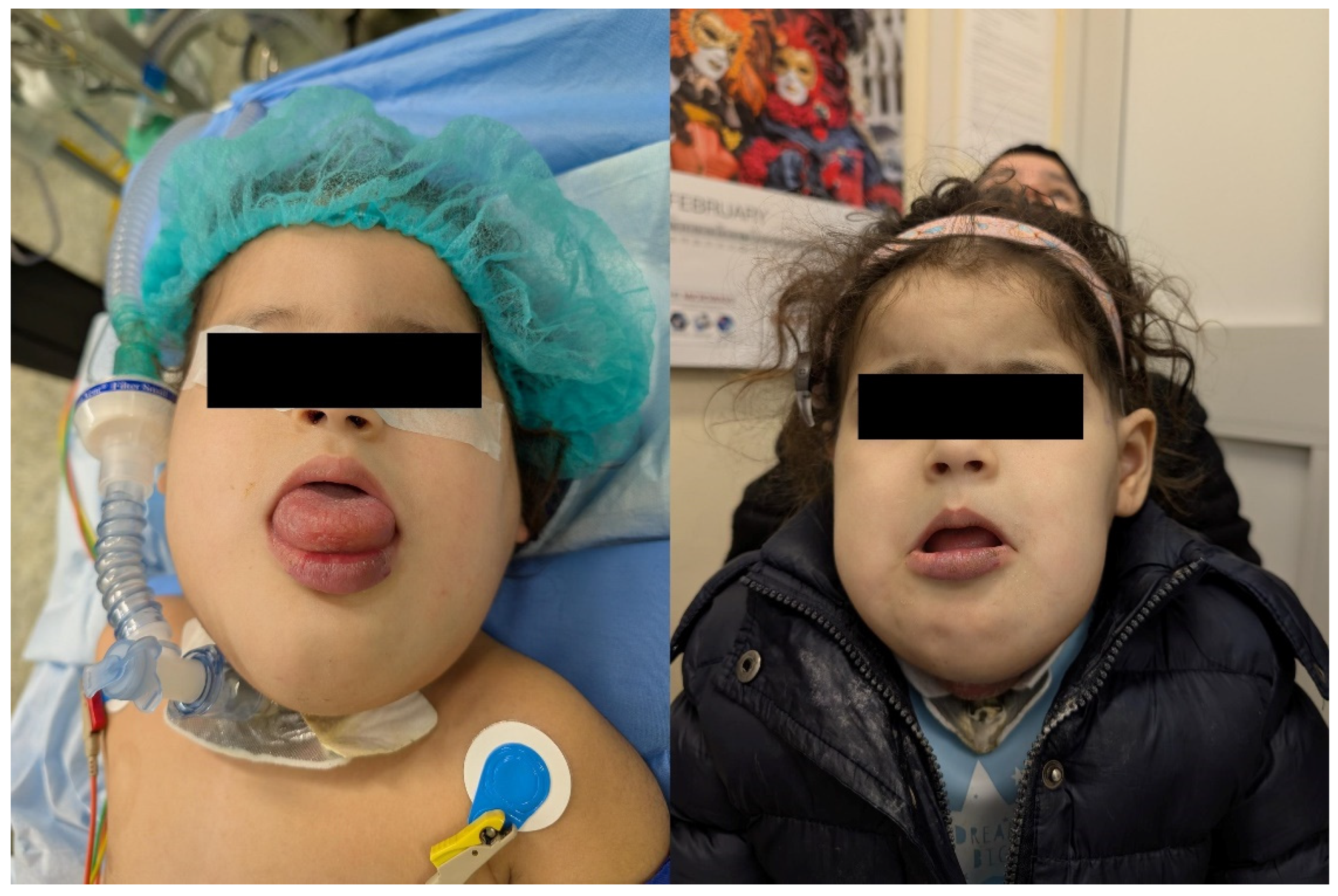
Figure 3), in the lower limbs in 2 patients (17%), in the subcutaneous tissue of the plantar face of the right foot in 2 patients (17%), in the right knee, subcutaneous tissue of the right buttock, and in the lumbosacral region in one (8%) patient respectively. Interestingly, two out of 12 patients (17%) presented with a large macrocystic lymphatic malformation on the left side of the neck and with diffuse mixed macrocystic and microcystic lymphatic malformations of the entire neck involving the tongue respectively (
Figure 4). Remarkably, only two patients (17%) experienced a self-limiting skin dyschromia and a hematoma of the tongue in the postoperative period followed by a full recovery in both cases.
Five out of twelve patients (42%) were discharged home the same day of the procedure. All patients underwent a clinical evaluation of the malformation 1 month after the procedure. A clinical and ultrasonographic evaluation of the malformation was performed 3 months after the procedure to determine whether to repeat BEST. In cases of clinical resolution, a second ultrasonographic evaluation was performed 6 months after the procedure. Notably, patients with venous malformations in the right lower limb and lumbosacral region required two procedures. In addition, two patients with large lymphatic malformations of the neck have required four procedures to date.
A minimum follow-up of 6 months was performed for 6 patients with a complete recovery in all cases, and a 5-month follow-up was performed for two patients after the last bleomycin injection, revealing a good reduction in the malformation volume and satisfying aesthetic results. Four out of 12 patients are still being followed and treated at our institution to date.
Detailed data about patients enrolled in our prospective project to date are reported in
Table 1.
Discussion
The term electrochemotherapy was first coined in 1991 by a group of researchers at the biochemical laboratory of the Institute Gustave-Roussy in France. Their study described the effect of reversible electroporation of cell membranes obtained through local application of electric pulses combined with highly cytotoxic drugs with limited or no transport across the plasma membrane. Accordingly, a hydrophilic antibiotic with endonuclease activity, such as bleomycin, is considered an optimal choice [
6,
7,
8]. Given the antitumor effectiveness of BEST demonstrated in preclinical studies, this approach was consequently included in the European guidelines for treating melanoma and colorectal cancer metastases and skin tumors [
8]. The use of BEST to treat venous malformations in the pediatric population has also been initiated, with encouraging results. Hence, the International Network for Sharing Practices on Electrochemotherapy (InspECT) has recently created a dedicated working group to standardize the procedure and promote clinical trials. Moreover, studies on BEST for treating peripheral low-flow vascular malformations in children are rare, and few have reported results in both pediatric and adult populations [
5,
9,
10].
This preliminary study focused exclusively on pediatric patients, investigating BEST as a treatment opportunity for peripheral low-flow vascular malformations. A positive result was obtained regarding aesthetic aspects, with no potentially severe post-procedural complications. In particular, consistent with the findings of a recent study by Schmidt and colleagues [
5], two patients in our case series experienced a post treatment side effect that resolved spontaneously without any sequelae. Remarkably, based on our preliminary experience, we learned that in lesions with microcystic aspects where intralesional bleomycin injection is challenging due to the small cyst size intravenous bleomycin injection with electroporation 10 minutes after injection initiation should be considered and could improve results. Moreover, we noted that previous treatments and/or pharmacological therapies do not appear to be a contraindication for Bleomycin electrosclerotherapy. In details, one of our patients underwent administration of intralesional OK-432 (Picibanil) while one of them is on oral therapy with Sirolimus.
The main limitation of this study is the small sample size, which limits the generalizability of our findings. Furthermore, long-term follow-up could provide significant information regarding patient outcomes.
BEST appears to be a promising and safe option for treating peripheral low-flow vascular malformations in children. Further studies with a greater number of patients and longer follow-up periods are needed to confirm our preliminary experience.
Submission declaration
Conceptualization: E.G., A.B., and J.S.; methodology: E.G. and A.B.; investigation: Z.Z., M. P-A., S.M., and D.O.; data curation: M.I., M-G. S., and D.C.; formal analysis: E.G., A.B., S.M., and D.C.; validation: A.B., D.C., and J.S.; writing—original draft preparation: Z.Z, M.P-A., M.I., M-G. S., S.M. and D.O.; writing—review and editing: E.G., A.B., D.C., and S.M.; supervision: J.S.; this work has not been published previously and is not under consideration for publication elsewhere. All the authors approved the final version of the manuscript and attest that they meet the ICMJE criteria for authorship.
Funding Source
This work was supported by the Italian Ministry of Health, through the contribution given to the Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste – Italy.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Availability of data and material
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgements
The authors would like to thank Mr. Dennis Chinello for their support as Application Specialist during the procedures performed in the operating theatre.
Conflict of Interest Statement
None declared.
Statement of ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Dasgupta R, Fishman SJ. ISSVA classification. Semin Pediatr Surg. 2014 Aug;23(4):158-61. Epub 2014 Jun 19. PMID: 25241091. [CrossRef]
- Penington A, Phillips RJ, Sleebs N, Halliday J (2023) Estimate of the prevalence of vascular malformations. J Vasc Anomalies. [CrossRef]
- Tofanelli L, Napolitano M, Baraldini V, Moneghini L. Venous malformations in children: comparison between magnetic resonance imaging and histopathological findings. Pediatr Radiol. 2024 Aug;54(9):1497-1506. Epub 2024 Jul 4. PMID: 38963573. [CrossRef]
- Hage AN, Chick JFB, Srinivasa RN, Bundy JJ, Chauhan NR, Acord M, et al. Treatment of Venous Malformations: The Data, Where We Are, and How It Is Done. Tech Vasc Interv Radiol. 2018 Jun;21(2):45-54. Epub 2018 Mar 8. PMID: 29784122. [CrossRef]
- Schmidt VF, Cangir Ö, Meyer L, Goldann C, Hengst S, Brill R, et al. Outcome of bleomycin electrosclerotherapy of slow-flow malformations in adults and children. Eur Radiol. 2024 Apr 16. Epub ahead of print. PMID: 38627287. [CrossRef]
- Mir LM, Orlowski S, Belehradek J Jr, Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer. 1991;27(1):68-72. PMID: 1707289. [CrossRef]
- Mir LM, Tounekti O, Orlowski S. Bleomycin: revival of an old drug. Gen Pharmacol. 1996 Jul;27(5):745-8. PMID: 8842674. [CrossRef]
- Cemazar M, Sersa G. Recent Advances in Electrochemotherapy. Bioelectricity. 2019 Dec 1;1(4):204-213. Epub 2019 Dec 12. PMID: 34471824; PMCID: PMC8370294. [CrossRef]
- Wohlgemuth WA, Müller-Wille R, Meyer L, Wildgruber M, Guntau M, Heydt SV, et al. Bleomycin electrosclerotherapy in therapy-resistant venous malformations of the body. J Vasc Surg Venous Lymphat Disord. 2021 May;9(3):731-739. Epub 2020 Oct 9. PMID: 33045393. [CrossRef]
- Muir T, Bertino G, Groselj A, Ratnam L, Kis E, Odili J, et al. Bleomycin electrosclerotherapy (BEST) for the treatment of vascular malformations. An International Network for Sharing Practices on Electrochemotherapy (InspECT) study group report. Radiol Oncol. 2023 Jun 21;57(2):141-149. PMID: 37341196; PMCID: PMC10286891. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).