1. Introduction
Postharvest losses of tropical fruits represent a major challenge for the agri-food sector due to their high perishability and susceptibility to microbial decay. Bananas (
Musa paradisiaca) are highly vulnerable to postharvest deterioration, experiencing rapid changes in firmness, color, weight, and nutritional quality during storage. These issues are especially critical in tropical producing countries such as Ecuador, where losses can reach up to 30% [
1,
2,
3]. Such losses have considerable economic implications for exporters and small-scale producers alike, limit international trade opportunities, and contribute to global food waste and insecurity [
4,
5].
Ecuador is one of the world’s leading banana exporters, and its
Musa paradisiaca cultivars are internationally recognized for their high quality and nutritional value [
6]. The fruits are harvested at the green-mature stage and packed under controlled conditions to ensure stability during transport and commercialization [
7]. Postharvest practices commonly involve the use of plastic packaging, which can affect ripening behavior and fruit quality [
8,
9]. These packaging systems serve as protective barriers that reduce moisture loss and limit air exposure, thereby delaying senescence and minimizing microbial spoilage during storage and transit [
10]. In recent years, HDPE and LDPE films have gained attention for their ability to prolong shelf life by modulating the internal atmosphere within the packaging [
11,
12]
To mitigate these challenges, the development of low-cost and sustainable preservation technologies is essential [
13]. Edible coatings based on natural biopolymers have emerged as effective strategies to extend shelf life by forming semi-permeable films that regulate moisture loss, gas exchange, and microbial growth [
8,
14]. Notably, combinations of whey, agar, starch, and glycerol have demonstrated promising performance in fruit preservation [
15,
16]. Whey protein contributes antimicrobial properties and film-forming capacity, while agar and starch improve barrier properties and structural stability [
17]. Glycerol, a common plasticizer, enhances film flexibility and reduces brittleness, helping to preserve fruit texture and integrity during handling [
18,
19].
Additionally, incorporating essential oils (EOs) into these coatings further boosts their antifungal activity, targeting pathogens such as
Fusarium spp.,
Colletotrichum musae, and
Rhizopus stolonifer, which are prevalent in banana spoilage [
20,
21]. These coatings have been effective in reducing respiration rates and delaying senescence in fruits such as mangoes and papayas, although their application in bananas under refrigerated conditions is still under development [
19,
22,
23]. However, some authors question the scalability of these treatments due to variability in oil composition and potential sensory impacts [
24,
25,
26].
Recent methodologies employ antifungal edible coatings enriched with essential oils such as cinnamon oil at controlled concentrations (e.g., 400 ppm), combined with standardized packaging materials like 0.1 mm PE films [
27,
28]. These coatings are typically applied via immersion or brushing, followed by a drying step at room temperature to ensure uniform adhesion to the fruit surface [
6,
7,
19]. After treatment, bananas are stored at refrigerated conditions (13 ± 1 °C and 93 ± 2% relative humidity) to evaluate the performance of coating and packaging combinations over time [
4,
29,
30].
Another widely used postharvest strategy is polyethylene (PE) packaging. High-density (HDPE) and low-density polyethylene (LDPE) films act as physical barriers that limit oxygen and moisture exchange, thereby slowing down ripening and microbial proliferation. LDPE, due to its flexibility, is particularly suited for delicate fruits like bananas, while HDPE offers greater mechanical protection [
11,
31,
32,
33]. Despite their benefits, controversies remain regarding the relative effectiveness of each type under different storage conditions, and their interaction with bioactive coatings has not been sufficiently explored [
32,
34,
35].
Moreover, recent discussions have focused on the need for integrative postharvest systems that consider not only physicochemical preservation but also microbiological safety, consumer acceptance, and environmental sustainability [
36]. A growing body of research highlights the role of these natural coatings and biodegradable packaging materials in reducing environmental impact and complying with new international standards for green packaging and reduced carbon footprint [
37]. Studies also emphasize the importance of maintaining organoleptic quality—such as taste, aroma, and texture—which directly influence consumer satisfaction and marketability [
14,
38]. In this context, the use of biopolymers and essential oils aligns with the principles of circular economy and sustainable agriculture [
39].
This study aims to assess the combined effect of an antifungal coating composed of whey, agar, starch, and glycerol, enriched with cinnamon essential oil, together with polyethylene packaging (HDPE and LDPE), on the physicochemical quality and microbial stability of bananas during refrigerated storage [
40]. The evaluated parameters include weight loss, firmness, peel color, pH, titratable acidity, soluble solids content, and respiratory rate— indicators of fruit senescence, metabolic activity, and consumer acceptability. By integrating both treatments, this work explores their synergistic potential to improve postharvest management strategies for banana conservation, especially in tropical supply chains [
36,
41]. The findings contribute to the current discussion on sustainable postharvest technologies and may help reduce food waste while maintaining fruit quality and safety.
2. Materials and Methodes
Bananas (
Musa paradisiaca) at commercial maturity stage 1 (completely green, uniform in size, and free from defects) were manually harvested in Machala, El Oro province, Ecuador. After selection, the fruits were transported under ventilated conditions to the laboratory of the DECAB at Escuela Politécnica Nacional (EPN) [
7]. Once sanitized, fruits were stored at 13 ± 1 °C and 95% relative humidity. A total of eight treatments were established in a completely randomized design
2.1. Coating Design
For the development of the antifungal coating used in this study, food-grade ingredients commonly employed in the formulation of functional films were selected. Whey powder (Agropur INC., Eden Prairie, MN, USA) served as the protein matrix, agar-agar (Sigma-Aldrich, St. Louis, MO, USA) was used as a natural gelling agent, and native cassava starch (Industrias Lojanas de Alimentos, Loja, Ecuador) provided the structural polysaccharide. Glycerol (Merck, Darmstadt, Germany; ≥99.5% purity) was incorporated as a plasticizer to enhance film flexibility [
7].
Cinnamon essential oil (
Cinnamomum verum) was obtained from Green Harmony (Quito, Ecuador) and extracted by steam distillation from dried bark sourced from Sri Lanka. This method is widely recognized for preserving the integrity of bioactive volatile compounds. The oil was incorporated at a final concentration of 600 ppm. Its major components, cinnamaldehyde (60–70%) and eugenol (5–15%), are known for their potent antimicrobial and antioxidant properties [
42].
The coating solution was prepared using a total solid concentration of 30 g/L, composed of 16.7% whey, 16.7% agar, 33.3% cassava starch, and 33.3% glycerol. All components were dissolved in distilled water and heated to 85 °C under constant agitation using a magnetic stirrer (Thermo Fisher Scientific, Massachusetts, USA) for 20 minutes until complete homogenization. Cinnamon essential oil was added after heating, followed by vigorous stirring to ensure uniform dispersion. The solution was then cooled to room temperature (~25 °C) [
7]. Bananas were immersed in the coating solution for 30 seconds, allowed to drain, and air-dried at room temperature for approximately 15 minutes prior to packaging.
Following the preparation of the coating solution, bananas were assigned to eight experimental treatments that combined the application of an antifungal coating and polyethylene packaging of different densities and commercial sources. Treatments T01 to T06 consisted of fruits coated with the antifungal formulation and packed in either low-density polyethylene (LDPE) or high-density polyethylene (HDPE) bags, sourced from three different suppliers (designated as Companies A, B, and C). Treatment T07 included bananas packed in polyethylene bags without antifungal coating, serving as the plastic-only control. Treatment T08 served as the absolute control, involving fruits without any coating or packaging.
All treatments were evaluated on day 0 and after 28 days of storage under controlled environmental conditions (13 ± 1 °C and 95% relative humidity). Temperature and humidity were continuously monitored using a digital thermo-hygrometer (Testo 608-H2; accuracy ±2% RH and ±0.5 °C), ensuring consistency across replicates and allowing for reliable assessment of the coating and packaging effects. A detailed description of each treatment is presented in
Table 1.
During the 28-day storage period, a comprehensive set of physicochemical, microbiological, and quality-related parameters were assessed to evaluate treatment efficacy. The variables measured included: weight loss, fruit firmness, peel color (L*, a*, b*), fruit length, peel thickness, titratable acidity (expressed as % citric acid), pH, and soluble solids content (°Brix). Additionally, respiration rate (expressed as mg CO₂ kg⁻¹ h⁻¹) was measured using gas chromatography. Sensory evaluation was performed on day 28 using a 10-point hedonic scale with 18 semi-trained panelists. Microbiological quality was assessed by quantifying total coliforms, mesophilic aerobic bacteria, and yeasts and molds. Data were statistically analyzed by one-way analysis of variance (ANOVA), and means were compared using Tukey’s HSD post-hoc test at a 95% confidence level (α = 0.05).
2.2. Physical Analysis
Physical measurements were conducted weekly using ten banana samples per treatment, in accordance with standardized analytical protocols.
2.2.1. Weight Variation
Each banana was individually weighed using a BPS 51 Plus precision balance (Boeco, Hamburg, Germany), featuring a 0.01 g resolution and a maximum capacity of 510 g. Initial weights were recorded immediately after the coating application, and subsequent measurements were conducted at regular intervals throughout the storage period. Weight loss was expressed as a percentage of the initial mass [
43].
This parameter evaluate the effectiveness of each treatment in minimizing water loss and controlling dehydration—factors in maintaining postharvest quality and extending shelf life [
44]. The calculation was performed using the following equation [
45]:
where
is the initial weight and
is the weight at time t.
2.2.2. Fruit Dimensions
Banana length and thickness were measured using a digital caliper (Truper, CALDI-6MP, Mexico) with an accuracy of 0.01 mm. The total fruit length was measured from the stem to the distal end, while thickness was recorded at the widest part of the fruit, typically around the central region. These dimensional parameters were monitored over the storage period to assess morphological changes and to determine their association with the different treatments applied [
14].
2.2.3. Firmness
Peel firmness was assessed using a McCormick FT327 penetrometer (Forlì, Italy), fitted with an 8 mm diameter plunger. The device has a measurement capacity of up to 13 kgf and a resolution of 0.1 kgf. This parameter served as an indicator of structural integrity and the peel’s protective role during storage [
46].
A consistent force was applied to the fruit surface, allowing the plunger to penetrate slightly into the peel [
47]. The recorded force reflected the peel's resistance to compression, offering reliable information on fruit firmness and the progression of ripening across treatments and storage time [
48].
2.2.4. Color
Peel color was evaluated using a Minolta CR-400 tristimulus colorimeter (Konica Minolta, Japan), which measures the L* (lightness), a* (red, green), and b* (yellow, blue) color coordinates. Readings were performed in triplicate at three evenly distributed locations on each fruit, carefully avoiding any areas with visible defects or discoloration to ensure consistency [
46,
49].
The meaning of these values was used to characterize surface color, providing an objective indicator of ripening stage, visual appeal, and the effectiveness of the treatments in preserving fruit appearance during storage.
2.3. Chemical Analysis
Performed in triplicate following accepted protocols, to monitor the ripening and assess the impact of each treatment on fruit quality attributes.
2.3.1. pH
pH values were measured using a digital pH meter (Mettler Toledo SG2-FK, Schwerzenbach, Switzerland) with a resolution of ±0.01 pH units. Prior to each measurement, the instrument was calculated with buffer solutions at pH 4.00 and 7.00 to ensure precision.
Juice was extracted from individual fruits using manual or mechanical pressing, and the electrode was directly immersed into the sample for reading. The procedure followed AOAC Official Method 981.12 [
50], which is standardized for pH determination in food matrices. The results were used to evaluate ripening progression and chemical stability of the fruit during storage [
38].
2.3.2. Titratable Acidity
Titratable acidity was determined following AOAC Method 942.15 [
51], using 0.1 N sodium hydroxide (NaOH) and expressing results as the percentage of citric acid. The titration was performed with a Brand Titrette digital burette (±0.01 mL accuracy) to ensure precise volume delivery.
Banana juice samples were titrated with NaOH until reaching the endpoint, indicated by a persistent color change upon the addition of phenolphthalein, signaling complete neutralization [
52]. All measurements were conducted in triplicate, and mean values were used to quantify citric acid concentration, providing insight into the acidity profile and metabolic activity of the fruit during storage.
2.3.3. Soluble Solids
Soluble solids content was determined using a digital refractometer (Atago PAL-1, Japan) with a measurement range of 0.0 to 53.0 °Brix and an accuracy of ±0.2 °Brix, in accordance with AOAC Method 932.12 [
53]. The results were expressed in degrees Brix (°Brix), reflecting the proportion of dissolved solids—primarily sugars—in the banana juice. This parameter served as a reliable indicator of sweetness, ripening progression, and compositional changes during the storage period.
2.4. Respiration Rate
Was measured using a Horiba infrared gas analyzer (Marca Post Harvest Research, model CG-100, Davis), which quantifies carbon dioxide (CO₂) production in a sealed environment. For this purpose, approximately 500 g of bananas were placed in a hermetically sealed chamber to ensure an isolated system, preventing the external air ingress and ensuring that any change in gas concentration was solely attributed to the metabolic activity of the fruit. CO₂ production was expressed in µL CO₂ kg⁻¹ h⁻¹, representing the amount of carbon dioxide released per kilogram of fruit per hour [
54].
The gas analyzer has an accuracy of ±2% full scale (FS), allowing for precise detection of changes in CO₂ concentration. The method followed the recommended procedures for determining respiration rates in fruits and vegetables per triplicate, ensuring reliability and methodological consistency. This parameter is considered a key indicator of metabolic activity and is closely related to fruit freshness, ripening behavior, and overall postharvest quality [
55].
2.5. Microbiological Analysis
Microbiological evaluations were carried out at the end of the storage period, once the bananas had reached full ripening. The purpose of this analysis was to assess the effects of storage conditions and packaging type on the microbial quality of the fruit. Samples were taken from each treatment under aseptic conditions and processed in triplicate to ensure reproducibility and analytical reliability.
The presence of total coliforms was determined using the plate count method on selective agar, following the protocol described by Maturin and Peeler (FDA-BAM) [
56]. This parameter is commonly used as a hygiene indicator and reflects possible contamination during postharvest handling or storage.
The total aerobic mesophilic count was assessed using the standard pour plate technique, allowing for the quantification of viable aerobic microorganisms capable of growing at moderate temperatures. This analysis provides a general overview of the microbial load on the fruit surface and serves as a useful index of product quality and shelf-life stability.
To evaluate the presence of yeasts and molds, selective culture media were used according to the guidelines established by the Aerobic Plate Count (APC) method. This test is essential for monitoring fungal development during storage, as yeasts and molds are often associated with postharvest spoilage and can compromise the sensory and structural integrity of the fruit.
2.6. Sensory Evaluation
Sensory analysis was conducted on day 28 of storage to evaluate the organoleptic quality of bananas subjected to different postharvest treatments. A panel of 18 semi-trained participants was recruited and instructed in the evaluation of specific sensory attributes. The selected treatments for evaluation were T01, T03, T05, T06, T07, and T08. Each panelist assessed four out of the six treatments, following a rotational design to ensure balanced exposure and minimize positional or order bias. For instance, Panelist 1 evaluated samples 1–2–3–4, Panelist 2 evaluated 2–3–4–5, and so on, completing the cycle across all panelists [
57,
58].
The sensory attributes evaluated included color, odor, sweetness, texture, and overall acceptance. A 10-point hedonic scale was used, where 1 indicated "dislike extremely" and 10 indicated "like extremely." Evaluations were performed under controlled conditions to reduce variability and external influences. Each fruit sample was presented coded and randomized to prevent panelist bias. The test followed the guidelines established in ASTM E1871-10 for sensory analysis of food products [
59]. This methodological approach ensured reliable and reproducible data, offering a consistent basis for comparing consumer-perceived quality across the selected treatments in this study.
3. Results
The following section presents the results obtained during the 28-day storage of Musa paradisiaca fruits subjected to postharvest treatments combining antifungal coatings and polyethylene packaging. The study included a comprehensive evaluation of physicochemical properties—such as weight loss, peel thickness and fruit length reduction, firmness, color (L*, a*, b*), pH, titratable acidity, and soluble solids content (°Brix)—to assess the preservation of structural and compositional quality.
Additionally, respiration rate, microbiological load (total coliforms, aerobic mesophilic bacteria, yeasts, and molds), and sensory perception (color, odor, sweetness, texture, and overall acceptance) were analyzed. These combined indicators were used to determine the effectiveness of each treatment in maintaining postharvest quality and consumer acceptability throughout the storage period.
3.1. Physical Analysis
3.1.1. Weight Variation
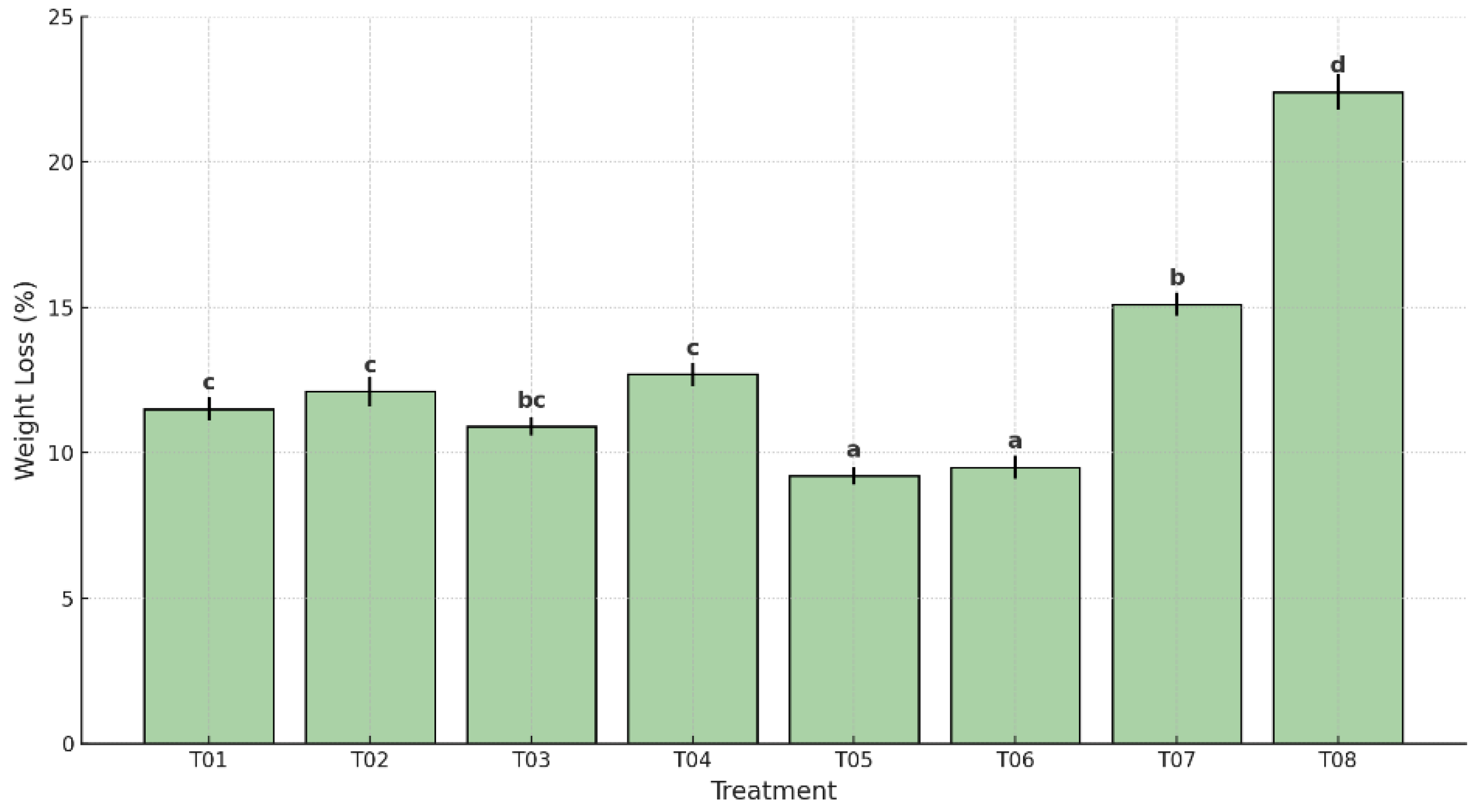
Figure 1, significant differences were observed among treatments (p < 0.05). The highest weight loss occurred in the uncoated and unpackaged control group (T08), with an average of 22.8%, followed by the plastic-only control (T07), which showed 15.1%. In contrast, the lowest values were recorded in samples T05 (9.3%) and T06 (9.5%), both of which combined the antifungal coating with polyethylene packaging—low and high density, respectively. These two treatments were statistically similar and significantly more effective in minimizing moisture loss compared to all other treatments.
Intermediate values were recorded in T01 (11.5%), T02 (12.0%), T03 (10.9%), and T04 (12.8%), showing statistically significant differences from the controls, but less efficacy than T05 and T06. According to Tukey’s HSD test, treatments were grouped into distinct statistical categories, confirming the enhanced performance of coated and packed samples, particularly T05 and T06, in preserving fruit mass during storage.
3.1.2. Fruit Dimensions
Peel thickness is a structural trait that contributes to moisture retention and protects the fruit from mechanical damage and microbial invasion. The treatments showed significant differences in thickness loss (p < 0.05), as shown in
Figure 2.
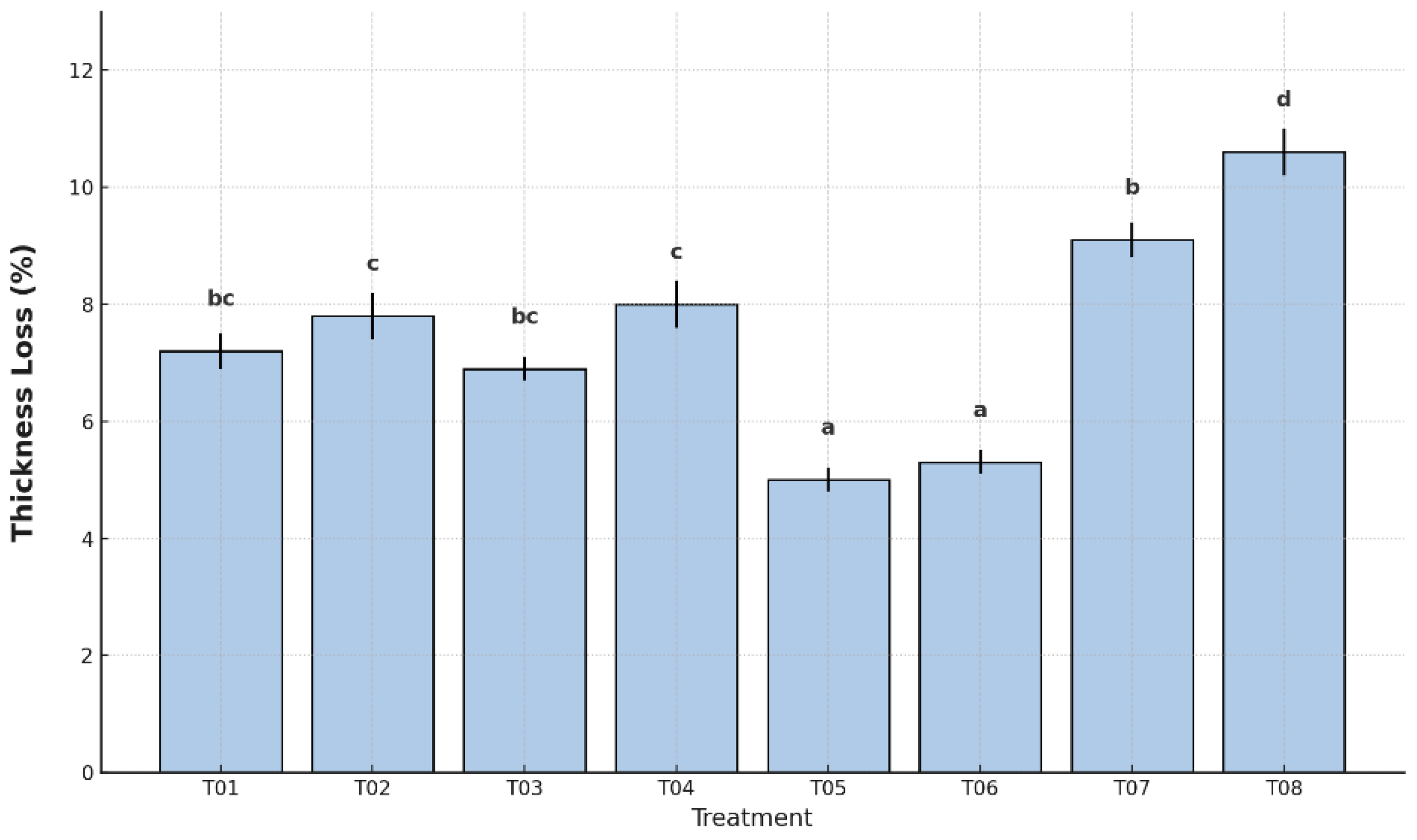
The greatest reduction was observed in the uncoated and unpackaged control (T08), which exceeded 10.5%, followed by the plastic-only control (T07) with 9.1%. In contrast, the lowest peel thickness losses were recorded in T05 (5.0%) and T06 (5.3%), both treatments that combined antifungal coating with polyethylene packaging.
Treatments T01 (7.2%), T02 (7.8%), T03 (6.9%), and T04 (8.0%) showed intermediate values. According to Tukey’s HSD test, treatments were grouped into four distinct statistical categories, with T05 and T06 forming the group with the best performance.
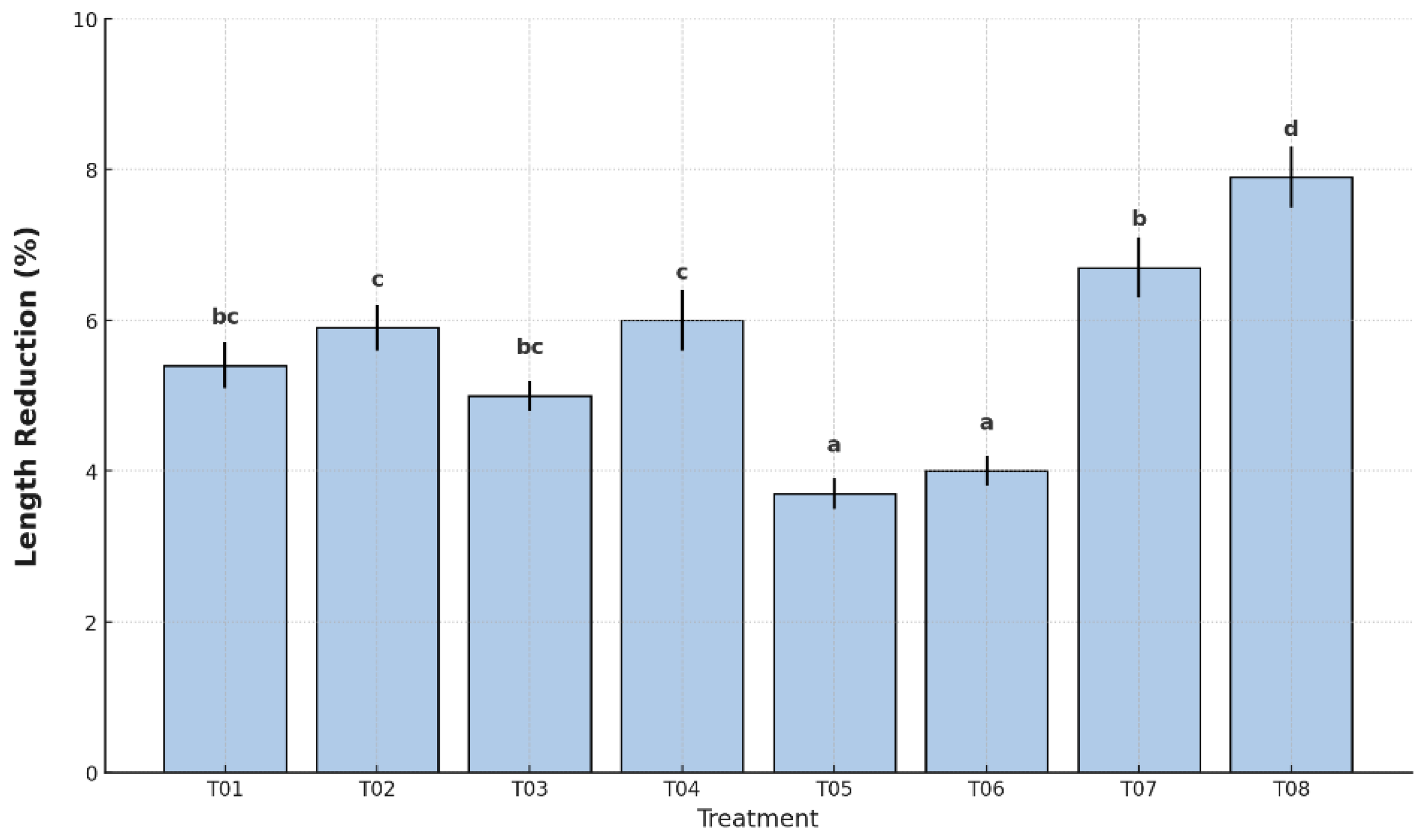
Fruit length is a structural parameter affected by dehydration and tissue softening during storage. Reduction in length reflects changes in turgor pressure and overall integrity of the fruit. As shown in
Figure 3, the treatments resulted in statistically significant differences in length reduction (p < 0.05).
The greatest decrease was observed in the uncoated and unpackaged control (T08), with a mean reduction of 7.9%, followed by the plastic-only control (T07), which recorded 6.8%. In contrast, treatments T05 and T06 showed the smallest reductions, with 3.7% and 4.0%, respectively, and were statistically grouped in the lowest category according to Tukey’s HSD test. Intermediate length losses were recorded in T01 (5.4%), T02 (5.9%), T03 (5.2%), and T04 (6.0%), all of which showed moderate structural preservation.
3.1.3. Firmness
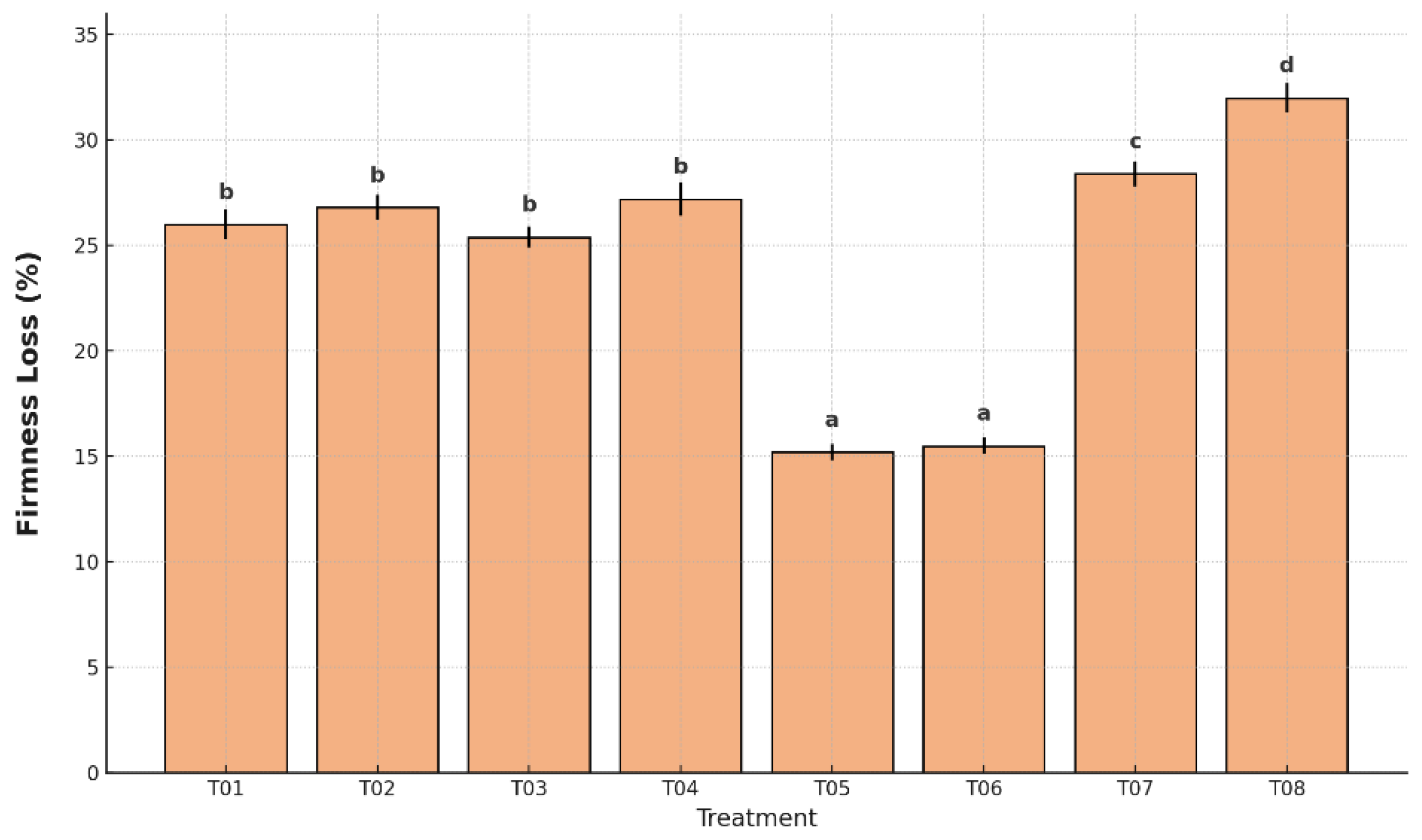
Loss of firmness typically results from enzymatic degradation of cell walls and starch-to-sugar conversion during ripening. As shown in
Figure 4, firmness loss varied significantly among treatments (p < 0.05). The uncoated and unpackaged control (T08) exhibited the greatest firmness reduction (32.2%), followed by the plastic-only control (T07) at 28.5%, both indicating accelerated softening and advanced ripening.
In contrast, the lowest firmness losses were recorded in T05 (15.2%) and T06 (15.5%), which included the antifungal coating and polyethylene packaging. These treatments significantly reduced tissue degradation and were statistically grouped in the lowest category. Intermediate firmness losses were found in T01 (26.0%), T02 (26.8%), T03 (25.5%), and T04 (27.3%), all significantly higher than T05 and T06.
3.1.4. Color Parameters
The L* parameter in the CIELAB color space represents surface lightness, with higher values indicating brighter and fresher appearance. A reduction in L* typically corresponds to peel browning or darkening, often associated with ripening and oxidative changes. As shown in
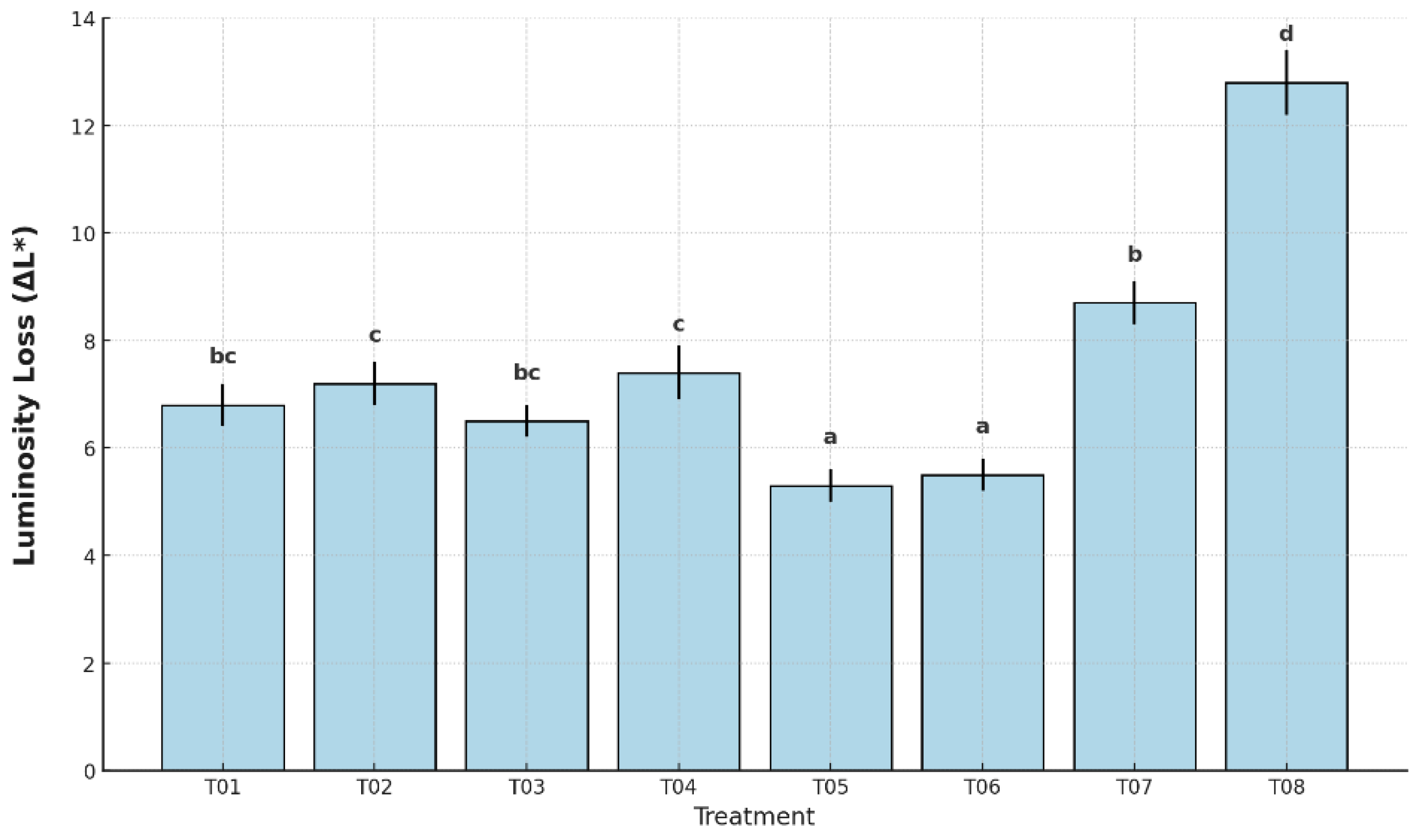
Figure 5, luminosity loss differed significantly among treatments (p < 0.05). The highest ΔL* was observed in the uncoated, unpackaged control (T08), reaching 12.8%, followed by the plastic-only control (T07) with 8.7%.
These values reflect the accelerated darkening of the banana peel. In contrast, the lowest losses occurred in T05 (5.3%) and T06 (5.5%), both of which maintained better surface lightness due to the protective effect of the antifungal coating combined with packaging. Intermediate luminosity losses were found in T01 (6.9%), T02 (7.3%), T03 (6.6%), and T04 (7.4%), with statistically distinguishable groupings based on Tukey’s HSD test.
The a* color parameter represents the red–green chromaticity axis, where an increase in Δa* indicates a shift toward reddish hues, typically associated with advanced ripening and senescence in bananas. Significant differences were observed among treatments (p < 0.05), as shown in
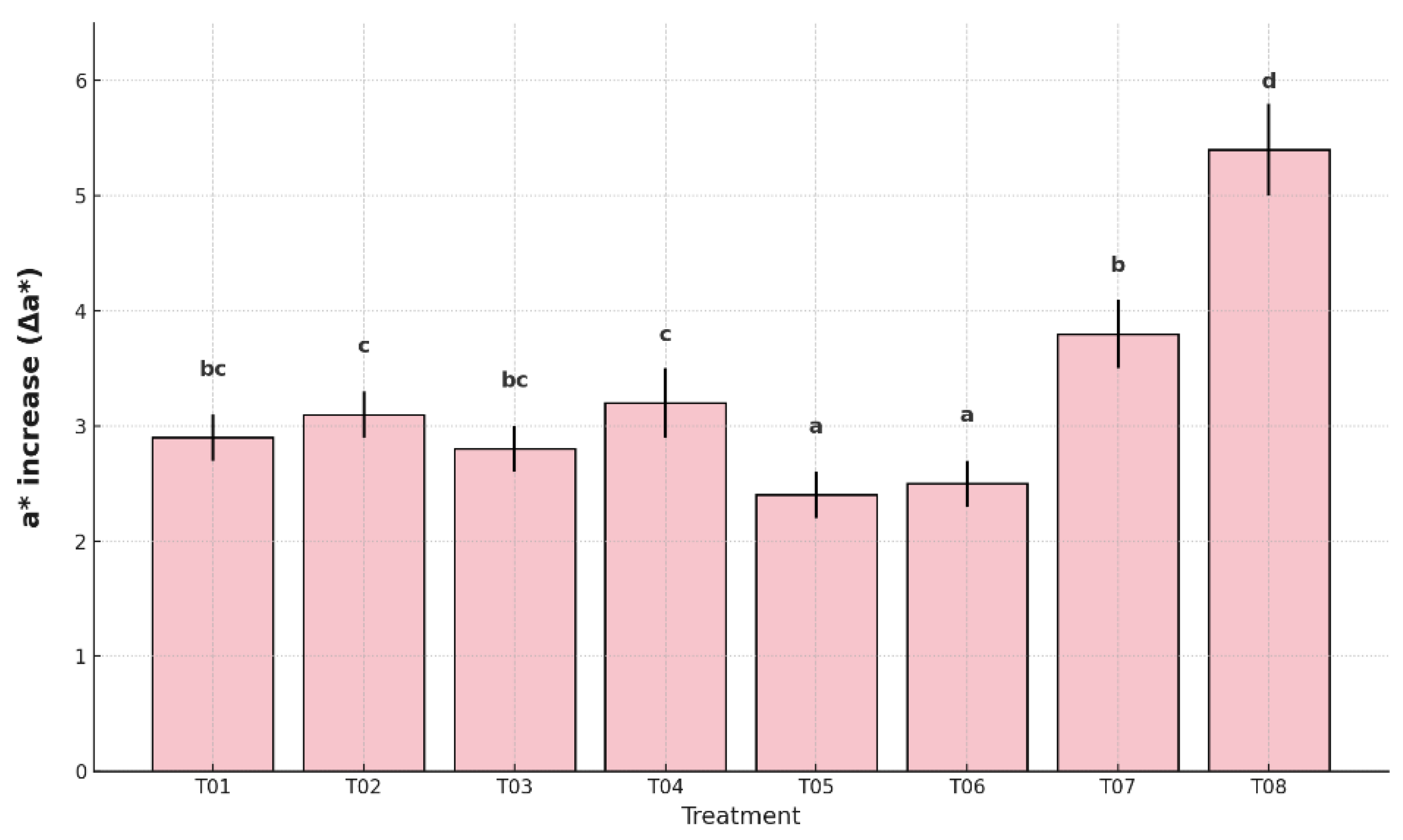
Figure 6. The uncoated and unpackaged control (T08) showed the highest Δa* increase (5.4%), followed by the plastic-only control (T07) with 3.8%, both indicating more advanced ripening stages.
The lowest values were recorded in T05 (2.4%) and T06 (2.5%), suggesting that the combined use of antifungal coating and packaging was effective in delaying pigment changes related to ripening. Intermediate values were observed in T01 (2.9%), T02 (3.1%), T03 (2.8%), and T04 (3.2%), which were statistically different from the controls but less effective than T05 and T06. The grouping analysis confirmed that T05 and T06 preserved peel greenness more efficiently, contributing to visual freshness and delayed senescence.
The b* parameter reflects the yellow–blue chromaticity of banana peel color, where a decrease in Δb* values is associated with loss of yellow tones and browning due to pigment degradation during senescence. As shown in
Figure 7, significant differences were found among treatments (p < 0.05). The uncoated and unpackaged control (T08) showed the highest Δb* decrease (6.2%), followed by the plastic-only control (T07) with 4.7%, indicating accelerated color loss.
On the other hand, T05 (3.1%) and T06 (3.3%) exhibited the lowest b* decreases, suggesting better preservation of the characteristic yellow color in coated and packed fruits. Intermediate values were recorded in T01 (3.7%), T02 (3.9%), T03 (3.5%), and T04 (4.0%). Tukey’s HSD grouping confirmed that T05 and T06 were statistically different from the controls and demonstrated superior color retention.
3.2. Chemical Analysis
3.2.1. pH
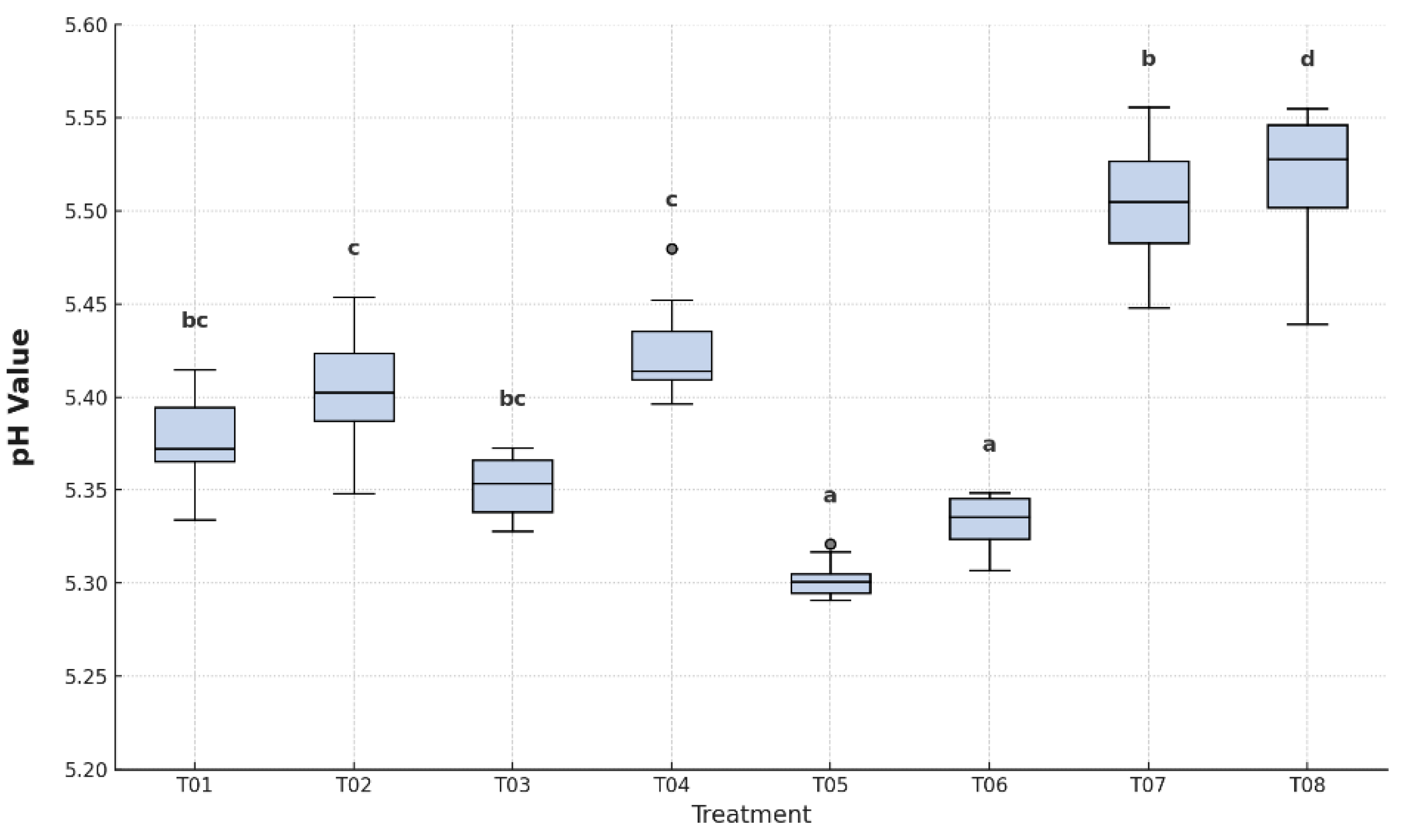
As shown in
Figure 8, statistically significant differences were observed among treatments (p < 0.05). The highest pH values were recorded in T08 (uncoated, unpackaged control) and T07 (plastic-only control), with mean values of 5.55 and 5.52, respectively, indicating more advanced ripening. In contrast, T05 (5.30) and T06 (5.33) showed the lowest pH values, suggesting a delay in ripening and preservation of acid content.
Intermediate pH values were observed in T01 (5.38), T02 (5.41), T03 (5.36), and T04 (5.42), with significant variation among treatments. According to Tukey’s HSD analysis, samples grouped into four distinct categories, confirming the ability of coatings—especially when combined with LDPE or HDPE.
3.2.2. Titratable Acidity
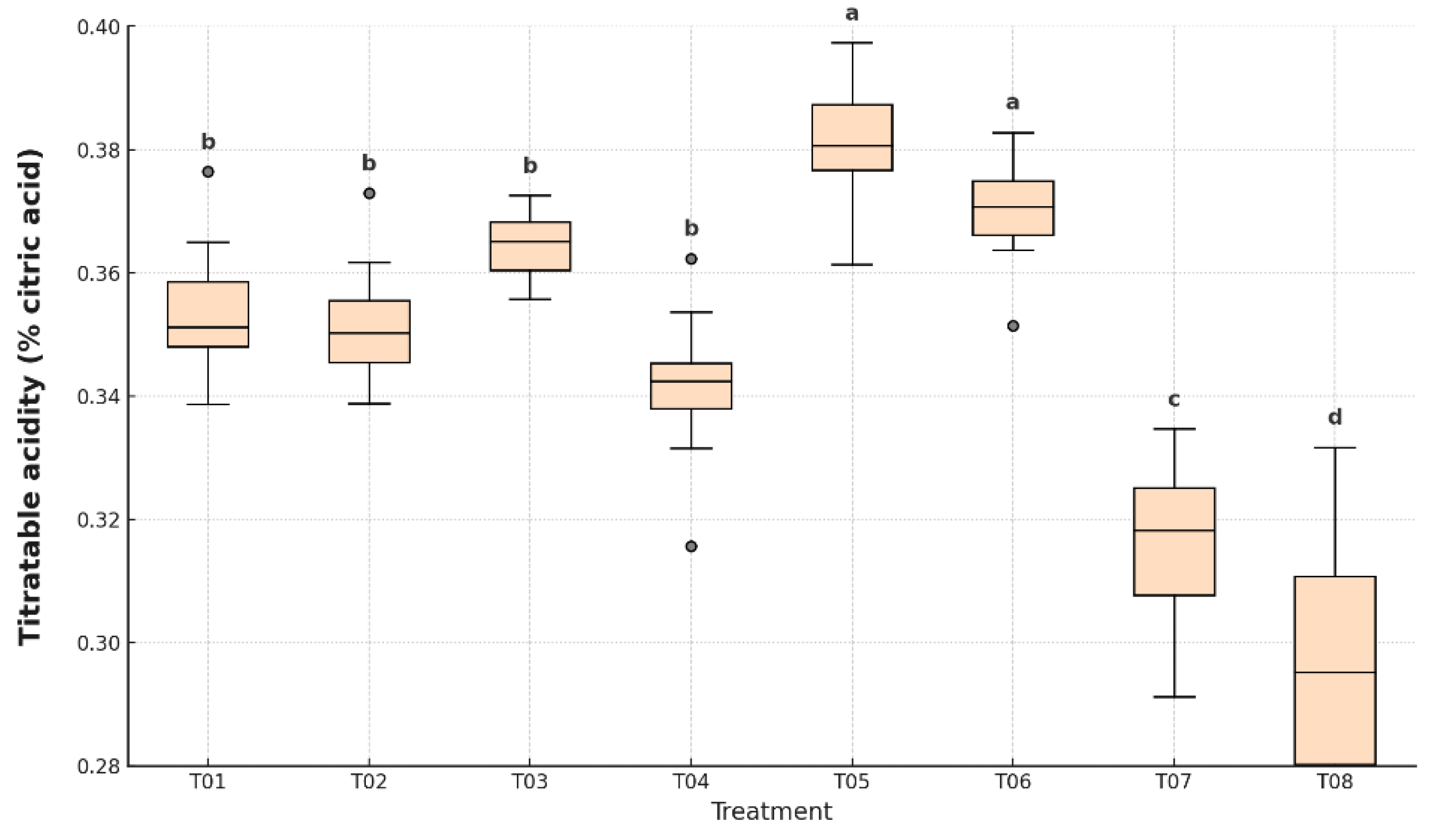
Figure 9, statistically significant differences (p < 0.05) were found among treatments.
The lowest titratable acidity values were observed in T07: 0.32% and T08: 0.30%, indicating a more advanced ripening state. In contrast, the highest values were recorded in T05 (0.39%) and T06 (0.38%), both significantly different from all other treatments. These samples preserved higher acid content, suggesting delayed ripening and enhanced metabolic stability. Intermediate values were detected in T01 (0.35%), T02 (0.35%), T03 (0.36%), and T04 (0.34%), with statistical differences according to Tukey’s HSD test.
3.2.3. Soluble Solids
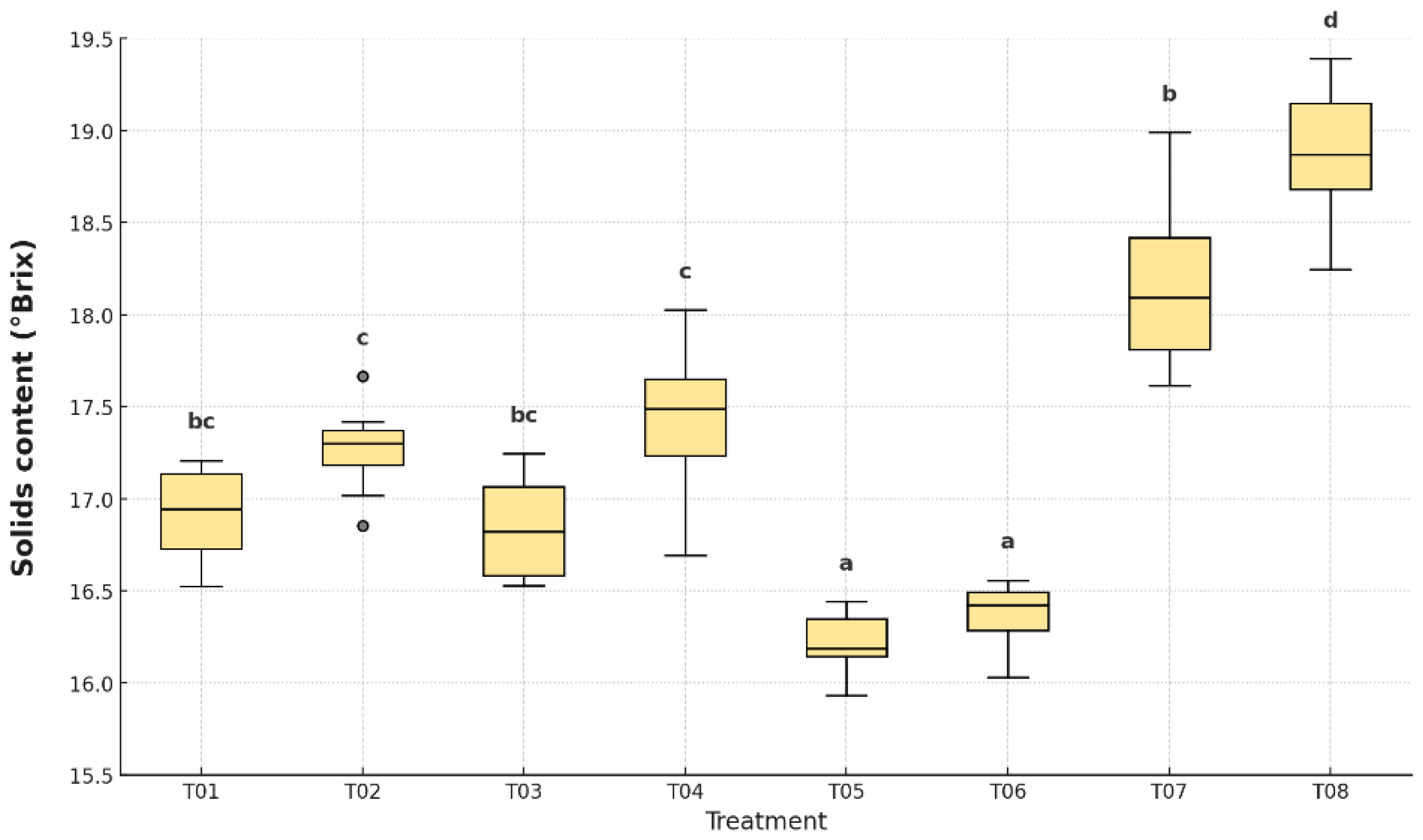
As shown in
Figure 10, there were significant differences in soluble solids content among treatments (p < 0.05). The highest values were observed in the uncoated control (T08), with an average of 19.1 °Brix, followed by the plastic-only control (T07), which reached 18.2 °Brix. These results are consistent with the accelerated ripening associated with the absence of coating. The lowest °Brix values were found in T05 (16.3 °Brix) and T06 (16.4 °Brix), treatments that combined antifungal coating with polyethylene packaging. These samples showed significantly slower sugar accumulation, indicating delayed ripening. Intermediate values were recorded for T01 (17.0 °Brix), T02 (17.3 °Brix), T03 (17.0 °Brix), and T04 (17.6 °Brix), all statistically distinguishable.
3.3. Respiration Rate
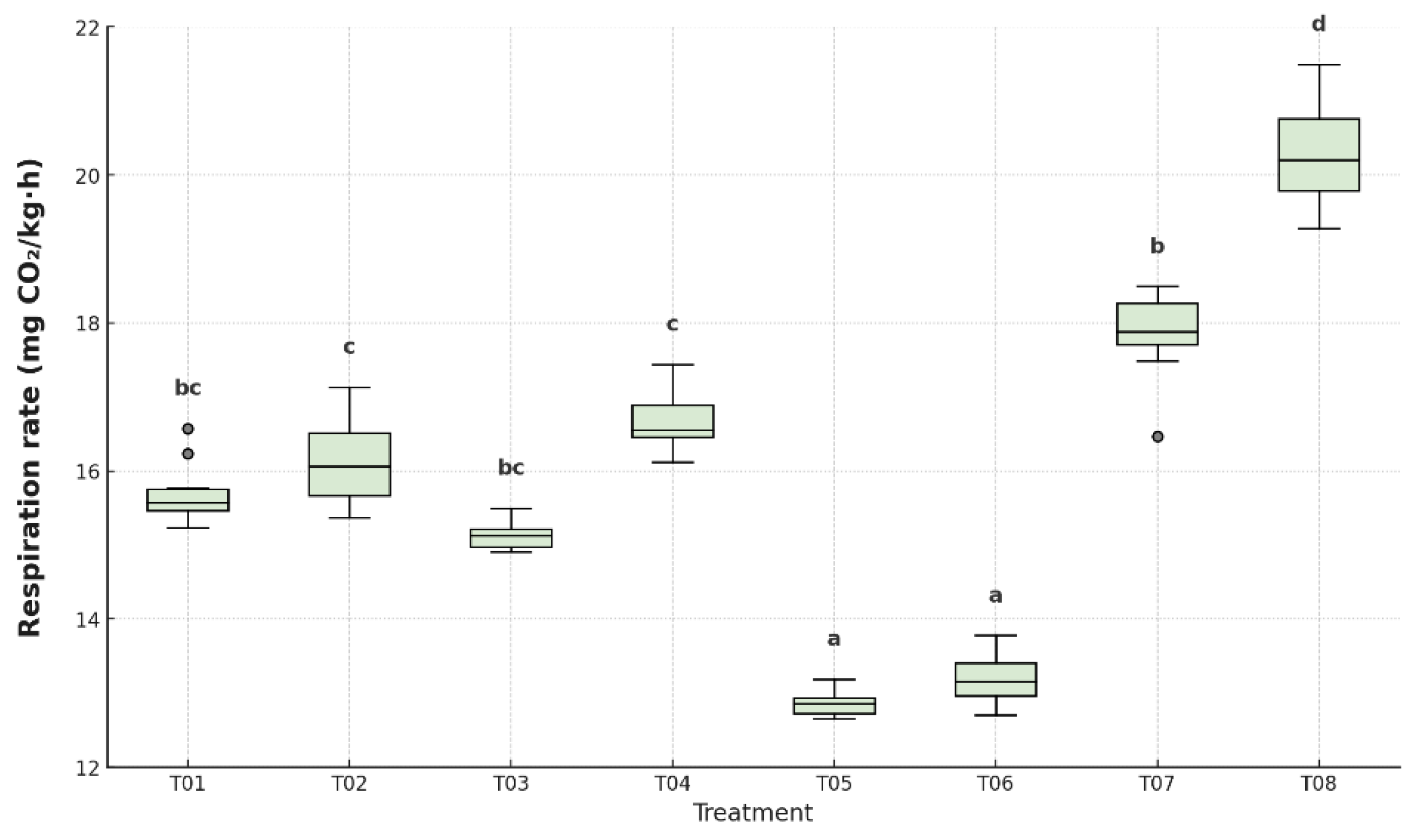
Figure 11, significant differences were found between treatments (p < 0.05).
The control group without coating or packaging (T08) exhibited the highest respiration rate (20.4 mg CO₂/kg·h), followed by T07 (18.1 mg CO₂/kg·h), confirming the rapid metabolic progression in unprotected fruits. In contrast, T05 and T06 demonstrated the lowest respiration rates (13.2 and 13.4 mg CO₂/kg·h, respectively), suggesting effective inhibition of climacteric respiration due to the antifungal coating combined with LDPE or HDPE. Intermediate values were recorded in T01 (15.6), T02 (16.2), T03 (15.1), and T04 (16.6), all of which were significantly lower than the controls but higher than T05 and T06. These findings confirm that the combination of coating and plastic packaging was able to suppress CO₂ emission rates, thereby extending the physiological shelf life of the fruit.
3.4. Microbiological Quality
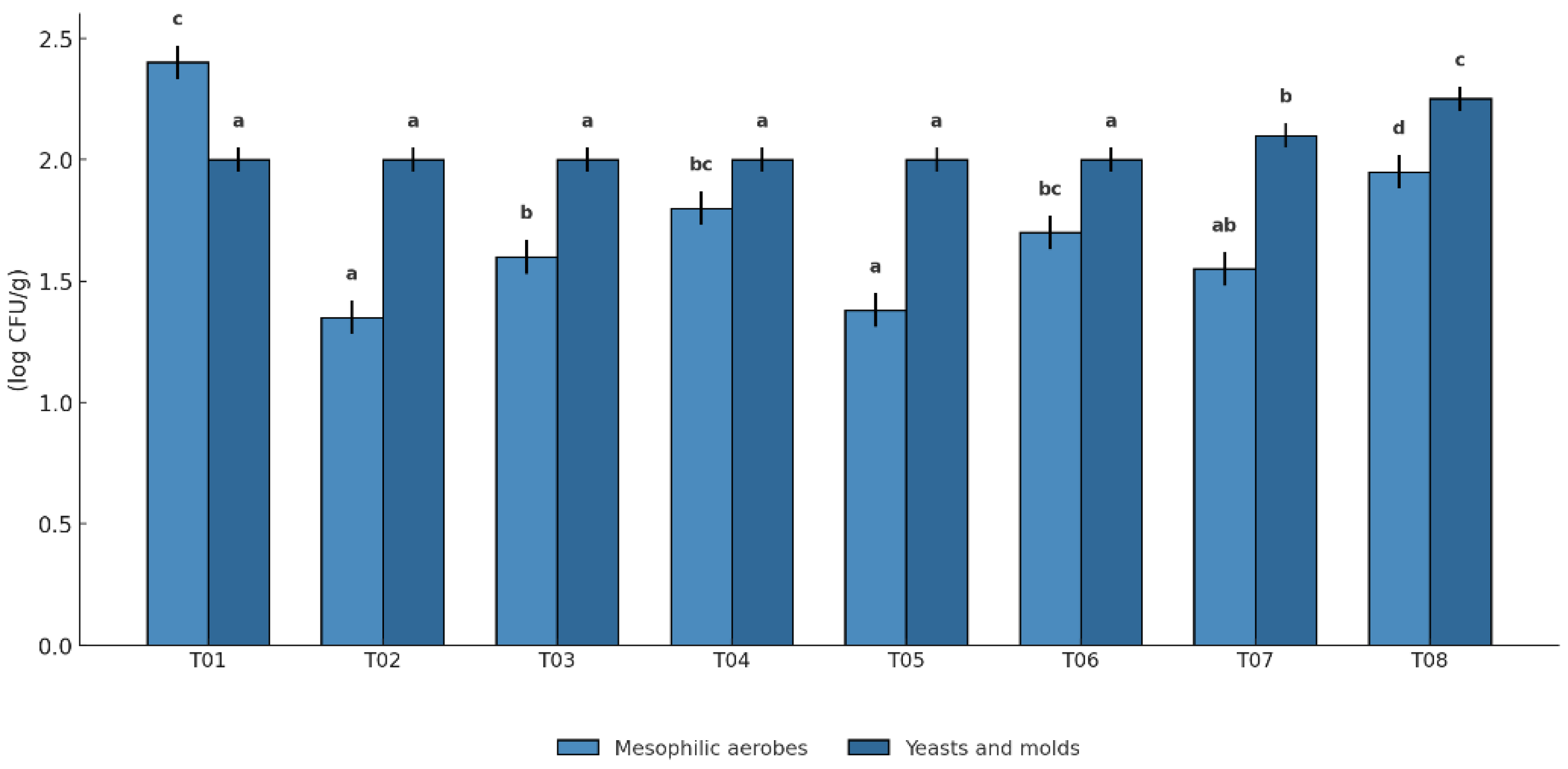
Microbial counts varied significantly among treatments after 28 days of storage (p < 0.05). As shown in
Figure 12, T05 and T06 exhibited the lowest mesophilic aerobic counts (1.41 ± 0.04 and 1.38 ± 0.05 log CFU/g, respectively), and yeast and mold counts remained below 2.0 log CFU/g. These results indicate strong microbial control in these coated and packaged fruits. In contrast, the highest mesophilic load was detected in T01 (2.40 ± 0.06 log CFU/g), despite also being coated and packaged in LDPE. This may suggest batch-specific variability in coating integrity or packaging effectiveness. The uncoated controls, T07 and T08, showed increased yeast and mold counts (2.10 ± 0.06 and 2.25 ± 0.06 log CFU/g, respectively), consistent with the absence of antimicrobial protection. Intermediate microbial levels were recorded in T02, T03, and T04, with values between 1.6 and 2.0 log CFU/g, depending on polyethylene type and supplier.
3.5. Sensory Evaluation
Consumer acceptance is a factor in postharvest studies, as it directly influences marketability.
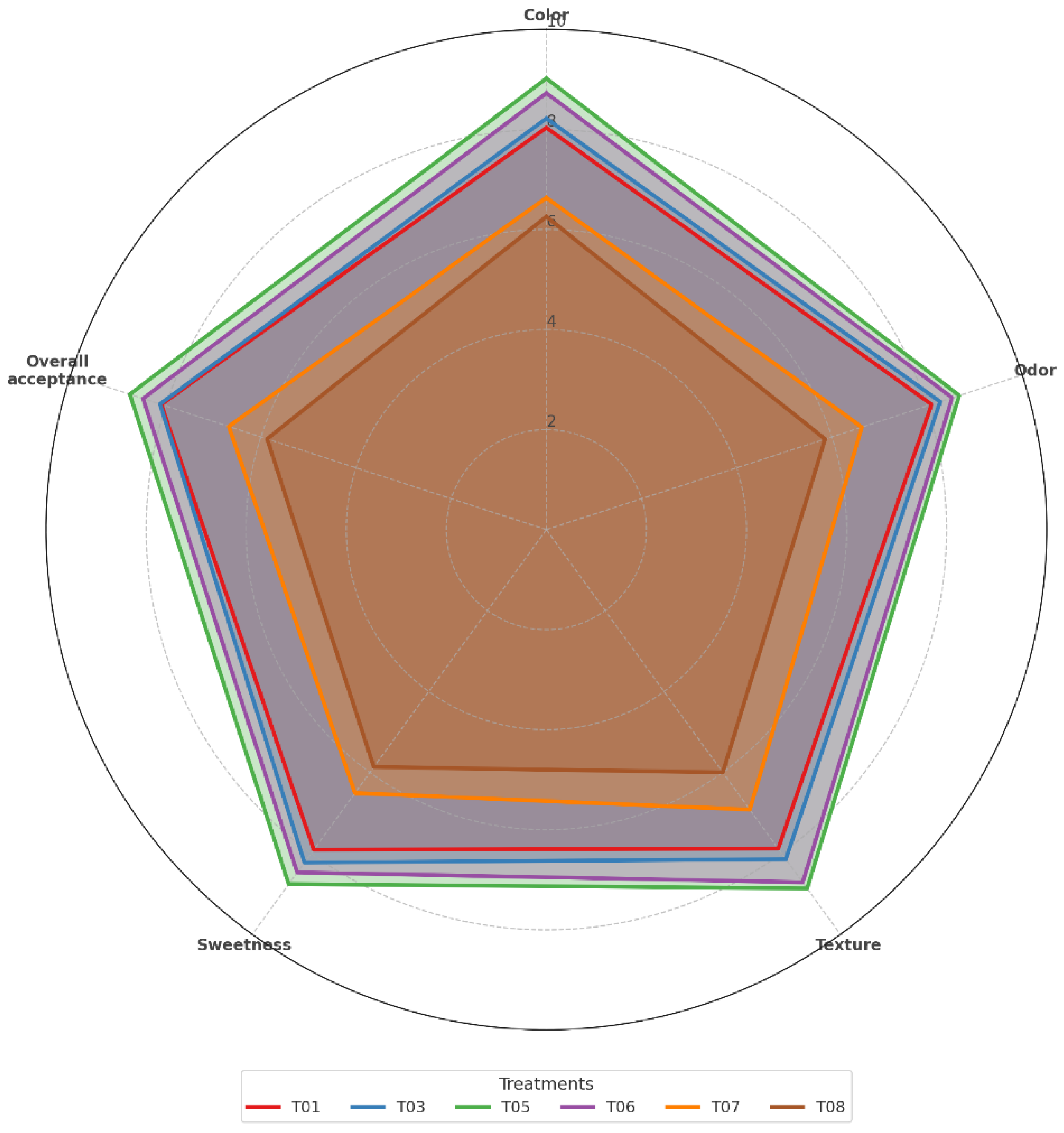
Figure 13 presents the sensory evaluation of the six best-performing treatments (T01, T03, T05, T06, T07, and T08) as assessed by 18 semi-trained panelists using a 10-point hedonic scale. Each panelist evaluated four fruit samples following a rotating design to minimize bias.
The evaluated attributes are color, odor, texture, sweetness, and overall acceptance. The highest scores were consistently associated with treatments T05 and T06, which combined the antifungal coating with polyethylene packaging (LDPE and HDPE, respectively). These treatments received the best ratings across all attributes, particularly in overall acceptance (mean > 9.0), indicating sensory preservation and consumer preference.
Treatments T01 and T03 also achieved favorable scores, although slightly lower than T05 and T06. In contrast, the control treatments (T07: plastic-only; T08: no coating or plastic) received significantly lower scores in all sensory parameters, especially in odor and texture, which are critical to consumer perception of freshness and ripeness.
4. Discussion
The results obtained in this study demonstrate that the application of coatings had a significant effect on preserving the physicochemical properties of Musa paradisiaca fruits during storage, in comparison to the untreated control. The coatings contributed to maintaining structural integrity and slowing down biochemical changes associated with ripening and senescence. The following section provides a comprehensive discussion of the main findings, highlighting the behavior of each evaluated parameter
4.1. Physical Analysis
4.1.1. Weight Variation
All treatments exhibited progressive weight loss during storage. However, T05, consisting of a coating combined with low-density polyethylene packaging, achieved the lowest cumulative loss (9.4% ± 0.3). This result contrasts sharply with the uncoated and unpackaged control (T08), which recorded a significantly higher loss (22.8% ± 0.6), followed by T07 (plastic-only control) at 15.1% ± 0.4, confirming the effectiveness of the study.
The performance of T05 can be attributed to the synergy of its biopolymeric matrix. Cassava starch and agar act as film-forming agents with low water vapor permeability, while glycerol provides flexibility without compromising structural cohesion [
60]. Whey proteins contribute emulsifying and antioxidant properties, supporting cellular integrity and delaying senescence [
17,
61]. Moreover, the antifungal and antioxidant activity of
Cinnamomum verum essential oil may have reduced oxidative stress and respiration, further limiting mass loss.
T01 to T04, which also included coatings and packaging, showed intermediate weight loss values (10.8%–12.8%), with statistically significant differences depending on the polymer-plastic combination. Despite also containing coating, T06 was slightly less effective than T05. These findings are in line with previous reports where starch-based coatings reduced weight loss in papaya and banana by up to 40% under refrigerated conditions [
22]. Likewise, blends of chitosan and essential oils have been shown to delay dehydration and ripening by modulating gas exchange [
62,
63].
ANOVA results confirmed a highly significant treatment effect (F = 182.3, p < 0.0001). Overall, the data validates the hypothesis that combining bioactive coatings and LDPE packaging effectively minimizes postharvest weight loss in Musa paradisiaca, offering a viable alternative to conventional plastic-only methods.
4.1.2. Fruit Dimensions
The reduction in fruit dimensions—specifically length and peel thickness—is a critical indicator of structural integrity and water retention during postharvest storage. In the present study, all treatments exhibited measurable shrinkage, although the extent varied significantly depending on the type of coating and packaging (p < 0.05).
Length reduction was lowest in T05 (3.7% ± 0.2) and T06 (4.0% ± 0.2), both of which combined the antifungal coating with LDPE and HDPE from company C, respectively. In contrast, the uncoated and unpackaged control (T08) reached the highest reduction (7.9% ± 0.3), followed by T07 (6.7% ± 0.3). Intermediate values were recorded for T01 to T04, ranging from 5.1% to 6.0%. These findings confirm the capacity of the coating to limit longitudinal shrinkage, likely by maintaining turgor pressure and reducing dehydration through a semi-permeable barrier [
64].
Similarly, peel thickness loss followed a comparable pattern, the lowest values were found in T05 (5.0% ± 0.2) and T06 (5.3% ± 0.2), statistically different from the controls and most other treatments (p < 0.05). The highest value again corresponded to T08 (10.6% ± 0.3), highlighting the accelerated desiccation in the absence of any protective barrier. T02 and T04 showed moderate performance. These trends are consistent with previous studies in papaya and mango, where starch-based coatings contributed to dimension stabilization by reducing surface transpiration and cellular collapse during ripening [
19].
4.1.3. Firmness
Firmness loss is directly associated with cell wall degradation and moisture loss. In this study, firmness varied significantly across treatments (p < 0.05). The untreated control T08 exhibited the highest value 32.0%, followed by T07 with 28.5%, indicating advanced softening and textural degradation during storage. T05 and T06 were the most effective, registering significantly lower values 15.3% and 15.5%, respectively. These coatings maintained the highest textural stability, likely due to reduced enzymatic activity and delayed cell wall breakdown [
46].
The incorporation of agar and starch into the coating matrix has previously been linked to the retardation of pectin solubilization, a mechanism in the softening process of climacteric fruits. Furthermore, the presence of cinnamon essential oil (
Cinnamomum verum), known for its antimicrobial and antioxidant effects, may have contributed to the inhibition of ethylene-mediated ripening and the reduction of oxidative stress [
18].
T01–T04 exhibited intermediate firmness losses (25–27%), suggesting that although the coating provided partial protection, variations in plastic type or film permeability may have limited its efficacy. Overall, the results confirm that coatings based on whey–agar–cassava starch–glycerol, especially in combination with LDPE or HDPE, enhance firmness retention during storage, offering a promising strategy for maintaining banana quality in the postharvest supply chain.
4.1.4. Color Parameters
Critical indicator of ripening and senescence in bananas, as it reflects chlorophyll breakdown and carotenoid accumulation. In this study, all color parameters (ΔL*, Δa*, and Δb*) showed statistically significant differences among treatments (p < 0.05), confirming that the application of antifungal coatings and polyethylene packaging influenced the visual quality of
Musa paradisiaca during storage [
7,
45].
ΔL* was significantly higher in the uncoated control T08, reaching an average of 12.8, indicative of advanced senescence and browning. In contrast, treatments T05 and T06 showed the lowest ΔL* values (5.3 and 5.5, respectively), indicating superior retention of surface brightness. The effectiveness of these coatings can be attributed to their semi-permeable nature, which reduces oxygen diffusion and enzymatic browning reactions
Δa* (green-to-red) values followed a similar trend. T05 (2.4) and T06 (2.5) exhibited minimal shifts, whereas T08 reached a* values above 5.3, confirming advanced peel discoloration. Red color development is associated with chlorophyll degradation and anthocyanin synthesis, processes accelerated by ethylene exposure and oxidative stress. T05 and T06 likely acted as ethylene barriers, delaying this transition, as supported by similar findings in mangoes coated with alginate-based films enriched with essential oils [
53].
Δb* (yellow, blue) was also less pronounced in T05 (3.1) and T06 (3.3), while the greatest decline occurred in T08 (6.2). This suggests that color retention in coated bananas was not only linked to reduced oxidative stress but also to slower degradation of carotenoid pigments, b* reduction leads to dull appearance and lower market acceptability.
Collectively, these results confirm the positive role of the coating (especially in T05) in maintaining peel color during postharvest storage. The balanced composition offered an effective barrier to gas exchange and moisture loss, which are critical triggers of pigment transformation. Future work could explore the inclusion of natural antioxidants (e.g., green tea extract, rosemary) to further enhance color stability during storage [
65].
4.2. Chemical Analysis
4.2.1. pH
pH is associated with the degradation of organic acids during postharvest storage. The results revealed statistically significant differences between treatments (p < 0.05), with values ranging from 5.30 to 5.54. These findings confirm that coatings influenced acid metabolism and helped regulate ripening kinetics in
Musa paradisiaca [
66].
T05 and T06 showed the lowest pH values (5.30 and 5.34, respectively), indicating a delayed degradation of organic acids. This result is consistent with their higher values, suggesting that preserved metabolic stability by creating a semi-permeable barrier to gas exchange, limiting oxygen diffusion and reducing respiratory stress. Similar trends were reported with alginate–cinnamon oil coatings maintained lower pH in coated tomatoes due to reduced ethylene production and enzymatic activity [
54].
T08 exhibited the highest pH (5.54), followed closely by T07 (5.52), confirming acid reduction. This behavior is characteristic of rapid senescence and corresponds with higher respiration rates observed in these treatments. T01 to T04 showed intermediate pH values (5.36 to 5.44), with T04 being higher than T01 and T03. The presence of glycerol may have allowed moderate acid retention, but less effectively than T05 and T06.
These results suggest that the formulation of T05 was particularly effective in delaying internal biochemical shifts associated with ripening.
4.2.2. Titratable Acidity
Reflects the concentration of organic acids in fruits and is a critical indicator of flavor and postharvest metabolism. All treatments displayed statistically significant differences in TA values (p < 0.05), confirming the influence of edible coatings on acid preservation.
T05 and T06 maintained the highest levels (0.382 and 0.371 % citric acid, respectively), suggesting a delay in the degradation of organic acids. These results agree with the corresponding low pH values observed for these treatments, indicating reduced respiratory and enzymatic activity. The effectiveness of T05 may be attributed to its cohesive matrix composed, which likely formed a semi-permeable barrier, regulating gas exchange and maintaining metabolic stability [
67].
T08 exhibited the lowest acidity (0.294 ± 0.01 %), followed by T07 (0.319 ± 0.01 %), demonstrating a more advanced ripening stage with accelerated acid consumption. These findings aligned with previous studies reported that untreated bananas exhibited faster acid decline due to higher respiration rates and ethylene production [
55]. In contrast, coatings composed of starch and essential oils have been shown to maintain higher acidity in mango and guava, delaying senescence [
68]. Intermediate titratable acidity values were observed in T01–T04 (0.340–0.370 %), with T03 slightly outperforming the others, compared to T05 and T06.
4.2.3. Soluble Solids
The total soluble solids content of bananas showed significant variation across treatments, with the uncoated control (T8) presenting the highest concentration (19.1 ± 0.4 °Brix), followed by T7 (18.2 ± 0.6 °Brix), both statistically different from the coated treatments (p < 0.0001). In contrast, T5 and T6 exhibited the lowest °Brix values (16.2 ± 0.3 and 16.4 ± 0.3, respectively), suggesting delayed sugar accumulation [
36]. These results are consistent with a slower ripening process in coated samples, particularly in T5.
The semipermeable nature of the coating likely reduced oxygen availability and ethylene diffusion, thus limiting the activity of enzymes responsible for starch hydrolysis, such as amylases and invertases [
69]. Lower enzymatic conversion rates from starch to soluble sugars have previously been reported in bananas treated with edible coatings containing proteins and polysaccharides. The reduced °Brix in T5 aligns with its lower respiration rate, since sugar degradation and synthesis are linked to metabolic activity.
Treatments with higher °Brix values, such as T7 and T8, indicate accelerated ripening and increased respiration, leading to greater breakdown of cell wall polysaccharides and soluble sugar release [
70]. Similar trends have been reported with starch-based coatings with essential oils reduced °Brix accumulation in mangoes during storage [
70]. Likewise, cassava starch films slowed °Brix increase in guava by 28% compared to controls [
71]. These studies reinforce the hypothesis that composite coatings enriched with bioactive compounds can modulate carbohydrate metabolism and delay senescence [
72]. The effectiveness of T5 in delaying sugar accumulation may be beneficial for extending shelf life and preserving flavor.
4.3. Respiration Rate
Significantly affected by the application of antifungal coatings and polyethylene packaging, showing a strong correlation with delayed ripening and reduced metabolic activity. T5 registered the lowest value (12.8 mg CO₂ kg⁻¹ h⁻¹), T6 (13.3 mg CO₂ kg⁻¹ h⁻¹), both of which were statistically different from the control (T8), which exhibited the highest rate (20.7 mg CO₂ kg⁻¹ h⁻¹). These findings highlight the effectiveness of T5 in reducing metabolic heat production. The film’s semipermeable nature limited O₂ ingress and CO₂ egress, thereby slowing oxidative processes and ethylene synthesis [
34].
Previous studies have shown that coatings based on polysaccharides and proteins can effectively regulate gas exchange in climacteric fruits such as banana and mango, reducing respiration and delaying ripening [
17]. In contrast, the untreated control (T8) and the uncoated polyethylene treatment (T7) exhibited elevated respiration rates, consistent with their accelerated ripening and greater physicochemical deterioration. T2 and T4 showed moderately elevated value, these results reinforce the hypothesis that a balanced combination of biopolymers and plasticizers, coupled with bioactive agents, enhances film performance by forming a cohesive matrix with selective permeability. This not only protects against microbial contamination but also mitigates metabolic acceleration.
4.4. Microbiological Quality
The microbiological results demonstrate the effectiveness of antifungal coatings in reducing microbial loads on Musa paradisiaca. Treatments T05 and T06, which combined the antifungal coating with LDPE and HDPE packaging respectively, showed superior performance in suppressing both mesophilic aerobes and surface yeasts and molds. These findings are consistent with their high performance across physicochemical and sensory parameters. The active coating, enriched with Cinnamomum verum essential oil, likely disrupted microbial membrane integrity and inhibited fungal growth.
Despite being coated and packaged, T01 showed unexpectedly high mesophilic counts (2.40 ± 0.06 log CFU/g). This result may be explained by batch-related factors, such as reduced adhesion or coverage of the coating matrix on those specific fruits, or lower-quality LDPE packaging from supplier A, with suboptimal oxygen barrier properties. Such conditions may have favored aerobic microbial activity despite the antifungal formulation. The findings reinforce that the combined quality and compatibility of the coating and packaging materials are essential to achieve optimal microbial control. While all coated treatments reduced microbial counts below the threshold for fresh produce (3.0 log CFU/g), T05 and T06 were the most consistent and reliable in preserving microbiological safety and fruit quality.
4.5. Sensory Evaluation
Revealed differences in perception of Musa paradisiaca fruit, T5 received the highest scores across all attributes—color, odor, texture, sweetness, and overall acceptance— after of T6 and together surpassed to all the treatments. even the uncoated plastic control (T07) and the untreated control (T08). This confirms that the antifungal coating formulated not only preserved the physicochemical integrity of the fruit but also maintained its sensory appeal. Panelists rated T5 high in terms of texture and sweetness, likely due to its lower water loss and better firmness retention, as confirmed by instrumental analysis.
The formulation provided a cohesive and semi-permeable film that delayed ripening-related degradation, reducing enzymatic softening and sugar concentration peaks that may result in off-flavors or textural breakdown [
7,
35,
61]. The coating contributes positively to preservation, plasticizer content plays a crucial role in the sensory perception, possibly by influencing the rate of metabolic changes and aroma compound diffusion.
T07 and T08 showed significantly lower acceptance, particularly for odor and overall quality. This aligns with the higher respiration rates and microbial activity recorded in these treatments, which are known to accelerate senescence and lead to off-odors and texture deterioration. The other treatments achieved low and have acceptable sensory scores, reinforcing the role of the coating matrix itself as a functional barrier. This confirms prior research indicating that biopolymer-based coatings can maintain or even improve perception of tropical fruits during storage when compared to traditional packaging [
8]. These results demonstrate that the antifungal coating strategy did not compromise sensory quality, but rather enhanced it, supporting its potential for commercial application.
4.6. Statistical Analysis
All experimental data were analyzed using Statgraphics Centurion XVII, applying a one-way analysis of variance (ANOVA) to determine the effect of treatments on the physicochemical, microbiological, respiratory, and sensory parameters of Musa paradisiaca fruits. Results are expressed as mean ± standard deviation, and Tukey’s Honest Significant Difference (HSD) test (α = 0.05) was used for multiple comparisons among treatment means. The sensory data were obtained from 18 semi-trained panelists following a rotational evaluation design. Statistically significant differences (p < 0.05) between treatments are indicated by different lowercase letters in the graphical representations.
5. Conclusions
The combined application of an antifungal coating based on whey, agar, cassava starch, glycerol, and cinnamon essential oil (600 ppm), together with polyethylene packaging, demonstrated significant effectiveness in preserving the postharvest quality of Musa paradisiaca.
Among the evaluated treatments, T05 (coating + LDPE) and T06 (coating + HDPE) consistently exhibited the most favorable results across physicochemical, microbiological, respiratory, and sensory parameters. These treatments achieved the lowest weight and firmness loss, minimized peel shrinkage, preserved color and titratable acidity, and maintained high consumer acceptance.
The bioactive coating functioned as a semi-permeable barrier, reducing water loss, gas exchange, and microbial proliferation, while the LDPE and HDPE provided complementary environmental protection. The presence of cinnamon essential oil further contributed to microbial control, particularly of mesophilic aerobes and surface yeasts and molds.
These findings support the potential of combining bioactive coatings and polyethylene packaging, especially T05 and T06, as a practical and scalable solution for extending banana shelf life.
Future research should explore the integration of these systems with biodegradable films, evaluate commercial logistics conditions, and assess long-term consumer response to coated and packaged bananas.
Author Contributions
All the mentioned authors have significantly contributed to the development and writing of this article. All authors have read and accepted the published version of the manuscript.
Funding
This research was funded by the Escuela Politécnica Nacional.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable. This study did not involve human participants.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the support of DECAB – Escuela Politécnica Nacional.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Capa Benítez LB, Alaña Castillo TP, Benítez Narváez RM. Importancia de la producción de banano orgánico. Caso: Provincia El Oro, Ecuador. Revista Universidad y Sociedad 2016;8:64–71.
- Agronomía C. Tecnología Poscosecha. Agronomía Costarricense 2005;29:207–9.
- Kabir J, Kore V, Tawade S. Application of Edible Coatings on Fruits and Vegetables. ResearchGate 2017;3.
- Ruiz Medina MD, Ruales J. Post-Harvest Alternatives in Banana Cultivation. Agronomy 2024;14:2109. [CrossRef]
- Palma RMM, Pérez AAF, Padilla MC. Recubrimientos comestibles para extender la vida de anaquel de productos hortofrutícolas. Ciencia Latina Revista Científica Multidisciplinar 2021;5:4605–25. [CrossRef]
- Akhtar M, Khan MR, Hussain S. Impact of edible coatings on the preservation of postharvest fruit quality. International Journal of Food Science and Technology 2020;55:3424–31. [CrossRef]
- Ruiz Medina MD, Quimbita Yupangui Y, Ruales J. Effect of a Protein–Polysaccharide Coating on the Physicochemical Properties of Banana (Musa paradisiaca) During Storage. Coatings 2025;15:812. [CrossRef]
- Moradinezhad F, Adiba A, Ranjbar A, Dorostkar M. Edible Coatings to Prolong the Shelf Life and Improve the Quality of Subtropical Fresh/Fresh-Cut Fruits: A Review. Horticulturae 2025;11:577. [CrossRef]
- Lin Y, Lin H, Fan Z, Wang H, Lin M, Chen Y, et al. Inhibitory effect of propyl gallate on pulp breakdown of longan fruit and its relationship with ROS metabolism. Postharvest Biology and Technology 2020;168:111272. [CrossRef]
- Castellanos DA, Algecira NA, Villota CP. Aspectos relevantes en el almacenamiento de banano en empaques con atmósferas modificadas. Revista Iberoamericana de Tecnología Postcosecha 2011;12:114–34.
- Vargas A, Valle H, González M. Efecto del color y de la densidad del polietileno de fundas para cubrir el racimo sobre dimensiones, presentación y calidad poscosecha de frutos de banano y plátano. Agronomía Costarricense 2010;34:269–85. [CrossRef]
- Peña-Baracaldo FJ, Chaparro HN, Orjuela-Matta HM, Romero-Guerrero G, Peña-Baracaldo FJ, Chaparro HN, et al. Respuesta en poscosecha de frutos de agraz (Vaccinium meridionale Swartz) a almacenamiento en dos tipos de plástico. Revista UDCA Actualidad & Divulgación Científica 2020;23. [CrossRef]
- Chiriboga J. Hacia una agricultura más sostenible: recubrimientos comestibles naturales para extender la vida útil de las frutas. VITSC 2025;2:18–32. [CrossRef]
- Ruiz Medina M, Ávila J, Ruales J. DISEÑO DE UN RECUBRIMIENTO COMESTIBLE BIOACTIVO PARA APLICARLO EN LA FRUTILLA (Fragaria vesca) COMO PROCESO DE POSTCOSECHA. Revista Iberoamericana de Tecnología Postcosecha 2016;17:276–87.
- Bello-Lara JE, Balois-Morales R, Universidad Autónoma de Nayarit, Juárez-López P, Alia-Tejacal I, Universidad Autónoma del Estado de Morelos, et al. Coatings based on starch and pectin from ‘Pear’ banana (Musa ABB), and chitosan applied to postharvest ‘Ataulfo’ mango fruit. Rchsh 2016;XXII:209–18. [CrossRef]
- Arce Ortiz KL, Ortega Villalba KJ, Ochoamartinez CI, Vélez Pasos C. Postharvest properties of banana gross michel coated with whey protein and chitosan. Vitae 2016;23:S749–53.
- Elsayed N, Hassan AA, Abdelaziz SM, Abdeldaym EA, Darwish OS. Effect of Whey Protein Edible Coating Incorporated with Mango Peel Extract on Postharvest Quality, Bioactive Compounds and Shelf Life of Broccoli. Horticulturae 2022;8:770. [CrossRef]
- Malmiri H, Osman A, Ping C, Abdul R. EFECTOS DE LOS RECUBRIMIENTOS SUPERFICIALES COMESTIBLES (CARBOXIMETILCELULOSA SÓDICA, CASEINATO SÓDICO Y GLICEROL) EN LA CALIDAD DE ALMACENAMIENTO DEL PLÁTANO BERANGAN (MUSA SAPIENTUM CV. BERANGAN) UTILIZANDO LA METODOLOGÍA DE SUPERFICIE DE RESPUESTA - JAFARIZADEH MALMIRI - 2012 - Revista de Procesamiento y Conservación de Alimentos - Biblioteca en Línea de Wiley 2011. [CrossRef]
- Sanchez-Tamayo M, Plaza-Dorado JL, Ochoa-Martínez C. Influence of Composite Edible Coating of Pectin, Glycerol, and Oregano Essential Oil on Postharvest Deterioration of Mango Fruit. Food Science & Nutrition 2024;12:10646–54. [CrossRef]
- Anjos IV dos, Melo SS de, Gilio TAS, Kreitlow JP, Neves SMA da S, AraÃojo KL, et al. Molecular Characterization of Isolates of Fusarium spp. Associated With Wilt in Capsicum spp. Journal of Agricultural Science 2024;11:519–519.
- Aquino-Martínez JG, Vázquez-García LM, Reyes-Reyes BG. Biocontrol in vitro e in vivo de Fusarium oxysporum Schlecht. f. sp. dianthi (Prill. y Delacr.) Snyder y Hans. con Hongos Antagonistas Nativos de la Zona Florícola de Villa Guerrero, Estado de México. Revista mexicana de fitopatología 2008;26:127–37.
- Ali A, Muhammad MTM, Sijam K, Siddiqui Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chemistry 2011;118:620–6. [CrossRef]
- Barrera Necha LL, García Barrera LJ. Actividad antifúngica de aceites esenciales y sus compuestos sobre el crecimiento de Fusarium sp. aislado de papaya ( Carica papaya). Revista Científica UDO Agrícola 2008;8:33–41.
- Allagui MB, Moumni M, Romanazzi G. Antifungal Activity of Thirty Essential Oils to Control Pathogenic Fungi of Postharvest Decay. Antibiotics 2024;13:28. [CrossRef]
- Ruiz Medina MD, Ruales J. Essential Oils as an Antifungal Alternative to Control Several Species of Fungi Isolated from Musa paradisiaca: Part III. Microorganisms 2025;13:1663. [CrossRef]
- Abd Rashed A, Rathi D-NG, Ahmad Nasir NAH, Abd Rahman AZ. Antifungal Properties of Essential Oils and Their Compounds for Application in Skin Fungal Infections: Conventional and Nonconventional Approaches. Molecules 2021;26:1093. [CrossRef]
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—A review. Food and Chemical Toxicology 2008;46:446–75. [CrossRef]
- Martínez M, Gómez S. Antifungal properties of essential oils in fruit preservation. Food Research International 2020;127:108679. [CrossRef]
- Aboboreira M. Principales labores del cultivo de banano. Primera. Costa Rica: Finca Comercial; 1994.
- Galan V, Rangel A, Lopez J, Hernandez JBP, Sandoval J, Rocha HS. Propagación del banano: técnicas tradicionales, nuevas tecnologías e innovaciones. Rev Bras Frutic 2018;40. [CrossRef]
- Oms-Oliu G, Soliva-Fortuny R, Martín-Belloso O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT - Food Science and Technology 2008;41:146–56. [CrossRef]
- Verma S, Giri S, Yadav S. Effect of polyethylene packaging on the storage of tropical fruits. Food Packaging and Shelf Life 2019;22:100422. [CrossRef]
- Zhao L, Wu J, Li J. Application of polysaccharide-based edible coatings for the preservation of fresh fruits. Food Hydrocolloids 2019;87:221–30. [CrossRef]
- Brackmann A, Steffens CA, Sestari I, Neuwald DA, Giehl RFH. Modified and controlled atmosphere storage of “Prata” banana with ethylene scrubbing. Ciênc Agrotec 2006;30:914–9. [CrossRef]
- Nischitha R, Shravya, Pooja DV, Mahesh S. (PDF) Impact of Edible Coating in Extending the Shelf life of Post-harvested Banana under Storage Condition. ResearchGate n.d. https://www.researchgate.net/publication/378011325_Impact_of_Edible_Coating_in_Extending_the_Shelf_life_of_Post-harvested_Banana_under_Storage_Condition (accessed May 26, 2025).
- Al-Dairi M, Pathare PB, Al-Yahyai R, Jayasuriya H, Al-Attabi Z. Postharvest quality, technologies, and strategies to reduce losses along the supply chain of banana: A review. Trends in Food Science & Technology 2023;134:177–91. [CrossRef]
- Aroca K, Regalado O, Acosta S. Estudio de la conservación de frutas en “Gamma IV” con la aplicación de un recubrimiento biodegradable-activo. Ecuador es Calidad 2018;5.
- Caicedo W, Vargas JC, Uvidia H, Samaniego E, Valle S, Flores L, et al. Physicochemical, biological and organoleptic indicators in banana silage (Musa sapientum) for pig feeding. Revista Cubana de Ciencia Agrícola 2017;51:85–92.
- Alzate Acevedo S, Díaz Carrillo ÁJ, Flórez-López E, Grande-Tovar CD. Recovery of Banana Waste-Loss from Production and Processing: A Contribution to a Circular Economy. Molecules 2021;26:5282. [CrossRef]
- Ruiz Medina MD, Ruales J. Essential Oils as an Antifungal Alternative to Control Several Species of Fungi Isolated of Musa paradisiaca: Part II 2025. [CrossRef]
- Lieu DM, Dang TTK, Nguyen HT. Protein and polysaccharide edible coatings: A promising approach for fruits preservation - recent advances. Food Chemistry: X 2025;27:102388. [CrossRef]
- Ruiz Medina MD, Ruales J. Essential Oils as an Antifungal Alternative for the Control of Various Species of Fungi Isolated from Musa paradisiaca: Part I 2025. [CrossRef]
- Fernández NM, Echeverria DC, Mosquera SA, Paz SP. ESTADO ACTUAL DEL USO DE RECUBRIMIENTOS COMESTIBLES EN FRUTAS Y HORTALIZAS. Biotecnología en el Sector Agropecuario y Agroindustrial 2017;15:134–41. [CrossRef]
- Villalta R, Pérez L, Guzmán M. Estimación de la pérdida de peso en frutos de banano (Musa AAA) empacado con destino al mercado europeo. CORBANA 2019;45:19–32.
- Ulloa L, Sáenz M, Castro J. Efecto del almacenamiento a diferentes temperaturas sobre el desarrollo de color externo y la calidad de frutos de piña cv. Dorada extra dulce 2015;39.
- Castellanos D, Algecira N. Modelling change in color and firmness of baby banana (Musa acuminata AA) in modified atmosphere packaging. Agronomía Colombiana 2012;30:84–94.
- Vásquez-Castillo W, Racines-Oliva M, Moncayo P, Viera W, Seraquive M, Vásquez-Castillo W, et al. Calidad del fruto y pérdidas poscosecha de banano orgánico Musa acuminata en el Ecuador. Enfoque UTE 2019;10:57–66. [CrossRef]
- Standardization IO for. ISO 11036:2017: Fruits and vegetables — Measurement of firmness 2017.
- Standardization IO for. ISO 11037:1997: Fruit and vegetable products — Determination of color 1997.
- International A. AOAC 981.12: pH of food. 15th ed. AOAC International: Official Methods of Analysis of AOAC International; 1981.
- International A. AOAC 942.15: Titratable acidity in fruits. 16th ed. AOAC International: Official Methods of Analysis of AOAC International; 1997.
- Davara PR, Patel NC. Assessment of post harvest losses in banana grown in Gujarat. Journal of Horticultural Sciences 2009;4:187–90. [CrossRef]
- International A. Official Methods of Analysis of AOAC International. 16th ed. Arlington, VA, USA: Association of Official Analytical Chemists; 1997.
- Ladino A, Valencia S. Estudio del manejo poscosecha del maíz tierno (Zea mays L.) procedente del cantón San Miguel de la provincia de Bolívar. Revista EPN 2014;33:1–11.
- Kandsamy P. Respiration rate of fruits and vegetables for modified atmosphere packaging: a mathematical approach. ResearchGate 2022. https://www.researchgate.net/publication/358532606_Respiration_rate_of_fruits_and_vegetables_for_modified_atmosphere_packaging_a_mathematical_approach (accessed July 16, 2025).
- Maturin L. Bacteriological Analytical Manual Chapter 3 Aerobic Plate Count 2018.
- Kemp SE, Hort J, Hollowood T. Descriptive Analysis in Sensory Evaluation. John Wiley & Sons; 2018.
- Lawless HT, Heymann H. Sensory Evaluation of Food: Principles and Practices. Springer Science & Business Media; 2010.
- ASTM E1871-10 - Standard Guide for Serving Protocol for Sensory Evaluation of Foods and Beverages. iTeh Standards n.d. https://standards.iteh.ai/catalog/standards/astm/06e76b44-4e1b-41ff-8ffc-cb1c938aded6/astm-e1871-10 (accessed July 16, 2025).
- Samarakoon KW, Thong PH, Jeewanthi RKC. Evaluation of antifungal activity of cassia and holy basil essential oils against postharvest banana pathogens. Chemical Papers 2020;74:3113–21. [CrossRef]
- Hwang S, Lee J. Effect of whey protein-based edible coatings on the shelf life of bananas. Food Chemistry 2016;210:120–5.
- Anaya-Esparza LM, Pérez-Larios A, Ruvalcaba-Gómez JM, Sánchez-Burgos JA, Romero-Toledo R, Montalvo-González E, et al. Funcionalización de los recubrimientos a base de quitosano para la conservación postcosecha de frutas y hortalizas. TIP Revista especializada en ciencias químico-biológicas 2020;23. [CrossRef]
- Uscocovich Á, Zambrano E, Proaño M, Díaz E, Bosquez A, Travez F. Influencia del recubrimiento con quitosano en la calidad física del banano en poscosecha 2023;7. [CrossRef]
- Nischita R, Shravya, Pooja D, Shetty M. (PDF) Impact of Edible Coating in Extending the Shelf life of Post-harvested Banana under Storage Condition. ResearchGate n.d. https://www.researchgate.net/publication/378011325_Impact_of_Edible_Coating_in_Extending_the_Shelf_life_of_Post-harvested_Banana_under_Storage_Condition (accessed July 16, 2025).
- Dávila M. RM, Cortés R. M, Gil G. JH. CAMBIOS FÍSICOS Y FISICOQUÍMICOS DURANTE EL ALMACENAMIENTO EN PLÁTANO IMPREGNADO AL VACÍO CON SOLUCIONES ANTIOXIDANTES. Biotecnología en el Sector Agropecuario y Agroindustrial 2016;14:125–34. [CrossRef]
- Dussán-Sarria S, Gaona-Acevedo AF, Hleap-Zapata JI. Efecto del Uso de Antioxidantes en Plátano Verde Dominico-Hartón (Musa AAB Simmonds) Cortado en Rodajas. Información Tecnológica 2017;28:03–10. [CrossRef]
- Dussán-Sarria S, Camacho-Tamayo JH, Álvarez-Herrera JG. Los cambios en atributos de calidad y comportamiento fisiológico de banano entero (Musa acuminata cv. ‘cavendish’) durante el almacenamiento comercial. Revista Brasileira de Ciências Agrárias 2024;19:e3469–e3469. [CrossRef]
- Fernández-Valdés D, García-Pereira A, Hernández-Gómez A, Monzón-Monrabal LL. Evaluación del daño mecánico producido por cargas estáticas de compresión en guayaba (Psidium guajaba L.) variedad enana roja EEA-123. Científica 2012;16:91–8.
- Alvarez LFA, Maya CFH, Troya ETT, Luzuriaga SAG. ESTUDIO DEL EFECTO DEL PARDEAMIENTO ENZIMÁTICO EN LA CALIDAD NUTRICIONAL DEL BANANO (musa paradisiaca l.). RECIENA n.d.;3:15–21. [CrossRef]
- García JC. Efecto del 1-Metilciclopropeno (1-MCP) en el comportamiento poscosecha de banano bocadillo (Musa acuminata AA, Simmonds) 2015.
- Singh G, Maurya S, de Lampasona MP, Catalan CAN. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food and Chemical Toxicology 2007;45:1650–61. [CrossRef]
- Borges CV, Amorim EP, Leonel M, Gomez 0Gomez HA, Santos TPR dos, Ledo CA da S, et al. Post-harvest physicochemical profile and bioactive compounds of 19 bananas and plantains genotypes. Bragantia 2018;78:284–96.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).