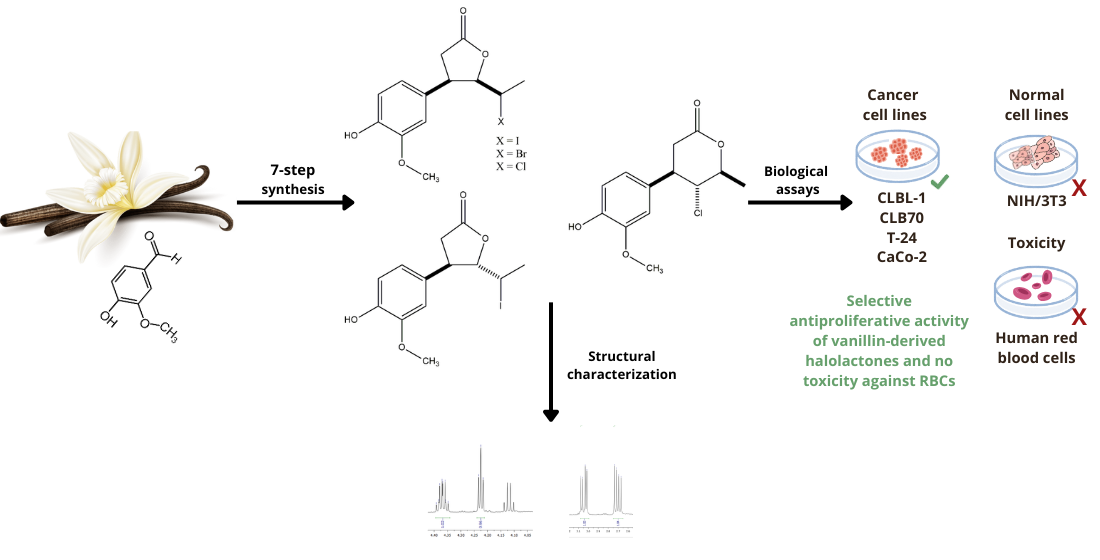
1. Introduction
Lactones are intramolecular esters of hydroxy acids, found in both natural and synthetic sources. Five- and six-membered lactone rings predominate in nature due to their favorable thermodynamic stability [
1]. Natural compounds containing lactone rings are produced mainly by plants [
2,
3,
4], but also by insects [
5], microorganisms [
6], and marine organisms [
7,
8], where they function as secondary metabolites.
This structurally diverse group of compounds exhibits a wide range of biological activities, including antibacterial [
9,
10,
11], anti-inflammatory [
12,
13], antifeedant [
14,
15,
16], and antifungal properties [
17,
18,
19]. Due to their broad spectrum of bioactivities, lactones are applied in economically important sectors such as the food industry, medicine, and agriculture. Their multifunctional nature makes them attractive candidates for the development of bioactive agents and functional materials.
However, particular attention has been paid to their anticancer properties [
20,
21,
22,
23]. Numerous studies have been conducted to demonstrate the cytotoxic activity of lactones containing an aromatic substituent against cancer or normal cell lines [
24,
25,
26,
27,
28,
29]. Selected β-aryl-δ-halo-γ-lactones synthesized from simple aromatic aldehydes exhibit cytotoxicity against human (e.g., Jurkat, HL-60, AGS) and canine (e.g., D17, GL-1, CLBL-1) cancer cell lines [
30,
31,
32,
33]. The mechanism of action of these compounds involves the induction of apoptosis via a mitochondrial-dependent pathway and caspase activation, as demonstrated for cuminaldehyde-derived δ-iodo-γ-lactones [
34]. Enantiomeric
trans β-aryl-δ-iodo-γ-lactones derived from 2,5-dimethylbenzaldehyde were shown to trigger cancer cell apoptosis by downregulating the anti-apoptotic proteins Bcl-2 and Bcl-xL. In the canine cell lines used in the study, the tested compounds also activated the receptor-mediated apoptotic pathway through fragmentation of the Bid protein, thereby enhancing their pro-apoptotic effects [
35]. To gain deeper insight into their biophysical behavior and anticancer mechanisms, enantiomeric piperonal-derived
trans β-aryl-δ-iodo-γ-lactones have been extensively studied for their ability to induce apoptosis and interact with biological systems, including cancer cell membranes and biomacromolecules. Membrane interaction studies revealed a reduction in membrane fluidity, correlating with a marked induction of apoptosis by these lactones [
36].
Cis β-aryl-δ-iodo-γ-lactones containing phenyl or
p-alkyl-substituted phenyl rings exhibited cytotoxic activity against HeLa (human cervix carcinoma) and MCF-7 (human breast adenocarcinoma) cancer cell lines, and significantly disrupted the antioxidative/oxidative balance in the NHDF (normal human dermal fibroblasts) cell line [
37].
Continuing our studies on the biological activity of lactones bearing an aromatic ring, we designed and synthesized a series of novel β-arylhalolactones using vanillin
(1) as the starting material. This naturally occurring aromatic aldehyde has a well-documented spectrum of biological properties, including gastro- and cardioprotective, diuretic, anti-organ toxicity, antimicrobial, anti-inflammatory, neuroprotective, and anti-infective effects, among others [
38,
39,
40]. Numerous research groups have also reported significant anticancer activity of vanillin
(1) against various human cancer cell lines, like MCF-7 (breast cancer) [
41], HepG2 (hepatocellular carcinoma) [
42], HT-29 (colorectal adenocarcinoma) [
43], NCI-H460 (non-small cell lung carcinoma) [
44], as well as A2058 (malignant melanoma) cell lines [
45].
Due to the presence of various reactive functional groups, vanillin
(1) is also a versatile building block for the synthesis of novel derivatives, including a wide range of heterocyclic systems such as pyrimidines, quinoxalines, imidazoles, and thiazoles, which are highly relevant in medicinal and pharmaceutical chemistry [
46]. Vanillin-derived compounds have demonstrated a broad spectrum of pharmacological activities. Notably, hydrazone derivatives of vanillin
(1) exhibit antimicrobial, anticancer, and enzyme inhibition properties, making them promising candidates for therapeutic applications [
47]. Additionally, derivatives bearing tacrine or naphthalimido moieties have been developed as multi-target agents for Alzheimer’s disease therapy. These compounds act as antioxidants and inhibit acetylcholinesterase (AChE) as well as β-amyloid peptide aggregation, both of which are key pathological features of Alzheimer’s disease [
48]. Other vanillin-based derivatives have been designed as dual inhibitors targeting both AChE and butyrylcholinesterase (BuChE), thereby enhancing their potential efficacy in neurodegenerative conditions [
49]. Regarding anticancer activity, certain vanillin derivatives-such as compounds based on the SBE13 scaffold significantly outperform the parent compound by potently inducing apoptosis and reducing Plk1 kinase activity in HeLa cells, indicating strong antiproliferative effects [
50]. This further emphasizes the potential of vanillin as a versatile scaffold for designing targeted anticancer therapeutics.
The presence of phenolic fragment is an advantageous factor to influence the potential of vanillin-derived halolactones as a promising bioactive compound with different therapeutic properties including cytotoxic, antioxidant, antiinflammatory which can be of high relevance in oncology. Here we would like to present the synthesis and studies on the cytotoxicity of new halolactones obtained from vanillin (1) towards different cancer cell lines. Due to the frequently observed high toxicity of anticancer agents towards healthy cells, we also decided to investigate the hemolytic properties of the synthesized halolactones towards human red blood cells (RBCs). Owing to their simple structure and crucial physiological functions, these cells are a simple and well established model to evaluate general hemolytic potential, as erythrocyte membranes are highly sensitive to chemical induced damage and serve as a convenient indicator of membrane disruptive properties of bioactive compounds.
2. Results and Discussion
2.1. Synthesis of Vanillin-Derived Halolactones
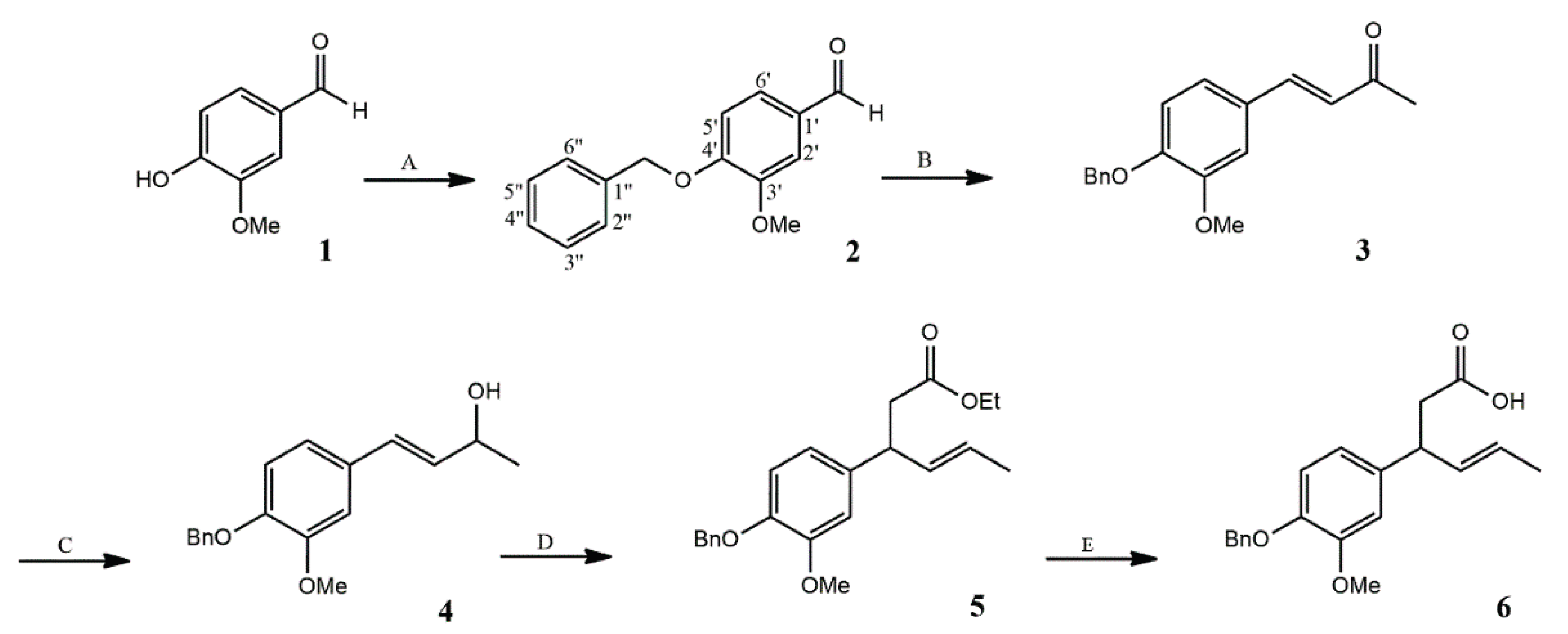
The presence of a reactive phenolic group in the benzene ring of vanillin (1) dictated our synthetic strategy, which included the benzyl protection of vanillin (1), the synthesis of the key intermediate γ,δ-unsaturated carboxylic acid 6, and subsequent halolactonization reactions, followed by a final deprotection step to obtain the target lactones. The use of benzyl protection was essential to prevent side reactions involving the phenolic OH group during the multistep synthesis.
2.1.1. Synthesis of α,β-Unsaturated Carboxylic Acid 6
The five-step synthesis of γ,δ-unsaturated carboxylic acid
6 is shown on
Scheme 1.
Benzylation of vanillin (1) was carried out using an ethanolic solution of benzyl bromide in the presence of potassium carbonate. Benzylvanillin 2 was obtained in an 88% yield after crystallization from ethanol. The presence of the benzyl group was confirmed by the 1H NMR spectrum, which showed multiplets in the range of 7.32–7.47 ppm corresponding to the aromatic protons, and a distinctive singlet at 5.21 ppm attributed to the benzylic protons of the methylene group. These characteristic signals provided clear evidence of successful benzyl protection, an essential step for subsequent synthetic transformations.
In the next step, benzylvanillin 2 was converted to the α,β-unsaturated ketone 3 in high yield (84%) via Claisen–Schmidt condensation with acetone under alkaline conditions. The coupling constant between the olefinic protons observed in the 1H NMR spectrum (J = 16.2 Hz) confirmed the E-configuration of the C3–C4 double bond.
Reduction of ketone (3) by sodium borohydride afforded exclusively the corresponding allylic alcohol 4. Spectroscopic data confirmed the selective reduction of the carbonyl group, indicated by a multiplet at 4.47 ppm from H-2 and a singlet corresponding to the hydroxyl group at 1.64 ppm, as well as a strong band at 3384 cm⁻¹ attributed to O–H stretching vibrations. The retention of the E-configured double bond was confirmed by the coupling constant between the H-3 and H-4 protons (J = 15.8 Hz).
Alcohol 4 was subjected to the Johnson–Claisen rearrangement using triethyl orthoacetate in the presence of a catalytic amount of propionic acid at 138 °C. Consistently with the mechanism of Johnson - Claisen rearrangement, E configuration of double bond in γ,δ-unsaturated ester 5 was retained, as indicated by the coupling constant (J = 15.3 Hz) between olefinic protons H-4 and H-5. Ester 5, after two-step purification (first by gravity chromatography and then by flash chromatography) was hydrolyzed in a 10% ethanolic NaOH solution under reflux to afford the γ,δ-unsaturated carboxylic acid 6.
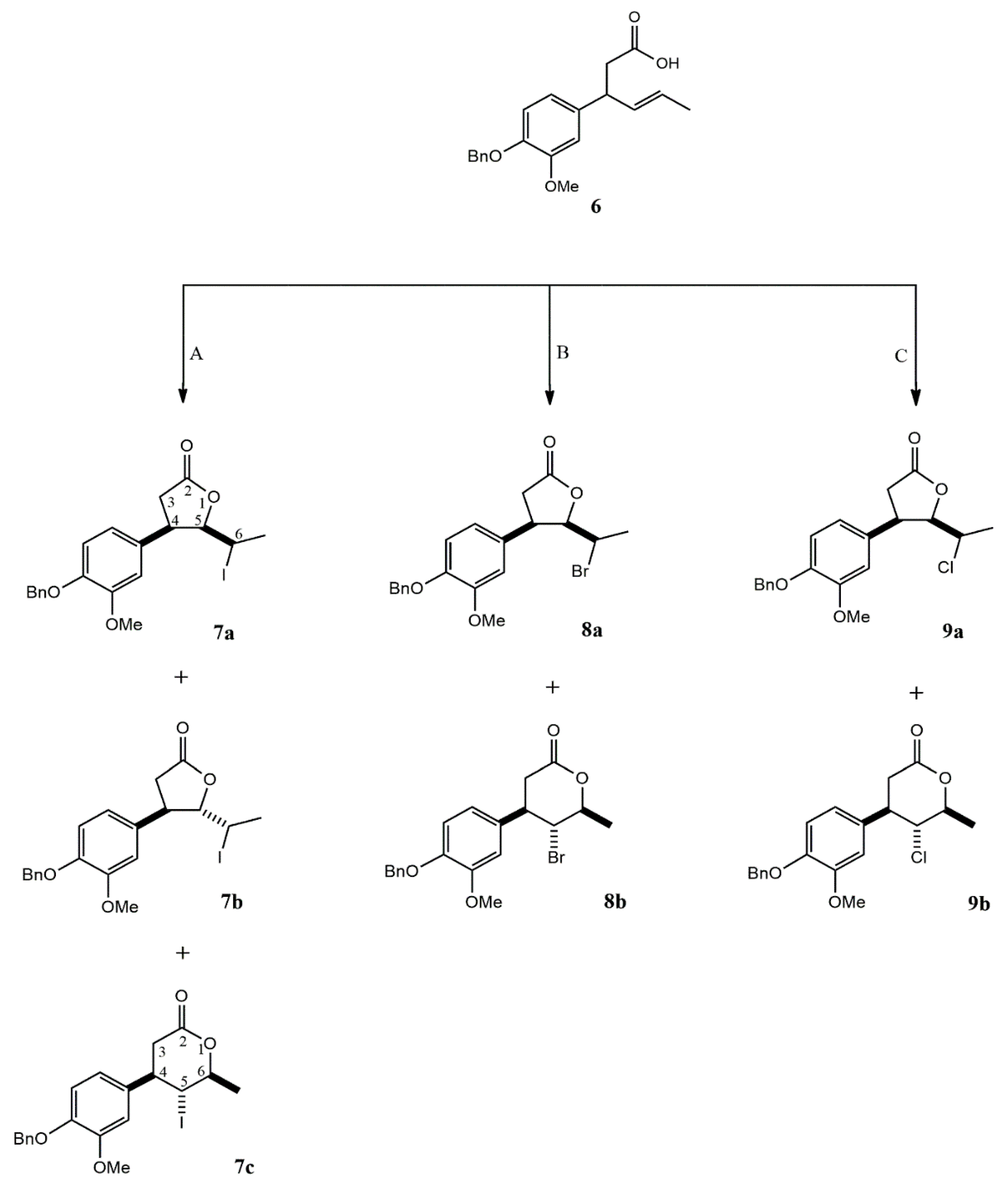
2.1.2. Halolactonizations of γ,δ-unsaturated Carboxylic Acid 6
Acid
6 was subjected to halolactonization reactions, leading to the formation of iodo-, bromo-, and chlorolactones as illustrated on
Scheme 2.
The iodolactonization reaction was carried out using iodine in potassium iodide in a biphasic system Et2O/NaHCO₃. After reaction, three products were successfully isolated and purified by flash chromatography.
The major isolated products were two γ-lactones obtained as a result of 5-exo cyclization. Spectroscopic analysis (1H NMR) revealed significant differences in the chemical shifts and multiplicities of the H-4, H-5, and H-6 protons, enabling confirmation of the relative orientation of substituents at C-4 and C-5 of the γ-lactone ring. For example, in the case of the cis isomer 7a, the signal from the H-6 proton was significantly shifted upfield (3.47 ppm) compared to its chemical shift in the trans isomer 7b (4.36 ppm), which resulted from the location of H-6 within the shielding cone of the aromatic ring. The shape of the multiplet (doublet of quartets) and the coupling constant between H-6 and H-5 (J = 10.9 Hz) indicated an antiperiplanar orientation of the C5–H5 and C6–H6 bonds. For the trans δ-iodo-γ-lactone 7b, a characteristic triplet at 4.23 ppm from H-5 (J = 5.3 Hz) was observed instead of the doublet of doublets at 4.78 ppm found in the spectrum of the cis isomer.
In the case of the trans δ-iodo-γ-lactone, additional minor signals were observed in the
1H and
13C NMR spectra, resulting from a conformational equilibrium between two conformers: a predominant syn conformer in which the C–I and C–O bonds adopt a “gauche” conformation, and a minor anti conformer where these bonds are arranged in an antiperiplanar orientation. This phenomenon was previously reported and elucidated based on crystallographic analysis by Gładkowski et al. (2013) for analogs of lactone
7b, namely trans δ-iodo-γ-lactones bearing an unsubstituted phenyl ring at the β-position [
31].
In the case of the minor isolated product of 5-endo iodolactonization, γ-iodo-δ-lactone 7c, the most informative signal was the triplet from the H-5 proton at 4.00 ppm. The coupling constant (J = 10.6 Hz) between the H-4 and H-6 protons observed in the 1H NMR spectrum unequivocally indicated a trans orientation of the iodine at C-5 relative to both the benzene ring at C-4 and the methyl group at C-6.
Bromo- and chlorolactonization of acid 6 were carried out using N-bromosuccinimide (NBS) and N-chlorosuccinimide (NCS), respectively, in anhydrous tetrahydrofuran with a catalytic amount of acetic acid. Both cis δ-bromo-γ-lactone 8a and cis δ-chloro-γ-lactone 9a, as well as their corresponding six-membered analogues 8b, 9b, were isolated from the product mixtures. However, attempts to isolate the pure stereoisomers of the trans γ-lactones were unsuccessful.
1H NMR data showed a high degree of resemblance in the shapes and coupling constant values of the signals from protons H-4, H-5, and H-6 compared to the corresponding signals observed in the spectra of the analogous iodolactones
7a and
7c. Among the halogens, the greatest deshielding effect for protons H-5 and H-6 was observed for iodine, followed by bromine and chlorine, which is consistent with literature data for analogous β-aryl-δ-halo-γ-lactones and β-aryl-γ-halo-δ-lactones [
31].
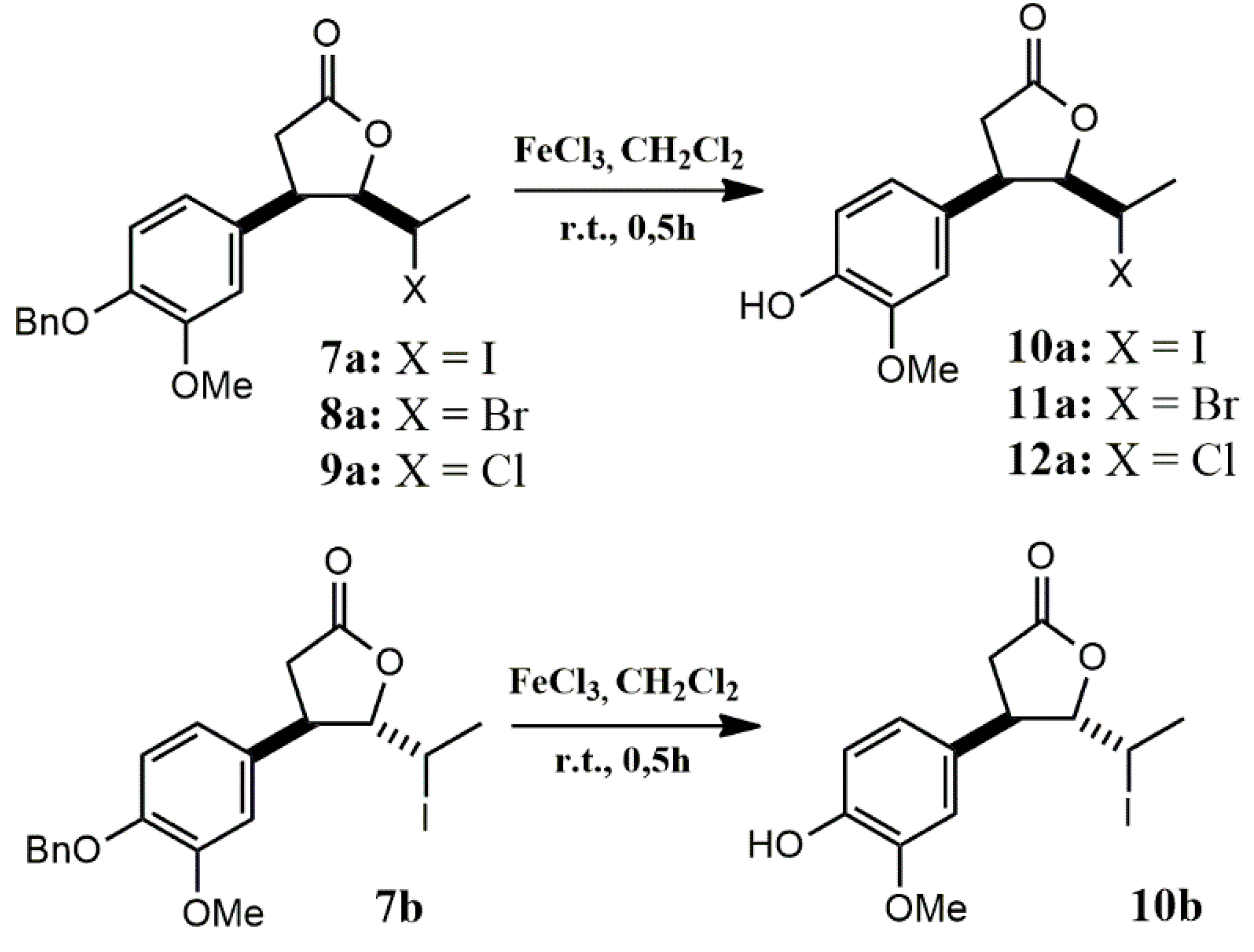
2.1.3. Benzyl Deprotection of Halolactones 7a-c, 8a,b, 9a,b
The final stage of the synthesis involved the removal of the benzyl protecting group. To identify a selective and efficient method, various deprotection strategies were tested using
cis δ-iodo-γ-lactone
7a as a model substrate. The first approach, catalytic hydrogenolysis over Pd/C, was chosen due to its general reliability and operational simplicity in benzyl group removal [
51]. However, in this case, the reaction led to a mixture of more polar products than the starting iodolactone
7a. In search of greater selectivity,
7a was treated with 33% HBr in acetic acid [
52], but the reaction yielded a complex mixture, and GC and NMR analysis indicated isomerization of
7a to its
trans isomer
7b. The resulting products were inseparable by standard purification techniques. Similarly, treatment with concentrated HCl under reflux [
53] resulted in a mixture of highly polar products. The use of aqueous NaBrO₃ and Na₂S₂O₃ solutions [
54] also produced a mixture of more and less polar compounds relative to
7a, that were difficult to separate. Ultimately, the only effective deprotection method involved the use of anhydrous FeCl₃ in dry dichloromethane at room temperature under a nitrogen atmosphere [
55]. This approach enabled successful removal of the benzyl group, though the initial isolated yield of the final lactone
10a after flash chromatography was only 10%, likely due to complexation of the phenolic product with Fe³⁺ ions. To improve the yield, during the work-up procedure reaction mixture was washed with 1% phosphoric(V) acid, following the protocol described by Giri et al. (2020) for FeCl₃-mediated deprotection of Boc-protected amino acids and peptides [
56]. This modification increased the isolated yield of
10a to 28%, significantly enhancing the efficiency of the deprotection process.
Following this procedure, successful debenzylation of
cis δ-halo-γ-lactones
7a-
9a and
trans δ-iodo-γ-lactone
7b was carried out, affording the corresponding deprotected lactones
10a-
12a and
10b, respectively
(Scheme 3). The removal of the benzyl group was confirmed by the disappearance of the aromatic proton signals in the range of 7.31–7.47 ppm and the singlet at 5.14–5.15 ppm corresponding to the benzylic methylene protons in the
1H NMR spectra. In place of these, a new singlet appeared at approximately 5.61–5.62 ppm, along with a broad band in the IR spectrum in the range of 3333–3422 cm⁻¹, corresponding to O–H stretching vibrations confirming the presence of a free hydroxy group. Moreover, the successful deprotection was additionally evidenced by the expected molecular masses of the target compounds determined by HRMS analysis. Together, these results provide strong evidence for the complete and selective removal of the benzyl protecting group under the optimized reaction conditions.
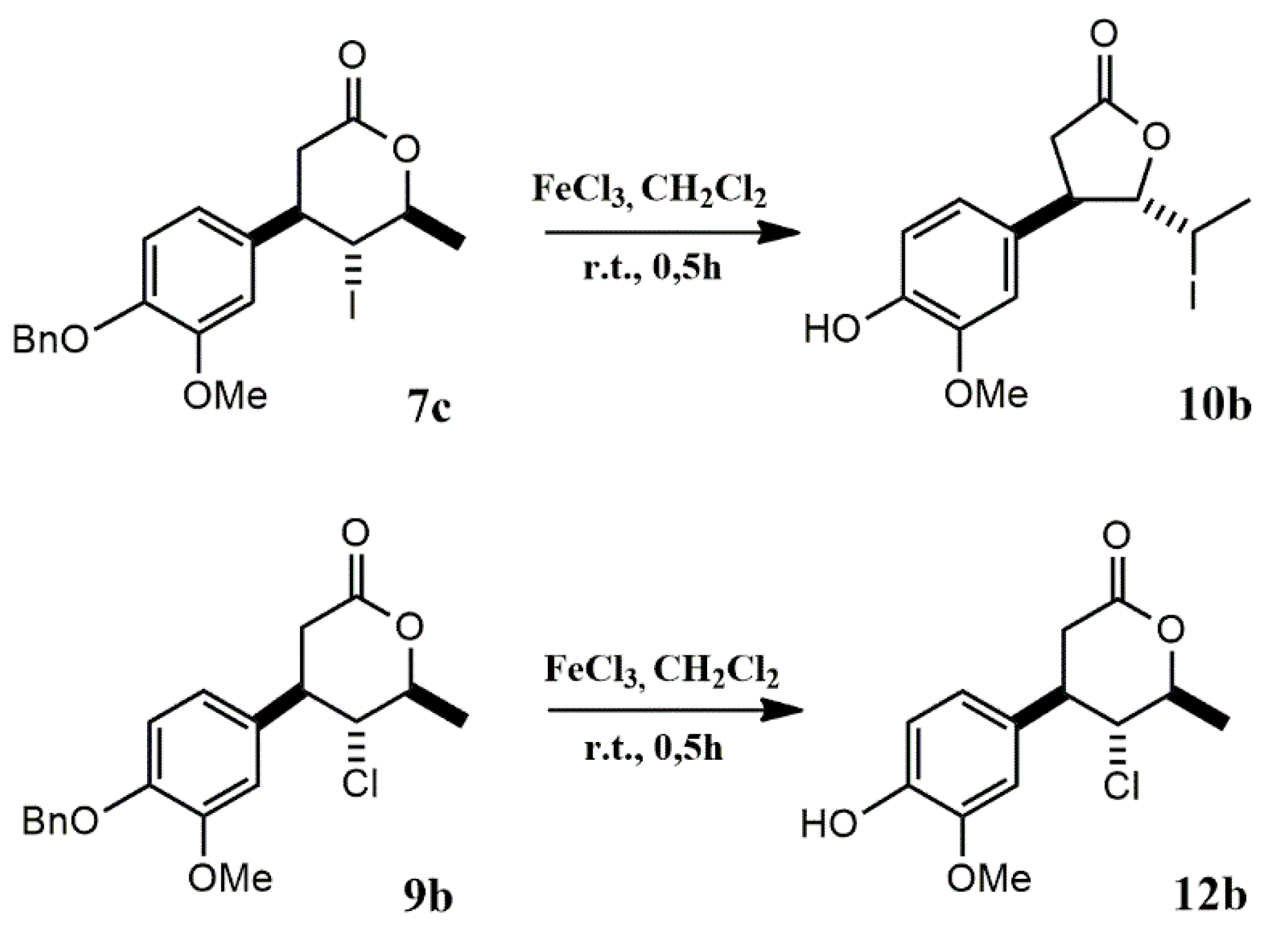
An analogous procedure was applied for the deprotection of γ-halo-δ-lactones
7c,
8b, and
9b. Among these, only in the case of γ-chloro-δ-lactone
9b was a successful deprotection achieved, leading to the isolation of the expected deprotected δ-lactone
12b as the sole product. Interestingly, NMR analysis of the product obtained from the deprotection of γ-iodo-δ-lactone
7c clearly confirmed the structure of
trans δ-iodo-γ-lactone
10b, suggesting the occurrence of an intramolecular rearrangement through a translactonization process (
Scheme 4). In contrast, for the bromo analogue
8b, TLC and GC analyses revealed the slow formation of two inseparable products, indicating a more complex and less selective reaction course.
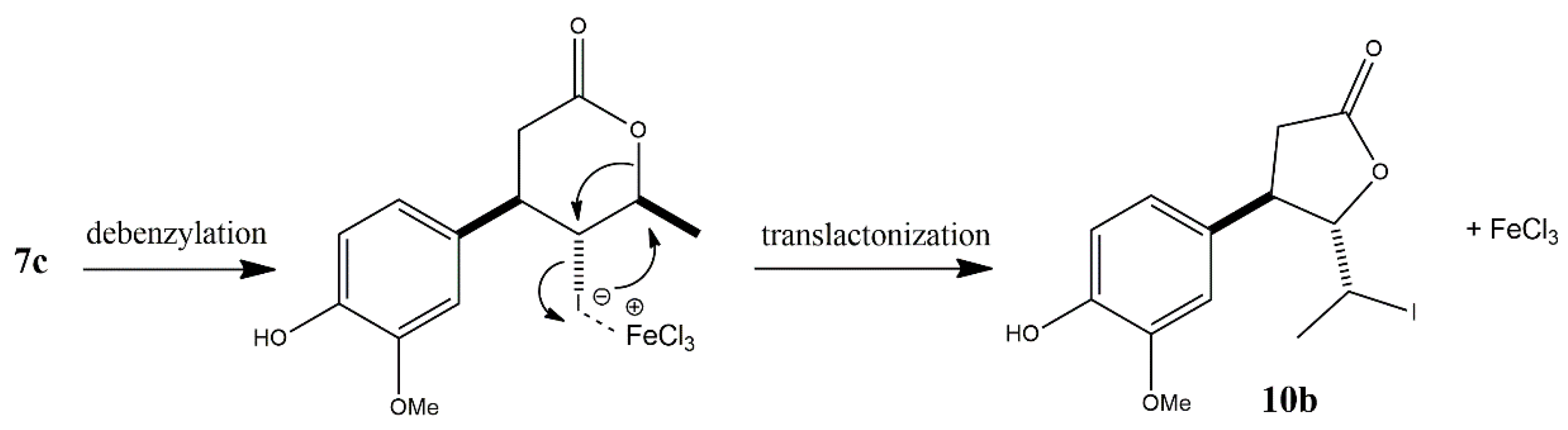
The proposed mechanism of the translactonization process, which occurs during FeCl₃-mediated debenzylation of γ-iodo-δ-lactone
7c, is illustrated in
Scheme 5.
The rearrangement appears to proceed in a single step via intramolecular nucleophilic substitution, facilitated by FeCl₃. Acting as a Lewis acid, FeCl₃ promotes the transposition of the iodine atom from the C-5 to the C-6 position, accompanied by a simultaneous nucleophilic attack of the lactone oxygen on the C-5 carbon, resulting in the formation of a five-membered ring. The exclusive formation of the trans isomer strongly suggests that the substitution follows an SN2-type mechanism, in which the oxygen nucleophile approaches from the side opposite to the departing iodine atom.
Translactonization converting δ-lactones into γ-lactones has been previously reported in the literature, both under chemical and microbiological halolactonization conditions. Kamizela et al. (2019) observed the unexpected formation of δ-hydroxy-γ-lactones among the products of iodolactonization of 3-methyl-5-arylpent-4-enoic acids using I₂/KI in a biphasic NaHCO₃/Et₂O aqueous system. This phenomenon was attributed to the rearrangement of initially formed γ-iodo-δ-lactones. In that case, the reaction was proposed to proceed via an S
N1-like mechanism, as evidenced by the formation of both cis and trans isomers of the δ-hydroxy-γ-lactones. The process involved a dehalogenation-lactonization sequence with two simultaneous nucleophilic substitutions: replacement of the iodine atom at C-5 by the lactone oxygen, and nucleophilic attack of water at C-6 [
24]. Interestingly, in one of our previous studies, a similar rearrangement was observed during the biotransformation of a chalcone-derived γ-bromo-δ-lactone in the culture of Penicillium frequentans AM 359. In contrast to the chemically induced rearrangement, the microbial process proceeded via an S
N2 mechanism, which led exclusively to the formation of a single trans isomer of the hydroxylactone [
57].
The translactonization of γ-halo-δ-lactones reported previously typically involved the participation of water, leading to the formation of δ-hydroxy-γ-lactones. In contrast, the FeCl₃-mediated debenzylation of γ-iodo-δ-lactone 7c was carried out under strictly anhydrous conditions, which favored an intramolecular SN2-type reaction and resulted in the selective formation of the trans δ-iodo-γ-lactone. To the best of our knowledge, this type of rearrangement has not been previously reported and may be attributed to the higher thermodynamic stability of five-membered lactone rings compared to six-membered ones. With regard to the relative reactivity of halogens in the presence of FeCl₃, the order can be classified as I > Br > Cl. This trend corresponds to the bond strength between the carbon and halogen atoms, iodine forms the weakest bond, rendering γ-iodo-δ-lactones less stable and more prone to rearrangement. Consequently, no translactonization was observed for γ-chloro-δ-lactone 9b, and the process occurred only very slowly for its bromo analogue 8b. Even after 2 h of reaction, TLC analysis revealed the presence of two distinct products, corresponding to the unrearranged δ-lactone and the newly formed γ-lactone.
2.2. Antiproliferative Activity
To evaluate their antiproliferative potential, synthesized vanillin-derived halolactones
10a,
b,
11a and
12a,
b as well starting vanillin
(1) were subjected to
in vitro MTT assay against panel of selected cancer cell lines: two lines representing hematopoietic canine cancers: canine B-cell lymphoma cell line (CLBL-1) and chronic B-cell leukemia (CLB70) and two human cancer lines: bladder carcinoma (T-24) and colorectal adenocarcinoma (CaCo-2). Normal cell line of mouse embryonic fibroblasts (NIH/3T3) was also used in these studies. The results, expressed as IC
50 values are shown in
Table 1.
All tested compounds exhibited measurable antiproliferative activity against the canine cancer cell lines, whereas only three compounds showed notable effects against the human cancer cell lines (T-24 or CaCo-2). This observation suggests a higher susceptibility of hematopoietic canine cancer lines compared to the relatively greater resistance displayed by the human cancer cells towards the tested compounds.
Among the tested compounds, the highest antiproliferative activity was observed for trans γ-iodo-δ-lactone 10b against the CLBL-1 cell line, with an IC₅₀ value of 46.26±4.07 µg/mL, and for vanillin (1) against the T-24 cell line (IC₅₀ = 48.46±13.16 µg/mL). Moderate activity was also noted for cis γ-bromo-δ-lactone 11a against CLBL-1 and for compound 10b against T-24. In all other cases, the tested compounds exhibited lower activity, with IC₅₀ values exceeding 70 µg/mL. Importantly, none of the compounds showed cytotoxic effects toward the normal NIH/3T3 fibroblast cell line, indicating a degree of selectivity toward cancer cells.
Comparing the antiproliferative activity of vanillin (1) with its halolactone derivatives against the CLBL-1 cell line, the trans γ-iodo-δ-lactone 10b and cis γ-bromo-δ-lactone 11a exhibited notably higher activity. In assays with CaCo-2 cells, vanillin (1) was inactive, whereas cis γ-bromo-δ-lactone 11a, cis γ-chloro-δ-lactone 12a, and γ-chloro-δ-lactone 12b demonstrated moderate activity. Conversely, vanillin (1) proved to be the most active compound against the T-24 cell line. For the CLB70 line, the antiproliferative activities of vanillin (1) and all tested vanillin-derived lactones were comparable.
When evaluating the impact of the halogen substituent on the biological activity of the lactones, iodolactones 10a and 10b demonstrated superior activity against the T-24 bladder carcinoma line compared to their bromo- and chlorolactone counterparts (11a, 12a and 12b). Conversely, for the CaCo-2 colorectal adenocarcinoma line, the trend was reversed, with bromo- and chlorolactones exhibiting relatively higher efficacy than the iodolactones.
Comparing the antiproliferative activity of cis isomers of γ-lactones against hematopoietic cell lines, the highest activity was observed for bromolactone
11a against the CLBL-1 cell line. In contrast, the activity of all tested cis δ-halo-γ-lactones (
10a,
11a, and
12a) against the CLB70 cell line was comparable. These results clearly indicate that the influence of the halogen substituent on the antiproliferative activity of lactones is strongly dependent on the specific cancer cell line. Similar observations were reported in our previous studies. For instance, when studying a series of racemic halolactones bearing phenyl, p-methylphenyl, and p-isopropylphenyl substituents at the β-position of the lactone ring, bromolactones exhibited higher antiproliferative activity compared to their iodo- and chloro- analogues against the Jurkat cell line (human T-cell leukemia) [
31]. In another study, β-aryl-δ-iodo-γ-lactones containing a 2’,5’-dimethylphenyl substituent showed greater activity against the CLBL-1 and D17 (canine osteosarcoma) cell lines, but lower activity against GL-1 (B-cell leukemia) and Jurkat cell lines when compared to their bromo analogs [
33].
Considering the effect of the spatial structure of iodolactones on their antiproliferative properties, it is evident that trans δ-iodo-γ-lactone
10b exhibited considerably higher activity than its cis isomer
10a against the CLBL-1 and T-24 cell lines, whereas the activities of both isomers against the CLB-70 and CaCo-2 lines were comparable. A higher antiproliferative activity of trans isomers of β-aryl-δ-iodo-γ-lactones, bearing 2,5-dimethylphenyl and 1,3-benzodioxole substituents, compared to their cis isomers was also reported for analogs tested against Jurkat, GL-1, CLBL-1, and D17 cell lines [
32].
In summary, among all tested halolactones, the most promising antiproliferative properties were exhibited by trans δ-iodo-γ-lactone
10b. It showed the highest activity toward the CLBL-1 and T-24 cell lines, and comparable activity against the CLB-70 cell line relative to the other tested halolactones. It is also worth mentioning that structurally related compounds, such as δ-aryl-γ-halo-δ-lactones synthesized from aryl bromides and 3-methylcrotonaldehyde [
25], or trans-crotonaldehyde [
24], exhibited cytotoxic effects not only toward some cancer cell lines but also against the L929 mouse fibroblast normal cell line. In contrast, in the present study, all tested vanillin-derived halolactones displayed potent and selective antiproliferative activity against cancer cells without affecting the normal NIH/3T3 fibroblast cell line. This favorable selectivity profile makes this group of lactones, particularly lactone
10b, a promising candidate for further development as a safe anticancer agents.
2.3. Cytotoxicity Toward Red Blood Cells (RBCs)
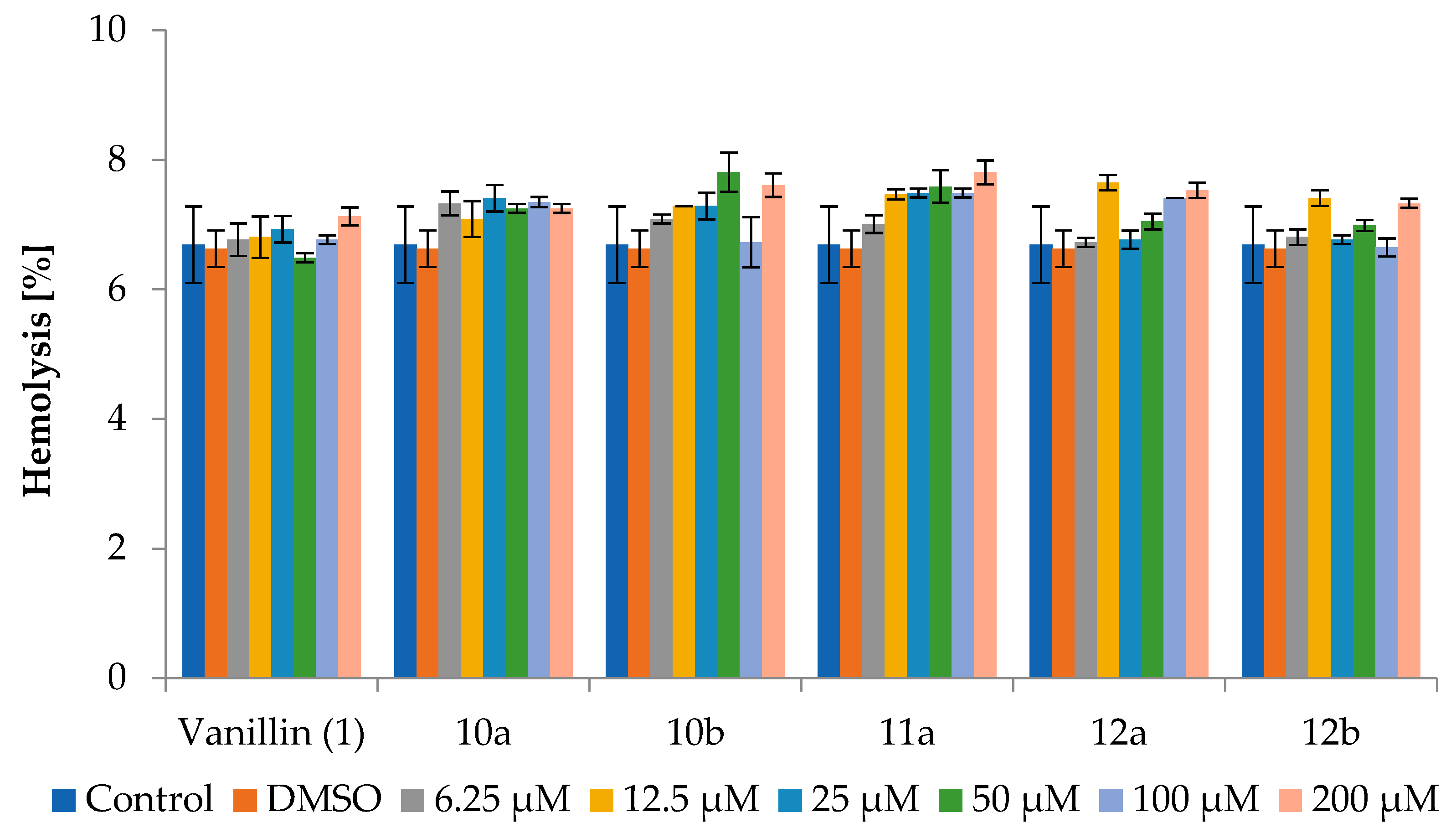
The next stage of the study focused on determining the toxicity of the tested lactones and vanillin (1) toward human red blood cells (RBCs) across a wide concentration range (0 to 200 μM) and at different incubation times (2 h, 24 h, and 72 h).
The results of these analyses are presented as the percentage of hemolysis relative to the control sample without the addition of the tested compound and the sample containing only the solvent - DMSO, allowing a precise assessment of the compounds' impact. Detailed results are shown in
Figure 1 and
Table 2.
The results showed that incubation of red blood cells with vanillin (1) and the tested lactones 10a,b, 11a, 12a,b for 2 h showed hemolysis between 6.65±0.14% and 7.81±0.18%, regardless of the concentration, with hemolysis levels for DMSO and control sample 6.69±0.59% and 6.63±0.28%, respectively.
A slight increase in the percentage of hemolysis was observed after 24 h of incubation, with values ranging from 7.30±0.19% to 8.99±0.69%, especially for 10a and 11a at the highest concentrations. However, that the level of hemolysis for the control and with DMSO was similar to each other, with values of 7.65±0.44% and 7.92±0.07%, respectively.
After 72 h of incubation, hemolysis increased slightly in all samples to above 9%. It was also observed that for three lactones - 10b, 11a and 12b at the highest concentration, the level of hemolysis was slightly higher compared to the other compounds and the control and amounted to 11.00±0.90%, 10.34±1.20% and 10.52±0.75%, respectively. The results obtained indicate a slight hemolytic effect associated with the prolonged incubation and very high concentration (200 μM) of the tested compounds. Nevertheless, the percentage of hemolysis increased by less than 2% compared to the control and DMSO.
The results of the study demonstrate that over a wide range of concentrations (exceeding those used in cell line studies), both vanillin and all tested vanillin-derived halolactones did not exhibit a significant increase in hemolysis compared to the control and DMSO after 2 and 24 h of exposure. Although prolonged exposure to high concentrations caused minor hemolytic effects for some halolactones, the values remained only slightly elevated relative to the control and DMSO, suggesting that the tested compounds should be considered non-toxic to erythrocytes. It should also be emphasized that hemolysis values for both DMSO and the control remained at the same level, confirming that the solvent itself had no impact on erythrocyte integrity.
The hemolytic activity profile of the tested lactones observed in this study is strongly supported by previous investigations of structurally related compounds, which likewise demonstrated no-toxic effects on erythrocyte membranes. Włoch et al. (2020) examined racemic β-aryl-δ-iodo-γ-lactones with various aromatic substituents and showed that bromolactone analogues, bearing either a methyl group or no substituent on the benzene ring, did not induce hemolysis in erythrocytes even after 48 h of incubation [
37].
In another study, Włoch et al. (2025) also evaluated the toxicity of enantiomeric piperonal-derived trans β-aryl-δ-iodo-γ-lactones toward human red blood cells (RBCs) across a wide range of concentrations and various incubation times [
36]. In this case as well, the results demonstrated a lack of toxicity at the tested concentrations and extended incubation periods. However, it should be noted that in both cited studies the concentration range examined (up to 100 µM) was significantly lower than in the present work. Furthermore, the incubation time in our study was longer (up to 72 h) compared to those previous reports (up to 48 h).
3. Materials and Methods
2.3. Chemicals
Vanillin (99% purity), benzyl bromide (98% purity), anhydrous methylene chloride (≥99.8% purity), anhydrous iron(III) chloride (98% purity), N-bromosuccinimide (NBS) (95% purity), N-chlorosuccinimide (NCS) (98% purity), sodium borohydride (≥96% purity), anhydrous tetrahydrofuran (THF) (≥99% purity), triethyl orthoacetate (97% purity), were purchased from Merck (Darmstadt, Germany). Other common reagents and solvents were purchased from Chempur (Piekary Śląskie, Poland or Idalia Radom, Poland).
3.2. Analysis and Purification
The reaction progress was monitored by Thin Layer Chromatography (TLC) using 0.2 mm aluminum plates coated with silica gel 60 F254 (Merck, Darmstadt, Germany). Chromatograms were visualized by spraying the plates with a 1% solution of Ce(SO4)2 and 2% H3[P(Mo3O10)4] in 10% H2SO4, followed by heating the plates at 120-200 °C.
Gas Chromatography (GC) analysis was conducted using an Agilent Technologies 6890N (Santa Clara, CA, USA) instrument equipped with an autosampler, split injection (50:1), and an FID detector, employing a DB5-HT column (Agilent, Santa Clara, CA, USA), polyimide-coated fused silica tubing, 30 m x 0.25 mm x 0.10 μm with hydrogen as the carrier gas. For analysis of lactones 10a,b, 11a, 12a,b, the following temperature program was used: injector and detector (FID) temperatures 300 °C, the column temperature was programmed from 100 °C to 200 °C at a rate of 20 °C/min, followed by a ramp from 200 °C to 300 °C at 30 °C/min, with a hold at 300 °C for 8 minutes. For intermediate product analysis 1-5, the initial column temperature was 100 °C, followed by a temperature increase from 100 °C to 200 °C at a rate of 20 °C/min, then from 200 °C to 300 °C at a rate of 30 °C/min, and finally a 1-minute hold at 300 °C.
Nuclear Magnetic Resonance (NMR) spectra, including
1H NMR,
13C NMR,
13C DEPT 135, COSY, HMQC and HMBC were recorded using either a Jeol 400 MHz Year Hold Magnet spectrometer (Jeol Ltd., Tokyo, Japan) or NMR Avance III HD 600 MHz spectrometer (Bruker Biospin GmbH, Rheinstetten, Germany). They are available in the
Supplementary Materials (Figures S1–S102). Samples were dissolved in CDCl
3 (≥ 99%) or CD
3OD (≥ 99%) and chemical shifts were referenced to the residual solvent signal (δ
H = 7.26, δ
C = 77.00 or δ
H = 3.31, δ
C = 49.00).
Infrared (IR) spectra were acquired using a Nicolet iS10 FTIR Spectrometer (Thermo Fisher Scientific™, Waltham, MA, USA), equipped with a monolithic diamond ATR crystal attachment. All recorded IR spectra are available in the
Supplementary Materials (Figures S103-S119).
High Resolution Mass Spectra (HRMS) were recorded on either a Bruker Daltonics ESI-Q-TOF maXis impact mass spectrometer (Bruker, Billerica, MA, USA) or a Waters Xevo G2 mass spectrometer (Waters, Milford, MA, USA), with positive or negative electrospray ionization (ESI) techniques.
Flash chromatography was conducted using the puriFlash® SX520 Plus system (Interchim, Montluçon, France), which includes a gradient pump, UV detector, and fraction collector. Samples were dry loaded on a pre-column (puriFlash®) and the compounds were separated on puriFlash® SIHP F0012 or F0040 30 µm columns by gradient elution with hexane/ethyl acetate mixtures (flow rate: 26 mL/min; pressure: 15 mbar).
The melting points (uncorrected) were determined on the Boetius apparatus (Nagema, Dresden, Germany).
3.3. Preparation of Benzylvanillin (2)
Vanillin (1) (5.00 g, 33.86 mmol), potassium carbonate (5.26 g, 38.06 mmol), and benzyl bromide (4.90 mL, 41.27 mmol) were dissolved in ethanol (150 mL) and stirred at room temperature for 48 h. The reaction mixture was then filtered to remove solid impurities, and the solvent was evaporated under reduced pressure. The resulting residue was treated with 30 mL of 5% aqueous NaOH and the product was extracted with methylene chloride (3 × 30 mL). The combined organic layers were dried over anhydrous MgSO₄, filtered, and the solvent was removed under reduced pressure. The crude product was dissolved in hot ethanol and recrystallized by cooling at –20 °C for 72 h. The resulting crystals were collected by vacuum filtration using a Büchner funnel, washed with cold ethanol, and dried to afford pure benzylvanillin (2), which was characterized by the following physical and spectral data:
Yield 7.01 g (88%); light yellow crystals; mp 58-60 °C (lit. 61-63 °C) [
58]; R
f = 0.45 (hexane/ethyl acetate 3:1,
v/v);
1H NMR (400 MHz, CD
3OD): δ 3.90 (s, 3H, -OCH
3), 5.21 (s, 2H, -OCH
2Ph), 7.17 (d,
J = 8.2 Hz, 1H, H-5’), 7.32 (m, 1H, H-4’’), 7.35 – 7.40 (m, 2H, H-3’’, H-5’’), 7.43 – 7.47 (m, 3H, H-2’, H-2’’ and H-6’’), 7.49 (dd,
J = 8.2 and 1.9 Hz, 1H, H-6’), 9.80 (s, 1H, -CHO);
13C NMR (100 MHz, CD
3OD): δ 56.44 (-OCH
3), 71.82 (-OCH
2Ph), 111.00 (C-2), 113.91 (C-5), 127.49 (C-6), 128.69 (C-2’,C-6’), 129.17 (C-4’), 129.59 (C-3’,C-5’), 131.71 (C-1), 137.84 (C-1’), 151.50 (C-3), 155.33 (C-4), 192.94 (-CHO); IR (ATR): ν
max = 2840, 1676, 1584, 1506, 1262, 1134, 748 cm
-1; HRMS (ESI):
m/z calcd for C
15H
14O
3 [M+Na]
+: 265.0835; found: 265.0837.
3.4. Preparation of Ketone 3 via Claisen–Schmidt Condensation
A solution of benzylvanillin (2) (7.01 g, 28.96 mmol) in acetone (230 mL) was stirred in a water bath. A 10% aqueous NaOH solution (20 mL) was added dropwise to the reaction mixture. The reaction was stirred at room temperature for 24 h, after which the mixture was acidified to pH = 2 using 1 M HCl. The resulting product was extracted with methylene chloride (3 × 40 mL). The combined organic layers were washed with brine, dried over anhydrous MgSO₄, and filtered. The solvent was removed under reduced pressure, and the crude product was purified by flash chromatography (gradient elution from hexane to hexane/ethyl acetate 7:1, v/v) to afford pure (E)-4-(4'-(benzyloxy-3'-methoxyphenyl)but-3-en-2-one (3), which was characterized by the following physical and spectral data:
Yield 6.86 g (84%); yellow crystals; mp 91-93 °C (lit. 92-93 °C) [
59]; R
f = 0.21 (hexane/ethyl acetate 4:1,
v/v);
1H NMR (400 MHz, CDCl
3): δ 2.36 (s, 3H, CH
3-1), 3.92 (s, 3H, -OCH
3), 5.19 (s, 2H, -OCH
2Ph), 6.59 (d,
J = 16.2 Hz, 1H, H-3), 6.88 (d,
J = 8.3 Hz, 1H, H-5’), 7.05 (dd,
J = 8.3 Hz and 2.0 Hz, 1H, H-6’), 7.09 (d,
J = 2.0 Hz, 1H, H-2’), 7.31 (m, 1H, H-4’’), 7.35-7.40 (m, 2H, H-3’’, H-5’’), 7.41-7.45 (m, 2H, H-2’’, H-6’’), 7.44 (d,
J = 16.2 Hz, H-4);
13C NMR (100 MHz, CDCl
3): δ 27.31 (C-1), 55.95 (-OCH
3), 70.78 (-OCH
2Ph), 110.12 (C-2’), 113.34 (C-5’), 122.74 (C-6’), 125.31 (C-3), 127.16 (C-2’’,C-6’’), 127.61 (C-1’), 128.01 (C-4’’), 128.61 (C-3’’, C-5’’), 136.44 (C-1’’), 143.46 (C-4), 149.76 (C-3’), 150.43 (C-4’), 198.35 (C-2); IR (ATR): ν
max = 1661, 1637, 1514, 1255, 1142, 974, 839, 806, 744, 697 cm
-1; HRMS (ESI):
m/z calcd for C
18H
18O
3 [M+Na]
+: 305.1148; found: 305.1155.
3.5. Preparation of Allylic Alcohol 4
Ketone 3 (6.86 g, 24.32 mmol) was dissolved in methanol (200 mL), and the solution was cooled in an ice bath. An aqueous solution of NaBH₄ (2.05 g in 12 mL H₂O) was added dropwise to the stirred reaction mixture. The reaction was stirred for 1 h in the ice bath and then for 23 h at room temperature. After this time, hot water (150 mL) was added, and the product was extracted with methylene chloride (3 × 30 mL). The organic layer was washed with brine, dried over anhydrous MgSO₄, and filtered. The solvent was removed under reduced pressure to afford pure (E)-4-(4'-benzyloxy-3'-methoxyphenyl)but-3-en-2-ol (4), which was characterized by the following physical and spectral data:
Yield 5.67 g (82%); white solid; mp 55-57 °C; Rf = 0.25 (hexane/acetone 3:1, v/v); 1H NMR (400 MHz, CDCl3): δ 1.37 (d, J = 6.4 Hz, 3H, CH3-1), 1.64 (s, 1H, -OH), 3.91 (s, 3H, -OCH3), 4.47 (m, 1H, H-2), 5.16 (s, 2H, -OCH2Ph), 6.13 (dd, J = 15.8 Hz and 6.6 Hz, 1H, H-3), 6.48 (d, J = 15.8 Hz, 1H, H-4), 6.82 (d, J = 8.2 Hz, 1H, H-5’), 6.85 (dd, J = 8.2 and 1.6 Hz, 1H, H-6’), 6.96 (d, J = 1.6 Hz, 1H, H-2’), 7.30 (m, 1H, H-4’’), 7.33-7.39 (m, 2H, H-3’’, H-5’’), 7.40-7.46 (m, 2H, H-2’’, H-6’’); 13C NMR (100 MHz, CDCl3): δ 23.44 (C-1), 55.91 (OCH3), 69.02 (C-2), 70.92 (-OCH2Ph), 109.25 (C-2’), 113.80 (C-5’), 119.52 (C-6’), 127.19 (C-2’’, C-6’’), 127.81 (C-4’’), 128.52 (C-3’’, C-5’’), 129.19 (C-4), 130.15 (C-1’), 131.75 (C-3), 136.99 (C-1’’), 147.91 (C-4’), 149.62 (C-3’); IR (ATR): νmax = 3384, 1513, 1264, 1139, 969, 798, 858, 744, 699 cm-1; HRMS (ESI): m/z calcd for C18H20O3 [M+Na]+: 307.1305; found: 307.1317.
3.6. Preparation of Ester 5 by Johnson-Claisen Rearrangement
A mixture of alcohol 4 (5.67 g, 19.94 mmol), triethyl orthoacetate (230 mL, 1.27 mol), and a few drops of propionic acid was heated at 138 °C for 6 h with continuous distillation of ethanol. Upon completion of the reaction (monitored by TLC), the mixture was subjected to silica gel column chromatography to remove excess triethyl orthoacetate (eluent: hexane/acetone 10:1, v/v). The crude product was further purified by flash chromatography (gradient elution from hexane to hexane/ethyl acetate 19:1, v/v) to afford pure (E)-3-(4'-benzyloxy-3'-methoxyphenyl)hex-4-enoate (5), which was characterized by the following physical and spectral data:
Yield 3.32 (47%); light yellow oily liquid; Rf = 0.64 (hexane/ethyl acetate (3:1, v/v); 1H NMR (400 MHz, CDCl3): δ 1.17 (t, J = 7.1 Hz, 3H, -OCH2CH3), 1.65 (d, J = 6.0 Hz, 3H, CH3-6), 2.62 (dd, J = 14.8 and 7.5 Hz, 1H, one of CH2-2), 2.66 (dd, J = 14.8 and 8.0 Hz, 1H, one of CH2-2), 3.73 (m, 1H, H-3), 3.88 (s, 3H, -OCH3), 4.04-4.10 (two q, J = 7.1 Hz, 2H, -OCH2CH3), 5.12 (s, 2H, -OCH2Ph), 5.48 (dq, J = 15.3 and 6.0 Hz, 1H, H-5), 5.57 (ddq, J = 15.3, 7.1 and 1.1 Hz, 1H, H-4), 6.68 (dd, J = 8.2 and 2.0 Hz 1H, H-6’), 6.75 (d, J = 2.0 Hz, 1H, H-2’), 6.81 (d, J = 8.2 Hz, H-5’), 7.29 (m, 1H, H-4’’), 7.32-7.39 (m, 2H, H-3’’, H-5’’), 7.40-7.45 (m, 2H, H-2’’, H-6’’); 13C NMR (100 MHz, CDCl3): δ 14.19 (-OCH2CH3), 17.89 (C-6), 41.18 (C-2), 44.57 (C-3), 55.98 (-OCH3), 60.26 (-OCH2CH3), 71.07 (-OCH2Ph), 111.38 (C-2’), 114.06 (C-5’), 119.12 (C-6’), 125.42 (C-5), 127.22 (C-2’’, C-6’’), 127.73 (C-4’’), 128.47 (C-3’’, C-5’’), 133.17 (C-4), 136.61 (C-1’), 137.32 (C-1’’), 146.75 (C-4’), 149.53 (C-3’), 172.03 (C-1); IR (ATR): νmax = 1731, 1511, 1259, 1137, 1025, 967, 852, 802, 734, 696 cm-1; HRMS (ESI): m/z calcd for C22H26O4 [M+Na]+: 377.1723; found: 377.1740.
3.7. Preparation of Acid 6
Ester 5 (3.32 g, 9.37 mmol) was heated under reflux for 5 h in a 10% ethanol solution of NaOH (230 mL). After evaporating the solvent under reduced pressure, the residue was diluted with water, and organic impurities were removed by extraction with diethyl ether (3 × 30 mL). The aqueous layer was acidified with 1M HCl, and the product was extracted with methylene chloride (3 × 30 ml). The combined organic layers were washed with brine, dried over anhydrous MgSO4, and the solvent was evaporated in vacuo to afford pure (E)-3-(4'-benzyloxy-3'-methoxyphenyl)hex-4-enoic acid (6) with the following physical and spectral data:
Yield 2.51 g (82%); white solid; mp 95-98 °C; Rf = 0.05 (hexane/ethyl acetate 3:1, v/v); 1H NMR (400 MHz, CDCl3): δ 1.66 (d, J = 5.8 Hz, 3H, CH3-6), 2.68 (dd, J = 15.6 and 7.4 Hz, 1H, one of CH2-2), 2.72 (dd, J = 15.6 and 8.0 Hz, 1H, one of CH2-2), 3.72 (m, 1H, H-3), 3.87 (s, 3H, -OCH3), 5.12 (s, 2H, -OCH2Ph), 5.50 (dq, J = 15.4 and 5.8 Hz, 1H, H-5), 5.57 (ddq, J = 15.4, 6.8 and 1.2 Hz, 1H, H-4), 6.68 (dd, J = 8.2 and 2.0 Hz 1H, H-6’), 6.74 (d, J = 2.0 Hz, 1H, H-2’), 6.82 (d, J = 8.2 Hz, H-5’), 7.29 (m, 1H, H-4’’), 7.33-7.39 (m, 2H, H-3’’, H-5’’), 7.40-7.45 (m, 2H, H-2’’, H-6’’); 13C NMR (100 MHz, CDCl3): δ 17.91 (C-6), 40.67 (C-2), 44.06 (C-3), 55.98 (-OCH3), 71.05 (-OCH2Ph), 111.36 (C-2’), 114.02 (C-5’), 119.06 (C-6’), 125.68 (C-5), 127.23 (C-2’’, C-6’’), 127.76 (C-4’’), 128.49 (C-3’’, C-5’’), 132.88 (C-4), 136.25 (C-1’), 137.26 (C-1’’), 146.86 (C-4’), 149.54 (C-3’), 177.57 (C-1); IR (ATR): νmax = 2500-3500, 1702, 1518, 1236, 1142, 965, 855, 791, 744, 696 cm-1; HRMS (ESI): m/z calcd for C20H22O4 [M+Na]+: 349.1410; found: 349.1413.
3.8. Preparation of Iodolactones 7a-c
A solution of acid 6 (2.69 g, 7.70 mmol) in diethyl ether (80 mL) and saturated NaHCO₃ solution (80 mL) was stirred for 1 h at room temperature. Then, a solution of I₂ (12.70 g, 50.01 mmol) and KI (3.97 g, 23.92 mmol) in water (20 mL) was added dropwise to the stirred mixture until a stable brown color was observed. After 19 h, the reaction mixture was diluted with diethyl ether and washed with aqueous Na₂S₂O₃. The aqueous phase was shaked with diethyl ether (3 × 30 mL), and the combined organic layers were washed with saturated NaHCO₃, brine, then dried over anhydrous MgSO₄. After evaporation of the solvent under reduced pressure, the mixture of iodolactones was separated by flash chromatography (gradient elution from hexane to hexane/ethyl acetate 5:1, v/v). Physicochemical and spectral data of the isolated iodolactones 7a–c are given below:
3.8.1. Cis-4-(4'-benzyloxy-3'-methoxyphenyl)-5-(1-iodoethyl)dihydrofuran-2-one (7a)
Yield 1.81 g (52%); white solid; mp 116-118 °C; Rf = 0.27 (hexane/ethyl acetate 4:1, v/v); 1H NMR (400 MHz, CDCl3): δ 2.01 (d, J = 6.8 Hz, 3H, CH3-7), 2.70 (d, J = 17.5 Hz, 1H, one of CH2-3), 3.10 (dd, J = 17.5 and 8.4 Hz, 1H, one of CH2-3), 3.47 (dq, J = 10.9 and 6.8 Hz, 1H, H-6), 3.84 (dd, J = 8.4 and 5.0 Hz, 1H, H-4), 3.89 (s, 3H, -OCH3), 4.78 (dd, J = 10.9 and 5.0 Hz, 1H, H-5), 5.14 (s, 2H, -OCH2Ph), 6.71 (dd, J = 8.3 and 2.1 Hz, 1H, H-6’), 6.80-6.86 (m, 2H, H-2’, H-5’), 7.31 (m, 1H, H-4’’), 7.34-7.40 (m, 2H, H-3’’, H-5’’), 7.41-7.46 (m, 2H, H-2’’, H-6’’); 13C NMR (100 MHz, CDCl3): δ 23.70 (C-6), 25.53 (C-7), 38.83 (C-3), 44.54 (C-4), 56.04 (-OCH3), 70.98 (-OCH2Ph), 87.87 (C-5), 112.80 (C-2’), 113.87 (C-5’), 120.26 (C-6’), 127.31 (C-2’’, C-6’’), 127.93 (C-4’’), 128.57 (C-3’’, C-5’’), 130.05 (C-1’), 136.86 (C-1’’), 147.81 (C-4’), 149.31 (C-3’), 176.64 (C-2); IR (ATR): νmax = 1789, 1518, 1239, 1148, 1012, 954, 857, 751, 700, 538 cm-1; HRMS (ESI): m/z calcd for C20H21IO4 [M+Na]+ 475.0377; found: 475.0390.
3.8.2. Trans-4-(4'-benzyloxy-3'-methoxyphenyl)-5-(1-iodoethyl)dihydrofuran-2-one (7b)
Yield 0.87 g (25%); white solid; mp 80-82 °C; Rf = 0.16 (hexane/ethyl acetate 4:1, v/v); 1H NMR (400 MHz, CDCl3): major signals from syn conformer: δ 1.86 (d, J = 7.1 Hz, 3H, CH3-7), 2.64 (dd, J = 18.4 and 6.6 Hz, one of CH2-3), 3.12 (dd, J = 18.4 and 10.0 Hz, 1H, one of CH2-3), 3.56 (ddd, J = 10.0, 6.6 and 5.3 Hz, 1H, H-4), 3.89 (s, 3H, -OCH3), 4.23 (t, J = 5.3 Hz, 1H, H-5), 4.36 (qd, J = 7.1 and 5.3 Hz, 1H, H-6), 5.14 (s, 2H, -OCH2Ph), 6.73 (dd, J = 8.2 and 2.1 Hz, 1H, H-6’), 6.75 (d, J = 2.1 Hz, 1H, H-2’), 6.85 (d, J = 8.2 Hz, 1H, H-5’), 7.31 (m, 1H, H-4’’), 7.34-7.40 (m, 2H, H-3’’, H-5’’), 7.41-7.45 (m, 2H, H-2’’, H-6’’); 13C NMR (100 MHz, CDCl3): δ 23.44 (C-7), 28.45 (C-6), 37.67 (C-3), 45.17 (C-4), 56.14 (-OCH3), 71.04 (-OCH2Ph), 89.56 (C-5), 110.54 (C-2’), 114.35 (C-5’), 119.06 (C-6’), 127.23 (C-2’’, C-6’’), 127.93 (C-4’’), 128.57 (C-3’’, C-5’’), 134.31 (C-1’), 136.85 (C-1’’), 147.69 (C-4’), 150.15 (C-3’), 174.88 (C-2); minor signals from anti conformer: 1H NMR: δ 1.97 (d, J = 7.1 Hz, 3H, CH3-7), 2.76 (dd, J = 18.1 and 9.5 Hz, one of CH2-3), 3.07 (dd, J = 18.1 Hz and 9.5 Hz, 1H, one of CH2-3), 3.46 (td, J = 9.5 and 7.1 Hz, 1H, H-4), 3.73 (dd, J = 7.1 and 2.4 Hz, 1H, H-5), 3.90 (s, 3H, -OCH3), 5.30 (s, 2H, -OCH2Ph), 6.85 (d, J = 8.1 Hz, 1H, H-5’); 13C NMR: δ 25.42 (C-7), 29.02 (C-6), 37.04 (C-3), 47.41 (C-4), 89.27 (C-5), 110.70 (C-2’), 119.26 (C-6’), 132.07 (C-1’), 174.80 (C-2); IR (ATR): νmax = 1786, 1517, 1454, 1254, 1142, 1030, 970, 848, 804, 746, 697, 644, 536 cm-1; HRMS (ESI): m/z calcd for C20H21IO4 [M+Na]+: 475.0377; found: 475.0378.
3.8.3. 4-r-(4'-Benzyloxy-3'-methoxyphenyl)-5-t-iodo-6-c-methyltetrahydropyran-2-one (7c)
Yield 0.38 g (11%); white solid; mp 109-112 °C; Rf = 0.15 (hexane/ethyl acetate 4:1, v/v); 1H NMR (400 MHz, CDCl3): δ 1.74 (d, J = 6.2 Hz, 3H, CH3-7), 2.65 (dd, J = 17.6 and 10.6 Hz, 1H, one of CH2-3), 2.98 (dd, J = 17.6 and 6.6 Hz, 1H, one of CH2-3), 3.43 (td, J = 10.6 and 6.6 Hz, 1H, H-4), 3.90 (s, 3H, -OCH3), 4.00 (t, J = 10.6 Hz, H-5), 4.75 (dq, J = 10.6 and 6.2 Hz, 1H, H-6), 5.15 (s, 2H, -OCH2Ph), 6.65 (dd, J = 8.0 and 2.2 Hz, 1H, H-6’), 6.67 (d, J = 2.2 Hz, 1H, H-2’), 6.87 (d, J = 8.0 Hz, 1H, H-5’), 7.31 (m, 1H, H-4’’), 7.35-7.40 (m, 2H, H-3’’, H-5’’), 7.42-7.46 (m, 2H, H-2’’, H-6’’); 13C NMR (100 MHz, CDCl3): δ 22.30 (C-7), 34.90 (C-5), 37.72 (C-3), 48.22 (C-4), 56.10 (-OCH3), 70.99 (-OCH2Ph), 81.00 (C-6), 110.46 (C-2’), 113.99 (C-5’), 118.90 (C-6’), 127.27 (C-2’’, C-6’’), 127.90 (C-4’’), 128.56 (C-3’’, C-5’’), 135.42 (C-1’), 136.90 (C-1’’), 147.79 (C-4’), 149.84 (C-3’), 169.73 (C-2); IR (ATR): νmax = 1714, 1518, 1377, 1261, 1141, 1029, 971, 851, 810, 740, 699, 574 cm-1; HRMS (ESI): m/z calcd for C20H21IO4 [M+Na]+: 475.0377; found: 475.0383.
3.9. Preparation of Bromolactones 8a,b
A solution of acid 6 (2.51 g, 7.70 mmol) and N-bromosuccinimide (1.89 g, 10.62 mmol) in THF (80 mL), with a few drops of acetic acid, was stirred at room temperature for 24 h. The mixture was then diluted with diethyl ether (40 mL) and washed with saturated NaHCO₃ solution and brine. The organic layer was dried over anhydrous MgSO₄. After solvent evaporation under reduced pressure, the product mixture was purified by flash chromatography (gradient elution from hexane to hexane/ethyl acetate 5:1, v/v). Two bromolactones, 8a and 8b, were isolated and characterized by the following data:
3.9.1. Cis-4-(4'-benzyloxy-3'-methoxyphenyl)-5-(1-bromoethyl)dihydrofuran-2-one (8a)
Yield 1.12 g (35%); white solid; mp 125-128 °C; Rf = 0.39 (hexane/chloroform/methanol 8:2:1, v/v); 1H NMR (400 MHz, CDCl3): δ 1.76 (d, J = 6.6 Hz, 3H, CH3-7), 2.71 (d, J = 17.5 Hz, 1H, one of CH2-3), 3.09 (dd, J = 17.5 and 8.4 Hz, 1H, one of CH2-3), 3.45 (dq, J = 10.4 and 6.6 Hz, 1H, H-6), 3.81 (dd, J = 8.4 and 5.1 Hz, 1H, H-4), 3.87 (s, 3H, -OCH3), 4.64 (dd, J = 10.4 and 5.1 Hz, 1H, H-5), 5.14 (s, 2H, -OCH2Ph), 6.68 (dd, J = 8.3 and 2.1 Hz, 1H, H-6’), 6.79 (d, J = 2.1 Hz, 1H, H-2’), 6.84 (d, J = 8.3 Hz, 1H, H-5’), 7.31 (m, 1H, H-4’’), 7.34-7.40 (m, 2H, H-3’’, H-5’’), 7.41-7.47 (m, 2H, H-2’’, H-6’’); 13C NMR (100 MHz, CDCl3): δ 23.26 (C-7), 38.29 (C-3), 43.64 (C-4), 45.54 (C-6), 56.02 (-OCH3), 70.97 (-OCH2Ph), 86.71 (C-5), 112.58 (C-2’), 113.83 (C-5’), 120.10 (C-6’), 127.30 (C-2’’, C-6’’), 127.93 (C-4’’), 128.57 (C-3’’, C-5’’), 130.19 (C-1’), 136.87 (C-1’’), 147.82 (C-4’), 149.34 (C-3’), 176.42 (C-2); IR (ATR): νmax = 178, 1518, 1254, 1146, 1015, 966, 780, 747, 699 cm-1; HRMS (ESI): m/z calcd for C20H21BrO4 [M+Na]+: 427.0515; found: 427.0522.
3.9.2. 4-r-(4'-Benzyloxy-3'-methoxyphenyl)-5-t-bromo-6-c-methyltetrahydropyran-2-one (8b)
Yield 0.26 g (8%); white solid; mp 132-134 °C; Rf = 0.33 (hexane/chloroform/methanol 8:2:1, v/v); 1H NMR (400 MHz, CDCl3): δ 1.65 (d, J = 6.2 Hz, 3H, CH3-7), 2.70 (dd, J = 17.6 and 9.4 Hz, 1H, one of CH2-3), 3.05 (dd, J = 17.6 and 6.9 Hz, 1H, one of CH2-3), 3.40 (td, J = 9.4 and 6.9 Hz, 1H, H-4), 3.90 (s, 3H, -OCH3), 3.91 (t, J = 9.4 Hz, H-5), 4.63 (dq, J = 9.4 and 6.2 Hz, 1H, H-6), 5.14 (s, 2H, -OCH2Ph), 6.68 (dd, J = 8.1 and 2.1 Hz, 1H, H-6’), 6.71 (d, J = 2.1 Hz, 1H, H-2’), 6.87 (d, J = 8.1 Hz, 1H, H-5’), 7.31 (m, 1H, H-4’’), 7.34-7.40 (m, 2H, H-3’’, H-5’’), 7.41-7.46 (m, 2H, H-2’’, H-6’’); 13C NMR (100 MHz, CDCl3): δ 20.62 (C-7), 37.64 (C-3), 46.78 (C-4), 54.51 (C-5), 56.10 (-OCH3), 71.00 (-OCH2Ph), 79.59 (C-6), 110.66 (C-2’), 114.05 (C-5’), 119.00 (C-6’), 127.25 (C-2’’, C-6’’), 127.90 (C-4’’), 128.57 (C-3’’, C-5’’), 133.91 (C-1’), 136.93 (C-1’’), 147.81 (C-4’), 149.88 (C-3’), 169.45 (C-2); IR (ATR): νmax = 1721, 1517, 1375, 1225, 1141, 1001, 972, 804, 758, 704 cm-1; HRMS (ESI): m/z calcd for C20H21BrO4 [M+Na]+: 427.0515; found: 427.0517.
3.10. Preparation of Chlorolactones 9a,b
A solution of acid
6 (2.51 g, 7.70 mmol) and
N-chlorosuccinimide (2.32 g, 17.37 mmol) in THF (80 mL), with a few drops of acetic acid, was stirred at room temperature for 24 h. The work-up procedure and isolation described in
Section 3.9 were applied to isolate chlorolactones
9a and
9b, which were characterized by the following physical and spectral data:
3.10.1. Cis-4-(4'-benzyloxy-3'-methoxyphenyl)-5-(1-chloroethyl)dihydrofuran-2-one (9a)
Yield 0.28 g (10%); white solid; mp 81-84 °C; Rf = 0.37 (hexane/chloroform/methanol 8:2:1, v/v); 1H NMR (400 MHz, CDCl3): δ 1.56 (d, J = 6.5 Hz, 3H, CH3-7), 2.71 (d, J = 17.6 and 0.8 Hz, 1H, one of CH2-3), 3.07 (dd, J = 17.6 and 8.4 Hz, 1H, one of CH2-3), 3.43 (dq, J = 10.1 and 6.5 Hz, 1H, H-6), 3.80 (dd, J = 8.4 and 5.2 Hz, 1H, H-4), 3.87 (s, 3H, -OCH3), 4.49 (dd, J = 10.1 and 5.2 Hz, 1H, H-5), 5.14 (s, 2H, -OCH2Ph), 6.66 (dd, J = 8.3 and 2.2 Hz, 1H, H-6’), 6.76 (d, J = 2.2 Hz, 1H, H-2’), 6.84 (d, J = 8.3 Hz, 1H, H-5’), 7.31 (m, 1H, H-4’’), 7.35-7.40 (m, 2H, H-3’’, H-5’’), 7.42-7.46 (m, 2H, H-2’’, H-6’’); 13C NMR (100 MHz, CDCl3): δ 22.38 (C-7), 37.94 (C-3), 43.13 (C-4), 53.93 (C-6), 56.02 (-OCH3), 70.97 (-OCH2Ph), 86.58 (C-5), 112.42 (C-2’), 113.82 (C-5’), 120.03 (C-6’), 127.29 (C-2’’, C-6’’), 127.92 (C-4’’), 128.57 (C-3’’, C-5’’), 130.30 (C-1’), 136.87 (C-1’’), 147.81 (C-4’), 149.37 (C-3’), 176.34 (C-2); IR (ATR): νmax = 1789, 1516, 1454, 1238, 1144, 1019, 973, 780, 745, 698, 680 cm-1; HRMS (ESI): m/z calcd for C20H21O4Cl [M+H]+: 361.1207; found: 361.1206.
3.10.2. 4-r-(4'-Benzyloxy-3'-methoxyphenyl)-5-t-chloro-6-c-methyltetrahydropyran-2-one (9b)
Yield 0.5 g (18%); white solid; mp 93-96 °C; Rf = 0.24 (hexane/chloroform/methanol 8:2:1, v/v); 1H NMR (400 MHz, CDCl3): δ 1.60 (d, J = 6.2 Hz, 3H, CH3-7), 2.72 (dd, J = 17.7 and 9.5 Hz, 1H, one of CH2-3), 3.06 (dd, J = 17.7 and 7.0 Hz, 1H, one of CH2-3), 3.29 (td, J = 9.5 and 7.0 Hz, 1H, H-4), 3.83 (t, J = 10.0 Hz, H-5), 3.90 (s, 3H, -OCH3), 4.50 (dq, J = 10.0 and 6.2 Hz, 1H, H-6), 5.14 (s, 2H, -OCH2Ph), 6.70 (dd, J = 8.1 and 2.2 Hz, 1H, H-6’), 6.72 (d, J = 2.2 Hz, 1H, H-2’), 6.87 (d, J = 8.1 Hz, 1H, H-5’), 7.31 (m, 1H, H-4’’), 7.35-7.40 (m, 2H, H-3’’, H-5’’), 7.42-7.46 (m, 2H, H-2’’, H-6’’); 13C NMR (100 MHz, CDCl3): δ 19.66 (C-7), 37.21 (C-3), 46.31 (C-4), 56.09 (-OCH3), 62.47 (C-5), 71.00 (-OCH2Ph), 79.35 (C-6), 110.78 (C-2’), 114.08 (C-5’), 119.10 (C-6’), 127.24 (C-2’’, C-6’’), 127.90 (C-4’’), 128.57 (C-3’’, C-5’’), 133.13 (C-1’), 136.93 (C-1’’), 147.82 (C-4’), 149.89 (C-3’), 169.33 (C-2); IR (ATR): νmax = 1732, 1511, 1258, 1219, 1139, 1016, 973, 796, 738, 696, 642 cm-1; HRMS (ESI): m/z calcd for C20H21O4Cl [M+H]+: 361.1207; found: 361.1205.
3.11. General Procedure for Benzyl Deprotection of Halolactones 7a-c, 8a,b and 9a,b
Lactone 7-9 (1 equiv) was placed in a two-necked flask equipped with a nitrogen-filled balloon and dissolved in 10 mL of anhydrous methylene chloride. The flask was placed on a magnetic stirrer, and after complete dissolution of the substrate, anhydrous FeCl3 (3 equiv) was added. The mixture was stirred at room temperature. After complete reaction of the substrate (30 min), the crude reaction mixture was filtered under reduced pressure through a Schott funnel, transferred to a separatory funnel, and washed with 1% orthophosphoric acid solution (3 × 10 mL). After phase separation, the aqueous layer was further washed with methylene chloride (3 × 15 mL). All combined organic layers were neutralized with saturated NaCl solution and dried over anhydrous MgSO4. After filtration, the solvent was evaporated under reduced pressure using a rotary evaporator. The concentrated crude product was purified by flash chromatography. Gradient elution from hexane to hexane/ethyl acetate 9:2 (v/v) was used for lactones 10a, 10b, 12a, and 12b, while for lactone 11a a gradient from hexane to hexane/ethyl acetate 3:1 (v/v) was applied. Reaction yields and the physical and spectral data of the deprotected halolactones are as follows:
3.11.1. Cis-4-(4'-hydroxy-3'-methoxyphenyl)-5-(1-iodoethyl)dihydrofuran-2-one (10a)
Obtained from lactone 7a (1.81 g, 4 mmol) in yield 0.41 g (28%); white solid; mp 56-59 °C; Rf = 0.24 (hexane/ethyl acetate 3:1, v/v), 1H NMR (400 MHz, CDCl3): δ 2.01 (d, J = 6.8 Hz, 3H, CH3-7), 2.70 (d, J = 17.5 Hz, 1H, one of CH2-3), 3.11 (dd, J = 17.5 and 8.4 Hz, 1H, one of CH2-3), 3.48 (dq, J = 10.9 and 6.8 Hz, 1H, H-6), 3.83 (dd, J = 8.4 and 5.0 Hz, 1H, H-4), 3.89 (s, 3H, -OCH3), 4.78 (dd, J = 10.9 and 5.0 Hz, 1H, H-5), 5.62 (s, 1H, -OH), 6.74 (dd, J = 8.1 and 2.0 Hz, 1H, H-6’), 6.78 (d, J = 2.0 Hz, 1H, H-2’), 6.86 (d, J = 8.1 Hz, 1H, H-5’); 13C NMR (100 MHz, CDCl3): δ 23.72 (C-6), 25.53 (C-7), 38.91 (C-3), 44.66 (C-4), 56.01 (-OCH3), 87.93 (C-5), 111.45 (C-2’), 114.64 (C-5’), 121.20 (C-6’), 128.94 (C-1’), 145.33 (C-4’), 146.34 (C-3’), 176.67 (C-2); IR (ATR): νmax = 3367, 1762, 1518, 1178, 1129, 1033, 966, 846, 597, 527 cm-1; HRMS (ESI): m/z calcd for C13H15O4I [M+H]+: 363.0093; found: 363.0094.
3.11.2. Trans-4-(4'-hydroxy-3'-methoxyphenyl)-5-(1-iodoethyl)dihydrofuran-2-one (10b)
Obtained from lactone 7b (0.87 g, 2.40 mmol) in yield 0.11 g (16%) and from lactone 7c (0.38 g, 1.05 mmol) in yield 0.05 g (18%); white solid; mp 39-41 °C; Rf = 0.16 (hexane/ethyl acetate 3:1, v/v); 1H NMR (600 MHz, CDCl3): δ 1.87 (d, J = 7.1 Hz, 3H, CH3-7), 2.64 (dd, J = 18.4 and 6.6 Hz, one of CH2-3), 3.13 (dd, J = 18.4 and 10.1 Hz, 1H, one of CH2-3), 3.56 (ddd, J = 10.1, 6.6 and 5.3 Hz, 1H, H-4), 3.90 (s, 3H, -OCH3), 4.23 (t, J = 5.3 Hz, 1H, H-5), 4.37 (qd, J = 7.1 and 5.3 Hz, 1H, H-6), 5.61 (s, 1H, -OH), 6.71 (d, J = 2.1 Hz, 1H, H-2’), 6.76 (dd, J = 8.1 and 2.1 Hz, 1H, H-6’), 6.89 (d, J = 8.1 Hz, 1H, H-5’); 13C NMR (150 MHz, CDCl3): δ 23.48 (C-7), 28.38 (C-6), 37.81 (C-3), 45.26 (C-4), 56.05 (-OCH3), 89.70 (C-5), 109.25 (C-2’), 115.00 (C-5’), 119.91 (C-6’), 133.26 (C-1’), 145.24 (C-4’), 147.07 (C-3’), 174.89 (C-2); IR (ATR): νmax = 3406, 1770, 1516, 1269, 1148, 1026, 969, 816, 757, 596 cm-1; HRMS (ESI): m/z calcd for C13H15O4I [M+H]+: 363.0093; found: 363.0094.
3.11.3. Cis-5-(1-bromoethyl)-4-(4'-hydroxy-3'-methoxyphenyl)dihydrofuran-2-one (11a)
Obtained from lactone 8a (1.12 g, 3.55 mmol) in yield 0.24 g (28%); white solid; mp 43-46 °C; Rf = 0.27 (hexane/chloroform/methanol 8:2:1, v/v); Rt = 6.84 min; 1H NMR (600 MHz, CDCl3): δ 1.76 (d, J = 6.6 Hz, 3H, CH3-7), 2.71 (d, J = 17.3 Hz, 1H, one of CH2-3), 3.10 (dd, J = 17.3 and 8.5 Hz, 1H, one of CH2-3), 3.46 (dq, J = 10.4 and 6.6 Hz, 1H, H-6), 3.81 (dd, J = 8.5 and 5.1 Hz, 1H, H-4), 3.88 (s, 3H, -OCH3), 4.64 (dd, J = 10.4 and 5.1 Hz, 1H, H-5), 5.62 (s, 1H, -OH), 6.71 (dd, J = 8.1 and 2.1 Hz, 1H, H-6’), 6.73 (d, J = 2.1 Hz, 1H, H-2’), 6.87 (d, J = 8.1 Hz, 1H, H-5’); 13C NMR (150 MHz, CDCl3): δ 23.26 (C-7), 38.36 (C-3), 43.74 (C-4), 45.56 (C-6), 55.97 (-OCH3), 86.76 (C-5), 111.23 (C-2’), 114.59 (C-5’), 121.00 (C-6’), 129.08 (C-1’), 145.32 (C-4’), 146.34 (C-3’), 176.47 (C-2); IR (ATR): νmax = 3333, 1737, 1519, 1132, 967, 777, 703, 624 cm-1; HRMS (ESI): m/z calcd for C13H15O4Br [M+H]+: 315,0232; found: 315.0230.
3.11.4. Cis-5-(1-chloroethyl)-4-(4'-hydroxy-3'-methoxyphenyl)dihydrofuran-2-one (12a)
Obtained from lactone 9a (0.28 g, 0.78 mmol) in yield 0.06 g (28%); white solid; mp 86-89 °C; Rf = 0.13 (hexane/chloroform/methanol 8:2:1, v/v); 1H NMR (600 MHz, CDCl3): δ 1.57 (d, J = 6.5 Hz, 3H, CH3-7), 2.72 (d, J = 17.6 Hz, 1H, one of CH2-3), 3.08 (dd, J = 17.6 and 8.5 Hz, 1H, one of CH2-3), 3.45 (dq, J = 10.1 and 6.5 Hz, 1H, H-6), 3.80 (dd, J = 8.5 and 5.2 Hz, 1H, H-4), 3.88 (s, 3H, -OCH3), 4.50 (dd, J = 10.1 and 5.2 Hz, 1H, H-5), 5.61 (s, 1H, -OH), 6.69-6.70 (two m, 2H, H-2’ and H-6’), 6.88 (d, J = 8.4 Hz, 1H, H-5’); 13C NMR (150 MHz, CDCl3): δ 22.38 (C-7), 38.02 (C-3), 43.30 (C-4), 53.96 (C-6), 55.97 (-OCH3), 86.65 (C-5), 111.10 (C-6’), 114.60 (C-5’), 120.96 (C-2’), 129.21 (C-1’), 145.35 (C-4’), 146.41 (C-3’), 176.33 (C-2); IR (ATR): νmax = 3422, 1763, 1517, 1266, 1188, 1128, 1018, 974, 838, 785, 632, 600 cm-1; HRMS (ESI): m/z calcd for C13H15O4Cl [M-H]-: 269.0581; found: 269.0573.
3.11.5. 5-t-Chloro-4-r-(4'-hydroxy-3'-methoxyphenyl)-6-c-methyltetrahydropyran-2-one (12b)
Obtained from lactone 9b (0.50 g, 1.39 mmol) in yield 0.07 g (19%); white solid; mp 52-55 °C; Rf = 0.06 (hexane/chloroform/methanol 8:2:1, v/v); 1H NMR (600 MHz, CDCl3): δ 1.60 (d, J = 6.2 Hz, 3H, CH3-7), 2.73 (dd, J = 17.8 and 10 Hz, 1H, one of CH2-3), 3.06 (dd, J = 17.8 and 7.0 Hz, 1H, one of CH2-3), 3.27 (td, J = 10.0 and 7.0 Hz, 1H, H-4), 3.83 (t, J = 10.0 Hz, H-5), 3.90 (s, 3H, -OCH3), 4.50 (dq, J = 10.0 and 6.2 Hz, 1H, H-6), 5.62 (s, 1H, -OH), 6.68 (d, J = 2.0 Hz, 1H, H-2’), 6.72 (dd, J = 8.1 and 2.0 Hz, 1H, H-6’), 6.91 (d, J = 8.1 Hz, 1H, H-5’); 13C NMR (150 MHz, CDCl3): δ 19.76 (C-7), 37.33 (C-3), 46.44 (C-4), 55.97 (-OCH3), 62.64 (C-5), 79.37 (C-6), 109.66 (C-2’), 115.04(C-5’), 119.84 (C-6’), 132.08 (C-1’), 145.30 (C-4’), 146.83 (C-3’), 169.30 (C-2); IR (ATR): νmax = 3394, 1728, 1518, 1215, 1054, 1030, 975, 735, 643, 578 cm-1; HRMS (ESI): m/z calcd for C13H15O4Cl [M+H]+: 271.0737; found: 271.0740.
3.12. Antiproliferative Activity
3.12.1. Chemicals for Biological Tests
The RPMI-1640 (Roswell Park Memorial Institute), McCoy’s 5A, Eagle’s Minimum Essential Medium (EMEM), Dulbecco’s Modified Eagle’s Medium High Glucose (DMEM HG), L-glutamine (L-Glu), DMSO (biological grade), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich® (Steinheim, Germany). The Fetal Bovine Serum (FBS) and advanced RPMI medium were purchased from Gibco/ThermoFisher (Waltham, MA, USA). Antibiotics penicillin-streptomycin (PS) was purchased from Corning (NY, USA). The T-75 cell culture flasks (EasYFlask Nunclon Delta Surface) and 96-well plates were purchased from NUNC (Kamstrupvej, Denmark).
3.12.2. Cell Lines and Cell Cultures
The CLB70 cell line (canine chronic B-cell lymphocytic leukemia) was established at the Department of Pharmacology and Toxicology, Wrocław University of Environmental and Life Sciences [
60]. The CLBL-1 cell line (canine B-cell lymphoma) was kindly provided by Dr. Barbara C. Rütgen from the Institute of Immunology, Department of Pathobiology, University of Veterinary Medicine, Vienna [
61]. The T-24 (human bladder carcinoma), Caco-2 (human colorectal adenocarcinoma), and NIH/3T3 (mouse embryonic fibroblasts) cell lines were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA).
Cell cultures were maintained in their respective growth media, with fetal bovine serum (FBS) concentrations ranging from 10% to 20%, depending on the cell line. Penicillin-streptomycin (PS) and L-glutamine (L-Glu) were consistently added at 1% each to all media. CLB70 cells were cultured in RPMI ADVANCE medium supplemented with 20% FBS, while CLBL-1 cells were maintained in RPMI-1640 medium with 20% FBS. T-24 cells were cultured in McCoy’s 5A medium with 10% FBS, and Caco-2 cells in Eagle's Minimum Essential Medium (EMEM) with 20% FBS. NIH/3T3 cells were maintained in high-glucose DMEM (DMEM HG) with 10% FBS. All cell cultures were grown in T-75 flasks under a humidified atmosphere at 37°C and 5% CO₂ and passaged every 3 days to maintain optimal cell density (70–80% confluence).
3.12.3. MTT Assay
Stock solutions used in the experiments were freshly prepared for each trial by dissolving the tested compounds in biological-grade DMSO. These stock solutions were then diluted with the culture medium appropriate for each specific cell line to achieve the desired final concentrations, which ranged from 3.125 to 100 µM. The final DMSO concentration in each well was maintained below 1%, a level generally considered non-toxic to cells [
62]. The MTT assay was employed to evaluate the antiproliferative and cytotoxic effects of the compounds on cancer cell lines, following a previously described protocol with minor modifications [
31]. Cells were seeded in 96-well plates at the following densities: 1.5 × 10⁴ cells/mL (NIH/3T3), 2 × 10⁴ cells/mL (T-24, Caco-2), and 3 × 10⁵ cells/mL (CLB70, CLBL-1). Each plate included control wells with untreated cells and wells with vehicle controls (culture medium specific to each cell line).
After 72 h of treatment with the tested compounds, 10 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL) was added to each well. The cells were subsequently incubated with MTT for an additional 3 h to allow the formation of formazan crystals. Following this, 40 µL of lysis buffer (composed of 46 g sodium dodecyl sulfate, 150 mL dimethylformamide, and 183.2 mL Milli-Q water) was added to each well to dissolve the formazan. After a 24 h incubation at room temperature, the optical density (OD) of the resulting formazan product was measured using a spectrophotometric microplate reader (Spark, Tecan, Männedorf, Switzerland) at 570 nm. To correct for background absorbance and minimize potential measurement artifacts, a reference wavelength of 630 nm, at which formazan exhibits negligible absorbance, was used.
The results were determined from three independent experiments (four wells each) and presented as mean IC50 value±SD from at least four independent experiments, each performed in quadruplicate. IC50 values (the concentration of the compound required to inhibit 50% of cell growth) were calculated from dose-response curves.
3.13. Cytototoxicity Toward Red Blood Cells (RBCs)
To evaluate the hemolytic potential and cytotoxicity of the tested compounds, a quantitative hemolysis assay was conducted using human red blood cells (RBCs), following the method described by Pruchnik et al. (2018), with minor modifications [
63].
RBCs were obtained from healthy donors through the Blood Donation Center in Wrocław. In accordance with Polish law, the use of erythrocytes from blood centers for experimental purposes does not require approval from an ethics committee. The collected RBCs were gently resuspended and washed three times with cold isotonic phosphate-buffered saline (PBS, pH 7.4) to remove plasma and cellular debris.
All tested compounds were dissolved in biological-grade DMSO. A series of working solutions was prepared by diluting the stock solutions to final concentrations of 6.25, 12.50, 25, 50, 100, and 200 μM. The DMSO content in all experimental and control samples did not exceed 1% (v/v). Control samples contained no tested compounds, while vehicle control samples contained DMSO at the same concentration as the treated samples. Positive control samples (A₁₀₀%) were prepared by inducing complete hemolysis with Milli-Q water. All experiments were performed in triplicate, with three independent replicates for each condition.
The erythrocyte suspension was adjusted to a final hematocrit of 1.2% in all test tubes, with a total sample volume of 1 mL. Samples were incubated at 37 °C under static conditions for 2, 24, and h to assess both short- and long-term cytotoxic effects.
Following incubation, all samples were centrifuged at 5000 rpm for 3 minutes at room temperature. The supernatants were carefully transferred to a 96-well plate, and the absorbance of free hemoglobin was measured at 540 nm using a UV-Vis microplate spectrophotometer (EPOCH, BioTek, Santa Clara, CA, USA). The degree of
in vitro hemolysis was calculated based on the amount of released hemoglobin relative to that in the completely hemolyzed positive control sample, according to the following formula:
where
H [%] is the percentage of erythrocyte hemolysis,
As is absorbance of hemoglobin in supernatant at 540 nm, and
A100% refers to the absorbance of hemoglobin in the supernatant following complete hemolysis of the erythrocytes.