1. Introduction
The combustion of fossil fuels continues to be a major driver of greenhouse gas emissions, particularly carbon dioxide (CO
2), which contributes to long-term climate change. In the quest for low-carbon alternatives, hydrogen (H
2) has emerged as a particularly promising energy carrier due to its high energy content (143 GJ ton
−1) and zero carbon emissions upon combustion [
1]. Unlike carbon-based fuels, H
2 releases only water vapor when burned, avoiding the formation of CO
2, acid rain, and other pollutants [
2].
Currently, most H
2 is produced via thermochemical or electrochemical methods, including steam methane reforming (SMR), pyrolysis, and water electrolysis. These techniques offer advantages in terms of H
2 yield and process control but are often energy-intensive and carbon-emitting unless paired with carbon capture technologies [
3]. For instance, SMR typically requires 142–164 MJ/kg H
2, while electrolysis methods such as alkaline electrolysis and proton exchange membrane electrolysis require approximately 180–216 MJ/kg H
2, with efficiencies ranging from 58–82% based on lower heating value [
4,
5].
In contrast, biological H
2 production, such as dark fermentation (DF), presents a more sustainable, though technically complex, alternative. This process leverages microbial consortia to metabolize organic substrates anaerobically, generating H
2 alongside various byproducts, including CO
2 and volatile fatty acids (VFAs) [
6]. While often characterized by lower yields (1.5—3.5 mol H
2/mol hexose) and slower microbial metabolism, DF offers a substantial energy advantage. For example,
Clostridium spp. can produce up to 3.0 mol H
2/mol glucose at rates of 1.5—4.0 L H
2/L/day with an energy requirement of ~20 MJ/kg H
2 [
7]. Even higher performance is achievable using engineered
Clostridium butyricum strains, reaching 3.5 mol H
2/mol glucose and ~18 MJ/kg H
2 [
8].
Microbial Electrolysis Cells (MECs), a hybrid of biological and electrochemical processes, have demonstrated theoretical yields up to 12 mol H
2/mol hexose with significantly lower energy requirements than conventional electrolysis, though practical scalability remains limited [
9,
10].
These comparisons underscore the performance gap but also the sustainability trade-offs between conventional and biological H2 production methods. While SMR and electrolysis produce more H2 per unit substrate, they incur higher energy and carbon costs unless decarbonized. DF, though limited in yield, offers a viable low-energy route for renewable H2, especially when integrated with innovative feedstocks and in situ applications.
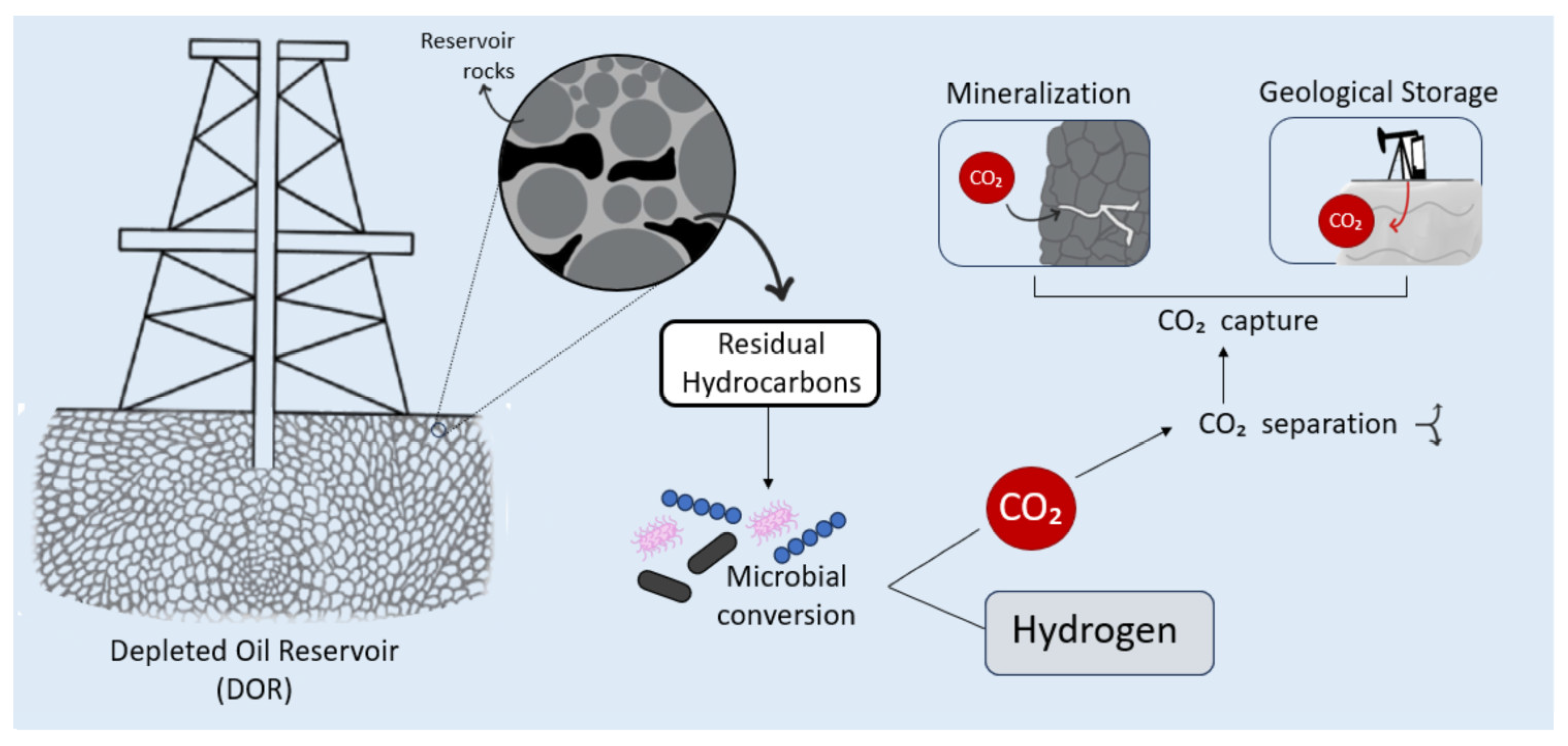
One particularly innovative solution is the use of depleted oil reservoirs (DORs) as in situ bioreactors. These subsurface environments contain significant amounts of RH and indigenous microbial communities, and they benefit from pre-existing infrastructure such as wells, pipelines, and geological data [
11,
12,
13,
14]. Depending on the recovery method previously applied, up to 60% of the original oil in place may remain as residual hydrocarbons (RH), representing an untapped substrate for microbial conversion into H
2 [
15,
16].
This process, recently termed Gold Hydrogen (H
2Au), refers specifically to biologically produced H
2 from residual fossil hydrocarbons via in situ anaerobic microbial activity. First promoted by R&D initiatives such as Cemvita, H
2Au differs from green H
2 (e.g., produced via electrolysis with renewable electricity), blue H
2 (e.g., SMR with carbon capture), and grey H
2 (e.g., SMR without carbon capture) in both raw material for microbial activity and production method [
17].
While the use of DORs for microbial H
2 production has compelling advantages, including scalability, potential cost savings from oil industry infrastructure reuse, and intrinsic containment for CO
2, there are notable challenges. These include the possibility of generation of hydrogen sulfide (H
2S), methane (CH
4), and CO
2, as well as reservoir souring, fracture risks, and the need for precise control of microbial populations. Coupling microbial H
2 production with carbon capture, utilization, and storage (CCUS) strategies, such as mineral trapping, biological fixation, and microalgal uptake, can enhance both carbon neutrality and operational safety (
Figure 1).
Economically, while leveraging existing oilfield infrastructure can reduce upfront costs, H
2 production from DORs remains in early-stage development. Substrate injection, microbial stimulation, gas recovery, and process monitoring introduce significant capital and operational expenditures. Therefore, despite its potential, this process cannot yet be considered economically viable without further rigorous studies, which are still scarce in the industry, despite recent announcements of supposedly commercial-scale pilot tests [
18].
This article reviews the scientific and technological basis of using DORs as bioreactors for H2 production. It explores the current state of knowledge, key microbial and geochemical processes, carbon management strategies, and the techno-economic framework necessary for feasibility. This work aims to inform researchers, engineers, and energy stakeholders about the transformative potential and limitations of this emerging paradigm in renewable H2 production.
2. Microbial Basis for H2 Production in Deplete Oil Reservoirs
H
2 production through DF has traditionally relied on carbohydrate-rich substrates; however, recent research has expanded this scope by exploring hydrocarbon fractions derived from petroleum as alternative feedstocks. These hydrocarbons, particularly saturated alkanes and aromatic compounds, are chemically inert and poorly soluble in water, presenting significant metabolic challenges under anaerobic conditions. Nevertheless, certain obligate and facultative anaerobic microorganisms have evolved specialized enzyme systems and pathways capable of activating and converting these compounds into central metabolic intermediates, which ultimately support H
2 production [
19,
20]. This concept underpins a promising biotechnological strategy wherein DF is applied to petroleum reservoirs for in situ energy recovery. DF is an anaerobic microbial process that converts organic substrates into H
2, CO
2, and soluble metabolic products such as VFAs and alcohols, in the absence of light. In petroleum systems, DF aligns with the acidogenic phase of anaerobic digestion, enabling the valorization of RH for renewable energy generation.
The microbial conversion of hydrocarbons begins with anaerobic activation, a crucial step due to the stability of hydrocarbon molecules. For alkanes, activation typically involves fumarate addition, catalyzed by alkylsuccinate synthase (assA), forming alkylsuccinates that undergo β-oxidation-like pathways to yield acetyl-CoA and reduced cofactors [
21,
22]. Similarly, aromatic hydrocarbons like toluene are activated via fumarate addition by benzylsuccinate synthase (bssA), producing benzylsuccinate and eventually benzoyl-CoA, a key intermediate in anaerobic aromatic metabolism [
23]. These reactions are often mediated by sulfate-reducing and nitrate-reducing bacteria such as
Desulfatibacillum aliphaticivorans [
24] and
Thauera aromatica [
25], which can initiate hydrocarbon degradation even in the absence of oxygen.
While H
2 producing microbes such as
Clostridium spp. are central to DF, they lack the enzymatic machinery, such as assA and bssA, for direct hydrocarbon activation [
26,
27,
28]. Therefore, H
2 production from residual petroleum as feedstock typically requires a syntrophic system where hydrocarbon-degrading bacteria generate intermediates such as acetate, propionate, lactate, and ethanol, which are then fermented by hydrogenogenic microbes such as
Clostridium butyricum,
Paraclostridium,
Ethanoligenens harbinense, and
Enterobacter aerogenes [
29,
30]. These consortia can operate under the strongly reducing, anoxic conditions of oil reservoirs, where alkanes, aromatics, and even asphaltenes are slowly oxidized to hydrogenogenic intermediates by primary fermenters. Alternatively, bioaugmentation or the genetic engineering of H
2 producing strains with hydrocarbon degradation genes is being investigated to bridge this metabolic gap. However, real-world implementation suffers another layer of complexity related to the use of engineered organisms in the environment.
Once hydrocarbons are converted to intermediates such as acetyl-CoA and pyruvate, these compounds enter fermentative pathways. Pyruvate is oxidized to acetyl-CoA by pyruvate:ferredoxin oxidoreductase, simultaneously reducing ferredoxin, which is then oxidized by [FeFe]- or [NiFe]-hydrogenases to release molecular H
2 [
31]. Acetyl-CoA may be converted into acetate for ATP generation or diverted into more reduced products like ethanol or butyrate, which act as redox sinks. Maintaining redox balance is therefore essential for maximizing H
2 yields [
32,
33].
The enzymes involved in these processes are sensitive to environmental factors: assA and bssA, involved in hydrocarbon activation, belong to the radical s-adenosyl methionine enzyme family and are typically encoded in operons regulated by substrate-inducible systems like assR (alkyl succinate synthase regulator) and bssR (benzyl succinate regulator) [
34]. Hydrogenase activity is influenced by pH, metal ions (Fe, Ni, Mo), and especially H
2 partial pressure, which thermodynamically inhibits further H
2 production [
35,
36]. Continuous removal of H
2 from the fermentation environment, via sparging, gas stripping or vacuum degassing is thus necessary to sustain high yields. Competition from hydrogenotrophic organisms such as methanogens, and homoacetogens that consume H
2 and produce acetic acid, must be suppressed through pretreatment methods like heat-shocking or the use of specific inhibitors.
DORs provide a unique anaerobic setting for H
2 production. These environments are typically deficient in terminal electron acceptors such as sulfate and nitrate, resulting in strongly reduced redox conditions. The oxidation-reduction potential becomes a critical control parameter, with optimal H
2 production occurring at values between -400 to -544 mV [
37]. Such conditions enhance hydrogenase activity and maintain a high NADH/NAD
+ ratio, both essential for reductive metabolism. They also protect redox-sensitive enzymes like hydrogenases and pyruvate:ferredoxin oxidoreductase from oxidative inactivation [
38]. In addition to redox control, reservoir-specific tuning methods, such as thermal desorption, chemical oxidation and biosurfactant application can improve hydrocarbon solubilization, enhancing microbial access and bioconversion efficiency [
39].
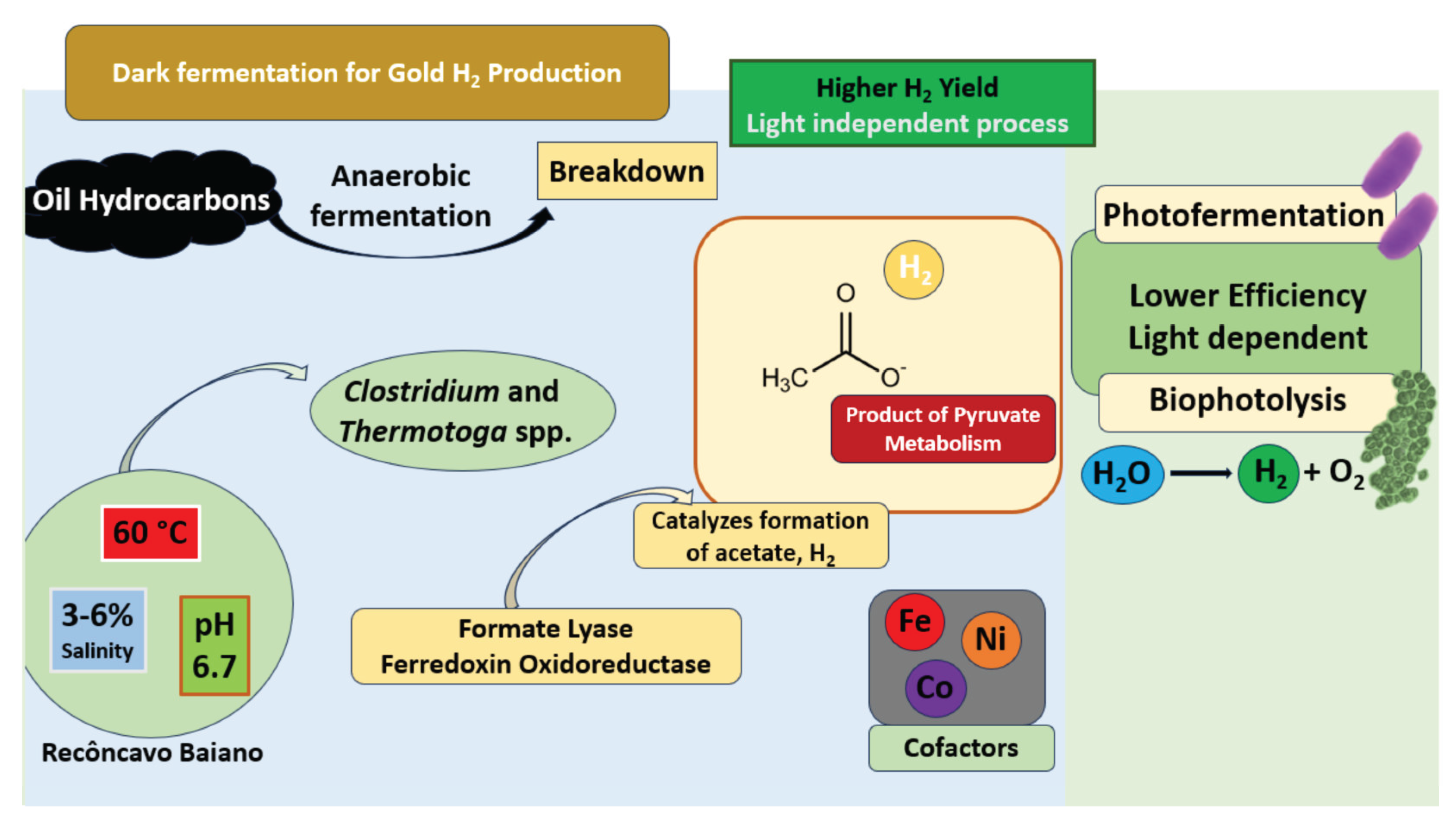
To stabilize these redox conditions, selective nutrient supplementation is key. The carbon to nitrogen ratio influences microbial growth and metabolism, with ammonium nitrogen supporting biomass development but requiring controlled dosing to prevent fermentation inhibition. Ammonium bicarbonate is commonly used as a nitrogen source and pH buffer [40]. Phosphorus, indispensable for ATP generation, is often supplemented during inoculum development [41,42]. Trace metals such as Fe
2+, Ni, Mo, Co, Mn, Zn, and Cu are critical cofactors in enzymes like hydrogenases and ferredoxins (
Figure 2). Nanoparticle-based additives such as cobalt ferrite, manganese ferrite, and alkali-based magnetic nanosheets can enhance trace metal bioavailability, stimulate
Clostridium enrichment, and improve emulsification and surface interactions with hydrocarbon droplets [42,43].
A systematic microbial management strategy is also necessary to suppress methanogens and sustain hydrogenogenic consortia. These include: thermal pretreatment, which selectively eliminates methanogens while preserving spore-forming H
2 producers like
Clostridium and
Bacillus spp. [
44,
45]; pH control, favoring H
2 producing bacteria at mildly acidic conditions (pH 5.0–6.5), though requiring monitoring to avoid over-acidification [
46]; chemical inhibitors, such as 2-bromoethanesulfonate, chloroform, and Poly(diallyldimethylammonium chloride), which are effective but must be managed due to cost and potential toxicity [
47]; hydraulic retention time optimization, which limits methanogen proliferation by favoring faster-growing hydrogenogens [
48]; and reactor design adaptations, such as biofilm reactors and MECs, which stabilize microbial communities and lower H
2 partial pressure [
49]. MECs can also further oxidize VFAs produced in DF with minimal external voltage input, offering staged energy recovery and improving overall H
2 yield [
50].
The versatility of DF extends to a wide range of substrates beyond petroleum, including glucose, food waste [
51], lignocellulosic biomass, and microalgae [
52,
53]. Novel strains like
Clostridium populeti FZ10, acclimated to cellulose, and
Thermotoga species capable of in situ hydrocarbon fermentation have expanded the scope of potential feedstocks, particularly in petroleum-associated environments [
54,
55]. Other promising substrates include glycerol, alcohol stillage (utilized by
Clostridium beijerinckii 6A1), and syngas, though the latter poses challenges such as toxicity limitations on conversion performance [
56].
Microbial consortia, especially those dominated by
Clostridium and
Thermotoga spp., are commonly employed due to their synergistic interactions [
57]. Process conditions such as pH and temperature are critical: pH 5.5 favors H2 production, while neutral pH can lead to methanogenesis. Thermophilic conditions (55–65 °C) benefit species like
Clostridium stercorarium [
58]. VFAs such as acetate and butyrate positively correlate with H
2 output, whereas methanogens consume H
2, although inhibitors such as 2-bromoethanesulfonate enhance yields by up to 25% [
51]. Nanoparticles of hematite, nickel and titanium dioxide can further improve performance by enhancing microbial surface interactions, pH stability, and enzyme activity [
59,
60].
Other biological routes include photo-fermentation, with an even lower energy footprint (~40.1 MJ/kg H
2), and two-stage fermentation, which can reduce energy use to 39.3 MJ/kg H
2 [
61]. MECs offer another avenue, suppressing methanogens while enhancing H
2 production, often utilizing
Desulfovibrio spp. [
62]. Methanogenesis remains important in biogas and biohythane (a mixture of H
2, CH
4 and CO
2) production, with methanogenic archaea like
Methanosarcina thermophila utilizing acetate and H
2/CO
2 via hydrogenotrophic and acetoclastic pathways [
63,
64].
In DF, hydrogenases, which are metal-dependent enzymes, catalyze the reversible reaction 2H
+ + 2e
− → H
2. Organic substrates are metabolized through intermediates like benzoyl-CoA and succinyl-CoA to acetyl-CoA, fueling hydrogen evolution [
65]. Reservoir-based stimulation strategies require careful modulation of the redox landscape, often depleted in terminal electron acceptors. Selective nutrient supplementation and microbial inhibition are key to promoting hydrogenogenic populations while suppressing hydrogenotrophs and mitigating issues like microbiologically influenced corrosion [
66,
67,
68]. Some microbes enhance hydrocarbon uptake by producing biosurfactants such as rhamnolipids, which improve solubilization and emulsification of oil droplets, however the low surface-to-volume ratio of these droplets remains a challenge. Additionally, certain bacteria use extracellular electron transfer via nanowires and dehydrogenases, rather than cytochromes, mechanisms susceptible to inhibitors like cyanide and CO, which selectively block hydrogenases without affecting formate dehydrogenase activity [
69,
70]. Despite the complexity, advances in microbial engineering, reactor optimization and syntrophic system design, petroleum-derived H
2 production represents a feasible and sustainable approach within the circular bioeconomy paradigm. The co-generation of H
2 and soluble microbial products also exhibits potential for bioplastic, microbial oil, or platform chemical production, further enhancing the value of DF in petroleum reservoir systems.
3. Depleted Oil Reservoir Conditions and Technological Challenges
Oil reservoirs are considered extreme environments due to their high temperatures, salinity, pressure, and toxicity, as well as the low wettability of reservoir rocks and the heterogeneity of water activity across zones. Reservoir temperatures, typically ranging between 36 °C and 80 °C or more, play a critical role in determining the types of microorganisms capable of inhabiting these systems [
71]. Among the most ubiquitous microbial groups across these temperature gradients are
Proteobacteria and
Euryarchaeota, while other taxa tend to be associated with specific temperature conditions.
In the Recôncavo Baiano basin, oil reservoirs exhibit average temperatures of approximately 60 °C. The reservoir fluids have salinities ranging from 3% to 6%, although salinity can reach up to 16% in specific fields such as Araçás. These fluids are slightly acidic, with an average pH of 6.7, and the oil wells are typically found at depths between 900 and 1200 meters. Within such environments, biofilm formation is recognized as the dominant microbial organizational strategy, enhancing nutrient uptake, promoting syntrophic interactions and offering protection against environmental stressors [
72].
The heterogeneity of water activity across different reservoir zones has direct implications for microbial distribution and activity. Variability in pore space geometry, saturation levels, and nutrient gradients can create distinct micro-niches that favor different metabolic groups. This spatial heterogeneity complicates process control, such as microbial stimulation for enhanced oil recovery or H2 production. Managing this complexity may involve implementing targeted microbial injections or using multiple injection and production points to ensure optimal distribution of nutrients and microbial consortia across various reservoir zones.
Although viruses are known to be abundant in oil reservoirs, their influence on microbial community structure remains poorly understood. Nevertheless, they are presumed to play a role in modulating microbial population dynamics [
73]. To address this knowledge gap, targeted research directions such as viral metagenomics and host-virus interaction studies are warranted. These approaches could help uncover how viruses affect microbial syntrophy, horizontal gene transfer or biofilm stability, factors critical to microbial-mediated processes such as hydrogen production or hydrocarbon degradation.
Experimental microcosm studies using diverse microbial consortia with crude oil have demonstrated notable changes in the activity of syntrophic and methanogenic hydrocarbonoclastic bacteria [
74], underscoring the metabolic interdependencies in these communities. Syntrophic microorganisms are particularly important due to their metabolic flexibility. They can produce or consume H
2 and facilitate interspecies electron transfer via formate, depending on the substrates available [
75]. In reservoir samples, complex microbial communities have been observed, composed of populations engaged in a variety of metabolic functions, often maintained through symbiotic, antagonistic or competitive relationships.
Much of what is known about oilfield microbiota has been derived from metagenomic analyses, in which DNA extracted from formation water serves as a proxy for the reservoir’s microbial community. However, comparisons between different phases, such as water and oil, have revealed significant differences in microbial composition [
76]. This variability may be further influenced by the sampling process itself. Microorganisms introduced during drilling or production operations may alter the native microbial profile, complicating the interpretation of results.
In extreme subsurface environments, applying advanced techniques such as next-generation sequencing and culture-based methods presents practical challenges. Sampling from depths exceeding 900 meters requires the maintenance of anaerobic conditions and in situ pressure and temperature during retrieval, since depressurization or oxygen intrusion can shift microbial community structure. Moreover, the risk of contamination during drilling or during transit through pipelines must be mitigated through rigorous aseptic protocols and the use of in-line filtration systems. Despite these difficulties, next-generation sequencing of the 16S rRNA gene remains a powerful tool for resolving taxonomic and functional profiles, particularly when complemented by the sequencing of hydrogenase and dehydrogenase genes to detect microbial populations specifically involved in H2 production.
Culture-based methods, though limited to cultivable strains, remain essential for validating functional capabilities and for monitoring key hydrocarbon-fermenting or H2 producing populations under controlled conditions. These integrative approaches, when carefully adapted to the operational realities of oilfield sampling, offer a more accurate and practical-driven view of the complex microbiological dynamics within petroleum reservoirs.
4. Infrastructure Adaptation for H2 Production in DORs
Repurposing DORs for H
2 production offers a promising pathway toward sustainable energy recovery and decarbonization. Reservoirs such as those in the Recôncavo Baiano region of Brazil possess extensive pre-existing infrastructure, including wells, pipelines and surface facilities, which can be strategically adapted for microbial H
2 production via DF. This reuse minimizes capital expenditure, accelerates implementation timelines, and aligns with circular economy principles by extending the functional lifespan of fossil fuel infrastructure [
77].
Many of these reservoirs feature structurally sound vertical and directional wells capable of facilitating microbial inoculation and nutrient injection, eliminating the need for new drilling, and thereby reducing environmental impacts. Existing surface infrastructure, such as gas-liquid separators, storage tanks, and pump stations, can be retrofitted to handle H2 rich gas mixtures, while the established ~400 km gas pipeline network supports efficient collection and transport. Additionally, historical subsurface datasets (e.g., seismic surveys and well logs) are valuable for identifying optimal inoculation zones, modeling flow dynamics and optimizing nutrient delivery. Operational equipment previously used for enhanced oil recovery (e.g., injection systems and pumps for rheological modifiers, surfactants, and others) can also be repurposed to manage nutrient distribution, pressure control, and microbial activity.
Depleted reservoirs often retain RH and harbor resilient indigenous microbial communities shaped by prior oil operations. These microorganisms can be stimulated under anaerobic conditions through the injection of biodegradable substrates to produce H2 in situ. However, microbial H2 production faces several limitations, including low yields, the formation of H2S and the risk of pore plugging. Pore plugging, caused by biomass accumulation, biofilm formation, mineral precipitation (e.g., calcium carbonate or iron sulfide) or the production of extracellular polymeric substances, can severely impair permeability and reduce H2 recovery. Mitigation strategies include careful filtration and pH adjustment of injection fluids, periodic backflushing, microbial consortia selection favoring planktonic phenotypes, and targeted well stimulation techniques to maintain flow pathways.
Recent cost estimates for H
2 production in depleted reservoirs exceed USD 25/kg H
2, primarily due to low conversion efficiencies, extended fermentation cycles, and the high costs of retrofitting infrastructure [
78]. For example, reinforcing steel casing with corrosion-resistant alloys (e.g., Inconel or duplex stainless steel) may range from
$300 to
$800 per meter of wellbore, while acid-resistant cement replacements like geopolymer or supplementary cementitious materials enhanced formulations can add
$100–
$200 per ton compared to Portland cement. Zonal isolation tools (e.g., retrievable packers) and advanced injection systems further contribute to capital expenditure, typically increasing retrofitting costs by 20–40% over standard enhanced oil recovery upgrades.
By contrast, Cemvita’s widely cited target of USD 1/kg H2 reflects a long-term techno-economic aspiration under highly optimized conditions and is likely contingent upon high microbial productivity, short fermentation cycles and economies of scale. Public sources suggest this figure was based on controlled laboratory or limited field trial conditions and may not reflect full-system lifecycle costs in heterogeneous, real-world reservoirs. In addition, Cemvita’s estimates likely assume synergistic revenue from CO2 capture and utilization, which offsets production costs. The Technology Readiness Level (TRL) for DF in oil reservoirs remains at TRL 4–5, while commercial-scale validation under representative reservoir conditions is lacking. Therefore, while long-term cost reductions are theoretically possible through bioprocess intensification and infrastructure optimization, achieving USD 1/kg H2 in depleted oil fields likely requires overcoming substantial technical bottlenecks, particularly those related to low microbial conversion rates, pore plugging, and corrosion control.
The intrinsic properties of H
2, including low density, high diffusivity, and microbial reactivity, introduce unique subsurface challenges. It can displace brine and RH, elevate formation pressure, and react with microbial populations to generate corrosive byproducts like H
2S. These effects may compromise rock integrity and reduce storage capacity. Ensuring well integrity is therefore paramount. Class G Portland cement, the industry standard, is susceptible to degradation from CO
2, H
2S, and potentially H
2. While some studies indicate structural stability, others report increased permeability and porosity. To address this, alternative materials, such as silica-enhanced cement, foamed cement, geopolymers, and supplementary cementitious materials, are under active development [
79].
Alternative in situ H
2 generation methods, including thermal reforming, pyrolysis, and steam-assisted conversion, offer higher yields but require substantial energy input and sophisticated gas separation. Demonstrated conversion efficiencies in depleted gas reservoirs remain low (e.g., 5.8% CH
4 to H
2), while coke deposition and H
2S formation remain operational barriers. Despite higher TRLs (7–9), these methods also demand further validation at field scale [
78].
Comprehensive technical adaptations are necessary to convert depleted reservoirs into viable H
2 systems. Key priorities include reinforcement of wells, advanced microbial containment, modular injection mechanisms, and real-time monitoring tools. Microbial fermentation requires strict anaerobic isolation, often achieved using retrievable packers and downhole thermal systems to maintain optimal fermentation temperatures (30–70 °C) [
78].
Surface systems must support precise microbial inoculum delivery, continuous nutrient injection and gas-phase monitoring. H
2 purification is critical: gas-liquid separators feed into purification units (e.g., pressure swing adsorption, membrane systems), often enhanced with palladium-based membranes and catalytic water-gas shift zones. Advanced setups integrate temperature swing adsorption/pressure swing adsorption units to remove trace contaminants and enable high-purity H
2 for downstream use and concurrent CO
2 capture [
80,
81,
82,
83]. Hybrid fermentation phototrophic systems may offer further process integration and CO
2 valorization [
84].
These technical adaptations, when paired with strategic retrofitting, illustrate how legacy oil infrastructure can be transformed into sustainable H
2 production assets. From a cost-benefit perspective, retrofitting remains more economical than new drilling and facilitates faster deployment. Still, techno-economic viability hinges on improving microbial efficiency, reducing system complexity, and developing robust solutions to corrosion and plugging. Pilot-scale demonstrations in Recôncavo Baiano and similar basins are essential to validate these integrated systems and resolve remaining knowledge gaps (
Table 1).
5. Carbon Capture, Utilization, and Storage
CCUS is a crucial strategy for addressing climate change, enabling the reduction of greenhouse gas emissions while supporting sustainable energy transitions. This approach plays a dual role in H
2 production from DORs: enhancing the carbon neutrality of H
2 production and ensuring the long-term sequestration of CO
2 generated during microbial hydrocarbon conversion [
1]. H
2 generation in situ from RH using native microbial communities produces not only H
2 but also CO
2. Therefore, integrated strategies for CO
2 capture, whether biological, geological or chemical, are essential for maximizing environmental benefits and ensuring the viability of the process.
Table 2 summarizes the approaches encompassing geological, mineralization, adsorption and microalgae-based techniques for CCUS, highlighting their advantages, drawbacks, and current bottlenecks for real-world implementation.
Biological CO
2 capture within the reservoir is primarily mediated by microbial pathways. Hydrogenotrophic methanogens, for example, can metabolize CO
2 and H
2 to form CH
4 via methanogenesis, an undesirable process. However, when supercritical CO
2 is co-injected into the reservoir, it lowers the pH of the formation water, creating acidic conditions that inhibit methanogenic activity while stimulating fermentative, H
2-producing microbes [
118]. This pH-mediated shift suppresses CH
4 formation and favors H
2 production, leaving more CO
2 available for sequestration. In this system, dissolved hydrocarbons serve as substrates for microbial metabolism, allowing for simultaneous H
2 generation and CO
2 retention.
In parallel, CO
2 may undergo mineralization reactions with reservoir rocks containing calcium or magnesium silicates, leading to the formation of stable carbonates [
119,
120]. These processes, though naturally occurring over geological timescales, can be enhanced under engineered conditions to accelerate CO
2 sequestration (
Figure 1). Engineered mineralization within DORs can be achieved through several strategies: co-injection of finely ground reactive minerals (e.g., olivine or serpentine) to increase surface area and reactivity; acidification via CO
2 dissolution to enhance mineral carbonation kinetics; or stimulation of indigenous or introduced microbial consortia that promote microbially induced carbonate precipitation through metabolic processes such as ureolysis or sulfate reduction. Additionally, controlled manipulation of formation temperature and pressure may further accelerate geochemical reactions, promoting the rapid formation of solid carbonates and enhancing CO
2 permanence in the reservoir environment.
In other microbial systems, such as microbially induced carbonate precipitation, microbial metabolism indirectly contributes to carbonate formation, though such strategies are more commonly studied in surface applications than within petroleum reservoirs [
121,
122].
While CO
2 sequestration within the reservoir is the primary objective, additional strategies exist for managing CO
2 if it reaches the surface during production. DORs are inherently well-suited for CO
2 storage due to their geological properties. Structural trapping occurs when CO
2 is confined beneath impermeable caprock layers, while capillary trapping immobilizes CO
2 within pore spaces in the reservoir rock [
123]. Solubility trapping involves CO
2 dissolving into formation water to form carbonated brine, which is denser and less mobile than pure CO
2 gas [
124]. Mineral trapping, in contrast, entails chemical reactions between CO
2 saturated brines and reservoir minerals, forming solid carbonates and providing durable, long-term sequestration [
125]. These mechanisms may work simultaneously [
125] under conditions of microbial H
2 production, where CO
2 solubility is naturally high and microbial processes suppress its escape.
If the CO
2 is produced alongside H
2 at the surface, separation and capture technologies can be employed to recover it for reuse or storage. Chemical absorption using solvents like monoethanolamine is a well-established method for capturing CO
2 from gas mixtures [
101]. Physical solvents such as Selexol or Rectisol are also effective, particularly at high pressures typical of reservoir outputs. Adsorption techniques using solid sorbents like activated carbon or metal-organic frameworks can selectively retain CO
2, often in pressure swing adsorption systems commonly used for H
2 purification [
126]. Membrane-based separation offers an energy-efficient alternative, leveraging selective permeability to separate CO
2 from H
2 streams. Cryogenic separation, though energy-intensive, may also be used to liquefy and isolate CO
2 at low temperatures [
127].
The products generated during microbial H
2 production provide insight into the feasibility of integrated carbon management. H
2 is the primary energy product, with simulated yields ranging from 154 to 1,670 kg per well over 100 days, particularly near injection zones where microbial activity is highest and residual oil concentrations are lowest [
128]. CO
2, as a co-product is effectively retained in situ due to its solubility, the acidic conditions that inhibit methanogenesis, and the presence of multiple trapping mechanisms [
128]. Methane formation is limited under these conditions, further enhancing the stability of CO
2 sequestration. Other microbial byproducts include organic acids and H
2S, which may present operational challenges, particularly microbial souring, and must be monitored and mitigated [
129].
However, attention must be paid to site conditions, since methanogenesis may convert 13 to 19% of the injected CO
2 during CO
2 enhanced oil recovery [
130], compromising the intended goal. This highlights the need for careful evaluation and monitoring of CO
2 behavior under real-world conditions in depleted reservoirs for H
2 production, as well as the proper inhibition of methanogenic microorganisms and the enhancement of hydrogenogenic activity to prevent the loss of CO
2 intended for conversion.
The inherent geological and chemical properties of DORs, combined with the microbiological dynamics under engineered conditions, support both H
2 generation and permanent CO
2 retention [
77]. Acidic formation conditions induced by CO
2 injection not only suppress undesirable microbial pathways (like methanogenesis) but also enhance H
2 yields and CO
2 solubility. This unique alignment of microbial behavior, geochemical trapping, and reservoir characteristics makes in situ CO
2 capture an intrinsic and feasible component of H
2 production systems, contributing significantly to the development of low-carbon, renewable energy solutions.
While microalgae-based carbon capture is not an in situ strategy, it provides a promising surface-level CCUS method to manage CO
2 co-produced during H
2 production. Due to their high photosynthetic efficiency, rapid growth rate, and ability to produce valuable biomass, microalgae are particularly relevant for integrating into surface operations following H
2 recovery [
131,
132]. They directly capture CO
2 from atmospheric or industrial sources and convert it into biomass that can be used to produce third-generation biofuels, including H
2 itself [
131,
133].
This biological CO
2 conversion pathway is particularly attractive because it is low-cost, does not require expensive catalysts, and directly fixes CO
2 using sunlight [
134]. Importantly, microalgae exhibit CO
2 capture rates 10–50 times higher than terrestrial plants [
132,
133]. These systems are especially useful in closed photobioreactor configurations that can be integrated with fermentation or gasification units to recapture CO
2 emitted during upstream H
2 production [
133].
While the generation of CO2 during H2 production is well-recognized and the CO2 fixing capabilities of microalgae are well-established, their role in this context is best understood as part of a broader surface-level CO2 management strategy that enhances the environmental sustainability of integrated H2 systems.
6. Safety Aspects of H2Au Production
Production of H2Au in subsurface environments, particularly in repurposed oil and gas reservoirs, presents unique safety challenges. These encompass wellbore and reservoir integrity, uncontrolled microbial activity, seal effectiveness, microbial H2S production, and explosion or fire hazards. Addressing these risks requires integrated engineering, microbiological and geochemical strategies for safe and reliable H2 generation and containment.
Maintaining long-term wellbore integrity is paramount in aged or depleted reservoirs repurposed for H
2 production. Structural degradation, corroded casing, compromised cement and failed zonal isolation can lead to unintended migration of gases such as H
2, H
2S, and CO
2, threatening freshwater aquifers and ecosystems. Failures may result from single-barrier degradation (casing or cement) or multibarrier failure, especially in wells exposed to corrosive fluids or microbial activity [
135,
136].
Corrosion is a primary concern, particularly near perforated production zones or areas exposed to injected CO
2 and formation fluids. Localized corrosion driven by microbial activity or CO
2 rich brines can perforate casing, prompting well abandonment [
137]. Legacy well studies confirm accelerated degradation in zones with poor cement bonding. Cyclic mechanical loads and thermal stresses further contribute to fatigue-induced failures, compromising sealing and structural capacity [
138].
Cement degradation via debonding, cracking or chemical alteration in acidic environments creates micro-annuli and fluid pathways, threatening containment. Although remediation methods such as cement squeezing and nanomaterial injection exist, they offer limited and context-dependent success [
139]. Older wells are especially vulnerable due to prolonged exposure to carbonation and mechanical stress [
140].
Artificial intelligence tools, particularly artificial neural networks, have shown promise in predicting casing corrosion based on well data, minimizing the need for frequent field testing [
141]. Integrating such models into H
2 operations enables real-time monitoring and preventive maintenance by analyzing pH, sulfate concentrations, and microbial signatures.
Comprehensive well integrity management is essential and should include periodic mechanical testing, real-time pressure and gas monitoring, geomechanical modeling, corrosion forecasting and premium-grade casing materials. These elements must be aligned with microbial H2 generation steps (nutrient injection timing, gas collection cycles and pressurization protocols) to prevent gas leaks, water contamination or system failure.
Uncontrolled microbial metabolism can lead to excessive gas accumulation (H
2, CO
2, CH
4, H
2S), risking caprock fracturing and fault reactivation. This biogenic pressurization differs from engineered gas injections, making pressure prediction challenging in heterogeneous reservoirs. At the Tvrdonice underground hydrogen storage site in the Czech Republic, microbial activity consumed 50% of injected H
2 and 12.5% of CO
2 within 40 days, significantly altering pressure profiles and reservoir chemistry [
142].
Microbial gas generation complicates pressure control and may result in caprock damage, micro-annuli formation, and gas escape through faults or wellbores [
143]. Microbial-induced changes in rock wettability and infrastructure corrosion further elevate mechanical risks. For instance, sulfate-reducing bacteria (SRB) activity shifted rock wettability from water-wet to neutral-wet in under 20 hours [
142].
Dynamic modeling that integrates biological, geochemical, mechanical, and thermal processes is essential for pressure management. Real-time monitoring tools, pressure sensors, gas analyzers and microbial activity detectors must be deployed. Nutrient supply should be finely controlled to limit excessive microbial growth, with selective inhibitors or competitive microbial strains used to suppress undesired gas generation. Accurate pressure predictions and responsive operational adjustments hinge on these coupled models.
Cap rock integrity is critical for trapping H
2 and CO
2. Shales and clays act as low-permeability seals, but their capacity can be challenged by fault zones or pressure-induced fractures. Field projects like the CS-D experiment at Mont Terri (Switzerland) demonstrated that CO
2 saturated brine injected into clay faults caused minimal fluid migration and no seismicity, supporting caprock effectiveness [
144].
CO
2 interactions can promote mineral trapping and enhance seal performance. Regulatory frameworks now require thorough geomechanical and geochemical assessment of seals before CO
2 storage [
145]. However, the small molecular size of H
2 and its high diffusivity increase leakage risks, especially through microfractures. H
2 may also alter redox conditions, stimulating microbial activity that changes seal mineralogy, an area needing further study [
146].
Despite these risks, faulted clay formations can still function as effective seals if adequately characterized. Monitoring systems and integrated models are essential to assess and mitigate leakage risks in microbial H2 production, especially by accounting for dynamic gas evolution and microbial interactions.
Reservoir souring, driven by SRB, results in H
2S production, posing severe health, environmental and operational hazards [
147]. SRB reduce sulfate to H
2S, particularly in nutrient-amended systems. Souring degrades infrastructure, compromises well integrity and increases treatment costs. Interestingly, SRB also exhibit high hydrogenase activity and can produce H
2 fermentatively under sulfate-limited conditions.
Desulfovibrio vulgaris Hildenborough yielded up to 560 mL H
2/L using formate as a substrate, with complete substrate conversion [
148]. This duality positions SRB as both a hazard and a biocatalytic asset, especially in second-stage H
2 systems converting DF effluents.
Uncontrolled SRB activity in oilfields, especially between injection and production wells in waterflooded zones, can lead to biosouring. Environmental factors (temperature, salinity, pH, nitrogen, phosphorus) affect H
2S generation. Modeling efforts that integrate microbial dynamics with fluid transport and H
2S scavenging mechanisms (by iron minerals) are necessary for accurate prediction [
149].
Active management strategies include substrate optimization (favoring formate), adjusting sulfate levels to steer microbial pathways, and fostering competitive microbial guilds such as nitrate-reducing bacteria. Environmental manipulation, pH, redox potential, electron donors, can suppress H2S while promoting H2 production, although this balance remains technically complex.
Technologies like gas-stripping reactors also offer promise. In packed-bed SRB bioreactors, using N
2 as a strip gas, improved H
2S recovery and sulfate conversion, with pH shifts enhancing H
2S output by over 60% [
150]. H
2S can then be converted into valuable products such as elemental sulfur or H
2 fuel.
Mitigation tools include nitrate or perchlorate injection, which inhibit SRB or promote competition, and biocide treatments, though these pose ecological risks. Early detection using chemical and microbial markers is key to tracking active souring zones and deploying targeted countermeasures.
The extreme flammability and low ignition energy (0.02 mJ) of H
2 make it a high-risk gas in oilfield settings. Its wide flammable range (4–75% in air) and nearly invisible flame increase the likelihood and severity of fires and secondary damage [
151,
152].
In microbial H2 systems, H2 can accumulate in enclosed areas like pipelines and tanks, forming stratified gas pockets near ceilings. Despite its buoyancy, confined spaces heighten the danger of explosive concentrations. Mitigation measures include spark-proof equipment, anti-static materials and robust ventilation systems. Continuous H2 detectors, especially in elevated indoor locations or along leak-prone paths, are essential for early warning. These should trigger alarms when concentrations approach the 4% lower flammable limit.
Safety protocols must include leak testing, equipment grounding, personnel training and clear emergency procedures. Integration of real-time detection and engineering controls, ventilation, static control and ignition prevention into H2 facility design is critical to reduce the risk of catastrophic events.
As discussed, hydrogen production from DORs offers a promising renewable pathway, but entails complex safety challenges spanning reservoir integrity, microbial behavior, chemical reactivity and explosive potential. A safe operation demands multidisciplinary integration of advanced monitoring, predictive modeling, microbial management and stringent engineering controls to ensure secure H2 containment and sustainable energy delivery.
7. Economic Aspects of the Process of Obtaining H2 Using DORs as Bioreactors
The use of DORs as bioreactors for H
2 production presents a promising alternative to conventional bioprocessing infrastructure by leveraging existing subsurface assets. From an economic standpoint, this approach offers both cost-saving opportunities and substantial challenges that must be evaluated within a robust techno-economic framework [
153].
One of the clearest economic advantages lies in the reuse of existing infrastructure, including wells, pipelines, and reservoir access systems, which significantly reduces capital expenditures (CAPEX) typically required for building surface-based bioreactors and containment systems [
154]. Moreover, oil reservoirs inherently provide anaerobic and pressurized environments favorable for hydrogenogenic microbial activity, potentially lowering operational expenditures (OPEX) by reducing the need for pH, temperature and pressure regulation [
153,
155].
Additionally, the large subsurface volume and containment properties of reservoirs offer opportunities for process scale-up with a minimal surface footprint. This addresses a critical bottleneck in traditional H
2 production, limited reactor size, by enabling continuous or semi-continuous production cycles that bypass the need for expensive vessel fabrication and maintenance [
156,
157]. However, these advantages must be critically weighed against several economic challenges, which require clearer quantification to evaluate feasibility.
Tailoring native microbial consortia or engineering synthetic communities to enhance H2 yields can require substantial R&D investments. Monitoring biological activity in inaccessible reservoirs requires advanced sensor arrays, real-time monitoring software and geochemical probes, which can add considerable costs per site during pilot-scale implementations.
Fermentable substrates such as biomass hydrolysates or organic acids must be injected under pressure. High-pressure injection systems and pumping infrastructure, along with feedstock procurement and processing, can result in high energy and logistics demands, depending on reservoir depth and injection rates.
Finally, recovering H
2 from the reservoir involves retrofitting existing extraction systems and installing surface separation units. These costs must be reconciled with H
2 pricing benchmarks. Cemvita’s publicized goal of achieving
$1/kg H
2 production cost in DORs [
153] stands in stark contrast to current general H
2 costs, which range from
$10–
$20/GJ, or approximately
$3.30–
$6.60/kg H
2, assuming 1 GJ ≈ 33 kg H
2. Cemvita’s estimate is believed to be based on early-stage modeling and small-scale field trials in highly favorable reservoir conditions, where infrastructure reuse, ideal microbial consortia and government-funded research helped reduce costs. However, scaling these conditions across varied geological settings remains a major challenge. Reaching the
$1/kg threshold would likely require simultaneous breakthroughs in substrate optimization, microbial efficiency, H
2 recovery and regulatory facilitation, none of which are guaranteed or fully demonstrated at commercial scales.
Therefore, while Cemvita’s cost target may be technically plausible under narrow, optimized conditions, it is not currently representative of average H2 production economics. Without robust validation across diverse reservoir types, the $1/kg figure should be regarded as a future-oriented projection, not a baseline assumption.
From a market perspective, the value of H
2 is becoming increasingly recognized in the context of the global energy transition. For example, Japan’s Strategic Roadmap for H
2 and Fuel Cells and the EU H
2 Strategy [
158] have both set ambitious targets and funding schemes to accelerate H
2 adoption, including production subsidies, tax credits, and carbon pricing mechanisms. In the U.S., the Inflation Reduction Act (IRA) of 2022 [
159] offers tax credits of up to
$3/kg H
2 for clean H
2 production, significantly improving the economics of low-emission pathways (including biological routes) when lifecycle carbon intensity thresholds are met. However, the pending repeal of the IRA and its H
2 incentive provisions may negate such benefits [
160].
Moreover, using and permanently sealing off depleted reservoirs for H2 production aligns with carbon credit schemes aimed at repurposing fossil infrastructure and mitigating subsurface methane emissions. These credits, while still under regulatory development, could provide additional revenue streams that partially offset process costs.
While the deployment of DORs as bioreactors introduces considerable technical and economic complexities, the potential for cost-effective and large-scale H2 production remains compelling, but only under clearly defined conditions supported by rigorous techno-economic analysis. A transparent evaluation that incorporates site-specific geological data, realistic cost modeling and market incentive structures is essential for assessing the true feasibility and profitability of this emerging bioprocessing paradigm.
8. Pilot Projects and Case Studies
8.1. CEMVITA - H2 Production from DORs - The “H2Au” Approach
CEMVITA, a Houston-based biotechnology company, is at the forefront of developing sustainable H
2 production through its innovative H
2Au platform, which harnesses microbial processes to recover H
2 from DORs. This approach, grounded in synthetic biology and detailed in a suite of patents, aims to convert RH into H
2, thereby transforming abandoned oil fields into clean energy sources and supporting decarbonization strategies in heavy industries [
161].
At the core of CEMVITA’s technology is the use of modified or naturally occurring microbes capable of degrading residual oil underground. These microorganisms convert hydrocarbons into H
2 and CO
2 through proprietary subsurface bioreactions initiated by the injection of circulating water, microbial inocula and metabolic enhancers into the formation reservoirs [
161,
162]. The residual hydrocarbons act as a carbon source, while the produced H
2 is extracted through the wellbore. The co-produced CO
2 is sequestered in situ to ensure carbon neutrality.
A landmark field trial in 2022 at the Permian Basin demonstrated the technical feasibility of in situ H
2 generation [
163,
164], with the company targeting a production cost of approximately
$1/kg—markedly lower than microbial H
2 costs from other pathways [
162,
163].
This approach offers numerous environmental and economic benefits. It provides a carbon-neutral source of H
2 [
163], repurposes legacy oil infrastructure [
77], extends the useful life of oil fields, and offers transitional economic value to fossil fuel-dependent regions [
165]. Additionally, it converts oilfield waste into a usable energy resource, thereby improving recovery efficiency from RH [
165].
However, the platform also faces significant challenges. While the process captures CO
2, it still generates emissions [
163], and the overall recovery model remains in early stages with limited independent validation beyond Cemvita’s own reports. Subsurface microbial H
2 production is subject to efficiency constraints and extended production timelines, mirroring broader issues in crude oil gasification which depend heavily on local geochemical and thermodynamic conditions [
166].
A central technical challenge remains H
2 productivity. Low H
2 concentrations are typical in microbial oil degradation, complicating extraction at scale. Optimizing dynamic extraction and separation systems remains an engineering bottleneck [
167], compounded by the fact that microbial H
2 generation primarily occurs at oil-water interfaces in aqueous zones.
Economically, the viability of this process is still unproven. Current estimates place H
2 costs between
$10–
$20 per GJ, substantially above the competitive threshold of
$0.33/GJ [
168]. Cemvita has not yet disclosed reproducible data regarding specific productivity (e.g., Nm
3/h or kg/day), making comparisons to other systems, such as dark fermentation (typically reporting 0.015–1.084 mmol/L·h, or up to 10.26 mmol/L·h in biomass-fed setups), not directly applicable to subterranean reservoirs [
166]. This absence of quantitative transparency extends to scientific literature, where Cemvita appears in several articles [
163], yet lacks detailed empirical data.
A rare production estimate was disclosed in an interview with CEO Moji Karimi, who claimed the process could yield up to 3 kg of H
2 per barrel of residual oil [
169]. While promising, this figure raises critical questions about in situ oil availability, microbial conversion rates and flow dynamics under operational conditions. H
2-View has reported speculative production rates of up to 2.5 tonnes of H
2 per well per day across 120 wells [
170], and GlobalSpec has cited possible field-scale yields of 20–50 tonnes [
171], though neither claim is currently supported by peer-reviewed validation or third-party documentation.
To bring clarity to the technology’s framework, CEMVITA’s patents provide valuable insights into the design and operation of its microbial H
2 platform. The process is described as a subterranean enclosed bioreactor system embedded directly within hydrocarbon-rich environments such as depleted oil wells or landfills [
172,
173]. This in situ reactor is engineered to optimize microbial hydrogenogenesis, with environmental parameters such as pH (5–9, ideally 6–7), temperature and pressure tightly regulated to sustain microbial activity [
173,
174,
175].
A key component of the technology is the injection of hydrogen-producing microorganisms, either indigenous or genetically modified, into the formation [
174,
175]. Preferred microbial genera include
Clostridium,
Syntrophobacter,
Thermoanaerobacter, and
Thermotoga [
172,
176]. These microbes are supported by a nutrient-rich formulation, including phosphates, sugars, yeast extract, biomass and corn steep liquor [
174,
176].
An innovative aspect of the system is electrochemical stimulation, where an electrical potential between subsurface electrodes enhances microbial H
2 production [
174,
175]. Power may be derived from renewable sources like solar and wind [
172]. The patented infrastructure also details a modular processing chain, including: I) A three-phase separator to divide oil, gas, and water [
174]; II) A water treatment system to remove inhibitory compounds [
175]; III) A mobile fermentation unit for on-site microbe cultivation [
174]; IV) A hydrogen separation unit (e.g., membrane-based) for gas purification [
173]; and V) Storage tanks for secure H
2 collection [
172].
The goal of the platform is to improve H
2 productivity by at least 20% over baseline methods through in-line monitoring and dynamic process control [
172,
175]. The system also proposes to recycle the co-produced CO
2 into other bioprocesses like algal biomass production, contributing to a circular carbon economy [
175].
CEMVITA has announced additional strategies for cost-effective oil field repurposing that integrate H
2-generating microbes with on-site CO
2 capture systems, estimating H
2 production costs under
$1/kg—dramatically below the
$16.8/kg typical of green H
2 via electrolysis [
3].
Yet technical barriers remain. Microbial H
2 production competes with methanogenic and sulfidogenic pathways, necessitating biocontrol strategies to suppress unwanted microbial populations [
177,
178]. Until transparent, peer-reviewed performance data are released, CEMVITA’s H
2Au technology, while scientifically compelling and supported by extensive intellectual property, remains conceptually promising but not yet empirically validated or scalable at commercial levels.
8.2. Recôncavo Baiano Studies: Application Potential of Cemvita-Inspired H2Au Production
The Recôncavo Baiano basin in Northeast Brazil, historically a cornerstone of national petroleum production, contains a high density of mature and abandoned oil wells, particularly in fields such as Buracica. These reservoirs retain significant volumes of RH and present a unique opportunity for biotechnological valorization aligned with the principles of the circular bioeconomy. Inspired by Cemvita’s H
2Au approach, which harnesses engineered microbes to recover H
2 from depleted oil fields [
163], the Recôncavo Baiano
’s favorable geological and microbiological conditions make it a compelling candidate for similar in situ H
2 initiatives.
Cemvita’s technology utilizes a proprietary subsurface bioprocess involving the injection of water, microbes and inhibitory agents into oil reservoirs, where engineered microbial consortia convert RH into H
2 and CO
2 [
162]. The co-produced CO
2 is sequestered underground, ensuring carbon neutrality. Applying this model in the Recôncavo Baiano would allow the transformation of legacy fossil resource infrastructure into renewable energy generation, leveraging the basin’s extensive existing assets, such as well-preserved infrastructure, long-standing geological data and close integration with Bahia’s 400 km natural gas pipeline network [
179], to facilitate H
2 distribution and reduce implementation costs.
At the regional level, the Laboratory of Biotechnology and Ecology of Microorganisms (LABEM) at the Federal University of Bahia (UFBA) has led pioneering efforts to characterize the indigenous microbiota of these reservoirs. Metagenomic analyses of formation waters and crude oil samples from Buracica and other fields revealed the presence of fermentative bacteria and hydrocarbon degraders with hydrogenogenic potential [
180,
181,
182]. This native microbial diversity can be harnessed or augmented through synthetic biology, following Cemvita
’s strategy of optimizing microbial performance via genome editing and nutrient modulation [
165].
Reservoir-specific physicochemical parameters, temperatures around 60 °C, salinity levels between 3% and 6%, and pH near 6.7, are conducive to thermophilic anaerobic consortia suitable for DF, the microbial pathway central to H
2 production. Functional gene targets such as assA, bssA, hydA (hydrogenase synthase), and fdh (putative formate dehydrogenase synthase) [
71,
76,
183,
184] can be employed to assess microbial metabolic capacity and guide bioaugmentation strategies. Combining hydrocarbon-degraders (
Thauera aromatica,
Desulfatibacillum aliphaticivorans) with thermophilic H
2 producers like
Clostridium thermocellum and
Thermotoga maritima may reproduce or even surpass the performance gains reported by Cemvita, which cited microbial productivity improvements of up to 6.5 times under engineered conditions [
164].
As in in Cemvita’s system, productivity in Recôncavo Baiano will depend on establishing syntrophic interactions: hydrocarbon degraders produce VFAs, which hydrogenogenic bacteria then ferment into H
2. Site-specific nutrient blends enriched with yeast extract, glycerol and trace metals (Fe, Ni, Mo) can stimulate microbial activity, while inhibitors like 2-bromoethanesulfonate and heat-shock treatments suppress methanogens and homoacetogens that consume H
2 [
21,
58].
The “push-pull” operational design, similar to Cemvita’s in-field protocol, involves injecting inoculated nutrient fluids (“push”), allowing incubation to stimulate hydrogenogenic activity, followed by gas extraction and microbiome monitoring [
72]. H
2 collection may be enhanced via gas-lift systems or degassing units, and the gas could be fed directly into Bahia’s natural gas network, supporting industrial and transportation applications.
However, the Recôncavo Baiano strategy must address the same challenges faced by Cemvita’s pilot projects, including limited H
2 yields and microbial competition. H
2 generation is often restricted to aqueous zones near the oil-water interface, and extraction efficiency is influenced by formation heterogeneity and microbial kinetics [
167]. Productivity metrics, such as the reported estimate by Cemvita of 3 kg H
2 per barrel of residual oil [
169], remain speculative without peer-reviewed validation. Thus, Recôncavo-based initiatives should prioritize transparent, measurable benchmarks such as Nm
3/h or kg/day of H
2, CO
2 retention capacity, and real-time microbiological shifts.
Moreover, the LABEM strategy must integrate continuous monitoring of gas composition (H
2, CH
4, CO
2), VFAs, and microbial profiles via real-time metagenomics and advanced reservoir simulation tools. These models should incorporate microbial metabolism, nutrient dispersion and redox gradients to guide process optimization and scale-up, while also mitigating operational risks such as microbiologically influenced corrosion [
66,
67,
68].
In the context of Brazil’s National H
2 Program (PNH
2) [
185] and evolving regulatory shifts by the National Agency of Petroleum, Natural Gas and Biofuels (ANP), which now restrict traditional fossil fuel activities, the repurposing of Recôncavo Baiano reservoirs offers a path to regional economic revitalization. It combines renewable H
2 production with job creation, technology development and environmental stewardship. By applying Cemvita’s model to Brazilian geological and microbiological realities, Recôncavo Baiano can emerge as a strategic node in the global green H
2 economy, contributing decisively to national decarbonization and circular bioeconomy frameworks.
Adopting Cemvita’s microbial H2 recovery concept in Recôncavo Baiano, adapted through Brazilian expertise in microbial ecology, reservoir engineering and biotechnology, could transform declining oil assets into productive bioreactors. The synergy between field-ready infrastructure, indigenous microbial diversity, and advanced monitoring systems offers a viable blueprint for clean energy transition in fossil-rich regions.
9. Relevance of H2 from DORs and Its Broader Impacts
The production of H2 in DORs represents a promising frontier for scientific advancement, technological innovation, and sustainable energy development. The research on H2 from depleted reservoirs addresses the urgent need to decarbonize the energy sector while offering a viable strategy for repurposing unproductive oil and gas fields. These fields, which often possess decades of geological, geochemical, and operational data, are ideal candidates for conversion into H2 production sites. This approach minimizes the environmental footprint compared to surface-based H2 reactors and allows for scale-up within existing infrastructure, reducing CAPEX and OPEX associated with new facility construction.
Advancing the understanding of H2 from DOR will significantly contribute to microbial reservoir ecology, particularly regarding the behavior and optimization of H2 producing anaerobes such as Clostridium, Desulfovibrio and hydrogenotrophic archaea under reservoir conditions (e.g., high pressure, temperature, salinity). Investigating the metabolic conversion of hydrocarbons to H2 under controlled lab-scale and simulated reservoir conditions (core flooding, high-pressure bioreactors) will yield crucial insights into reaction kinetics, electron donor/acceptor dynamics and H2 yield optimization.
Moreover, this research topic aligns with global efforts to mitigate climate change through circular carbon utilization. In situ microbial H2 production can be integrated with carbon management strategies, such as CO2 injection for enhanced H2 recovery and geologic carbon sequestration, further increasing the value and sustainability of this approach and the development of other required technologies.
H2 from DOR represents an opportunity to transform environmentally and economically marginal oil reservoirs into renewable energy assets. By coupling microbial biotechnology with geoscience and petroleum engineering, this strategy could pave the way for decentralized, low-emission H2 generation directly at the source of fossil energy legacies, unlocking a new paradigm for sustainable energy transitions.
10. Concluding Remarks
The urgent need to transition from fossil fuels to cleaner energy alternatives has placed H2 at the forefront of sustainable solutions. Among emerging strategies, H2Au production through biological processes in abandoned oil fields offers a promising, low-cost alternative that leverages existing infrastructure and RH. Notably, mature fields like those in Brazil’s Recôncavo Baiano region present a strategic opportunity for revitalizing local economies while promoting clean energy innovation. However, for this innovative paradigm to move beyond its conceptual and early pilot stages and achieve widespread commercial viability, several critical research and development priorities must be rigorously addressed.
First, fundamental advances in microbial engineering and complex ecosystem management are paramount to significantly enhance H2 yields and ensure process stability in subsurface environments. Current limitations of DF include inherently lower H2 yields (typically 1.5–3.5 mol H2/mol hexose) and slower microbial metabolism compared to conventional methods. A key challenge is that common H2 producing microbes, such as Clostridium spp., lack the enzymatic machinery (like assA and bssA) for direct hydrocarbon activation, necessitating complex syntrophic systems or the injection of engineered inocula. Furthermore, the process is thermodynamically inhibited by high H2 partial pressure, and competition from hydrogenotrophic organisms like methanogens and homoacetogens consumes produced H2, necessitating their suppression. Future research and development must focus on optimizing microbial consortia or engineering strains for more efficient, direct hydrocarbon conversion, alongside developing innovative methods for continuous H2 removal from the fermentation environment to alleviate product inhibition and sustain high yields. This requires sophisticated, real-time subsurface monitoring capabilities for microbial activity, gas composition, and nutrient dispersion to manage the complex and heterogeneous reservoir micro-environments.
Second, ensuring the long-term integrity of repurposed reservoir infrastructure is non-negotiable for safe and sustainable operations. DORs are extreme environments characterized by high temperatures, salinity, pressure and toxicity, posing significant challenges to wellbore and reservoir integrity. The intrinsic properties of H2, low density, high diffusivity and microbial reactivity, can lead to displacement of reservoir fluids, elevated formation pressure and the generation of corrosive byproducts such as H2S, which may compromise rock integrity and storage capacity. Critical R&D efforts should focus on developing advanced, corrosion-resistant materials for well casings and acid-tolerant cement formulations that can withstand the chemical alteration and degradation induced by CO2 rich brines and biogenic H2S. Moreover, dynamic modeling that integrates biological, geochemical and geomechanical processes is essential to accurately predict and control biogenic pressurization within heterogeneous reservoirs, thereby preventing caprock fracturing and unintended gas migration. Active management strategies for mitigating reservoir souring, a severe health, environmental and operational hazard resulting from H2S production, also require ongoing development and validation.
Finally, transparent, peer-reviewed techno-economic validation at commercial scale is indispensable to bridge the gap between aspirational cost targets and real-world profitability. While leveraging existing oilfield infrastructure offers significant CAPEX reductions, current cost estimates for H2 production from depleted reservoirs remain high, exceeding USD 25/kg H2, primarily due to low conversion efficiencies and extended fermentation cycles. Cemvita’s widely publicized target of USD 1/kg H2 is understood to be contingent upon achieving high microbial productivity, short fermentation cycles and economies of scale, conditions that have not yet been fully demonstrated or validated across diverse, heterogeneous field settings. Future research must prioritize rigorous pilot-scale demonstrations across varied geological conditions, accompanied by the public disclosure of standardized, quantitative performance metrics such as sustained H2 flow rates (e.g., Nm3/h or kg/day) and comprehensive lifecycle cost analyses. This will be crucial for attracting necessary investment and fostering broader adoption of this emerging technology.
Successfully advancing H2 production from DORs will require a truly multidisciplinary approach, integrating cutting-edge research in microbial ecology, synthetic biology, reservoir engineering and advanced real-time monitoring technologies. By focusing on these explicit, unsolved R&D challenges, the full potential of biologically produced H2 from fossil energy legacies can be unlocked, contributing decisively to global decarbonization efforts and the circular bioeconomy.
Author Contributions
Conceptualization, P.F.A. and I.C.F.S.; writing—original draft preparation, I.C.F.S. and I.V.L.M.; writing—review and editing, P.F.A., I.C.F.S., I.V.L.M., J.B.T.L.M. and C.M.J.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author Paulo F Almeida thanks the National Council for Scientific and Technological Development, CNPq for the financial support and technological development grant (CNPq process no. 302753/2020-6).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANP |
Brazilian National Agency of Petroleum, Natural Gas and Biofuels |
| CO2
|
Carbon dioxide |
| H2
|
Molecular H2
|
| CH4
|
Methane |
| VFAs |
Volatile fatty acids |
| MECs |
Microbial electrolysis cells |
| DORs |
Depleted oil reservoirs |
| CCUS |
Carbon capture, utilization, and storage |
| DF |
Dark fermentation |
| assA |
alkyl succinate synthase |
| bssA |
benzyl succinate synthase |
| assR |
alkyl succinate regulator |
| bssR |
benzyl succinate regulator |
| hydA |
hydrogenase synthase |
| fdh |
putative formate dehydrogenase synthase |
| TRL |
Technology readiness level |
| H2S |
Hydrogen sulfide |
| SRB |
Sulfate reducing bacteria |
| CAPEX |
Capital expenditures |
| OPEX |
Operational expenditures |
| PNH2
|
Brazil’s National H2 Program |
References
- S. Meherkotay, D. Das, Biohydrogen as a renewable energy resource—Prospects and potentials, International Journal of Hydrogen Energy 33 (2008) 258–263. [CrossRef]
- M. Yue, H. Lambert, E. Pahon, R. Roche, S. Jemei, D. Hissel, Hydrogen energy systems: A critical review of technologies, applications, trends and challenges, Renewable and Sustainable Energy Reviews 146 (2021) 111180. [CrossRef]
- G. Piggot, C. Verkuijl, H. Van Asselt, M. Lazarus, Curbing fossil fuel supply to achieve climate goals, Climate Policy 20 (2020) 881–887. [CrossRef]
- Q. Hassan, A.Z. Sameen, H.M. Salman, M. Jaszczur, Large-scale green hydrogen production using alkaline water electrolysis based on seasonal solar radiation, Energy Harvesting and Systems 11 (2024) 20230011. [CrossRef]
- S. Gouws, J. Mackay, Production of green hydrogen through PEM water electrolysis, Pure and Applied Chemistry 96 (2024) 1383–1401. [CrossRef]
- J.M. Filgueira, A.O. Pereira Júnior, R.S. Barbosa De Araújo, N.F.D. Silva, Economic and Social Impacts of the Oil Industry on the Brazilian Onshore, Energies 13 (2020) 1922. [CrossRef]
- R. Jain, N.L. Panwar, S.K. Jain, T. Gupta, C. Agarwal, S.S. Meena, Bio-hydrogen production through dark fermentation: an overview, Biomass Conv. Bioref. (2022). [CrossRef]
- Y. Cao, H. Liu, W. Liu, J. Guo, M. Xian, Debottlenecking the biological hydrogen production pathway of dark fermentation: insight into the impact of strain improvement, Microb Cell Fact 21 (2022) 166. [CrossRef]
- M.M. Albuquerque, W.J. Martinez-Burgos, G. De Bona Sartor, A.B.P. Medeiros, J.C. De Carvalho, C.R. Soccol, Microbial Electrolysis Cells in Biohydrogen Production, in: C.R. Soccol, S.K. Brar, K. Permaul, K. Pakshirajan, J.C. De Carvalho (Eds.), Biohydrogen - Advances and Processes, Springer Nature Switzerland, Cham, 2024: pp. 429–453. [CrossRef]
- B. Logan, Pennsylvania StateUniversity, Novel Microbial Electrolysis Cell Design for Efficient Hydrogen Generation from Wastewaters, (n.d.).
- D. Vieira Barboza, M. Jasmim Meiriño, S.R. Da Silveira Barros, R.L. Fernandes Bella, Towards the Sustainable Decommissioning of Fixed Platforms by Aligning Ecosystem Services and Wind Generation: A Brazilian Case, IJEEP 13 (2023) 235–242. [CrossRef]
- B.M. Storey, R.H. Worden, D.D. McNamara, J. Wheeler, J. Parker, A. Kristen, Reactivation of Abandoned Oilfields for Cleaner Energy Generation: Three-Dimensional Modelling of Reservoir Heterogeneity and Geometry, Processes 12 (2024) 2883. [CrossRef]
- A. Meenakshisundaram, O.S. Tomomewo, L. Aimen, S.O. Bade, A comprehensive analysis of repurposing abandoned oil wells for different energy uses: Exploration, applications, and repurposing challenges, Cleaner Engineering and Technology 22 (2024) 100797. [CrossRef]
- D.S. Oliver, K. Fossum, T. Bhakta, I. Sandø, G. Nævdal, R.J. Lorentzen, 4D seismic history matching, Journal of Petroleum Science and Engineering 207 (2021) 109119. [CrossRef]
- W.-L. Kang, B.-B. Zhou, M. Issakhov, M. Gabdullin, Advances in enhanced oil recovery technologies for low permeability reservoirs, Petroleum Science 19 (2022) 1622–1640. [CrossRef]
- J. Magill, Oil, Gas Companies Deploy AI In The Fight To Reduce Carbon Emissions, Forbes (n.d.). https://www.forbes.com/sites/jimmagill/2021/03/28/oil-gas-companies-deploy-ai-in-the-fight-to-reduce-carbon-emissions/ (accessed May 30, 2023).
- Gold Hydrogen | The Gold Standard for the Energy Transition, (n.d.). https://goldhydrogen.com/ (accessed June 28, 2025).
- T. Jacobs, Firm Says Microbes Strike Hydrogen Gold in California Oil Field, Journal of Petroleum Technology (2025). https://jpt.spe.org/firm-says-microbes-strike-hydrogen-gold-in-california-oil-field (accessed July 13, 2025).
- H. Wu, A. Li, H. Zhang, S. Li, C. Yang, H. Lv, Y. Yao, Microbial mechanisms for higher hydrogen production in anaerobic digestion at constant temperature versus gradient heating, Microbiome 12 (2024) 170. [CrossRef]
- K. Liang, Metabolic Pathways and Genetic Engineering of Anaerobic Bacteria for Biohydrogen Production, Be (2024). [CrossRef]
- M. Boll, ed., Anaerobic Utilization of Hydrocarbons, Oils, and Lipids, Springer International Publishing, Cham, 2020. [CrossRef]
- K. Laczi, Á. Erdeiné Kis, Á. Szilágyi, N. Bounedjoum, A. Bodor, G.E. Vincze, T. Kovács, G. Rákhely, K. Perei, New Frontiers of Anaerobic Hydrocarbon Biodegradation in the Multi-Omics Era, Front. Microbiol. 11 (2020) 590049. [CrossRef]
- H.R. Beller, A.M. Spormann, Analysis of the Novel Benzylsuccinate Synthase Reaction for Anaerobic Toluene Activation Based on Structural Studies of the Product, J Bacteriol 180 (1998) 5454–5457. [CrossRef]
- V. Grossi, C. Cravo-Laureau, A. Méou, D. Raphel, F. Garzino, A. Hirschler-Réa, Anaerobic 1-Alkene Metabolism by the Alkane- and Alkene-Degrading Sulfate Reducer Desulfatibacillum aliphaticivorans Strain CV2803T, Appl Environ Microbiol 73 (2007) 7882–7890. [CrossRef]
- H. Wilkes, W. Buckel, B.T. Golding, R. Rabus, Metabolism of Hydrocarbons in n-Alkane-Utilizing Anaerobic Bacteria, Microb Physiol 26 (2016) 138–151. [CrossRef]
- L. Cabrol, A. Marone, E. Tapia-Venegas, J.-P. Steyer, G. Ruiz-Filippi, E. Trably, Microbial ecology of fermentative hydrogen producing bioprocesses: useful insights for driving the ecosystem function, FEMS Microbiology Reviews 41 (2017) 158–181. [CrossRef]
- S. Gupta, A. Fernandes, A. Lopes, L. Grasa, J. Salafranca, Microbes and Parameters Influencing Dark Fermentation for Hydrogen Production, Applied Sciences 14 (2024) 10789. [CrossRef]
- F. Von Netzer, G. Pilloni, S. Kleindienst, M. Krüger, K. Knittel, F. Gründger, T. Lueders, Enhanced Gene Detection Assays for Fumarate-Adding Enzymes Allow Uncovering of Anaerobic Hydrocarbon Degraders in Terrestrial and Marine Systems, Appl Environ Microbiol 79 (2013) 543–552. [CrossRef]
- M.P. Díez, E. Villanueva-Galindo, I. Moreno-Andrade, E. Díaz, M.A. De La Rubia, A.F. Mohedano, M. Perez-Rangel, Enhanced hydrogen production from food waste via bioaugmentation with Clostridium and Lactobacillus, Biomass Conv. Bioref. (2025). [CrossRef]
- K.M. Muñoz-Páez, G. Buitrón, M. Vital-Jácome, Predicting metabolic pathways and microbial interactions in dark fermentation systems treating real cheese whey effluents, Bioresource Technology 413 (2024) 131536. [CrossRef]
- Y. Wang, X. Chen, K. Spengler, K. Terberger, M. Boehm, J. Appel, T. Barske, S. Timm, N. Battchikova, M. Hagemann, K. Gutekunst, Pyruvate:ferredoxin oxidoreductase and low abundant ferredoxins support aerobic photomixotrophic growth in cyanobacteria, eLife 11 (2022) e71339. [CrossRef]
- R. Biswas, T. Zheng, D.G. Olson, L.R. Lynd, A.M. Guss, Elimination of hydrogenase active site assembly blocks H2 production and increases ethanol yield in Clostridium thermocellum, Biotechnol Biofuels 8 (2015) 20. [CrossRef]
- L.C. De Souza, C.D. Herring, L.R. Lynd, Genetic investigation of hydrogenases in Thermoanaerobacterium thermosaccharolyticum suggests that redox balance via hydrogen cycling enables high ethanol yield, Appl Environ Microbiol 91 (2025) e01109-24. [CrossRef]
- S. Yadav, R. Haas, E.B. Boydas, M. Roemelt, T. Happe, U.-P. Apfel, S.T. Stripp, Oxygen sensitivity of [FeFe]-hydrogenase: a comparative study of active site mimics inside vs. outside the enzyme, Phys. Chem. Chem. Phys. 26 (2024) 19105–19116. [CrossRef]
- K. Schuchmann, N.P. Chowdhury, V. Müller, Complex Multimeric [FeFe] Hydrogenases: Biochemistry, Physiology and New Opportunities for the Hydrogen Economy, Front. Microbiol. 9 (2018) 2911. [CrossRef]
- H. Ogata, W. Lubitz, Y. Higuchi, Structure and function of [NiFe] hydrogenases, J Biochem 160 (2016) 251–258. [CrossRef]
- A.-R. Fleitas García, J.-S. Guez, C.G. Dussap, P. Fontanille, G. Christophe, Effect of redox potential on biohydrogen production during dark fermentation of food wastes in bioreactor, International Journal of Hydrogen Energy 141 (2025) 1199–1210. [CrossRef]
- X. Li, Y. Liu, X. Li, Z. Qin, Y. Su, S. Freguia, L. Feng, Y. Chen, Phenanthrene regulates metabolic pathways for hydrogen accumulation in sludge alkaline dark fermentation, Bioresource Technology 384 (2023) 129311. [CrossRef]
- X. Li, K. Sui, J. Zhang, X. Liu, Q. Xu, D. Wang, Q. Yang, Revealing the mechanisms of rhamnolipid enhanced hydrogen production from dark fermentation of waste activated sludge, Science of The Total Environment 806 (2022) 150347. [CrossRef]
- B. Zhao, S. Wang, Z. Dong, S. Cao, A. Yuan, H. Sha, N. Chen, Enhancing dark fermentative hydrogen production from wheat straw through synergistic effects of active electric fields and enzymatic hydrolysis pretreatment, Bioresource Technology 406 (2024) 130993. [CrossRef]
- S. Xue, D. Liang, J. Yan, Y. Xu, F. Wang, G. Lv, Geographic scale-based analysis of hydrogen production efficiency and mechanism in dark fermentation utilizing diverse inoculums, International Journal of Hydrogen Energy 89 (2024) 390–400. [CrossRef]
- Y. Fu, Y. Song, H. Chen, H. Chen, Y. Li, Q. Wu, Effects of biological carrier optimization on hydrogen production and biofilm formation in anaerobic circulating fluidized bed reactor via dark fermentation, International Journal of Hydrogen Energy 144 (2025) 85–95. [CrossRef]
- H. Sun, J. Shen, M. Hu, J. Zhang, Z. Cai, L. Zang, F. Zhang, D. Ji, Manganese ferrite nanoparticles enhanced biohydrogen production from mesophilic and thermophilic dark fermentation, Energy Reports 7 (2021) 6234–6245. [CrossRef]
- J.J. Rodríguez-Reyes, O. García-Depraect, S. Cantera, V. Mena-Navarro, E. León-Becerril, Assessment of the recovery of hydrogen production activity in dark fermentation reactors after a long period of shutdown, International Journal of Hydrogen Energy 144 (2025) 1134–1146. [CrossRef]
- G. Mohanakrishna, N.P. Sneha, S.M. Rafi, O. Sarkar, Dark fermentative hydrogen production: Potential of food waste as future energy needs, Science of The Total Environment 888 (2023) 163801. [CrossRef]
- A. Ouafa, D. Kerroum, P. Antonio, R. Lamis, B. Chaima, Z.-Z. Rania, B.-L. Mossaab, Coffee grounds and fruit and vegetable waste co-digestion in dark Fermentation: Evaluation of mixing ratio and hybrid pretreatments impact on bio-hydrogen production, Biomass and Bioenergy 199 (2025) 107896. [CrossRef]
- Y. Wei, Y. Jiao, H. Chen, Polydimethyldiallylammonium chloride inhibits dark fermentative hydrogen production from waste activated sludge, Bioresource Technology 393 (2024) 130003. [CrossRef]
- S. Feng, W. Guo, S. Zhang, G. Luo, H.T. Nguyen, N.C. Nguyen, D. Cheng, Y. Ye, H.H. Ngo, Optimization of hydraulic retention time in continuous orange peel crude enzyme - mediated dark fermentation for sustainable biohydrogen production from synthetic swine wastewater, Journal of Water Process Engineering 73 (2025) 107714. [CrossRef]
- P. Srivastava, E. García-Quismondo, J. Palma, C. González-Fernández, Coupling dark fermentation and microbial electrolysis cells for higher hydrogen yield: Technological competitiveness and challenges, International Journal of Hydrogen Energy 52 (2024) 223–239. [CrossRef]
- M. Hussien, H.O. Mohamed, D.A. Jadhav, A. Bahaa, S.-M. Jo, J.-H. Jang, J.-H. Kim, J.-Y. Kwon, E.T. Sayed, M.A. Abdelkareem, P. Castaño, K.-J. Chae, Synergistic integration of dark fermentation and microbial electrolysis cells for hydrogen production and sustainable swine manure treatment, International Journal of Hydrogen Energy 115 (2025) 299–309. [CrossRef]
- A. Jalil, Z. Yu, Impact of Substrates, Volatile Fatty Acids, and Microbial Communities on Biohydrogen Production: A Systematic Review and Meta-Analysis, Sustainability 16 (2024) 10755. [CrossRef]
- J. Zhang, B. Jiang, H. Zhang, S. Qian, T. Wei, Z. Zhang, L. Song, X. Yang, Fermentative Hydrogen Production from Lignocellulose by Mesophilic Clostridium populeti FZ10 Newly Isolated from Microcrystalline Cellulose-Acclimated Compost, Applied Sciences 12 (2022) 9562. [CrossRef]
- C.-H. Liu, C.-Y. Chang, C.-L. Cheng, D.-J. Lee, J.-S. Chang, Fermentative hydrogen production by Clostridium butyricum CGS5 using carbohydrate-rich microalgal biomass as feedstock, International Journal of Hydrogen Energy 37 (2012) 15458–15464. [CrossRef]
- M.J. Veshareh, M. Poulsen, H.M. Nick, K.L. Feilberg, A.A. Eftekhari, N. Dopffel, The light in the dark: In-situ biorefinement of crude oil to hydrogen using typical oil reservoir Thermotoga strains, International Journal of Hydrogen Energy 47 (2022) 5101–5110. [CrossRef]
- F.T. Jannat, K. Aftab, U. Kalsoom, M.A. Baig, A bibliometric analysis of the role of nanotechnology in dark fermentative biohydrogen production, Environ Sci Pollut Res 31 (2024) 24815–24835. [CrossRef]
- P. Kottenhahn, G. Philipps, S. Jennewein, Hexanol biosynthesis from syngas by Clostridium carboxidivorans P7 – product toxicity, temperature dependence and in situ extraction, Heliyon 7 (2021) e07732. [CrossRef]
- S. Maintinguer, B. Fernandes, I. Duarte, N. Saavedra, M. Adorno, M. Varesche, Fermentative hydrogen production by microbial consortium, International Journal of Hydrogen Energy 33 (2008) 4309–4317. [CrossRef]
- M. Černý, M. Vítězová, T. Vítěz, M. Bartoš, I. Kushkevych, Variation in the Distribution of Hydrogen Producers from the Clostridiales Order in Biogas Reactors Depending on Different Input Substrates, Energies 11 (2018) 3270. [CrossRef]
- H. Han, M. Cui, L. Wei, H. Yang, J. Shen, Enhancement effect of hematite nanoparticles on fermentative hydrogen production, Bioresource Technology 102 (2011) 7903–7909. [CrossRef]
- X.-J. Zheng, L.-F. Wei, Z.-H. Zhang, Q.-J. Jiang, Y.-J. Wei, B. Xie, M.-B. Wei, Research on photocatalytic H2 production from acetic acid solution by Pt/TiO2 nanoparticles under UV irradiation, International Journal of Hydrogen Energy 34 (2009) 9033–9041. [CrossRef]
- V. Beschkov, T. Parvanova-Mancheva, E. Vasileva, Experimental Study of Bio-Hydrogen Production by Clostridium beijerinckii from Different Substrates, Energies 16 (2023) 2747. [CrossRef]
- M. Martins, C. Mourato, I.A.C. Pereira, Desulfovibrio vulgaris Growth Coupled to Formate-Driven H2 Production, Environ. Sci. Technol. 49 (2015) 14655–14662. [CrossRef]
- N. Lackner, A. Hintersonnleitner, A.O. Wagner, P. Illmer, Hydrogenotrophic Methanogenesis and Autotrophic Growth of Methanosarcina thermophila, Archaea 2018 (2018) 1–7. [CrossRef]
- S. Saranya, L. Thamanna, V.P. Sreekutty, S. Dhayanithi, P. Chellapandi, Bioremediation of Oil and Natural Gas Industry Waste Using Methanogens: Current Status and Future Perspective to Biohythane Production, Arab J Sci Eng 50 (2025) 4457–4475. [CrossRef]
- M. Martins, I.A.C. Pereira, M. Pita, A.L. De Lacey, Biological Production of Hydrogen, in: J.J.G. Moura, I. Moura, L.B. Maia (Eds.), Enzymes for Solving Humankind’s Problems, Springer International Publishing, Cham, 2021: pp. 247–273. [CrossRef]
- G.M. Van Der Kraan, J. Bruining, B.P. Lomans, M.C.M. Van Loosdrecht, G. Muyzer, Microbial diversity of an oil-water processing site and its associated oil field: the possible role of microorganisms as information carriers from oil-associated environments, FEMS Microbiology Ecology 71 (2010) 428–443. [CrossRef]
- J. Xuan, L. He, W. Wen, Y. Feng, Hydrogenase and Nitrogenase: Key Catalysts in Biohydrogen Production, Molecules 28 (2023) 1392. [CrossRef]
- F.T. Mbow, A. Akbari, N. Dopffel, K. Schneider, S. Mukherjee, R.U. Meckenstock, Insights into the effects of anthropogenic activities on oil reservoir microbiome and metabolic potential, New Biotechnology 79 (2024) 30–38. [CrossRef]
- G.E. Thomas, J.L. Brant, P. Campo, D.R. Clark, F. Coulon, B.H. Gregson, T.J. McGenity, B.A. McKew, Effects of Dispersants and Biosurfactants on Crude-Oil Biodegradation and Bacterial Community Succession, Microorganisms 9 (2021) 1200. [CrossRef]
- C. Nikolova, T. Gutierrez, Biosurfactants and Their Applications in the Oil and Gas Industry: Current State of Knowledge and Future Perspectives, Front. Bioeng. Biotechnol. 9 (2021) 626639. [CrossRef]
- Y. Tian, S. Xue, Y. Ma, Comparative analysis of bacterial community and functional species in oil reservoirs with different in situ temperatures, Int Microbiol 23 (2020) 557–563. [CrossRef]
- M. Pannekens, L. Kroll, H. Müller, F.T. Mbow, R.U. Meckenstock, Oil reservoirs, an exceptional habitat for microorganisms, New Biotechnology 49 (2019) 1–9. [CrossRef]
- L. An, X. Liu, J. Wang, J. Xu, X. Chen, X. Liu, B. Hu, Y. Nie, X.-L. Wu, Global diversity and ecological functions of viruses inhabiting oil reservoirs, Nat Commun 15 (2024) 6789. [CrossRef]
- A. Sherry, N. Gray, C. Aitken, J. Dolfing, Microbial Oil Degradation Under Methanogenic Conditions, in: K.N. Timmis (Ed.), Handbook of Hydrocarbon and Lipid Microbiology, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010: pp. 3905–3917. [CrossRef]
- J.J. Hamilton, M. Calixto Contreras, J.L. Reed, Thermodynamics and H2 Transfer in a Methanogenic, Syntrophic Community, PLoS Comput Biol 11 (2015) e1004364. [CrossRef]
- V.V. Kadnikov, N.V. Ravin, D.S. Sokolova, E.M. Semenova, S.K. Bidzhieva, A.V. Beletsky, A.P. Ershov, T.L. Babich, M.R. Khisametdinov, A.V. Mardanov, T.N. Nazina, Metagenomic and Culture-Based Analyses of Microbial Communities from Petroleum Reservoirs with High-Salinity Formation Water, and Their Biotechnological Potential, Biology 12 (2023) 1300. [CrossRef]
- I.C.C. Hsia, I.A. Shouki, J. Li, Feasibility of Rejuvenating Depleted Oil Fields with New Energy: Biohydrogen, in: Offshore Technology Conference Asia, OTC, Kuala Lumpur, Malaysia, 2024: p. D021S005R004. [CrossRef]
- H. Alkan, J.F. Bauer, O. Burachok, P. Kowollik, M. Olbricht, M. Amro, Hydrogen from Depleted/Depleting Hydrocarbon Reservoirs: A Reservoir Engineering Perspective, Applied Sciences 14 (2024) 6217. [CrossRef]
- M.M. Madirisha, B.D. Ikotun, Hydrogen storage in depleted geological hydrocarbon reservoirs: Enhancing wellbore integrity and sustainability with geopolymer cement – Review, Journal of Energy Storage 84 (2024) 110834. [CrossRef]
- T. Chaubey, B.S. Grover, P. Tessier, Staged Membrane Process for High Pressure Hydrogen Production, US2014170061A1, 2014.
- D.J. Edlund, W. a Pledger, R.T. Studebaker, Hydrogen purification membranes, components and fuel processing systems containing the same, US6723156B2, 2004.
- D.J. Edlund, K. Hoshi, R.T. Studebaker, R.J. Studebaker, Hydrogen purification devices, US10717040B2, 2020.
- D.J. Edlund, Hydrogen generation assemblies and hydrogen purification devices, US9656215B2, 2017.
- Y. Li, Q. Zhang, Z. Zhang, Y. Jing, H. Zhang, J. Yue, D. Jiang, C. Lu, Y. Zhang, C. Wang, Biological hydrogen-alkane co-production fermentation system and method with negative carbon emission, CN114058479A, 2022.
- S.R. Gillick, M. Babaei, In-Situ Hydrogen Production from Natural Gas Wells with Subsurface Carbon Retention, SPE Journal 29 (2024) 2119–2129. [CrossRef]
- J.J. Abraham, A. Carvero, C. Teodoriu, M. AlMujalhem, M. Amani, Evaluating Potential Near Wellbore Integrity Issues in Cement and Casing Layers During Subsurface Hydrogen Storage and Production, in: GOTECH, SPE, Dubai City, UAE, 2025: p. D031S044R001. [CrossRef]
- » H2FIT Tubes: Paving the Way for Hydrogen Production, Storage, and Transport, (n.d.). https://www.centravis.com/en/news/h2fit-tubes-paving-the-way-for-hydrogen-production-storage-and-transport/ (accessed June 28, 2025).
- A. Naquash, A. Riaz, F. Yehia, Y.D. Chaniago, H. Lim, M. Lee, Hydrogen Purification through a Membrane–Cryogenic Integrated Process: A 3 E’s (Energy, Exergy, and Economic) Assessment, Gases 3 (2023) 92–105. [CrossRef]
- M. Yang, R. Hunger, S. Berrettoni, B. Sprecher, B. Wang, A review of hydrogen storage and transport technologies, Clean Energy 7 (2023) 190–216. [CrossRef]
- O. Massarweh, A.S. Abushaikha, CO2 sequestration in subsurface geological formations: A review of trapping mechanisms and monitoring techniques, Earth-Science Reviews 253 (2024) 104793. [CrossRef]
- H.S. Santos, H. Nguyen, F. Venâncio, D. Ramteke, R. Zevenhoven, P. Kinnunen, Mechanisms of Mg carbonates precipitation and implications for CO2 capture and utilization/storage, Inorg. Chem. Front. 10 (2023) 2507–2546. [CrossRef]
- W.H. Tay, K.K. Lau, A.M. Shariff, High frequency ultrasonic-assisted chemical absorption of CO 2 using monoethanolamine (MEA), Separation and Purification Technology 183 (2017) 136–144. [CrossRef]
- M. Sai Bhargava Reddy, D. Ponnamma, K.K. Sadasivuni, B. Kumar, A.M. Abdullah, Carbon dioxide adsorption based on porous materials, RSC Adv. 11 (2021) 12658–12681. [CrossRef]
- Y. Fan, W. Yu, A. Wu, W. Shu, Y. Zhang, Recent progress on CO2 separation membranes, RSC Adv. 14 (2024) 20714–20734. [CrossRef]
- M.G. De Morais, E.G. De Morais, J.H. Duarte, K.M. Deamici, B.G. Mitchell, J.A.V. Costa, Biological CO2 mitigation by microalgae: technological trends, future prospects and challenges, World J Microbiol Biotechnol 35 (2019) 78. [CrossRef]
- Case Studies of CO2 Storage in Depleted Oil and Gas Fields, IEAGHG (n.d.). https://ieaghg.org/publications/case-studies-of-co2-storage-in-depleted-oil-and-gas-fields/ (accessed June 24, 2025).
- Z.M. Shakor, N. Parsafard, E. Al-Shafei, Adsorption and separation modeling of CO2, hydrogen, and biogas: a mathematical review, J Mater Sci 60 (2025) 6850–6876. [CrossRef]
- A. Kamolov, Z. Turakulov, S. Rejabov, G. Díaz-Sainz, L. Gómez-Coma, A. Norkobilov, M. Fallanza, A. Irabien, Decarbonization of Power and Industrial Sectors: The Role of Membrane Processes, Membranes 13 (2023) 130. [CrossRef]
- M.M. Joachimski, J. Müller, T.M. Gallagher, G. Mathes, D.L. Chu, F. Mouraviev, V. Silantiev, Y.D. Sun, J.N. Tong, Five million years of high atmospheric CO2 in the aftermath of the Permian-Triassic mass extinction, Geology 50 (2022) 650–654. [CrossRef]
- R. Kumar, W.J. Chung, M.A. Khan, M. Son, Y.-K. Park, S.S. Lee, B.-H. Jeon, Breakthrough innovations in carbon dioxide mineralization for a sustainable future, Rev Environ Sci Biotechnol 23 (2024) 739–799. [CrossRef]
- N. Wang, D. Wang, A. Krook-Riekkola, X. Ji, MEA-based CO2 capture: a study focuses on MEA concentrations and process parameters, Front. Energy Res. 11 (2023) 1230743. [CrossRef]
- R. Prasad, S.K. Gupta, N. Shabnam, C.Y.B. Oliveira, A.K. Nema, F.A. Ansari, F. Bux, Role of Microalgae in Global CO2 Sequestration: Physiological Mechanism, Recent Development, Challenges, and Future Prospective, Sustainability 13 (2021) 13061. [CrossRef]
- S.V. Kiseleva, N.I. Chernova, M.S. Vlaskin, A.V. Grigorenko, E.A. Chunzhuk, S.Ya. Malaniy, E.A. Bakumenko, T.V. Rositskaya, Carbon Dioxide Absorption by Microalgae: Analysis of Technologies and Energy Costs, Therm. Eng. 71 (2024) 1038–1048. [CrossRef]
- Costs of CO2 sequestration, Thunder Said Energy (n.d.). https://thundersaidenergy.com/downloads/co2-disposal-in-geologic-formations-the-economics/ (accessed June 24, 2025).
- M. Mazzotti, J.C. Abanades, R. Allam, K.S. Lackner, F. Meunier, E. Rubin, J.C. Sanchez, K. Yogo, R. Zevenhoven, Mineral carbonation and industrial uses of carbon dioxide, in: IPCC Special Report on Carbon Dioxide Capture and Storage, Cambridge University Press, 2005. https://research.aalto.fi/en/publications/mineral-carbonation-and-industrial-uses-of-carbon-dioxide (accessed June 24, 2025).
- A. Raksajati, M.T. Ho, D.E. Wiley, Reducing the Cost of CO2 Capture from Flue Gases Using Aqueous Chemical Absorption, Ind. Eng. Chem. Res. 52 (2013) 16887–16901. [CrossRef]
- Cost of carbon capture by approach or technology, Statista (n.d.). https://www.statista.com/statistics/1304575/global-carbon-capture-cost-by-technology/ (accessed June 24, 2025).
- Is carbon capture too expensive? – Analysis, IEA (2021). https://www.iea.org/commentaries/is-carbon-capture-too-expensive (accessed June 24, 2025).
- J. Meng, W. Liao, G. Zhang, Emerging CO2-Mineralization Technologies for Co-Utilization of Industrial Solid Waste and Carbon Resources in China, Minerals 11 (2021) 274. [CrossRef]
- H. Zeng, X. Qu, D. Xu, Y. Luo, Porous Adsorption Materials for Carbon Dioxide Capture in Industrial Flue Gas, Front. Chem. 10 (2022) 939701. [CrossRef]
- E. Ruales, C. Gómez-Serrano, A. Morillas-España, C. González-López, M. Escolà Casas, V. Matamoros, M. Garfí, I. Ferrer, Resource recovery and contaminants of emerging concern mitigation by microalgae treating wastewater, Journal of Environmental Management 367 (2024) 121950. [CrossRef]
- Y.T. Yuen, P.N. Sharratt, B. Jie, Carbon dioxide mineralization process design and evaluation: concepts, case studies, and considerations, Environ Sci Pollut Res 23 (2016) 22309–22330. [CrossRef]
- M.H.J.-J. François, A. Grimstvedt, H.K. Knuutila, Iron Solubility Measurements in Aqueous MEA for CO2 Capture, Ind. Eng. Chem. Res. 64 (2025) 2318–2328. [CrossRef]
- F. Raganati, F. Miccio, P. Ammendola, Adsorption of Carbon Dioxide for Post-combustion Capture: A Review, Energy Fuels 35 (2021) 12845–12868. [CrossRef]
- C. Castel, R. Bounaceur, E. Favre, Membrane Processes for Direct Carbon Dioxide Capture From Air: Possibilities and Limitations, Front. Chem. Eng. 3 (2021) 668867. [CrossRef]
- IEA Greenhouse Gas R&D Programme, L. Stalker, K. Michael, C. Jenkins, K. Holland, L. Peeters, M. Myers, A. Ross, Reviewing the implications of unlikely but potential CO2 migration to the surface or shallow subsurface, IEAGHG, 2025. [CrossRef]
- M. Karimi, M. Shirzad, J.A.C. Silva, A.E. Rodrigues, Carbon dioxide separation and capture by adsorption: a review, Environ Chem Lett 21 (2023) 2041–2084. [CrossRef]
- S. Ray, C. Kuppam, S. Pandit, P. Kumar, Biogas Upgrading by Hydrogenotrophic Methanogens: An Overview, Waste Biomass Valor 14 (2023) 537–552. [CrossRef]
- S. Barbier, F. Huang, M. Andreani, R. Tao, J. Hao, A. Eleish, A. Prabhu, O. Minhas, K. Fontaine, P. Fox, I. Daniel, A Review of H2, CH4, and Hydrocarbon Formation in Experimental Serpentinization Using Network Analysis, Front. Earth Sci. 8 (2020) 209. [CrossRef]
- R. Kanaujiya, A.K. Metya, N. Choudhary, R. Kumar, T.K. Patra, Molecular dynamics insights into tetrahydrofuran-assisted formation of CH4, CO2, and H2 gas hydrates, Phys. Chem. Chem. Phys. (2025) 10.1039.D5CP01574J. [CrossRef]
- M. S, Carbonate and Sandstone Reservoirs in CO2 Sequestration: Assessing Porosity and Permeability for Enhanced Storage Potential, PPEJ 8 (2024) 1–12. [CrossRef]
- S. Ni, W. Lv, Z. Ji, K. Wang, CO2 Mineralized Sequestration and Assistance by Microorganisms in Reservoirs: Development and Outlook, Energies 16 (2023) 7571. [CrossRef]
- A. Askarova, A. Mukhametdinova, S. Markovic, G. Khayrullina, P. Afanasev, E. Popov, E. Mukhina, An Overview of Geological CO2 Sequestration in Oil and Gas Reservoirs, Energies 16 (2023) 2821. [CrossRef]
- Y. Chen, S. Chen, D. Li, X. Jiang, Density-Driven Convection for CO2 Solubility Trapping in Saline Aquifers: Modeling and Influencing Factors, Geotechnics 3 (2023) 70–103. [CrossRef]
- J.W. Johnson, J.J. Nitao, K.G. Knauss, Reactive transport modelling of CO2 storage in saline aquifers to elucidate fundamental processes, trapping mechanisms and sequestration partitioning, SP 233 (2004) 107–128. [CrossRef]
- Q. Chen, M. Ramdin, T.J.H. Vlugt, Solubilities of CO2, CH4, C2 H6, CO, H2, N2, N2 O, and H2 S in commercial physical solvents from Monte Carlo simulations, Molecular Simulation 49 (2023) 1341–1349. [CrossRef]
- D.V. Bhalani, B. Lim, Hydrogen Separation Membranes: A Material Perspective, Molecules 29 (2024) 4676. [CrossRef]
- J. Vilcáez, E. Chowdhury, Biogenic hydrogen production from oil hydrocarbons at geological carbon storage conditions, Energy Conversion and Management 325 (2025) 119438. [CrossRef]
- G.-C. Yang, L. Zhou, S.M. Mbadinga, J.-F. Liu, S.-Z. Yang, J.-D. Gu, B.-Z. Mu, Formate-Dependent Microbial Conversion of CO2 and the Dominant Pathways of Methanogenesis in Production Water of High-temperature Oil Reservoirs Amended with Bicarbonate, Front. Microbiol. 7 (2016). [CrossRef]
- R.L. Tyne, P.H. Barry, M. Lawson, D.J. Byrne, O. Warr, H. Xie, D.J. Hillegonds, M. Formolo, Z.M. Summers, B. Skinner, J.M. Eiler, C.J. Ballentine, Rapid microbial methanogenesis during CO2 storage in hydrocarbon reservoirs, Nature 600 (2021) 670–674. [CrossRef]
- Y.K. Leong, Y.-J. Huang, J.-S. Chang, Boosting CO2 capture and conversion to biohydrogen through enhanced microalgal biomass yield, International Journal of Hydrogen Energy 139 (2025) 998–1007. [CrossRef]
- M. Ashour, A.T. Mansour, Y.A. Alkhamis, M. Elshobary, Usage of Chlorella and diverse microalgae for CO2 capture - towards a bioenergy revolution, Front. Bioeng. Biotechnol. 12 (2024) 1387519. [CrossRef]
- A. Maghzian, A. Aslani, R. Zahedi, A comprehensive review on effective parameters on microalgae productivity and carbon capture rate, Journal of Environmental Management 355 (2024) 120539. [CrossRef]
- Y. Kikuchi, D. Kanai, K. Sugiyama, K. Fujii, Biogas Upgrading by Wild Alkaliphilic Microalgae and the Application Potential of Their Biomass in the Carbon Capture and Utilization Technology, Fermentation 10 (2024) 134. [CrossRef]
- G.E. King, D.E. King, Environmental Risk Arising From Well-Construction Failure—Differences Between Barrier and Well Failure, and Estimates of Failure Frequency Across Common Well Types, Locations, and Well Age, SPE Production & Operations 28 (2013) 323–344. [CrossRef]
- R.B. Jackson, The integrity of oil and gas wells, Proc. Natl. Acad. Sci. U.S.A. 111 (2014) 10902–10903. [CrossRef]
- J.D. Laumb, K.A. Glazewski, J.A. Hamling, A. Azenkeng, T.L. Watson, Wellbore corrosion and failure assessment for CO2 EOR and storage: Two case studies in the Weyburn field, International Journal of Greenhouse Gas Control 54 (2016) 479–489. [CrossRef]
- Pablo.G. Cirimello, J.L. Otegui, G. Carfi, W. Morris, Failure and integrity analysis of casings used for oil well drilling, Engineering Failure Analysis 75 (2017) 1–14. [CrossRef]
- N. Yousuf, O. Olayiwola, B. Guo, N. Liu, A comprehensive review on the loss of wellbore integrity due to cement failure and available remedial methods, Journal of Petroleum Science and Engineering 207 (2021) 109123. [CrossRef]
- M. Bai, Z. Zhang, X. Fu, A review on well integrity issues for CO 2 geological storage and enhanced gas recovery, Renewable and Sustainable Energy Reviews 59 (2016) 920–926. [CrossRef]
- D.A. Al-Shehri, Oil and Gas Wells: Enhanced Wellbore Casing Integrity Management through Corrosion Rate Prediction Using an Augmented Intelligent Approach, Sustainability 11 (2019) 818. [CrossRef]
- L. Wu, Z.-M. Hou, Z.-F. Luo, Y.-L. Fang, L.-C. Huang, X.-N. Wu, Q.-J. Chen, Q.-C. Wang, Impacts of microbial interactions on underground hydrogen storage in porous media: A comprehensive review of experimental, numerical, and field studies, Petroleum Science 21 (2024) 4067–4099. [CrossRef]
- K. Opoku Duartey, W. Ampomah, H. Rahnema, M. Mehana, Underground Hydrogen Storage: Transforming Subsurface Science into Sustainable Energy Solutions, Energies 18 (2025) 748. [CrossRef]
- A. Zappone, A.P. Rinaldi, M. Grab, Q.C. Wenning, C. Roques, C. Madonna, A.C. Obermann, S.M. Bernasconi, M.S. Brennwald, R. Kipfer, F. Soom, P. Cook, Y. Guglielmi, C. Nussbaum, D. Giardini, M. Mazzotti, S. Wiemer, Fault sealing and caprock integrity for CO2 storage: an in situ injection experiment, Solid Earth 12 (2021) 319–343. [CrossRef]
- IEA Greenhouse Gas R&D Programme, D. Bason, H. Nourollah, S. De Morton, M. Watson, G. Petho, I. Maffeis, M. Rieger, G. O’Brien, Geological Storage of CO2: Seal Integrity Review, IEAGHG, 2024. [CrossRef]
- N. Dopffel, B.A. An-Stepec, J.R. De Rezende, D.Z. Sousa, A. Koerdt, Editorial: Microbiology of underground hydrogen storage, Front. Energy Res. 11 (2023) 1242619. [CrossRef]
- Y. Sugai, Y. Owaki, K. Sasaki, Simulation Study on Reservoir Souring Induced by Injection of Reservoir Brine Containing Sulfate-Reducing Bacteria, Sustainability 12 (2020) 4603. [CrossRef]
- J. Hu, C. Li, Q. Zhang, Q. Guo, S. Zhao, W. Wang, D.-J. Lee, Y. Yang, Using chemical looping gasification with Fe2O3/Al2O3 oxygen carrier to produce syngas (H2+CO) from rice straw, International Journal of Hydrogen Energy 44 (2019) 3382–3386. [CrossRef]
- H.S. Hagar, J. Foroozesh, S. Kumar, D. Zivar, N. Banan, I. Dzulkarnain, Microbial H2S generation in hydrocarbon reservoirs: Analysis of mechanisms and recent remediation technologies, Journal of Natural Gas Science and Engineering 106 (2022) 104729. [CrossRef]
- M.J.L. McMahon, A.J. Daugulis, Gas phase H2S product recovery in a packed bed bioreactor with immobilized sulfate-reducing bacteria, Biotechnol Lett 30 (2008) 467–473. [CrossRef]
- Q. He, F. Kong, R. Sun, R. Li, J. Yang, Q. Min, Experimental study on dynamic response performance of hydrogen sensor in confined space under ceiling, Front. Energy Res. 12 (2024) 1456316. [CrossRef]
- H. Se, J. Jiang, C. Sun, K. Song, Hydrogen Leakage Detection in Confined Spaces with Drift Suppression Based on Subspace Alignment, in: H. Sun, W. Pei, Y. Dong, H. Yu, S. You (Eds.), Proceedings of the 10th Hydrogen Technology Convention, Volume 1, Springer Nature Singapore, Singapore, 2024: pp. 180–186. [CrossRef]
- J.J. Sheng, Techno-Economic Analysis of Hydrogen Generation in Hydrocarbon Reservoirs, SPE Journal 29 (2024) 5752–5760. [CrossRef]
- S. Sundeep, L. Sethuraman, D. Akindipe, L. Fingersh, Z. Wenrick, A. Munoz, Repurposing inactive oil and gas wells for energy storage: maximizing the potential via optimal drivetrain control, IET Conf. Proc. 2023 (2023) 486–493. [CrossRef]
- TresCantos, CAPEX y OPEX en proyectos de hidrógeno, ARIEMA (2023). https://www.ariema.com/capex-opex-proyectos-hidrogeno-ariema (accessed June 28, 2025).
- A. Ganguly, P. Sun, X. Liu, H.E. Delgado, L. Sun, A. Elgowainy, Techno-economic and life cycle analysis of bio-hydrogen production using bio-based waste streams through the integration of dark fermentation and microbial electrolysis, Green Chem. 27 (2025) 6213–6231. [CrossRef]
- G.M. Teke, B. Anye Cho, C.E. Bosman, Z. Mapholi, D. Zhang, R.W.M. Pott, Towards industrial biological hydrogen production: a review, World J Microbiol Biotechnol 40 (2024) 37. [CrossRef]
- N.Z.M. of F.A. and Trade, Japan: Strategic Hydrogen Roadmap - 30 October 2020, New Zealand Ministry of Foreign Affairs and Trade (2020). https://www.mfat.govt.nz/en/trade/mfat-market-reports/japan-strategic-hydrogen-roadmap-30-october-2020 (accessed June 28, 2025).
- Financial Incentives for Hydrogen and Fuel Cell Projects, Energy.Gov (n.d.). https://www.energy.gov/eere/fuelcells/financial-incentives-hydrogen-and-fuel-cell-projects (accessed June 28, 2025).
- R. Fujii-Rajani, S. Patnaik, What will happen to the Inflation Reduction Act under a Republican trifecta?, Brookings (2025). https://www.brookings.edu/articles/what-will-happen-to-the-inflation-reduction-act-under-a-republican-trifecta/ (accessed July 13, 2025).
- Cemvita Launches the Gold Hydrogen Program for Subsurface Biomanufacturing of Hydrogen | Gold Hydrogen, (n.d.). https://goldhydrogen.com/cemvita-launches-the-gold-hydrogen-program-for-subsurface-biomanufacturing-of-hydrogen/ (accessed June 28, 2025).
- V. Adomavicius, G. Simkoniene, Overview of innovations and recommendations for efficient operation of RES-based power plants, in: 2023. [CrossRef]
- K. Adeli, M. Nachtane, A. Faik, D. Saifaoui, A. Boulezhar, How Green Hydrogen and Ammonia Are Revolutionizing the Future of Energy Production: A Comprehensive Review of the Latest Developments and Future Prospects, Applied Sciences 13 (2023) 8711. [CrossRef]
- Cemvita’s Successful Field Test Demonstrates Gold HydrogenTM Production in Situ | Gold Hydrogen, (n.d.). https://goldhydrogen.com/cemvitas-successful-field-test-demonstrates-gold-hydrogen-production-in-situ/ (accessed June 28, 2025).
- J.D. Grinstein, Fighting Climate Change with Synthetic Biology: Cemvita Factory generates cost-effective, low-carbon solutions that net climate-positive results, Genetic Engineering & Biotechnology News 42 (2022) 12–13. [CrossRef]
- S. Ni, W. Lv, Z. Ji, K. Wang, Y. Mei, Y. Li, Progress of Crude Oil Gasification Technology Assisted by Microorganisms in Reservoirs, Microorganisms 12 (2024) 702. [CrossRef]
- I.M. Head, N.D. Gray, S.R. Larter, Life in the slow lane; biogeochemistry of biodegraded petroleum containing reservoirs and implications for energy recovery and carbon management, Front. Microbiol. 5 (2014). [CrossRef]
- S.F. Ahmed, N. Rafa, M. Mofijur, I.A. Badruddin, A. Inayat, M.S. Ali, O. Farrok, T.M. Yunus Khan, Biohydrogen Production From Biomass Sources: Metabolic Pathways and Economic Analysis, Front. Energy Res. 9 (2021) 753878. [CrossRef]
- P.D.S.J. of P. Technology, Biotech Firm To Create Gold Hydrogen by Injecting Microbes Into Depleted Oil Reservoirs, JPT (2022). https://jpt.spe.org/biotech-firm-to-create-gold-hydrogen-by-injecting-microbes-into-depleted-oil-reservoirs (accessed June 28, 2025).
- H2-View News: Start-up plans to generate low-cost hydrogen from old oil wells with microbes | H2 Energy Group, (2024). https://h2eg.com/h2-view-news-start-up-plans-to-generate-low-cost-hydrogen-from-old-oil-wells-with-microbes/ (accessed June 28, 2025).
- Microbes that eat oil and excrete cheap, clean hydrogen | GlobalSpec, (n.d.). https://insights.globalspec.com/article/19402/microbes-that-eat-oil-and-excrete-cheap-clean-hydrogen (accessed June 28, 2025).
- P.K. Kevin, L.B.D.S. Marcio, K. Mojtaba, A.G. Renata, A.H. Roger, C.T. Aaron, R.B. Zachary, K. Tahereh, L.A.L. Luiza, D.F.M. Barbara, W.R. Christian, Method. GB2613039A, 2023.
- Z.R. Broussard, K.P. Kincaid, M. Karimi, T. Karimi, S.M.L.B. Da, R.A. Gonçalv, R.A. Harris, A.C. Trevino, L.L.A. Lahme, B.D.F. Magalhães, C.W. Rimbau, Method for Microbiological Production of Hydrogen, US2024392324A1, 2024.
- R.B. Zachary, T. Tj, S. Michael, J.K. Edward, S.R. Raja, A.G. Renata, C.T. Aaron, W. Addien, D.F.M. Barbara, T. Edris, Process and plant, GB2629486A, 2024.
- Z.R. Broussard, T. Tidwell, M. Samuel, E.J. Koch, R.S. Ramanathan, R.A. Gonçalves, A. Trevino, A. Wray, B.D.F. Magalhães, Process and Plant, WO2024206528A2, 2024.
- T. Karimi, M. Karimi, Methods and Systems for Producing Organic Compounds in a Subterranean Environment, US2022282604A1, 2022.
- L. Shen, Q. Zhao, X. Wu, X. Li, Q. Li, Y. Wang, Interspecies electron transfer in syntrophic methanogenic consortia: From cultures to bioreactors, Renewable and Sustainable Energy Reviews 54 (2016) 1358–1367. [CrossRef]
- B. Hu, S. Chen, Pretreatment of methanogenic granules for immobilized hydrogen fermentation, International Journal of Hydrogen Energy 32 (2007) 3266–3273. [CrossRef]
- J.I. Ghignone, G.D. Andrade, General Geology and Major Oil Fields of Recôncavo Basin, Brazil1, in: Geology of Giant Petroleum Fields, American Association of Petroleum Geologists, 1970. [CrossRef]
- Universidade Federal da Bahia: Isolamento e identificação molecular de bactérias redutoras de sulfato de amostras de água produzida em campo de petróleo, (n.d.). https://repositorio.ufba.br/handle/ri/18451 (accessed March 18, 2025).
- S. Myhr, B.L.P. Lillebø, E. Sunde, J. Beeder, T. Torsvik, Inhibition of microbial H2S production in an oil reservoir model column by nitrate injection., Applied Microbiology and Biotechnology 58 (2002) 400–8. [CrossRef]
- S. Deng, B. Wang, S. Su, S. Sun, Y. She, F. Zhang, Dynamics of Microbial Community and Removal of Hydrogen Sulfide (H2 S) Using a Bio-Inhibitor and Its Application under the Oil Reservoir Condition, Energy Fuels 36 (2022) 14128–14135. [CrossRef]
- S. Sarma, D. Ortega, N.P. Minton, V.K. Dubey, V.S. Moholkar, Homologous overexpression of hydrogenase and glycerol dehydrogenase in Clostridium pasteurianum to enhance hydrogen production from crude glycerol, Bioresource Technology 284 (2019) 168–177. [CrossRef]
- G. Meshulam-Simon, S. Behrens, A.D. Choo, A.M. Spormann, Hydrogen Metabolism in Shewanella oneidensis MR-1, Appl Environ Microbiol 73 (2007) 1153–1165. [CrossRef]
- Programa Nacional de Hidrogênio - PNH2 — Ministério de Minas e Energia, (n.d.). https://www.gov.br/mme/pt-br/programa-nacional-do-hidrogenio-1 (accessed July 1, 2025).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).