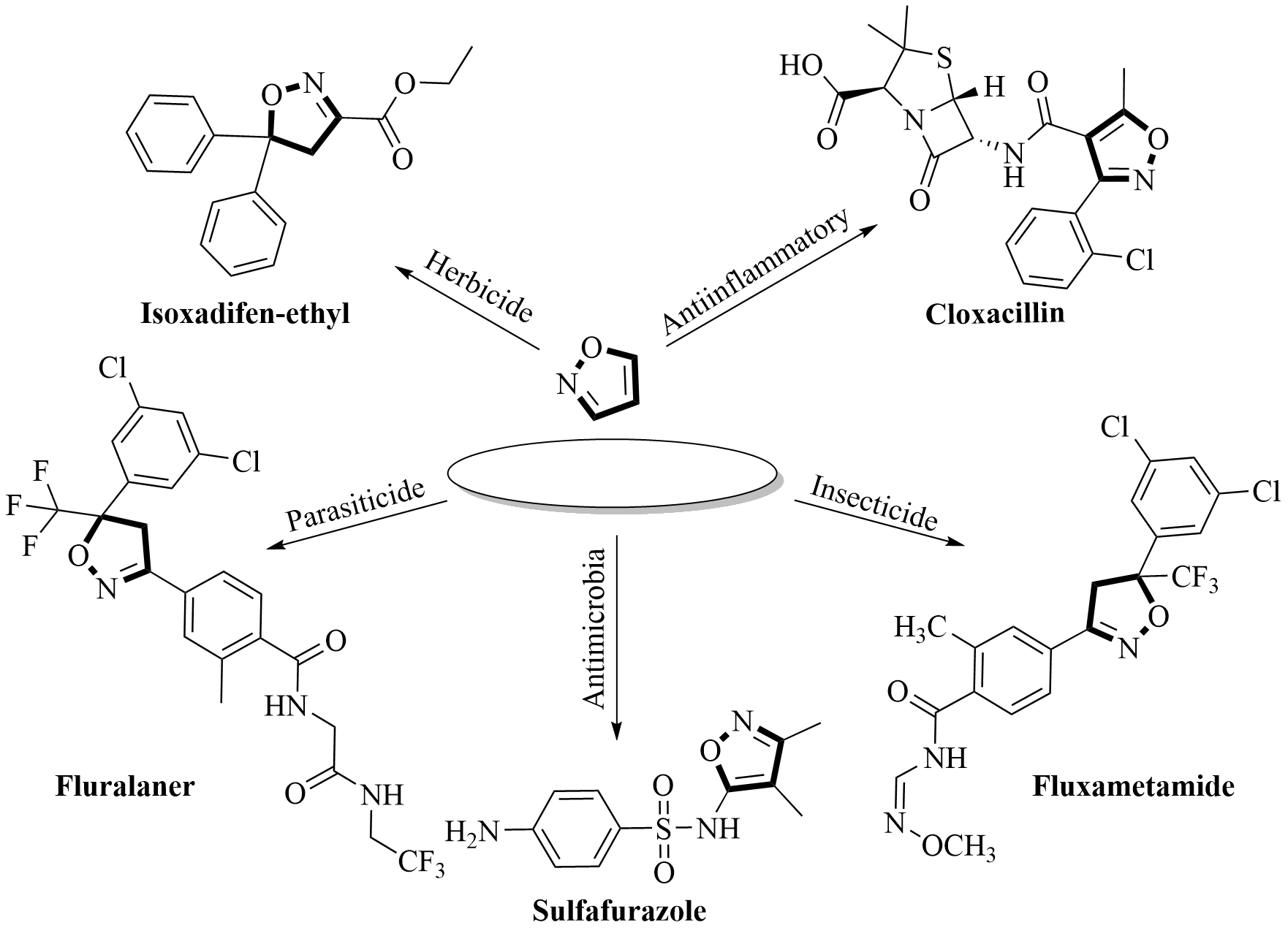
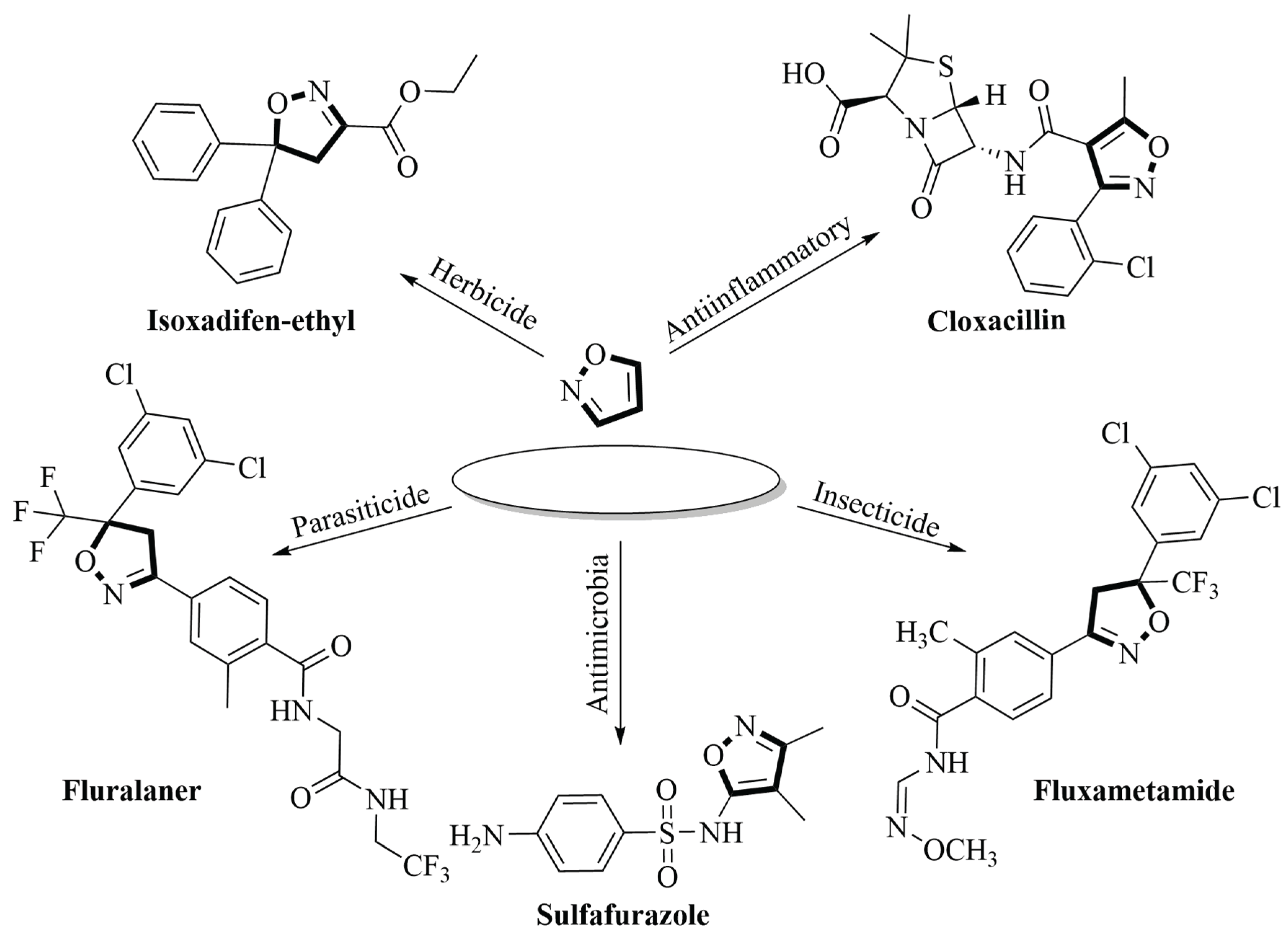
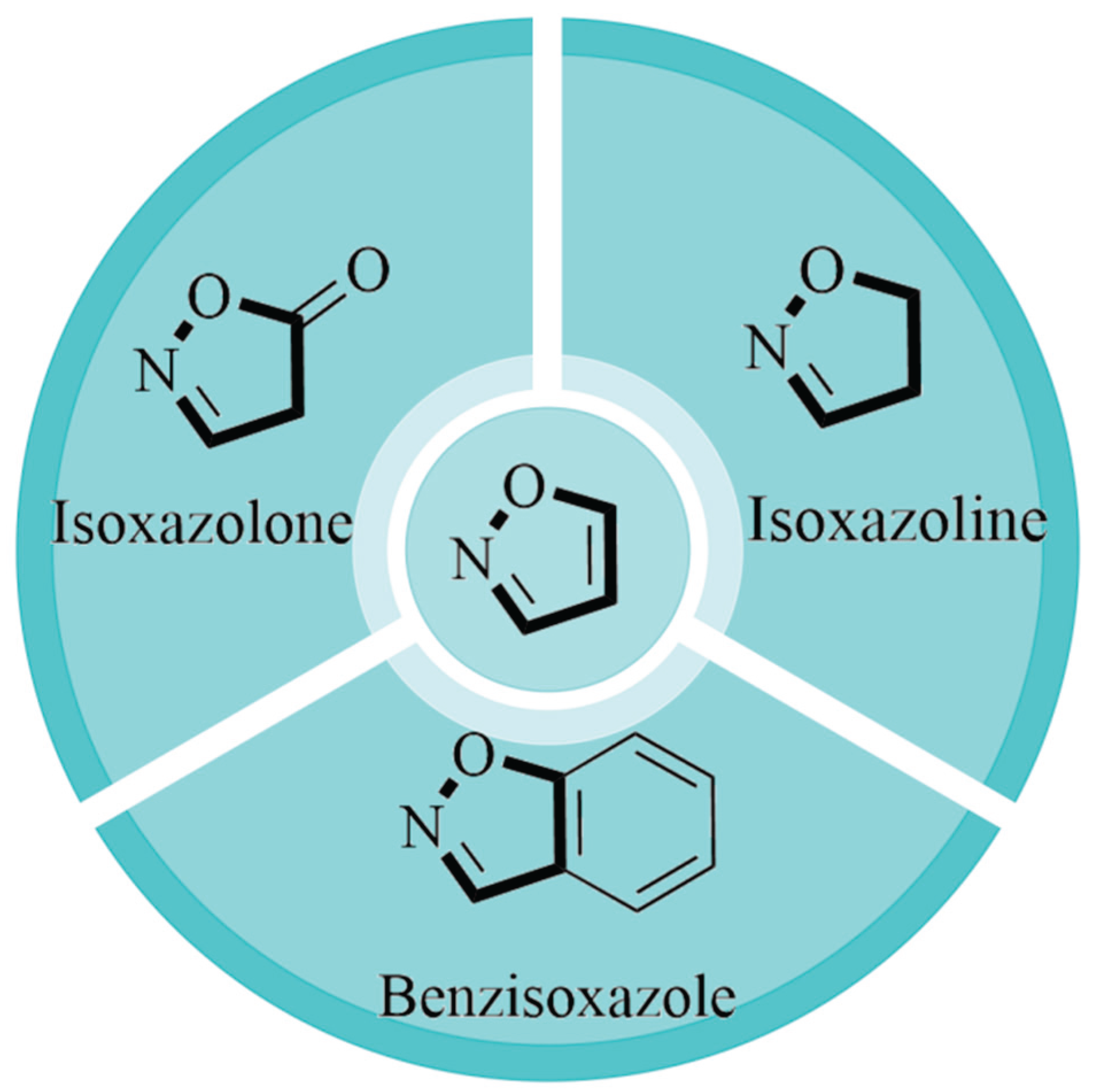
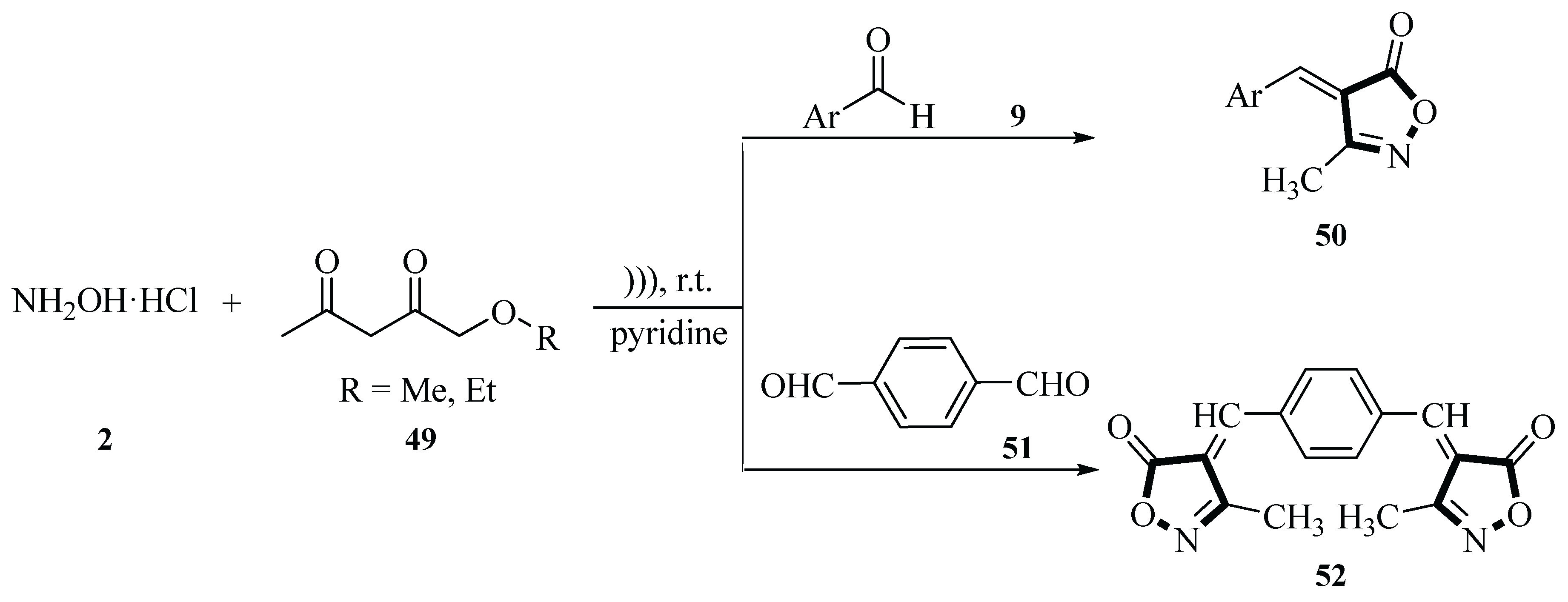
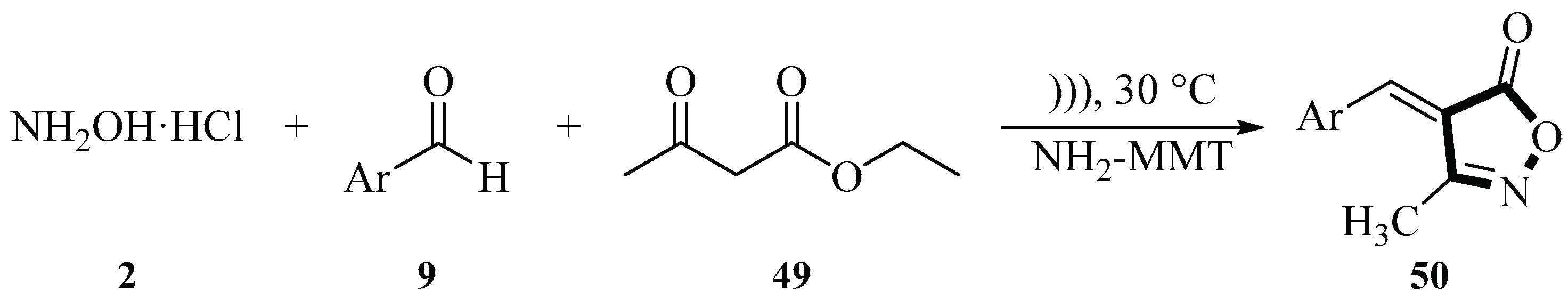
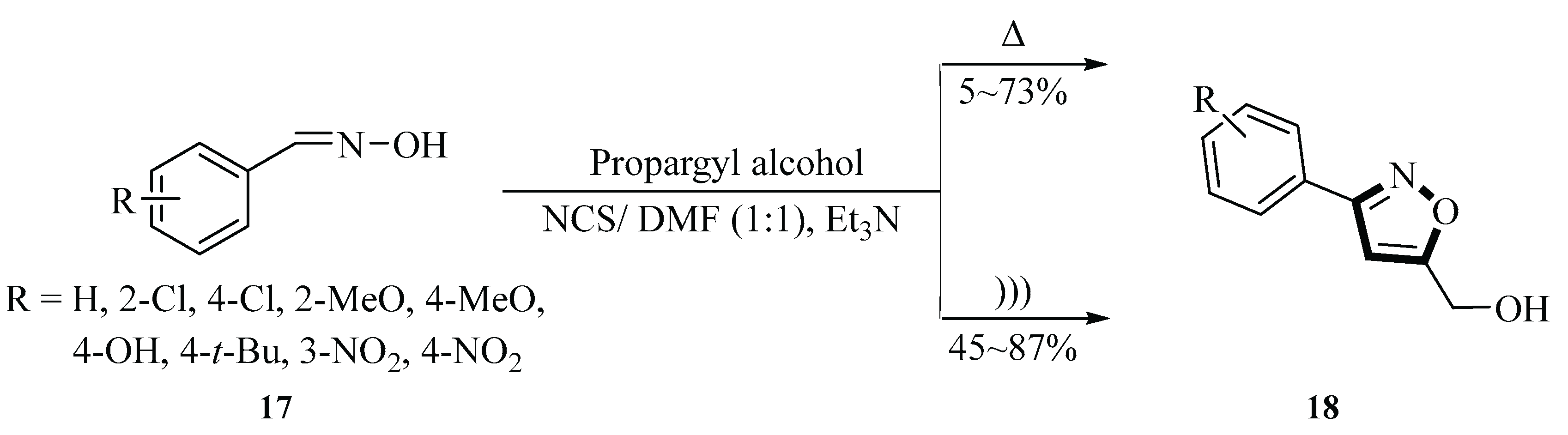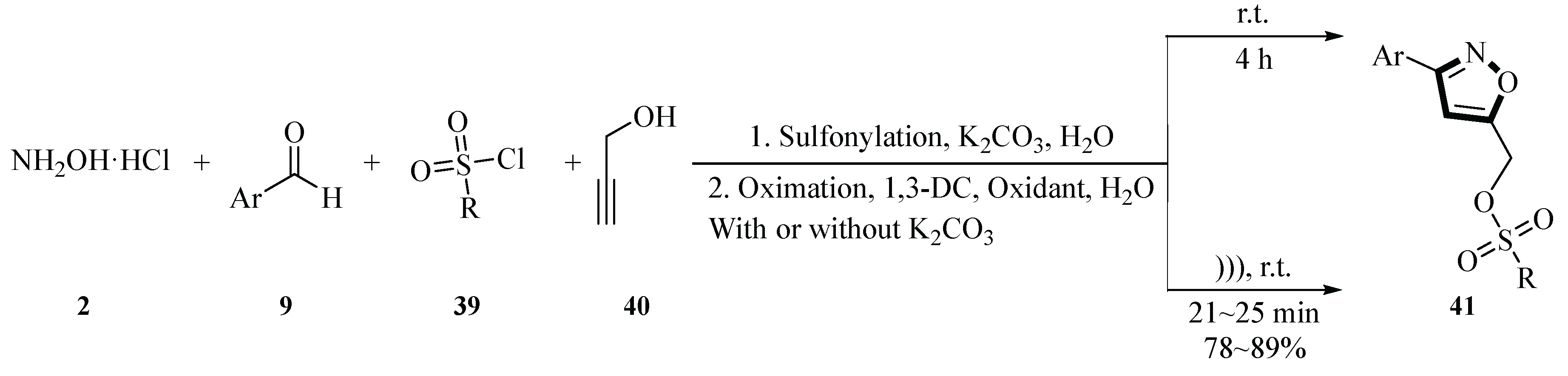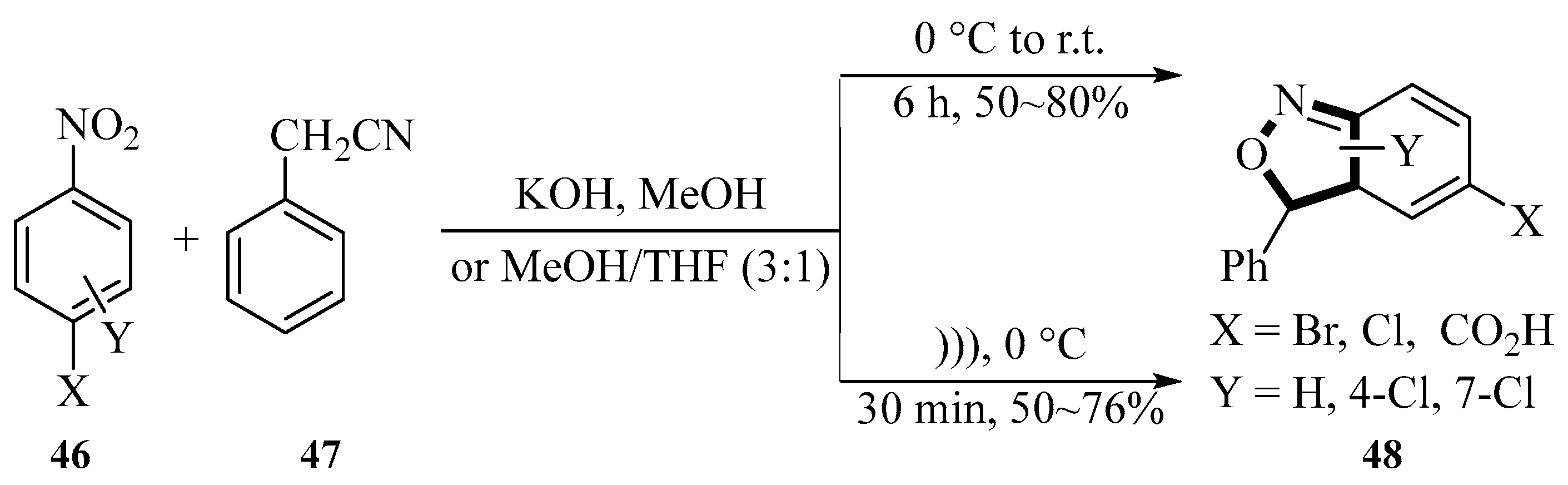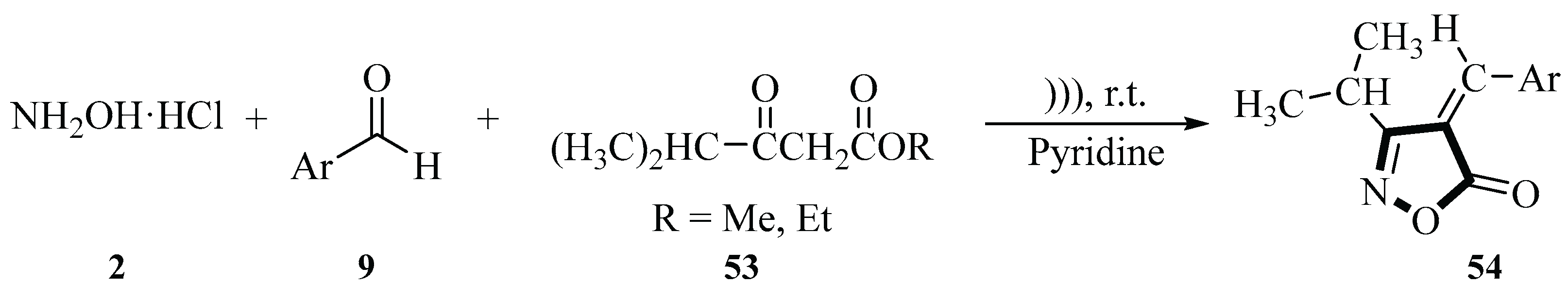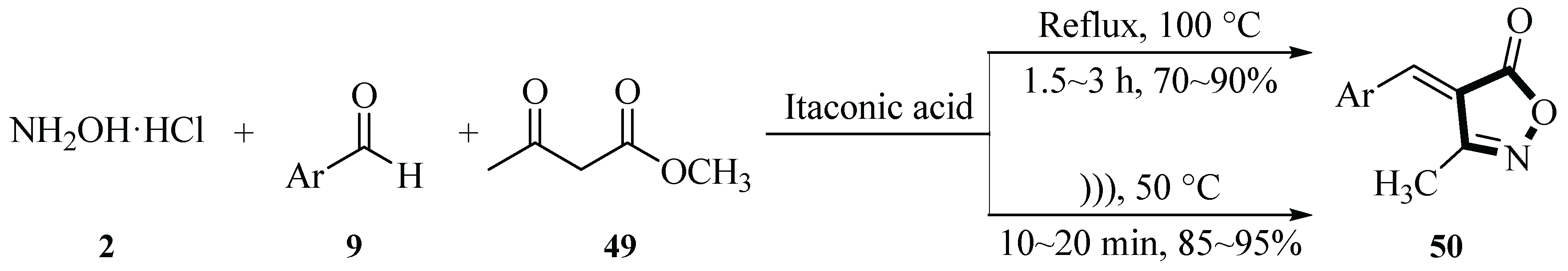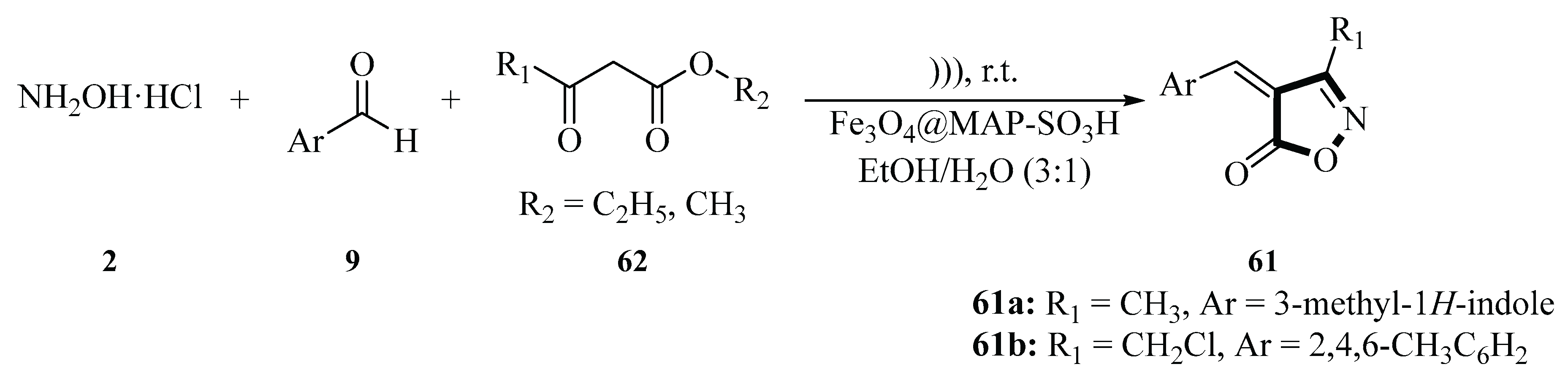2.1. Synthesis of Isoxazole-Based Derivatives
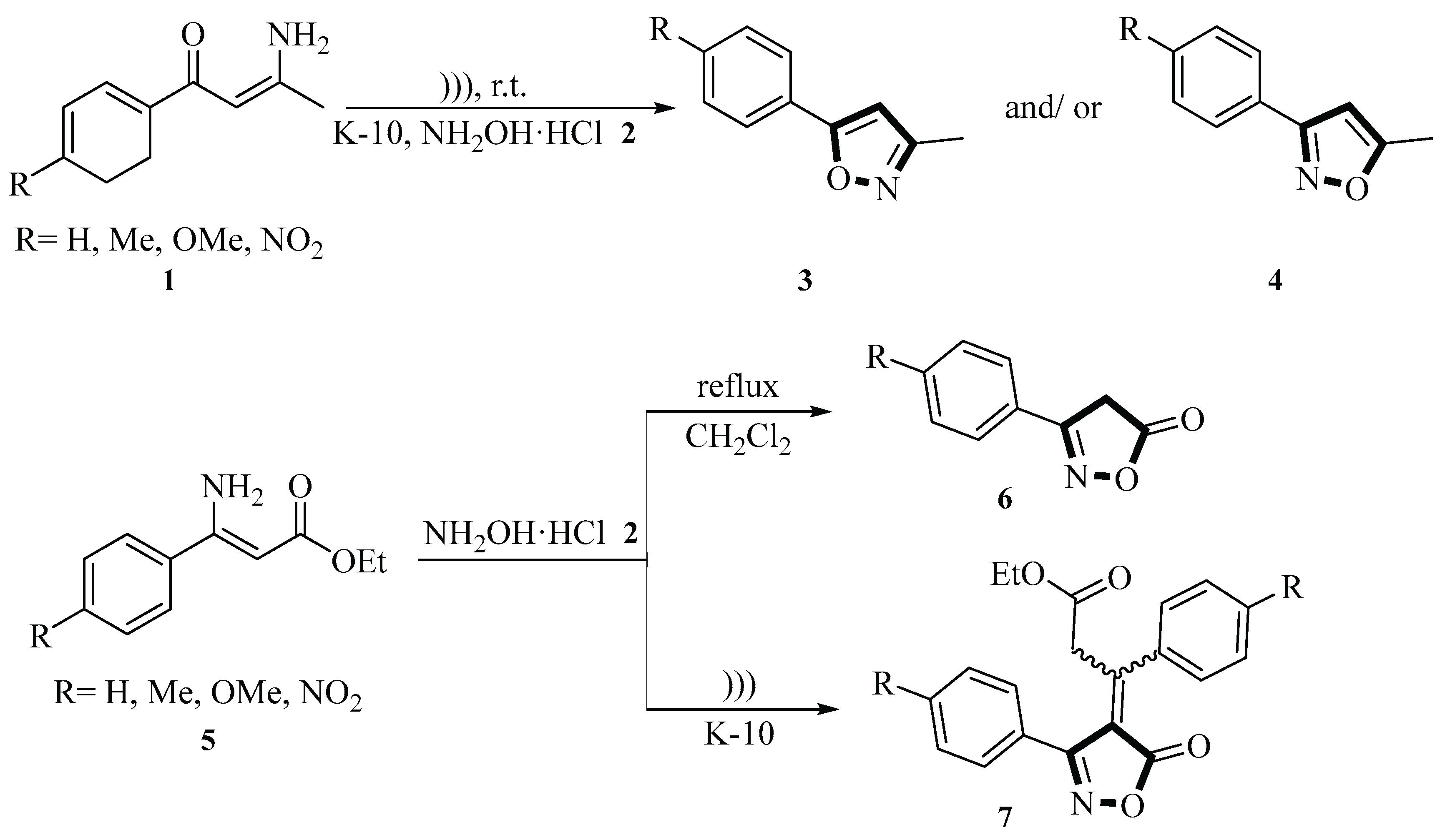
In 1999, the Valduga group reported a method in which a series of β-enamino compounds
1 and
5 underwent cyclization and addition reactions under mild conditions, catalyzed by montmorillonite K-10(MMT-K10), to generate 3-methyl-5-phenylisoxazole derivatives
3 and/or
4, 5-methyl-3-phenylisoxazole derivatives
6, and ethyl 3-[(4-phenyl-substituted)-5-oxo-4,5-dihydro-4-isoxazolyliden]-3-(4-phenyl-substituted)propanoates
7. The reactions were carried out in dichloromethane under ultrasound irradiation (50~60 Hz, 110/220 V, 1.0 A) for 3~6 h at room temperature. The yields of the products varied depending on the substrate and reaction conditions, ranging from 89% to 99% for isoxazoles
3 and/or
4 and 56% to 80% for 5-oxoisoxazolylidene derivatives
6 and
7. Notably, the use of K-10 and ultrasound significantly improved the reaction efficiency compared with traditional reflux methods, which required 20 h and often resulted in lower yields or incomplete reactions. This methodology provides a novel and efficient approach for the preparation of isoxazole and 5-isoxazolone derivatives, which are valuable intermediates for further organic synthesis. The strategy provides important insights into the reaction mechanisms and product characterization, highlights the potential of ultrasound-assisted reactions in enhancing reaction rates and yields under mild conditions, and serves as a valuable reference for subsequent research in organic synthesis, particularly in the development of green and sustainable catalytic methods (
Scheme 1) [
28].
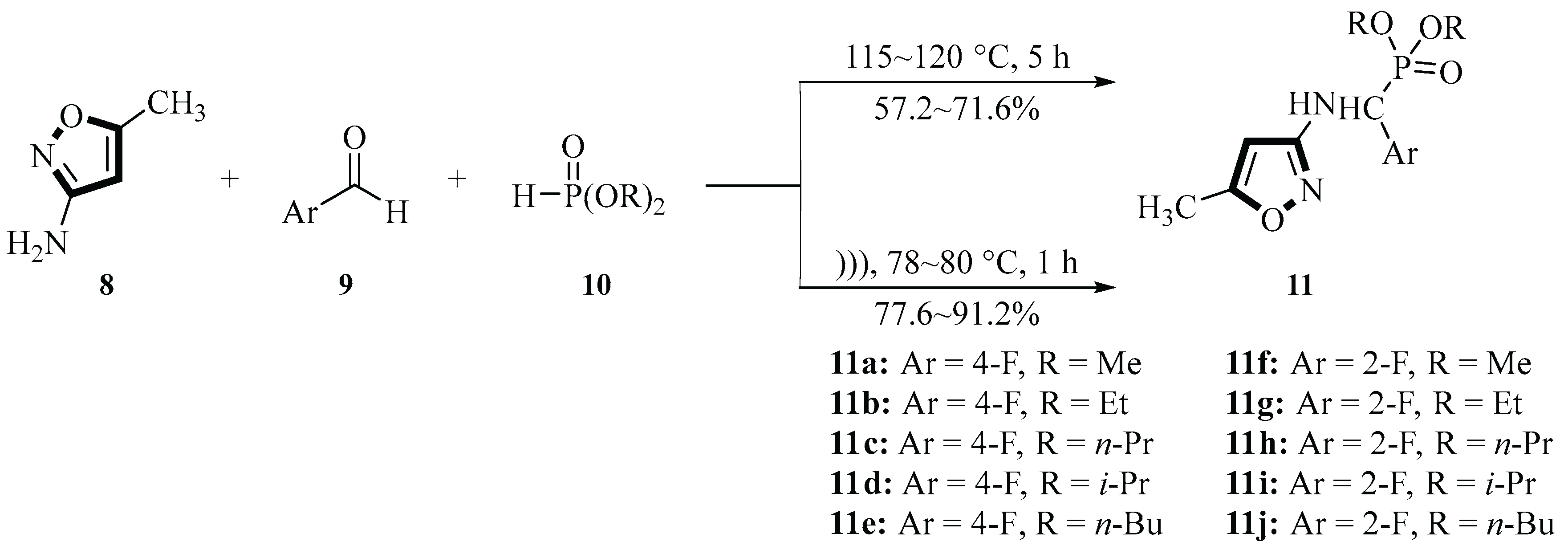
In 2005, the Song group successfully utilized 5-methylisoxazol-3-amine
8, 2-fluorobenzaldehyde or 4-fluorobenzaldehyde
9, and dialkyl phosphite
10 to synthesize a series of 1-[(5-methylisoxazol-3-yl)amino]-1-(2- or 4-fluorophenyl)methanephosphonate derivatives
11 under ultrasound irradiation. This reaction was based on a one-pot Mannich-type reaction conducted without solvent or catalyst. Ultrasonic irradiation (25 kHz, 500 W) significantly accelerated the reaction and improved the yield, increasing from 57.2%~71.6% under traditional heating (115~120 °C, 5 h) to 77.6%~91.2% under ultrasound conditions (78~80 °C, 1 h). The position of the fluorine substituent (2- or 4-) and the alkyl chain length influenced the yield. For the same fluorine substituent, the yield order was ortho > para. These compounds exhibited moderate anticancer activity against PC3 (prostate cancer) and A431 (epidermoid carcinoma) cells in MTT assays. Notably, the compounds
11a~
11e showed higher inhibition rates compared with
11f~
11j, with compound
11a demonstrating the strongest activity (78.3% and 69.0% inhibition of PC3 and A431 cells, respectively, at 10 µM). This synthesis method was simple, rapid, and high yielding, and marked the first report of anticancer bioactivity for these compounds (
Scheme 2) [
29]
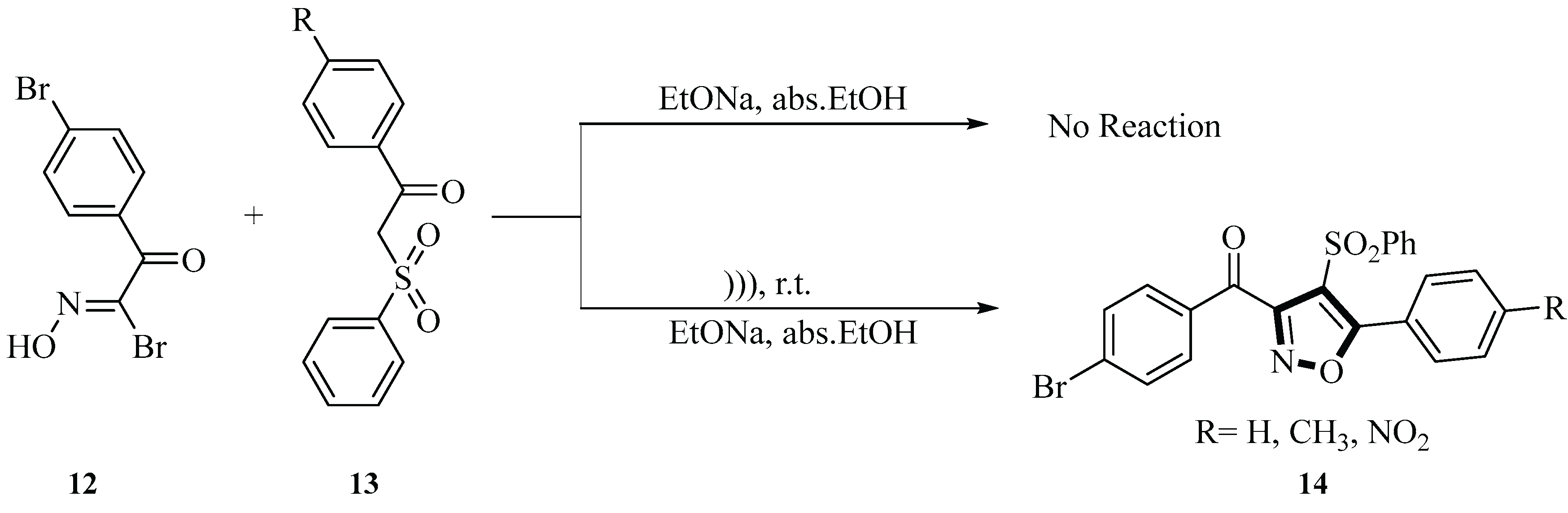
In 2009, Saleh et al. developed a method for synthesizing isoxazole derivatives
14 using 1-(4-bromophenyl)-2-bromo-2-hydroximinoethanone
12 and β-ketosulphone derivatives
13 under ultrasonic irradiation and with sodium ethoxide in absolute ethanol solution as a catalyst. The sodium ethoxide in ethanol solution acted as both a catalyst and a base in these reactions, facilitating the reaction by generating carbanion intermediates, neutralizing acidic by-products, and maintaining the alkaline environment of the reaction system. This step was crucial for the smooth progression and high yield of the reaction. The reaction was carried out at room temperature for approximately 45 min, with yields under ultrasound conditions ranging between 60% and 65%. The ultrasound-promoted reaction proved to be an effective method for synthesizing substituted isoxazoles containing sulfonyl groups. This approach not only reduced the reaction time but also improved the yield. The synthesized products exhibited high purity and did not require further purification (
Scheme 3) [
30].
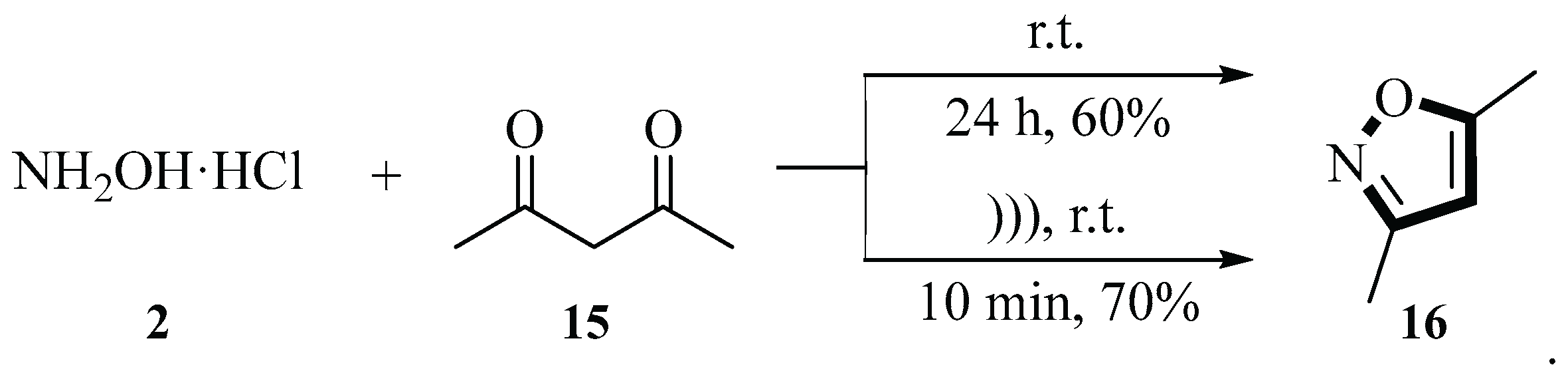
In 2009, the Silva group described a reaction for synthesizing 3,5-dimethylisoxazole
16 using 2,4-pentanedione
15 and hydroxylamine
2 under ultrasound conditions. Such reactions usually yielded only intermediates, but this process yielded the isoxazole product directly in one pot. This reaction was carried out under ultrasound irradiation for 10 min in aqueous media at room temperature, without the need for catalysts, acids, or a prolonged reaction time. They also conducted antioxidant activity tests on the product. The results showed that 3,5-dimethylisoxazole
16 did not exhibit significant antioxidant activity in either the oxygen radical absorbance capacity (ORAC) or 2,2-diphenylpicrylhydrazyl (DPPH) testing methods. This method not only shortened the reaction time (from 24 h under conventional conditions to 10 min) but also improved the yield (from 60% to 70%), providing a new and efficient approach for the synthesis of such heterocyclic derivatives (
Scheme 4) [
31].
In 2011, the Shen group reported the synthesis of (3-phenylisoxazol-5-yl) methanol derivatives
18 using benzaldehyde oxime
17,
N-chlorosuccinimide (NCS), dimethylformamide (DMF), triethylamine (Et
3N), and propargyl alcohol under ultrasound irradiation. NCS was used for the halogenation reaction, DMF served as the solvent, and Et
3N acted as the base. The reaction was carried out under ultrasonic conditions (250 W, 25 kHz) in one pot. The article also highlighted that the yield of electron-donating substituents on aromatic rings was higher than that of electron-withdrawing substituents. For example, when the substituent was a nitro group, the yield was very low. In contrast, when the substituent was an electron-donating group, the yield exceeded 70% under ultrasound conditions, especially when the hydroxyl group, which has strong electron-donating properties, was present, resulting in the highest product yield (87%). The yield of various (3-phenylisoxazol-5-yl)methanol derivatives
98 synthesized by the one-pot method under ultrasound irradiation ranged from 45% to 87%, with a reaction time of 90~270 min. Ultrasonic irradiation significantly accelerated the entire process, shortened the reaction time by over an hour, and increased the product yield by 14%~40% (
Scheme 5) [
32].
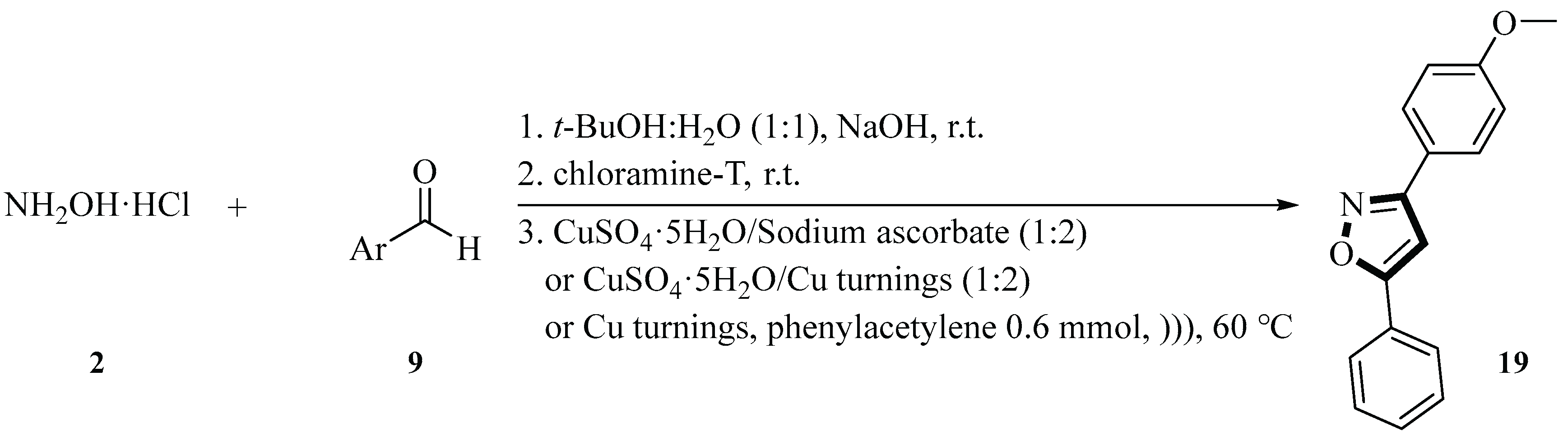
In 2014, the Koufaki group synthesized 3,5-disubstituted isoxazoles
19 using hydroxylamine hydrochloride
2, 4-methoxybenzaldehyde
9, chloramine-T, terminal alkyne, copper sulfate pentahydrate, and sodium ascorbate or copper turnings under ultrasound irradiation. The researchers employed a one-pot, three-step process based on a copper(I)-catalyzed cycloaddition reaction, where in situ-generated nitrile oxides (from corresponding aldehydes) react with alkynes. They used
t-BuOH/H
2O (1:1) at 60 ℃ for 60 min, and the ultrasound frequency was 40 kHz (ultrasound bath) and 20 kHz (probe). The experimental results indicated that the use of dual-frequency ultrasonic irradiation and copper catalyst could significantly improve the reaction efficiency and yield; in particular, the yield could reach 75% when the ultrasound bath was combined with the probe. Although the yield under metal-free conditions was low, it still had regioselectivity, indicating that this method had certain applicability under different conditions. By combining the copper turnings and copper sulfate pentahydrate as catalysts with the ultrasound bath and probe, the yield could reach 75% within 60 min (
Scheme 6) [
33].
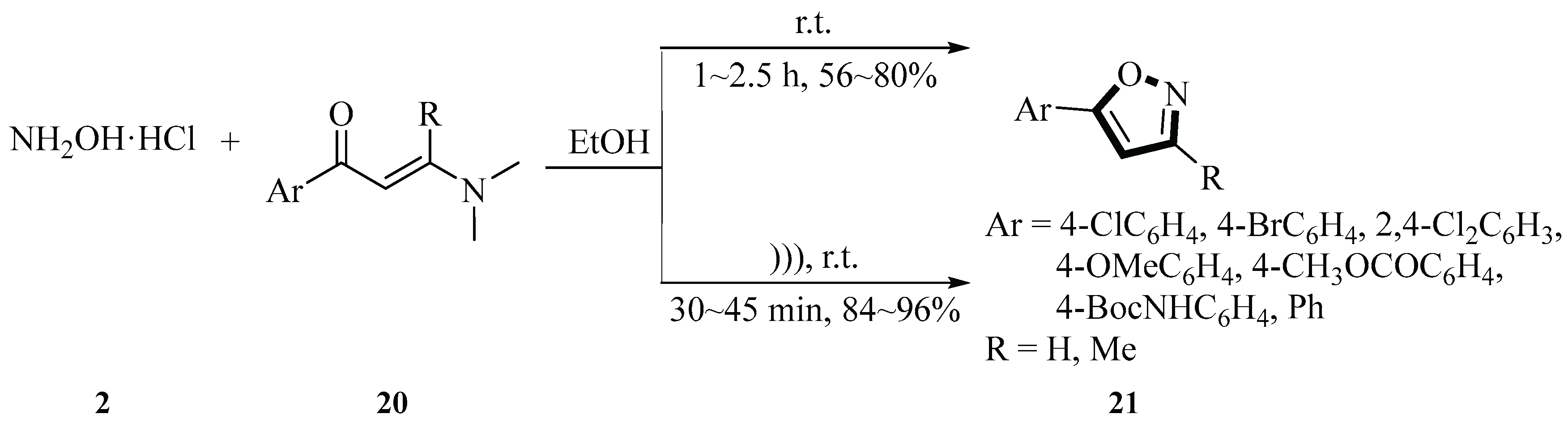
In 2014, Huang et al. synthesized a series of 5-arylisoxazole derivatives
21 via the reaction of 3-(dimethylamino)-1-arylprop-2-en-1-one
20 with hydroxylamine hydrochloride
2 under ultrasound irradiation using ethanol as the solvent. One of the most striking features of this reaction was that no catalyst was required. Under ultrasound irradiation, the yields of 5-arylisoxazole derivatives
21 ranged from 84% to 96%, whereas reactions without ultrasound irradiation provided lower yields (56%~80%). The reaction time was significantly shortened to 30~45 min under sonication, compared to 1~2.5 h under conventional thermal conditions. The reaction temperature was controlled by adjusting the water level in the ultrasonic bath (via addition or removal of H
2O), and the crude product was purified through recrystallization from ethanol. Under optimized conditions, various 5-arylisoxazole derivatives
21 were successfully synthesized, generating good yields regardless of whether the aromatic ring bore electron-withdrawing or electron-donating substituents. This method demonstrated advantages such as simple operation, mild reaction conditions, high yields, shorter reaction time, and an environmentally benign procedure (
Scheme 7) [
34].
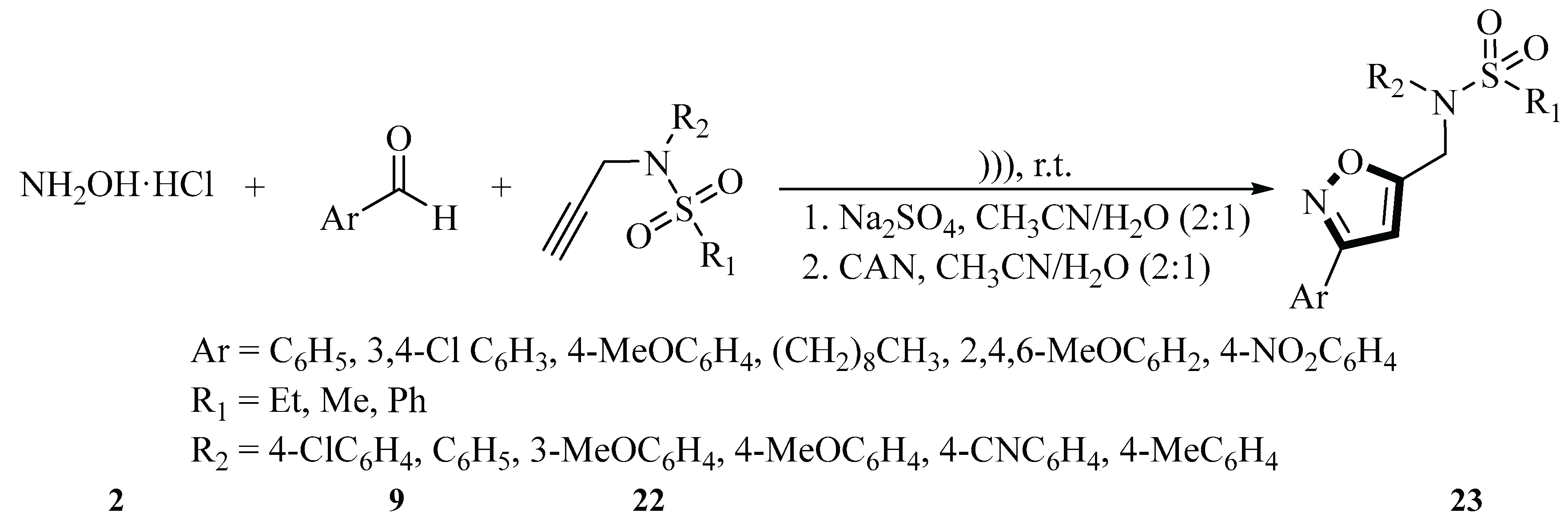
In 2017, Alaoui and colleagues reported the synthesis of a novel sulfonamide–isoxazole compound
23 using a one-pot reaction of various aldehydes
9 and alkynes–sulfonamides
22 under ultrasonic radiation. The reaction was carried out at room temperature using a solvent mixture of H
2O and acetonitrile (2:1 ratio), and ceric ammonium nitrate (CAN) was used as the key oxidizing agent. Hydroxylamine hydrochloride
2 was used as a precursor for imine formation, and sodium sulfate was used to facilitate the reaction. Under optimal reaction conditions, the sonication method could save 340 to 356 min compared with the conventional stirring method. For ultrasound assistance, two devices were used to increase the efficiency of the reaction: an ultrasonic scavenging bath (47 kHz) and an ultrasonic probe (20 kHz). The reaction could not proceed in the absence of an oxidizing agent, and when CAN was used as an oxidizing agent, the reaction in the ultrasonic scavenging bath took 20 min and yielded 88% of the product. The use of an ultrasonic probe reduced the reaction time to 4 min and increased the yield to 95%. Reducing the amount of CAN to 0.5 mmol resulted in incomplete conversion and lower yields. This one-pot synthesis strategy is carried out in an aqueous medium and uses inexpensive and environmentally friendly CAN as an oxidizing agent, in line with the principles of green chemistry. The process is simple, efficient and uses readily available starting materials, which are important for the further synthesis of 3,5-functionalized isoxazoles (
Scheme 8) [
35].
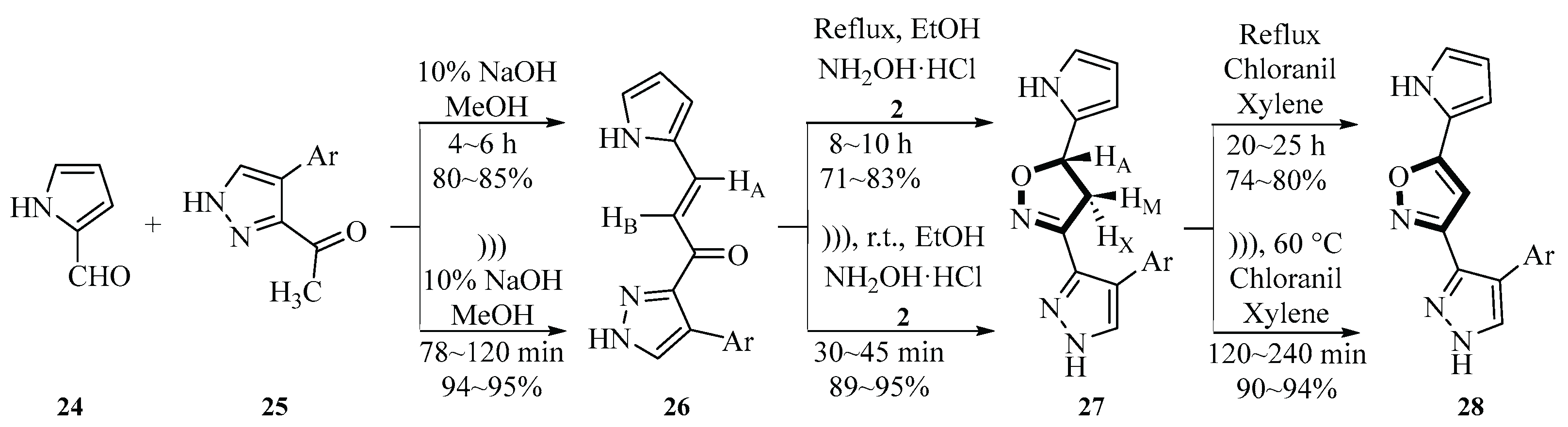
In 2018, the group used an ultrasound-assisted reaction of pyrrole-2-carboxaldehyde
24, 1-(4-aryl-1
H-pyrazol-3-yl)ethan-1-one
25, and hydroxylamine hydrochloride
2 to generate 3-(4-arylpyrazol-3-yl)-5-(pyrrol-2-yl)isoxazole
28 by using chloranil and xylene as mixed solvents. This was the first synthesis of a triheterocyclic system containing pyrrole, pyrazole, and isoxazole. They determined the antioxidant activity of the compounds
27 and
28 using DPPH, NO, and H
2O
2. Compound
28 exhibited significant activity in all three antioxidant activity tests, comparable to that of standard ascorbic acid, showing good antioxidant potential. The two studies exemplify a progression from methodological innovation to functionalized applications; the former focused on green processes while the latter explored structural diversity and bioactivity; together, they advanced sonochemistry in organic synthesis (
Scheme 9) [
36].
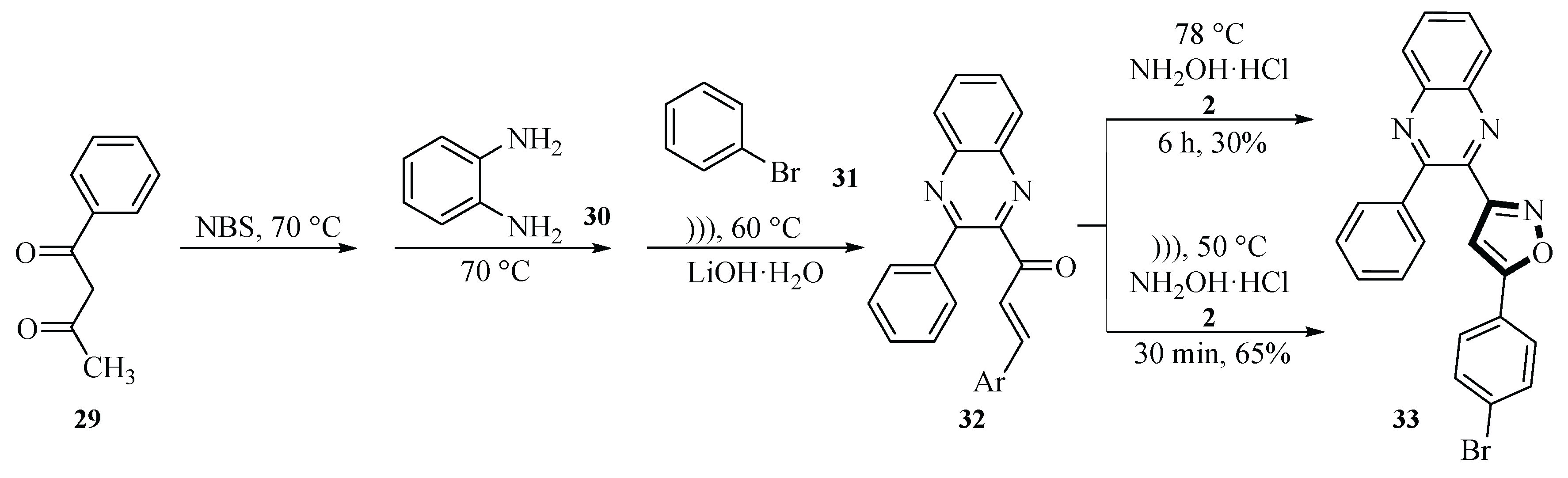
In 2017, the Salman group demonstrated the formation of isoxazole derivative
33, which was achieved by reacting chalcone-substituted quinoxaline derivative
32 with hydroxylamine hydrochloride
2 and sodium acetate under ultrasound irradiation. Hydroxylamine underwent a nucleophilic addition reaction with the α,β-unsaturated carbonyl groups of chalcone
29 to form an intermediate. The intermediate
32 was further cyclized to form an isoxazole ring. Ultrasound technology was used in two of these three reaction steps. The reaction process required heating to 50 ℃ in ethanol solvent for 85 min. The yield of the product obtained by this method was about 65%, which meant that the ultrasound-assisted method significantly improved the synthesis efficiency by about 35 percentage points. This strategy provided an efficient and green synthesis method, which has important reference value for the synthesis of nitrogen-containing heterocyclic compounds. The promotion of ultrasound-assisted synthesis technology is expected to be widely applied in the field of organic synthesis, especially in scenarios where reaction time needs to be shortened and product yield needs to be improved (
Scheme 10) [
37].
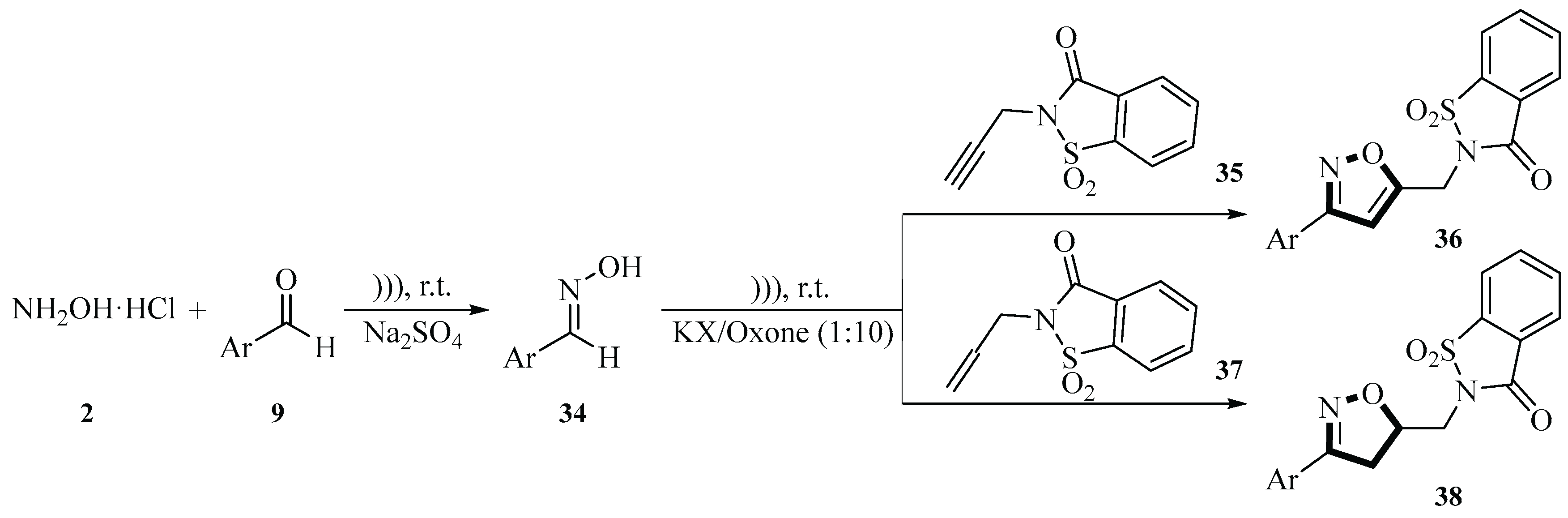
In 2019, the Tachallait group described a method for the preparation of novel
N-saccharin isoxazole derivative
36 and isoxazoline derivative
38 using KI/Oxone as a catalyst through a sonication-assisted cycloaddition reaction of hydroxylamine hydrochloride
2, aldehydes
9, and dipolarophiles promoted in water solvent. This is a one-pot three-step reaction utilizing in situ-generated nitrile oxides and dipolarophiles via a 1,3-dipolar cycloaddition reaction. The first step generates an oxime
34, the second generates a nitrile oxide, and the third undergoes a 1,3-dipolar cycloaddition to generate the target product
36 and
38. It is important to note that the first two steps do not require ultrasound, but the second step requires Oxone as the oxidizing agent. The third step in the reaction was carried out at room temperature for 30 min and the exact structure of the product depends on the aldehyde and olefin/alkyne substrate used. The yields ranged from 65% to 85% for isoxazoles
36 and 70% to 95% for isoxazoline
38, indicating that the synthetic method is highly efficient. This reflects the advantages of this green method for the synthesis of
N-sulfonamide isoxazole and isoxazoline derivatives. The method used H
2O as the solvent, avoiding the use of volatile organic solvents and conforming to the principles of green chemistry. The whole process was green, efficient, environmentally friendly, and sustainable (
Scheme 11) [
38].
In 2021, Talha and co-workers reported a one-pot four-component synthesis of 3,5-disubstitutedisoxazole-sulfonates and -sulfonamides
41 using hydroxylamine hydrochloride
2, aromatic aldehydes
9, sulfonyl chloride
39, and propargyl alcohol/amine
40 in water solvent under ultrasound irradiation. The reaction employed sodium dichloroisocyanurate (NaDCC) as a metal-free catalyst and oxidant, combined with ultrasonic activation (47 kHz) at room temperature, achieving completion within 20~28 min. This green methodology utilized H
2O as the solvent, avoided toxic organic reagents, and demonstrated broad substrate compatibility, with a product yield of 72%~89% depending on the substrate combination. The process proceeded via in situ sulfonylation, oximation, and 1,3-dipolar cycloaddition steps, generating four new bonds (S-O, C-N, C-O, and C-C). Compared with conventional magnetic stirring, ultrasound irradiation significantly enhanced reaction efficiency by improving mass transfer and reducing dimerization side reactions. This study not only provides an environmentally benign route for synthesizing isoxazole derivatives but also highlights the synergistic potential of ultrasound and heterogeneous catalysis in green chemistry and pharmaceutical development (
Scheme 12) [
39].
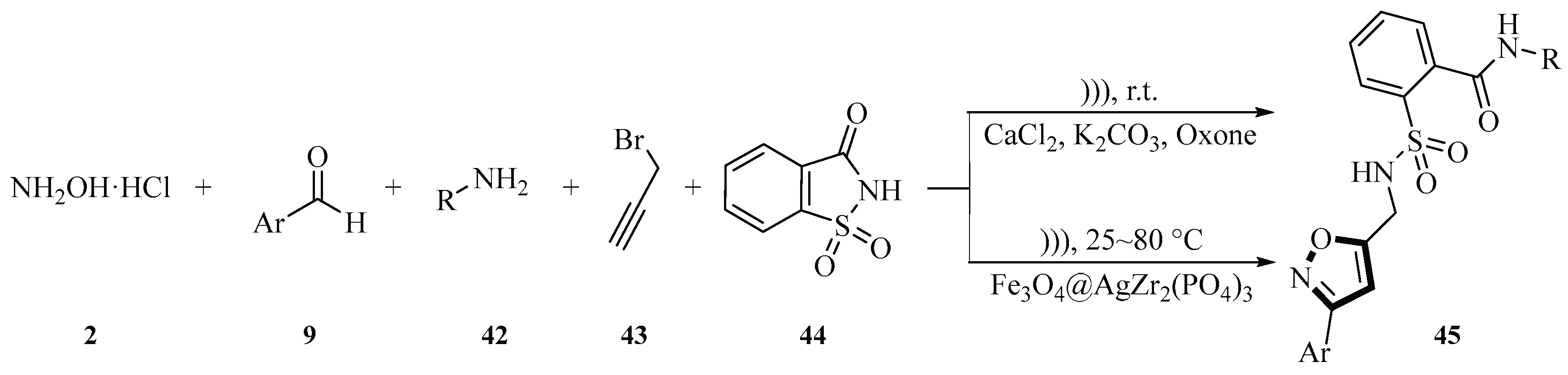
In recent years, ultrasound-assisted multicomponent reactions have emerged as powerful tools for sustainable heterocyclic synthesis. In 2022 and 2024, the Bougrin group published two articles describing the generation of isoxazole derivatives
45 in a one-pot, five-component reaction. In the 2022 article, they pioneered a one-pot five-component strategy using CaCl
2/K
2CO
3 as a synergistic catalytic system to synthesize 3,5-disubstituted isoxazole secondary sulfonamides
45 from hydroxylamine hydrochloride
2, aromatic aldehydes
9, primary amines
42, propargyl bromide
43, and saccharin
44. Their aqueous-phase protocol (MeOH/H
2O 4:1 optimized to pure H₂O) employed ultrasonic cavitation (sonotrode: 20 kHz, 130 W; bath: 37 kHz, 280 W) at 25°C with K
2CO
3 (3~4 mmol) and Oxone (2 mmol), achieving 75%~96% yields within 13~17 min while emphasizing green chemistry principles (Scheme 37) [
40].
Building on this, they developed an advanced Fe
3O
4@AgZr
2(PO
4)
3 nanocatalyst for a one-pot four-step cascade synthesizing isoxazole-linked 1,2,3-triazoles
45 from propargyl alcohols, benzenesulfonyl chloride, aryl aldoximes, sodium azide, and terminal alkynes in 2024. Their ultrasound-assisted aqueous system (20 kHz, 60% power) demonstrated remarkable efficiency improvements, reducing reaction time from 24 h to 75 min (135 min total) and boosting yields from 79% to 94% under temperature gradient conditions (25~80 °C). This ultimately resulted in 86% to 94% yields of all synthesized compounds
45 in a relatively short reaction time (135 min). Both protocols highlight ultrasonic cavitation's dual role in enhancing reaction kinetics and selectivity while employing H
2O as a green solvent. The Mokhi system further advanced sustainability through magnetic catalyst recyclability (five cycles without significant activity loss), contrasting with Mahmoudi's homogeneous base system (
Scheme 13) [
41]. These methodologies collectively exemplify progress in atom-economical heterocycle synthesis, addressing traditional limitations of toxic reagents, energy-intensive conditions, and poor regiocontrol through innovative catalyst design and mechanochemical activation.
2.3. Synthesis of Isoxazolone-Based Derivatives
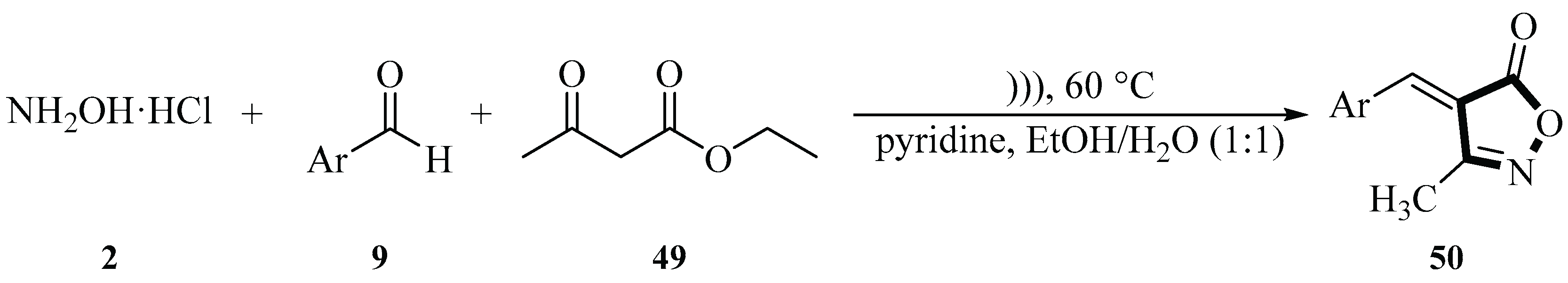
In 2009, the Cheng group reported a method for synthesizing 3-methyl-4-arylmethylene-isoxazol-5(4H)-one derivatives
50 through a three-component one-pot condensation reaction of hydroxylamine hydrochloride
2, aromatic aldehydes
9 (such as terephthalaldehyde), and methyl (or ethyl) acetoacetate
49 in aqueous media under ultrasonic irradiation. The reaction employed pyridine as an acid-binding agent to neutralize the hydrochloric acid released from hydroxylamine hydrochloride
2, maintaining a neutral or weakly basic environment, thereby reducing side reactions and improving reaction selectivity. Experimental results showed that the reaction proceeded smoothly at room temperature with moderate to good yields (51%~96%) regardless of whether the aryl ring was attached to electron-donor groups such as
p-dimethylamino and
p-methoxy, or to electron-withdrawing groups such as
p-nitro and
p-fluoro. The ultrasonic irradiation time ranged from 30 to 90 min. The reaction also proceeded well for ortho-substituted aldehydes, α,β-unsaturated aldehydes, and heteroaromatic aldehydes, with yields ranging from 47% to 71%. Additionally, terephthalaldehyde
51 underwent two condensation reactions with methyl acetoacetate
49 and hydroxylamine hydrochloride
2 to form the bis-isoxazolone derivative
52, with a yield of 63% after 90 min. It was experimentally demonstrated that the method requires mild conditions, has a short reaction time and a simple action, and is environmentally friendly (aqueous medium) compared with the traditional methods (e.g., heated reflux or stepwise reaction), which provides an efficient and green synthesis route for isoxazolone derivatives (
Scheme 15) [
43].
In 2012, the Ablajan group described a three-component, one-pot reaction of methyl hydroxylamine hydrochloride
2, aromatic aldehydes
9, and 4-methyl-3-oxovalerate
53, to synthesize 4-arylmethylidene-3-isopropylisoxazol-5-ones
54 under ultrasonic irradiation, catalyzed by pyridine in an aqueous medium at room temperature. The study highlighted the advantages of multicomponent reactions (MCRs) in organic synthesis, including straightforward procedures, experimental simplicity, and high product yields (63%~96%). The reaction sequence involved an initial 5-minute ultrasonic activation of the reactants (methyl 4-methyl-3-oxovalerate
53, hydroxylamine hydrochloride
2 and pyridine) in H
2O, followed by the addition of aromatic aldehyde
9 and continued ultrasonic irradiation for 40 min. Notably, the aqueous solvent system is aligned with green chemistry principles as it minimizes environmental impact and operational costs. Comparative experiments revealed that ultrasonic irradiation significantly improved yields compared with conventional heating methods. Structural characterization of the products confirmed the formation of the target isoxazol-5-ones
54, with electron-withdrawing substituents on the aromatic aldehyde enhancing reactivity. This methodology demonstrated efficiency and sustainability, consistent with green synthesis practices (
Scheme 16) [
44].
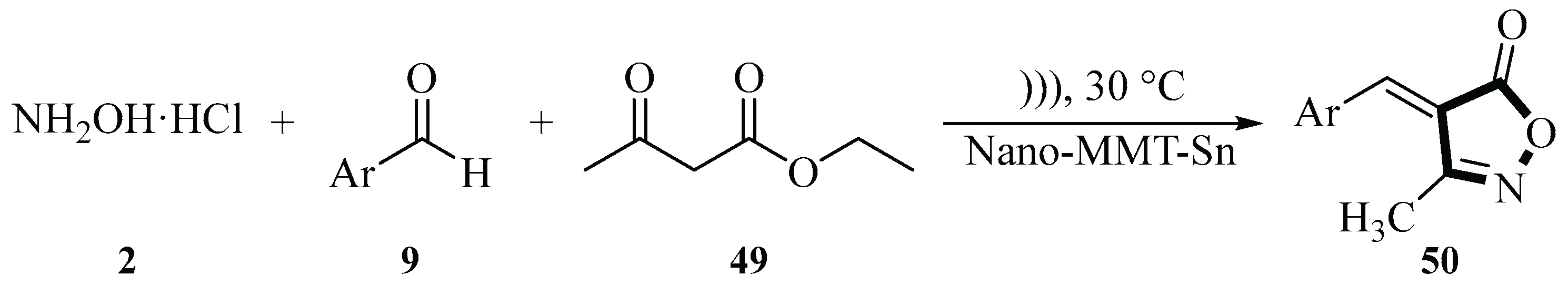
In 2016, the Safari group described an ultrasound-assisted reaction for synthesizing 3-methyl-4-arylmethylene isoxazole-5(4
H)-ones
50 using hydroxylamine hydrochloride
2, benzaldehyde derivatives
9, and ethyl acetoacetate
49 under ultrasound irradiation conditions with NH
2-MMT as the catalyst. MMT-K10 has received considerable attention as a catalyst and catalyst carrier in chemical synthesis. The group modified MMT by introducing 3-aminopropyltriethoxysilane (APTES), prepared NH
2-MMT, and used it as a nanocatalyst for chemical reactions. The reaction was conducted at 30℃ using H
2O as the solvent and NH
2-MMT as the catalyst, under irradiation with an ultrasonic power of 100 W. The reaction time varied between 10 and 55 min and the yield ranged from 80% to 97% depending on the substrate. The ultrasound-assisted synthesis method using NH
2-MMT as a catalyst had a shorter reaction time, easier post-treatment, higher yield, and a simpler experimental process. NH
2-MMT exhibited good recovery and reuse in the 6-cycle model reaction, with only a slight decrease in yield (
Scheme 17) [
45].
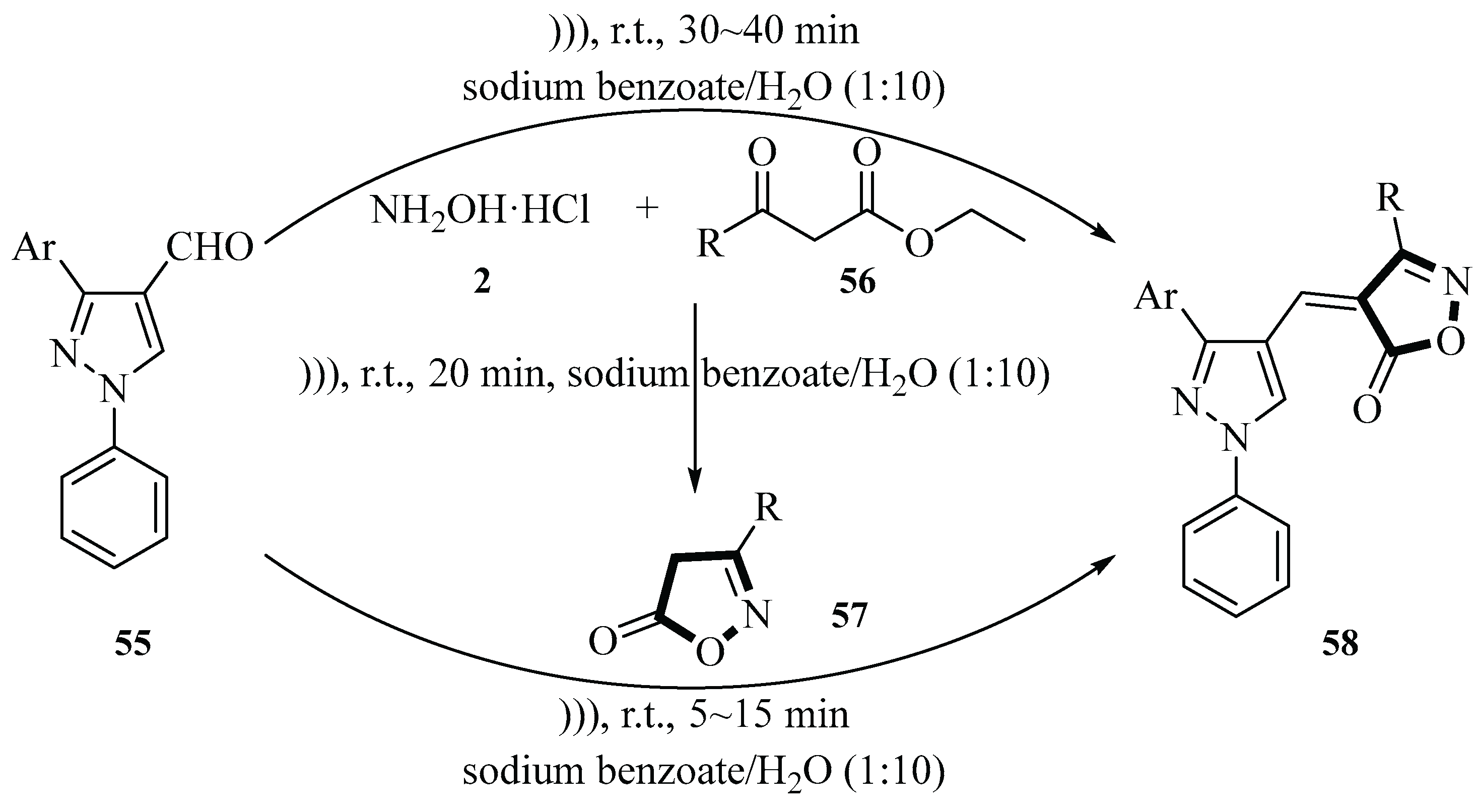
In 2017, the Konkala group published an article on the reaction of hydroxylamine hydrochloride
2, pyrazole-4-carbaldehyde
55, and β-ketoesters
56 to generate 4-pyrazolylmethylene-isoxazol-5(4
H)-ones derivatives
58 catalyzed by sodium benzoate using sonochemical methods. During the process, they first synthesized the starting material 1,3-diphenyl-1
H-pyrazole-4-carbaldehyde
57 via the Vilsmeier–Haack reaction, and then used this compound as the substrate for the one-pot three-component cyclocondensation reaction with the other two reactants. They screened various catalysts and solvents for the reaction. Experimental results indicated that only sodium benzoate, piperidine, and sodium acetate efficiently catalyzed the reaction, with sodium benzoate providing the highest yield (88%) in H
2O under ultrasonic irradiation. The reaction was conducted at room temperature, and the one-pot and step-by-step methods had similar times and yields. The one-pot method offers advantages such as operational simplicity, shorter reaction time, and scalability for industrial applications. The step-wise approach, though involving intermediate isolation, is suitable for mechanistic studies and reaction optimization. This protocol aligns with green chemistry principles by avoiding hazardous organic solvents, utilizing H
2O as a reaction medium, and enabling catalyst recycling. The method also features high efficiency, minimal by-products, and compatibility with diverse substrates, making it a viable strategy for Knoevenagel condensation reactions (
Scheme 18) [
46].
In 2018, an ultrasound-assisted method for synthesizing 3-methyl-4-(hetero)arylmethylene isoxazole-5(4
H)-ones
50 was reported by Ahmadzadeh et al. The target compounds
50 were synthesized via a one-pot multicomponent cyclocondensation of hydroxylamine hydrochloride
2, aromatic aldehydes
9, and ethyl acetoacetate
49 catalyzed by Sn
II-Mont K10. The catalyst exhibited recoverability through simple filtration and maintained consistent catalytic activity over six reuse cycles. Reaction optimization revealed the optimal conditions to be 0.01g of catalyst, 90 W ultrasonic power, 30 °C temperature, and 20 min reaction time in an aqueous medium, achieving a maximum yield of 96%. Substrates with electron-donating or electron-withdrawing groups demonstrated yields ranging from 87% to 96%. This study pioneered the synergistic combination of Sn
II-Mont K10 with ultrasound irradiation, leveraging cavitation effects to enhance reaction efficiency under mild, solvent-free conditions. The methodology emphasized green chemistry principles by eliminating organic solvents, reducing energy consumption, and enabling catalyst reuse, offering a novel approach for sustainable heterocyclic compound synthesis (
Scheme 19) [
47].
In 2019, the Kasar group reported a method of synthesizing 4
H-isoxazol-5-ones derivatives
50 via a multicomponent reaction of hydroxylamine hydrochloride
2, aromatic aldehydes
9, and ethyl acetoacetate
49 catalyzed by itaconic acid under ultrasonic irradiation. Optimization studies revealed that under conventional heating (100 °C, 3 h), the reaction achieved a 90% yield. In contrast, ultrasound-assisted conditions (50 °C, 15 min) significantly enhanced efficiency, delivering a 95% yield. Notably, the aqueous solution of itaconic acid could be reused for ten catalytic cycles without loss of activity. The protocol employs H
2O as a green solvent and ultrasonication as a non-conventional energy source, aligning with green chemistry principles by eliminating organic solvents, hazardous acids, and metal catalysts. This method demonstrates high efficiency, broad substrate compatibility, and excellent catalyst reusability, providing a sustainable approach for synthesizing biologically active isoxazole derivatives (
Scheme 20) [
48].
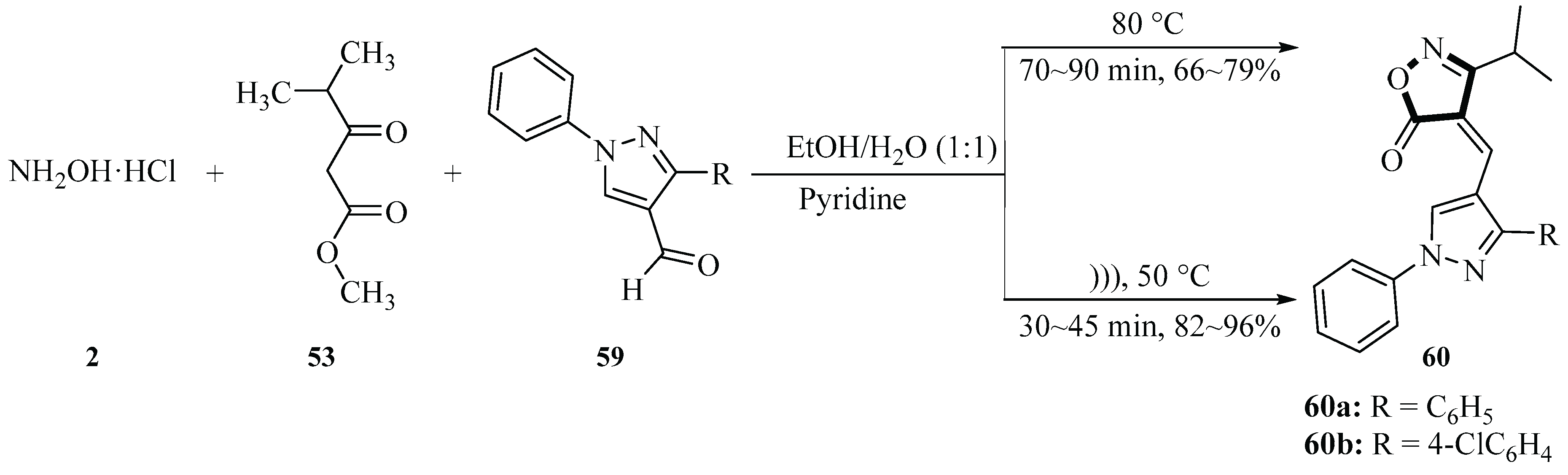
In 2019, Bhatt and co-workers synthesized 4-(substituted-1
H-pyrazol-4-yl) methylene)-3-isopropylisoxazol-5(4
H)-ones
60 via a one-pot, three-component reaction using hydroxylamine hydrochloride
2, methyl 4-methyl-3-oxovalerate
53 and substituted pyrazole aldehyde
59 under ultrasonic irradiation conditions. The ultrasound-assisted method (300 W, 20~60 kHz) was conducted at 50 °C for 30~45 min, with a product yield of 82%~96% with pyridine as the base. The synthesized compounds were evaluated for in vitro anticancer activity, demonstrating significant growth inhibitory effects against various tumor cell lines, particularly leukemia cell lines. Compared with the conventional heating method (70~90 min, 66%~79% yield), ultrasonic irradiation provided shorter reaction times (25~60 min), higher yields (3%~30%), and reduced impurity formation (
Scheme 21) [
49]. The synthesized compounds
60 were evaluated for their in vitro anticancer activity using a single high dose (10 μM) against a panel of 60 human tumor cell lines derived from nine cancer types. The results showed that most of the compounds exhibited significant growth inhibitory activity against human tumor cells, particularly the leukemia cell lines, which showed remarkable sensitivity to compounds
60a and
60b. Compound
60a demonstrated the most potent cytotoxic activity, especially against leukemia (HL-60(TB); GI
50 = -45.19), melanoma (LOX IMVI; GI
50 = 17.99), and colon cancer (HCT-116; GI
50 = 28.89) cell lines.
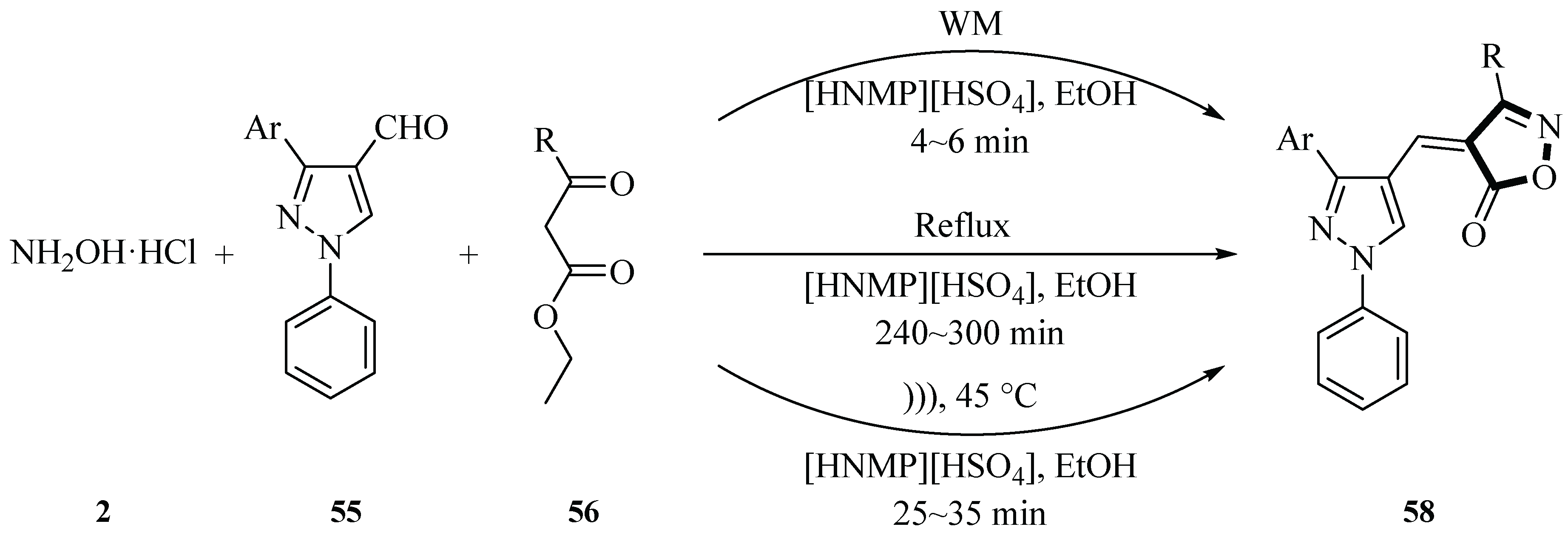
In 2020, the Shirole group investigated the reaction of 1,3-diaryl-1
H-pyrazole-4-carboxyaldehyde
56, β-keto ester
55, and hydroxylamine hydrochloride
2 to form 3-methyl-4-((3-aryl-1-phenyl-1
H-pyrazol-4-yl)methylene)isoxazol-5(4
H)-one derivatives
58 via a one-pot multicomponent reaction catalyzed by the ionic liquid [HNMP][HSO
4]. The reaction was conducted under both ultrasound irradiation (45 °C, 30 min) and microwave irradiation (210 W, 5 min), achieving yields of 80%~82% for the target products. The authors first optimized the model reaction by determining the optimal catalyst dosage (100 mg) and solvent (ethanol), with the conventional reflux method having a product yield of 70% under optimized conditions. The study emphasizes the green chemistry advantages of this protocol, including reduced environmental impact, shorter reaction times, and high efficiency, highlighting its significance for sustainable chemical synthesis and drug development (
Scheme 22) [
50].
In 2021, the Ren group investigated a method for the synthesis of 3-methyl-4-aromatic methylene isoxazole-5(4
H)-ketone
50 using ultrasonic radiation, with hydroxylamine hydrochloride
2, aromatic aldehydes
9, and methyl acetoacetate
49 as substrates, and pyridine as a catalyst to accelerate the reaction. The one-pot preparation simplified the post-reaction treatment steps. Results showed that a 96% yield was obtained with
p-dimethylaminobenzaldehyde as the aromatic aldehyde
9, while a 64% yield was achieved with benzaldehyde
9. By comparing the effects of different aromatic aldehydes on product yields, it was observed that aromatic aldehydes
9 with electron-donating groups provided higher yields, whereas those with electron-withdrawing groups resulted in lower yields. During their experiments, they found that the ethanol–water system reacted faster than pure water. The sonication reaction required approximately 60 min at 60 °C, with a product yield of 43%~96%. The incorporation of ultrasound technology significantly enhanced reaction rates, simplified operations, and improved yields while maintaining environmental friendliness; however, this method imposed limitations on solvent choices and generated minor by-products. The application of ultrasonic radiation in organic synthesis could be further improved through the optimization of reaction conditions, exploration of more efficient catalysts, and implementation of real-time monitoring tools (
Scheme 23) [
51].
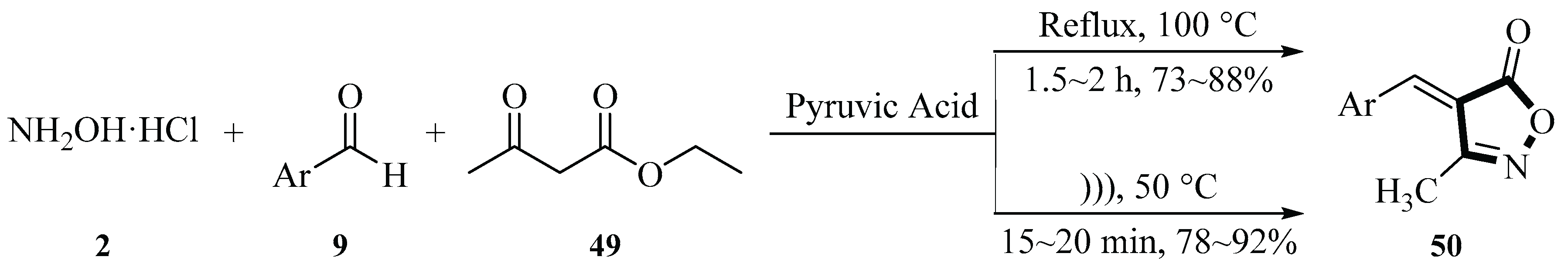
In 2022, the Deshmukh group reported a one-pot three-component synthesis of 3-methyl-4-arylmethylene isoxazol-5(4
H)-ones
50 using hydroxylamine hydrochloride
2, benzaldehyde derivatives
9, and ethyl acetoacetate
49 in aqueous medium catalyzed by pyruvic acid (5 mol%). The reaction mechanism involves pyruvic acid-mediated activation of ethyl acetoacetate
49, followed by oxime formation, keto-enol tautomerization, nucleophilic attack on the aldehyde
9, intramolecular cyclization, and deprotonation to yield the isoxazole
50 scaffold. They found that ultrasonic irradiation reduced the temperature by 50 °C compared to conventional heating methods; the formation rate increased by only 4%, but the time was reduced to one-tenth of that of the conventional method. The protocol demonstrated broad substrate compatibility, accommodating mono- and di-substituted aromatic aldehydes
9, with a product yield of 73%~92%. Key innovations include the use of H
2O as a green solvent, biodegradable pyruvic as an acid catalyst, and ultrasound technology to enhance efficiency. This method offers advantages such as short reaction times, avoidance of toxic reagents, and energy efficiency, highlighting its potential for sustainable synthesis of bioactive isoxazole derivatives (
Scheme 24) [
52].
In 2022, Maddila and his colleagues published a facile and efficacy preparation of isoxazole-5(4H)-one analogues
50 via multicomponent, single-step condensation of hydroxylamine hydrochloride
2, substituted aldehyde
9, and ethyl acetoacetate
49 in the presence of a recyclable catalyst under ultrasound-assisted reaction at room temperature. The reaction showed various benefits like simple handling, mild conditions, easy work-up procedure, short reaction times (not more than 10 min), safety, use of greener solvents, lack of toxic reagents, excellent product yields (not less than 94%), and recyclability. The main advantage of this method was that the solid catalyst was utilized for over eight cycles without any significant loss of activity (
Scheme 25) [
53].
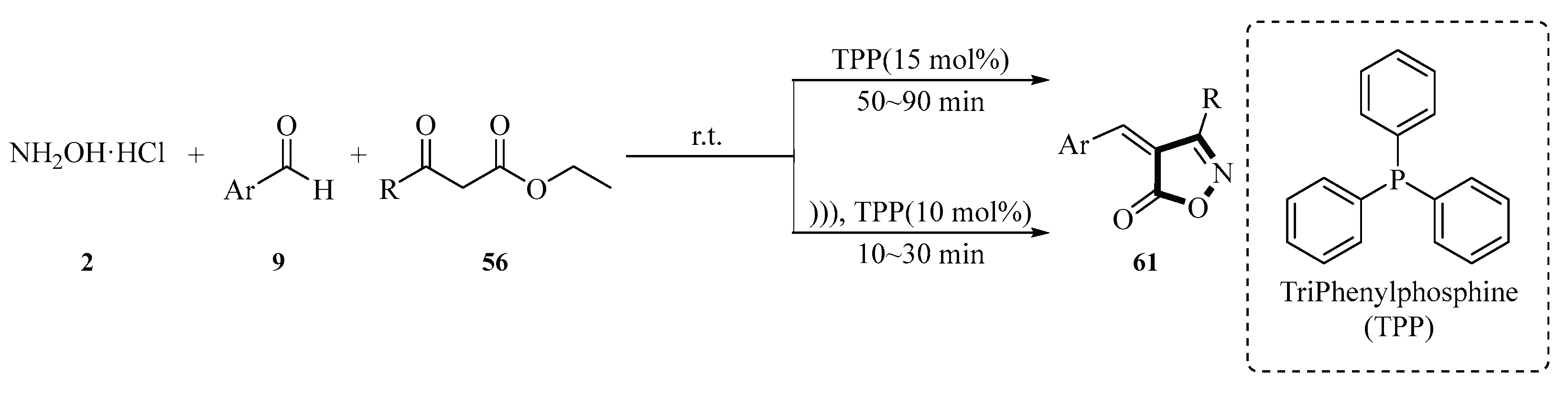
In 2023, Daroughehzadeh and Kiyani reported a three-component organocatalytic approach for the synthesis of 4-arylideneisoxazol-5(4
H)-ones
61 using triphenylphosphine (TPP) as a catalyst. The model reaction involved hydroxylamine hydrochloride
2, 4-hydroxy-3-methoxybenzaldehyde
9, and ethyl acetoacetate
56 under aqueous conditions. Optimization studies revealed that 15 mol% TPP at 25 °C in H
2O afforded the target product a 98% yield within 50 min. Sonochemical synthesis further enhanced the reaction efficiency, achieving a 99% yield with only 10 mol% TPP in 10 min under ultrasonic irradiation. Comparative analysis demonstrated the superiority of ultrasonication in reducing reaction time and catalyst loading. Notably, the study emphasized the green aspects of the protocol, including H
2O as a solvent and high atom economy, but did not report catalyst recyclability data. This work highlights the efficacy of TPP in multicomponent heterocyclization and the benefits of sonochemical activation (
Scheme 26) [
54].
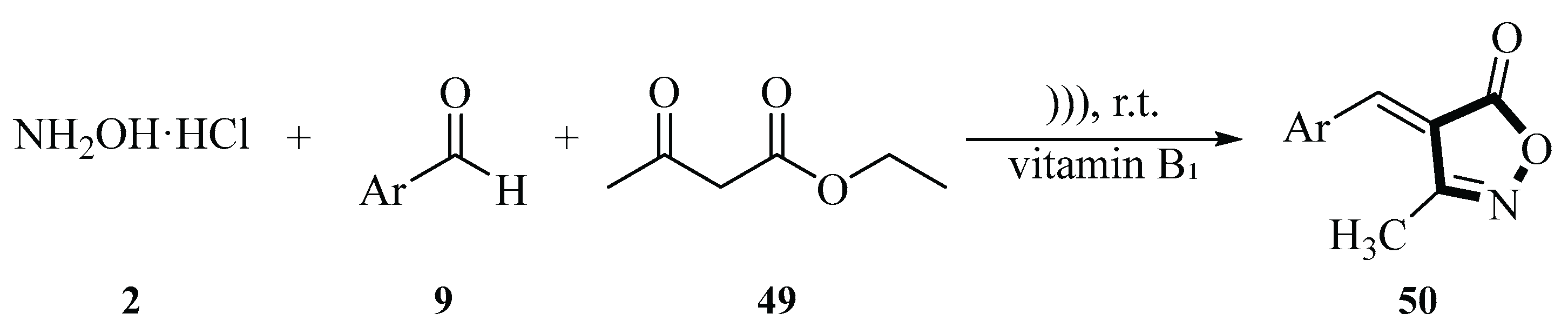
In 2023, the Zhang group developed an efficient method for the synthesis of 3-methyl-4-(arylmethylene)isoxazol-5(4
H)-ones
50 via a vitamin B₁-catalyzed cyclocondensation reaction under ultrasound radiation. The model reaction system employed hydroxylamine hydrochloride
2, 2-methoxybenzaldehyde
9, and ethyl acetoacetate
49 in H
2O as the solvent. Nucleophilic attack of hydroxylamine hydrochloride
2 on the ethyl acetoacetate
49 carbonyl group first leads to the formation of an oxime intermediate via dehydration. Next, vitamin B₁-mediated activation of the aldehyde group occurs in a Knöwenagel condensation reaction with the tautomerized intermediate. Finally, intramolecular cyclization and elimination of ethanol produces the target compound. The reaction achieved a 94% yield under optimized conditions (0.1 equiv vitamin B₁, 20 °C, 30 min). Ultrasound-assisted synthesis significantly improved yields (84~94%) and reduced reaction time compared to conventional stirring. Notably, vitamin B₁ exhibited excellent reusability, maintaining yields above 88% after five cycles. This method was metal-free, acid/base-free, and environmentally friendly, leveraging H
2O as a green solvent (
Scheme 27) [
55].
In 2023, the Nongrum group described a three-component condensation reaction comprising hydroxylamine hydrochloride
2, aromatic aldehydes
9, and β-ketoesters
62 using Fe
3O
4@MAP-SO
3H as a catalyst to synthesize 3-methyl-4-(phenyl)methylene-isoxazole-5(4
H)-one derivatives
61. The reaction was mediated by ultrasound irradiation in an ethanol–water (1:3) medium under a one-pot protocol. Optimization studies indicated that 20 mg of catalyst in an ethanol–water (1:3) medium under ultrasound irradiation provided the highest yield (92%) and significantly accelerated the reaction rate. The synthesized compounds
61 were evaluated for antifungal and antimycobacterial activity. The 3-chloromethyl-4-(phenyl)methylene-isoxazole-5(4
H)-one series (notably compound
61a) exhibited over 50% growth inhibition against Fusarium oxysporum at 100 µg/mL. Compounds
61a and
61b displayed antimycobacterial activity against Mycobacterium tuberculosis H37Rv strain, with MIC values (1.56 µg/mL) equipotent to the standard drug ethambutol. Additionally, these compounds demonstrated efficacy against both active and dormant forms of
M. tuberculosis in a nutrient starvation model. This green synthesis method features mild reaction conditions, easy catalyst recovery via an external magnet, short reaction time, and the use of eco-friendly solvents. The biological activities of the compounds highlight their potential as candidates for antifungal and antimycobacterial drug development (
Scheme 28) [
56].
2.4. Synthesis of Isoxazoline-Based Derivatives
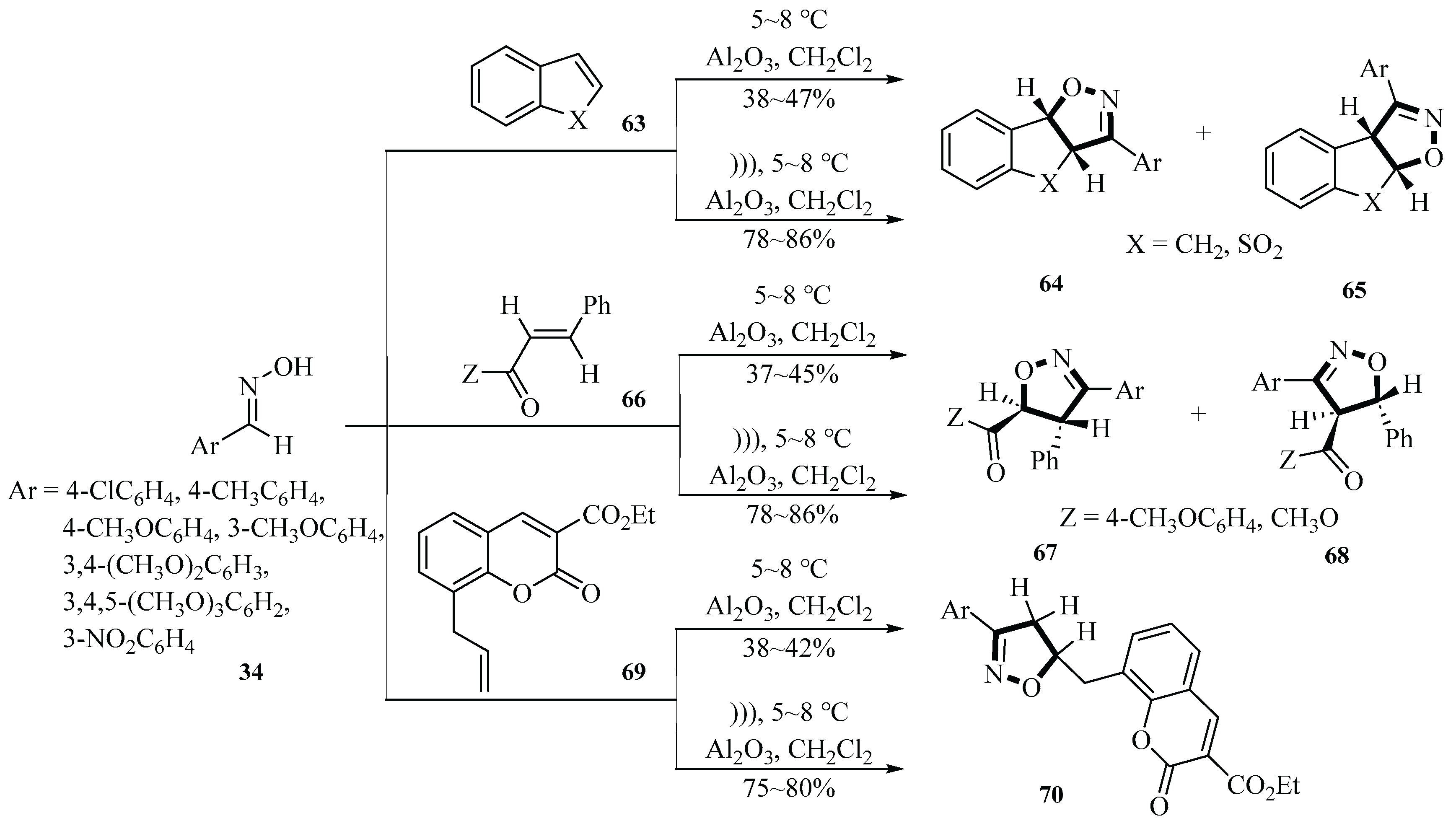
In 1999, the Syassi group published an article about synthesizing 4,5-dihydroisoxazoles derivatives
64,
65,
67,
68, and
70 using 1-sodium-3,5-dichloro-s-triazine-2,4,6-trione (SDCTT), arylaldoximes, and dipolarophiles under ultrasound irradiation conditions. The reaction utilized aluminum oxide as a catalyst and dichloromethane as a solvent. This method was activated by ultrasound in a solid–liquid two-phase system. They developed a new halogenating and oxidizing agent called SDCTT for the synthesis of the target compound. Arylaldoximes were used as substrates. The water bath temperature was maintained at 5~8 °C, and ultrasonic irradiation (47 kHz, 70 W) was applied for 15 min. Ultrasonic radiation had a significant impact on yield improvement. Specifically, ultrasound radiation increased the reaction yield from 37%~47% under magnetic stirring to 75%~86%. This indicated that ultrasonic radiation highly increased the synthesis yield of 4,5-dihydroisoxazoles, with an average yield increase of approximately 41.5%. This demonstrated that ultrasound radiation played an important role in promoting reaction progress and improving the yield (
Scheme 29) [
57].
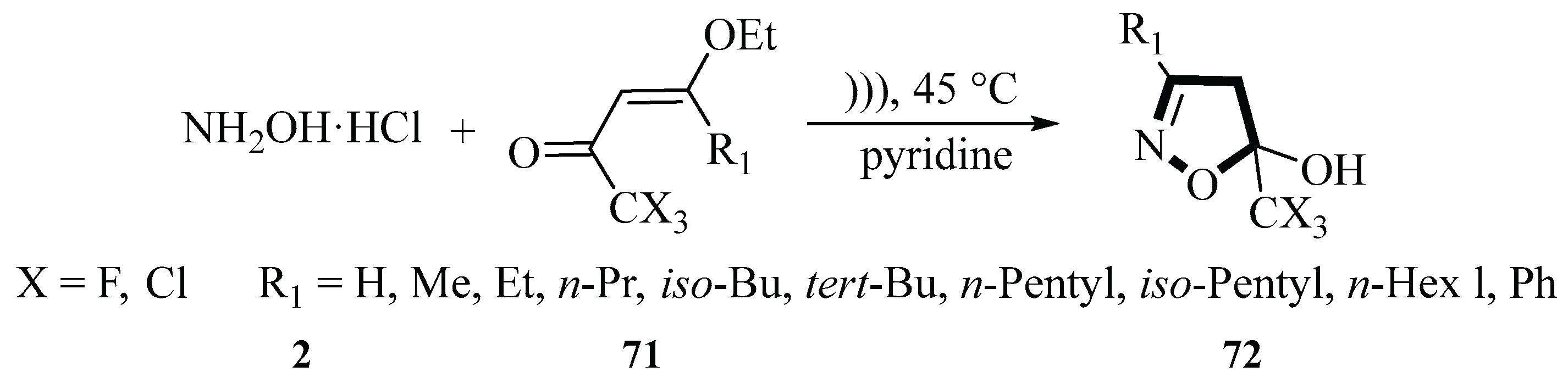
In 2006, the Martins group described a reaction in which 1,1,1-trihalo-4-alkoxy-3-alken-2-ones reacted
71 with hydroxylamine hydrochloride
2 and pyridine to generate 5-hydroxy-5-trihalomethyl-4,5-dihydroisoxazoles
72 under ultrasound irradiation in a water bath at 45 °C. Under ultrasound irradiation conditions (20 W), the yields of 5-hydroxy-5-trihalomethy1-4,5-dihydroisoxazolesranged
72 ranged between 30 and 90%, and the reaction time was 30 min. The use of ultrasound irradiation not only significantly shortened the reaction time and improved the yield but also utilized water as a solvent, thereby reducing environmental impact and cost. The product
72 had high purity and did not require further purification (
Scheme 30) [
58].
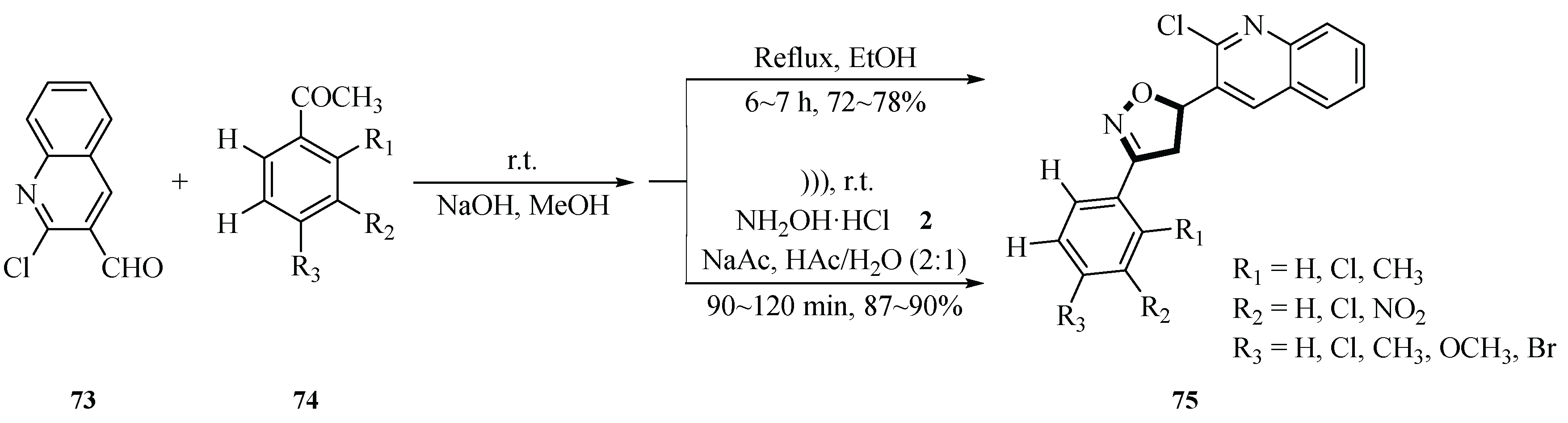
In 2011, Tiwari et al. presented an improved synthesis method for the preparation of 5-(2-chloroquinolin-3-yl)-3-phenyl-4,5-dihydroisoxazolines
75 using aqueous acetic acid under ultrasound irradiation, with chalcones and hydroxylamine hydrochloride
2 as substrates and sodium acetate as a base. The reaction was conducted at room temperature for 90~120 min under ultrasound irradiation (40 kHz, 250 W) in an ultrasonic cleaner. The optimized molar ratio of chalcone to hydroxylamine hydrochloride
2 was 1:3, yielding target compounds
75 with 80%~90% efficiency. Ultrasonic irradiation shortened the process to 240~330 min and increased the yield compared with the conventional thermal method (72%~78%). The method’s advantages were cost-effectiveness, operational simplicity, room temperature conditions, and environmental compatibility, highlighting its potential for practical applications (
Scheme 31) [
59].
In 2015, the Nikam group described a study in which a series of new isoxazoline derivatives
79 were synthesized from the corresponding chalcone
78 under ultrasonic radiation. The reaction was carried out in an ultrasonic bath at a frequency of 40 MHz and a power of 100 W. The reaction time was shortened from 2 h to a few min. The reaction yield for the generation of chalcone
78 was 78%, and the yields of isoxazoline derivatives
79 were 78%~81%. Some of the synthesized compounds
78 and
79 showed significant biological antimicrobial and antioxidant activities, with compounds
78a and
79a having excellent antimicrobial activity, while compounds
78a,
78b,
79a, and
79b having excellent antioxidant activity, with IC
50 values (21.0~26.5 μg/mL) surpassing those of ascorbic acid and BHT. Studies suggested interactions with fungal CYP51, and Absorption Distribution Metabolism Excretion and Toxicity (ADMET) predictions indicated favorable drug-like properties. This study provides a series of structurally diverse compounds with dual antimicrobial and antioxidant potential, offering promising leads for further development (
Scheme 32) [
60].
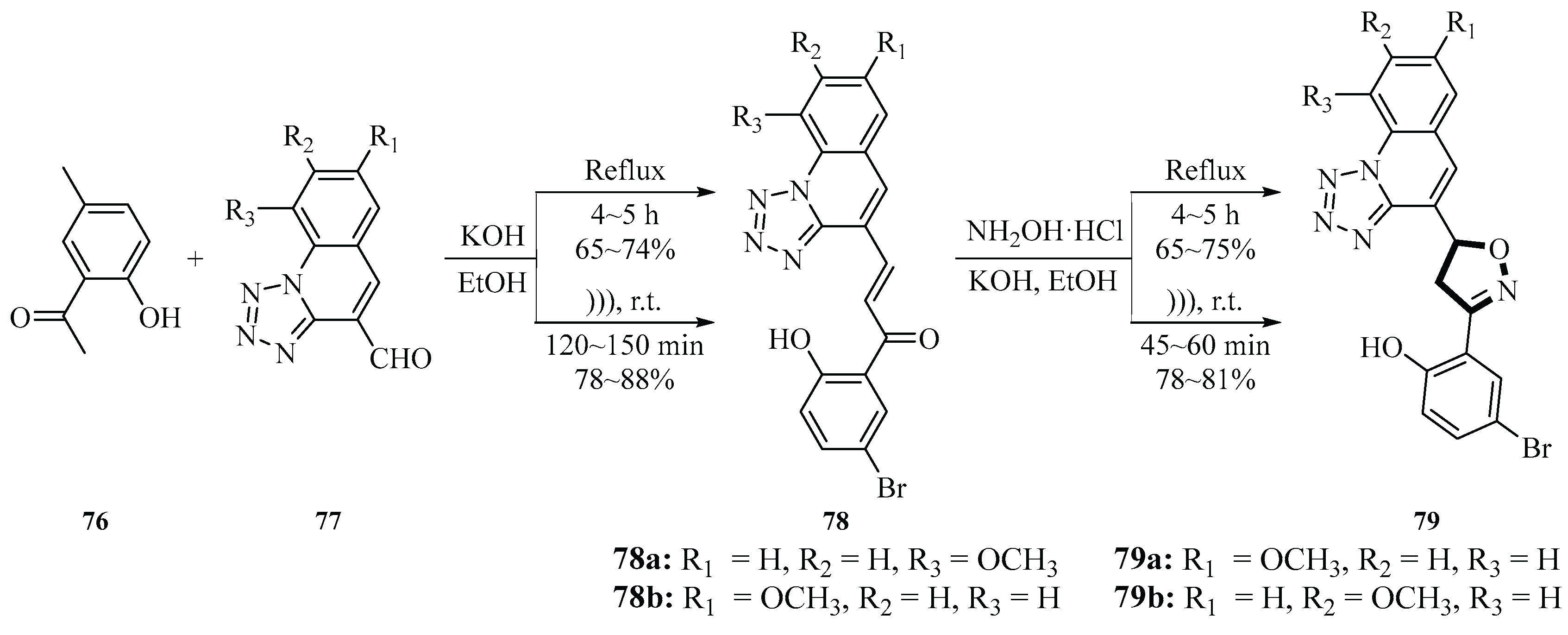
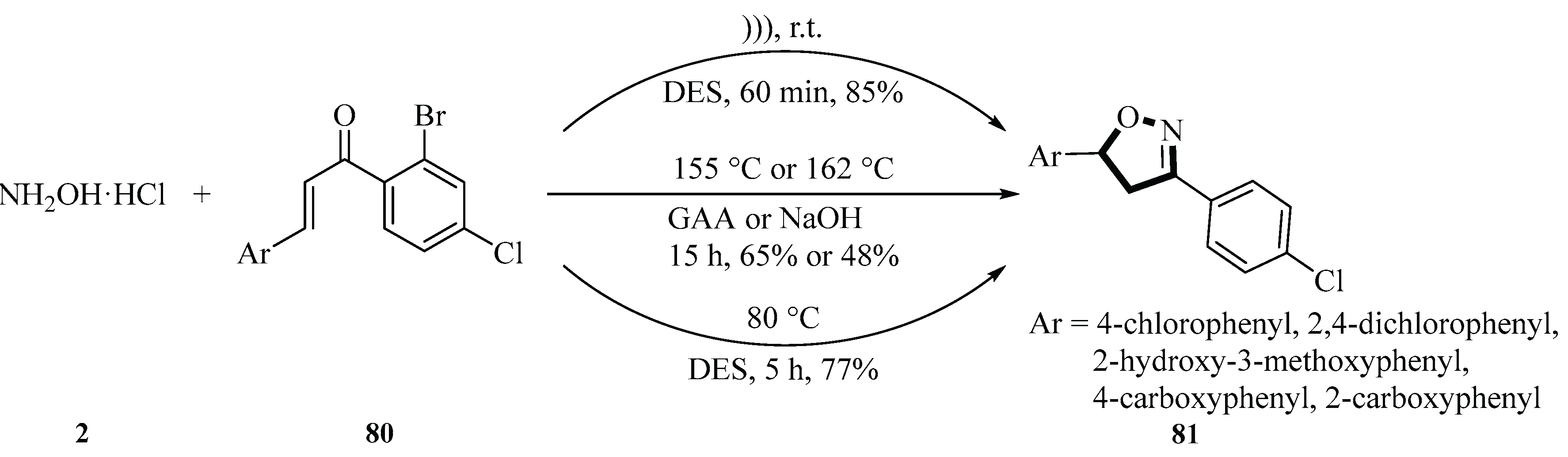
In 2016, Bakht et al. and co-workers developed an efficient method for the synthesis of isoxazoline derivatives
81 based on a DES prepared from benzalkonium chloride (BZK) and urea (1:2 molar ratio) in combination with ultrasonic irradiation (20 kHz, 130 W, 28~32 °C). The reaction was realized via cyclization of chalcone
80 with hydroxylamine hydrochloride
2. In this study, benzalkonium chloride-based DES was used for the first time in organic synthesis with ultrasonication in a green chemistry-oriented approach. DES was produced by mechanically mixing benzalkonium chloride with urea for 30 min at room temperature. The reaction time was drastically shortened from 15 h to 1 h, the product
81 yield was increased from 48%~65% to 78%~85%, and the energy consumption was reduced by 86%~88% compared with conventional solvents (glacial acetic acid/sodium hydroxide) as the reaction medium. Ultrasonication can accelerate molecular collision and mass transfer through the cavitation effect by generating localized high temperature (2000~5000 K) and high pressure (about 18,000 atm), which significantly improve reaction efficiency. In addition, DES showed good recyclability, with only a small decrease in yield after four cycles of reuse. This study provides a new strategy for the efficient, low-energy, and environmentally friendly green synthesis of isooxazoline (
Scheme 33) [
61].
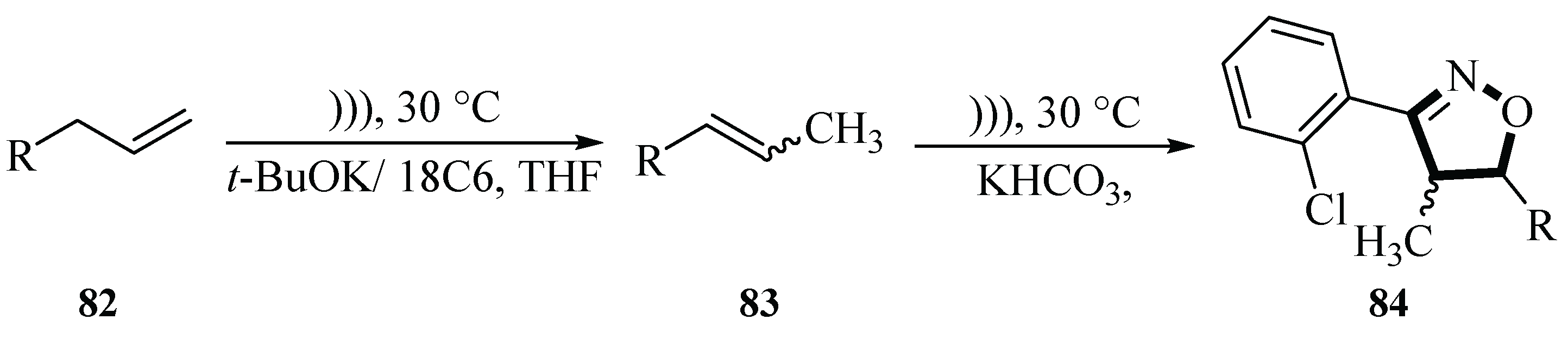
In 2017, the Krompiec group reported highly active, regioselective, and reusable crown ether/base catalytic systems (e.g., 15-Crown-5/NaOH, 18-Crown-6/KOH, dibenzo-18-Crown-6/
t-BuOK, and 18-Crown-6/
t-BuOK) for double bond migration in allylic compounds
82 and
83. These systems are capable of efficiently isomerizing a wide range of allyl substrates under mild conditions (typically 30 °C, 0.25~24 h). Remarkably, ultrasound assistance significantly accelerated reaction rates without compromising selectivity. The reactions proceeded in various solvents (THF, Et
2O, DME, toluene, etc.) or under solvent-free conditions, with full regioselectivity observed for C,O- and O,S-diallyl compounds, where only the less substituted allyl group underwent migration. Additionally, a one-pot synthesis of isoxazolines
84 was demonstrated by combining isomerization, nitrile oxide generation, and 1,3-dipolar cycloaddition. Ultrasonic synthesis shortened the reaction time, reduced the use of solvents, and kept the selectivity of the reaction unaffected (
Scheme 34) [
62].
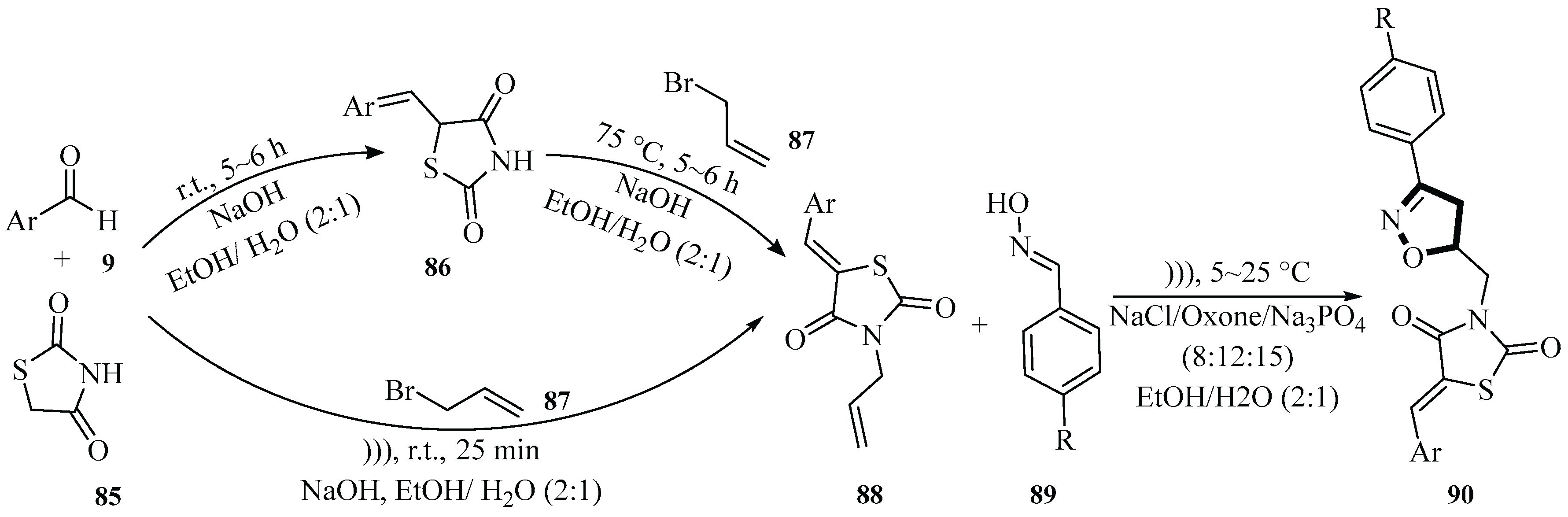
In 2020, Thari and co-workers reported a method for the synthesis of isoxazoline derivatives
90 from aryl aldehydes
9, thiazolidine-2,4-diones
85, allyl bromide
87, and sodium hydroxide via a one-pot, two-step reaction under ultrasonic irradiation, utilizing NaCl/Oxone/Na
3PO
4 in an aqueous medium as a chlorine source, oxidant, and catalyst. In the first step,
N-allyl-5-arylidenethiazolidine-2,4-diones
88 were synthesized from thiazolidine-2,4-dione
85, aryl aldehydes
9, and allyl bromide
87 through Knoevenagel condensation followed by
N-allylation using aqueous NaOH under ultrasonic conditions. This process avoids the separation of intermediates
86 required by conventional methods, and yields can be increased by 7% to 52% under ultrasonic conditions. The second step involved a regioselective 1,3-dipolar cycloaddition reaction using the NaCl/Oxone/Na
3PO
4 system under ultrasonic activation (130 W, 20 kHz) at 5~25 °C for 30 min in ethanol/water (2:1), yielding 69%~90% of the target isoxazoline derivatives
90. The catalyst system efficiently promoted cycloaddition while suppressing byproduct formation. Compared with traditional multi-step approaches, this ultrasound-assisted method simplifies the process, enhances reaction efficiency (24-fold acceleration in cycloaddition), and reduces environmental impact by employing aqueous media and recyclable reagents. The study presents an eco-friendly strategy for synthesizing
N-thiazolidine-2,4-dione-isoxazoline hybrids with potential pharmacological applications, emphasizing improved selectivity and scalability (
Scheme 35) [
63].
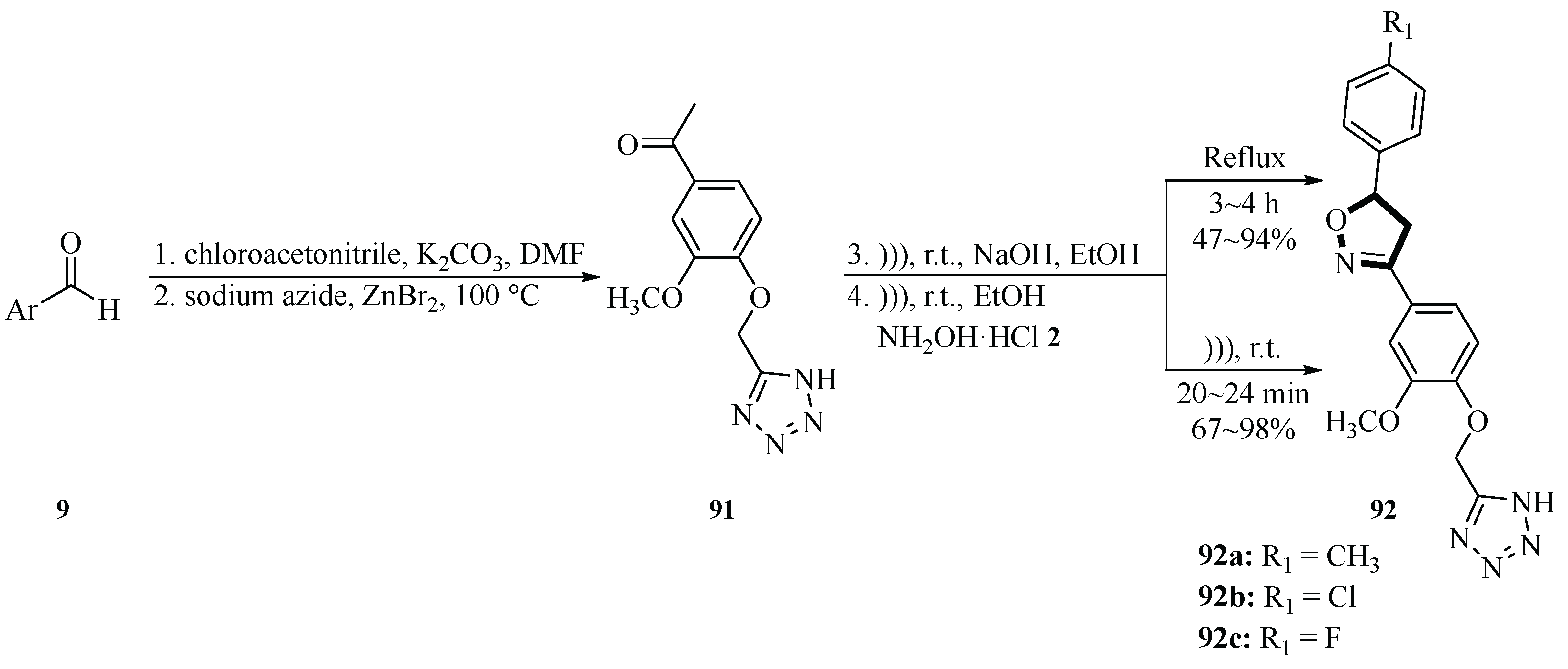
In 2020, the Dofe group developed an ultrasound-assisted method for the synthesis of tetrazole-based isoxazoline derivatives
92 using a multi-step protocol. Starting from 1-(4-hydroxy-3-methoxyphenyl)ethenone
9 alkylation with chloroacetonitrile in DMF using K
2CO
3 yielded 1-(4-(cyanomethoxy)-3-methoxyphenyl)ethanone
91 (90% yield). Subsequent cyclization with sodium azide (NaN
3) and ZnBr
2 in H
2O at 100 °C afforded the tetrazole precursor 1-(4-((1
H-tetrazol-5-yl)methoxy)-3-methoxyphenyl)ethanone
91 (87% yield non-ultrasound step). The final yield of 5-((4-(4,5-dihydro-5-phenylisoxazol-3-yl)-2-methoxyphenoxy)methyl)-1
H-tetrazole derivatives
92 was 67~98% yield, significantly outperforming conventional methods in both time reduction (hours to minutes) and yield improvement. MTT assays against A549, HepG2 (liver), and MCF-7 (breast) cancer cells revealed potent activity. The compound
92a exhibited the strongest inhibition against A549, with IC
50 = 0.78 µM, while
92b and
92c and showed significant tubulin polymerization inhibition, comparable to combretastatin A-4 (CA-4). Molecular docking confirmed binding at the colchicine site of tubulin, mimicking CA-4. This study highlights ultrasound-enhanced efficiency, broad anticancer activity, and the tubulin-targeting mechanism of tetrazole-isoxazoline hybrids, offering a robust platform for novel anticancer drug development (
Scheme 36) [
64].
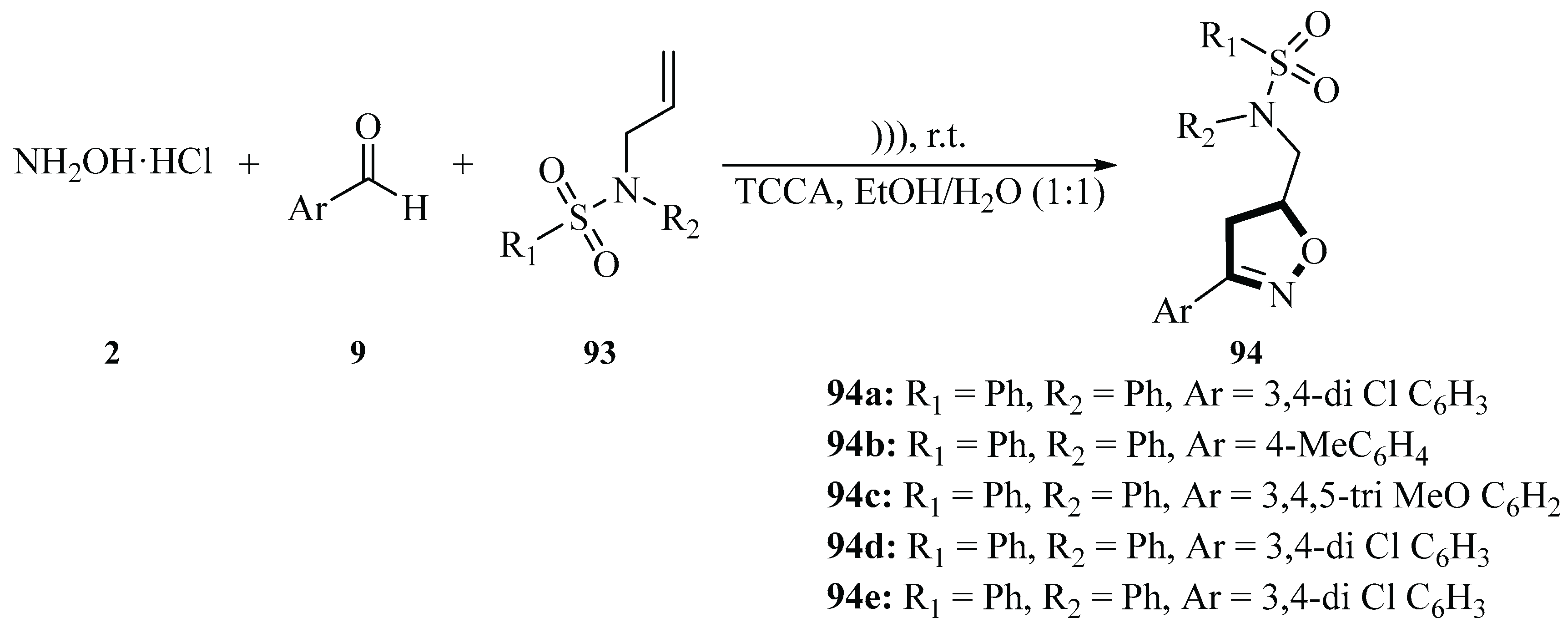
In 2021, the Talha group published an article on a novel ultrasound-assisted one-pot three-component method for the synthesis of sulfonamide-isoxazoline hybrids
94 in which the target compounds
94 were obtained through the reaction of hydroxylamine hydrochloride
2, aldehydes
9, alkenes, and trichloroisocyanuric acid (TCCA). The study proposed a dual reaction mechanism supported by controlled experiments. Under ultrasonic irradiation, TCCA undergoes homolytic cleavage of the N–Cl bond to generate chlorine radicals and dichloroisocyanuric acid radicals, which oxidize aldoximes to unstable biradical nitrile oxides. These intermediates undergo regioselective 1,3-dipolar cycloaddition with alkenes to form 4-substituted isoxazolines
94. Alternatively, TCCA may generate electrophilic chlorine atoms and cyanurate anions through mechanical effects, promoting an ionic pathway to nitrile oxides that similarly react with alkenes. The reaction was conducted in ethanol/water (1:1 v/v) under ultrasonic activation (80 W, 47 kHz) at 25 °C. TCCA served as both an oxidant and chlorinating agent, enabling aldehyde-to-nitrile oxide conversion without an additional base. Optimal conditions, using 0.5 mmol of TCCA, afforded products with 52%~89% yields within 12~22 min. The synthesized sulfonamide-isoxazoline hybrids were evaluated for antineoplastic activity against hematological malignancies (K562 and HL-60 cell lines). Compound 3h demonstrated significant activity with an EC
50 of 60~64 μM against HL-60 cells, inducing caspase-dependent apoptosis as evidenced by PARP and caspase-3 cleavage. Co-treatment with the pan-caspase inhibitor Q-VD-OPh fully restored cell viability, confirming the apoptotic mechanism. The marked difference in activity between
94a and
94e highlights the critical role of sulfonamide substituents in biological efficacy. This green, efficient methodology provides a valuable framework for developing novel antileukemic agents bearing sulfonamide-isoxazoline pharmacophores (
Scheme 37) [
65].
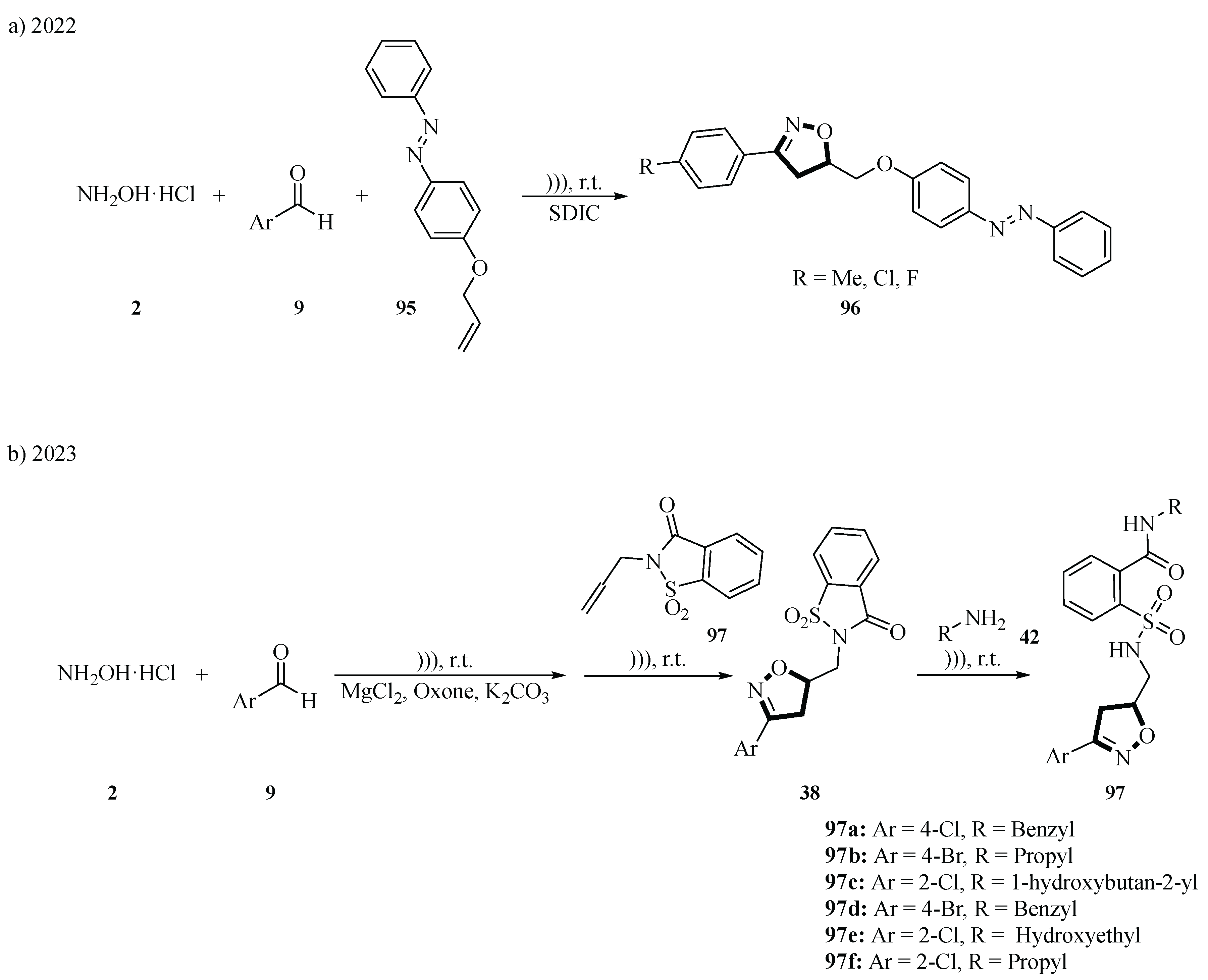
In 2022, the Mahmoudi group developed a green, one-pot, three-component method for the synthesis of novel azo-isoxazolines
96 via ultrasound-assisted 1,3-dipolar cycloaddition. The reaction involved hydroxylamine hydrochloride
2, aromatic aldehydes
9, and 4-(allyloxy)azobenzene
95 as substrates. The process began with the in situ generation of nitrile oxides from hydroxylamine hydrochloride
2 and aldehydes
9 under sodium dichloroisocyanurate (SDIC)-mediated oxidation in H
2O. These nitrile oxides then underwent regioselective 1,3-dipolar cycloaddition with 4-(allyloxy)azobenzene
95 under ultrasonic cavitation (47 kHz, 80 W) at room temperature, yielding 75%~90% azo-isoxazolines
96 within 25~30 min. The method utilized H
2O as a green solvent and SDIC as an eco-friendly, cost-effective oxidant, eliminating the need for toxic catalysts or organic solvents (
Scheme 38a) [
66].
The following year, Mahmoudi and co-workers successfully synthesized novel 3,5-disubstituted isoxazoline-sulfonamide derivatives
97 via a green ultrasound-assisted four-component reaction in the aqueous phase. The reaction involved hydroxylamine hydrochloride
2, aromatic aldehydes
9, pre-synthesized
N-allyl-saccharin
37, and primary amines as substrates. The reactions were consistent with the 2022 protocol in terms of temperature, ultrasound assistance, and solvent selection, with 70%~95% yields achieved in 21~26 min, highlighting their efficiency and mild conditions. Bioassays against
Sphodroxia maroccana larvae revealed exceptional larvicidal activity for halogenated derivatives
97a~
97f, with
97c showing an LC
50 of 0.18 mg/mL, outperforming fluralaner (LC
50 = 0.99 mg/mL). Both studies used experiments to confirm that the reactions were both somewhat regioselective. The first study focused on the global significance of this research, which was primarily concerned with the application of green chemistry in the field of chemical synthesis, while the second study focused on solving the problem of agricultural pests in a specific region, particularly
Sphodroxia maroccana Ley, a cork oak pest in Morocco (
Scheme 38b) [
67].
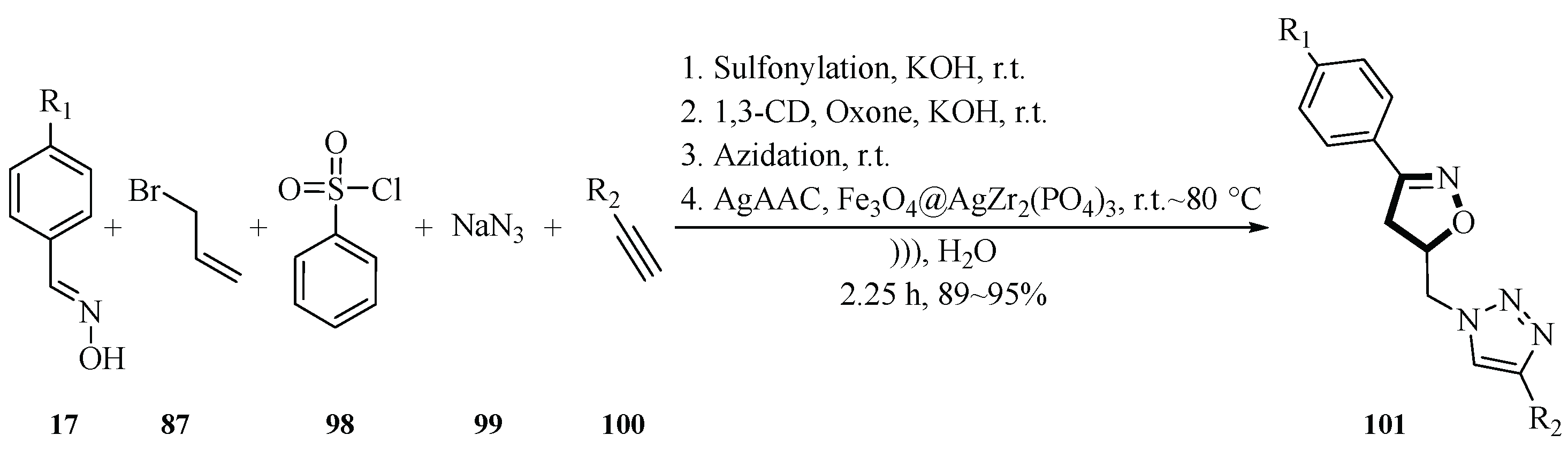
In 2024, the Bougrin group cleverly synthesized isoxazolines
101 through a one-pot five-component four-step reaction by modifying the reaction products based on their research on ultrasound-assisted and Fe
3O
4@AgZr
2(PO
4)
3 nanocatalyst synthesis of isoxazoles. This reaction employed benzenesulfonyl chloride
98 as the sulfonylation reagent and potassium hydroxide as the base, reacting with allyl alcohol in water at 25°C, and combined aryl aldehyde oxime
17, sodium azide
99 and terminal alkynes
100 to synthesize isoxazoline compounds
101. Their ultrasound-assisted aqueous system (20 kHz, 60% power) under temperature gradient conditions (25~80°C) reduced the reaction time from 24 h to 75 min (total duration 135 min), with yields of product
101 reaching 89%~95%. This method represents an advancement in atom-economical heterocyclic synthesis, addressing limitations such as high energy consumption and poor regioselectivity through innovative catalyst design and sonochemistry activation (
Scheme 39) [
41].