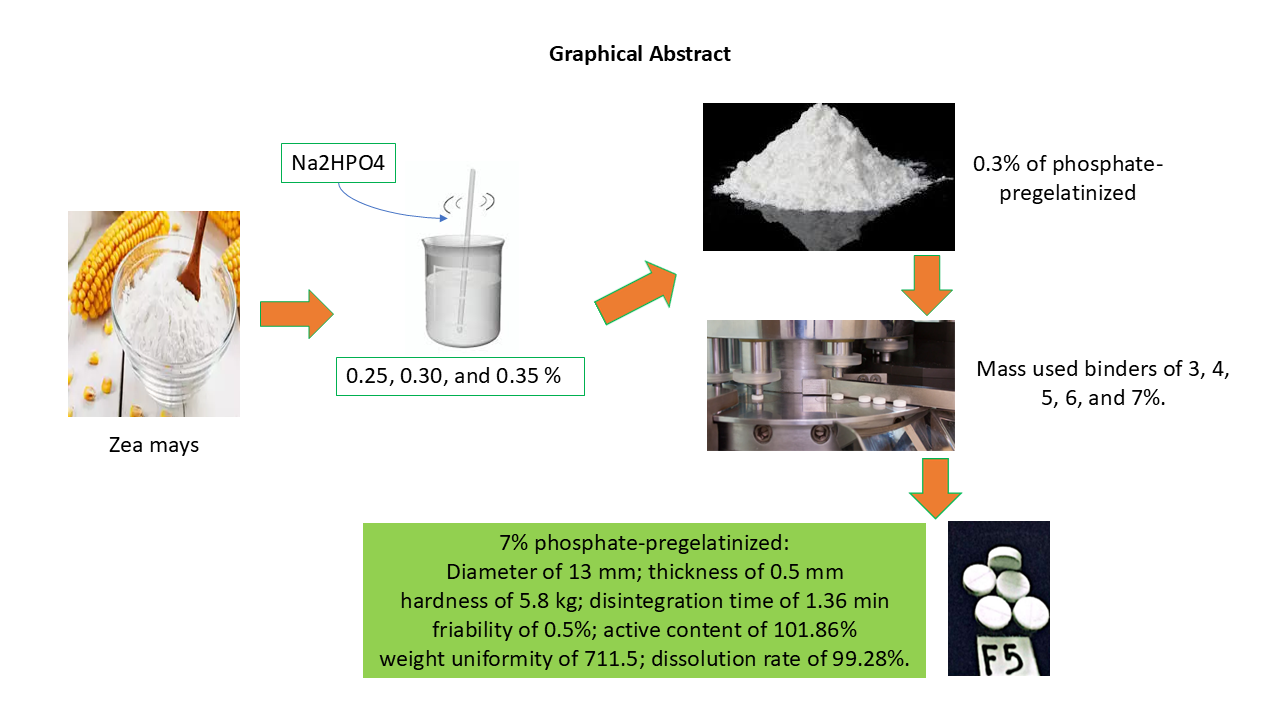
3.10.1. Preparation of Phosphate-Pregelatinized Cornstarch
To prepare phosphate-pregelatinized cornstarch, regular cornstarch (
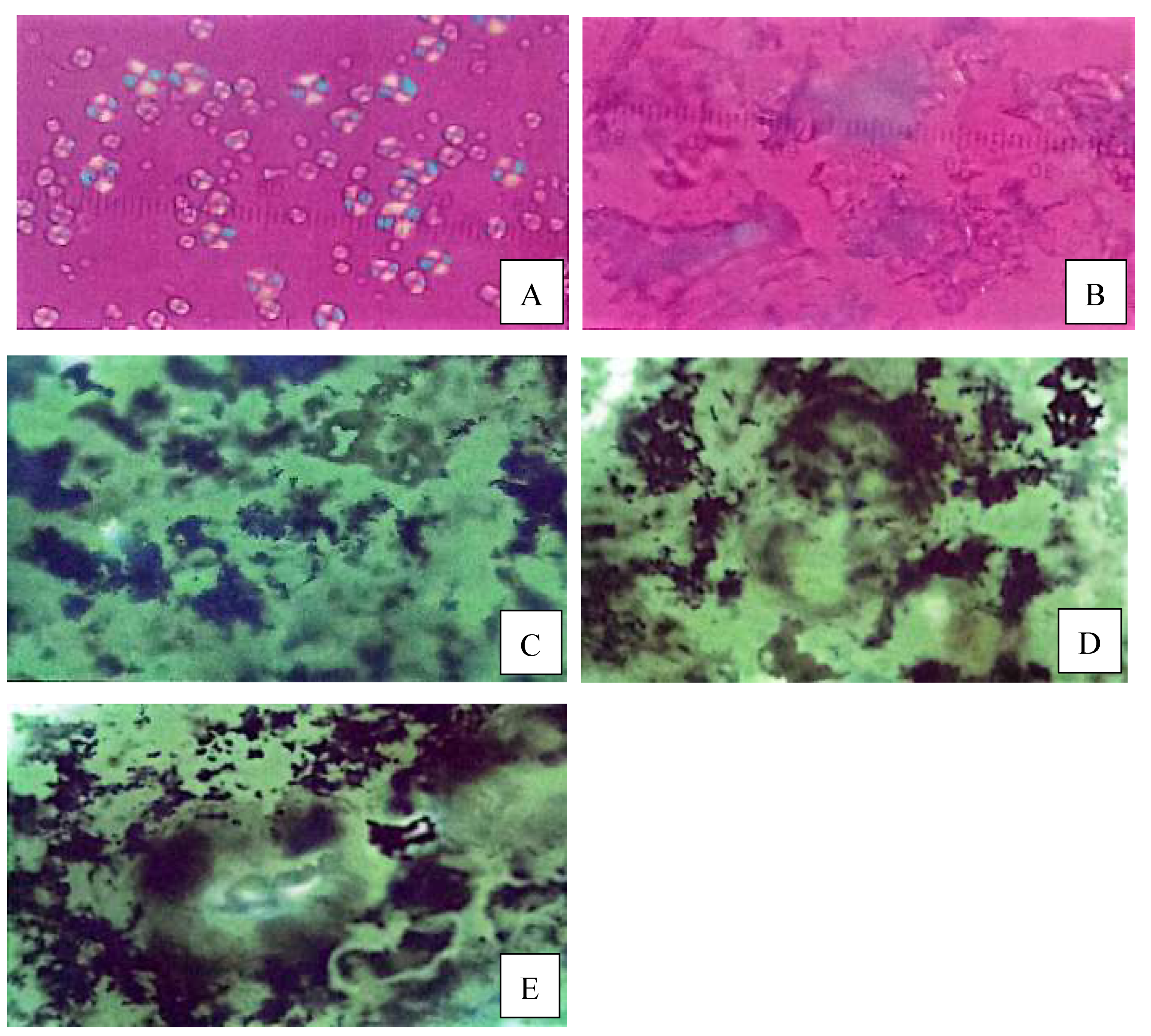
Figure 1A) must first be pregelatinized (
Figure 1B). This involves making a cornstarch paste at 72 °C and drying it with a double-drum dryer at a temperature above the gelatinization point of cornstarch, which is 80 °C. During paste formation, the starch granules swell, and as the paste passes through the double-drum dryer, the swollen granules break down, releasing amylose and amylopectin. This damages the granules’ structure and disrupts the hydrogen bonds, making it easier for water and phosphate molecules to penetrate the starch [
29,
30].
In this study, the addition of phosphate reagents led to the formation of starch diester phosphate. After the pregelatinization process, adding disodium hydrogen phosphate (Na2HPO4) allowed it to penetrate the starch molecules and form intramolecular bridges between them. These bonds are more durable than the hydrogen bonds between the hydroxyl groups in starch.
The reaction pH is crucial, as each reagent requires a specific pH to produce the desired result. Diester and triester phosphates generally form within a pH range of 8 to 12. Meanwhile, this study utilized Na
2HPO
4, which has a pH of 9. Therefore, before adding Na
2HPO
4 during the preparation of phosphate-pregelatinized cornstarch (
Figure 1C, D, and E), the starch paste was adjusted to pH 9 using NaOH to optimize the reaction [
9,
31].
3.10.2. Characterization of Phosphate-Pregelatinized Cornstarch
This study began with an examination of the microscopic form of cornstarch according to that described in the Indonesian Pharmacopoeia edition IV, which formed polygonal, angular, or round grains that, when viewed under polarized light, appear black and cross-shaped, cutting at the hilus [
11,
32].
During the pregelatinization process, the starch granules interact with water and heat; as a result, they swell and break, releasing amylose and amylopectin. Initially, starch granules reflect polarized light, appearing as black and white crystals under a microscope. This is referred to as birefringence. When the granules break, this birefringence will disappear.
Figure 1A and 1B prove that the structure of pregelatinized cornstarch changes as a result of the pregelatinization process [
33,
34].
Figure 1C, 1D and 1E show the microscopic shapes of the phosphate-pregelatinized cornstarch, which appear grouped and irregular due to the rebinding of amylose and amylopectin molecules that were previously broken down by the pregelatinization process involving the phosphate group. As a result, a phosphate diester bond forms, or the molecules undergo cross-linking [
31].
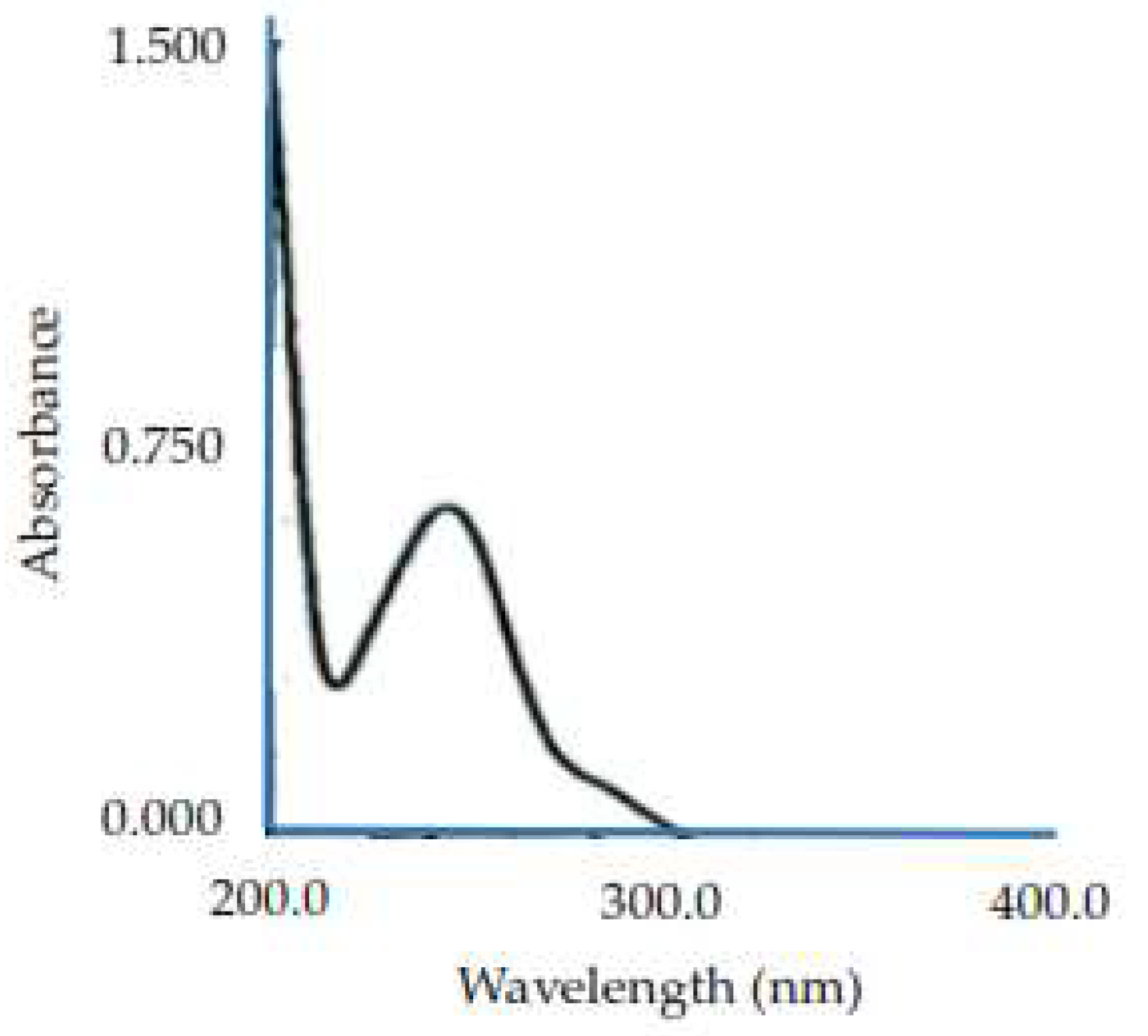
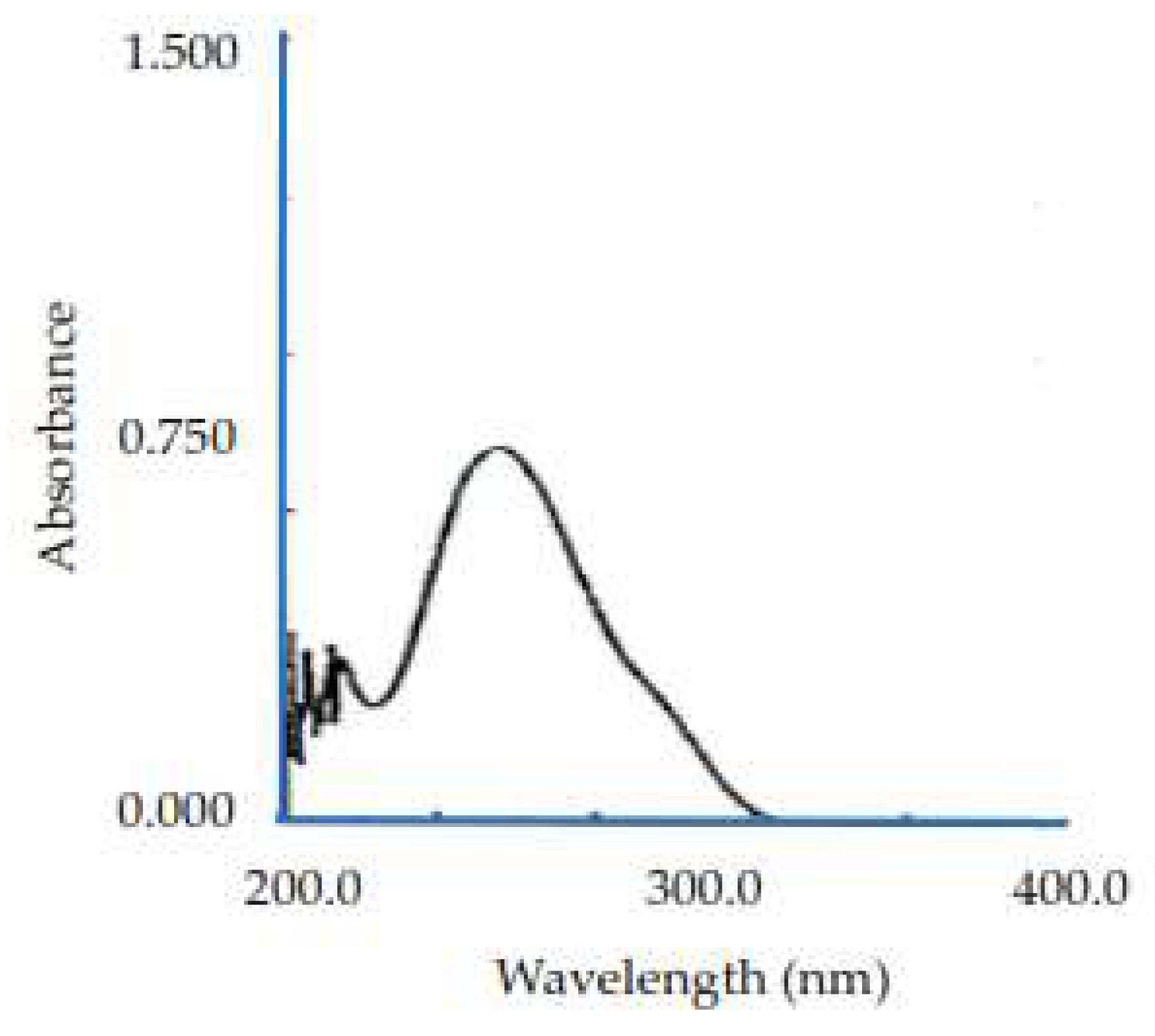
The results of the viscosity examination show that the cornstarch dispersion has very low viscosity, close to that of water. Cornstarch is practically insoluble in water and possesses few hydroxyl groups that can bind to water; thus, its viscosity value is small or undetectable. Pregelatinized cornstarch has higher viscosity than phosphate-pregelatinized cornstarch because it contains more hydroxyl groups, allowing it to bind with water through hydrogen bonding, which increases viscosity. Meanwhile, phosphate-pregelatinized cornstarch has a lower viscosity value than pregelatinized cornstarch because the hydroxyl group in phosphate-pregelatinized cornstarch links to the phosphate group. Although the viscosity is lower, the bond of the starch diester phosphate is more durable, has a more stable viscosity, and produces strong granules, but is easily dispersed in water. Phosphate-pregelatinized cornstarch with a Na2HPO4 concentration of 0.3% was used as a binder because it has a higher viscosity value than that with Na2HPO4 concentrations of 0.25 and 0.35%. A high viscosity will produce strong granules by creating stronger bonds between particles. The results are shown in
Table 2 [
35].
The chemical identification test confirmed that the white powder used was starch (
Table 2). The test used the iodine reagent and produced a substance with a light purple color. In contrast, pregelatinized cornstarch phosphate reacted to form a blue color. The difference in color results from variations in the length of the amylose chains present in different starches—the longer the amylose chain, the darker the resulting color [
36].
The acidity-level examination showed that cornstarch, pregelatinized cornstarch, and phosphate-pregelatinized cornstarch all met the necessary requirements, ranging from 4.5 to 7.0 (
Table 2). The pH of phosphate-pregelatinized cornstarch is increased due to the neutralization process (resulting from the addition of acidic reagents during the manufacturing process) [
35].
The results of the whiteness examination showed that phosphate-pregelatinized cornstarch had a higher whiteness percentage than cornstarch and pregelatinized cornstarch (
Table 2). The greater the number of phosphate reagents used, the whiter the starch obtained. This improved color could be attributed to PO
4-
3 ions, which block the reducing sugar group, inhibiting the reaction with free amino acids, which can produce brown pigments [
37].
The results of the loss on drying (LOD) examination of cornstarch, pregelatinized cornstarch, and phosphate-pregelatinized cornstarch are within the requirements, namely, no more than 15% (
Table 2). The LOD test aims to determine the starch water content. The variation in LOD values among different starches is due to the rotation speed of the double-drum dryer during the drying process. Rotation that is too fast results in uneven starch breakdown and the formation of thin sheets that resemble wood shavings, but if the rotation speed decreases, a fine powder will be produced [
38].
3.10.4. Tablet Preparation
Based on the literature, the concentration of cornstarch used as a binder was 5-25%, and for pregelatinized cornstarch, it was 5-10%. Previous studies utilizing various types of phosphate-pregelatinized cornstarch employed concentrations ranging from 1 to 5% [
41].
After testing phosphate-pregelatinized cornstarch at a 3% concentration, the tablets produced did not exhibit sufficient strength due to their high fragility, although they did meet the dissolution requirements. To enhance tablet integrity, a higher concentration was necessary. Thus, this study used phosphate-pregelatinized cornstarch as a binder at concentrations ranging from 3 to 7%, with the 7% concentration as the comparative formula (
Table 1).
The process of preparing paracetamol tablets begins by weighing all the ingredients. Then, paracetamol, lactose, and cornstarch were mixed until homogeneous (Mass I). In another container, the modified cornstarch was dissolved in cold water until it formed a paste. Pregelatinized cornstarch requires 7 times the weight of the starch, while phosphate-pregelatinized cornstarch requires 4.5 times the weight of the starch.
If the water exceeds the limit, the mass will become soft and will be unable to be sieved. The paste is mixed in Mass I and stirred until homogeneous. The moist mass is sieved with an 8-mesh sieve, then dried in an oven at 50 °C for 8 hours. The dry granules are sieved again with an 18-mesh sieve. In a different container, primogel, talcum, and magnesium stearate are mixed, then reweighed according to the weight of the dry granule mass. The external phase is mixed into the dry granule mass and stirred until homogeneous. Then, the mass is ready to be compressed [
42].
3.10.5. Granule Evaluation
Testing begins with checking the water content. Quality granules have a residual moisture content of 3-5%. If the moisture content of the granules is more than 5%, the granules will become a place for microorganisms to grow, while if the moisture content is below 3%, the granules will become brittle and the strength of the binding material will decrease so that the granules can turn into powder. According to our test results, all the stated formulas meet the requirements.
Table 3 shows the water content of granules [
43].
The purpose of checking the water content is to measure the remaining water in the sample, whereas the loss on drying (LOD) test determines both the water content and the presence of volatile substances (
Table 3). Therefore, this test can be considered equivalent to the water content test [
44].
Before being compressed into tablets, granules must undergo a flow-rate test. This test determines the time required for 100 g of granules to pass through an aluminum funnel, which correlates with their ability to flow from the hopper to the die during tablet compression. The data in
Table 3 indicates a decrease in flow time from F1 to F5, attributed to the granules’ increasing compressibility, resulting in shorter flow times. The repose angle tests show that all formulas except F1 have perfect repose angle values. The data on repose angles is presented in
Table 4 [
45].
Granules are considered to be fragile if their brittleness value exceeds 20%. The results show that F1, F2, and F3 have fragile granules due to the use of insufficient binders (
Table 6) [
46]. Granule friability can be influenced by particle size distribution (PSD) (
Table 5). For example, granules with a broader PSD or smaller particles may be more prone to breaking or crumbling under stress. Understanding the relationship between PSD and friability is crucial for optimizing granule production and ensuring the quality of the final product [
47].
The granule compressibility test results indicate that all formulas exhibit low compressibility (
Table 4), allowing each formula to be efficiently compressed even with minimal pressure. Additionally, the percentage decrease is directly related to the binder concentration used [
48].
Granule homogeneity tests are performed to determine whether the active substances contained in the granules are homogeneous. This test involves taking granules from three different parts. In our study, we determined that paracetamol granules are homogeneous (
Table 4) [
49].
3.10.6. Tablet Evaluation
Tablet evaluation includes physical and chemical tests. The physical evaluation involves assessing the tablets’ appearance, size uniformity, hardness, friability, disintegration, organoleptic properties, and weight uniformity. The tablets’ chemical content, weight variation, and dissolution are determined via the chemical evaluation [
50].
a. Physical evaluation of tablets
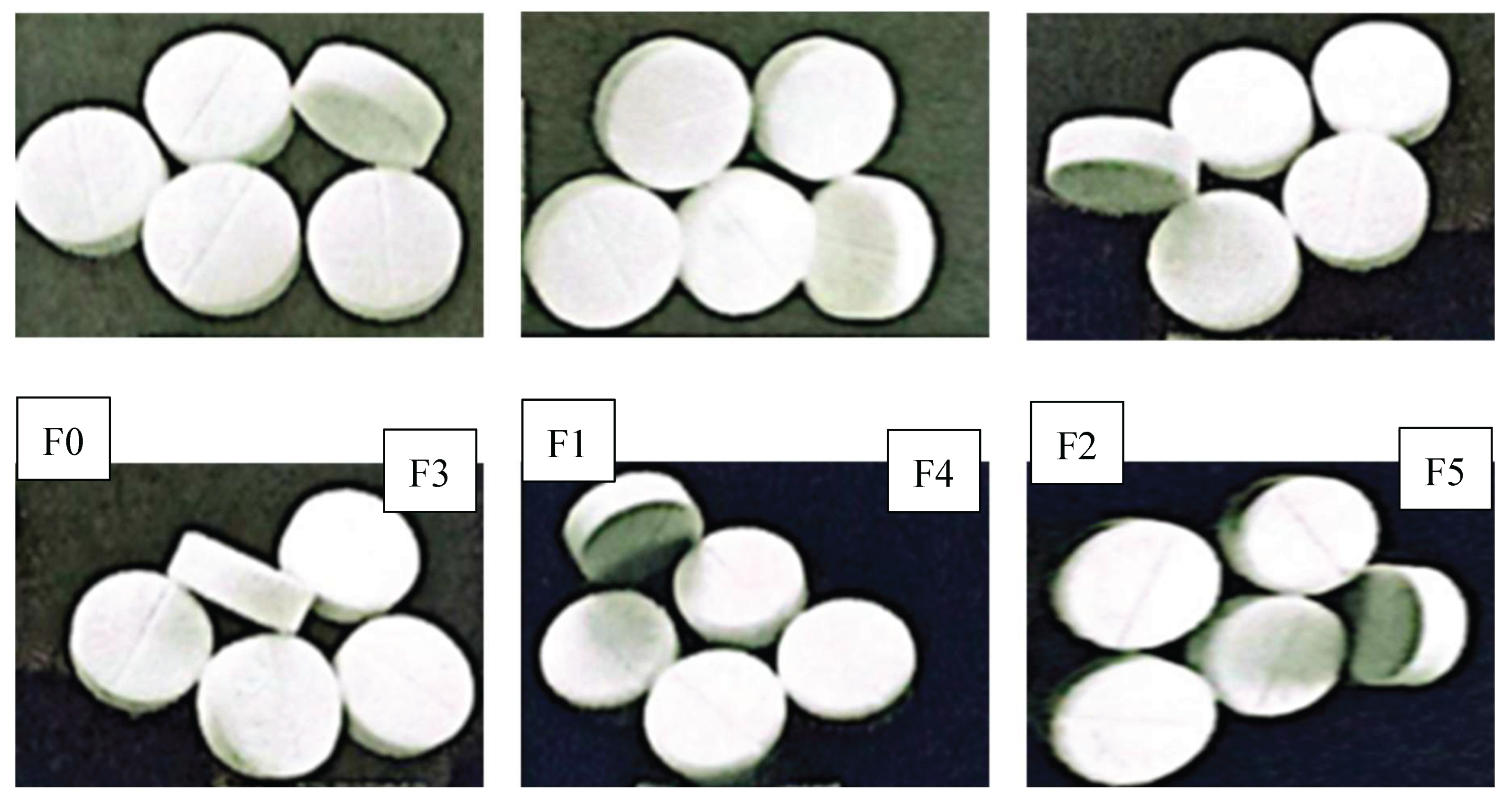
The evaluation begins with an assessment of the general appearance and organoleptic properties of each formula. In our experiments, the tablets were white and odorless, with a smooth and shiny surface and a slightly bitter taste, and did not feature the UHAMKA logo (
Figure 8) [
51,
52].
The diameter of all tablet formulas remains constant because the same tableting machine is used, with the tablet’s diameter determined by the punch and die size. However, tablet thickness varies due to compression pressure, granule particle size, and the quantity of granules entering the die. All tablet formulas meet the standards outlined in the Indonesian Pharmacopoeia Edition III, which specifies that the diameter should not be more than three times but should be at least 4/3 times the thickness of the tablet
Tablet friability is a parameter that indicates the strength of the tablet; namely, whether the tablet can erode or flake, and whether it is resistant to shocks, friction, packaging, and distribution. Friability testing on F1 and F2 did not meet the requirements because the results exceeded the specified percentage. In contrast, the friability of F0, F3, F4, and F5 adhered to the requirements. This is due to the insufficient amount of binder in F1 and F2, which caused a decrease in compactness. A good tablet has friability of less than 1% (
Table 7) [
51,
52].
The tablet disintegration time is another parameter that is used to assess the physical durability of a tablet. It tests the tablet’s ability to disintegrate into fine particles so that it is ready to dissolve and be absorbed by the body. According to the Indonesian Pharmacopoeia edition IV, uncoated tablets must have a disintegration time of less than 15 minutes. All of the formulas in our study met this requirement. The values obtained from each formula indicate no significant correlation between the increase in binder concentration and the tablet disintegration time (
Table 7).[
51,
52]
The results of the tablet hardness test indicate that each formula meets the requirements. Tablet hardness refers to tablets’ ability to withstand physical shocks that cause breakage, and a hardness value of 4-8 kg is considered good. The values obtained from each formula show that the tablet hardness increases, although the difference is not too significant with the addition of the concentration of the binder. These changes are due to the relationship between the contents of the die and the pressure that determines the hardness of the tablet (
Table 7) [
51,
52].
The tablet weight variation test showed that all formulas met the test requirements because the weight deviation percentage was less than 5% (
Table 7) [
51,
52].
b. Tablet chemical evaluation
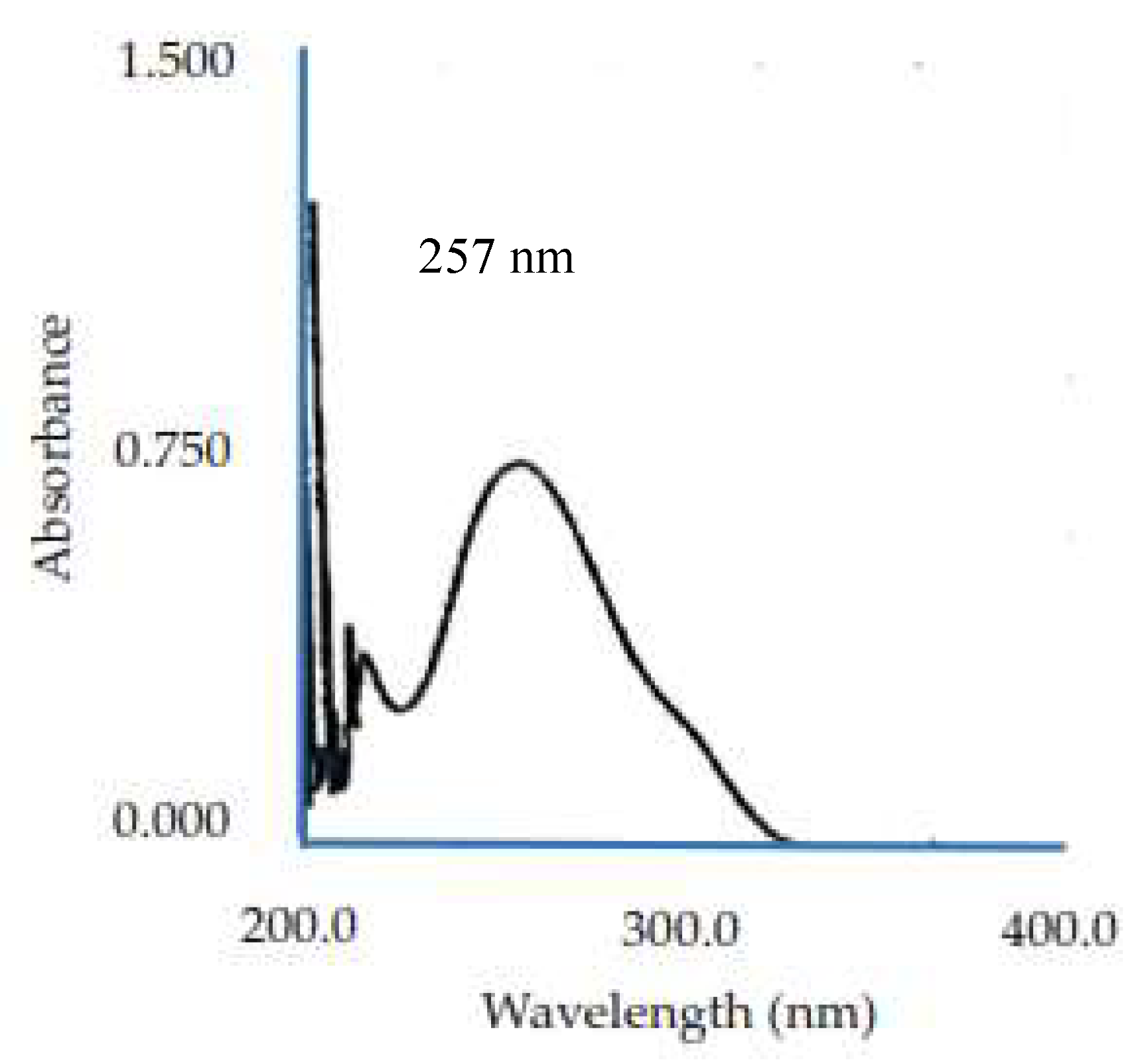
The test begins with a content examination performed using a UV-Vis spectrophotometer, which allows us to ascertain whether the tablets meet the requirements specified by the Indonesian Pharmacopoeia IV. The general requirements for levels in paracetamol tablets are no less than 90.0% and no more than 110.0% of the amount stated on the label. The use of 0.1N NaOH medium in determining the content of paracetamol tablets is as per the requirements stated in the British Pharmacopoeia volume II, while the use of a wavelength of 257 nm is based on the results of the examination of paracetamol raw materials and by the wavelength listed in Clarke’s book (1986). All of the tablet tests showed that all formulas met the requirements for determining the content (
Table 8) [
31,
39,
53].
The test results of the weight variation indicate that all formulas meet the previously established requirements. According to the Indonesian Pharmacopoeia IV, weight variation is used for soft capsule products containing liquids or in products containing 50 mg or more of active ingredients, which is 50% or more of the weight of the preparation unit calculated based on the determination of content with the assumption that the active ingredient is homogeneous (
Table 8).[
31]
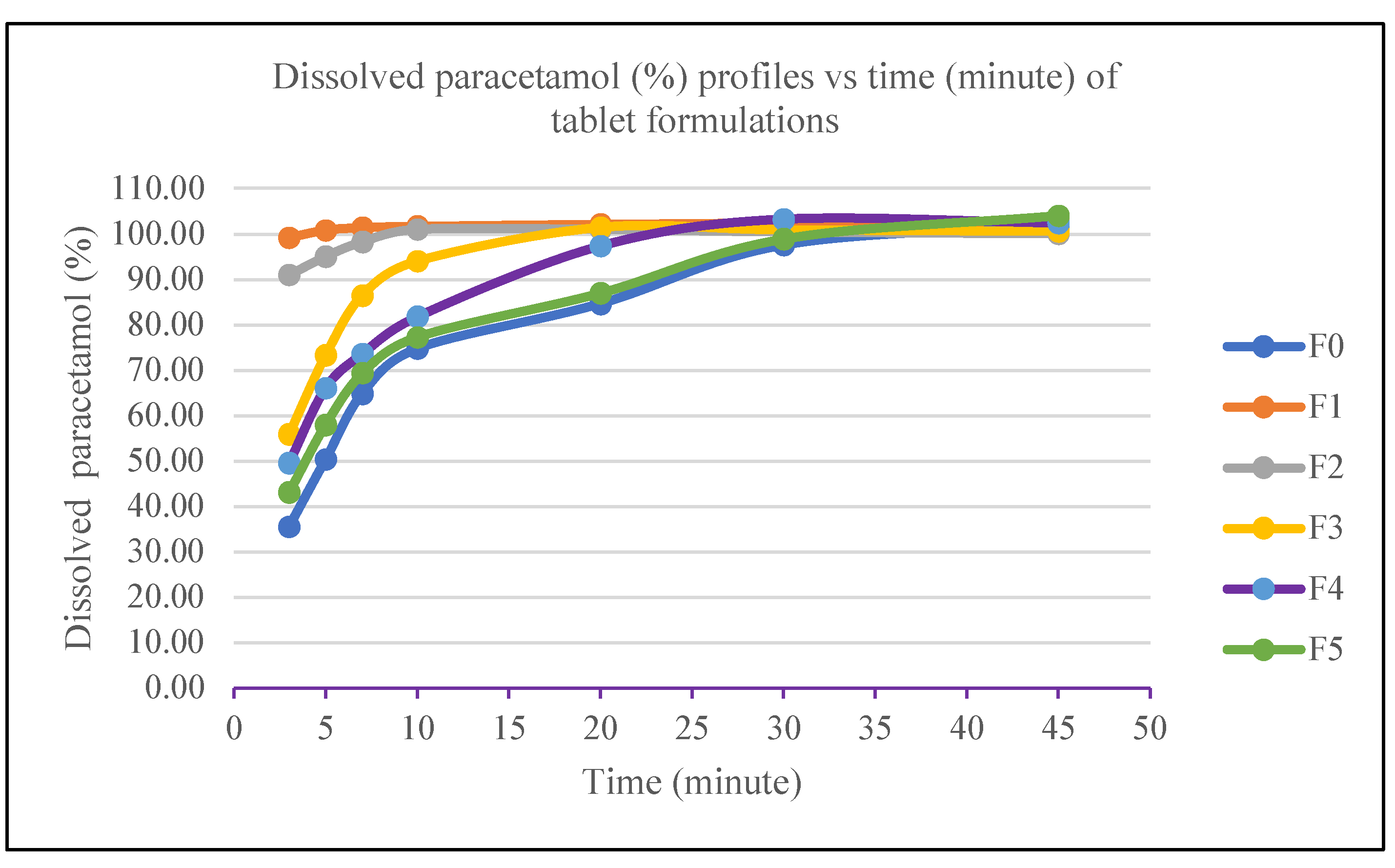
Dissolution testing determines the amount of active ingredients that dissolve within a specific time according to the requirements. We performed dissolution tests on paracetamol tablets using type 2 alt with phosphate-buffered medium (pH 5.8) in quantities as high as 900 mL, with a speed of 50 rpm at 37 °C for 45 minutes. The tolerance (Q) set for C8H9NO2 at 3 minutes is 80% of the amount stated on the label. The dissolution results at a specific time were used to create a dissolution profile curve between the percentage of dissolved substances and time (minutes). As a result, by the 30-minute mark, all formulas had met the requirements; namely, they achieved no less than Q + 5% (
Table 9).[
31]
The dissolution efficiency percentage was obtained from the percentage data after correction was performed in advance. Calculation with a correction factor will provide the actual percentage of dissolution levels because it does not rely on the experimental conditions, including the influence of removing the test solution from the chamber and adding new medium. The average dissolution efficiency did not differ significantly between F1 and F2. Although both have different dissolution profiles, there was little difference in the area under the curve. The binding power of the two tablets was deemed insufficient due to the concentrations of phosphate-pregelatinized cornstarch being 3% and 4%, respectively (
Table 10). This concentration is considered less effective at binding the active substance in the granules, causing the substance to be released and dissolve immediately in the medium [
54].
There is a difference in the percentage of dissolution efficiency between F5 (using phosphate-pregelatinized cornstarch) and F0 (using pregelatinized cornstarch) as a binder with the same concentration of 7%. Therefore, phosphate-pregelatinized cornstarch has properties that allow it to more easily bind with water, forming a gel, and dissolution test treatment, such as stirring and increasing the temperature, weakens the gel bond and releases the active substance faster so that paracetamol can dissolve in the medium (
Table 10) [
55].
The study found that pregelatinization and subsequent phosphate modification of the starch significantly improved its binding capabilities and solubility, leading to better tablet characteristics, particularly with a 7% concentration of the modified starch. The novelty lies in the specific method of phosphate pregelatinization and its successful application in tablet formulation, leading to improved paracetamol solubility. This approach is not commonly reported, especially the combination of pregelatinization and phosphate modification.
3.10.7. Statistical Analysis
Statistical analysis was performed using the SPSS (Statistical Product and Service Solution) program, starting with the Kolmogorov–Smirnov, non-parametric Kruskal–Wallis, and Daniel’s multiple tests [
56].
The Kolmogorov–Smirnov test assesses whether the data follows a normal distribution. Based on the asymptotic significance column, each formula has a significance value greater than 0.05 (α= previously predetermined), indicating that the dissolution efficiency data has a normal distribution.
The homogeneity of variance test aims to determine whether the five formulas have the same variance. The significant value (0.000) < α (0.05) suggests that the population of dissolution efficiency data does not have the same variance (not homogeneous). The data was processed using the equation log √(x), but the data variance is still not homogeneous with a significant value (0.000) < α (0.05). The homogeneity results indicate that the data do not meet the requirements for parametric testing, so the analysis continued using the Kruskal–Wallis non-parametric test.
Statistical analysis via the Kruskal–Wallis test showed that there were differences in dissolution efficiency among the formulas, as indicated by a significant value (0.000) < α (0.05). Statistical analysis was continued with Daniel’s multiple tests to see which formula was different. Daniel’s multiple test results showed that all formulas had significantly different dissolution percentages.