Submitted:
09 July 2025
Posted:
11 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Whole Genome Sequencing Analysis
2.2. Collateral Susceptibility to Carbapenems in the FDC-Resistant Mutant
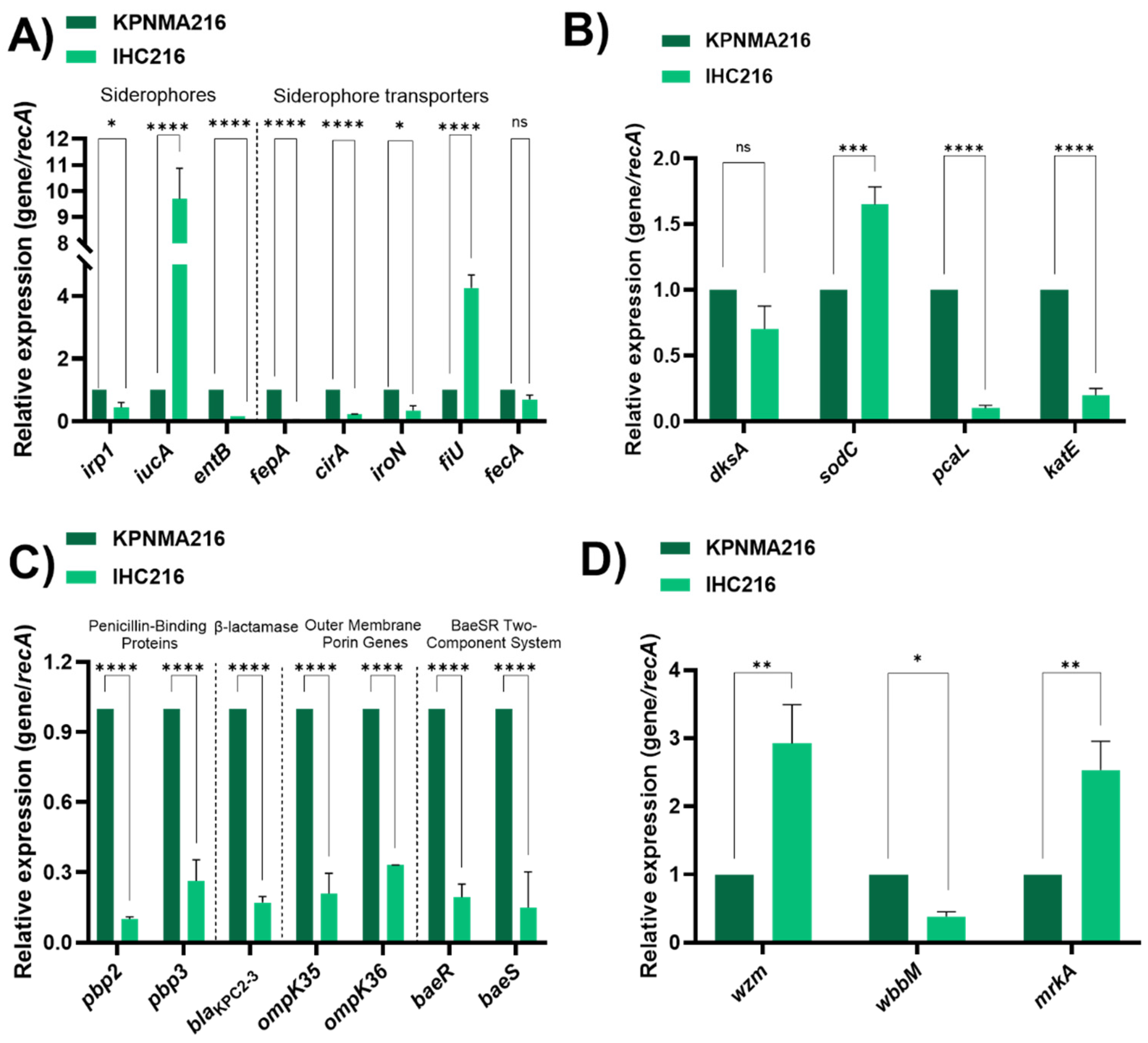
2.3. Molecular and Transcriptional Adaptations Underlying FDC Resistance in the IHC216 Mutant
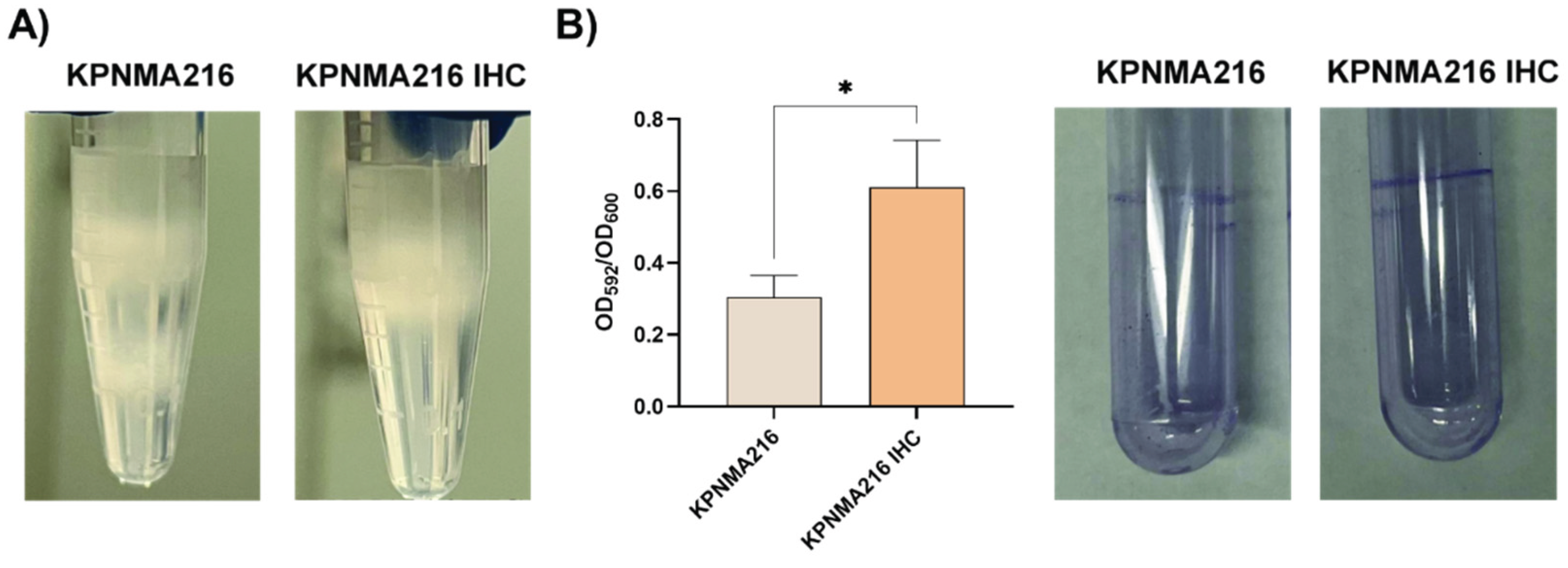
2.4. Increase Capsule and Biofilm Formation Was Seen in the FDC-Resistant Mutant
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Whole Genome Sequencing Analysis
4.3. RNA Extraction and Transcriptional Analysis Using Quantitative RT-qPCR
4.4. Antimicrobial Susceptibility Testing
4.5. Capsule and Biofilm
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kerneis, S.; Lucet, J.C.; Santoro, A.; Meschiari, M. Individual and collective impact of Klebsiella pneumoniae carbapenemase (KPC)-producing K. Pneumoniae Patients Admit. ICU. J Antimicrob Chemother. 2021, 76, i19–i26. [Google Scholar]
- Li, T.; Zhu, Y.; Xiang, G.; Xu, Z.; Yang, H.; Li, M.; et al. Adaptive evolution of extensive drug resistance and persistence in epidemic ST11 KPC-producing Klebsiella pneumoniae during antimicrobial chemotherapy. Antimicrob Agents Chemother. 2025, 69, e0123524. [Google Scholar] [CrossRef]
- Lee, G.C.; Burgess, D.S. Treatment of Klebsiella pneumoniae carbapenemase (KPC) infections: a review of published case series and case reports. Ann Clin Microbiol Antimicrob. 2012, 11, 32. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Golden, A.R.; Zelenitsky, S.; Wiebe, K.; Lawrence, C.K.; Adam, H.J.; et al. Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Drugs. 2019, 79, 271–89. [Google Scholar] [CrossRef]
- Bianco, G.; Boattini, M.; Cricca, M.; Diella, L.; Gatti, M.; Rossi, L.; et al. Updates on the Activity, Efficacy and Emerging Mechanisms of Resistance to Cefiderocol. Curr Issues Mol Biol. 2024, 46, 14132–53. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.A.; Cointe, A.; Jacquier, H.; Choudhury, A.; Magnan, M.; Courroux, C.; et al. Cross-resistance to cefiderocol and ceftazidime-avibactam in KPC beta-lactamase mutants and the inoculum effect. Clin Microbiol Infect. 2021, 27, 1172–e7. [Google Scholar] [CrossRef]
- Islam, M.M.; Jung, D.E.; Shin, W.S.; Oh, M.H. Colistin Resistance Mechanism and Management Strategies of Colistin-Resistant Acinetobacter baumannii Infections. Pathogens.
- Karakonstantis, S.; Rousaki, M.; Kritsotakis, E.I. Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance. Antibiotics (Basel).
- Bianco, G.; Boattini, M.; Iannaccone, M.; Cavallo, R.; Costa, C. Bloodstream infection by two subpopulations of Klebsiella pneumoniae ST1685 carrying KPC-33 or KPC-14 following ceftazidime/avibactam treatment: considerations regarding acquired heteroresistance and choice of carbapenemase detection assay. J Antimicrob Chemother. 2020, 75, 3075–6. [Google Scholar] [CrossRef]
- Ding, L.; Shen, S.; Chen, J.; Tian, Z.; Shi, Q.; Han, R.; et al. Klebsiella pneumoniae carbapenemase variants: the new threat to global public health. Clin Microbiol Rev. 2023, 36, e0000823. [Google Scholar] [CrossRef]
- Lin, T.L.; Lee, C.Z.; Hsieh, P.F.; Tsai, S.F.; Wang, J.T. Characterization of integrative and conjugative element ICEKp1-associated genomic heterogeneity in a Klebsiella pneumoniae strain isolated from a primary liver abscess. J Bacteriol. 2008, 190, 515–26. [Google Scholar] [CrossRef]
- Goetz, D.H.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002, 10, 1033–43. [Google Scholar] [CrossRef]
- Bachman, M.A.; Miller, V.L.; Weiser, J.N. Mucosal lipocalin 2 has pro-inflammatory and iron-sequestering effects in response to bacterial enterobactin. PLoS Pathog. 2009, 5, e1000622. [Google Scholar] [CrossRef]
- Polani, R.; De Francesco, A.; Tomolillo, D.; Artuso, I.; Equestre, M.; Trirocco, R.; et al. Cefiderocol Resistance Conferred by Plasmid-Located Ferric Citrate Transport System in KPC-Producing Klebsiella pneumoniae. Emerg Infect Dis. 2025, 31, 123–4. [Google Scholar] [CrossRef]
- Lan, P.; Lu, Y.; Liao, W.; Yu, Y.; Fu, Y.; Zhou, J. Cefiderocol-resistant hypervirulent Klebsiella pneumoniae with CirA deficiency and co-production of KPC-2 and SHV-12. Clin Microbiol Infect. 2024. [CrossRef] [PubMed]
- Kumar, A.; Chakravorty, S.; Yang, T.; Russo, T.A.; Newton, S.M.; Klebba, P.E. Siderophore-mediated iron acquisition by Klebsiella pneumoniae. J Bacteriol. 2024, 206, e0002424. [Google Scholar] [CrossRef] [PubMed]
- Tsuka, T.; Kumashiro, S.; Kihara, T.; Iida, T. Correlation between Polymerase Chain Reaction Identification of Iron Acquisition Genes and an Iron-Deficient Incubation Test for Klebsiella pneumoniae Isolates from Bovine Mastitis. Microorganisms.2022;10.
- Daoud, L.; Al-Marzooq, F.; Moubareck, C.A.; Ghazawi, A.; Collyns, T. Elucidating the effect of iron acquisition systems in Klebsiella pneumoniae on susceptibility to the novel siderophore-cephalosporin cefiderocol. PLoS One. 2022, 17, e0277946. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, R.P.; Sullivan, G.J.; Adams, F.G.; Shah, B.S.; Hawkey, J.; Delgado, N.; et al. DksA is a conserved master regulator of stress response in Acinetobacter baumannii. Nucleic Acids Res. 2023, 51, 6101–19. [Google Scholar] [CrossRef]
- Kim, N.; Son, J.H.; Kim, K.; Kim, H.J.; Kim, Y.J.; Shin, M.; et al. Global regulator DksA modulates virulence of Acinetobacter baumannii. Virulence. 2021, 12, 2750–63. [Google Scholar] [CrossRef]
- Switala, J.; Triggs-Raine, B.L.; Loewen, P.C. Homology among bacterial catalase genes. Can J Microbiol. 1990, 36, 728–31. [Google Scholar] [CrossRef]
- Carvalho, I.; Chenouf, N.S.; Carvalho, J.A.; Castro, A.P.; Silva, V.; Capita, R.; et al. Multidrug-resistant Klebsiella pneumoniae harboring extended spectrum beta-lactamase encoding genes isolated from human septicemias. PLoS One. 2021, 16, e0250525. [Google Scholar] [CrossRef]
- Ejaz, H. Analysis of diverse beta-lactamases presenting high-level resistance in association with OmpK35 and OmpK36 porins in ESBL-producing Klebsiella pneumoniae. Saudi J Biol Sci. 2022, 29, 3440–7. [Google Scholar] [CrossRef]
- Li, Y.; Ni, M. Regulation of biofilm formation in Klebsiella pneumoniae. Front Microbiol. 2023, 14, 1238482. [Google Scholar] [CrossRef] [PubMed]
- Shebl, R.I.; Elkhatib, W.F.; Badawy, M. Modulating the transcriptomic profile of multidrug-resistant Klebsiella pneumoniae biofilm formation by antibiotics in combination with zinc sulfate. Ann Clin Microbiol Antimicrob. 2023, 22, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Liu, Y.; Xu, M.; Yao, Z.; Zhang, X.; et al. Effects of chlorogenic acid on antimicrobial, antivirulence, and anti-quorum sensing of carbapenem-resistant Klebsiella pneumoniae. Front Microbiol. 2022, 13, 997310. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Polo, J.A.; Hernandez-Garcia, M.; Morosini, M.I.; Perez-Viso, B.; Soriano, C.; De Pablo, R.; et al. Outbreak by KPC-62-producing ST307 Klebsiella pneumoniae isolates resistant to ceftazidime/avibactam and cefiderocol in a university hospital in Madrid, Spain. J Antimicrob Chemother. 2023, 78, 1259–64. [Google Scholar] [CrossRef]
- Findlay, J.; Bianco, G.; Boattini, M.; Nordmann, P. High-level cefiderocol and ceftazidime/avibactam resistance in KPC-producing Klebsiella pneumoniae associated with mutations in KPC and the sensor histidine kinase EnvZ. J Antimicrob Chemother. 2025, 80, 1155–7. [Google Scholar] [CrossRef]
- Yang, C.; Wang, L.; Lv, J.; Wen, Y.; Gao, Q.; Qian, F.; et al. Effects of different carbapenemase and siderophore production on cefiderocol susceptibility in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2024, 68, e0101924. [Google Scholar] [CrossRef]
- Roemhild, R.; Andersson, D.I. Mechanisms and therapeutic potential of collateral sensitivity to antibiotics. PLoS Pathog. 2021, 17, e1009172. [Google Scholar] [CrossRef]
- Pournaras, S.; Kristo, I.; Vrioni, G.; Ikonomidis, A.; Poulou, A.; Petropoulou, D.; et al. Characteristics of meropenem heteroresistance in Klebsiella pneumoniae carbapenemase (KPC)-producing clinical isolates of K. pneumoniae. J Clin Microbiol. 2010, 48, 2601–4. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiology. 2008, 6, 199–210. [Google Scholar] [CrossRef]
- De Serrano, L.O.; Camper, A.K.; Richards, A.M. An overview of siderophores for iron acquisition in microorganisms living in the extreme. Biometals. 2016, 29, 551–71. [Google Scholar] [CrossRef]
- Page, M.G.P. The Role of Iron and Siderophores in Infection, and the Development of Siderophore Antibiotics. Clin Infect Dis. 2019, 69, S529–S37. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Pan, D.; Li, M.; Wang, Y.; Song, L.; Yu, D.; et al. Aerobactin-Mediated Iron Acquisition Enhances Biofilm Formation, Oxidative Stress Resistance, and Virulence of Yersinia pseudotuberculosis. Front Microbiol. 2021, 12, 699913. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Tapscott, T.; Fitzsimmons, L.F.; Liu, L.; Reyes, A.M.; Libby, S.J.; et al. Redox-Active Sensing by Bacterial DksA Transcription Factors Is Determined by Cysteine and Zinc Content. mBio. 2016, 7, e02161–e15. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, C.Y. Generally Stressed Out Bacteria: Environmental Stress Response Mechanisms in Gram-Positive Bacteria. Integr Comp Biol. 2020, 60, 126–33. [Google Scholar] [CrossRef]
- Sun, F.; Liang, H.; Kong, X.; Xie, S.; Cho, H.; Deng, X.; et al. Quorum-sensing agr mediates bacterial oxidation response via an intramolecular disulfide redox switch in the response regulator AgrA. Proc Natl Acad Sci U S A. 2012, 109, 9095–100. [Google Scholar] [CrossRef]
- Raffatellu, M.; Chessa, D.; Wilson, R.P.; Tukel, C.; Akcelik, M.; Baumler, A.J. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infect Immun. 2006, 74, 19–27. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; An, H.; Wang, J.; Ding, M.; Wang, L.; et al. Capsule type defines the capability of Klebsiella pneumoniae in evading Kupffer cell capture in the liver. PLoS Pathog. 2022, 18, e1010693. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014, 30, 2068–9. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernandez-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol Biol Evol. 2021, 38, 5825–9. [Google Scholar] [CrossRef]
- Lam, M.M.C.; Wick, R.R.; Watts, S.C.; Cerdeira, L.T.; Wyres, K.L.; Holt, K.E. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat Commun. 2021, 12, 4188. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001, 25, 402–8. [Google Scholar] [CrossRef]
- (CLSI), C.L.S.I. Performance standards for antimicrobial susceptibility testing: Thirty Edition informational supplement. CLSI Document M100‐S30:2020. Clinical Lab Standards Institute, 2020. [Google Scholar]
- Valcek, A.; Philippe, C.; Whiteway, C.; Robino, E.; Nesporova, K.; Bove, M.; et al. Phenotypic Characterization and Heterogeneity among Modern Clinical Isolates of Acinetobacter baumannii. Microbiol Spectr. 2023, 11, e0306122. [Google Scholar] [CrossRef]
- Martinez, J.; Fernandez, J.S.; Liu, C.; Hoard, A.; Mendoza, A.; Nakanouchi, J.; et al. Human pleural fluid triggers global changes in the transcriptional landscape of Acinetobacter baumannii as an adaptive response to stress. Sci Rep. 2019, 9, 17251. [Google Scholar] [CrossRef]
- Mezcord, V.; Escalante, J.; Nishimura, B.; Traglia, G.M.; Sharma, R.; Valle, Q.; et al. Induced Heteroresistance in Carbapenem-Resistant Acinetobacter baumannii (CRAB) via Exposure to Human Pleural Fluid (HPF) and Its Impact on Cefiderocol Susceptibility. International journal of molecular sciences. 2023, 24. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





