1. Introduction
Cor pulmonale is a syndrome characterized by pathological alterations of the right ventricle due to pulmonary arterial hypertension [
1,
2]. The disease is characterized by hypertrophy and dilation of the right ventricle, secondary to a lung disease that causes pulmonary arterial hypertension, as occurs in chronic lung diseases and several other conditions. The main clinical sign of the disease is generalized, severe, chronic and progressive subcutaneous edema, which mainly affects the submandibular and abdominal regions, chest and, occasionally, limbs. In cattle, pulmonary arterial hypertension poses a significant challenge to producers in mountainous regions of the United States. The high incidence of this high-altitude illness suggests that the anatomical structure of cattle lungs predisposes to the condition. Their lungs are relatively small and lobulated in comparison to their body weight, contributing to severe loss of functional lung capacity [
1,
2,
3].
In Brazil, four groups of toxic plants are known to cause chronic edema in cattle: 1) plants that cause chronic cardiac fibrosis, including
Ateleia glazioviana,
Niedenzuella acutifolia and
Niedenzuella multiglandulosa; 2) plants that cause hepatic fibrosis, including
Crotalaria spp. and
Senecio spp.; 3) plants that cause nephrosis, including
Combretum glaucocarpa,
Amaranthus spp., and
Metternichia princeps [
4]; 4)
Crotalaria pallida, the only plant described as a cause of pulmonary lesions with arterial hypertension [
4,
5].
For nearly 30 years, outbreaks of a disease of unknown etiology characterized by chronic subcutaneous edema, particularly affecting the head, dewlap, chest, and forelimbs, have been observed in cattle in Central-Northern and Central-Southern mesoregions of Bahia, affecting at least 12 municipalities [
7]. This region has a semi-arid climate, with high temperatures and scarce and poorly distributed rainfall. The average annual temperature is around 24ºC (maximum 29.2ºC and minimum 20.2ºC). The average annual rainfall is 672 mm, ranging from 323 to 1147 mm, with a rainy period from November to April and a dry period from May to October [
8]. This study aimed to describe the clinical, epidemiological and pathological aspects of a disease of unknown etiology, characterized by chronic edema, associated with
cor pulmonale in cattle in Bahia.
2. Material and Methods
Eight outbreaks of the disease were studied between October 2023 and April 2025. Additionally, another seven outbreaks reported by Veterinarians and/or farmers were analyzed through observations of photographs, videos and visits to farms.
Forty-six animals exhibiting edema were clinically examined, and blood and fecal samples were collected from these animals. Feces were collected from the rectal ampulla and subjected to egg count per gram of feces (EPG) using the MacMaster method. Blood samples were obtained by puncture of the coccygeal vein in 5 mL tubes containing sodium ethylenediaminetetraacetate (EDTA) in 10% aqueous solution to perform hemograms, and in 10 mL tubes containing clot activator to obtain serum after centrifugation at 1500 RCF for 10 min. The serum samples were transferred to Eppendorf’s® microtubes and stored at -20°C. Serum total protein, albumin, creatinine and urea were determined by the colorimetric method, while the serum activities of aspartate aminotransferase (AST), gamma glutamyl transferase (GGT) and creatine kinase (CK) was obtained by the kinetic method. Globulin was calculated by the difference between total protein and albumin.
Seven autopsies were performed, two after natural death and three after euthanasia. Fragments of brain, spinal cord, lung, heart, liver, kidney, spleen, esophagus, rumen, reticulum, omasum, abomasum, small intestine, large intestine and subcutaneous tissue with edema were collected and fixed in 10% phosphate-buffered formalin. After fixation, these materials were embedded in paraffin, cut at a thickness of 5 μm, and stained with hematoxylin-eosin (HE). To identify muscle fiber hyperplasia in the arterioles, pulmonary samples from cattle affected by edema and from a healthy slaughterhouse bovine were submitted to immunohistochemical (IHC) analysis using an anti–actin (smooth muscle / a-SMA) antibody (M0851, 1:100 dilution, Dako, Carpinteria, California, USA). Immunolabeling was visualized with 3,3’-diaminobenzidine (DAB), and sections were counterstained with Harris’ hematoxylin.
To determine the thickness of the medial layer of cardiac and pulmonary arteries and arterioles, five arteries or arterioles from the heart and five from the lung were randomly selected from 10 cattle with edema and 10 control cattle. The heart and lung of control animals were collected at a slaughterhouse located in the municipality of Feira de Santana, from cattle of similar age (adults) and breed (Nelore) to those of the affected animals. Using a camera attached to a microscope, images of arteries and arterioles in the heart and lung were obtained using the Yais® app and a 10x objective. These photographs were used for histomorphometry of the medial layer of these vessels. The images were analyzed using the ImageJ® application, in which the medial thickness was measured at four equidistant points (superior, inferior, right lateral, and left lateral). For statistical analysis, the data were transferred to an Excel® spreadsheet and subjected to a normal distribution assessment. Nonparametric analysis was performed using the Mann-Whitney test for thickness of the media of the arteries and arterioles. The data were plotted on a scatterplot and analyzed using Graphprism® software.
The pastures where the affected animals were grazing were inspected. The suspicious plants observed in large amounts in different outbreaks were collected and sent to the Herbarium of the State University of Feira de Santana (HUEFS) for botanical identification. In addition to the unknown suspicious plants, the toxic species already known were identified during the inspection of the pastures.
3. Results
Seven outbreaks occurred between October and December 2023 and 2024, in the municipalities of Mairi, Maracás, Irajuba, Mirangaba, Jaguaquara, Ruy Barbosa, Jacobina, Umburanas, Lajedinho, Itaberaba, Jaguaquara and Jequié, during periods of prolonged drought, when pastures had very little forage availability, and animals needed to be released into areas of natural forest. One outbreak occurred in April 2025, but also during a dry season. The first clinical signs (subcutaneous edema) were observed approximately two months after the introduction of the animals into the area. After the onset of the rains, outbreaks were no longer observed, with only a few cases occurring again on one of the farms in May 2024. Even so, the owner reported that the most severe presentation of the disease had also occurred on the farm at the end of the previous year. The periods of occurrence and other epidemiological characteristics observed in the 15 outbreaks are described in
Table 1.
In outbreaks 7, 8, 9, and 12, the disease was observed in crossbred cattle, while in the other outbreaks, only Nelore animals were affected, but this was the only breed present on these farms. The age of the affected animals ranged from 2 to 4 years. However, in outbreak 6, five calves aged 4-6 months were observed with severe clinical signs, and of these, three rapidly evolved to death. The sex of the affected animals varied according to the type of production. However, on farms 3, 8, 9 and 12, where male and female cattle were raised on the same pasture, the disease was observed in both sexes.
Owners and employees of farms 1, 4, 6, 7, 8, 11, and 12 reported that the disease had occurred previously during prolonged periods of drought in cattle grazing on native forests. Some farmers also reported that the disease only appeared in animals brought from other regions and that animals born in the region were not affected.
The main clinical signs included marked progressive subcutaneous edema of the dewlap, thorax, forelimbs, and occasionally the head, together with marked distention and pulsation of the jugular vein, and progressive weight loss (
Figure 1A-D). Edema of the inguinal region and udders and scleral edema (
Figure 1D) were observed in some cases. Ventral edema was a common clinical sign in all animals examined; however, they usually presented with apathy, progressive weight loss, and bristly, coarse, dull hairs before the development of edema. Lethargy and anorexia were also observed since the onset of the clinical signs. Other signs included altered heart sounds, and dyspnea.
Death occurred after a clinical manifestation period of 5-15 days; however, there were animals that showed gradual recovery when removed from the paddock with other pastures, and others that died suddenly even after the edema disappeared.
Results of hematologic values and serum biochemistry of 46 affected animals in farms 1-8 are presented in
Table 2 and
Table 3, respectively. Briefly, a normocytic normochromic anemia was the most frequent finding. The hematocrit, red blood cell count and hemoglobin concentration were below the reference values in 51% (22/43) of the cattle examined. In the serum biochemistry, hypoproteinemia was observed in 58% (25/43) of the animals evaluated, hypoalbuminemia in 88% (38/43), and hyperglobulinemia in 91% (39/43). In the evaluation of renal function, mean urea levels were below the reference value in 25% (11/43) of cases. On the other hand, creatinine levels were elevated in 30% (13/43) of affected cattle, but both parameters were not related. The serum liver enzymes activities showed decreased serum AST activity in 30% (13/43) of the animals, and elevation in 16% (7/43) of the cases. Serum GGT activities were elevated in 70% (30/43) of the cattle examined representing the most frequent alterations. Serum CK activities were elevated in 26% (12/46) of the animals examined. In the stool examination, there was absence or less than 50 helminth eggs per gram of feces.
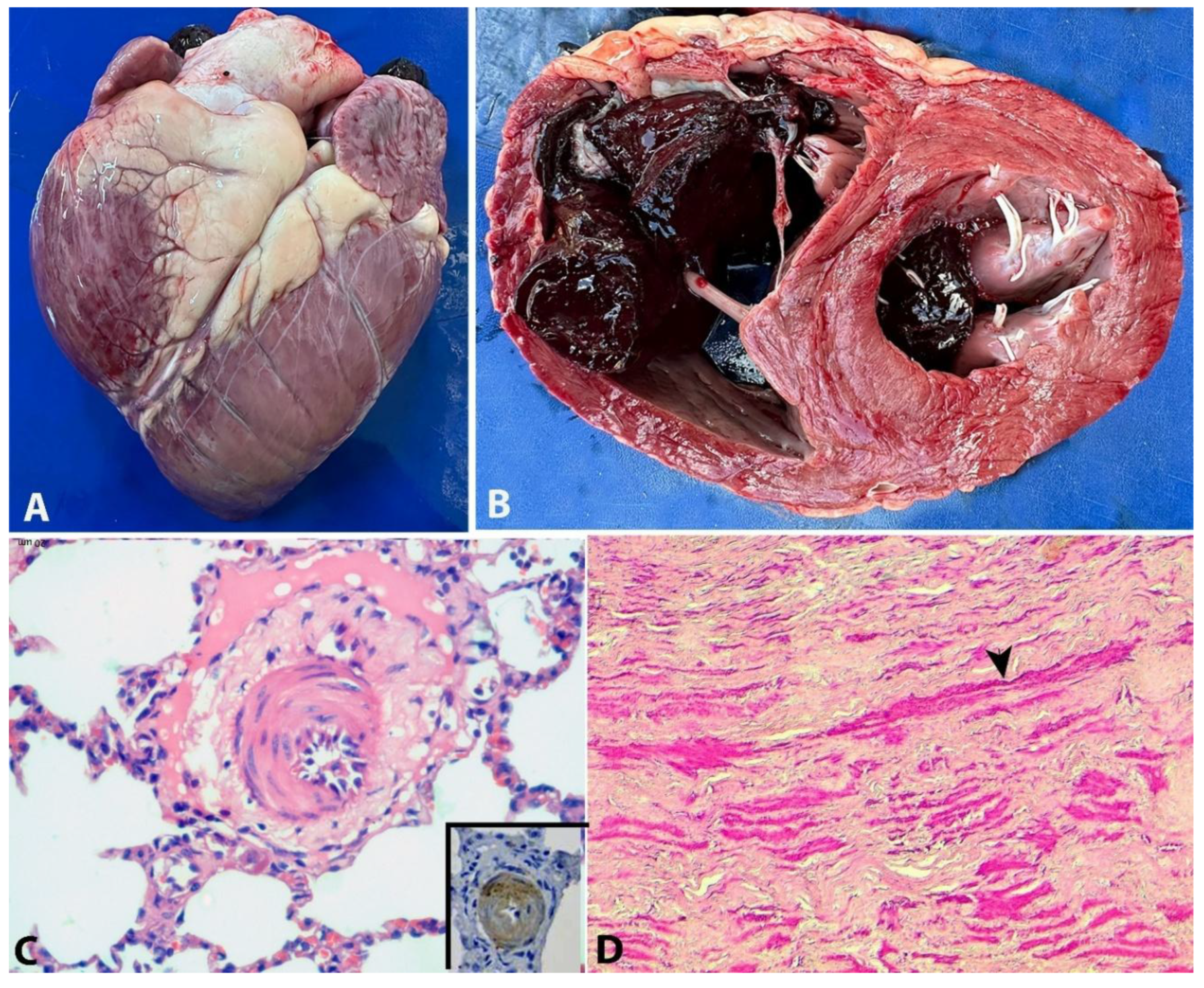
Postmortem examinations were performed on seven animals. Gross lesions, present in all animals, included subcutaneous edema, particularly in the thorax, dewlap, and facial regions, hydrothorax, hydropericardium, ascites, cachexia, and pallor of mucous membranes and organs. Hydrothorax was the most pronounced effusion encountered. Edema of the abomasum and small intestine, mainly in the duodenum was also observed. Pulmonary lesions consisted of pulmonary oedema with interlobular septal thickening. Cardiac findings included right ventricular dilatation (
Figure 2A and 2B). Serous fat atrophy was observed in some animals. The livers were moderately enlarged, with rounded edges and dark red or yellowish coloration. An accentuated lobular pattern (nutmeg appearance) was observed in 4 animals. The kidneys appeared normal, except for some pallor or redness.
Histologically, in the lungs, there was marked hypertrophy of smooth muscle cells in the medial layer of arteries and arterioles, sometimes with an eccentric, irregular, and asymmetric arrangement (
Figure 2C). On immunohistochemistry, the hyperplastic cells in the arteriolar media were intensely positive for α-SMA. (
Figure 2 inset). The tunica adventitia was thickened with increased deposition of collagen, and the intima was hyperplasic with endothelial cell proliferation (
Figure 2C). Additionally, degeneration of endothelial cells, duplication of the elastic lamina, periarteriolar fibrosis, fibrinoid necrosis or perivascular edema were occasionally observed. The alveolar septa were moderately thickened, occasionally with connective tissue proliferation and edema; multifocal hyperplasia of type II pneumocytes was also observed. Similar lesions were observed in some arterioles and arteries of the heart. The aorta and carotid arteries showed multifocal smooth muscle cells proliferation in the tunica media (
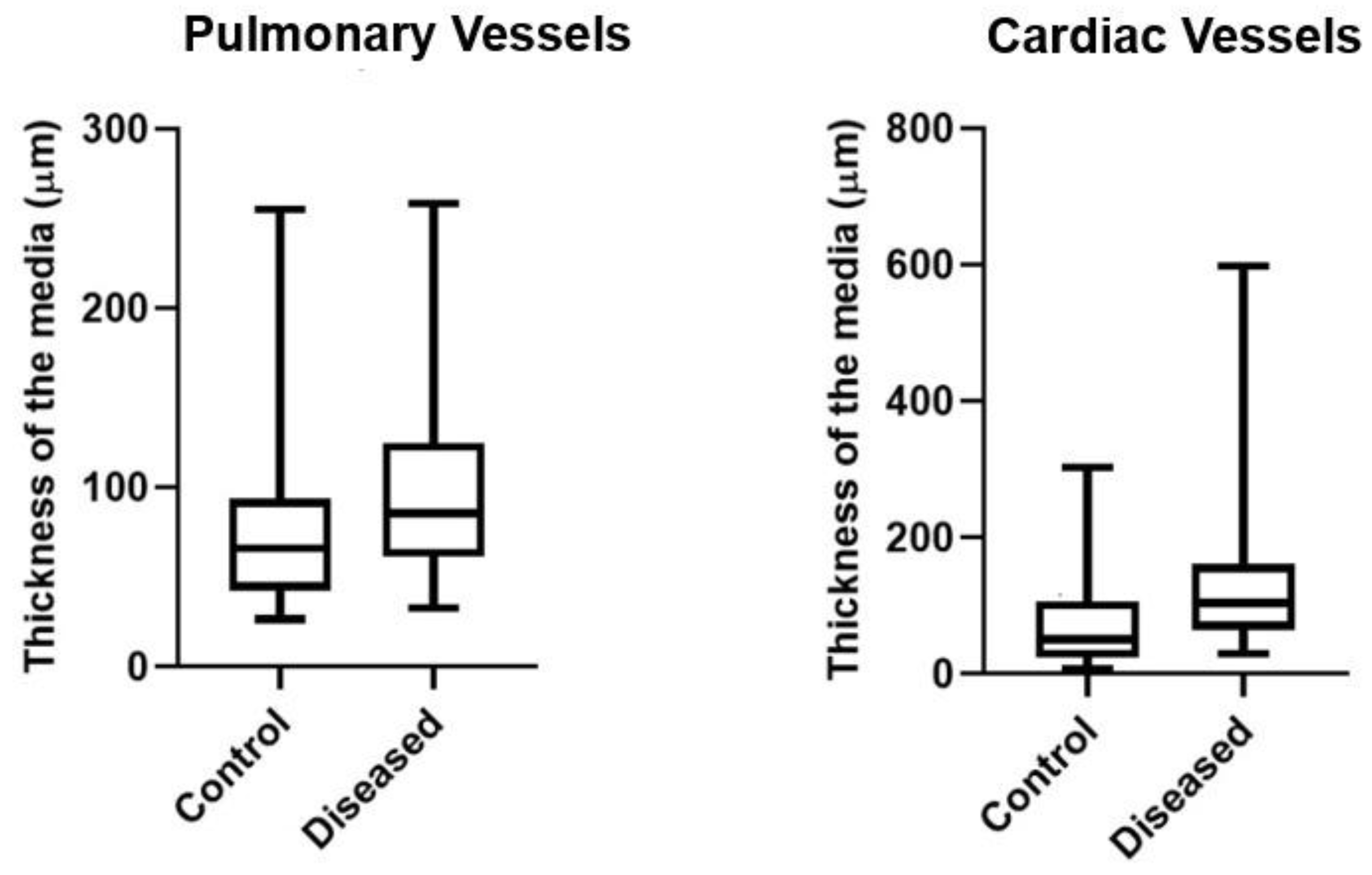
Figure 2D). Both the lung and heart arteries and arterioles of the affected cattle were significantly thicker (p<0.05) than those of the control animals (
Figure 3). Hepatic findings included vacuolization of hepatocytes, centrilobular or diffuse congestion, and centrilobular necrosis and loss of hepatocytes with replacement by fibrous tissue. In two animals few arteries had marked hypertrophy of smooth muscle cells in the tunica media.
Renal lesions involved glomerular changes characterized by proliferation of mesangial cells, occasionally with cytoplasmic vacuoles in the mesangial cells and moderate vascular congestion. In some glomeruli, cell proliferation resulted in synechiae with Bowman’s capsule, and the affected glomeruli occasionally showed fibroplasia (glomerulosclerosis). Multifocal areas of vacuolar degeneration and tubular epithelial necrosis were observed in two animals. Dilated tubules with epithelial regeneration were also observed occasionally. In one animal few arteries had marked hypertrophy of smooth muscle cells in the tunica media.
4. Discussion
The clinical-pathological picture reported in this study is characterized by generalized subcutaneous edema and right ventricular dilation with histological lesions of pulmonary arteries and arterioles, in particular hypertrophy of the smooth muscle cells of the medial layer. These lesions demonstrate that the edema is due to right heart failure, secondary to pulmonary arterial hypertension caused by lesions of the blood vessels of the lung (chronic
cor pulmonale). These results rule out the possibility of the disease being caused by any of the plants that cause edema as a result of liver fibrosis, kidney failure, or cardiac fibrosis mentioned in the introduction [
4].
In clinical pathology, although several altered values were recorded, none of these alterations were consistent in all animals tested, which suggests that they are not due to the primary cause of the disease. They are probably a consequence of anorexia and nutritional deficiencies, since the disease occurs in the dry season, when there is a shortage of forage. In some cases, it may be due the hepatic lesions, which were observed in some animals, probably as a consequence of heart failure.
We suggest that the chronic bovine edema disease (
cor pulmonale) reported here is caused by constriction of the blood vessels in the lungs with consequent increase in pulmonary arterial pressure and secondary lesions of the right ventricle. It is clinically and pathologically very similar to that known as Brisket disease, which occurs in areas located at high altitudes (around 2,000 m) [
3], although the disease has been reported at lower altitudes (1,369 m) in western Nebraska [
2]. However, the municipalities in Bahia where the disease has been observed are located at much lower altitudes (ranging from 368m a 1.069m), making it unlikely that the etiology is related to altitude. Plants of the
Astragalus and
Oxytropis genera (locoweeds), which contain swainsonine, may exacerbate the hypoxic condition in animals susceptible to pulmonary hypertension [
8]. In Northeast Brazil, plants of the
Ipomoea genus, which also contain swainsonine are well known, but no reports of edema caused by these plants exist; they are typically associated with neurological signs [
4,
9]. Furthermore, no plants of this genus were found on the farms where the outbreaks occurred.
Lesions of arteries and arterioles were also observed in other organs, mainly in the aorta and carotid arteries that showed multifocal smooth muscle cells proliferation. The clinical significance of this arterial lesions, observed in of all necropsied animals, is unknown. In humans, the proliferation and degradation of smooth muscle cells promote aortic diseases including aortic aneurysms and aortic dissection [
11].
The epidemiological characteristics of the disease (occurrence in specific areas of native forest, during the dry season, and affecting cattle of different ages, sexes and breeds) suggest that the disease is caused by a toxic plant. In an experiment,
Fredericia cinerea found on farms where the disease occurs, was administered in daily doses of 40 g/kg to a bovine for 60 days and no clinical signs were observed [
7]. Another plant
Banisteriopsis oxyclada was administered to one bovine at doses of 20g/kg during 40 days. The animal presented weight loss, loose feces (dirty perineum), jugular engorgement and positive venous pulse, but no edema was observed [
11]. New experiments should be carried out with other plants found in areas where the disease occurs.
Certain species of
Crotalaria that contain pneumotoxic pyrrolizidine alkaloids, that induce pulmonary hypertension and associated vascular changes, are often used as a model for experimental pulmonary hypertension in laboratory animals [
12]. In Brazil, lung lesions, such as thickening of the alveolar walls and arterioles with reduction of the lumen and periarteriolar fibrosis, were observed in animals experimentally poisoned with
Crotalaria mucronata [
5]. However, no significant amounts of
Crotalaria species were found in the areas where the disease occurred.
Another disease that induces pulmonary arterial hypertension is poisoning by
Pimelea spp., which contains a toxin (simplexin) that causes contraction of the muscular walls of blood vessels in the lungs. The disease occurs in Australia, where it is known as St. George disease. Vasoconstriction triggers an increase in pressure followed by effusion, right heart failure, and subcutaneous edema. In the liver, the toxin induces expansion of the sinusoids and portal venules (peliosis hepatis) and also affects the intestinal tract, causing diarrhea [
13].
Pimelea spp. does not occur in Brazil, and the clinical-pathological picture induced by simplexin, despite including right heart failure, appears to be different from that observed in the cattle in this study.
Parasitic causes of edema due to hypoproteinemia, including hemonchosis, ostertagiasis and fasciolosis, were ruled out by the results of fecal analyses and necropsies. Fasciolosis has not been reported in the region.
In conclusion, the disease results from pulmonary arterial hypertension induced by lesions of the pulmonary arterioles, with subsequent right ventricular failure. Such arterial lesions are caused, probably, by an unknown toxic plant. The disease causes significant economic losses in a large region of the state of Bahia, and it is necessary to know its etiology in order to determine prophylaxis and control measures. Farm visits during disease outbreaks to identify plants suspected of causing the disease and the administration of these plants to experimental cattle are necessary to determine the cause of the progressive generalized edema reported in this article.
Author Contributions
All authors contributed to study design, study execution, data analysis and interpretation, preparation of the manuscript and final approval of the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Laís G. Caymmia is a recipient of a student fellowship from the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (CAPES).
Institutional Review Board Statement
The experiment was approved by the Animal Use Ethics Committee of the Federal University of Bahia and registered under protocol number 29/2024.
Use of Artificial Intelligence
Generative AI was not used in the writing of this manuscript.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Rhodes, J. Comparative physiology of hypoxic pulmonary hypertension: Historical clues from brisket disease. J. Appl. Physiol, 2005, 98(3), 1092-1100. [CrossRef]
- Moxley, R.A.; Smith, D.R., Grotelueschen, D.M.; Edwards, T; Steffen, D.J.; Investigation of congestive heart failure in beef cattle in a feedyard at a moderate altitude in western Nebraska. J. Vet. Diag. Invest. 2019, 31(4), 509–522. [CrossRef]
- Holt, T.N.; Callan, R.J. Pulmonary arterial pressure testing for high mountain disease in cattle. Vet. Clin. North Am. Food Anim. Pract. 2007, 23(3), 575–96. [Google Scholar] [CrossRef] [PubMed]
- Riet-Correa, F.; Michelooud, J.; Machado, M.; Mendonça, F.; Schild, A.L.; Lemos, R.L. Intoxicaciones por plantas, micotoxinas y otras toxinas en rumiantes y équidos en Sudamérica Gurnee: Davis-Thompson Foundation; 2024. 507 p.
- Boghossian, M.R.; Peixoto, P.V.; Brito, M.F.; Tokarnia, C.H. Aspectos clínico-patológicos da intoxicação experimental pelas sementes de Crotalaria mucronata (Fabaceae) em bovinos. Pesq. Vet. Bras. 2007, 27(4), 149–156. [Google Scholar] [CrossRef]
- Dourado, C.S., Oliveira, S.R.M.; de Avila, A.M.H. Análise de zonas homogêneas em séries temporais de precipitação no Estado da Bahia. Bragantia, Campinas, 2013, 72 (2),192-198.
- Souza, A.Z.S.N. Edema crônico em bovinos no Estado da Bahia. Ms thesis. Federal University of Bahia. Bahia, Brazil, 2021; pp.93.
- James, L.F; Panter, K.E.; Broquist, H.P; Hartley, WJ. Swainsonine-induced high mountain disease in calves. Vet. Human Toxicol 1991, 33, 217–219. [Google Scholar]
- Oliveira,, C.A.; Riet-Correa, G.; Lima, E.; Medeiros, R.M.T,; Miraballes, C.; Pfister J.A,; Gardner, D.; Cook D.; Riet-Correa, F. Toxicity of the swainsonine-containing plant Ipomoea carnea subsp. fistulosa for goats and sheep. Toxicon 2021, 197, 40–47. [CrossRef]
- Caymmi, L.G. Perfil clínico-epidemiológico e anatomopatológico de uma doença caraterizada por edema crônico em bovinos no estado da Bahia. PhD thesis. Federal University of Bahia. Bahia, Brazil, 2025; pp.61.
- Hulsmans, M.; Nahrendorf, M. Proliferative, degradative smooth muscle cells promote aortic disease. J, Clin. Invest. 2021, 130(3), 1096-1098. [CrossRef]
- Lee, Y.S.; Byun, B.J.; Kim, J.A.; Lee, J.S.; Kim, K.L.; Suh, Y.L.; Kim, J.M.; Jang, H.S.; Lee, J.Y.; Suh, W.; Jeon, E.S.; Kim, D.K. Monocrotaline-induced pulmonary hypertension correlates with upregulation of connective tissue growth factor expression in the lung. Exp. Molec. Med. 2005, 37, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.J.; Hungerford, N.L.; Laycock, B.; Fletcher, M.T. A review on Pimelea poisoning of livestock. Toxicon 2020, 186, 46–57. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).