1. Introduction
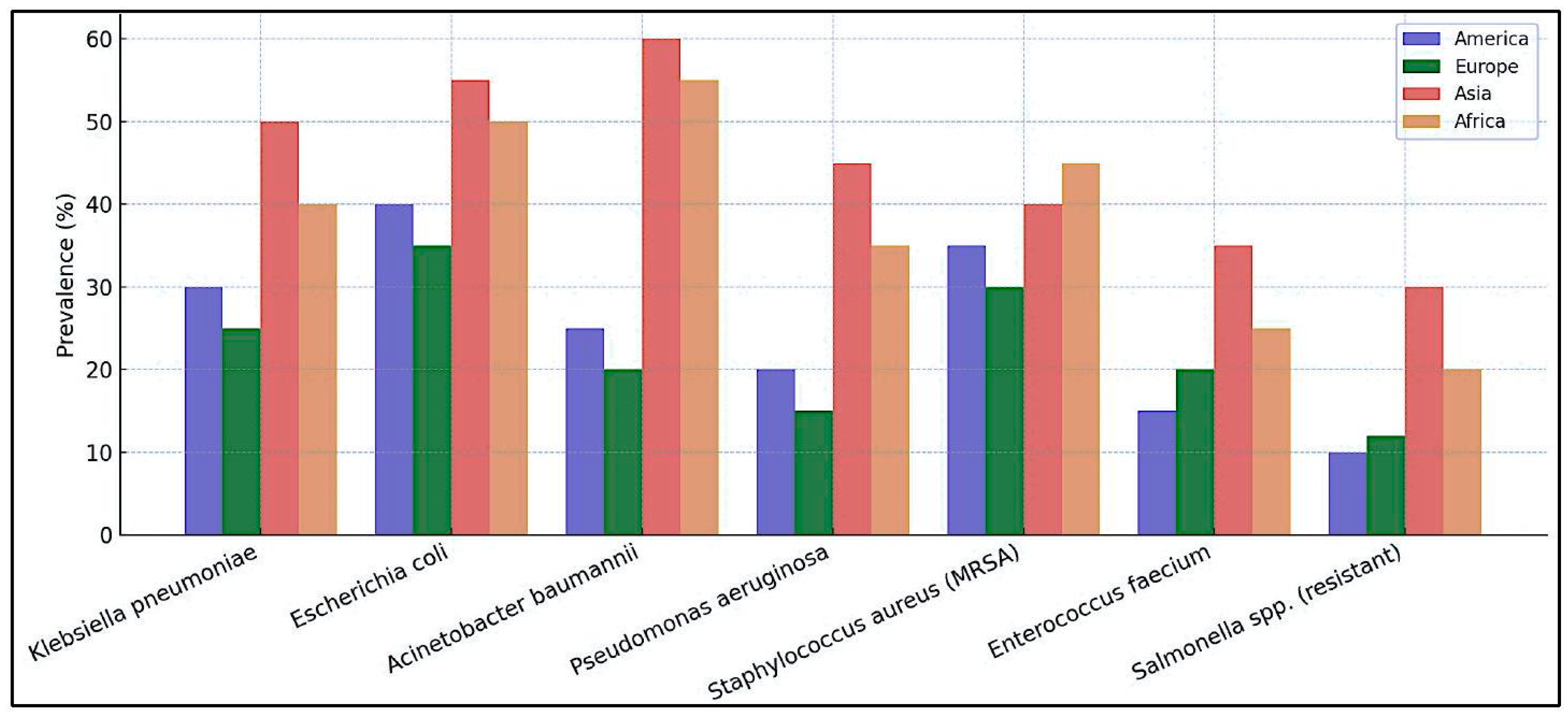
Superbacteria represent one of the most concerning threats to global public health (1, 2). According to the World Health Organization (WHO), antibiotic-resistant infections cause approximately 1.27 million direct deaths annually worldwide and contribute to nearly 5 million additional deaths (3). The most affected regions include South Asia and sub-Saharan Africa, where bacterial resistance rates reach critical levels (47). The most concerning superbacteria are Klebsiella pneumoniae, Escherichia coli, Acinetobacter baumannii, and Pseudomonas aeruginosa, all of which show increasing resistance to multiple drugs (8, 9). In India, it is estimated that over 200,000 deaths per year are related to resistant infections, whereas in China and Pakistan, the figures exceed 100,000 and 50,000, respectively. Resistance has been particularly observed in key antibiotics, such as colistin, carbapenems, and third-generation cephalosporins, limiting treatment options . These microorganisms exhibit extreme resistance to multiple antibiotics, which makes treatment with conventional drugs difficult or impossible. Their proliferation has been driven by the indiscriminate use of antibiotics in medicine and livestock as well as deficiencies in infection control within healthcare systems (12, 13).
Globally, some countries present a higher risk due to various factors such as high population density, limited access to adequate treatments, and the presence of healthcare systems with poor infrastructure (14, 15). Among the countries with the highest incidence of superbacterial infections are India, China, and Pakistan, where unrestricted access to antibiotics has fostered the emergence of antibiotic-resistant bacteria. In India, approximately
of bacterial infections are resistant to at least one last-line antibiotic. China faces a growing number of carbapenem-resistant
Acinetobacter baumannii infections, while Pakistan struggles with the spread of multidrug-resistant strains of
Escherichia coli. In India, approximately 57% of
Klebsiella pneumoniae cases show resistance to carbapenems, while in China,
of
Acinetobacter baumannii infections are resistant to multiple drugs. In Pakistan,
of Escherichia coli strains have developed resistance to third-generation cephalosporins (16-20). Likewise, nations in Latin America, such as Brazil and Mexico, face significant challenges owing to the lack of effective measures to control the spread of these microorganisms. In Latin America, Brazil and Mexico have reported a significant increase in resistant infections due to uncontrolled antibiotic use and the lack of strict regulations in their sale (21-24). In Africa, countries such as Nigeria and South Africa have reported alarming cases of resistance to colistin and carbapenems, with bacterial resistance rates exceeding
in hospital infections exacerbated by the scarcity of healthcare infrastructure and infection control programs, leaving patients without effective treatment options (6, 25, 26). Additionally, poor hospital infrastructure in these regions contributes to the rapid spread of nosocomial infections, making it difficult to contain superbacteria in clinical settings. The lack of access to adequate diagnostics and effective treatments remains a key challenge in these areas, exacerbating the antimicrobial resistance crisis (
Figure 1).
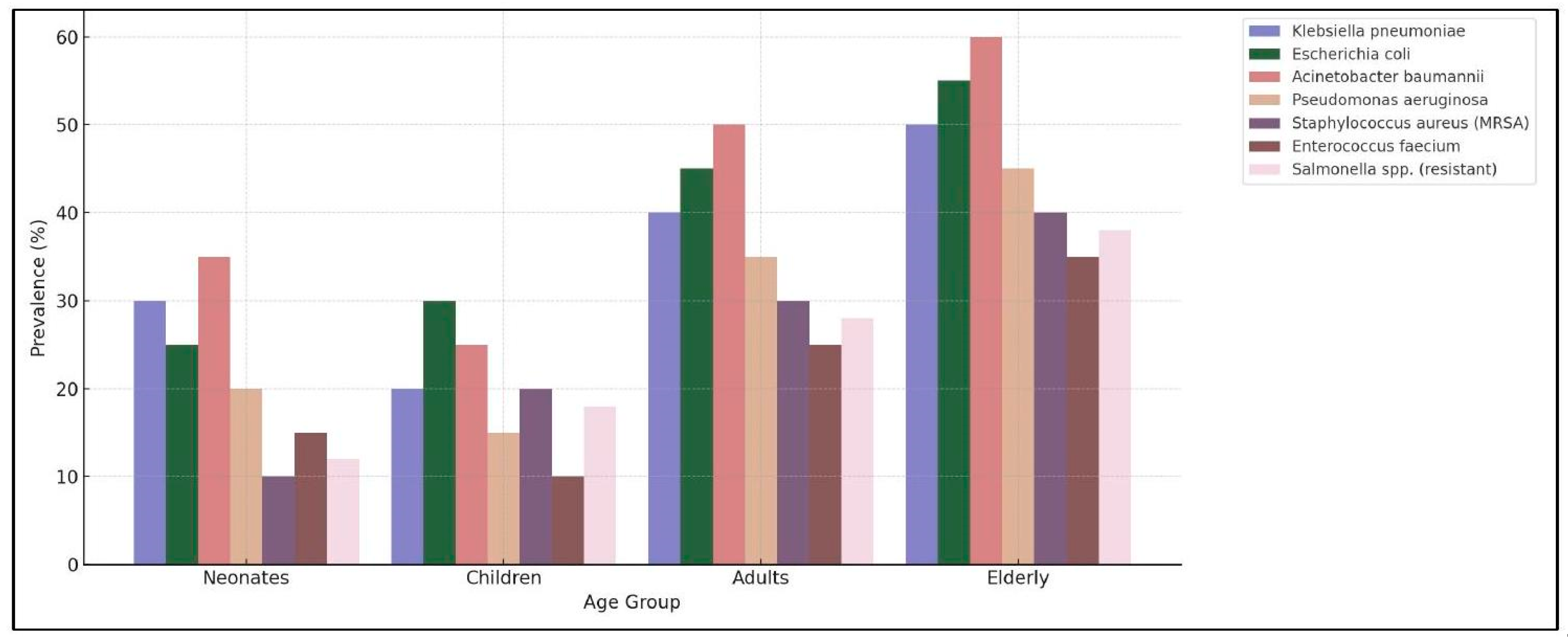
Studies on superbacteria are essential for the development of new prevention and treatment strategies. Superbacteria are microorganisms that have acquired resistance to multiple antibiotics through genetic mutations or the transfer of resistance genes between bacteria. Scientific research in this field has enabled the identification of new antibiotics and therapeutic alternatives, such as phage therapy (viruses that attack bacteria), antimicrobial peptides, and gene-editing therapies such as CRISPR-Cas9. Additionally, the implementation of rational antimicrobial use policies is fundamental for curbing the spread of resistance. It is also crucial to develop and apply rapid diagnostic tests that allow for precise identification of resistant infections and reduce unnecessary antibiotic use. The implementation of rational antimicrobial use policies, including appropriate antibiotic prescriptions, regulation of their sale, and monitoring of bacterial resistance, is key to the spread of these infections. In this context, the pediatric population, particularly neonates (newborns in their first 28 days of life), infants (up to the first year), and children (up to 12 years), represents a highly vulnerable group. The development of immune systems, immature gut microbiota, and increased exposure to hospital environments makes antibiotic-resistant infections especially dangerous. They face an elevated risk due to their immunological fragility and the need for invasive procedures, such as mechanical ventilation and intravenous catheters, which can facilitate the entry of resistant bacteria into their bodies. In neonatal intensive care units (NICUs), according to the WHO, resistant infections are responsible for approximately 214,000 neonatal deaths per year, with
Klebsiella pneumoniae and
Acinetobacter baumannii being the most common bacteria in these infections. Morbidity and mortality in this population increase significantly in resource-limited countries where access to last-line treatments is scarce. According to previous studies, in some regions, the mortality rate from resistant infections in neonates can exceed
, highlighting the urgent need to strengthen prevention and treatment measures aimed at this population (
Figure 2).
Considering this issue, it is crucial to strengthen epidemiological surveillance, promote awareness of the prudent use of antibiotics, and foster research into innovative treatments. Epidemiological surveillance should include the implementation of systems, such as the WHO’s Global Antimicrobial Resistance Surveillance System (GLASS), which allows for the monitoring and analysis of resistance trends globally. Additionally, it is essential to carry out public awareness campaigns, such as the World Antimicrobial Awareness Week (WAAW), which educates people about the proper use of these medications. Research into innovative treatments includes the development of new antibiotics, exploration of alternative therapies, such as bacteriophage use, and the design of vaccines to prevent bacterial infections. International cooperation and commitment of healthcare systems will be decisive in curbing the spread of these bacterial threats. Programs such as WHO’s Global Action Plan (GAP) on Antimicrobial Resistance and initiatives by the Global Antibiotic Research and Development Partnership (GARDP) are the key examples of collaborative efforts. Through a coordinated and multidisciplinary approach, it will be possible to protect future generations from the devastating effects of superbacteria.
2. Objective
The main objective of this research study is to conduct a revision and meta-analysis based on the available literature, to enlighten society about this problem and analyze the actual solutions that have been given, whether superbacteria are most common, especially in infants and baby populations. For this purpose, a systematic review and meta-analysis of research published between 1990 and 2024 was conducted to ensure focused and systematic assessment. The research question will be defined using the PICO framework: diseases produced by superbacteria in infants and neonatal populations worldwide.
3. Methodology
Literature Search
The initial step was to conduct a systematic review involving drafting a detailed protocol that outlined the objectives, eligibility criteria, and methodological approach. It has been conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines, which provide a standardized framework for protocol development (27-29). Accordingly, a systematic search was performed using the following databases: Cochrane Library (30), EMBASE (31), MEDLINE (32), and CINAHL (33). Scientific reports included in the study were obtained using the following MeSH search terms: “(superbacteria) AND (infants) AND (neonatal).”
Study Eligibility Criteria
The research question will be defined using the PICO framework, which involves population, intervention, comparator, and outcomes of interest. The following inclusion criteria were used for screening: (1) infants aged 0-6 years old; (2) superbacterial infections; (3) all countries included; and (4) articles published between 1990 and2024. The exclusion criteria were (1) patients over 7 years of age, (2) no bacterial infections, and (3) non-controlled trials.
Study Selection
Both the titles and abstracts of articles that met the criteria were independently screened by two authors of the present study using Rayyan software (Rayyan systematic review software, India). Subsequently, all authors acted as peer reviewers by independently assessing the full-text articles of potentially eligible studies. In the case of a lack of consensus between the two reviewers involved in the screening or assessment of the full texts, the authors discussed the conflict and reached an agreement. We documented the reasons for the exclusion of the full-text articles that we assessed.
Quality Assessment and Risk of Bias
Studies included in the meta-analysis were carried out using previously defined superbacterial cell lines. In this type of study, it was not possible to analyze bias following the methodology proposed in the Cochrane manual (30), as it is specific to the evaluation of clinical studies.
Statistical Analysis
Where feasible, a quantitative synthesis in the form of a meta-analysis will be conducted using software such as Review Manager (RevMan), R (meta package), or STATA. Effect measures will be chosen based on the outcome type: risk ratios (RR) or odds ratios (OR) with confidence intervals (CIs) for dichotomous outcomes and mean difference (MD) or standardized mean difference (SMD) for continuous outcomes. Model selection will depend on the degree of heterogeneity, with a fixed-effects model used for low heterogeneity and a random-effects model employed when heterogeneity was substantial. Heterogeneity will be assessed using the statistic and chi-squared test, with values of considered low, moderate, and over high. Subgroup analyses may be conducted based on study characteristics such as population or geographical region, and sensitivity analyses will be performed to evaluate the robustness of the findings.
Ethical Considerations
Because the review will be based entirely on previously published data, formal ethical approval is generally not required. Nonetheless, the review will adhere to ethical standards in research and reporting, including respect for data privacy and compliance with the accepted guidelines for the ethical publication of scientific work.
4. Results
The three outcomes pursued in the present study were: (1) determine whether the effect of each superbacterium on the diseases and their common resistance patterns; (2) analyze the effect of superbacteria around different countries; and (3) determine whether cell cycle arrest is the molecular target of grape polyphenols on leukemia cells.
A flowchart describing the process of selecting publications to be included in the study is presented in
Table 1, which summarizes the included articles and the information extracted to evaluate the three outcomes stated above. Once duplicate results were removed, 5131 articles were selected as a result of the search carried out. The Titles and Abstracts of these articles were reviewed by the authors against the previously established eligibility criteria. Accordingly, 5016 articles were excluded from the study because they did not fulfill the criteria. The full text of the remaining 115 articles was screened.
Overall, 75 studies met the criteria, but only 37 articles, which included quantitative data useful for statistical analysis, were selected for the meta-analysis (
Table 2) (36-72).
As illustrated in
Table 3, the following data are presented: first, the frequency of the different bacterial species identified in a specific study or analysis and second, the percentage of each species. The most prevalent species identified was Escherichia coli, with a frequency of nine cases, representing
of the total. Klebsiella pneumoniae was the second most prevalent species, with nine cases representing
of the total species. Pseudomonas aeruginosa was mentioned eight times, representing
of the findings.
Acinetobacter baumannii was documented on seven occasions, corresponding to
of the total population. Enterobacter cloacae and Staphylococcus aureus were identified in
of the samples. Group B
Streptococcus was documented at a frequency corresponding to
of the samples. Furthermore, the prevalence of vancomycin-resistant
Enterococcus was documented in four cases, accounting for
of the total observed cases. The fungal species
Candida auris and the bacterium
Clostridium difficile were identified in
of the cases, with a frequency of three occurrences each.
Table 4 presents the frequencies and percentages of various patient groups. The data indicated that the largest group of patients required intensive care, with 121 patients representing 17. This is equal to
of the total. The second largest group was that of NICU patients, with 101 cases, representing
of the total. The total number of pediatric patients with urinary tract infections (UTIs) was 81, representing
of the total. Community-acquired infections were observed in 71 patients, constituting
of all the cases. Six patients, which is
of the total, had neonatal sepsis. The study revealed that both liver transplant patients and those afflicted with ventilator-associated pneumonia constituted five patients each, thereby accounting for
of the total sample. Finally, the patient groups infected with the novel coronavirus (
) and those suffering from pediatric oncology (
) accounted for
of the total. The following table provides a concise summary of the distribution of various patient groups in a clinical setting.
The data presented in
Table 5 demonstrate the distribution of specific parameters in various countries along with their respective percentages. India is at the top of the list, with a score of 8, corresponding to
of the total. Spain had a score of 7, representing 13.0% of the total. Portugal and Tunisia were assigned the same score of 6, representing
each. China is the second-highest-ranked country, with a score of 5, which equates to
. Yemen and Pakistan had scores of 4 and 3, representing
and
, respectively. Romania also had a score of 3, equivalent to
. The table concludes with an overall figure of 10, which constitutes
of the total. This table provides a comparative overview of the selected countries in relation to a specific parameter, thus enabling an assessment of their performance or representation in this context.
Table 6 presents the prevalence of various antibiotic resistances among the sample population. The data indicates that beta-lactam resistance is the most prevalent, occurring in twelve cases, representing
of the total. Carbapenem resistance was identified in ten cases, constituting
of the total. Furthermore, the presence of Extended-Spectrum Beta-Lactamase (ESBL)-producing organisms was observed in nine cases, constituting
of the total.
Fluoroquinolone resistance has been documented in eight cases, constituting
of the total. As illustrated in the following table, other forms of resistance include vancomycin resistance in six cases (
) and macrolide resistance in five cases (8.6%). This table highlights the varying degrees of antibiotic resistance in the study group.
Taking all the data together, a more comparative and complete
Table 7 can be built to collect all these comparisons and observe the relationships between the superbacteria, their resistance, and the population affected in the different countries.
Escherichia coli (14.7%) and Klebsiella pneumoniae (13.2%) were the most frequently reported drug-resistant bacteria. Pseudomonas aeruginosa () and Acinetobacter baumannii () were also of significant concern. Regarding the most affected populations, several resistant bacteria are prevalent in neonates and children, increasing hospitalization risks, such as ICU patients () and NICU patients () are at the highest risk, and pediatric UTI cases () and community-acquired infections () are also major concerns. The potential use of Ceftolozane/Tazobactam in pediatric cases with severe multidrug-resistant infections is emerging. The most affected countries have widespread antibiotic resistance issues, and the resistance patterns differ across regions, emphasizing the need for localized surveillance. Focusing on the most concerning resistance types, most bacteria studied exhibit resistance to multiple antibiotic classes, making treatment difficult, such as beta-lactam resistance (), which is the most frequent, or carbapenem resistance, and ESBL production highlights severe antimicrobial threats. Other pathologies such as ventilator-associated pneumonia are commonly linked to Pseudomonas aeruginosa and Acinetobacter baumannii infections. Antibiotic resistance and the gut microbiome produced by early antibiotic exposure can increase the resistant bacterial populations in the gut. A significant presence of multidrug-resistant bacteria has also been detected in Liver Transplant Patients, with a high intestinal dominance by MDR organisms correlated with extraintestinal spread and infection. Pregnancy-related UTI risk: Pregnant women with MDR gram-negative UTIs have an increased risk of pyelonephritis. Candidemia in COVID-19 patients: Higher prevalence of multidrug-resistant Candida auris infections, especially in ICU settings.
5. Discussion
The emergence of multidrug-resistant (MDR) bacterial strains represents a significant public health concern, affecting diverse geographical areas and patient demographics, particularly those within healthcare facilities (73). The available data on antimicrobial resistance (AMR) indicate trends, particularly among hospitalized patients with compromised immune systems. Key pathogens, including Escherichia coli and Klebsiella pneumoniae, often cause urinary tract infections, bloodstream infections, and neonatal sepsis. The proliferation of extended-spectrum beta-lactamase (ESBL)-producing strains has led to a paucity of treatment options, rendering carbapenems the preferred choice for cases of severe infection. However, the emergence of carbapenem-resistant Enterobacteriaceae (CRE) has led to the need for alternative treatments such as colistin and tigecycline. Neonates and children exhibit heightened vulnerability owing to their underdeveloped immune systems and frequent exposure to hospital environments. This exposure, among other factors, contributes to elevated mortality rates from resistant infections in neonatal intensive care units (NICUs) (73-75).
Infections caused by vancomycin-resistant Staphylococcus aureus (VRSA) and methicillin-resistant Staphylococcus aureus (MRSA) pose a particularly high risk to immunocompromised pediatric patients and liver transplant recipients (76-78). Ventilator-associated pneumonia (VAP) has been observed to demonstrate resistance issues analogous to those of bacteria, such as Pseudomonas aeruginosa. The horizontal transfer of resistance genes, such as the vanA operon from resistant Enterococcus, exacerbates this problem (79-81). The dissemination of MDR bacteria from healthcare facilities to communities is a matter of significant concern, particularly in light of inadequate regulatory oversight regarding antibiotic utilization. The emergence of resistant strains such as Neisseria gonorrhoeae and Shigella underscores the need for comprehensive public health strategies (73, 82-85).
Exposure to antibiotics during early life has been shown to play a significant role in the development of antibiotic-resistant bacteria within the gut microbiota. This phenomenon not only increases the likelihood of future infections, but also promotes the transfer of resistance genes within communities. The increasing global burden of AMR is exacerbated by inappropriate antibiotic use, especially in resource-limited areas. Escherichia coli has demonstrated notable resistance to various antibiotics, with a high prevalence of ESBL-producing strains observed in multiple countries. Methicillin-resistant Staphylococcus aureus (MRSA) remains a major concern, particularly in resource-constrained environments (86-92). Recent advancements in antibiotic therapies, including ceftaroline and tedizolid, hold promise for combating resistant infections. However, the emergence of resistant strains highlights the need for continuous surveillance and vigilance. Innovative treatment options such as bacteriophage therapy and antimicrobial peptides are currently under investigation. A multifaceted strategy is imperative to address this challenge, encompassing international collaboration, enhanced local surveillance, and public education to promote judicious antibiotic usage. Key organizations, such as the World Health Organization (WHO), have initiated systems, such as the Global Antimicrobial Resistance Surveillance System (GLASS), to track resistance patterns and support global efforts against MDR bacteria (93-95). Strategies also include hospital-based Antimicrobial Stewardship Programs (ASPs) to ensure proper antibiotic use, reduce unnecessary prescriptions, and control the use of vital antibiotics such as carbapenems. Infection control practices, such as strict hand hygiene and sanitation in hospital environments, are of utmost importance (96-99). Moreover, the implementation of vaccination programmes targeting bacterial infections has led to a concomitant reduction in the utilization of antibiotics. The utilization of surveillance systems such as GLASS is imperative for the tracking and comprehension of resistance patterns.
This study underscores the need for expeditious diagnostic tests to identify resistant infections and curtail unwarranted broad-spectrum antibiotic prescriptions from animals. Furthermore, it underscores the necessity of national action plans to combat AMR and the imperative for enhanced infection control measures, precise antibiotic treatment, and comprehensive programs to address the escalating threat of MDR infections (100-104).
6. Conclusions
This research helps us to understand the complexity of the superbacteria resistance problem that we already suffer in some countries, especially in infants and the neonatal population, which has led us to identify a few of the most harmful superbacteria. Therefore, it is imperative to explore combination therapies and develop novel drugs to enhance the effectiveness of antibiotics. The increasing prevalence of multidrug-resistant (MDR) bacteria represents a significant global health threat, complicating the treatment of a range of infections caused by well-known pathogens such as Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus. These superbacteria have emerged in hospitals and communities, posing a significant challenge to the public health systems worldwide. The acceleration of this issue can be attributed to two factors: inadequate antibiotic stewardship and excessive use of antibiotics. The latter is particularly problematic in regions with limited resources. The dissemination of pathogens has expanded beyond healthcare facilities to community settings, thereby posing a threat to both healthy individuals and those with compromised immune systems. This situation emphasizes the need for comprehensive public health strategies, including enhanced infection control measures, educational campaigns to promote optimal antibiotic stewardship, and improved surveillance systems. To address these challenges, alternative treatments are currently being explored, including bacteriophage therapy in new diagnostic and therapeutic strategies, which happen to be essential. The use of rapid diagnostic tools has the potential to expedite the identification of resistant pathogens, thereby mitigating the need for the indiscriminate use of broad-spectrum antibiotics. There is a pressing need for sustained investment in research and international collaboration, fostering a dynamic and cooperative environment to effectively combat the ever-evolving resistance. The emergence of superbacteria can be mitigated through the implementation of prevention strategies, development of innovative treatments, and promotion of international collaboration, thereby ensuring public health on a global scale.
Author Contributions
Conceptualization, DJGM, CGRF; methodology, DJGM, ASM, CGRF formal analysis; DJGM, ASM, CGRF; data curation, DJGM, ASM, CGRF; writing- original draft preparation, DJGM; writing revision-ASM, CGRF; funding acquisition, DJGM. All the authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by the University Francisco De Vitoria, Madrid, Spain.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available because of the data protection and privacy policies of some collaborating entities in the research project that funded this study.
Acknowledgments
The authors wish to thank the University of Francisco de Vitoria, Madrid, Spain.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Adnan F KNKMAMMHSMN. Changing Trends Of Multidrug Resistant Bacteria: A Six Year Experience From A Tertiary Care Hospital.
- Nemeth J LBPBNAKSRCKSP. Multidrug-resistant bacteria in travellers hospitalized abroad: Prevalence, characteristics, and influence on clinical outcomes.
- Abat C FPEJMTRJMRD. Extremely pandrug-resistant bacterial extra-deaths: myth or reality?
- Suwantarat N CKC. Epidemiology and molecular characterization of multidrugresistant gram-negative bacteria in Southeast Asia.
- Veldman K, KADCvE-ZAWBMD. Enterobacteriaceae resistant to third-generation cephalosporins and quinolones in fresh culinary herbs imported from Southeast Asia.
- Chipangura Jk CTKMNV. Prevalence of antimicrobial resistance in bacterial cultures and susceptibility records from horse samples in South Africa.
- Ekwanzala Md DJBKIMMNB. A systematic review in South Africa revealed that antibiotic resistance genes are shared between clinical and environmental settings.
- Bak, G.; Lee, J.; Suk, S.; Kim, D.; Young Lee, J.; Kim, K.S.; et al. Identification of novel sRNAs involved in biofilm formation, motility, and fimbriae formation in Escherichia coli. Scientific reports 2015, 5, 15287. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, H.J.; Kim, G.W.; Cho, K.; Takahashi, S.; Koshino, H.; et al. Isolation of coralmycins A and B and potent anti-gram-negative compounds from myxobacteria Corallococcus coralloides M23. Journal of Natural Products 2016, 79, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Hoashi K HBSMSATKETTKODDYH. Comparison of the Treatment Outcomes of Piperacillin-Tazobactam versus Carbapenems for Patients with Bacteremia Caused by Extended-Spectrum β-Lactamase-Producing Escherichia coli in Areas with Low Frequency of Co-production of OXA-1: A Preliminary Analysis.
- Jia Y, SBPYFCDLACKBSJAKB. Kinetics, thermodynamics, and structural Effects of Quinoline-2-Carboxylates, Zinc-Binding Inhibitors of New Delhi Metallo-β-lactamase-1 Re-sensitizing Multidrug-Resistant Bacteria for Carbapenems.
- Minerdi D LDSP. Monooxygenases and Antibiotic Resistance: A Focus on Carbapenems.
- Petersen Mw PASFJABSMAJSAMPF-M. Piperacillin/tazobactam vs. carbapenems for patients with bacterial infection: Protocol for a systematic review.
- Xiao Y. Antimicrobial Stewardship in China: Systems, Actions and Future Strategies.
- Mai W LYMQXJWJ. Bacterial Epidemiology and Antimicrobial Resistance Profiles of Respiratory Specimens of Children with Pneumonia in Hainan, China.
- Rapp Rp UC. Klebsiella pneumoniae carbapenemases in Enterobacteriaceae: History, evolution, and microbiological concerns.
- Petersen-Morfin S, B-IPM-ORG-GEP-GHRG. New Delhi Metallo-BetaLactamase (NDM-1)-producing Klebsiella pneumoniae isolated from a burn patient.
- Zeng L YCZJHKZJLJWJHWYLZX. An Outbreak of Carbapenem-Resistant Klebsiella pneumoniae in an Intensive Care Unit of a Major Teaching Hospital in Chongqing, China.
- Jalal Na A-GAMMAMASSBFBFSHSHJAKB. Prevalence and Antibiogram Pattern of Klebsiella pneumoniae in a Tertiary Care Hospital in Makkah, Saudi Arabia: An 11-Year Experience.
- Ghenea Ae CRDAIȚENVCMMAȚCGSAIP. Prevalence and Antimicrobial Resistance of Klebsiella Strains Isolated from a County Hospital in Romania.
- Silva Ggdc CEHVPCSNSFLLELGPENG. Occurrence of KPC-Producing Escherichia coli in Psittaciformes Rescued from Trafficking in Paraíba, Brazil.
- Lemos Ev DlHRFANQECOLY. [Acinetobacter baumannii - related mortality in intensive care units in Colombia].
- Ocampo Am VCASPMCAVJJN. [Molecular characterization of an outbreak of carbapenem-resistant Klebsiella pneumoniae in a tertiary care hospital in Medellín, Colombia].
- Garza-González E, L-DJMB-PFJGGM. Prevalence of multidrug-resistant bacteria at a tertiary-care teaching hospital in Mexico: a special focus on Acinetobacter baumannii.
- Koch, A.; Mizrahi, V. Mycobacterium tuberculosis. Trends Microbiology 2018, 26, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Barragán A MSEKKKXGSHSJMJBJM. Predominance of multidrug-resistant bacteria causing tract infections among symptomatic patients in East Africa: A call for action.
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMAP) 2015: Elaboration and explanation. Bmj 2015, 349, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 2009, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hutton B, Salanti G, Caldwell D, Chaimani A, Schmid C, Cameron C, et al. The PRISMA Extension Statement for Reporting Systematic Reviews Incorporation Network Meta-analyses of Health Care Interventions: Checklist and Explanation Annals of Internal Medicine, 2015.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 In: Green S, editor. www.cochrane-handbook.org.: The Cochrane Collaboration; 2011. p. 1-639.
- Elsevier. EMBASE library 2018 [Available from: https://www.elsevier.com/solutions/embase-biomedical-research.
- Unidos BNdMdlE. MedLine database 2018 [Available from: https://medlineplus.gov/spanish/.
- EBSCO. The CINAHL database 2018 [Available from: https://www.ebscohost.com/nursing/products/cinahl-databases/cinahl-complete.
- Moher, D.; Shamseer, L.; Fau-Clarke, M.; Clarke, M.; Fau-Ghersi, D.; Ghersi, D.; Fau Liberati, A.; Liberati, A.; Fau-Petticrew, M.; Petticrew, M.; Fau-Shekelle, P.; et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moher D, Shamseer L, Fau - Clarke M, Clarke M, Fau - Ghersi D, Ghersi D, et al. Syst Rev. 2015;4(1):1.
- Hartoyo E FFV. A 30-Day-Old Infant with Necrotizing Fasciitis of the Perineal Region Involving the Scrotum Due to Methicillin-Resistant Staphylococcus aureus (MRSA) and Extended-Spectrum β-Lactamase (ESBL)-Producing Klebsiella pneumoniae: A Case Report.
- Hertting O LJGCGBREM. Acute infection as cause of hospitalization of asylum-seeking children and adolescents in Stockholm, Sweden 2015-2016.
- Zhang Y LSLYZJDY. Analysis and risk factors of deep vein catheterization-related bloodstream infections in neonates.
- Romandini A PASPAPGACDGCSF. Antibiotic Resistance in Pediatric Infections: Global Emerging Threats, Predicting the Near Future.
- Petca A ŞFNSDMCDDANTMCFMMP. Antimicrobial Resistance Profile of Group B Streptococci Colonization in a Sample Population of Pregnant Women from Romania.
- Burns Je RPDLYGGWGFJGJJPSJCB. Assessment of the impact of inpatient infectious events in pediatric patients with newly diagnosed acute leukemia at Dr. Robert Reid Cabral Children’s Hospital, Dominican Republic.
- Huynh Bt K-DEHPPMRFMJFHH. Bacterial Infections in Neonates, Madagascar, 2012-2014.
- Bhuyan Gs, HMASSKRAIMTHTNBNQSKMAK. Bacterial and viral pathogen spectra of acute respiratory infections in under-5 children in hospital settings in Dhaka city.
- Denoble A, RHWKMRHSSWKHPRD-KS. Bad bugs: antibiotic-resistant bacteriuria in pregnancy and risk of pyelonephritis.
- Moin S FJRSNNZSJK. C. auris and non-C. auris candidemia in hospitalized adult and pediatric COVID-19 patients; single-center data from Pakistan.
- Tfifha M FAMMMNASBJ. Carriage of multidrug-resistant bacteria among pediatric patients before and during their hospitalization in a tertiary pediatric unit in Tunisia.
- Zhou B LXWJBJBZYY. Clinical and microbiological profile of babies born with risk of neonatal sepsis.
- Dammeyer Ah, HSAACNLSLZMHKKBFM. Clinical relevance of colonization with antimicrobial-resistant bacteria (AMRB) and methicillin-susceptible Staphylococcus aureus (MSSA) for mothers during pregnancy.
- Stoesser N XSVMPKEIDOECCDWN. Colonization with Enterobacteriaceae producing ESBLs in children attending preschool childcare facilities in the Lao People’s Democratic Republic.
- Milic M SMCVJMPVMMMJSMMV. Colonization with Multidrug-Resistant Bacteria in the First Week of Life among Hospitalized Preterm Neonates in Serbia: Risk Factors and Outcomes.
- Chakraborty M SSDRBMMMTMMCBSMAN. Current Trends in Antimicrobial Resistance Patterns in Bacterial Pathogens among Adult and Pediatric Patients in the Intensive Care Unit in a Tertiary Care Hospital in Kolkata, India.
- Salamat S EHZAJH. Detection of AmpC β-lactamase producing bacteria isolated in neonatal sepsis.
- Girona-Alarcón M FEG-GAB-PSBMFAEMEJ. Device-associated multidrug-resistant bacteria surveillance in critically ill children: 10 years of experience.
- Tian D WBZHPFWCSYSY. Dissemination of the blaNDM-5 Gene via IncX3Type Plasmid among Enterobacteriaceae in Children.
- Béranger A CCLFFABNGMOMRSDD. Early Bacterial Infections After Pediatric Liver Transplantation in the Era of Multidrug-resistant Bacteria: Nine-year Single-center Retrospective Experience.
- Foglia Ee FVJEAM. Effect of Nosocomial Infections Due to Antibiotic-Resistant Organisms on Length of Stay and Mortality in the Pediatric Intensive Care Unit.
- Papan C M-BMLGHJ. Evaluation of the multiplex PCR based assay Unyvero implant and tissue infection application for pathogen and antibiotic resistance gene detection in children and neonates.
- Wacharachaisurapol N PCSWPWCAWSC. Greater optimisation of pharmacokinetic/pharmacodynamic parameters through a loading dose of intravenous colistin in paediatric patients.
- Sardzikova S AKSPBGKLMGBVKASK. Gut diversity and the resistome as biomarkers of febrile neutropenia outcome in pediatric oncology patients undergoing hematopoietic stem cell transplantation.
- Sturød K DADURVDFPFC. Impact of narrow-spectrum penicillin V on the oral and faecal resistome in a young child treated for otitis media.
- Dahdouh E F-TLC-BER-CGSCA-DML. Intestinal Dominance by Multidrug-Resistant Bacteria in Pediatric Liver Transplant Patients.
- Huespe I PESICNDLSRESJ. Kinetics of procalcitonin in infections caused by multidrug-resistant bacteria.
- Martin-Mateos R M-ALC-GÁALCVSMMAA. Multidrug-resistant bacterial infections after liver transplantation: prevalence, impact, and risk factors.
- Salah A A-SIHAAAAARFWSDAOAT. Neonatal sepsis in Sana’a ity, Yemen: a predominance of Burkholderia cepacia.
- McDonald Lc, WMCLAMASMGPMPJWR. Outbreak of Acinetobacter spp. bloodstream infections in a nursery associated with contaminated aerosols and air conditioners.
- Tamma Sm HAJTPD. Prescribing Ceftolozane/Tazobactam for Pediatric Patients: Current Status and Future Implications.
- Zhu Zy YJFNYHYWFWJWML. Retrospective analysis of tigecycline shows that it may be an option for children with severe infections.
- Perween N RSNSKSK, 2nd. Retrospective Analysis of Urinary Tract Infection in the Pediatric Population at a Tertiary Care Centre.
- Zhao L, YXLYZZDYWYCBWJJCZGLZZL. Temporal development and potential interactions between the gut microbiome and resistome in early childhood.
- Sanches B GRDJCMGA. [The Age of Multidrug Resistance: Ten Year Incidence in a Neonatal Intensive Care Unit].
- Huang H WKCHWJCLLZLS. Impact of the COVID-19 pandemic on nosocomial infections: A retrospective analysis in a tertiary maternal and child healthcare hospital.
- Rammaert B AFNS. [Ventilator-associated pneumonia and chronic obstructive pulmonary disease] COPD).
- Vivas R BAATDSSJS. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview.
- Agga Ge SJWATM. Antimicrobial-Resistant Fecal Bacteria from CeftiofurTreated and Nonantimicrobial-Treated Comingled Beef Cows at a Cow-Calf Operation.
- Obasi A NSUEKCGABVPYSI. Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae from Pharmaceutical Wastewaters in South-Western Nigeria.
- Ali Alghamdi B and A-JIA-SJMMAHA-OBGAKY. Antimicrobial resistance of methicillin-resistant Staphylococcus aureus.
- Willis Ja, CVCSSJMKGBKCBVSdFPY. Breaking down antibiotic resistance in methicillin-resistant Staphylococcus aureus: Combining antimicrobial photodynamic and antibiotic treatments.
- Shen W CJZRCJ. An 11-year linezolid-resistant Staphylococcus capitis clone was disseminated with a similar cfr-carrying plasmid in China.
- Arvanitis M ATKTKZPDDAME. Impact of antimicrobial resistance and aging on VAP outcomes: Experience from a large tertiary care center.
- Bailey Kl KAC. Ventilator-Associated Pneumonia (VAP) with multidrug-resistant (MDR) Pathogens: Optimal Treatment.
- Loyola-Cruz MÁ D-MEMC-CCM-VLMB-AJCC. Characterization of ESKAPE bacteria revealed the presence of Acinetobacter baumannii and Pseudomonas aeruginosa outbreaks in COVID-19/VAP patients.
- Alsaab Fm DSNBSAGGvHML. Computationally Designed AMPs with Antibacterial and Antibiofilm Activity against MDR Acinetobacter baumannii.
- Osman M YIHMAMHGTABBCKJMJYH. Emergence of Extended-Spectrum Cephalosporin- and Colistin-Resistant Enterobacterales in Otherwise Healthy University Students.
- Roszak M DBC-HESNJJGB. Bacteriophage-ciprofloxacin combination effectiveness depends on the growth model of Staphylococcus aureus-Candida albicans dual-species communities.
- Yap Psx AKACCWNSTTCSJ. Genomic Insights into Two Colistin-Resistant Klebsiella pneumoniae Strains Isolated from the Stool of Preterm Neonate During the First Week of Life.
- Selvarajan R OCSTAALKLH. Evolution and Emergence of Antibiotic Resistance in Given Ecosystems: Possible Strategies for Addressing the Challenge of Antibiotic Resistance.
- Shooraj M TMK-YM. A Review on the Antibiotic Resistance of Shigella Strains in Iran.
- Cheng H JHFJZC. Antibiotic Resistance and Characteristics of Integrons in Escherichia coli Isolated from Penaeus vannamei at a Freshwater Shrimp Farm in Zhejiang Province, China.
- Khan S BTKKCW. Relationship between antibiotic and disinfectant resistance profiles of bacteria harvested from tap water.
- Brooks Bd BAE. Therapeutic strategies to combat antibiotic resistance.
- Janarthanan S RSHS. Myconanoparticles Break Antibiotic Resistance in Staphylococcus aureus and Acinetobacter baumannii.
- Jeje O EAJJ-FLKGJL, Jr. Serving Two Masters: Effect of Escherichia coli Dual Resistance on Antibiotic Susceptibility.
- Collignon P PJHCTMA-KAAFM. World Health Organization Ranking of Antimicrobials According to Their Importance in Human Medicine: A Critical Step for Developing Risk Management Strategies to Control Antimicrobial Resistance From Food Animal production.
- Gu Y KM. How can we fight against antimicrobial-resistant bacteria in the World Health Organization Western Pacific Region?
- Sulis G SSKSBNGIYLHCMATEYS. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug-resistant bacteria: a systematic review and meta-analysis.
- van Huizen P KLRPLCCJ. The nurses’ role in antimicrobial stewardship: A scoping review.
- Han Ks GLRVCSS-HK. Antimicrobial stewardship approach: Prevalence of antimicrobial resistant bacteria at a regional hospital in South Africa.
- Turner Mm CH. Communicating Commitment to Antibiotic Stewardship: an Effective Strategy for Responding to Online Patient Reviews.
- Friedman ND. Antimicrobial Stewardship: The Need to Cover All Bases.
- Hazards EPanel oB, Ricci A AABDCMDRFEPSGRK. Risk for the development of Antimicrobial Resistance (AMR) due to feeding of calves with milk containing residues of antibiotics.
- Hess As SMJJKTKARMCNGASMDJHAD. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria.
- Mane Mb BVMBKRVV. Destroying antimicrobial resistant bacteria (AMR) and difficult, opportunistic pathogen using cavitation and natural oils/plant extract.
- Phillips C CBAACCAPEJR-SRJSBAMCP. A scoping review of factors potentially linked with antimicrobial-resistant bacteria from turkeys (iAM.AMR Project). 104. Schirò G GDMFVMGAPGAFLFCG. Antimicrobial Resistance (AMR) of Bacteria Isolated from Dogs with Canine Parvovirus (CPV) infection: The need for rational use of antibiotics in anion animal health.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).