Holotype. MNRJ 56004, 23.4 mm SL, Brazil, Espírito Santo State. Aracruz, Rio Riacho basin. Swamp area on Sertão do Riacho Stream, -19,73992S -40,04357W, 26 Jan 2035, B. Pinheiro.
Paratypes. MNRJ 56005, 9, 16.4–23.4 mm SL, Brazil, Espírito Santo State. Aracruz, Rio Riacho basin, Swamp area on Sertão do Riacho Stream, -19,73992S -40,04357W, 26 Jan 2025, B. Pinheiro, collected with the holotype.
Diagnosis. Xenurolebias tupinikin is distinguished from remaining congeners by adult males presenting in life a dark blotch overlapping the median portion of the last stripe on caudal peduncle (
Figure 2). Further differs from congeners, except X. myersi, by presenting the dorsal and anal fin borders with black outline. Additionally distinct from X. myersi by body depth 25.9- 27.9 in SL (vs. 29.0- 31.4 in SL).
Differs from X. izecksohni and X. cricarensis by the caudal fin in males with 5-6 bars (vs. 7- 14 bars) and by male head depth 64.5-76.9% of HL (vs. 81.2- 85.6% of head length in males). Further differs from X. pataxo and X. myersi by no yellow spots on distal half of dorsal fin in males (vs. yellow spot present).
Description. Morphometric data available in
Table 1. Maximum adult size 23.9 mm SL. Body slender, sub-cylindrical anteriorly, slightly deeper than wide, to compressed posteriorly; greatest body depth at level of pelvic-fin base. Dorsal and ventral profiles gently convex from snout to end of dorsal and anal-fin bases, nearly straight on caudal peduncle. Jaws short, snout blunt. Eye small, positioned on dorsal portion of head side. Extremity of dorsal fin pointed and long in males, rounded to slightly pointed in females; tip of anal fin pointed in males, rounded in females; in males, one or two filamentous rays on tip of dorsal and anal fins reaching vertical between base and middle of caudal fin. Caudal fin rounded or sub-lanceolate in shape. Pectoral fin elliptical, posterior margin reaching vertical between base of third and sixth anal-fin rays in males, reaching urogenital papilla in females. Pelvic fin small, tip reaching base of second or third anal-fin ray in males, reaching base of third anal-fin ray in females. Pelvic-fin bases medially united. Dorsal-fin origin on vertical through base of eighth or nineth anal-fin ray in both sexes. Dorsal-fin rays 17 in males, 12-13 in females; anal-fin rays 21 in males, 21-23 in females; caudal-fin rays 21; pectoral-fin rays 13; pelvic-fin rays 6. Scales small, cycloid. Body and head entirely scaled, except ventral surface of head. Body squamation extending over anterior 25% of caudal-fin base; few scales slightly extending on middle of anal-fin base; no scales on dorsal-fin base. Scales arranged in regular transverse pattern. Two small supraorbital scales. Longitudinal series of scales 27; transverse series of scales 9; scale rows around caudal peduncle 12. Contact organ on each scale of ventral portion of flank in male. Minute papillate contact organs on 2 dorsal-most rays of pectoral fin in males. Single neuromast on each scale of lateral line; 2 neuromasts on caudal-fin base. Cephalic neuromasts: supraorbital 10-12, parietal 1, anterior rostral 1, posterior rostral 1, infraorbital 2 + 16-18, preorbital 3, otic 1, post-otic 2, supratemporal 1, median opercular 1, ventral opercular 2, pre-opercular plus mandibular 23-24, lateral mandibular 5-7, paramandibular 1.
Table 1.
Morphometric data of Xenurolebias tupinikin, sp. nov.
Table 1.
Morphometric data of Xenurolebias tupinikin, sp. nov.
| |
Xenurolebias tupinikin |
Holotype
male |
Male
(n=5) |
Female (n=10) |
| Standard length (mm) |
23.3 |
20.3-23.3 |
16.5-23.9 |
| Percents of standard length |
|
|
|
| Body depth
|
26.9 |
25.9-27.9 |
19.0-32.9 |
| Caudal peduncle depth
|
14,3 |
11.4-14.4 |
6.6-14.7 |
| Predorsal length
|
59.0 |
56.6-64.1 |
48.8-73.9 |
| Prepelvic length
|
44.0 |
42.7-49.4 |
44.1-56.2 |
| Length of dorsal-fin base
|
24.2 |
24.2-29.2 |
09.1-16.8 |
| Length of anal-fin base
|
51.0 |
47.6-56.1 |
17.8-26.1 |
| Caudal-fin length
|
40.7 |
37.3-40.7 |
29.2-39.5 |
| Pectoral-fin length
|
32.9 |
27.8-33.2 |
15.8-29.7 |
| Head length
|
29,9 |
29.2-31.5 |
28.5-33.8 |
| Percents of head length |
|
|
|
| Head depth
|
76.8 |
64.4-76.8 |
61.27-85.5 |
| Lower jaw length
|
25.5 |
18.4-25.5 |
15.1-21.6 |
| Eye diameter
|
32.2 |
31.5-46.1 |
10.8-34.8 |
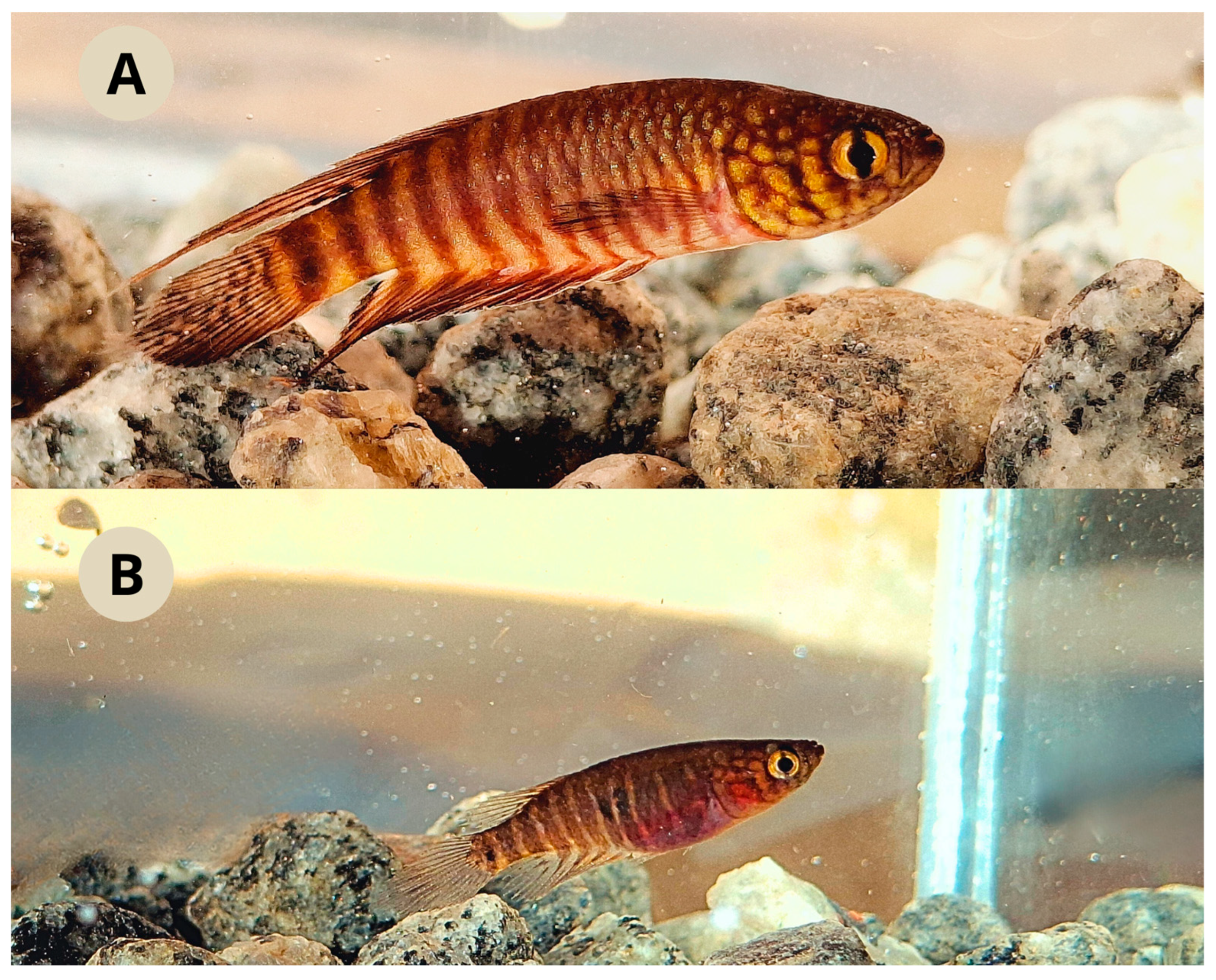
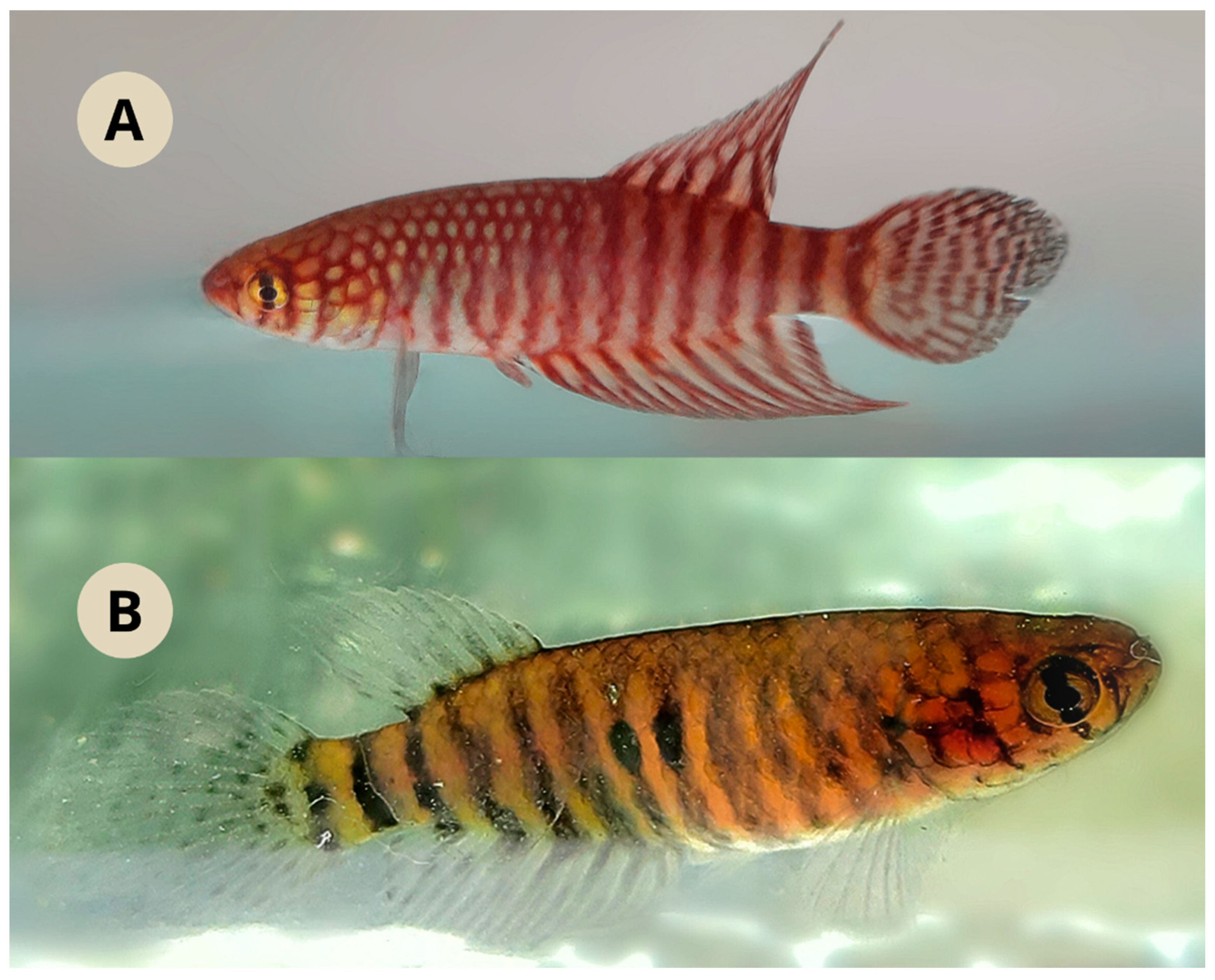
Live coloration. Male (
Figure 3a). Side of body brownish yellow, with 12-13 dark brown bars; caudal peduncle with a dark spot united to posterior-most bar. Dorsum pale brown. Venter pale pink. Opercular and infraorbital region yellowish, with two difuse dark bars. Iris light yellow with dark reddish brown bar on middle part. Dorsal fin dark brown with scattered irregular marks and fin margin becoming dark towards its tip. Anal fin dark brown to dark red, with irregular marks. Caudal fin pale yellow, with 5-6 dark irregular bars. Pectoral fin hyaline with a browinsh border. Pelvic fin light brown.
Female (
Figure 3b). Side of body light brown, with 10-11 dark grey bars; one or two black blotches on anterocentral part of flank. Dorsum pale brown. Venter light pink. Opercular region yellowish. Iris light yellow, with dark brownish bar. Unpaired fins hyaline with small dark brownish spots. Paired fins hyaline.
Distribution. The Riacho River corresponds to the first independent coastal river basin south of the Rio Doce. Its tributaries are mostly small watercourses, with predominantly sandy substrate and banks covered by riparian vegetation in different states of conservation. The largest tributary of Rio Riacho corresponds to the Rio dos Comboios, which runs in parallel to coast line until meeting the River Riacho close to its mouth.
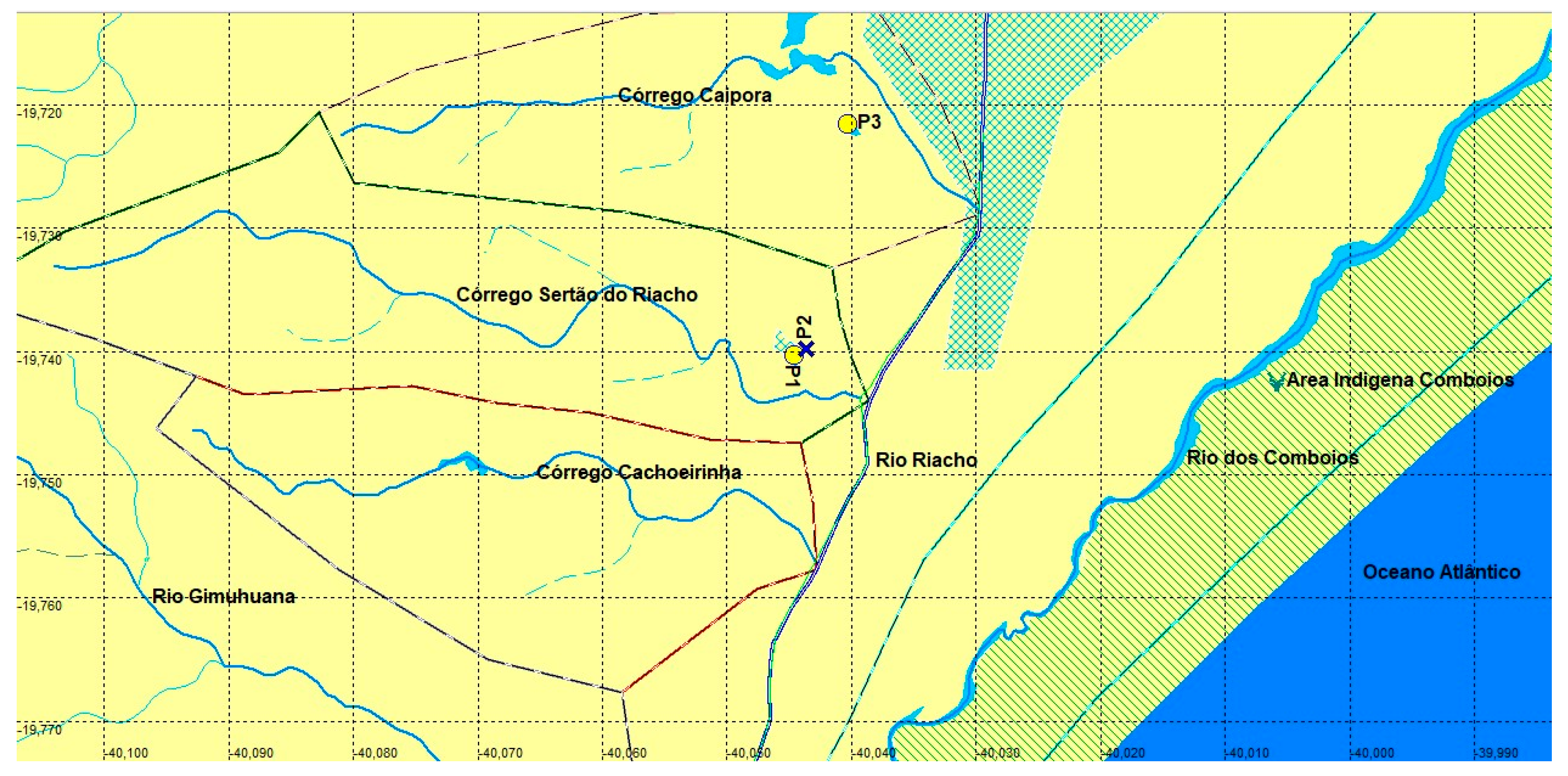
The new species is known from three localities, on two tributaries of the rio Riacho, central north of Espírito Santo, Brazil (
Figure 4- P1 to P3). The Sertão do Riacho stream and also found in neighbor stream, the Caipora stream, all contributors to the right margin of the Rio Riacho, a coastal river drainage south of the Rio Doce, Espirito Santo (
Figure 4).
Ecological notes. The Sertão do Riacho isolated pool, identified as an extensive swamp area, with approximately 2 km². Completely covered by dense emerging vegetation, formed mainly by grasses and ferns. The substrate is formed by emerging vegetation, decomposing plant material and sand (
Figure 5). Abiotic information. Dark tea colored waters in a lentic environment. Water depth at pool between 40 cm to 60 cm, Humidity: 60% / Water temperature: 27.1ºC / pH: 4.3-6.2 / Salinity (ppm): 0 (
Table 2).
The swampy area at type locality (
Figure 4- blue X) is about 100 m from point 1 (
Figure 4- yellow circle). It features dense emergent vegetation, marginal vegetation, and floating macrophytes. The swamp showed an abundance of Xenurolebias tupinikin, adults and juveniles, scattered throughout the swampy area, found mainly under vegetation.
Sampling was carried out in various parts of the pool, between edges and central part, with some limitation due to the dense vegetation that emerged from the bottom of the pool. Only adult individuals were captured, three females and one male for record. Additional specimens were immediately returned to the environment. As for the accompanying fauna, aquatic insects in a wide variety and a specimen of Callichthys callichthys was captured.
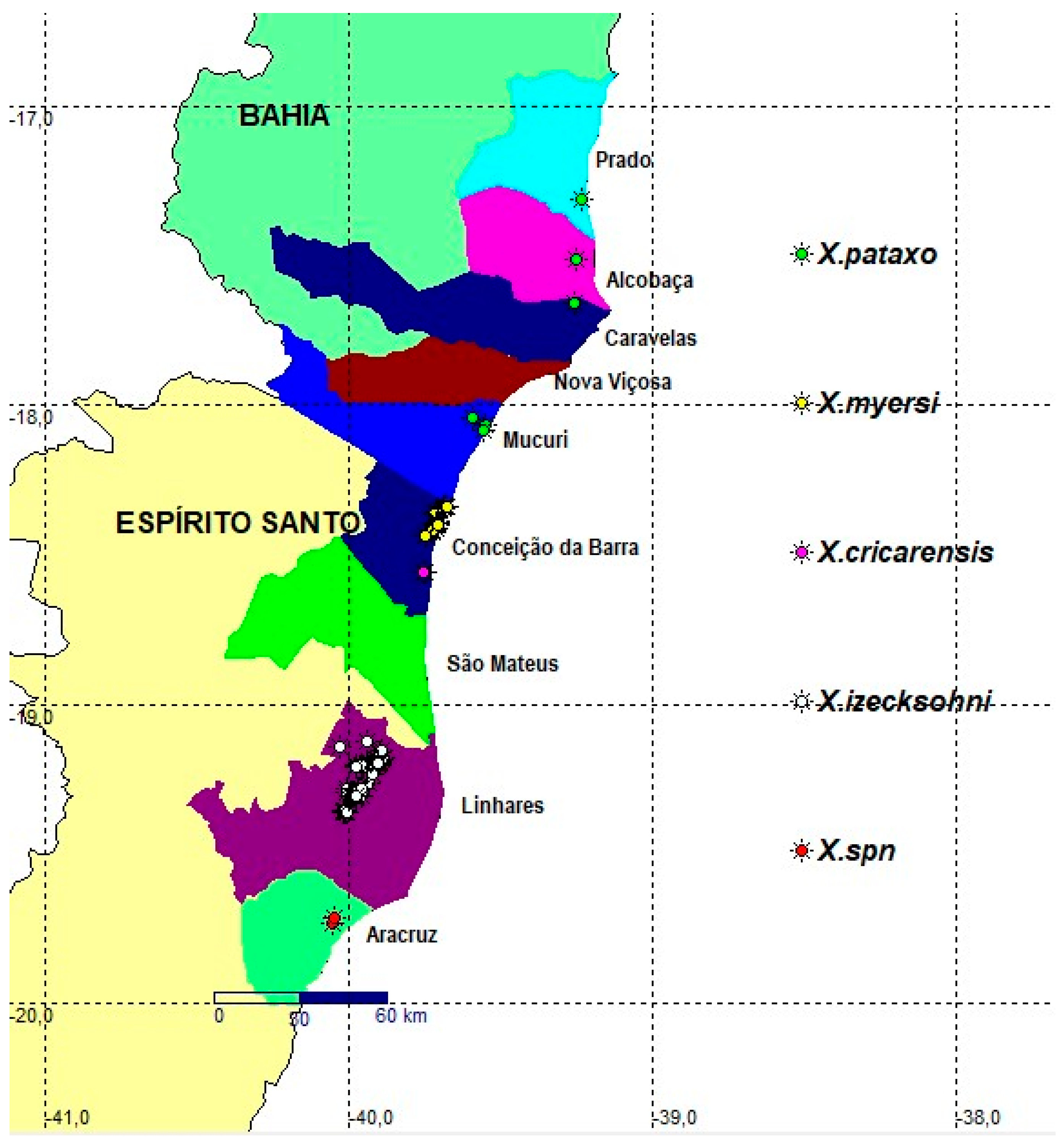
Etymology. The specific name is a reference to the Tupiniquim indigenous people, inhabitants of lowlands at central north of Espírito Santo. On left margin of the Rio Comboios is located the indigenous land Comboios (
Figure 4- green stripes near coast), which correspond to the nearest human occupation relative to the environments inhabited by these fishes. A noun in apposition.
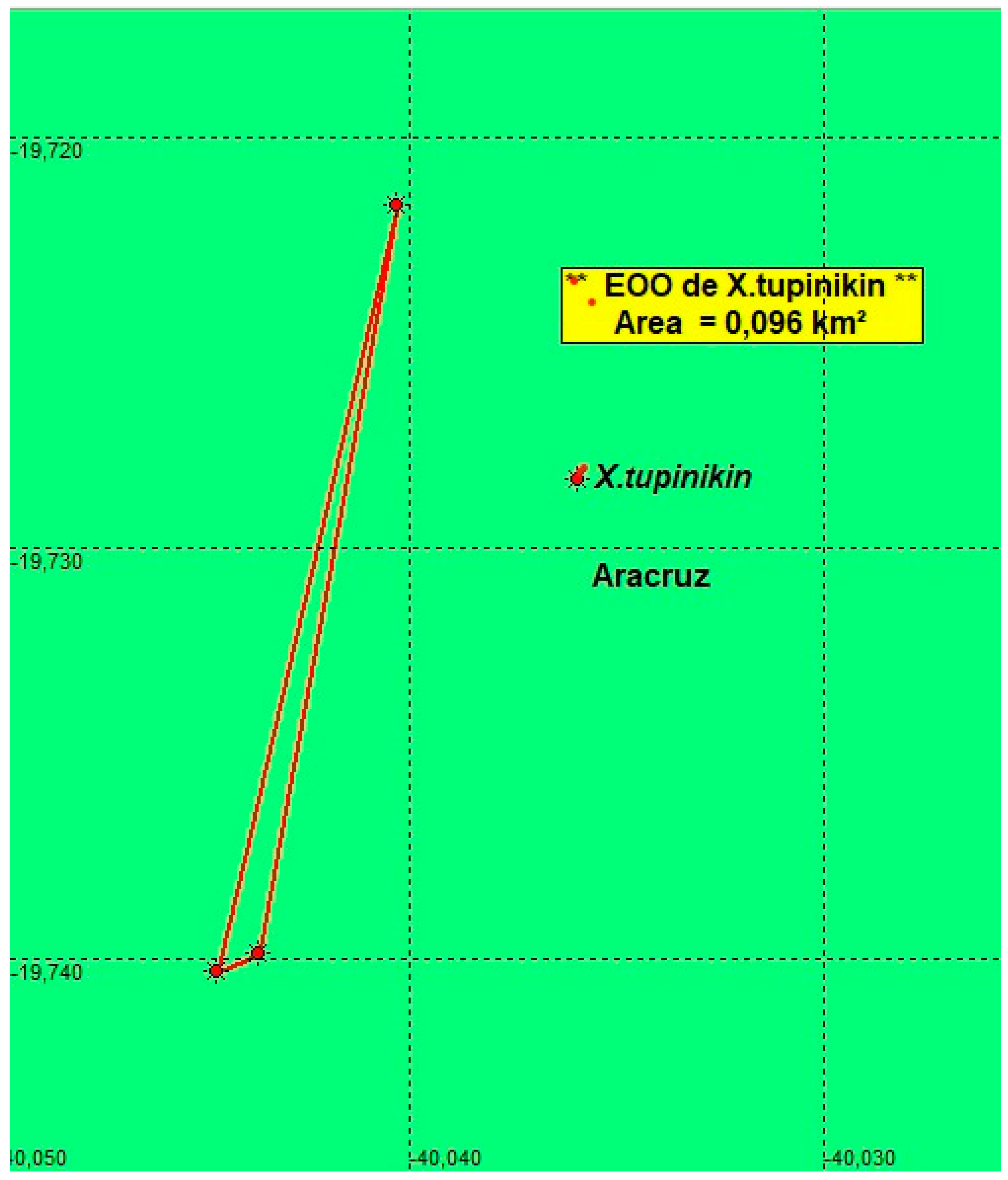
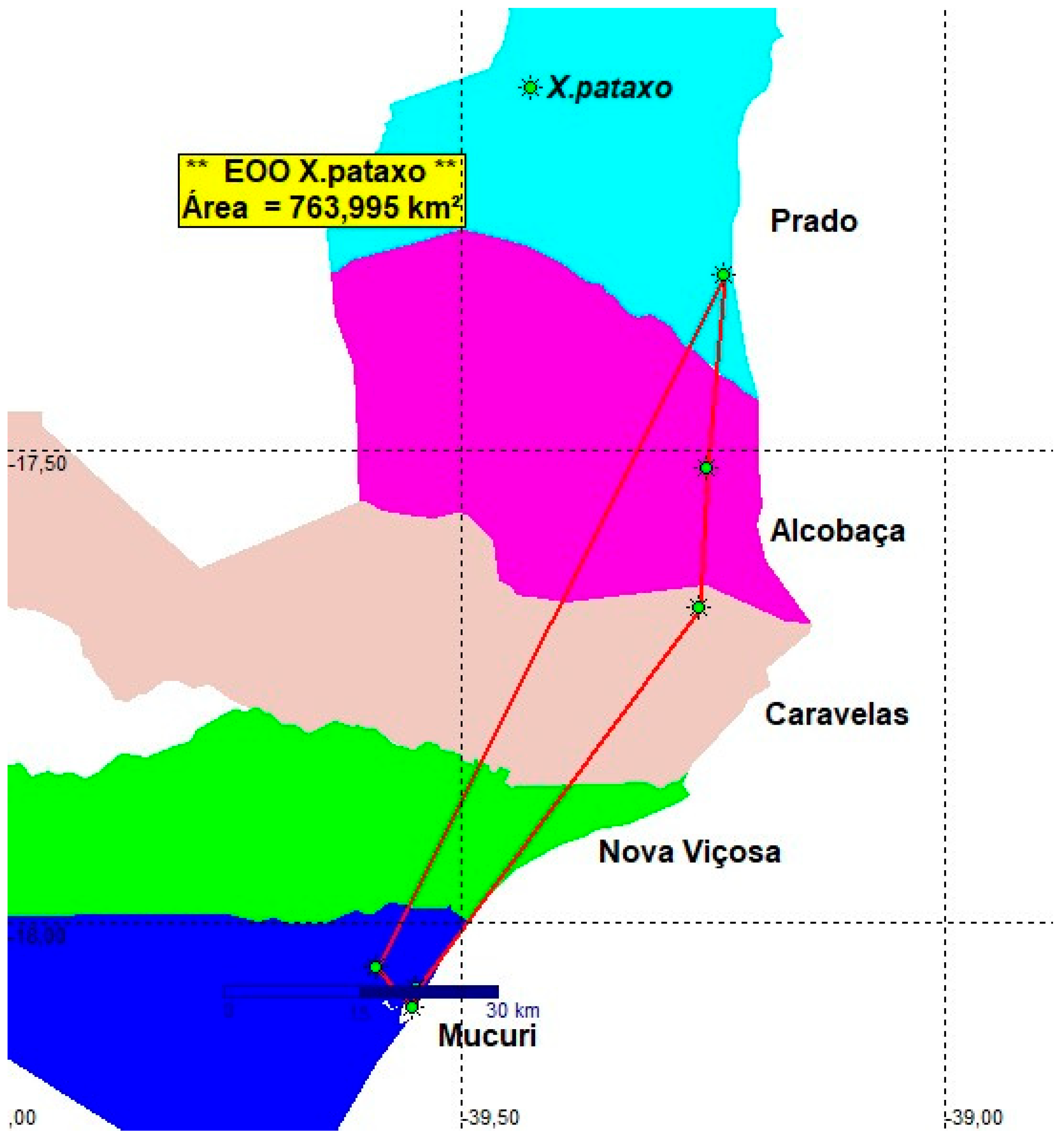
Conservation concerns. The estimated Extent of occupancy (EOO) of the population found in Riacho river basin was defined as 0.096 Km2 (two locations)(
Figure 6). From May until September, the pools where the rivulids were captured become dry.The Sertão do Riacho pool is near the road to the village of Riacho, and quite anthropized. At the site, garbage dumping points (solid waste) and debris (civil construction waste) were identified, in addition to sand extraction. The distance from the pool to the sea is approximately 4 km in straight line (
Figure 6).
Xenurolebias izecksohni was described for the Barra Seca river basin, at the locality of Canto Grande, Farias, within the Vale Natural Reserve (RNV), a private preserved area [
13,
14]. Until now, the distribution of the species was limited to the Barra Seca river basin. Between 2023 and 2025, field campaigns were carried out in search of populations. New populations were found at the Doce River basin, considerably expanding its area of occurrence to include two river basins- Doce and Barra Seca (see map on
Figure 7). The collections were carried out in different periods, covering both the dry and rainy seasons, which allowed the measurement of important data hitherto unknown for the species. It was observed that the life cycle of the species is configured as biannual, and not annual, as expected. That is, throughout the year, the puddles dry up and reestablish themselves twice, usually between November and March the first cycle, and between May and August the second, and there may be variations according to the climatic conditions of each year. In addition, it was found that puddles completely exposed to the sun dry out more quickly, while those with vegetation cover tend to persist for longer, and may even remain flooded throughout the year, which maintain populations alive in these environments.
Most of the new populations of X. izecksohni registered are distributed in private areas, including rural properties and lands of the Linhares Agroindustrial Plant - LASA, an ethanol producing company. In addition to places around the RNV, in the vicinities of the type locality. The number of individuals per pond varied between a few specimens (1 to 5) and concentrations of more than fifty individuals. The morphometric analysis revealed a considerable variation in the standard length (SL), with values higher than those recorded in the literature for the species (30–43 mm)[
14]. Males with up to 54.24 mm and females with up to 41.36 mm were measured, evidencing the occurrence of specimens larger than those previously described. Behaviors observed in the field indicate a preferential occupation of the edges of the puddles by Xenurolebias izecksohni, especially under vegetation cover or between submerged leaves. The color of the individuals varied according to the shade of the water, which, in many cases, presented a dark color due to the presence of tannins from the decomposition of organic matter. Under these conditions, the fish showed equally darkened coloration, which may indicate phenotypic plasticity and adaptation to the environment, possibly related to camouflage mechanisms and protection against predators.
Native Vegetation and Landscape. In view of the new records, it was possible to verify that the habitats occupied by Xenurolebias izecksohni present considerable variation in their structural aspects. Individuals have been found in different types of temporary aquatic environments, including puddles, ponds, and even intermittent streams. Such environments varied in terms of vegetation cover, with conditions of total exposure, partial shading or complete vegetation cover. In addition, the composition of the vegetation was also diverse, with records of marginal, floating and emerging plants. The puddles, specifically, showed great variability in terms of morphology (circular, elongated and amoeboid shapes), substrate (sandy, leafy or mixed), depth and color of the water, revealing a considerable ecological plasticity of the species. Puddles in open areas exposed to the sun showed clear waters and emergent herbaceous or aquatic vegetation, while those in areas shaded by shrub or forest vegetation exhibited darker waters and substrate rich in organic matter. Abiotic information. Dark tea colored waters in a lentic environment. Water depth at pool between 20 cm to 70 cm, Humidity: 60%-88% / Water temperature: 22ºC- 37ºC / pH: 3.5 – 5.6 / Salinity (ppm): 0 – 2 (
Table 2).
Similar to what occurs with many species of rivulids (15-16), Xenurolebias izecksohni occupies ephemeral habitats, with very peculiar characteristics, becoming inhospitable to most fish species. This condition was observed throughout the expeditions, in which records of coexistence of X. izecksohni with other species of ichthyofauna were rare. In less than 10% of the sampled points, specimens of other species, such as Astyanax spp., were collected. Hoplias malabaricus, Hoplerythrinus unitaeniatus and Callichthys callichthys, usually in larger, newly formed puddles, and close to springs or perennial watercourses. In these cases of coexistence with other fishes, the density of X. izecksohni was considerably reduced, suggesting a possible negative impact of the presence of predators or competition for resources. In contrast, in the smaller, more isolated pools, in which X. izecksohni was often the only species recorded, the population density was significantly higher, indicating a possible adaptive specialization to environments with low biotic pressure.
In newly formed puddles after rain events, newly hatched fish were recorded, which indicates recent reproductive episodes and reinforces the importance of these temporary environments ass nursery for the reproductive success of the species. In addition, an unusual observation was the simultaneous capture of individuals at different stages of development, including fry and adults living in the same environment. Such coexistence is uncommon among rivulids, whose life cycle usually involves synchronization between hatching and periods of temporary flooding, with no overlap of generations [
17,
18]. These data may indicate local phenological variations or plastic responses to changes in environmental conditions.
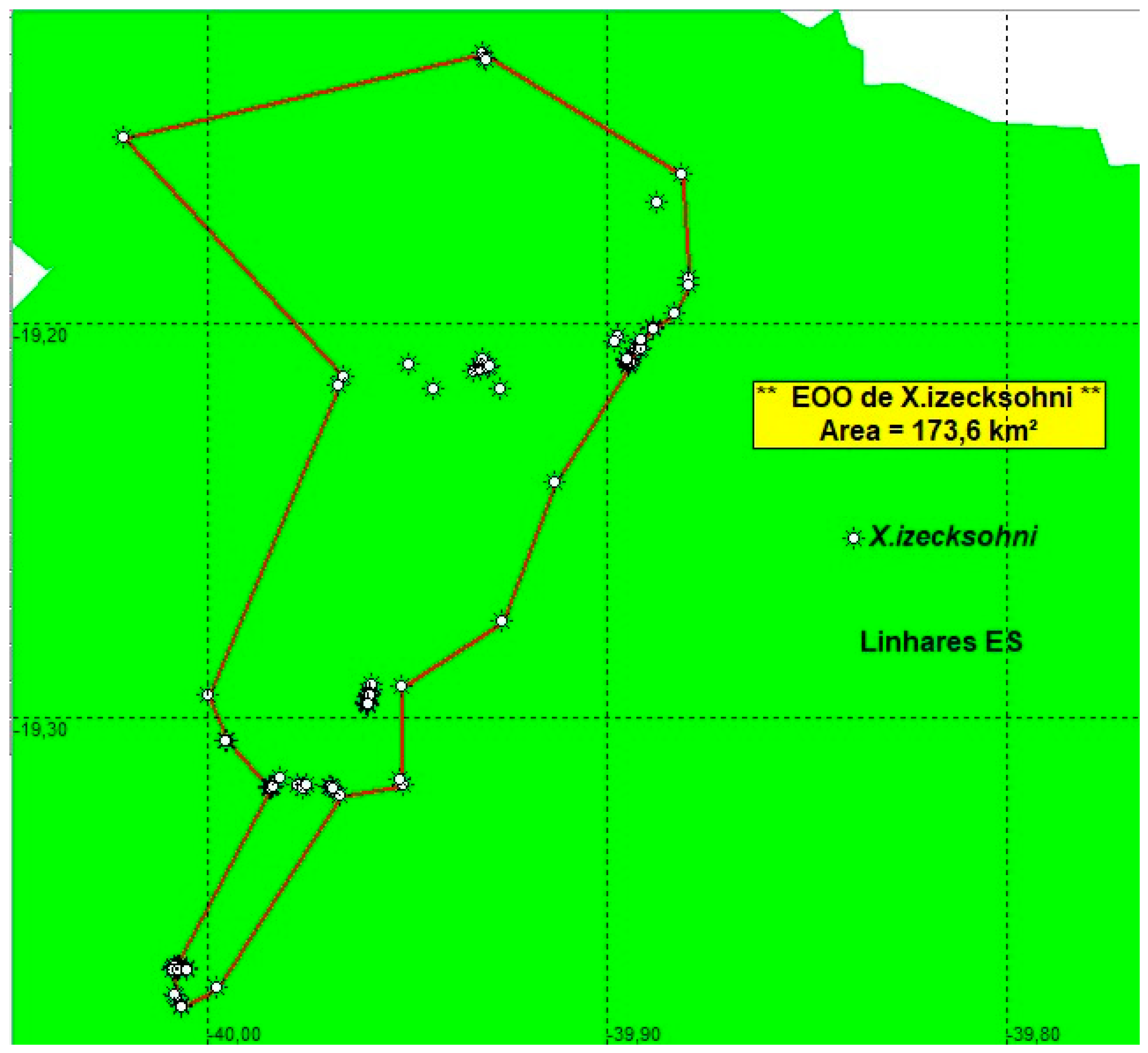
Conservation concerns. The estimated Extent of occupancy (EOO) of the population found in both Doce and Barra Seca river basins was defined as 173.6 Km2 (more than 5 locations)(
Figure 10). The pools where the rivulids occurs were full of water at least twice a year. The current estimated distribution area is approximately 385 km², with a sampling perimeter of 95.6 km, demonstrating a significant expansion of the previously known distribution. Xenurolebias izecksohni is listed as "Vulnerable" by the [
19]. Otherwise, the new information obtained indicates that its situation may be still delicate for its environments than previously estimated, especially in the face of intense anthropogenic pressure on unprotected private areas, where the species was widely recorded during this study.
Xenurolebias cricarensis is known to occur in its type locality only, a temporary swamp located inside a farm, near road from BR-101 to Conceição da Barra city, north of Espírito Santo.
In 2024, two expeditions were carried out in search of the species. During dry season, on June, an expedition visited the type locality, no specimens were found, since the environment was completely dry. Other points nearby were visited, but were unsuccessful. A second expedition was carried out on wet season, between November 23 and 29, 2024, in the same region. This time the pond was flooded, and populations were recorded, the first findings since the species description.
During the wet season field campaign, 14 specimens of X. cricarensis, were collected, along with the accompanying fauna composed of aquatic insects, tadpoles and mollusks. Specimens of Oreochromis niloticus were also captured. Additional areas, with potential occurrence were visited, but the only with populations is theat single puddle in the sub-basin of the São Domingos stream.
Considering that X. cricarensis is dependent on temporary pools, its persistence is directly related to climatic conditions and the integrity of these environments. Degradation can lead to the local extinction of the species, which highlights the need for urgent actions, such as the creation of conservation units and the awareness of landowners about the ecological importance of these systems.
Native Vegetation in the Landscape. The environment in São Domingos is a large temporary swamp, with approximately 3 km². with dark colored acidic waters, clay soil, and a substrate with decomposing leaves and branches. These characteristics refer to the initial portion of the puddle, which is easier to access, located next to the road. The bottom portion was not accessible and remains to be investigated. Abiotic information. Dark tea colored waters in a lentic environment. Water depth at pool between 50 cm to 60 cm, Humidity: 68% / Water temperature: 24.6ºC / pH: 5.6 (
Table 2).
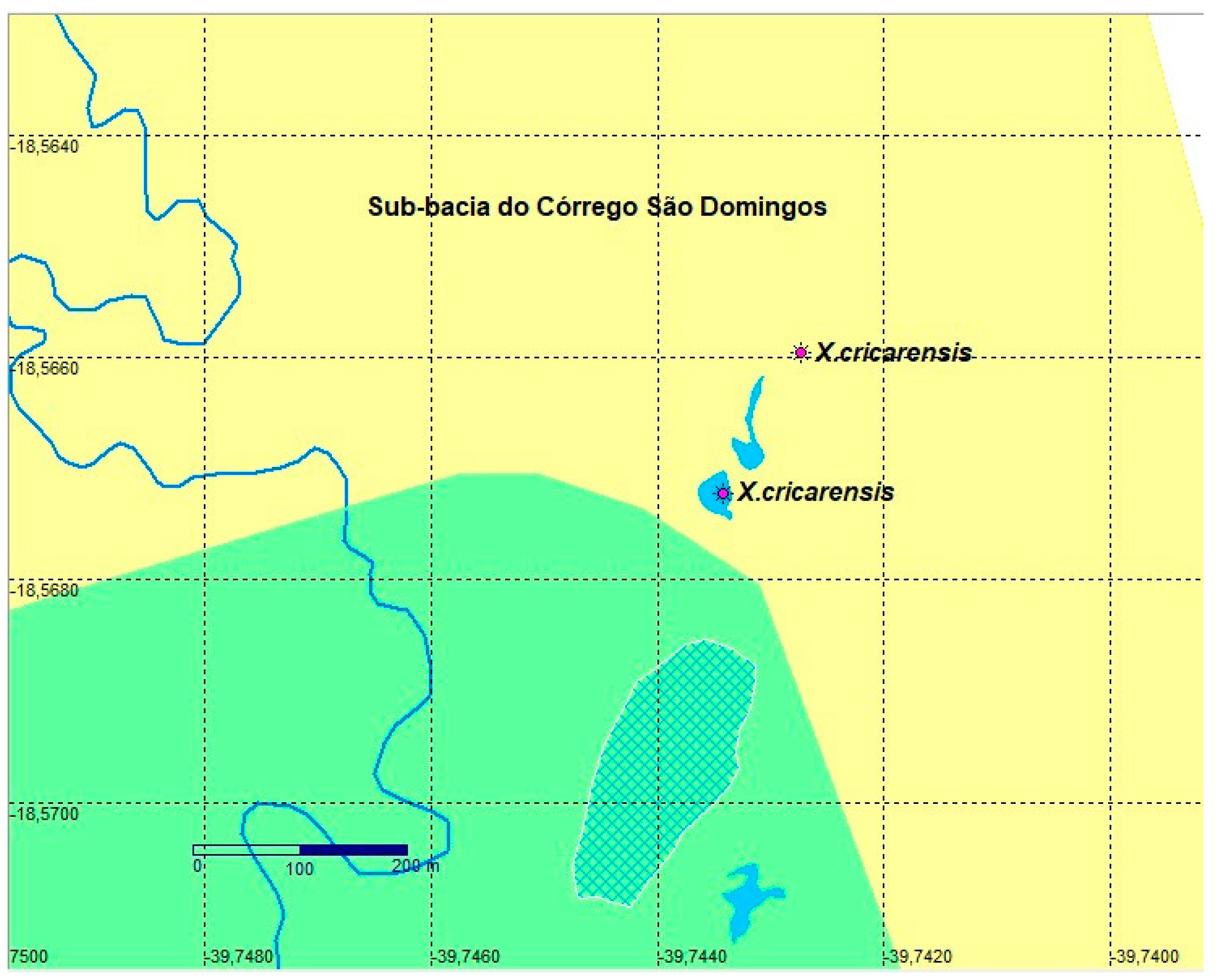
Conservation concerns. The estimated Extent of occupancy (EOO) of the population found in São Mateus river basin corresponds to a single location (
Figure 13). Only one pool have populations. From May until the beginning of the rainy season, the pool was completely dry. Categorized as "Data Deficient" (DD) by the [
8], the recent data obtained reinforces that Xenurolebias cricarensis needs to be categorized as threatened with extinction. The species has only been found in a single puddle in the sub-basin of the São Domingos Stream with approximately 85 thousand square meters, totalling an approximate occupation area of 0.15 km².
This small area is largely anthropized, with the detection of the practice of cattle grazing in the wetlands. Additionally, such puddle is in the vicinities of the city, and subject to urban pressure. These evidences reinforces the urgent need to create protected areas around the local wetlands habitats. Coexistence with exotic species, such as tilapia, may also compromises population viability due to competition and habitat degradation.
The Itaúnas cloud fish inhabits the sub-basins of the Velha Antônia stream, Moças stream, in the Itaúnas river basin and additionally in the Limo stream, a tributary of Doce creek basin [
20,
21]. These bright colored fishes are sexually dimorphic. Lives in peculiar environments, where water is transparent, translucent, in a yellowish or dark orange tone, without turbidity.
Native Vegetation in the Landscape. The species inhabits seasonal swamps in open areas of taboal (Typha spp.) or in restinga forests. They are found in temporary freshwater floods, of varying size, from small defined puddles with tens of meters to very extensive swamp areas, with a few square kilometers. Such environments are in the floodplain of the rivers, some very close to the sea, just over a hundred meters from the beach. The puddles are shallow, between 20 and 70 cm deep. The bed of the marsh is composed of a triple layer of substract: leaf litter, red mud, which occupies about 20 centimeters or more deep, and then sand underneath. Abiotic information. Dark tea colored waters in a lentic environment. Water depth at pool between 40 cm to 110 cm, Humidity: 60% / Water temperature: 27.1ºC / pH: 3.5-5.6/ Salinity (ppm): 0 (
Table 2).
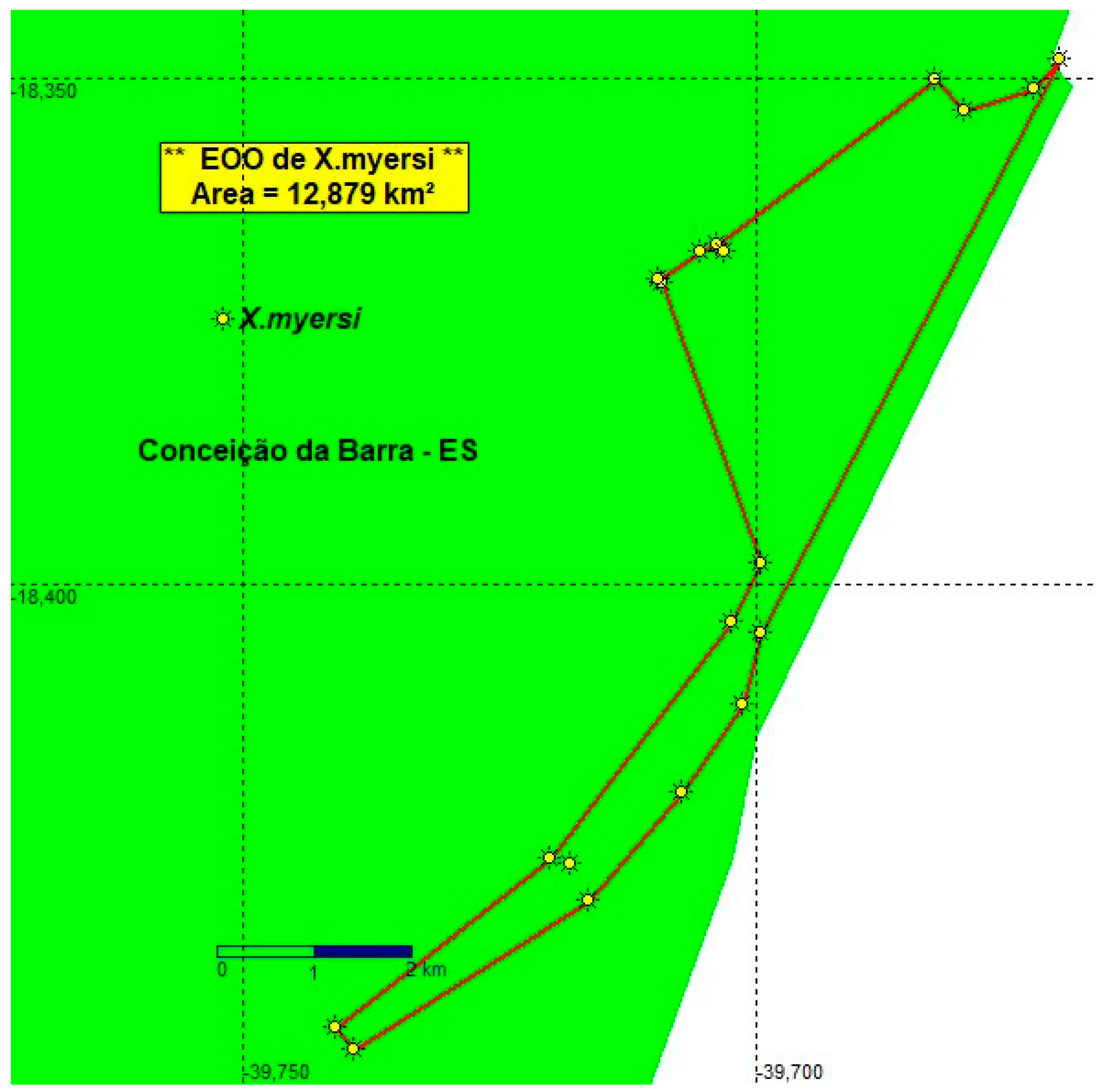
Conservation concerns. The estimated Extent of occupancy (EOO) of the population found in both Itaúnas and Riacho Doce river basins was defined as 12.879 Km2 (more than 5 locations)(
Figure 16). The pools where the rivulids occurs were full of water at least twice a year. Xenurolebias myersi is endemic to coastal lowland swamps and restinga environments at Itaúnas village and the Riacho Doce locality. Categorized as "Endangered" (EN), in both on the national red list [
8] and on the State red list of Espírito Santo [
18]. The species is also included in the Action Plan to conservation of Rivulidae fishes [
22].
Its noticeably that few populations were recorded inside the Itaúnas State Park, and mostly of them are found in environments outside the limits of the park, are susceptible to impacts, running imminent risks of extirpation. Attention should be given to the village of Itaúnas, that recently undergone several modifications, driven by tourism and immobiliary pression, urban expansion as well as the pressures of monocultural Eucalyptus plantations in its surroundings.