1. Introduction
Cervical intraepithelial neoplasia (CIN) remains one of the most common forms of precancerous lesions of the cervix. CIN is a clinically significant but often diagnostically complex category of gynecological pathology. CIN has the high grade of morphological, colposcopic, and molecular heterogeneity, which makes it difficult to stratify risk early and justify individualized patient management tactics. In most cases, CIN 1 is prone to spontaneous regression within 12-24 months [
1,
2,
3], whereas dysplasia of high grades (CIN 2 and CIN 3) is associated with the significantly higher risk of transformation into invasive cervical cancer [
4,
5]. In recent years, there has been the paradigm shift towards the organ-preserving personalized approach to the treatment of this pathology, especially in young women with preserved reproductive potential and the absence of high-risk factors [
6].
Cases of persistent and recurrent CIN are of particular clinical importance. In these cases, neoplastic changes persist or reappear after treatment. Such forms can occur both in the presence and absence of persistent HPV infection [
7] and are often associated with an increased risk of therapeutic failure, re-progression, and malignant transformation [
8,
9,
10]. Against this background, there is an increasing need for verified morphological and molecular markers that can improve the accuracy of predicting the clinical behavior of neoplasia and help in choosing a personalized follow-up strategy.
The vast majority of diagnostic approaches are focused primarily on epithelial changes, despite the development of cytological, colposcopic and biopsy methods. At the same time, the subepithelial stroma remains significantly underestimated diagnostically. Meanwhile, the extracellular matrix (ECM) of the stroma is not only the architectural framework, but also the actively regulating element of tissue homeostasis. It controls proliferation, migration, apoptosis, and differentiation of epithelial cells by mediating signaling interactions between stroma and epithelium. Modifications of the extracellular matrix composition and mechanical properties during carcinogenesis are crucial for the onset and progression of a tumor [
11,
12]. In addition, the restructuring of the extracellular matrix is an important element of chronic inflammation and epithelial-stromal interaction, i. e. processes of critical importance for CIN persistence [
13,
14]. Accumulated data demonstrate that changes in the microenvironment of the cervical stroma include increased activity of matrix metalloproteinases, which leads to excessive degradation of collagen [
15,
16,
17,
18,
19]. Such structural and molecular changes contribute to the preservation and prolongation of adverse changes, which can lead to stable changes in the extracellular matrix.
The study aim was the morphological assessment and comparative analysis of histological patterns of the extracellular matrix of the cervical stroma in normal conditions, at CIN of varying severity, as well as at the persistence or progression of the disease in repeated biopsies.
2. Materials and Methods
2.1. Tissue Samples and Selection of the Study Group
This single-center retrospective cohort study consistently included biopsy and surgical cervical samples from women of reproductive age with cervical intraepithelial neoplasia (CIN) 1-3. The samples were obtained as the result of diagnostic or therapeutic intervention in Karaganda Regional Clinical Hospital, the Clinic of Karaganda Medical University and Multidisciplinary Center for Mother and Child of Temirtau (Kazakhstan) from January 2017 to December 2024.
The exclusion criteria were as follows: 1) the histological diagnosis other than CIN 1-3 (for example, invasive carcinoma, cervical ectopia); 2) the presence of expressed artifact damage in the area of interest (for example, the absence of the subepithelial stroma <3 mm); 3) expressed inflammatory or necrotic changes that interfere with morphological assessment; 4) previous destructive cervical therapy (laser vaporization, diathermocoagulation, cryodestruction or photodynamic treatment) until the time of the test material receiving; 5) lack of subsequent clinical follow-up within ≥6 months after the initial biopsy or excision intervention; 6) unavailability of clinical data on HPV-status or the results of follow-up examinations (cytology, colposcopy, histology); 7) refusal of the patient from further observation or treatment, as well as the severe somatic condition that prevents standard management tactics.
All samples included in the study were independently re-examined by two experienced pathologists (with experience in gynecological pathology). These pathologists acted independently, blindly, and did not know the clinical history. All samples were anonymized prior to the start of the study.
The sample size was calculated using standard statistical methods [
20] and verified using G*Power software, version 3.1 (Heinrich-Heine-Universität Düsseldorf, Germany) [
21].
The control group (defined as the relative physiological norm) included cervical tissue samples obtained during routine gynecological procedures and autopsies without morphological signs of inflammatory, dysplastic, or neoplastic changes.
The CIN 1-3 groups included women of reproductive age (18-49 years old) who underwent the planned diagnostic or therapeutic biopsy of the cervix in a hospital at the presence of histologically confirmed CIN, diagnosed according to the current classification [
22,
23].
The study included 120 patients with CIN 1, CIN 2, and CIN 3 who regularly underwent follow-up cytological examination and HPV-testing. None of these patients have been vaccinated.
Follow-up was performed at 6-month intervals using cytology in the first year and annually thereafter.
CIN persistence was defined as the histologically confirmed presence of CIN of the same grade upon repeated biopsy or excision within 6-12 months after the initial diagnosis, with no signs of remission during interim clinical or cytological follow-up or cytological verification of LSIL/HSIL (for CIN 1)/HSIL (for CIN 2).
The progression of CIN was defined as the increase in the grade of dysplasia (for example, from CIN 1 to CIN 2 or 3) according to repeated histological examination during the follow-up period.
No subsequent confirmation: cases without repeated biopsy or clinical cytological follow-up for ≥12 months were classified as under-controlled and excluded from the analysis.
HPV-testing was performed at 6-month intervals in the first year after treatment and annually thereafter.
2.2. Clinical Data
Clinical data was collected from the patients' medical records using software in an integrated medical information system. The following clinical data were collected: clinical and pathological data, including а patient's age, body mass index, type of pathology, HPV-strain, subsequent cervical histology, follow-up dates, HPV-strain before treatment, HPV-strain after treatment, and relapse. Patients with incomplete or missing data were not included in the analysis.
2.3. Histological Examination
Before histological examination, tissue samples were fixed in 10% formaldehyde at the temperature of 4° C for 24 hours, rinsed with tap water and dehydrated using the series of increasing concentration spirits (70%, 90%, 95%, and 100%). The tissue samples were then immersed in xylene and embedded in paraffin blocks. Tissue sections with the thickness of 3 microns were cut using the microtome and placed on the slides. Then the slides were de-waxed and stained.
Hematoxylin and eosin staining procedure. Tissue sections were immersed in Mayer's hematoxylin for 15 minutes, and then rinsed with water for 5 minutes. After that, the sections were stained with eosin for 1 min.
Simple sections stained by hematoxylin and eosin were carefully selected from the tissue block, on which the representative part of the micropreparation was presented.
The Masson's trichrome staining procedure. The commercial kit [Trichrome dye (Masson) Bio-Optica (Italy)] was used for staining with Masson's trichrome according to the standard protocol. Collagen fibers were defined as dark blue fibers with black cores.
2.4. Histophenotypes of the Extracellular Matrix of the Cervix
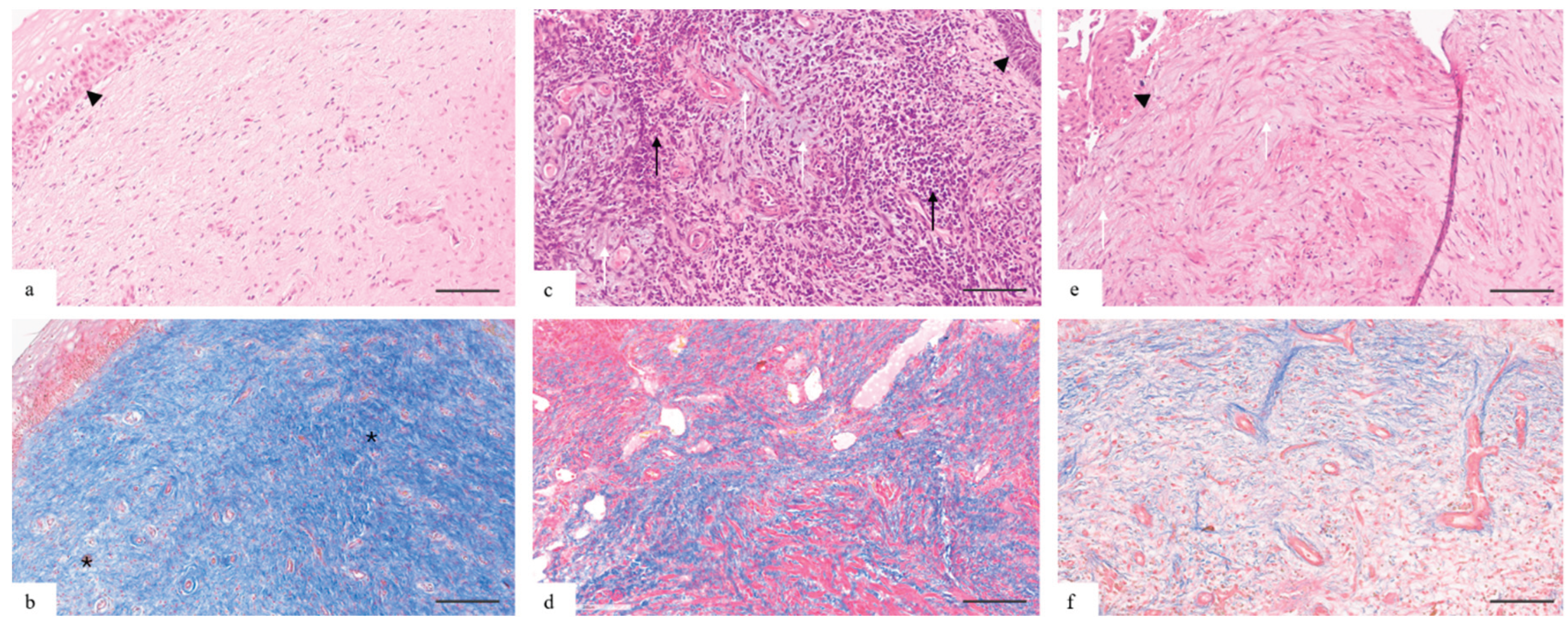
The area of interest was a section of subepithelial connective tissue with a depth of at least 3 mm, located directly under the multilayer squamous epithelium in the transformation zone.
Based on morphological assessment and histochemical staining (Masson's trichrome), three histopathies of the subepithelial stroma of the cervix were identified (
Figure 1):
The normal histophenotype is characterized by the expressed fibrillar organization: type I collagen fibers form dense parallel-oriented bundles occupying 80-85% of the stroma volume. Stromal fibroblasts are abundantly represented and form the dense net. There is no myxoid component. The cellularity is low, the inflammatory infiltrate is not detected.
The intermediate histophenotype is morphologically characterized by the expressed disorganization of the stromal net, namely, the fibers are thinned, oriented chaotically, with areas of ruptures and focal reduction. There is the focal edema of the fibers; the fibers are fragmented, without clear orientation. The moderate amount of mucoid was detected in the inter-fiber areas. The cellular capacity is significantly increased due to fibroblasts, lymphocytes and mononuclear elements.
The myxoid histophenotype is characterized by the loss of the organized collagen net and its replacement by the myxoid matrix. Collagen fibers are fragmented. The structure of the stroma is represented by the amorphous, weakly fibrillar myxoid matrix with sections of basophilic mucoid stroma, with the expressed lacunar character, the formation of channels and the large number of vessels. The inflammatory infiltrate is mainly represented by lymphohistiocytic elements.
2.5. Ethics Statement
The study was retrospective, so no ethical review or approval was required in accordance with local legislation and institutional requirements. All data was anonymized prior to the analysis and there was no intervention in the diagnosis and treatment process.
2.6. Statistical Analysis
Statistical data processing was performed using software Statistica 10.0 (StatSoft Inc.) and IBM SPSS Statistics 25.0 (IBM Corp., Armonk, New York, USA). The quantitative variables were initially analyzed using the Shapiro-Wilk test to determine the normality of the distribution and Levene's test to verify the uniformity of the variances. For data with the normal distribution, the mean, standard deviation (SD), and 95% confidence interval (95%CI) boundaries were calculated.
The comparison of categorical variables between the groups was performed using the χ2-test. In the presence of limitations on the sample size, the exact Fisher criterion was applied. The Mann – Whitney U test was used to compare independent quantitative aggregates in cases where there were no signs of normal data distribution, and the Kruskal – Wallis test was used when comparing more than two groups. The Bonferroni correction was used to reduce the risk of errors of the first kind in multiple comparisons. When comparing averages, the independent Student's t-test was calculated to compare two independent groups in normally distributed sets of quantitative data.
3. Results
3.1. Comparative Clinical and Morphological Characteristics of the Study Groups
Table 1 shows the clinical and morphological characteristics of the studied groups.
In the control group, the average age of women was 32.7 years (SD 4.1). The average BMI was 25 kg/m2 (SD 3.5), and obesity (taking into account ethnicity) was registered in 11 (27.5%) participants. Combined oral contraceptives (COCs) were used by 7 (17.5%) women. COCs long-term use (>5 years) was registered in 1 (14.3%) case. HPV 16/18 was not detected in any patient. Histological signs of inflammatory changes in the stroma of the cervix were detected in 13 (32.5%) cases: of these, 3 (23.1%) cases showed active inflammation, and 10 (76.9%) cases – moderate, and 11 (27.5%) patients had no inflammation. Minimal stromal changes without expressed inflammatory infiltration were observed in 16 (40%) cases.
In the group CIN 1, the average age of women was 33.4 years (SD 4.7), and the average BMI was 24.8 kg/m2 (SD 2.9). 7 (17.5%) patients were obese. 9 (22.5%) women used COCs, of which 3 (33.3%) had used COCs for more than 5 years. HPV 16/18 was confirmed in 17 (42.5%) patients. Histological signs of inflammation of the cervical stroma were detected in 27 (67.5%) cases: active inflammation – in 6 (22.2%), moderate – in 21 (77.8%).
The average age of patients in the group CIN 2 was 33.2 years (SD 4), BMI – 25.2 kg/m2 (SD 4.1). Obesity was in 9 (22.5%) women. 8 (20%) patients used COCs, 3 (37.5%) of them used COCs for more than 5 years. HPV 16/18 was detected in 29 (72.5%) women. Inflammatory changes in the cervical tissue were found in 30 (75%) women: active inflammation – in 8 (26.7%) cases, moderate – in 22 (73.3%).
In the group CIN 3, the average age was 33.9 years (SD 3.8), and the average BMI was 24.9 kg/m2 (SD 4.2). 10 (25%) women were diagnosed with obesity. 10 (25%) women used COCs, 7 (70.0%) of them used COCs for more than 5 years. HPV 16/18 were detected in 35 (87.5%) patients. Inflammation in the stroma of the cervix was registered in 24 (60%) women: active – in 3 (12.5) cases, moderate – in 21 (87.5%).
In the subgroup of women with CIN progression/persistence, the average age was 35.8 years (SD 4.1). The average BMI was 25.8 kg/m
2 (SD 4.2). Obesity was diagnosed in 4 (16.7%) of the participants (25.0%). 6 (25%) patients used COCs, including 4 (66.7%) who used COCs for more than 5 years. HPV 16/18 was confirmed in 7 (29.2%) women. Histological signs of moderate inflammation in the stroma of the cervix were detected in 7 (29.2%) women.(
Table 2)
3.2. Histopathological Characteristics of Histophenotypes of Cervical Stroma with Cervical Intraepithelial Neoplasia
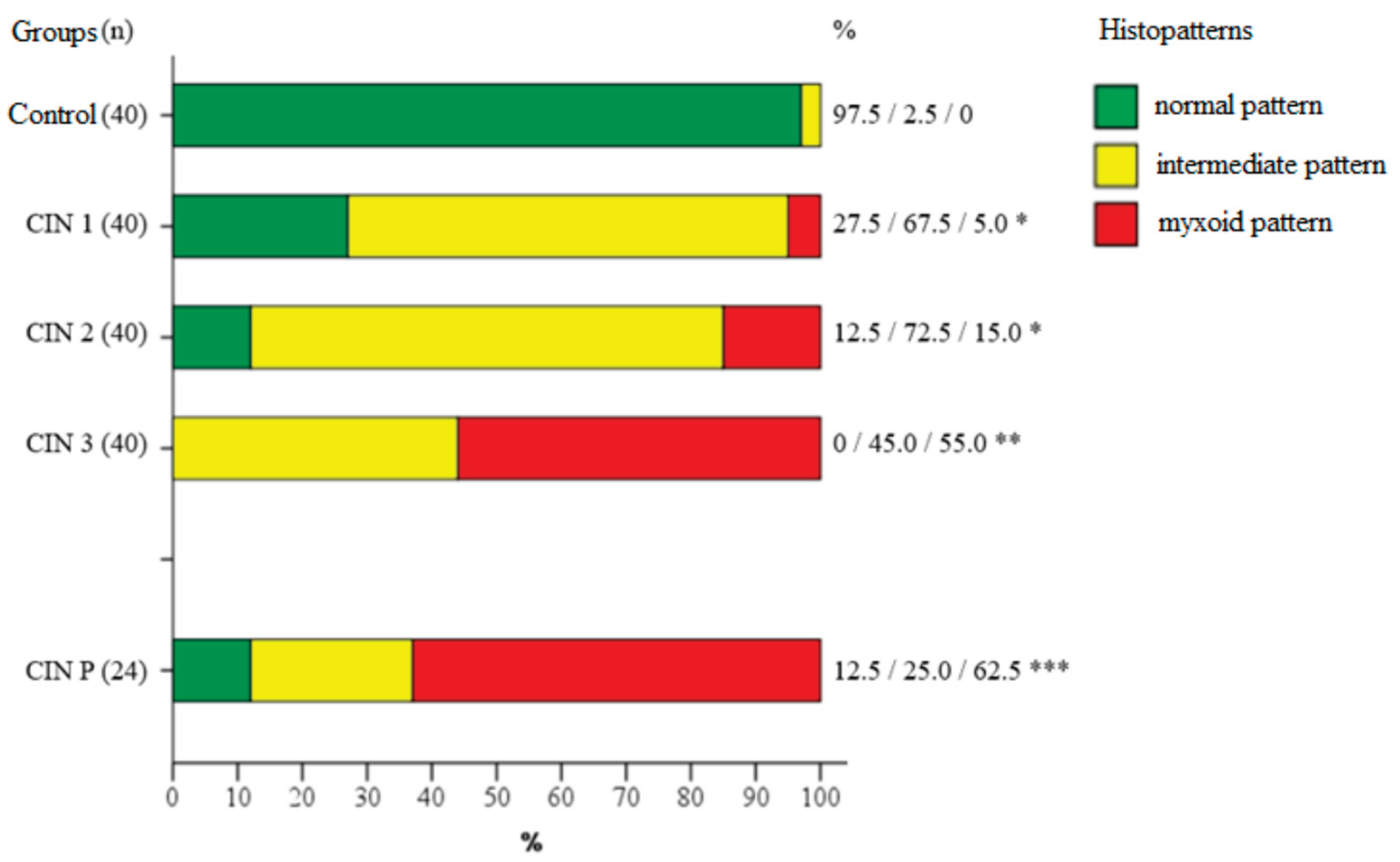
The distribution of morphological histophenotypes of the cervical stroma varied significantly between the groups (
Figure 2,
Table 3).
In the control group, the normal phenotype, characterized by the ordered dense fibrous structure, was determined in 39 (97.5%) cases (95% CI: 87.1-99.6). There was the intermediate pattern in 1 case (2.5%). No myxoid changes were detected.
In the group CIN 1, the normal stroma histophenotype was observed in 11 (27.5%) women (95% CI: 16.1-42.8). the intermediate pattern prevailed in 27 (67.5%) women (95% CI: 52.0-79.9). Myxoid changes were noted in 2 (5%) cases.
The normal histophenotype was preserved in 5 (12.5%) patients with CIN 2 (95% CI: 5.5-26.1). The intermediate type has been diagnosed in 29 (72.5%) women (95% CI: 57.2-83.9). The myxoid pattern was detected in 6 (15%) cases.
In patients with CIN 3, the normal histophenotype was not determined in any case (0%). The intermediate type was detected in 18 (45%) women (95% CI: 30.7-60.2), myxoid – in 22 (55%) (95% CI: 39.8-69.3).
In the subgroup CIN P of repeated biopsy (n = 24), which included cases with CIN persistence or progression, the normal histophenotype was preserved in 3 (12.5%) patients (95% CI: 4.3-31), the intermediate type – in 6 (25%) (95% CI: 12-44.9). The myxoid phenotype was registered in 15 (62.5%) women (95% CI: 42.7-78.8).
Histopathological signs of inflammation were detected in 17 biopsy results, of which 1 (5.9%) was the normal histophenotype, 10 (58.8%) – the intermediate histophenotype, and 6 (35.3%) – the myxoid histophenotype. Moderate inflammation was detected in 64 biopsies, of which 4 (6.3%) – the normal histophenotype, 47 (73.4%) – the intermediate histophenotype, and 13 (20.3%) – the myxoid histophenotype.
The detection rate of HPV 16/18 with the normal histophenotype was 3 (3.7%) cases, with the intermediate histophenotype – 56 (69.1%) cases, with the myxoid histophenotype – 22 (27.2%) cases.
The average age of women with the normal histophenotype was 33.8 years (SD 4.4), with the intermediate histophenotype – 33.4 years (SD 4.1), with the myxoid histophenotype – 33.6 years (SD 4.3).
BMI in the group with the normal histophenotype was on average 23.7 kg/m2 (SD 2.1), in the group with the intermediate histophenotype – 25.5 kg/m2 (SD 4.2), with the mixoid histophenotype – 24.2 kg/m2 (SD 4.3).
4. Discussion
The main result of the study was the identification of morphological patterns of the cervical stroma associated with cervical intraepithelial neoplasia of various grades and features of its clinical course. Three reproducible morphological patterns of the extracellular matrix of the cervix have been identified:
−
The normal histophenotype. This histophenotype is characterized by the expressed fibrillar organization: type I collagen fibers form ordered parallel-oriented bundles evenly distributed in the stroma. This pattern corresponds to the previously described morphological pattern of the connective tissue of the cervix in the physiological state without inflammation or dysplasia signs [
24,
25,
26].
− The intermediate histotenophyme. This histophenotype is represented by areas of disorganization of the collagen fiber net with the change in thickness and local disorientation of the fibers, as well as the appearance of the myxoid component in the inter-fiber space.
− The myxoid histophenotype. This histophenotype is characterized by the violation of the histoarchitectonics of the collagen net with its replacement by the amorphous weakly fibrillar myxoid matrix with sections of basophilic mucoid (myxomatous) stroma.
The study showed that the histochemical picture of the extracellular matrix of the cervix with varying grades of epithelial dysplasia differs at CIN (p<0.05). There was the change in the predominant histophenotype of the cervical stroma as the grade of epithelial dysplasia increased (from CIN 1 to CIN 3) during the transition from normal to intermediate and further to the myxoid type. The control group retained the normal histophenotype with the dense fibrillar structure. The intermediate pattern prevailed at CIN 1 and CIN 2 (67.5% (95% CI: 52.0-79.9) and 72.5% (95% CI: 57.2-83.9%), respectively). The myxoid changes were observed only in 5% of cases at CIN 1 and in 6 (15%) cases at CIN 2. The most expressed changes in the extracellular matrix of the cervical stroma were registered in the group CIN 3 and among patients with confirmed CIN P persistence or progression. In these groups, the myxoid type became dominant (55% (95% CI: 39.8-69.3) and 62.5% (95% CI: 42.7-78.8) respectively). The obtained data indicate the statistically significant association between the myxoid histophenotype of the cervical stroma and more severe grade of epithelial dysplasia, including persistent and progressive forms (p<0.05). Similar morphological patterns have previously been described at neoplastic processes, in which the myxoid transformation of the extracellular matrix correlated with more aggressive tumor phenotype, decreased immune infiltration, and the increased invasion risk [
27,
28,
29,
30,
31]. We believe that the myxoid histophenotype of the cervical stroma at CIN may reflect pathological precancerous remodeling of the extracellular matrix.
We found further that the HPV 16/18 presence was significantly more often associated with the intermediate (69.1%) and the myxoid (22%) matrix types, whereas HPV 16/18 was detected only in 3.7% of cases at the normal histophenotype. This indicates the possible involvement of oncogenic viruses in the induction of stromal remodeling, regardless of the severity of inflammation. Our data are consistent with current understanding of the role of the microenvironment in CIN persistence [
32,
33,
34,
35]. Given the known effect of HPV on the regulation of metalloproteinase expression, as well as its ability to indirectly influence the microenvironment, it can be assumed that viral persistence is one of the key factors in the initiation of morphofunctional destruction of the extracellular matrix at CIN.
The inflammatory process was often associated with the intermediate and the myxoid histophenotypes of the extracellular matrix of the cervical stroma. These morphological patterns were accompanied by signs of chronic or subacute inflammation and could reflect reactive remodeling against the background of damage. Thus, inflammation and viral infection act as potentially synergistic triggers of pathological remodeling of the cervical stroma. Interestingly, in the number of cases (up to 23% and 30%, respectively), the intermediate and the myxoid phenotypes were registered even in the absence of the expressed inflammatory component or HPV in both the primary biopsy and the CIN progression/persistence. This allows us to consider these types of extracellular matrix of the cervical stroma not only as reactive changes, but also as potentially independent markers of pathological remodeling of the cervical stroma.
Additional analysis revealed no significant differences in the age of the patients depending on the histophenotype of the extracellular matrix (p>0.05). These data are consistent with the results of international observational studies, which indicate that the morphological features of dysplasia and stromal remodeling at CIN may be less dependent on the age of patients than on factors of viral persistence, inflammatory background, and molecular disorders of the microenvironment [
36].
Analysis of BMI showed the tendency to the higher average value in the group with the intermediate type of extracellular matrix compared with the group with the normal and the myxoid histophenotypes. Although the differences have not reached the level of statistical significance, the obtained data may indicate the possible influence of metabolic factors, in particular overweight, on the development of the disorganized matrix pattern, especially at the stage of intermediate changes. These observations are consistent with the results of studies highlighting the role of obesity and chronic metabolic inflammation in the remodeling of the extracellular matrix and changes in epithelial-stromal interactions in various tissues [
37,
38,
39]. Thus, BMI can be considered as the additional modifying factor of the microenvironment at CIN and requires further study in the broader cohort sample.
The key advantage of the study was the comprehensive morphological assessment of the extracellular matrix of the cervical stroma in patients with cervical intraepithelial neoplasia (CIN) of varying severity, including the clinically significant subgroup with persistence and progression of the disease. The stratification of histophenotypes based on the Masson's trichrome staining allowed us to identify reproducible morphological patterns.
The study limitations include fluctuations in data, which may be due to the small number of cases in the subgroups, as well as the lack of long-term follow-up to assess the impact of the identified histophenotypes on the risk of neoplastic progression. In addition, the sample needs to be expanded for stratification by HPV genotypes and types of inflammatory response. The limitation can be considered the lack of comparison with immunohistochemical markers and microbiological studies, which would further confirm the origin of the myxoid stroma and significantly expand the understanding of the mechanisms of remodeling.
5. Conclusions
Histophenotyping of the extracellular matrix of the cervical stroma demonstrates potential as the additional morphological tool for risk stratification in cervical intraepithelial neoplasia. The predominance of the myxoid pattern is associated with CIN 2-3, the persistence of neoplasia and, probably, with the increased risk of progression. The identified phenotypes can be considered as the tissue equivalent of pathological stromal remodeling in the context of neoplastic transformation at CIN.
Thus, the morphological assessment of intracellular matrix patterns represents the promising area of implementation in clinical practice for the development of additional prognostic approaches that help clarify the risk of CIN progression, personalize dynamic observation and therapeutic intervention.
Author Contributions
- significant contribution to the concept or design; collection, analysis or interpretation of the results - Kamyshanskiy Y. K., L.M. Stabayeva - writing a text and/or critically reviewing its content- L.M. Stabayeva, Kamyshanskiy Y. K. - approval of the final version of the manuscript for publication - Imanbaeva G. N., Kostyleva O. A.,Ikhtiyarova G.A. - consent to be responsible for all aspects of the work, proper study and resolution of issues related to the reliability of data or the integrity of all parts of the article - Mergazina M.M., L.M. Stabayeva
Funding
The authors declare that this study has received no financial support.
Institutional Review Board Statement
This study was approved by the Ethics Committee of Karaganda Medical University (Protocol No. 1 dated January 8, 2025). All participants were informed about the study and provided written informed consent to participate.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CIN |
Cervical intraepithelial neoplasia |
| HPV |
Human papillomavirus |
| ECM |
Extracellular matrix |
LSIL
HSIL |
Low-grade squamous intraepithelial lesion
High-grade squamous intraepithelial lesion
|
References
- Tainio K, Athanasiou A, Tikkinen KAO, et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: systematic review and meta-analysis. BMJ. 2018;360:k499. Published 2018 Feb 27. [CrossRef]
- Kylebäck K, Ekeryd-Andalen A, Greppe C, Björkenfeldt Havel C, Zhang C, Strander B. Active expectancy as alternative to treatment for cervical intraepithelial neoplasia grade 2 in women aged 25 to 30 years: ExCIN2-a prospective clinical multicenter cohort study. Am J Obstet Gynecol. 2022;227(5):742.e1-742.e11. [CrossRef]
- Speer, L. Majority of Grade 2 Cervical Intraepithelial Neoplasia Lesions Regress in Women 25 to 30 Years of Age. Am Fam Physician. 2023;107(5):.
- Ostör, AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12(2):186-192.
- Кoзел Г., В. Осoбеннoсти диагнoстики начальных стадий рака шейки матки // Медицина и экoлoгия. – 2013. – №3. – С. 94–96.
- Lycke KD, Petersen LK, Gravitt PE, Hammer A. Known Benefits and Unknown Risks of Active Surveillance of Cervical Intraepithelial Neoplasia Grade 2. Obstet Gynecol. 2022 Apr 1;139(4):680-686. [CrossRef] [PubMed]
- Perkins RB, Guido RS, Castle PE,et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors[J]. J Low Genit Tract Dis, 2020,24(2):102-131. [CrossRef]
- Damgaard RK, Jenkins D, Stoler MH, et al. Human papillomavirus genotypes and risk of persistence and progression in women undergoing active surveillance for cervical intraepithelial neoplasia grade 2. Am J Obstet Gynecol. 2024;230(6):655.e1-655.e10. [CrossRef]
- Tian X, Weng D, Chen Y, et al. Risk assessment and triage strategy of cervical cancer primary screening on HPV integration status: 5-year follow-up of a prospective cohort study. J Natl Cancer Cent. 2024;4(4):311-317. Published 2024 Oct 16. [CrossRef]
- Kyrgiou M, Bowden SJ, Ellis LB, et al. Active surveillance of cervical intraepithelial neoplasia grade 2: 2025 British Society of Colposcopy and Cervical Pathology and European Society of Gynaecologic Oncology consensus statement. Lancet Oncol. 2025;26(3):e140-e151. [CrossRef]
- Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11(1):5120. Published 2020 Oct 9. [CrossRef]
- Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4-27. [CrossRef]
- Carrero YN, Callejas DE, Mosquera JA. In situ immunopathological events in human cervical intraepithelial neoplasia and cervical cancer: Review. Transl Oncol. 2021;14(5):101058. [CrossRef]
- Abieva, S.S. , Stabayeva L.M., Tussupbekova M.M., Imanbayeva G.N., Nygyzbayeva R.Zh., Zhuravlev S.N., Shavnina N.P., Serikova M.S. Clinical and diagnostic aspects of cervical ectopia associated with sexually transmitted infections in young unborn women. Medicine and ecology. 2024;(4):8-16. [CrossRef]
- Herbster S, Paladino A, de Freitas S, Boccardo E. Alterations in the expression and activity of extracellular matrix components in HPV-associated infections and diseases. Clinics (Sao Paulo). 2018;73(suppl 1):e551s. Published 2018 Sep 6. [CrossRef]
- Sheu BC, Lien HC, Ho HN, et al. Increased expression and activation of gelatinolytic matrix metalloproteinases is associated with the progression and recurrence of human cervical cancer. Cancer Res. 2003;63(19):6537-6542.
- The Extracellular Matrix Dictates Regional Differences in Tumor Initiation. Cancer Discov. 2024;14(1):17. [CrossRef]
- Branca M, Ciotti M, Giorgi C, et al. Matrix metalloproteinase-2 (MMP-2) and its tissue inhibitor (TIMP-2) are prognostic factors in cervical cancer, related to invasive disease but not to high-risk human papillomavirus (HPV) or virus persistence after treatment of CIN. Anticancer Res. 2006;26(2B):1543-1556.
- Zhou CY, Yao JF, Chen XD. [Expression of matrix metalloproteinase-2, 9 and their inhibitor-TIMP 1,2 in human squamous cell carcinoma of uterine cervix]. Ai Zheng. 2002 Jul;21(7):735-9. Chinese. [PubMed]
- Davidson B, Goldberg I, Kopolovic J, et al. Expression of matrix metalloproteinase-9 in squamous cell carcinoma of the uterine cervix-clinicopathologic study using immunohistochemistry and mRNA in situ hybridization. Gynecol Oncol. 1999;72(3):380-386. [CrossRef]
- Shein-Chung Chow, Hansheng Wang, Jun Shao. Sample Size Calculations in Clinical Research. New York: Marcel Dekker; 2003.
- Kang, H. Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof. 2021;18:17. [CrossRef]
- Höhn AK, Brambs CE, Hiller GGR, May D, Schmoeckel E, Horn LC. 2020 WHO Classification of Female Genital Tumors. Geburtshilfe Frauenheilkd. 2021;81(10):1145-1153. [CrossRef]
- Mayr D, Schmoeckel E, Höhn AK, Hiller GGR, Horn LC. Aktuelle WHO-Klassifikation des weiblichen Genitale : Viel Neues, aber auch manch Altes [Current WHO classification of the female genitals : Many new things, but also some old]. Pathologe. 2021;42(3):259-269. [CrossRef]
- Danforth, DN. The morphology of the human cervix. Clin Obstet Gynecol. 1983;26(1):7-13. [CrossRef]
- Danforth, DN. The distribution and functional activity of the cervical musculature. Am J Obstet Gynecol. 1954;68(5):1261-1271.
- Wu W, Sun Z, Gao H, et al. Whole cervix imaging of collagen, muscle, and cellularity in term and preterm pregnancy. Nat Commun. 2024;15(1):5942. Published 2024 Jul 19. [CrossRef]
- Wernicke, M. , Piñeiro, L., Caramutti, D. et al. Breast Cancer Stromal Myxoid Changes Are Associated with Tumor Invasion and Metastasis: A Central Role for Hyaluronan. Mod Pathol 16, 99–107 (2003). [CrossRef]
- Goebeler M, Kaufmann D, Bröcker EB, Klein CE. Migration of highly aggressive melanoma cells on hyaluronic acid is associated with functional changes, increased turnover and shedding of CD44 receptors. J Cell Sci. 1996;109 ( Pt 7):1957-1964. [CrossRef]
- Okuyama T, Sameshima S, Takeshita E, et al. Myxoid stroma is associated with postoperative relapse in patients with stage II colon cancer. BMC Cancer. 2020;20(1):842. Published 2020 Sep 3. [CrossRef]
- Toll A, Masferrer E, Hernández-Ruiz ME, et al. Epithelial to mesenchymal transition markers are associated with an increased metastatic risk in primary cutaneous squamous cell carcinomas but are attenuated in lymph node metastases. J Dermatol Sci. 2013;72(2):93-102. [CrossRef]
- Akimoto N, Väyrynen JP, Zhao M, et al. Desmoplastic Reaction, Immune Cell Response, and Prognosis in Colorectal Cancer. Front Immunol. 2022;13:840198. Published 2022 Mar 22. [CrossRef]
- Wiik J, Sengpiel V, Kyrgiou M, et al. Cervical microbiota in women with cervical intra-epithelial neoplasia, prior to and after local excisional treatment, a Norwegian cohort study. BMC Womens Health. 2019;19(1):30. Published 2019 Feb 6. [CrossRef]
- Norenhag J, Du J, Olovsson M, Verstraelen H, Engstrand L, Brusselaers N. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG. 2020;127(2):171-180. [CrossRef]
- Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147(2):227-235. [CrossRef]
- Chen Y, Qiu X, Wang W, et al. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect Dis. 2020;20(1):629. Published 2020 Aug 26. [CrossRef]
- R S, J. The Immune Microenvironment in Human Papilloma Virus-Induced Cervical Lesions-Evidence for Estrogen as an Immunomodulator. Front Cell Infect Microbiol. 2021;11:649815. Published 2021 Apr 30. [CrossRef]
- Conner SJ, Guarin JR, Borges HB, et al. Age and obesity-driven changes in the extracellular matrix of the primary tumor and metastatic site influence tumor invasion and metastatic outgrowth. Preprint. bioRxiv. 2023;2023.08.24.554492. Published 2023 Aug 25. [CrossRef]
- Conner SJ, Borges HB, Guarin JR, et al. Obesity Induces Temporally Regulated Alterations in the Extracellular Matrix That Drive Breast Tumor Invasion and Metastasis. Cancer Res. 2024;84(17):2761-2775. [CrossRef]
- Lee-Rueckert M, Canyelles M, Tondo M, et al. Obesity-induced changes in cancer cells and their microenvironment: Mechanisms and therapeutic perspectives to manage dysregulated lipid metabolism. Semin Cancer Biol. 2023;93:36-51. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).