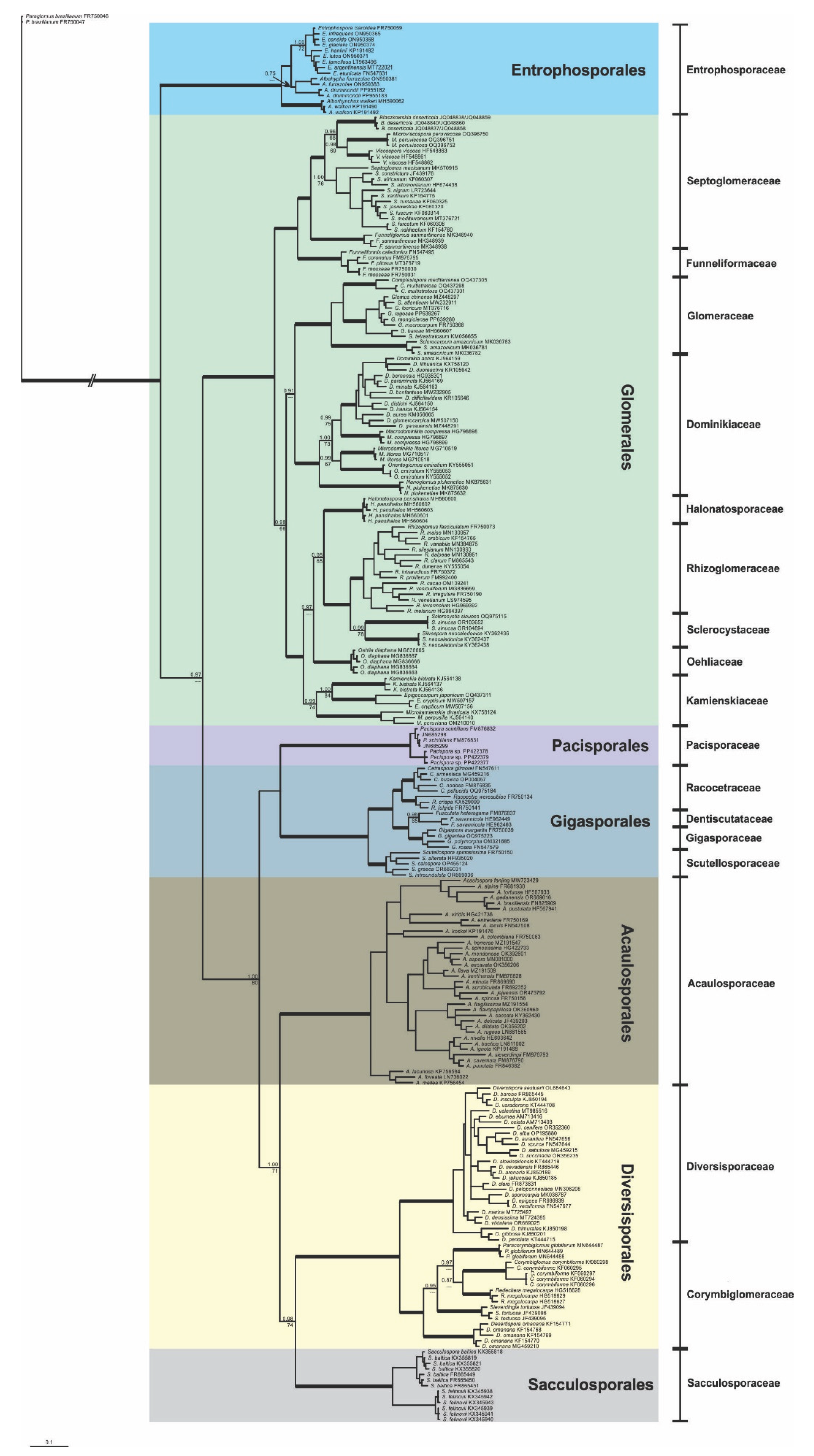
3.3. Taxonomy of the Glomeromycetes
Glomeromycetes Caval.-Sm., Biol. Rev. Cambridge Philos. Soc. 73: 246. 1998.
Mycobank MB 90168
Emended description: see Oehl et al. [
4]
Type order: Glomerales J.B. Morton and Benny, emend. Błaszk., B.T. Goto, and Magurno
Other orders:
Acaulosporales Sieverd., Silva G.A. & Oehl,
Entrophosporales Błaszk., Sánchez-García, B.T. Goto & Magurno,
Gigasporales Sieverd., G.A. Silva, B.T. Goto & Oehl,
Pacisporales Oehl, Sieverd., G.A. Silva,
Sacculosporales Silva G.A., Sieverd. & Oehl.
Glomerales J.B. Morton and Benny, Mycotaxon 37: 473. 1990.
MycoBank MB 90425
Emended description: see Błaszkowski et al. [
11] and Silva et al. [
14]
Type family: Glomeraceae Piroz. & Dalpé emend. Oehl, G.A. Silva & Sieverd.
Other families:
Dominikiaceae G.A. Silva, Sieverd. & Oehl,
Funneliformaceae Oehl, Sieverd. & G.A. Silva,
Halonatosporaceae Oehl, Sieverd. & G.A. Silva,
Kamienskiaceae G.A. Silva, Sieverd. & Oehl,
Oehliaceae Sieverd. & G.A. Silva,
Rhizoglomeraceae Sieverd., G.A. Silva & Oehl,
Sclerocystaceae Oehl, G.A. Silva & Sieverd.,
Septoglomeraceae Oehl, G.A. Silva & Sieverd.
Glomeraceae Piroz. & Dalpé, Symbiosis 7: 19. 1989.
MycoBank MB 82026
Emended description: see Oehl et al. [
4] and Silva et al. [
14]
Type genus: Glomus Tul. & C. Tul.
Other genera:
Complexispora Błaszk., B.T. Goto, Niezgoda & Magurno,
Sclerocarpum B.T. Goto, Błaszk., Niezgoda, Kozłowska & Jobim,
Simiglomus Sieverd., G.A. Silva & Oehl.
Glomus Tul. & C. Tul., G. bot. ital. 2(1): 63. 1845. [1844].
MycoBank MB 20244
Description: see Silva et al. [
14]
Type species: Glomus macrocarpum Tul & C. Tul., G. bot. ital. 2(1): 63. 1845. [1844].
MycoBank MB 240247
≡ Endogone macrocarpa (Tul. & C. Tul.) Tul. & C. Tul., Fungi Hypog.: 182. 1851.
MycoBank MB 218537
≡ Endogone guttulata E. Fisch., Ber. Schweiz. Bot. Ges. 32: 13. 1923.
MycoBank MB 266684
≡ Endogone nuda Petch., Ann. R. Bot. Gdns Peradeniya 9: 322. 1925.
MycoBank MB 223365
≡ Endogone pampaloniana Bacc., Nuovo Giorn. Bot. Ital., n.s. 10: 90. 1903.
MycoBank MB 223215
≡ Paurocotylis fulva var. zealandica Cooke, Grevillea 8: 59. 1879.
MycoBank MB 522257
Complexispora Błaszk., B.T. Goto, Niezgoda & Magurno, Mycol. Prog. 22(5, no. 34): 7. 2023.
MycoBank MB 847607
Description: see Błaszkowski et al. [
40] and Silva et al. [
14]
Type species: Complexispora multistratosa Błaszk., B.T. Goto, Niezgoda & Magurno,
Mycol. Prog. 22(5, no. 34): 6. 2023.
MycoBank MB 847608
Sclerocarpum B.T. Goto, Błaszk., Niezgoda, Kozłowska & Jobim, Mycol. Prog. 18(3): 375. 2019.
MycoBank MB 828316
Description: see Jobim et al. [
41] and Silva et al. [
14]
Type species: Sclerocarpum amazonicum B.T. Goto, Błaszk., Niezgoda, Kozłowska & Jobim, Mycol. Prog. 18: 377. 2019.
MycoBank MB 828317
Simiglomus Sieverd., G.A. Silva & Oehl, Mycotaxon 116: 104. 2011.
MycoBank MB 518435
Description: see Oehl et al. [
24]
Type species: Simiglomus hoi (S.M. Berch & Trappe) G.A. Silva, Oehl & Sieverd., Mycotaxon 116: 104 (2011).
MycoBank MB 518461
Basionym: Glomus hoi S.M. Berch & Trappe, Mycologia 77: 654. 1985.
MycoBank MB 105333
Dominikiaceae G.A. Silva, Sieverd. & Oehl, Taxonomy 4: 773. 2024.
MycoBank MB 855469
Description: see Silva et al. [
14]
Type genus: Dominikia Błaszk., Chwat & Kovács
Other genera:
Delicatispora Błaszk., Niezgoda & B.T. Goto,
Macrodominikia Oehl, Sieverd. & G.A. Silva,
Microdominikia Oehl, Corazon-Guivin & G.A. Silva,
Nanoglomus Corazon-Guivin, G.A. Silva & Oehl,
Orientoglomus G.A. Silva, Oehl & Corazon-Guivin.
Dominikia Błaszk., Chwat & Kovács, Nova Hedwigia 100(1–2): 228. 2014 [2015].
MycoBank MB 808255
Emended description: see Silva et al. [
14]
Type species: Dominikia minuta (Błaszk., Tadych & Madej) Błaszk., Chwat & Kovács,
Nova Hedwigia 100: 230. 2014 [2015].
MycoBank MB 808256
Basionym: Glomus minutum Błaszk., Tadych & Madej. Mycotaxon 76: 189. 2000.
Delicatispora Błaszk., Niezgoda & B.T. Goto, MycoKeys 112: 261. 2025.
Mycobank MB 856187
Description: see Błaszkowski et al. [
38]
Type species: Delicatispora indica (Błaszk., Wubet & Harikumar) Błaszk., Niezgoda & B.T. Goto, MycoKeys 112: 261. 2025.
Mycobank MB 856189
Basionym: Glomus indicum Błaszk., Wubet & Harikumar, Botany 88: 134. 2010.
Mycobank MB 515245.
≡ Dominikia indica (Błaszk., Wubet & Harikumar) Błaszk., G.A. Silva & Oehl, Nova Hedwigia 101 (1-2): 71. 2014.
Mycobank MB 808258
Macrodominikia Oehl, Sieverd. & G.A. Silva, Taxonomy 4: 774. 2024.
MycoBank 855470
Description: see Silva et al. [
14] and Błaszkowski et al. [
38]
Type species: Macrodominikia compressa (Sieverd., Oehl, Palenz., Sánchez-Castro & G.A. Silva) Oehl, Sieverd. & G.A. Silva, Taxonomy 4: 774. 2024.
MycoBank MB 855471
Basionym: Glomus compressum Sieverd., Oehl, Palenz., Sánchez-Castro & G.A. Silva. Nova Hedwigia 99: 433. 2014.
MycoBank MB 807530
≡ Dominikia compressa (Sieverd., Oehl, Palenz., Sánchez-Castro & G.A. Silva) Oehl, Palenz., Sánchez-Castro & G.A. Silva. Nova Hedwigia. 101: 71. 2014 [2015].
MycoBank MB 809861
Microdominikia Oehl, Corazon-Guivin & G.A. Silva, Mycol. Prog. 18(12): 1400. 2019.
MycoBank MB 831098
Description: see Silva et al. [
14]
Type species: Microdominikia litorea (Błaszk. & Kozłowska) Oehl, Corazon-Guivin & G.A. Silva, Mycol. Prog. 18(12): 1400. 2019.
MycoBank MB 831099
Basionym: Dominikia litorea Błaszk. & Kozłowska, Phytotaxa 338(3): 246. 2018.
MycoBank MB 823832
Nanoglomus Corazon-Guivin, G.A. Silva & Oehl, Mycol. Prog. 18(12): 1398. 2019.
Description: see Silva et al. [
14]
MycoBank MB 831096
Type species: Nanoglomus plukenetiae Corazon-Guivin, G.A. Silva & Oehl, Mycol.
Prog. 18(12): 1398. 2019.
MycoBank MB 831097
Orientoglomus G.A. Silva, Oehl & Corazon-Guivin, Mycol. Prog. 18(12): 1400. 2019.
MycoBank MB 831100
Description: see Silva et al. [
14]
Type species: Orientoglomus emiratium (Błaszk., Kozłowska, Mullath, AlDhaheri & Al-Yahya’ei) G.A. Silva, Oehl & Corazon-Guivin, Mycol. Prog. 18(12): 1403. 2019.
MycoBank MB 831101
Basionym: Dominikia emiratia Błaszk., Kozłowska, Mullath, AlDhaheri & Al-Yahya’ei, Botany 95(7): 632. 2017.
MycoBank MB 819815
Funneliformaceae Oehl, Sieverd. & G.A. Silva, fam. nov.
Mycobank MB 860129
Description: Spores formed within soil or rarely roots, singly or sometimes in sporocarps with one, a few to several spores per sporocarp only; the conspicuous SH is concolorous with spore wall color (or slightly lighter in color), SH is species-specific and generally funnel-shaped to rarely cylindrical. Wall differentiation and pigmentation may continue over long distances from the spore base (often > 50–250 μm), then mycelium generally is hyaline. Pore regularly closed by a conspicuous septum in some distance from spore base, or with some genera at spore base. Septum arises species-specifically from the structural wall layer, from an additional adherent innermost, (semi-)flexible lamina, or from both but not by introverted wall thickening, which is lacking. Forming typical vesicular-arbuscular mycorrhiza, with mycorrhizal structures that stain blue to dark blue with trypan blue.
Type genus: Funneliformis C. Walker & A. Schüssler, emend. Oehl, G.A. Silva & Sieverd.
Funneliformis C. Walker & A. Schüssler, The Glomeromycota—a species list with new families and genera: 13. 2010.
MycoBank MB 542894
Description: see Oehl et al. [
24] and Silva et al. [
14]
Type species: Funneliformis mosseae (T.H. Nicolson & Gerd.) C. Walker & A. Schüssler, The Glomeromycota—a species list with new families and genera: 13. 2010.
MycoBank MB 542895
Basionym: Endogone mosseae T.H. Nicolson & Gerd., Mycologia 60: 314. 1968.
MycoBank MB 330367
≡ Glomus mosseae (T.H. Nicolson & Gerd.) Gerd. & Trappe, Mycol. Mem. 5: 40. 1974.
MycoBank MB 314604
Halonatosporaceae G.A. Silva, Oehl & Sieverd., fam. nov.
Mycobank MB 860134
Description: Spores formed in loose clusters or singly in soil and frequently in roots. SH wall continuous with the SW and for a certain distance concolorous with the SW or slightly lighter in color. Outer SW layer strongly swells in PVLG, forming a halo with radiate columns. SH cylindrical or slightly flared, sometimes slightly constricted at spore base, straight or slightly curved. Pore open or closed by a septum at spore base. SW shows more than one, generally two to three (and up to five) distinct layers. Forming vesicular–arbuscular mycorrhizae, whose fungal structures stain blue to dark blue with trypan blue.
Type genus: Halonatospora Błaszk., Niezgoda, B.T. Goto & Kozłowska
Halonatospora Błaszk., Niezgoda, B.T. Goto & Kozłowska, Botany 96(11): 743. 2018.
MycoBank MB 826963
Description: see Halonatosporaceae, Silva et al. [
14] and Błaszkowski et al. [
42]
Type species: Halonatospora pansihalos (S.M. Berch & Koske) Błaszk., Niezgoda, B.T. Goto & Kozłowska, Can. J. Bot. 96(11): 743 (2018)
MycoBank MB 826964
Basionym: Glomus pansihalos S.M. Berch & Koske. Mycologia 78: 832, 1986.
MycoBank MB 358213
Kamienskiaceae G.A. Silva, Sieverd. & Oehl, Taxonomy 4: 772. 2024.
MycoBank MB 855468
Description: see Silva et al. [
14]
Type genus: Kamienskia Błaszk., Chwat & Kovács
Other genera:
Epigeocarpum Błaszk., B.T. Goto, Jobim, Niezgoda & Marguno,
Microkamienskia Corazon-Guivin, G.A. Silva & Oehl
Kamienskia Błaszk., Chwat & Kovács, Nova Hedwigia 100(1–2): 230. 2014 [2015].
MycoBank MB 808260
Description: see Silva et al. [
14]
Type species: Kamienskia bistrata (Błaszk., D. Redecker, Koegel, Symanczik, Oehl & Kovács) Błaszk., Chwat & Kovács, Nova Hedwigia 100(1–2): 230. 2014 [2015].
MycoBank MB 808261
Basionym: Glomus bistratum Błaszk., D. Redecker, Koegel, Symanczik, Oehl & Kovács,
Botany 87: 267. 2009.
MycoBank MB 512540
Epigeocarpum Błaszk., B.T. Goto, Jobim, Niezgoda & Marguno, Frontiers in Microbiology 12(no. 655910): 10. 2021.
MycoBank MB 838879
Description: see Błaszkowski et al. [
43] and Silva et al. [
14]
Type species: Epigeocarpum crypticum Jobim, Błaszk., Niezgoda, Magurno & B.T. Goto, Frontiers Microbiol. 12(no. 655910): 14. 2021.
MycoBank MB 838880
Microkamienskia Corazon-Guivin, G.A. Silva & Oehl, Nova Hedwigia 109: 359. 2019. MycoBank MB 830814
Description: see Silva et al. [
14]
Type species: Microkamienskia perpusilla (Błaszk. & Kovács) Corazon-Guivin, G.A. Silva & Oehl. Nova Hedwigia 109: 361. 2019.
MycoBank MB 830815
Basionym: Glomus perpusillum Błaszk. & Kovács. Mycologia 101: 249. 2009.
MycoBank MB 512346
≡ Kamienskia perpusilla (Błaszk. & Kovács) Błaszk., Chwat & Kovács. Nova Hedwigia 100: 231. 2015.
MycoBank MB 808264
Oehliaceae Sieverd. & G.A. Silva, fam. nov.
Mycobank MB 860132
Description: Spores formed in loose sporocarps, in clusters or singly in soil and frequently also intraradical singly or in loose sporocarps and clusters. When formed in sporocarps, they are not organized around a central plexus of hyphae. SH wall continuous with the SW and for a certain distance concolorous with the SW or slightly lighter in color. SH cylindrical or seldom slightly funnel-shaped at spore base. Pore at spore base regularly closed by a septum. SW shows more than one, generally two to three (and up to five) distinct layers. Forming vesicular–arbuscular mycorrhizae, fungal structures stain blue to dark blue with trypan blue.
Type genus: Oehlia Błaszk., Kozłowska & Dalpe
Oehlia Błaszk., Kozłowska & Dalpe, Nova Hedwigia 107(3–4): 507. 2018.
MycoBank MB 824689
Description: see Oehliaceae, and Błaszkowski et al. [
44] and Silva et al. [
14] for
Oehlia
Type species: Oehlia diaphana (J.B. Morton & C. Walker) Błaszk., Kozłowska & Dalpe,
Nova Hedwigia 107(3–4): 507. 2018.
MycoBank MB 824693
Basionym: Glomus diaphanum J.B. Morton & C. Walker. Mycotaxon 21: 433, 1984.
MycoBank MB 106161
Rhizoglomeraceae Sieverd., G.A. Silva & Oehl, fam. nov.
Mycobank MB 860131
Description: Spores formed in loose sporocarps, in clusters or singly in soil and frequently also intraradical in loose sporocarps and clusters. When formed in compact sporocarps, they are not organized around a central plexus of hyphae. SH wall continuous with the SW and for a certain distance concolorous with the SW or slightly lighter in color. SH cylindrical or seldom slightly funnel-shaped at spore base. Pore at spore base regularly open, rarely closed by a septum. SW shows more than one, generally two to three (and up to five) distinct layers, of which one or several of the outermost layers may easily separate when pressure is applied to spores. Forming vesicular–arbuscular mycorrhizae, the fungal structures of which stain blue to dark blue in trypan blue.
Type genus: Rhizoglomus Sieverd., G.A. Silva & Oehl
Rhizoglomus Sieverd., G.A. Silva & Oehl, Mycotaxon 129: 377. 2015.
MycoBank MB 803191
Description: see Rhizoglomeraceae and Silva et al. [
14]
Type species: Rhizoglomus intraradices (N.C. Schenck & G.S. Sm.) Sieverd., G.A. Silva & Oehl, Mycotaxon 129(2): 378. 2015 [2014].
MycoBank MB 803192
Basionym: Glomus intraradices N.C. Schenck & G.S. Sm., Mycologia 74: 78. 1982.
MycoBank MB 110704
≡ Rhizophagus intraradices (N.C. Schenck & G.S. Sm.) C. Walker & A. Schüssler, The Glomeromycota—a species list with new families and genera: 19. 2010.
MycoBank MB 542910
Sclerocystaceae Oehl, G.A. Silva, & Sieverd., Taxonomy 4: 770. 2024.
MycoBank MB 855467
Description: see Silva et al. [
14]
Type genus: Sclerocystis Berk. & Broome
Other genera:
Silvaspora Błaszk., Niezgoda, B.T. Goto, Crossay & Magurno,
Parvocarpum Magurno.
Sclerocystis Berk. & Broome, J. Linn. Soc., Bot. 14(no. 74): 137. 1873. [1875].
MycoBank MB20512
Emended description: see Silva et al. [
14]
Type species: Sclerocystis coremioides Berk. & Broome, J. Linn. Soc., Bot. 14(no. 74):
137. 1873. [1875].
MycoBank MB 213141
≡ Glomus coremioides (Berk. & Broome) D. Redecker & J.B. Morton, Mycologia 92:
284. 2000.
MycoBank MB 464612
Parvocarpum Magurno, MycoKeys 107: 283. 2024.
Emended description: see Silva et al. [
14]
Type species: Parvocarpum badium (Oehl, Redecker & Sieverd.) Magurno, MycoKeys 107: 284. 2024.
MycoBank MB 853560
Basionym: Glomus badium Oehl, D. Redecker & Sieverd., J. Appl. Bot. Food Qual. 79: 39. 2005.
MycoBank MB 341387
≡ Funneliformis badius (Oehl, D. Redecker & Sieverd.) C. Walker & A. Schüssler, The Glomeromycota—a species list with new families and genera: 13. 2010.
MycoBank MB 541897
Silvaspora Błaszk., Niezgoda, B.T. Goto, Crossay & Magurno, Frontiers in Microbiology 12(no. 655910): 14. 2021.
MycoBank 838881
Description: see Silva et al. [
14]
Type species: Silvaspora neocaledonica (D. Redecker, Crossay & Cilia) Błaszk., Niezgoda, B.T. Goto, Crossay & Magurno, Frontiers in Microbiology 12(no. 655910): 14 (2021)
MycoBank MB 838882
Basionym: Rhizophagus neocaledonicus D. Redecker, Crossay & Cilia, Mycol. Prog. 17: 739. 2018.
MycoBank MB 820537
≡ Rhizoglomus neocaledonicum (D. Redecker, Crossay & Cilia) Oehl, A. Turrini & Giovann., Mycol. Prog. 17: 1216 (2018).
MycoBank MB 827095
Septoglomeraceae Oehl, G.A. Silva, Sieverd., Taxonomy 4: 768. 2024.
Mycobank MB 855466
Emended description: Spores formed in soil and sometimes in roots, terminally on or intercalarily in hyphae, either singly, in loose spore clusters or in sporocarps. Spores with one mono- to multiple-layered wall. SH wall conspicuously continuous and concolorous with the SW or slightly lighter in color than the SW; SH funnel-shaped, cylindrical or constricted, rarely inflated; straight, curved or flared; forming typical vesicular–arbuscular mycorrhiza with mycorrhizal structures that stain blue to dark blue with trypan blue.
Type genus: Septoglomus Sieverd., G.A. Silva & Oehl
Other genera:
Blaszkowskia G.A. Silva & Oehl,
Funneliglomus Corazon-Guivin, G.A. Silva & Oehl,
Melanoglomus B.T. Goto, Błaszk., Sieverd., G.A. Silva & Oehl,
Microviscospora Oehl, Corazon-Guivin, Błaszk., B.T. Goto, Sieverd. & G.A. Silva,
Viscospora Sieverd., Oehl & G.A. Silva.
Septoglomus Sieverd., G.A. Silva & Oehl, Mycotaxon 116: 105. 2011.
MycoBank MB 518436
Emended description: see Oehl et al. [
24]
Type species: Septoglomus constrictum (Trappe) Sieverd., G.A. Silva & Oehl. Mycotaxon 116: 105. 2011.
MycoBank MB 518462
Basionym: Glomus constrictum Trappe, Mycotaxon 6: 361. 1977.
MycoBank MB 314589
≡ Funneliformis constrictus (Trappe) C. Walker & A. Schüssler, The Glomeromycota— a species list with new families and genera: 14. 2010.
MycoBank MB 542904
Blaszkowskia G.A. Silva & Oehl, Mycol. Prog. 22(11, no. 74): 5. 2023.
MycoBank MB 847414
Description: see Silva et al. [
14]
Type species: Blaszkowskia deserticola (Trappe, Bloss & J.A. Menge) Oehl & G.A.
Silva. Mycol. Prog. 22(11, no. 74): 5. 2023.
MycoBank MB 847415
Basionym: Glomus deserticola Trappe, Bloss & J.A. Menge, Mycotaxon 20: 123. 1984.
MycoBank MB 106847
≡ Septoglomus deserticola (Trappe, Bloss & J.A. Menge) G.A. Silva, Oehl & Sieverd.
Mycotaxon 116: 106. 2011.
MycoBank MB 518463
Funneliglomus Corazon-Guivin, G.A. Silva & Oehl, Sydowia 71: 19. 2019.
MycoBank MB 829266
Description: see Silva et al. [
14]
Type species: Funneliglomus sanmartinense Corazon-Guivin, G.A. Silva & Oehl, Sydowia 71: 21. 2019
MycoBank MB 830216
Melanoglomus B.T. Goto, Błaszk., Sieverd., G.A. Silva & Oehl, Mycol. Prog. 2025. In review.
MycoBank MB 857542
Description: see Goto et al. [
45]
Type species: Melanoglomus titan (B.T. Goto & G.A. Silva) B.T. Goto, Błaszk., Sieverd., G.A. Silva & Oehl
MycoBank MB 857543
Basionym: Septoglomus titan B.T. Goto & G.A. Silva, Mycotaxon 124: 105. 2013.
MycoBank MB564321
Microviscospora Oehl, Corazon-Guivin, Błaszk., B.T. Goto, Sieverd. & G.A. Silva, Mycol. Prog. 2025. In review.
MycoBank MB 857544
Description: see Goto et al. [
45]
Type species: Microviscospora peruviscosa Oehl, Corazon-Guivin, Błaszk., B.T. Goto, Sieverd. & G.A. Silva
MycoBank MB 857544
Basionym: Viscospora peruviscosa Corazon-Guivin, G.A. Silva & Oehl, J. Appl. Bot. Food Quality - Angew. Botan. 96: 118. 2023.
MycoBank MB 857545
Viscospora Sieverd., Oehl & G.A. Silva, Mycotaxon 116: 108. 2011.
MycoBank MB 518439
Emended description: see Goto et al. [
45]
Type species: Viscospora viscosa (T.H Nicolson) Sieverd., Oehl & G.A. Silva, Mycotaxon
116: 108. 2011.
MycoBank MB 518471
Basionym: Glomus viscosum T.H. Nicolson, Mycol. Res. 99: 1502. 1995.
MycoBank MB 413125
≡ Septoglomus viscosum (T.H. Nicolson) C. Walker, D. Redecker, D. Stiller & A. Schüssler, Mycorrhiza 23: 524. 2013
MycoBank MB 550089
Acaulosporales Sieverd., Silva G.A. & Oehl, ord. nov.
Mycobank MB 860126
Description: Spores formed laterally on or intrahyphally within hyphal stalk of a terminally or intercalary formed sporiferous saccule, in same distance to the sporiferous saccule termini, or rarely even within the inflated sporiferous saccule terminus; spores have three walls: an outer spore wall, a middle wall and an inner wall. One or a few layers of the outer spore wall are continuous with the wall of the stalk and the sporiferous saccule. The outer hyaline to subhyaline layers are often evanescent, the inner layers of the outer spore wall are permanent. When the connections of the hyphal stalk break off, the spore appears with one to two cicatrices which are closed by the permanent sublayers of the inner layers of the outer spore wall. The middle wall is thin and flexible. The inner wall consists of several thin layers of which the outer layer is ornamented having a characteristic' beaded' appearance. The second layer of the inner wall often stains deep purple in Melzer's reagent. The inner wall functions as germinal wall, a germination orb may be formed on the outer surface. Forming vesicular-arbuscular mycorrhizae whose fungal structures in the roots stain significantly blue with trypan blue.
Type family: Acaulosporaceae J.B. Morton & Benny, Mycotaxon 37: 479. 1990.
Mycobank MB 82037
Emended description; see description for the order here, directly above.
Type genus: Acaulospora Gerd. & Trappe
Other genus:
Kuklospora Oehl & Sieverd., J. Appl. Bot. Food Qual. 80: 74. 2006.
Mycobank MB 29042, current synonym of Acaulospora
Acaulospora Gerd. & Trappe, Mycologia Memoirs 5: 31. 1974.
Mycobank MB 20003
Emended description; see description for the order here directly above.
Type species: Acaulospora laevis Gerd. & Trappe, Mycologia Memoirs 5: 33. 1974.
MycoBank MB 308078
Diversisporales C. Walker & A. Schüssler, Mycological Research 108 (9): 981. 2004.
Mycobank MB 90593
Emended description: Spore formation diversisporoid, otosporoid, or tricisporoid, redeckeroid or (para-)corymbiglomoid. Diversisporoid spores formed singly, in clusters, or in disorganized sporocarps with up to very high spore numbers (up to several hundreds). In pigmented spores, SH conspicuously change color, becoming hyaline to white behind the septum, (immediately or at a very short distance from this septum); SH generally straight, cylindrical, in some species constricted or inflated. Spores with 13 wall layers; pore often closed with a septum that may arise from innermost wall lamina, an overlaying laminate layer, or from both; SH pore rarely open. Otosporoid and tricisporoid spores with two multiple-layered walls; otosporoid spores formed laterally on the persistent neck of a terminal or intercalary sporiferous saccule at some distance from the saccule terminus; spore pore generally closed by a septum at spore base. Tricisporoid spores formed within the evanescent neck of a tightly attached terminal or intercalary sporiferous saccule, closely attached to the saccule terminus which is often smaller in size than the mature spores attached, rarely equal in size; tricisporoid spores with two cicatrices formed by the outer wall pigmented structural layer. Para-)corymbiglomoid spores include several generic clades of Glomeromycetes fungi, such as Corymbiglomus, Paracorymbiglomus and Sieverdingia species forming mono-to-bi-walled glomoid-like spores, either corymbiforme or with a dense hyphal mantle on cylindrical SH, or Desertispora, forming glomoid-diversisporoid-like spores staining purple on the spore wall, which is not known for Diversispora species, or redeckeroid spores, which form mono-walled spores disorganized in large and compact sporocarps, containing hundreds to thousands of spores per sporocarp; spores, with 2 to rarely 3 wall layers; SH generally broad at spore base and with a conspicuous, thick and broad septum that arises from the inner lamina of the structural wall layer; structural layer generally continue over very short distances into SH; SWL1 fragile, usually inflating in a short distance to the spore base where SWL2 becomes invisible in the SH (= characteristically ‘redeckeroid’).
Type family: Diversisporaceae C. Walker & A. Schüssler
Other family: Corymbiglomeraceae G.A. Silva, Sieverd. & Oehl.
Diversisporaceae C. Walker & A. Schüssler, Mycological Research 108 (9): 981. 2004.
Mycobank MB 82125
Emended description: Spore formation diversisporoid, otosporoid, or tricisporoid. Diversisporoid spores formed singly, in clusters, or in disorganized sporocarps with up to very high spore numbers (up to several hundreds). In pigmented spores, SH conspicuously change color, becoming hyaline to white behind the septum, (immediately or at a very short distance from this septum); SH generally straight, cylindrical, in some species constricted or inflated. Spores with 1–3 wall layers; pore often closed with a septum that may arise from innermost wall lamina, an overlaying laminate layer, or from both; SH pore rarely open. Otosporoid and tricisporoid spores with two multiple-layered walls; otosporoid spores formed laterally on the persistent neck of a terminal or intercalary sporiferous saccule at some distance from the saccule terminus; spore pore generally closed by a septum at spore base. Tricisporoid spores formed within the evanescent neck of a tightly attached terminal or intercalary sporiferous saccule, closely attached to the saccule terminus which is often smaller in size than the mature spores attached, rarely equal in size; tricisporoid spores with two cicatrices formed by the outer wall pigmented structural layer.
Type genus: Diversispora C. Walker & A. Schüssler
Other genera:
Otospora Oehl, Palenzuela & N. Ferrol, Mycologia 100(2): 297. 2008.
Mycobank MB 506746, current synonym of Diversispora
Tricispora Oehl, Sieverd., G.A. Silva & Palenz., Mycotaxon 117: 310. 2012.
Mycobank MB 561642, current synonym of Diversispora
Diversispora C. Walker & A. Schüssler, Mycol. Res. 108 (9): 982. 2004.
Mycobank MB 28884
Emended description: see description for the family Diversisporaceae here, directly above.
Type species: Diversispora spurca (C.M. Pfeiff., C. Walker & Bloss) C. Walker & A. Schüssler, Mycological Research 108 (9): 982. 2004.
Mycobank MB 487795
Basionym: Glomus spurcum C.M. Pfeiff., C. Walker & Bloss, Mycotaxon 59: 374. 1996.
Mycobank MB 415789
Corymbiglomeraceae G.A. Silva, Sieverd. & Oehl fam. nov.
Mycobank MB 860135
Description: This new family includes several generic clades of Glomeromycetes fungi, such as Corymbiglomus, Paracorymbiglomus and Sieverdingia species forming mono-to-bi-walled glomoid-like spores, either corymbiforme or with a dense hyphal mantle on cylindrical SH, or Desertispora, forming glomoid-diversisporoid-like spores staining purple on the spore wall, which is not known for Diversispora species, or Redeckera species, which form mono-walled spores disorganized in large and compact sporocarps, containing hundreds to thousands of spores per sporocarp; spores, with 2 to rarely 3 wall layers; SH generally broad at spore base and with a conspicuous, thick and broad septum that arises from the inner lamina of the structural wall layer; structural layer generally continue over very short distances into SH; SWL1 fragile, usually inflating in a short distance to the spore base where SWL2 becomes invisible in the SH (= characteristically ‘redeckeroid’).
Type genus: Corymbiglomus (Błaszk. & Chwat) emend. Błaszk., Niezgoda & B.T.Goto
Other genera:
Desertispora Błaszk., Kozłowska, Ryszka, Al-Yahya’ei & Symanczik,
Paracorymbiglomus Błaszk., Niezgoda & B.T. Goto,
Redeckera C. Walker & A. Schüssler,
Sieverdingia Błaszk., Niezgoda & B.T. Goto.
Corymbiglomus Błaszk. & Chwat, Glomeromycota 1: 272. 2012.
Mycobank MB 564566
Description: see Błaszkowski [
46],
Emended description: Błaszkowski et al. [
37,
39]
Type species: Corymbiglomus corymbiforme (Blaszk.) Błaszk. & Chwat, The Glomeromycota 1: 274. 2012.
Mycobank MB 564567
Basionym: Glomus corymbiforme Blaszk., Mycologia 87(5): 732. 1995.
Mycobank MB 413122
Desertispora Błaszk., Kozłowska, Ryszka, Al-Yahya’ei & Symanczik, Mycological Progress 17 (4): 444. 2018.
Mycobank MB 823563
Description: see Symanczik et al. [
47]
Type species: Desertispora omanana (Symanczik, Błaszk. & Al-Yahya’ei) Symanczik, Błaszk., Kozłowska & Al-Yahya’ei, Mycological Progress 17 (4): 444. 2018.
Mycobank MB 830800
Basionym: Diversispora omanana Symanczik, Błaszk. & Al-Yahya’ei, Mycologia 106 (2): 247. 2014.
Mycobank MB 830799
Paracorymbiglomus Błaszk., Niezgoda & B.T. Goto, Mycokeys 117: 178. 2025.
Mycobank MB 858391
Description: see Błaszkowski et al. [
39]
Type species: Paracorymbiglomus globiferum (Koske & C. Walker) Błaszk., Niezgoda & B.T. Goto, MycoKeys 117: 178. 2025.
MycoBank No: 858393
Basionym: Glomus globiferum Koske & C. Walker, Mycotaxon 26. 133. 1986.
Synonym: Corymbiglomus globiferum (Koske & C. Walker) Błaszk. & Chwat, Acta Mycol. 48 (1): 99. 2013.
MycoBank No 622179
Redeckera C. Walker & A. Schüssler, The Glomeromycota—a species list with new families and new genera: 44. 2010.
MycoBank MB 542402
Description: see Oehl et al. [
24]
Type species: Redeckera megalocarpa (D. Redecker) C. Walker & A. Schüssler, The Glomeromycota—a species list with new families and new genera: 44. 2010.
MycoBank MB 542403
Basionym: Glomus megalocarpum D. Redecker, Mycological Progress 6 (1): 38. 2007.
Mycobank MB 529715
Sieverdingia Błaszk., Niezgoda & B.T. Goto, Mycological Progress 18 (11): 1368. 2019.
Mycobank MB 832298
Description: see Błaszkowski et al. [
48]
Type species: Sieverdingia tortuosa (N.C. Schenck & G.S. Sm.) Błaszk., Niezgoda & B.T. Goto, Mycological Progress 18 (11): 1369. 2019.
MycoBank MB 832299
Basionym: Glomus tortuosum N.C. Schenck & G.S. Sm., Mycologia 74 (1): 83. 1982.
MycoBank MB 110706
Entrophosporales Błaszk., Sánchez-García, B.T. Goto & Magurno, Frontiers in Microbiology 13 (no. 962856): 10. 2022.
MycoBank MB 846043
Description: see Błaszkowski et al. [
11] and Silva et al. [
15]
Type family: Entrophosporaceae Oehl & Sieverd.
Entrophosporaceae Oehl & Sieverd., J. Appl. Bot. Food Qual. 80: 73. 2006.
MycoBank MB 521877
Description: see Błaszkowski et al. [
11] and Silva et al. [
15]
Type genus: Entrophospora R.N. Ames & R.W. Schneid.
Other genera:
Albahypha Oehl, G.A. Silva, B.T. Goto & Sieverd.,
Alborhynchus Oehl, B.T. Goto, Corazon-Guivin, Sieverd. & G.A. Silva
Entrophospora R.N. Ames & R.W. Schneid., Mycotaxon 8 (2): 347. 1979.
MycoBank MB 20223
Emended description: see Błaszkowski et al. [
11] and Silva et al. [
15]
Type species: Entrophospora infrequens (I.R. Hall) R.N. Ames & R.W. Schneid., Mycotaxon 8 (2): 348. 1979.
Mycobank MB 313899
Basionym: Glomus infrequens I.R. Hall, Transactions of the British Mycological Society 68 (3): 345. 1977.
MB 314599
Albahypha Oehl, G.A. Silva, B.T. Goto & Sieverd., Mycotaxon 117: 308. 2011.
Mycobank MB 561639
Emended description: see Silva et al. [
15]
Type species: Albahypha drummondii (Blaszk. & Renker) Sieverd., Oehl, B.T. Goto & G.A. Silva, Mycotaxon 117: 308. 2012.
Mycobank MB 561640
Basionym: Glomus drummondii Blaszk. & Renker, Mycological Research 110 (5): 559. 2006.
Mycobank MB 510049
≡ Entrophospora drummondii (Błaszk. & Renker) Błaszk., Niezgoda, B.T. Goto & Magurno, Frontiers in Microbiology 13 (no. 962856): 13. 2022.
Mycobank MB 836247
Alborhynchus Oehl, B.T. Goto, Corazon-Guivin, Sieverd. & G.A. Silva, J Fungi 11(2, no 97): 6. 2025.
Mycobank MB 856948
Description: see Silva et al. [
15]
Type species: Alborhynchus walkeri (Blaszk. & Renker) Oehl, B.T. Goto, Corazon-Guivin, Sieverd. & G.A. Silva, J Fungi 11(2, no 97): 7. 2025.
Mycobank MB 856949
Basionym: Glomus walkeri Blaszk. & Renker, Mycological Research 110 (5): 563. 2006.
Mycobank MB 510050
≡ Entrophospora walkeri (Błaszk. & Renker) Błaszk., Niezgoda, B.T. Goto & Magurno, Frontiers in Microbiology 13 (no. 962856): 13. 2022.
Mycobank MB 836252
Gigasporales Sieverd., G.A. Silva, B.T. Goto & Oehl, Mycotaxon 116: 373. 2011.
MycoBank MB 519688
Description: see Oehl et al. [
4]
Type family: Gigasporaceae Morton & Benny
Other families:
Dentiscutataceae F.A. Souza, Oehl & Sieverd.,
Intraornatosporaceae B.T. Goto & Oehl,
Racocetraceae Oehl, Sieverd. & F.A. Souza,
Scutellosporaceae Sieverd., F.A. Souza & Oehl.
Gigasporaceae Morton & Benny, Mycotaxon 37: 483. 1990.
MycoBank MB 82038
Description: see Morton & Benny [
9]
Emended description: see Oehl et al. [
49] for Gigasporaceae
Type genus: Gigaspora Gerd. & Trappe, Mycologia Memoirs 5: 25. 1974.
MycoBank MB 20239
Type species: Gigaspora gigantea (T.H. Nicolson & Gerd.) Gerd. & Trappe, Mycologia Memoirs 5: 29. 1974.
MycoBank MB 314484
Basionym: Endogone gigantea T.H. Nicolson & Gerd., Mycologia 60 (2): 321. 1968.
MycoBank MB 330364
Scutellosporaceae Sieverd., F.A. Souza & Oehl, Mycotaxon 106. 330. 2009 [2008].
Mycobank MB 511945
Emended description: Sporocarps are unknown. Spores generally singly formed on bulbous sporogenous cells that are formed terminally on subtending hyphae (=sporogenous hyphae; SH) that arise from mycelia hyphae in soil. Spores have 3 walls, an outer, a middle and an inner, germinal wall. Outer wall with 3 or several layers, middle wall with 1–2 layers, and inner wall with 2-4. A single germination shield, rarely two shields, are formed on the outer surface or between the outer and the subsequent layer of the inner wall. Germination shields are transparent, or hyaline to subhyaline, seldom light yellow, generally bi-lobed, sometimes mono-lobed; often violin-shaped or oval to ovoid, rarely cardioid, circular or coiled; only a few folds cover the shield surface where in general one germ tube initiation (gti) is formed per lobe. Generally from one gti, seldom from both gti, a germ tube arises and penetrates the outer spore walls. SH form one to several septa in some distance to the sporogenous cells. Forming typical arbuscular mycorrhizae without vesicle formation in roots; knobby extraradical auxiliary cells branching from the hyphal mycelium without spines on the surface.
Type genus: Scutellospora C. Walker & F.E. Sanders
Other genera:
Bulbospora Oehl & G.A. Silva,
Orbispora Oehl, G.A. Silva & D.K. Silva.
Scutellospora C. Walker & F.E. Sanders, Mycotaxon 27: 179. 1986.
Mycobank MB 25074
Emended description: Sporocarps unknown. Spores formed on sporogenous cells that form terminally on a hypha which arises from mycelia hyphae in soil. Outer spore wall generally is three-layered and continuous with the wall of the sporogenous cell. Outer layer of the outer spore wall generally rigid, second layer laminate and third layer thin, often membranous, tightly adherent to the laminate layer and thus, often difficult to observe. Pore between the spore and sporogenous cell is narrow and usually closed by a plug formed by spore wall material. Two hyaline walls (‘MW’ and ‘IW’) form de novo during spore formation and have 1–2 and 2–4 layers, respectively. The IW is two to three-layered forming a germination shield on its outer surface or between the outer and the subsequent layer of IW. Germination shield is transparent, or hyaline to subhyaline, seldom light yellow, bi-lobed; often violin-shaped to oval to ovoid to more rarely cardioids or coiled and then, either circular or apparently broad ellipsoid to irregular; only a few folds cover the shield surface where 1–2 rounded germ tube initiations (‘gti’, about 2–4 μm in diam) are visible from where the germ tubes arise which penetrate the outer spore wall layers. Sometimes one to a few so-called false lobes are visible, which never bear a gti, and usually develop artificially by pressure on the spore, e.g., through pressure on the cover slides under the microscope; mycelia hyphae form one to several septa in some distance to the sporogenous cells. Auxiliary cells in the hyphal mycelium, as far as known, are knobby without spines on the surface. Forming typical arbuscular mycorrhizae without vesicle formation in roots; knobby extraradical auxiliary cells branching from the hyphal mycelium without spines on the surface.
Type species: Scutellospora calospora (T.H. Nicolson & Gerd.) C. Walker & F.E. Sanders, Mycotaxon 27: 180. 1986.
Mycobank MB 128413
Basionym: Endogone calospora T.H. Nicolson & Gerd., Mycologia 60 (2): 322. 1968.
Mycobank MB MB 330363
≡ Gigaspora calospora (T.H. Nicolson & Gerd.) Gerd. & Trappe, Mycologia Memoirs 5: 28. 1974.
Mycobank MB 314482
Bulbospora Oehl & G.A. Silva, Sydowia 66. 315. 2014.
Mycobank MB 809231
Description: see Marinho et al. [
50]
Type species: Bulbospora minima Oehl, Marinho, B. T. Goto & G. A. Silva, Sydowia 66. 315. 2014.
MycoBank MB 809232
Orbispora Oehl, G.A. Silva & D.K. Silva, Mycotaxon 116. 163. 2011.
MycoBank MB 519533
Emended description: Sporocarps unknown. Spores 100-300 µm, formed on sporogenous cells that form terminally on hyphae arising from mycelia in soil. Outer spore wall (OW) generally (2–)3-layered and continuous with the wall of the sporogenous cell. Two hyaline walls (‘MW’ and ‘IW’) form de novo during spore formation and have 1–2 and 2–3 layers, respectively. A germination orb is formed on the outer IW surface or between the outer and the subsequent layer of IW. Germination orb is transparent, or hyaline to subhyaline, seldom light yellow, mono-lobed; coiled and then, either circular or apparently broad ellipsoid to rarely irregular; with one rounded germ tube initiation in the outer periphery of the lobe. One (rarely two) germ tube arises from this gti to penetrate the outer spore wall layers.
Type species: Orbispora pernambucana (Oehl, D.K. Silva, N. Freitas & L.C. Maia) Oehl, G.A. Silva & D.K. Silva, Mycotaxon 116. 166. 2011.
MycoBank MB 519535
Basionym: Scutellospora pernambucana Oehl, D.K. Silva, N. Freitas & L.C. Maia, Mycotaxon 106: 363. 2009 [2008].
MycoBank MB 512130
Racocetraceae Oehl, Sieverd. & F.A. Souza, Mycotaxon 106: 333. 2009 [2008].
MB 511946
Description: see Oehl et al. [
49]
Type genus: Racocetra Oehl, F.A. Souza & Sieverd.
Other genus: Cetraspora Oehl, F.A. Souza & Sieverd.
Racocetra Oehl, F.A. Souza & Sieverd., Mycotaxon 106: 334. 2009 [2008].
MB 511947
Description: see Oehl et al. [
49]
Type species: Racocetra coralloidea (Trappe, Gerd. & I. Ho) Oehl, F.A. Souza & Sieverd., Mycotaxon 106: 336. 2009 [2008].
MB 511948
Basionym: Gigaspora coralloidea Trappe, Gerd. & I. Ho, Mycologia Memoir No. 5:
30. 1974.
≡ Scutellospora coralloidea (Trappe, Gerd. & I. Ho) C. Walker & F.E. Sanders, Mycotaxon 27: 181. 1986.
Cetraspora Oehl, F.A. Souza & Sieverd., Mycotaxon 106: 337. 2009 [2008].
MB 511957
Description: see Oehl et al. [
49]
Type species: Cetraspora gilmorei (Trappe & Gerd.) Oehl, F.A. Souza & Sieverd., Mycotaxon 106: 338. 2009 [2008].
MycoBank MB 511958
Basionym: Gigaspora gilmorei Trappe & Gerd., Mycologia Memoir No. 5: 27. 1974.
≡ Scutellospora gilmorei (Trappe & Gerd.) C. Walker & F.E. Sanders, Mycotaxon 27: 181. 1986.
Dentiscutataceae F.A. Souza, Oehl & Sieverd., Mycotaxon 106. 340. 2009 [2008].
MycoBank MB 511962
Description: see Oehl et al. [
49]
Type genus: Dentiscutata Sieverd., F.A. Souza & Oehl
Other genera:
Fuscutata Oehl, F.A. Souza & Sieverd.,
Quatunica F.A. Souza, Sieverd. & Oehl.
Dentiscutata Sieverd., F.A. Souza & Oehl, Mycotaxon 106. 340. 2009 [2008].
MycoBank MB 511968
Description: see Oehl et al. [
49]
Type species: Dentiscutata nigra (J.F. Redhead) Sieverd., F.A. Souza & Oehl, Mycotaxon 106. 342. 2009 [2008].
MycoBank MB 511969
Basionym: Gigaspora nigra J.F. Redhead, Mycologia 71: 187. 1979.
MycoBank MB 314489
≡ Scutellospora nigra (J.F. Redhead) C. Walker & F.E. Sanders, Mycotaxon 27: 181. 1986.
MycoBank MB 128421
Fuscutata Oehl, F.A. Souza & Sieverd., Mycotaxon 106. 342. 2009 [2008].
MycoBank MB 511963
Description: see Oehl et al. [
49]
Type species: Fuscutata heterogama Oehl, F.A. Souza, L.C. Maia & Sieverd., Mycotaxon 106. 342. 2009 [2008].
MycoBank MB 511964
Quatunica F.A. Souza, Sieverd. & Oehl, Mycotaxon 106. 347. 2009 [2008].
MycoBank MB 511976
Description: see Oehl et al. [
49]
Type species: Quatunica erythropus (Koske & C. Walker) F.A. Souza, Sieverd. & Oehl, Mycotaxon 106. 348. 2009 [2008].
MycoBank MB 511977
Basionym: Gigaspora erythropus Koske & C. Walker, Mycologia 76: 250. 1984.
MycoBank MB 318956
≡ Scutellospora erythropus (Koske & C. Walker) C. Walker & F.E. Sanders, Mycotaxon 27: 181. 1986.
MycoBank MB318960
Intraornatosporaceae B.T. Goto & Oehl, Mycotaxon 119: 121. 2012.
MycoBank MB 563599
Description: see Goto et al. (2012)
Type genus: Intraornatospora B.T. Goto, Oehl & G.A. Silva, Mycotaxon 119: 122. 2012.
Other genus: Paradentiscutata B.T. Goto, Oehl & G.A. Silva
Intraornatospora B.T. Goto, Oehl & G.A. Silva, Mycotaxon 119: 122. 2012.
MycoBank MB 563600
Description: see Goto et al. [
51]
Type species: Intraornatospora intraornata (B.T. Goto & Oehl) B.T. Goto, Oehl & G.A. Silva, Mycotaxon 119: 122. 2012.
MycoBank MB 563601
Basionym: Racocetra intraornata B.T. Goto & Oehl, Mycotaxon 109: 485. 2009.
MycoBank MB 513428
Paradentiscutata B.T. Goto, Oehl & G.A. Silva, Mycotaxon 119: 122. 2012.
MycoBank MB 563602
Description: see Goto et al. [
51]
Type species: Paradentiscutata bahiana Oehl, Magna, B.T. Goto & G.A. Silva, Mycotaxon 119: 123. 2012.
MycoBank MB 563603
Pacisporales Oehl, Sieverd., G.A. Silva, ord. nov.
MycoBank MB 860128
Description: Spores formed singly in soils or rarely in roots, terminally on SH, with two spore walls (OW & IW), while only the outer wall is continuous with the mycelia and SH, but the inner wall forms de novo during spore differentiation. Spore pore usually is closed by a septum at the spore base arising from the inner wall layers of the outer spore wall. IW functions as germinal wall; germ tubes emerge from the germinal wall directly or from the center of a multiple-lobed germination structure consisting of several lobes positioned around this center. Forming typical vesicular-arbuscular mycorrhizal fungi, whose fungal structures in the roots stain significantly blue with trypan blue, and additionally extraradical auxiliary cells in the rhizosphere of mycorrhizal roots, rarely intraradically, as also known for Gigasporales.
Type family: Pacisporaceae C. Walker, Błaszk., A. Schüssler & Schwarzott, Mycol. Res. 108 (9): 981. 2004.
MycoBank MB 82126
Emended description: see above for Pacisporales
Type genus: Pacispora Sieverd. & Oehl
Pacispora Sieverd. & Oehl, J. Appl. Bot. 78: 74. 2004.
MycoBank MB 28842
Emended description: see above for Pacisporales
Type species: Pacispora scintillans (S.L. Rose & Trappe) Sieverd. & Oehl ex C. Walker, Vestberg & A. Schüssler, Mycological Research 111 (3): 254. 2007.
MycoBank MB 510506
Basionym: Glomus scintillans S.L. Rose & Trappe, Mycotaxon 10 (2): 417. 1980.
MycoBank MB 113776
Sacculosporales Silva G.A., Sieverd. & Oehl, ord. nov
Mycobank MB 860127
Description: Sporocarps unknown. Spores formed within the hyphal neck of closely adherent terminal or intercalary formed sporiferous saccules. Spores have two walls: outer and inner. At least two layers (including the outer wall structural layer) are continuous with the sporiferous saccule wall. After the hyphal neck connections break off, spores show two, often opposite, cicatrices that are closed by the permanent sublayers of the outer wall structural layer. Inner wall forms de novo. Inner wall consists of several layers, none of which has a granular, 'beaded' appearance, and does not stain in Melzer’s reagent.
Type family: Sacculosporaceae Oehl, Sieverd., G.A. Silva, B.T. Goto, I.C. Sánchez & Palenzuela, Mycotaxon 117: 310. 2012.
MycoBank MB 561645
Emended description: see above for the order Sacculosporales and Willis et al. [
52]
Type genus: Sacculospora Oehl, Sieverd., G.A. Silva, B.T. Goto, I.C. Sánchez & Palenzuela
Sacculospora Oehl, Sieverd., G.A. Silva, B.T. Goto, I.C. Sánchez & Palenzuela, Mycotaxon 117: 311. 2011.
MycoBank MB 561646
Description: see above for the order Sacculosporales and Willis et al. [
52]
Type species: Sacculospora baltica (Blaszk., Madej & Tadych) Oehl, Palenzuela, I.C. Sánchez, B.T. Goto, G.A. Silva & Sieverd., Mycotaxon 117: 311. 2012.
MycoBank MB 561647
Basionym: Entrophospora baltica Blaszk., Madej & Tadych, Mycotaxon 68: 167. 1998.
Mycobank MB 444868