Submitted:
27 July 2025
Posted:
28 July 2025
You are already at the latest version
Abstract
Keywords:
1. Extremely Low Exposure Levels and Regulatory Comparisons
2. Trace-Level Concentrations Are Below Selection Thresholds
3. Lack of Evidence for Cross-Resistance or Transferability
Additional Considerations
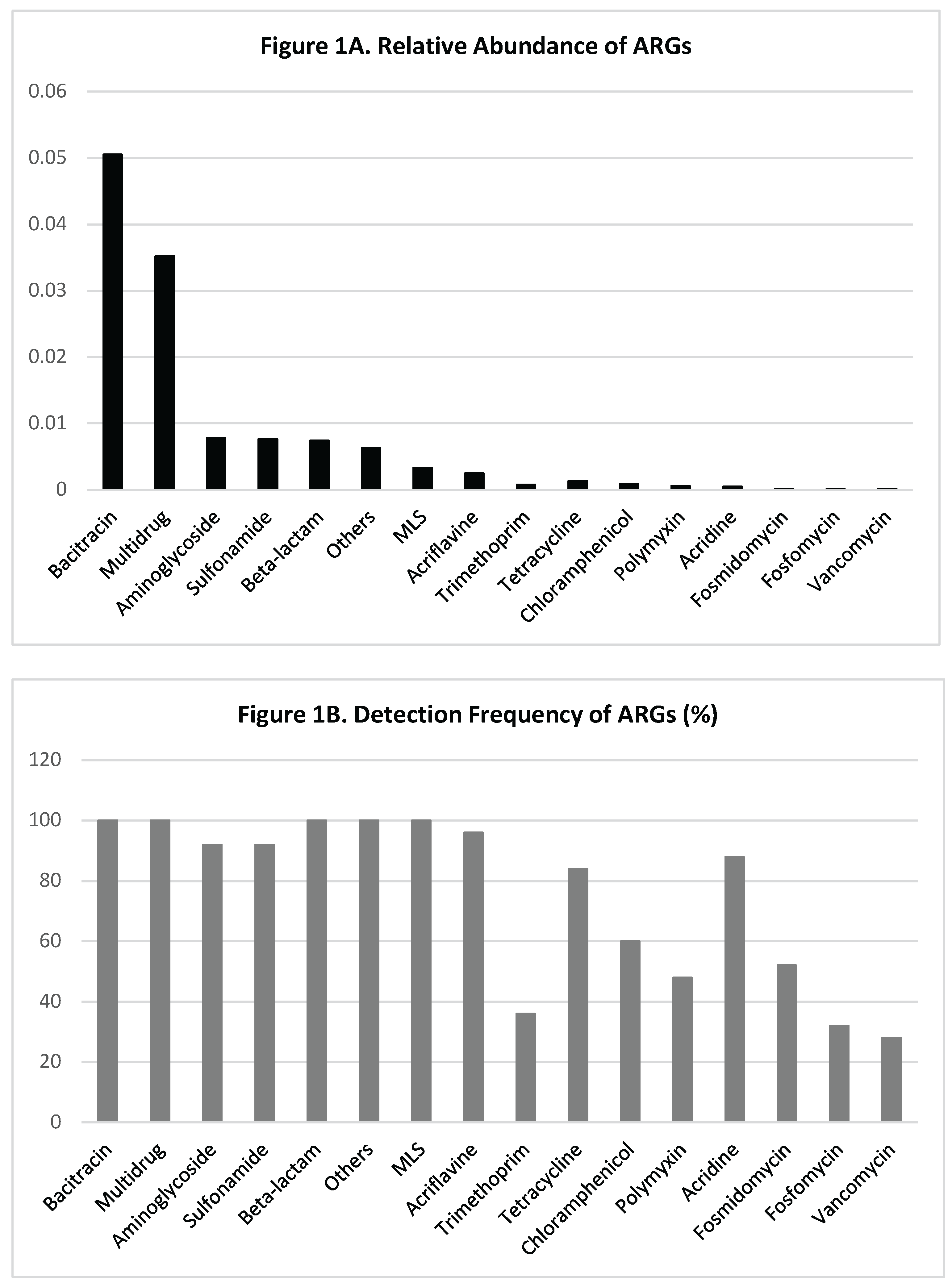
- The levels of bacitracin present in subtilisin preparations do not raise safety concerns.
- Antimicrobial resistance (AMR) cannot develop at bacitracin concentrations below its minimum inhibitory concentration (MIC); levels in subtilisin are nearly 60-fold below the MIC and approximately 4000-fold below the minimal selective concentration (MSC) referenced by EFSA.
- Bacitracin does not induce cross-resistance with human medicinal antibiotics or other marketed antimicrobials.
- The transferability of bacitracin resistance via plasmids or other mobile genetic elements is unlikely.
- Environmental concentrations of bacitracin in groundwater and soil are significantly higher than those found in subtilisin preparations.
- Acceptable residue limits for bacitracin in meat, eggs, and milk, as established by regulatory bodies, are far higher than the trace levels present in food processed with subtilisin.
- Therefore, the likelihood that the use of subtilisin in food processing contributes to AMR development is considerably lower than other existing exposure pathways.
| Source or Threshold | Bacitracin Concentration (ng/mL or ng/g) |
Reference / Note |
|---|---|---|
| Subtilisin-processed food (estimated) | ≤ 0.002 | 4000× below MSC; 60× below MIC |
| Minimal Selective Concentration (MSC) | 8 | Hypothetical threshold (EFSA) |
| Minimum Inhibitory Concentration (MIC) | 2000 | Effective bacterial inhibition [11] |
| EU MRL in milk (‡) | 50,400 | 1.2 IU/mL × 42 (†)[9,10] |
| EU MRL in meat | 29,400 | 0.7 IU/g × 42 [9,10] |
| EU MRL in eggs | 201,600 | 4.8 IU/g × 42 [9,10] |
| Groundwater/Drinking water (natural presence) | Up to 10 | Detected environmental levels [27,28] |
| Animal feed (therapeutic use) | 100,000–200,000 | Used as additive [14] |
Acknowledgments
References
- Buijs, N.P.; Vlaming, H.C.; Kotsogianni, I.; Arts, M.; Willemse, J.; Duan, Y.; Alexander, F.M.; Cochrane, S.A.; Schneider, T.; Martin, N.I. A Classic Antibiotic Reimagined: Rationally Designed Bacitracin Variants Exhibit Potent Activity against Vancomycin-Resistant Pathogens. Proc Natl Acad Sci U S A 2024, 121, e2315310121. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.J.; Strominger, J.L. Mechanism of Action of Bacitracin: Complexation with Metal Ion and C55-Isoprenyl Pyrophosphate. Proc Natl Acad Sci U S A 1971, 68, 3223. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Shafique, M.; Saleem, F.; Azim, M.K.; Jabeen, N.; Naz, S.A. Characterization of the Genome and Serine Protease of a Novel Bacillus Subtilis Isolate. Antonie van Leeuwenhoek, International Journal of General and Molecular Microbiology 2022, 115, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lambré, C.; Lampi, E.; Mengelers, M.; et al. Taxonomic Identity of the Bacillus Licheniformis Strains Used to Produce Food Enzymes Evaluated in Published EFSA Opinions. EFSA Journal 2024, 22, e8770. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, C.A.; Kwon, S.W.; Rooney, A.P.; Kim, S.J. Bacillus Paralicheniformis Sp. Nov., Isolated from Fermented Soybean Paste. Int J Syst Evol Microbiol 2015, 65, 3487–3492. [Google Scholar] [CrossRef] [PubMed]
- Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; Mortensen, A.; et al. Safety Evaluation of the Food Enzyme Subtilisin from the Non-genetically Modified Bacillus Paralicheniformis Strain DP-Dzx96. EFSA Journal 2023, 21, e08155. [Google Scholar] [CrossRef] [PubMed]
- Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; Mortensen, A.; et al. Safety Evaluation of the Food Enzyme Subtilisin from the Non-genetically Modified Bacillus Paralicheniformis Strain LMG S-30155. EFSA Journal 2023, 21, e07910. [Google Scholar] [CrossRef] [PubMed]
- Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; Mortensen, A.; et al. Safety Evaluation of the Food Enzyme Subtilisin from the Non-genetically Modified Bacillus Paralicheniformis Strain AP-01. EFSA Journal 2024, 22, e8873. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA) Bacitracin: JECFA Evaluation Summary – Food Safety and Quality; 1968.
- European Commission EU Regulation 37/2010 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin; Brussels, 2010.
- Gu, Y.; Song, S.; Zhu, Q.; Jiao, R.; Lin, X.; Yang, F.; Veen, S. van der Bacitracin Enhances Ceftriaxone Susceptibility of the High-Level Ceftriaxone-Resistant Gonococcal FC428 Clone. Microbiol Spectr 2023, 11, e02449-23. [Google Scholar] [CrossRef] [PubMed]
- Axelsen, P.H. A Chaotic Pore Model of Polypeptide Antibiotic Action. Biophys J 2008, 94, 1549. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect Dis 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I. The Use of Bacitracin as a Growth Promoter in Animals Produces No Risk to Human Health. Journal of Antimicrobial Chemotherapy 1999, 44, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Li, Q.; Kohut, B.; Biedenbach, D.J.; Bell, J.; Turnidge, J.D. Contemporary Antimicrobial Activity of Triple Antibiotic Ointment: A Multiphased Study of Recent Clinical Isolates in the United States and Australia. Diagn Microbiol Infect Dis 2006, 54, 63–71. [Google Scholar] [CrossRef] [PubMed]
- A.L. Laboratories, Inc. Environmental Assessment for BMD® 514.1(b)10. Supplement to 21 CFR 558.76.; 1989.
- Alpharma AS, A.A.H.D. Bacitracin Zinc – New Evidence: Resistance and Safety to Human Health. Internal Report 1999.
- Alpharma Animal Health Division Public Health Impact of the Use of Bacitracin Zinc in Animals. Scientific Dossier for the Re-Examination of the Decision in Reg. (EC) 2821/98 Regarding the Use of Zinc Bacitracin in Animal Feeds. Internal Report 2000.
- Mathers, J. Bacitracin — Natural Peptide with Minimal Resistance Issues: A Comprehensive Overview Including Its Efficacy Against Clostridium Perfringens. In Proceedings of the Proceedings of Alpharma’s Swine Enteric Health Symposium; Alpharma Animal Health, 2008.
- Thibodeau, A.; Quessy, S.; Guévremont, E.; Houde, A.; Topp, E.; Diarra, M.S.; Letellier, A. Antibiotic Resistance in Escherichia Coli and Enterococcus Spp. Isolates from Commercial Broiler Chickens Receiving Growth-Promoting Doses of Bacitracin or Virginiamycin. Canadian Journal of Veterinary Research 2008, 72, 129. [Google Scholar] [PubMed]
- Walton, J.R.; Laerdal, O.A. The Effect of Dietary Zinc Bacitracin on the Resistance Status of Porcine Strains of Escherichia Coli. In Proceedings of the Proceedings of the 1980 International Pig Veterinary Society Congress; International Pig Veterinary Society , 1980.
- Helmuth, R.; Bulling, E. Proceedings of the Symposium “Criteria and Methods for the Microbiological Evaluation of Growth Promotors in Animal Feeds.” In Proceedings of the VetMed-Hefte; 1989.
- Walton, J.R. The Effect of Dietary Zinc Bacitracin on the Resistance Status of Intestinal Escherichia Coli and Enterococci from Broiler Chickens. Zentralblatt für Veterinärmedizin Reihe B 1984, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mathers, J.J. Individual and Combined in Vitro Effects of Bacitracin and Chlortetracycline in Swine-Derived Clostridium Spp. In Proceedings of the Abstracts of the American Society for Microbiology Annual Meeting (ASM Ann Meet); American Society for Microbiology, 2004; p. “Poster A-150”.
- Nguyen, R.; Khanna, N.R.; Safadi, A.O.; Patel, P.; Sun, Y. Bacitracin Topical. StatPearls 2024. [Google Scholar]
- Chang, T.-W.; Gorbach, S.L.; Bartlett, J.G.; Saginur, R. Bacitracin Treatment of Antibiotic-Associated Colitis and Diarrhea Caused by Clostridium Dilficile Toxin. Gastroenterology 1980, 78, 1584–1586. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Xu, B.; Chen, H.; Zhao, X.; Li, G.; Zheng, Y.; Qiu, W.; Zheng, C.; Duan, L.; Wang, W. Occurrence and Distribution of Antibiotics in Groundwater, Surface Water, and Sediment in Xiong’an New Area, China, and Their Relationship with Antibiotic Resistance Genes. Science of The Total Environment 2022, 807, 151011. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, B.; Jiang, X.T.; Wang, Y.L.; Xia, Y.; Li, A.D.; Zhang, T. Catalogue of Antibiotic Resistome and Host-Tracking in Drinking Water Deciphered by a Large Scale Survey. Microbiome 2017, 5, 154. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




