Submitted:
10 July 2025
Posted:
11 July 2025
You are already at the latest version
Abstract
Keywords:
Introduction
PFAS in the Environment
One Health Imperative
Materials and Methods
Soil Sampling and Characterization
- Particle size distribution: Laser diffraction (Mastersizer 3000, Malvern Instruments) [8].
- pH and electrical conductivity: 1:2.5 soil:water suspension, measured with an Orion Star A211 pH/conductivity meter.
- Organic matter: Loss-on-ignition at 550 °C for 4 h.
- Cation exchange capacity (CEC): Ammonium acetate extraction.
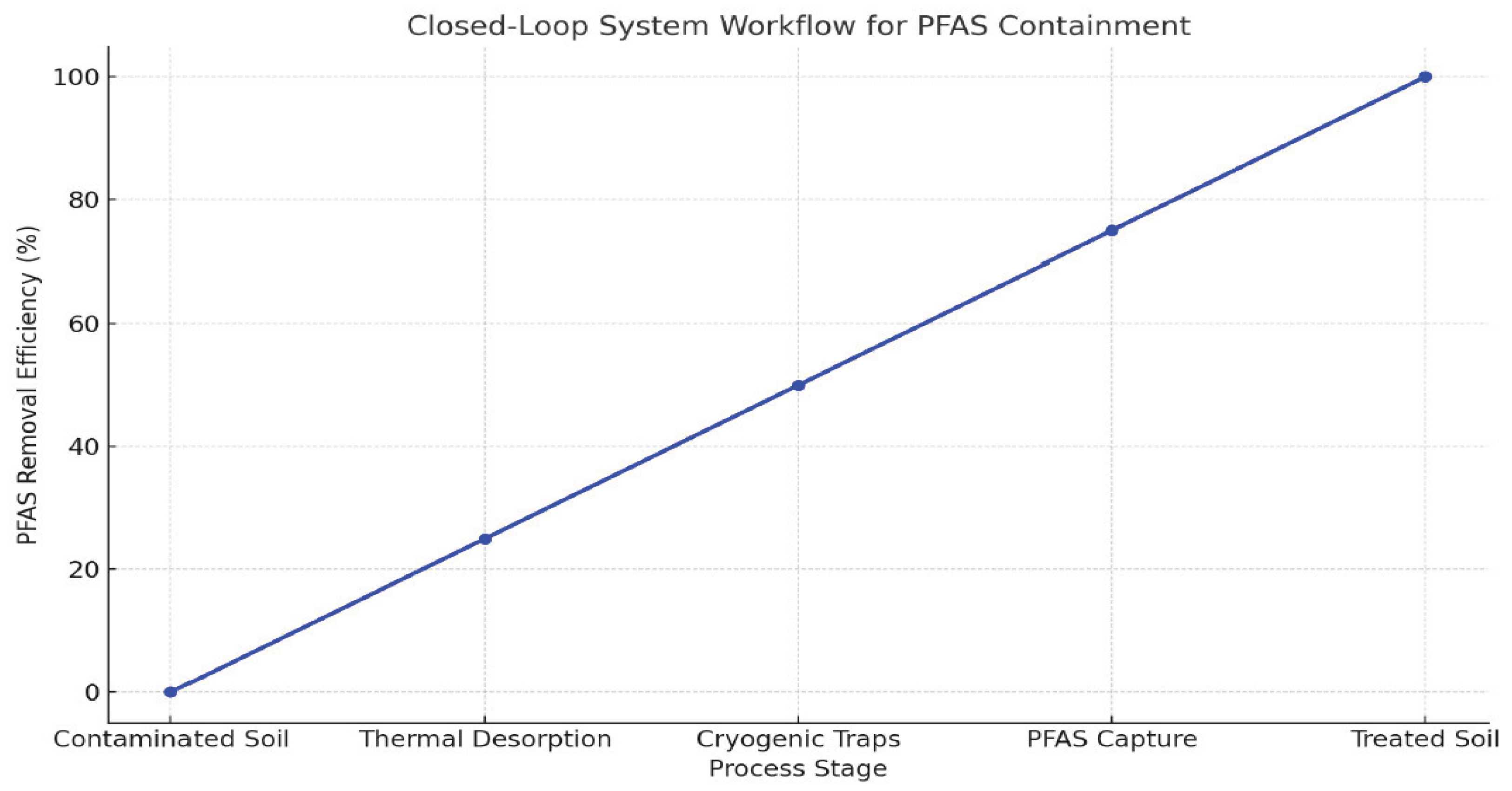
Ultrasound Pre-Treatment Setup
Electrokinetic Extraction Protocol
Cold Plasma Oxidation Reactor
Engineered Microbial Consortia Preparation
Analytical Techniques
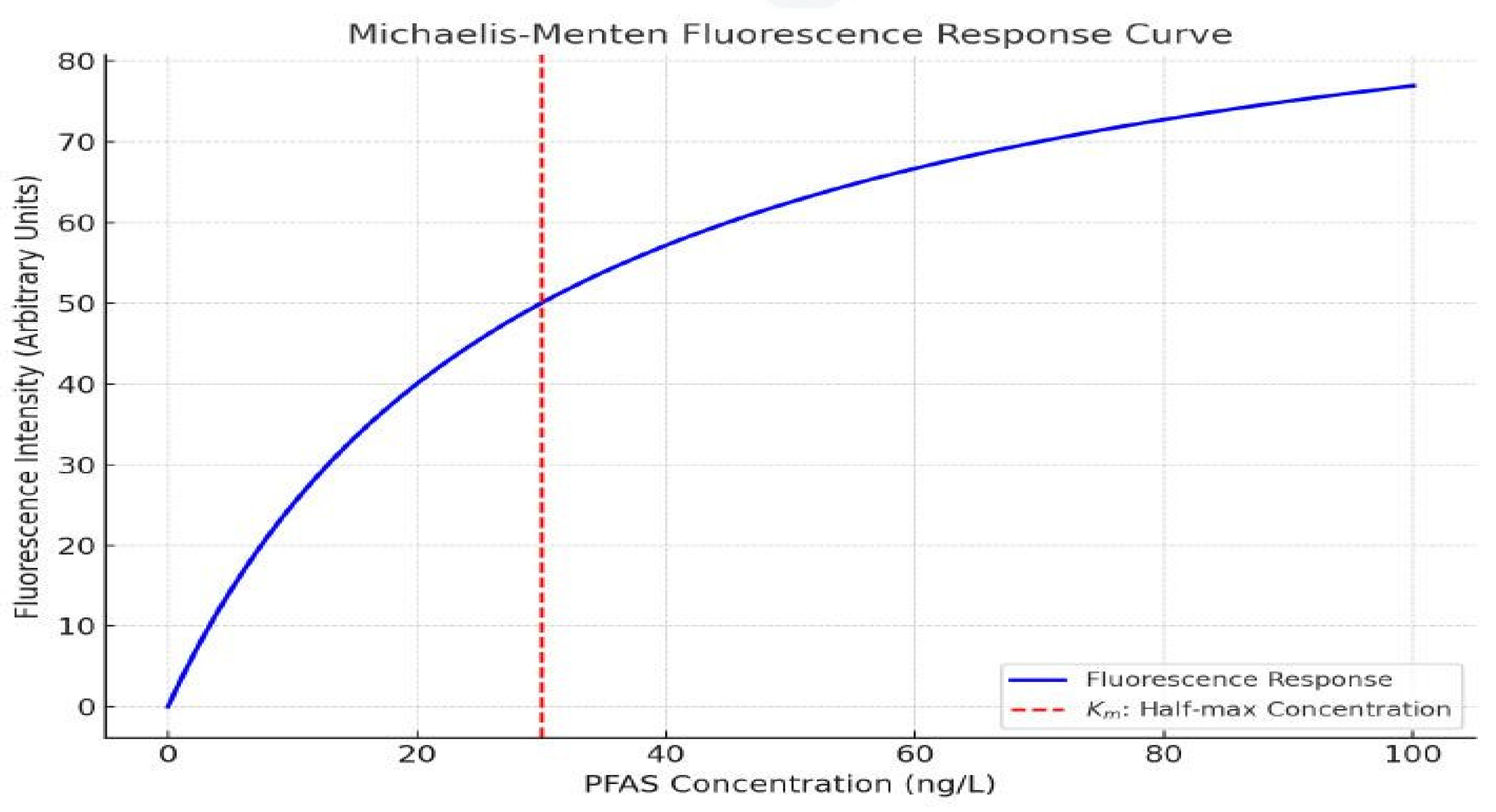
- High-Resolution Mass Spectrometry (HR-MS): PFAS quantification employed LC–HR- MS (Q Exactive) with a C18 column (2.1 × 100 mm, 1.7 µm), gradient elution (5–95 % MeOH in 10 mM ammonium acetate), flow rate 0.3 mL/min. Data were acquired in negative ESI full-scan (m/z 200–1200) at 70,000 resolution [9,10].
- Total Organic Carbon (TOC): Measured using TOC analyzer (Shimadzu TOC-L) on filtered (0.45 µm) samples to assess mineralization.
- Defluorination Efficiency: Fluoride ion concentration determined colorimetrically using a fluoride ion-selective electrode (Thermo Fisher) [10].
- Acute Toxicity Assays: Aliquots of treated and control solutions were tested using Vibrio fischeri bioluminescence inhibition (Microtox 500), with EC50 values calculated after 30 min exposure [13].
- Soil Health Indicators: Post-treatment soil pH, CEC, and dehydrogenase activity (triphenyl tetrazolium chloride reduction) were measured to evaluate ecological impact [8].
Results
PFAS Mobilization Efficiency
Plasma-Mediated Defluorination Rates
Biodegradation Kinetics of Residuals
Soil Health Indicators (pH, CEC, Microbial Activity)
Comparative Performance vs. Single-Method Approaches
Discussion
Implications for Environmental and Public Health
Recommendations and Future Work
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
Abbreviations
| PFAS | Per- and polyfluoroalkyl substances |
| PFOS | Perfluorooctane Sulfonate |
| PFBA | Perfluorobutanoic acid |
| LC–HR-MS | Liquid chromatography-high resolution-mass spectrometry |
| AI | Artificial Intelligent |
| kWh | The kilowatt-hour /unit of measurement for energy |
| AFFF | Aqueous Film-Forming Foam |
| HDPE | High-density polyethylene |
| CEC | Cation exchange capacity |
| DBD | Dielectric barrier discharge |
| HR-MS | High-Resolution Mass Spectrometry |
| TOC | Total Organic Carbon |
References
- Rahman, F.; Peldszus, S.; Anderson, W.B. Behavior and fate of PFAS in the environment: A review. Environmental Toxicology and Chemistry 2014, 33, 1573–1590. [Google Scholar] [CrossRef]
- Ling, J.; Zhu, L. Recent advances in PFAS soil remediation technologies. Critical Reviews in Environmental Science and Technology 2021, 51, 2579–2616. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Duan, J. Electrokinetic remediation of PFAS-contaminated soil: Laboratory investigation. Chemosphere 2020, 244, 125497. [Google Scholar] [CrossRef]
- Smith, R.; Jeffries, T.W. Sequential multi-modal remediation for PFAS: A One Health perspective. Science of The Total Environment 2022, 838, 156691. [Google Scholar] [CrossRef]
- Aoude; McIntyre, C.R. Biological degradation of PFAS by microorganisms: Progress and perspectives. Applied Microbiology and Biotechnology 2019, 103, 4651–4663. [Google Scholar] [CrossRef]
- University of Rhode Island STEEP. Sources, Transport, Exposure & Effects of PFAS: Cape Cod Case Study; Technical Report; University of Rhode Island, 2024; Available online: https://web.uri.edu/steep/communities/cape-cod/ (accessed on 2 June 2025).
- Moran. Study: Toxic contamination at Joint Base Cape Cod could persist for centuries. WBUR. 2023. Available online: https://www.wbur.org/news/2023/05/15/pfas-water-joint-base- cape-cod (accessed on 4 June 2025).
- Guo, Y.; Zhong, D. Ultrasonic-assisted soil remediation: Mechanisms and applications. Journal of Hazardous Materials 2017, 322, 27–36. [Google Scholar] [CrossRef]
- Wang, Z.; Thompson, J. Role of advanced analytical techniques in PFAS determination. Journal of Chromatography A 2018, 1547, 1–16. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Q Exactive Orbitrap MS Operator’s Manual, 2016.
- Sonics; Materials Inc. VCX-750 ultrasonic processor specifications, 2015.
- Yang, Y.; Kabor, J. Cold plasma oxidation for PFAS degradation: A review. Plasma Processes and Polymers 2020, 17, 1900195. [Google Scholar] [CrossRef]
- Microbiotox Systems. Microtox 500 User Manual. 2014.
- The Impact of PFAS on the Public Health and Safety of Future Food Supply in Europe: Challenges and Sustainable Solutions Paper #2570, 25, Research Gate. 20 January. [CrossRef]
- Sasaki, K.; Cai, Y. Adsorption materials for PFAS removal: Graphene oxide and biochar. Environmental Science: Water Research & Technology 2022, 8, 946–959. [Google Scholar] [CrossRef]
- Adamopoulos, I.P.; Valamontes, A.; Karantonis, J.T.; Syrou, N.F.; Damikouka, I.; Dounias, G. The Impact of PFAS on the Public Health and Safety of Future Food Supply in Europe: Challenges and AI-Driven Solutions. European Journal of Sustainable Development Research 2025. [CrossRef] [PubMed]
- US EPA. Technical Brief: PFAS Soil Remediation; Technical Report EPA-820-F-20-XXX; US Environmental Protection Agency, 2020. [Google Scholar]
- Adamopoulos, I.; Valamontes, A.; Tsirkas, P.; Dounias, G. Predicting Workplace Hazard, Stress and Burnout Among Public Health Inspectors: An AI-Driven Analysis in the Context of Climate Change. Eur. J. Investig. Health Psychol. Educ. 2025, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.C.; Dai, M.; Sun, J.M.; Sunderland, E.M. The utility of machine learning models for predicting chemical contaminants in drinking water: Promise, challenges, and opportunities. Current Environmental Health Reports 2023, 10, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Breitmeyer, S.E.; Williams, A.M.; Conlon, M.D.; Wertz, T.A.; Heflin, B.C.; Shull, D.R.; Duris, J.W. Predicted potential for aquatic exposure effects of per- and polyfluorinated alkyl substances (PFAS) in Pennsylvania’s statewide network of streams. Toxics 2024, 12, 921. [Google Scholar] [CrossRef] [PubMed]
- Tokranov, A.K.; Ransom, K.M.; Bexfield, L.M.; Lindsey, B.D.; Watson, E.; Dupuy, D.I.; Stackelberg, P.E.; Fram, M.S.; Voss, S.A.; Kingsbury, J.A.; Jurgens, B.C.; Smalling, K.L.; Bradley, P.M. Predictions of groundwater PFAS occurrence at drinking water supply depths in the United States. Science 2024, 386, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.; Park, S.; Mahajan, S.; Zhou, J.; Blotevogel, J.; Li, Y.; Tong, T.; Chen, Y. Elucidating governing factors of PFAS removal by polyamide membranes using machine learning and molecular simulations. Nature Communications 2024, 15, 10918. [Google Scholar] [CrossRef] [PubMed]
- Lazova-Borisova, İ.; Adamopoulos, I.P. Compliance of carminic acid application with european legislation for food safety and public health. International Journal of Agricultural and Natural Sciences 2024, 17, 89–99. [Google Scholar] [CrossRef]
- Adamopoulos, I.; Syrou, N.; Mpourazanis, G.; Constantinidis, T.C.; Dounias, G. The Association of the Global Climate Crisis with Environmental Risks and the Impact of Heat Stress on Occupational Safety, Health, and Hygiene. Med. Sci. Forum 2025, 33, 2. [Google Scholar] [CrossRef]
- Adamopoulos, I.; Frantzana, A.; Adamopoulou, J.; Syrou, N. Climate Change and Adverse Public Health Impacts on Human Health and Water Resources. Environ. Sci. Proc. 2023, 26, 178. [Google Scholar] [CrossRef]
- Adamopoulos, I.; Syrou, N. Climate Change, Air Pollution, African Dust Impacts on Public Health and Sustainability in Europe. European Journal of Public Health 2024, 34 (Suppl. 3), ckae144.1374. [Google Scholar] [CrossRef]
- Adamopoulos, I.P.; Syrou, N.F.; Adamopoulou, J.P.; Mijwil, M.M. Conventional water resources associated with climate change in the Southeast Mediterranean and the Middle East countries. EUR J SUSTAIN DEV RES. 2024, 8, em0265. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




