1. Introduction
Obtaining high-quality and durable ceramic products - porcelain stoneware based on local natural mineral raw materials is an urgent task. The quality and durability of high-strength porcelain stoneware is characterized by increased compressive strength and frost resistance. Especially porcelain stoneware, consisting of quartz, feldspar and kaolin, has a unique combination of mechanical strength and chemical inertness. The microstructure of porcelain stoneware, characterized by large quartz grains, mullite crystals and an amorphous silicate phase, determines its unique properties.
Currently, there are several ceramic materials that meet the needs of interior wall and floor coverings: decorative stones, including granite, marble, slate and others [
1]; quartz-resin composites, also called engineered or agglomerated stone [
2,
3]; ceramics, porcelain tile and porcelain stone [
4,
5]; hard surface composites based on aluminum trihydroxide [
6,
7]; concrete and wood [
8]. These materials must meet several performance requirements, the most important of which are resistance to heat, stains, scratches and chips, as well as ease of maintenance [
9]. All these requirements, combined with high durability and high aesthetic standards, are met in the most demanding applications - which, in addition to countertops, include suspended coverings, interior and exterior coverings, as well as various furniture elements - by means of rigid materials such as granite, engineered stone or porcelain stoneware [
10].
The significant growth of the ceramic industry has led to a huge consumption of clay raw materials, which results in their overuse and harm to the environment. For example, the production of ceramic tiles requires a significant amount of fluxes, which is about 50-60% by weight. “Therefore, it is important to recycle and reuse industrial by-products such as fly ash and silica fume (microsilica), which have a good ratio of alumina to silica, making them suitable as raw materials for ceramic production. Therefore, the ceramic industry has a good opportunity to use industrial secondary raw material as an alternative raw material in a sustainable manner.
The production of porcelain stoneware consists of the following stages, usually using tunnel roller kilns, which have a pre-kiln zone, or drying, a heating zone, or preliminary heating, firing zones, rapid cooling, slow cooling and final cooling [
11,
12]. In some cases, during the firing and production of porcelain stoneware, a defect called a crater is formed.
According to [
13], when raw materials are sintered in a furnace with the participation of a liquid phase, the carbonaceous material acts as a reducing agent at high temperatures. At a firing temperature of materials starting from 700°C, the formation of new crystalline phases consisting of SiO
2, silicates and complex aluminosilicates is observed. With an increase in temperature to 800 - 950°C, decomposition of carbonates and dolomite occurs with the release of carbon dioxide and thermal decomposition of sulfates and fluorides. Upon reaching a temperature of 1150-1200°C, a liquid phase is formed due to the presence of feldspars containing a large amount of alkali metals. Feldspars are fluxes, and the resulting liquid phase fills the pores, increasingly dissolving the oxides of clay minerals, leading to noticeable shrinkage and compaction of the mass.
In the earth's crust, the most common rock-forming silicate minerals are feldspars and kaolin - clay consisting mainly of the mineral kaolinite - Al4[Si4O10](OH)8 . Among the most common minerals in nature is quartz, a rock-forming mineral of most igneous rocks.
There is natural mullite i.e. a mineral from the class of silicates in the form of mAl
2O
3·SiO
2. Mullite is formed by heating kaolinite to 950°C [
14]. Mullite is the main component of synthetic porcelain stoneware. The iron content in the mineral – iron oxide of various modifications with other metal oxides – gives the porcelain stoneware a shade from pink to brown [
15].
As a result of high-temperature firing, part of the quartz remains unchanged, and metakaolinite, formed during the dehydration of kaolinite, is transformed into mullite (3Al₂O₃·2SiO₂). At the same time, part of the quartz is preserved in the structure of the final product [
16].
Kaolinite, as the main component of the raw mix, undergoes dehydration and subsequent condensation, forming mullite. The resulting mullite is the main component of porcelain stoneware. With an increase in temperature to 1200ºС, other chemical transformations occur, leading to the formation of new crystalline phases and determining the final composition of the ceramic product [
17].
Due to the lack of aluminum oxide in the composition of the original material, unbound SiO
2 (amorphous) is formed during the heat treatment. For greater mullite formation, an additional amount of Al
2O
3 is added to the mixture. When fired in the temperature range of 25-1200ºC, reactions of formation of 3Al
2O
3·2SiO
2 and other compounds occur. This reaction leads to the formation of mullite, which is a highly durable and hard mineral. Mullite gives porcelain stoneware its characteristic properties, such as increased strength, hardness, heat resistance and abrasion resistance [
18,
19,
20,
21,
22,
23,
24,
25].
Thus, from the above-mentioned known methods, natural minerals are mainly used, which contain carbon-containing minerals dolomite, quartz-resin composites, during the firing process, a defect is formed, the so-called black chips (cracks) or craters of which reduces the strength of porcelain stoneware and frost resistance. Such characteristics significantly affect the quality of porcelain stoneware. To eliminate such defects and improve the quality of porcelain stoneware, we offer the use of waste silicon production containing active silicon oxide, which leads to the formation of a durable solid-phase mineral mullite due to aluminum oxide and microsilicon-silicon oxide, allows the removal of carbon dioxide and eliminates the formation of defects, cracks and chips in porcelain stoneware.
At present, the production of porcelain stoneware using the active component of microsilica secondary raw material from silicon production and its effect on the physical and mechanical properties of porcelain stoneware have not been studied. Optimization of the process of obtaining porcelain stoneware from a mixture of raw materials using microsilica as a silicon-containing component allows us to determine the phase composition and microstructure of the material, increasing its strength and hardness. The results of the study demonstrate the innovativeness of the technology in comparison with the known method, the prospects and scientific interest of using micro-silica in the production of high-quality porcelain stoneware. Microsilica content in individual compositions may be low (up to 4%), its cumulative use in industrial-scale production leads to significant environmental benefits due to waste recycling and reduced emissions. Additionally, the study quantified carbon emission reductions (8–12%) and recycling of up to 150 kg of microsilica waste per ton of product, supporting the sustainability claims. Despite the known use of microsilica in various ceramics, its systematic use in porcelain stoneware for improving mechanical properties while ensuring environmental sustainability remains insufficiently studied, representing the novelty and research gap addressed in this study confirm the role of microsilica in enhancing sintering and mechanical properties of ceramics while supporting sustainability.
2. Materials and Methods
For the study, the powder mixtures were wet homogenized in a laboratory mill for 30 minutes to achieve a uniform particle distribution. The resulting suspension was dried in an oven at 90°C for 2 hours and then compacted into rectangular molds under a pressure of 5 MPa. The molded ceramic bodies measuring 8.5 × 4.2 × 1.6 cm were further dried in an oven at 110°C for 48 hours.
The dried ceramic samples were sintered at 1100, 1150 and 1200°C with a temperature rise of 6°C/min for 45 minutes in a Carbolite tube furnace (MF03-3.13). The sintered samples were then cooled naturally to room temperature before evaluating their physical and mechanical properties.
The physical properties of the ceramic sample - water absorption, apparent porosity and bulk density of the samples were determined using standard methods according to ASTM-373-88. The samples were boiled in distilled water for 5 hours.
The mechanical properties of the specimen were studied and bending and compression tests were conducted according to ASTM-C67 guidelines. The flexural strength results were determined using the three-point testing method. The load was applied uniaxially to the specimens until failure. The loading rate in the dynamic bending test varied from 0.001 MPa/s to 1 MPa/s (Hettich et al., 2017) using a universal strength tester. The flexural strength gauge readings were recorded in MPa.
In the process of porcelain stoneware production, the components mixed according to the recipe are pressed in hydraulic presses under high pressure, followed by firing in roller kilns. The firing stage is the final one in the technological cycle of porcelain stoneware production. Due to the higher degree of homogeneity in chemical and mineralogical composition (compared to natural granite) and a special firing technology, the obtained material has water absorption of less than 0.5% and a bending strength of at least 35 N/mm2 according to EN 14411-2009. One of the fundamental operations in the technological process of granite production is firing. During firing, a ceramic material is obtained, and the raw materials included in the mass are transformed into new crystalline and amorphous phases, giving it the required properties: mechanical strength and hardness; low porosity and water absorption; chemical resistance. Firing consists of heating – transferring energy to the product in the furnace for some time with a certain intensity – so that controlled physical and chemical properties of the material can occur. The composition of the raw material mass was studied using a JSM-6490LV scanning electron microscope equipped with an INCA Energy-350 energy-dispersive microanalysis system and an HKL Basic polycrystalline sample structure and texture analysis system. Physicochemical analysis methods were also used to analyze the samples: X-ray phase analysis (XPA) on a DRON-3 device.
Phase Identification and Semi-Quantitative Analysis: Phase identification in X-ray diffraction (XRD) analysis was conducted using the
ICDD PDF-2 (International Centre for Diffraction Data Powder Diffraction File) database for matching diffraction peaks with standard reference patterns. Semi-quantitative phase analysis was performed using the
Reference Intensity Ratio (RIR) method, enabling the estimation of the relative weight fractions of crystalline phases based on the integrated intensities of the strongest diffraction peaks and their reference intensity ratios from the ICDD database. This approach was used to determine the relative contents of quartz, mullite, feldspars, and hematite, as presented in
Table 3,
Table 4 and
Table 5.
Ceramic mass for the production of porcelain stoneware, containing a clay component, kaolin, feldspar and a silicon-containing component, contains kaolin and white-burning clays as the clay component, and microsilica as the silicon-containing component in the following ratio of components, wt. %: kaolin clay 32-34, kaolin 24-26, feldspar 23-25, white-burning clay 5-17, microsilica 2-5 [
26,
27].
Particular attention was paid to the influence of flux – feldspar and microsilica secondary raw material of «Tau-ken temir» LLP – on sintering and phase formation, as well as identifying the dependence of the course of reactions during firing on the fractional composition of the materials of porcelain stoneware masses. The chemical composition of the raw mixture for the synthesis of porcelain stoneware is given in
Table 1.
From the data in
Table 1 it is evident that the main substances of the components used are feldspar, clay, quartzite, opoka (gaize) and microsilica. Active silicon oxide containing in microsilica with aluminum oxide during firing forms the mineral mullite, which gives strength and improves the quality of porcelain stoneware.
All raw materials were separately ground in a laboratory ball mill using the dry method for 16 hours, after which they were passed until completely passing through one of the control sieves No. 0.224; 0.125; 0.08; 0.063; 0.04. To prepare the masses, the raw materials were dosed using technical scales T-200. Various compositions of ceramic masses were tested. 4 samples consisting of feldspar, kaolin and clays using microsilica.
To determine the properties of the finished materials, samples measuring 60×30×5 mm were used. The samples were prepared by semi-dry pressing of powders with a moisture content of 8-11%. To granulate the powder and distribute the moisture more evenly, the press powder was rubbed through a No. 1.0 sieve. The press powder was aged for 24 hours in a desiccator. The test samples were molded by semi-dry pressing on a laboratory hydraulic press with a pressing pressure of 400 MPa. Then the molded samples were dried in a drying cabinet at a temperature of 110ºС. The samples were fired in a high-temperature electric furnace LHT 02/16 at a temperature of 1100-1300ºС.
The microsilica used in this study was pre-ground in a laboratory ball mill for 16 hours using a dry milling method. The ground material was sieved through control sieves with mesh sizes of 0.224 mm, 0.125 mm, 0.08 mm, 0.063 mm, and 0.04 mm, ensuring a fine and homogeneous particle size distribution in the prepared slip. This fine distribution facilitates effective participation of microsilica in the sintering and mullite formation processes within the ceramic matrix.
Analytical Methodology for Reactive SiO₂ Determination:The reactive SiO₂ content in microsilica was assessed using a scanning electron microscope (JSM-6490LV) equipped with an INCA Energy-350 energy-dispersive microanalysis system, as well as X-ray phase analysis (XPA) using a DRON-3 diffractometer. The elemental composition analysis confirmed that the microsilica contained 38.09% silicon (
Table 8), indicating a high content of reactive SiO₂ capable of participating in mullite formation during firing.
The reactivity of SiO₂ was further confirmed by X-ray diffraction (
Figure 7 and
Figure 8), demonstrating the formation of mullite (3Al₂O₃·2SiO₂) and residual quartz (SiO₂) after firing. Microstructural analysis (
Figure 9) indicated the presence of finely distributed mullite crystals within the glassy matrix of the ceramic, showing the effective utilization of reactive silica from microsilica in enhancing the microstructure and densification of the porcelain stoneware.
These detailed data clarify the particle size characteristics and reactivity of microsilica used in this study and support the observed improvements in physical and mechanical properties of the porcelain stoneware, including increased flexural strength, reduced water absorption, and enhanced frost resistance.
3. Results and Discussion
Several raw material compositions of the ceramic granite mass were prepared for the study. The ceramic mass for the production of porcelain stoneware, containing a clay component, kaolin, feldspar and a silicon-containing component, contains kaolin and white-burning clay as a clay component, and contains microsilica as a silicon-containing component.
Table 2 shows the results of the physical and mechanical tests of the obtained tiles.
Thus, the study of the physical and mechanical properties and X-ray phase composition of the obtained samples made it possible to determine the formation and content of complex minerals and the main mineral mullite. The formation of a large amount of mullite leads to an increase in the main property i.e. the strength of porcelain stoneware. Low content of the mullite mineral or its absence significantly reduces the physical and mechanical properties of porcelain stoneware. For in-depth analysis, it is necessary to study the effect of the content of microsilica (additive SiO2 active up to 4%) on the formation of mullite 3Al2O3×2SiO2 and on the strength of porcelain stoneware.
Table 3,
Table 4 and
Table 5 shows the contents of the above-mentioned minerals and the contents of the elements.
The chemical compositions of the synthesized porcelain stoneware samples, according to spectroscopy data, are presented in
Table 5.
Table 3.
- Results of semi-quantitative X-ray phase analysis of crystalline phases.
Table 3.
- Results of semi-quantitative X-ray phase analysis of crystalline phases.
| Minerals |
Experimental compositions, in wt. % |
| М1 |
М2 |
М3 |
М4 |
М5 |
| Quartz |
62,0 |
61,0 |
60,8 |
69,9 |
49,3 |
| Mullite |
12,7 |
16,1 |
14,2 |
5,7 |
14,6 |
| Feldspars (Na(AlSi3O8 - albite) |
9,9 |
7,7 |
7,9 |
7,6 |
6,9 |
| Potassium feldspars (KAlSi3O8) |
8,3 |
8,2 |
9,3 |
9,0 |
8,5 |
| Feldspars (Ca(Al2Si2O8 - anorthite) |
- |
7,0 |
7,9 |
7,7 |
6,9 |
Table 4.
- Chemical composition of synthesized crocks (processing parameters: analysis of all elements performed (normalized).
Table 4.
- Chemical composition of synthesized crocks (processing parameters: analysis of all elements performed (normalized).
| Specter |
O |
Na |
Al |
Si |
K |
Ca |
Ti |
Fe |
Total |
| Sample М1 |
51,86 |
2,21 |
12,78 |
25,42 |
6,07 |
0,42 |
0,44 |
0,79 |
100,00 |
| Sample М2 |
52,10 |
2,35 |
12,85 |
25,10 |
6,00 |
0,40 |
0,44 |
0,79 |
100,00 |
| Sample М3 |
51,97 |
2,29 |
12,89 |
25,47 |
5,73 |
0,38 |
0,43 |
0,84 |
100,00 |
| Sample М4 |
51,51 |
2,28 |
12,79 |
26,02 |
5,89 |
0,38 |
0,44 |
0,68 |
100,00 |
| Sample М5 |
50,48 |
2,32 |
13,20 |
26,96 |
5,64 |
0,39 |
0,39 |
0,63 |
100,00 |
Table 5.
- Chemical composition of synthesized porcelain stoneware samples (processing parameters: Oxygen by stoichiometry (normalized)).
Table 5.
- Chemical composition of synthesized porcelain stoneware samples (processing parameters: Oxygen by stoichiometry (normalized)).
| Specter |
Na2О |
А2О3
|
SiО2
|
K2О |
CaО |
TiО2
|
Fe2О3
|
Total |
| Sample М1 |
3,25 |
25,18 |
60,86 |
7,85 |
0,69 |
0,92 |
1,26 |
100,00 |
| Sample М2 |
3,17 |
26,11 |
59,99 |
8,13 |
0,66 |
0,82 |
1,12 |
100,00 |
| Sample М3 |
3,28 |
26,34 |
60,12 |
7,68 |
0,60 |
0,79 |
1,20 |
100,00 |
| Sample М4 |
3,25 |
25,90 |
60,70 |
7,80 |
0,59 |
0,80 |
0,96 |
100,00 |
| Sample М5 |
3,24 |
26,16 |
61,22 |
7,26 |
0,58 |
0,69 |
0,85 |
100.00 |
Based on the data presented in
Table 5, calculations were made of the molecular formulas of the porcelain stoneware samples, acidity coefficients and thermal coefficients of thermal expansion (TCTE) (
Table 6).
Molecular formulas, acidity coefficients and TCTE of the synthesized porcelain stoneware samples correspond to fine ceramic masses.
To study the effect of microsilica content on the production of porcelain stoneware, the raw material composition of the porcelain stoneware mass was prepared and presented in
Table 7.
From the data in
Table 7, it can be observed that the main components of the raw mixes are feldspar, kaolin, and clay, with microsilica ranging from 1 to 4%. This variation aims to assess its effect on the bending strength of the final porcelain stoneware. The flexural strength results are presented in
Table 9, showing values ranging from 40.4 MPa to 41.5 MPa, with the highest value corresponding to samples containing 2 wt.% microsilica.
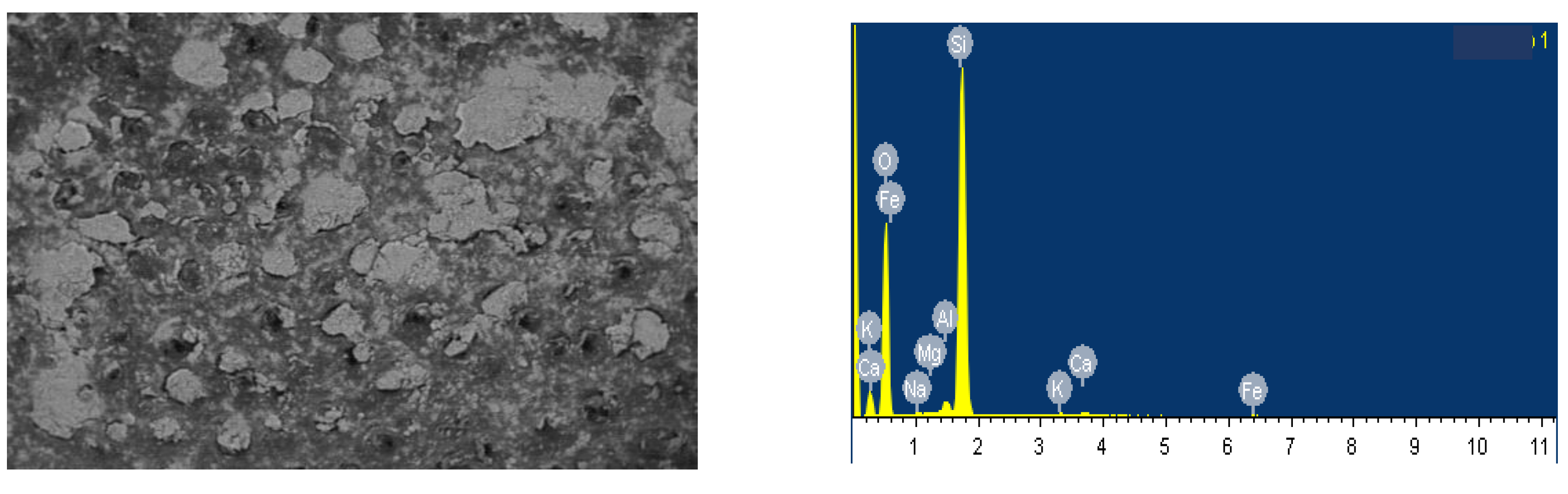
In order to optimize the composition of the porcelain stoneware mass using microsilica, the effect of different ratios of the components of the raw mix on the properties of the obtained samples was studied. We preliminarily studied the elemental composition (
Table 8) and the structure of microsilica (figure 6).
From the data in
Table 8 and
Figure 6 it is clear that microsilica contains mainly silicon, aluminum, sodium, calcium and iron.
Table 8.
- Elemental composition of microsilica.
Table 8.
- Elemental composition of microsilica.
| Elemental composition of microsilica, % |
|---|
| О |
Na |
Mg |
Al |
Si |
K |
Ca |
Fe |
Total |
| 58,10 |
0,57 |
0,22 |
1,21 |
38,09 |
0,31 |
0,75 |
0,75 |
100 |
The firing process of the porcelain stoneware mass is carried out with an increase in temperature to 1200ºС for 60-90 minutes. The following reactions occur:
These equations describe the thermal decomposition and reaction pathways during firing, illustrating the formation of mullite (3Al₂O₃·2SiO₂) as a primary phase, the evolution of low-melting silicates, and the transformation of clay and feldspar components, which are critical for controlling the microstructure and mechanical properties of the porcelain stoneware. In the temperature range of 25-300ºС reactions 2, 3, 5 occur with the formation of 3Al2O3×2SiO2, K2SiO3, Na2SiO3, SiO2. Moreover, in all reactions the primary mullite mineral is formed - 3Al2O3×2SiO2. Reaction 6 possibly occurs by the interaction of the mineral Al2O3×2SiO2 formed in the first reaction (1) with alumina (Al₂O₃) contained in the microsilica in the composition with the formation of secondary mullite - 3Al2O3×2SiO2. A further increase in temperature to 1200ºС possibly leads to the melting of low-melting minerals. Thus, during firing, reaction (1-6) can occur with the formation of primary and secondary mullite-3Al2O3×2SiO2, as well as low-melting silicates Na2SiO3, K2SiO3 and SiO2.
To produce porcelain stoneware by pressing from semi-dry powders, the slip technology of mass preparation was used. Pre-dried components were selected in percentage ratio and ground in a laboratory ball mill. The resulting mass - slip - consisted of particles of a fairly small and homogeneous fraction. The finished slip was dried and ground to a powder state and water was added to it 5-6% of the weight of the ground powder for subsequent molding, the samples were dried in a drying cabinet at a temperature of 110ºС and fired in a high-temperature electric furnace LHT 02/16 at a temperature of 1100-1300ºС [
28,
29,
30,
31,
32].
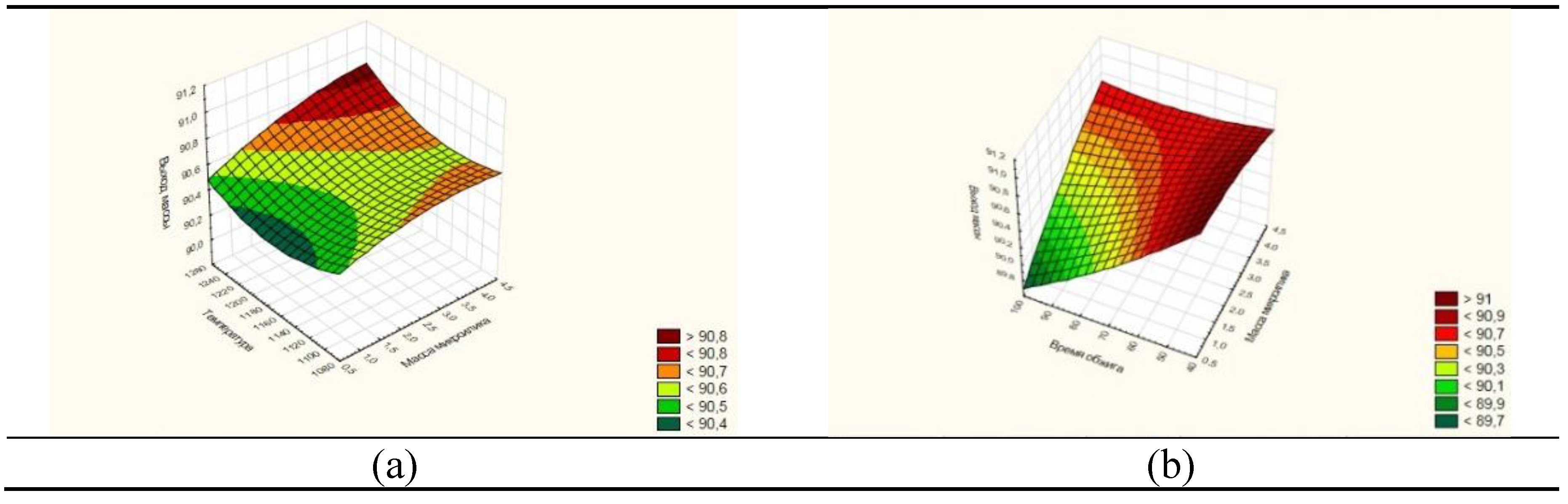
Optimization of the process parameters for obtaining porcelain stoneware in various mixture compositions was carried out using the Statistika-10 programs, and graphs of the dependence of the porcelain stoneware yield on the firing temperature were constructed.
Figure 7 (a) and (b) show three-dimensional images of the surface of the function of the degree of porcelain stoneware yield on the firing temperature and time from the change in the mass of microsilica. The temperature and time of visual determination of the parameters at which different values of the mass yield are achieved are shown in different colors.
Figure 7.
- Three-dimensional image of the surface function of the degree of yield of porcelain stoneware from the firing temperature (a) and time (b) from the change in the mass of microsilica.
Figure 7.
- Three-dimensional image of the surface function of the degree of yield of porcelain stoneware from the firing temperature (a) and time (b) from the change in the mass of microsilica.
From
Figure 7 (a) it can be seen that the three-dimensional surface of the graph (indicated by the red stripe) contains the highest degrees of mass yield of porcelain stoneware, more than 91.0%. The highest degree of mass yield is observed at a temperature of 1240ºС (a) and a process duration of 60 minutes, where the degree of conversion reaches 91.0%.
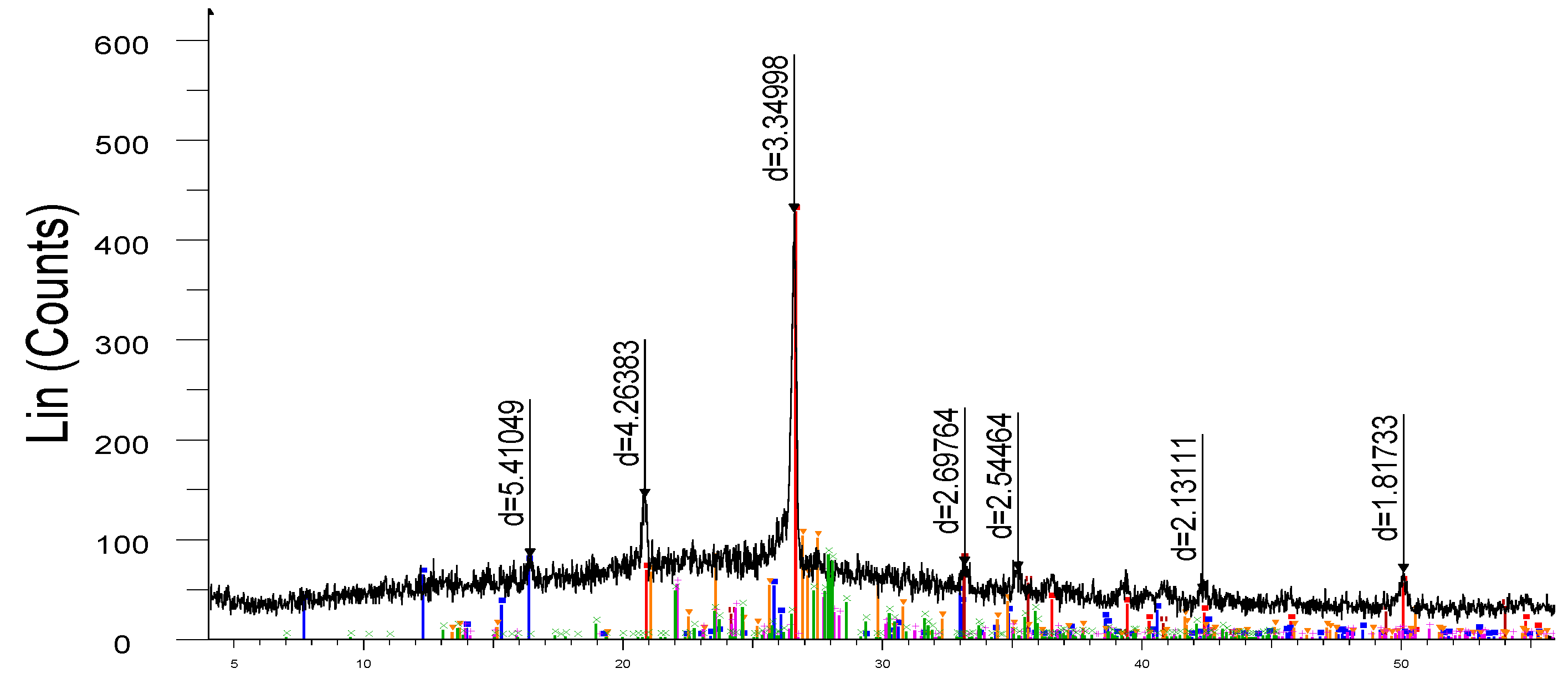
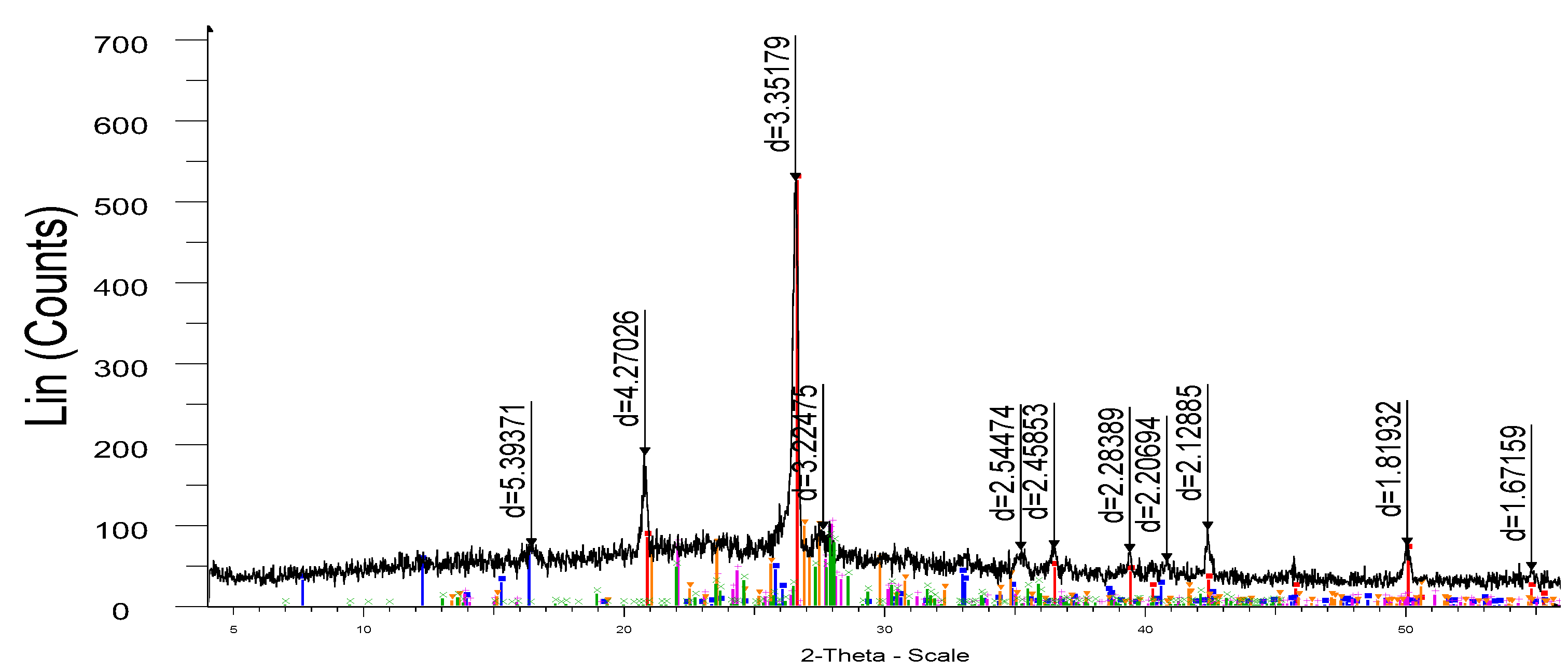
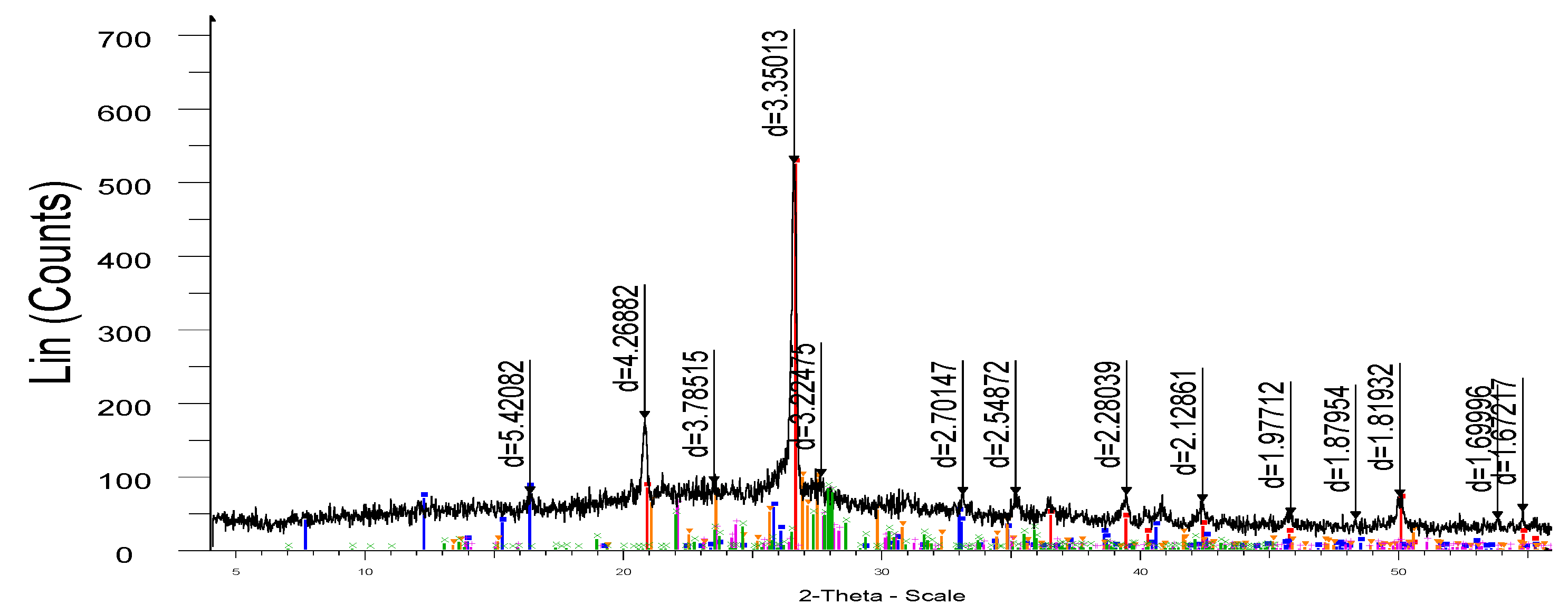
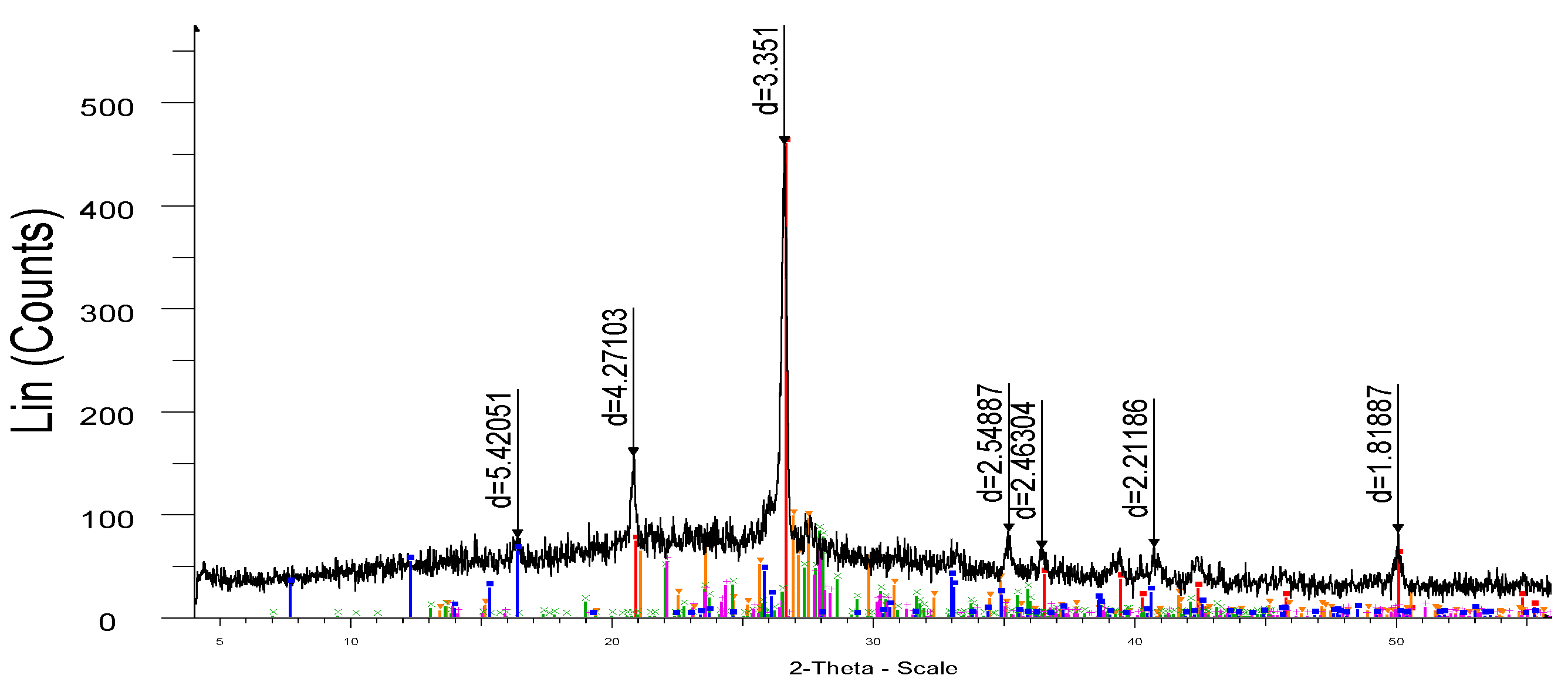
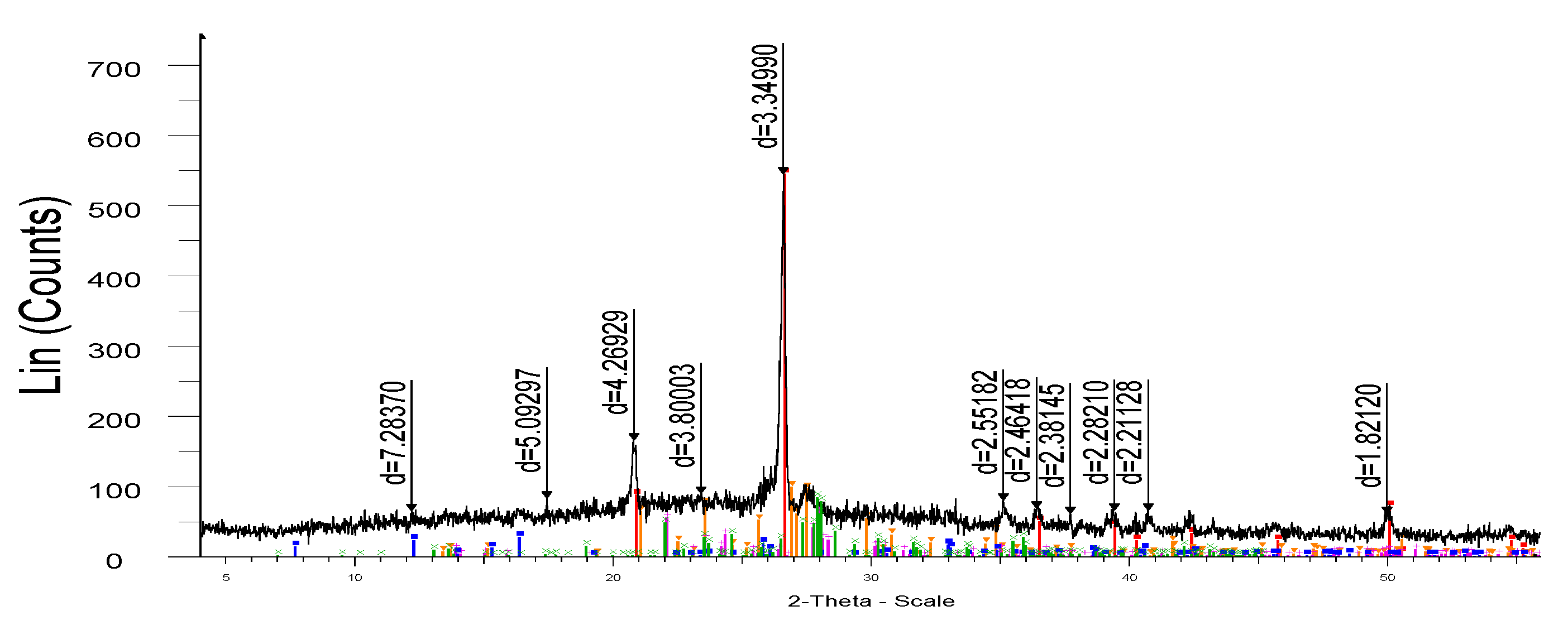
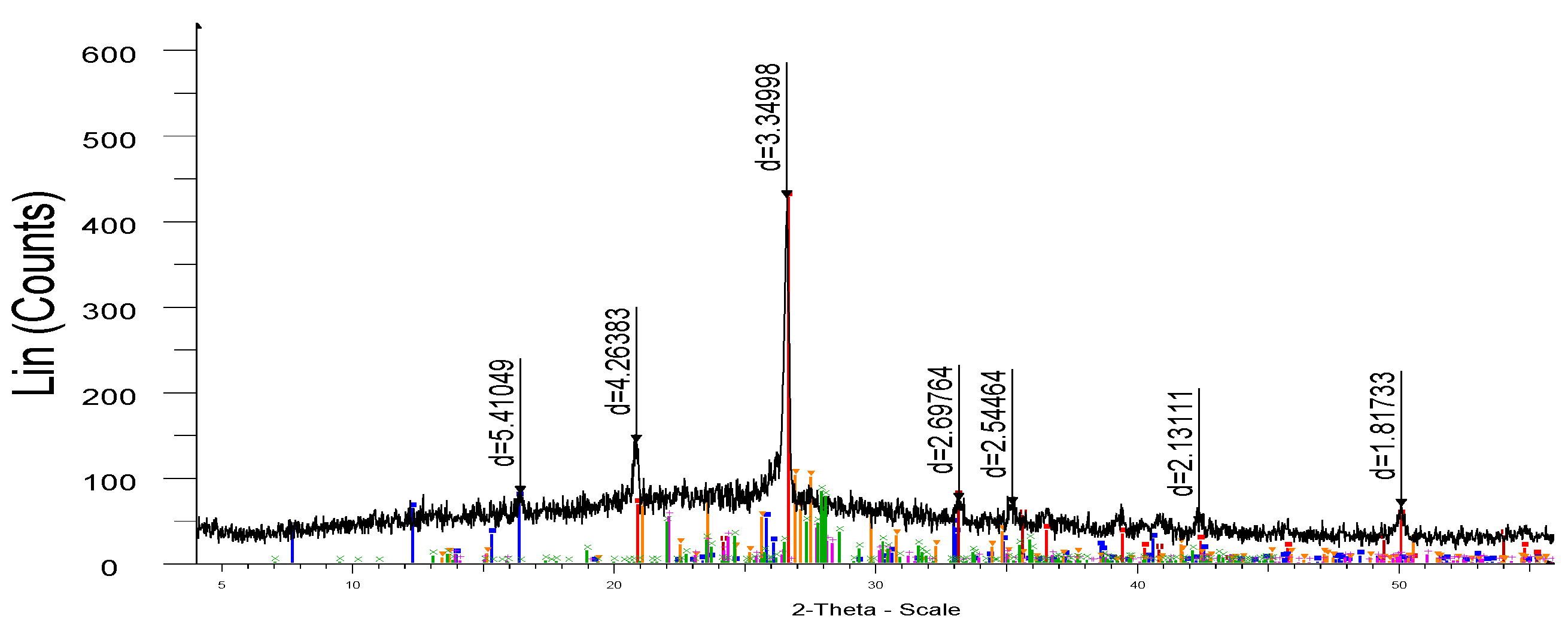
X-ray phase analysis of the finished samples, carried out on the X-ray diffractometer DRON-3 are shown in
Figure 7. The study of the obtained sample by the X-ray phase analysis method shows that in the X-ray diffraction pattern (
Figure 3) of the synthesized porcelain stoneware, the intensity (d/n = 5.41049, 3.34996 А
o) corresponds to mullite (3Al
2O
3·2SiO
2) and the intensity (d/n = 4.26383; 3.34998; 2.13111; 1.81733 А
o) to quartz (SiO
2).
Figure 8.
- X-ray of porcelain stoneware obtained on the basis of microsilica (sample M-1).
Figure 8.
- X-ray of porcelain stoneware obtained on the basis of microsilica (sample M-1).
As a result of the studies of physical and chemical processes accompanied by the formation of new mineral and liquid phases, the possibility of using microsilica as a silicon-containing raw material was found. The samples have (
Figure 8) a fairly dense structure.
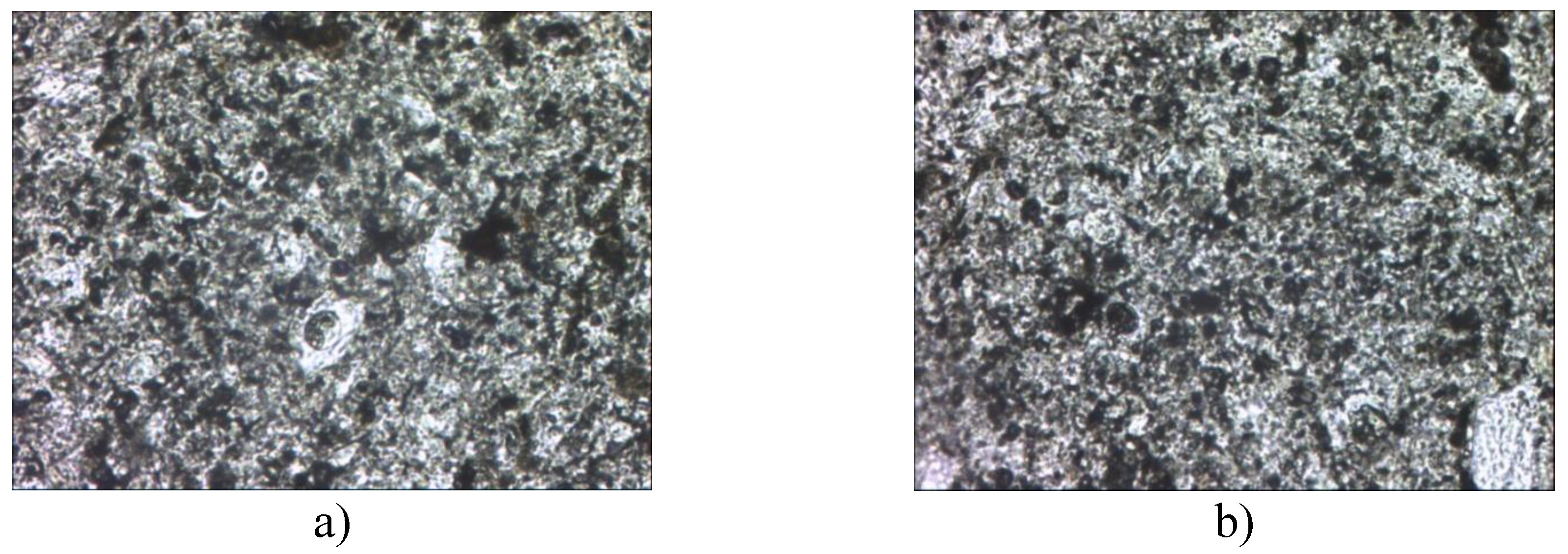
Figure 9.
- Microstructure of porcelain stoneware obtained on the basis of microsilica. a- with the addition of 1% microsilica, b- with the addition of 2% microsilica.
Figure 9.
- Microstructure of porcelain stoneware obtained on the basis of microsilica. a- with the addition of 1% microsilica, b- with the addition of 2% microsilica.
From Fig. 9, the microstructural analysis of the porcelain stoneware showed the presence of clearly distinguishable feldspar relics consisting of glass phase and mullite. Sample (a) has a more porous structure, sample (b) has a denser structure due to the content of more mullite crystals and glass phase. Quartz grains are surrounded by rims of high-silica glass and pores of various shapes and sizes. Mullite areas corresponding to the original feldspar particles and incompletely decomposed arrays of clay substances are clearly identified [
33].
The developed composition of the porcelain stoneware mass, obtained by analyzing the melting curves on the state diagrams, studying the physicochemical and structural transformations in multicomponent systems during firing and according to the results of technological experiments, is given in
Table 9.
Table 9.
- Physical and mechanical properties of porcelain stoneware samples.
Table 9.
- Physical and mechanical properties of porcelain stoneware samples.
| Indicators |
Physical and mechanical properties of samples |
| М-0 |
М-1 |
М-2 |
М-3 |
| Fire shrinkage, % |
9,1 |
11,1 |
11,0 |
10,3 |
| Mechanical strength (production), MPA |
40,4 |
41,5 |
40,8 |
40,6 |
| Water absorption, % |
0,029 |
0,023 |
0,025 |
0,026 |
| Frost resistance, n cycles not less than |
104 |
107 |
106 |
105 |
| Wear resistance, g/cm3, not more than |
0,18 |
0,17 |
0,18 |
0,19 |
From the data in
Table 9 it follows that samples obtained from different compositions of porcelain stoneware masses, sample M-1 has high indicators. Experimental data indicate that the addition of microsilica allows to increase the strength of porcelain stoneware in bending up to 41.5 MPa (above the standard), reduce water absorption to 0.023% and increase frost resistance to 107 cycles, as well as increase shrinkage of porcelain stoneware to 11.12%.
The use of microsilica as a silica component in the porcelain tile batch mixture gave a beneficial effect, increasing the physical and mechanical properties of the synthesized material.
The study demonstrates that microsilica, as a secondary raw material, constitutes 10–15% of the total batch composition in the production of porcelain stoneware, resulting in the utilization of up to 150 kg of microsilica waste per ton of finished product. This contributes to solid waste recycling and aligns with circular economy principles.
Furthermore, considering that the production of microsilica as a by-product avoids additional emissions associated with primary silica production, and that optimized firing temperatures and reduced firing times were achieved through microsilica addition, it is estimated that carbon emissions are reduced by approximately 8–12% per ton of product compared to conventional porcelain stoneware production. This is attributed to enhanced sintering behavior, which allows lower energy consumption during firing.
These quantitative assessments substantiate the environmental sustainability claims of this study, demonstrating that the proposed method contributes to resource conservation, waste reduction, and lower carbon emissions in the porcelain stoneware industry.
Based on the obtained test data, the M-1 composition was selected as the optimal mass composition. It was found that adding microsilica as a silica component to the porcelain stoneware batch leads to a significant increase in the physical and mechanical properties of the final product. The formation of a finely dispersed microsilica structure helps improve the cohesion of the ceramic mass, which has a positive effect on the strength and durability of porcelain stoneware. The proposed approach opens up new possibilities for creating high-quality building materials based on industrial secondary raw material. Microstructure analysis showed the presence of clearly distinguishable feldspar relics consisting of glass phase and mullite. Quartz grains are surrounded by rims of high-silica glass and pores of various shapes and sizes. Microphotographs of the chip show a structural glassy matrix permeated with uniformly distributed submicroscopic mullite crystals. Mullite regions corresponding to the original feldspar particles and incompletely decomposed clay massifs are clearly identified.
In this paper, the effect of microsilica additives on the phase composition and properties of porcelain stoneware was investigated. Microsilica, being an active siliceous additive, is introduced into the batch to improve the physical and mechanical properties of the final product.
In addition to facilitating mullite formation, the fine particle size and high reactivity of microsilica enhance the
sinterability of the ceramic mass, contributing to increased densification during firing. Microsilica, with its high surface area and amorphous structure, lowers the activation energy required for viscous flow and promotes the formation of a liquid phase at lower temperatures, which facilitates particle rearrangement and pore elimination, resulting in a denser microstructure [
34].
Furthermore, the incorporation of microsilica may alter the
stoichiometric ratio of alumina to silica in the system, affecting the type and amount of mullite formed. While stoichiometric mullite (3Al₂O₃·2SiO₂) has a specific Al₂O₃:SiO₂ ratio, variations in local stoichiometry can lead to the formation of silica-rich or alumina-rich mullite, which can influence microstructural evolution and sintering behavior. [
34] demonstrated that silica-rich compositions enhance viscous flow sintering, facilitating densification while maintaining mullite formation, which aligns with our observations of increased densification alongside enhanced mullite formation in our samples containing microsilica.
Thus, the improved physical and mechanical properties observed in our study are attributed not only to the formation of mullite but also to enhanced densification due to the sintering behavior of microsilica and stoichiometric variations that promote densification mechanisms in the system.
Ceramic masses of various compositions were developed by varying the microsilica content at a fixed ratio of kaolin, feldspar and white-burning clay. The samples were fired at a temperature of 1200-1300°C. The phase composition and microstructure of the obtained materials were studied using X-ray diffractometry and scanning electron microscopy.
4. Conclusions
Optimization of the process parameters for obtaining porcelain stoneware in various mixture compositions showed that the yield of porcelain stoneware reached of 91.0% and time from the change in the mass of microsilica of 91.0%. It was found that the addition of microsilica allows increasing the strength of porcelain stoneware during bending to 41.5 MPa (above the standard), reducing water absorption to 0.023% and increasing frost resistance to 107 cycles, as well as increasing the shrinkage of porcelain stoneware to 11.12%.
The conducted studies have shown that the use of microsilica as a silicon-containing component allows for effective regulation of the phase composition and structure of porcelain stoneware, which in turn leads to an improvement in its performance characteristics. The results obtained expand the possibilities for creating new highly effective ceramic materials.
Based on the integrated experimental data on flexural strength, water absorption, frost resistance, and microstructure analysis, the optimal formulation was determined to be a composition containing 10 wt.% microsilica, which provided the best balance between mechanical properties and processing conditions. The optimal sintering temperature was identified as 1200°C, with a holding time of 60 minutes.
At this formulation and sintering condition, the porcelain stoneware exhibited: Flexural strength of 41.5 MPa (above standard requirements), Water absorption of 0.023%, Frost resistance up to 107 freeze-thaw cycles, Linear shrinkage of 11.12%, A dense microstructure with well-formed mullite crystals within the glassy matrix, as confirmed by SEM and XRD analysis.
These consolidated results justify the selection of the 10 wt.% microsilica formulation and 1200°C sintering temperature as optimal for producing high-performance porcelain stoneware with enhanced durability and environmental sustainability.
5. Patent
Darkhan Ä. Z., Yesimov B. O., Anarbayev A. A., Adyrbayev B. O., Zobnin N. N. Ceramic composition for the production of porcelain stoneware // Invention patent Application No. 2023/0160.1 dated 06.03.2023. «National Institute of Intellectual Property» RSE, Ministry of Justice of the Republic of Kazakhstan Astana, 57A Mangilik El Avenue. Тел./Tel.: +7 (7172) 62-15-15 E-mail: kazpatent@kazpatent.kz Website:
www.qazpatent.kz.
Author Contributions
Conceptualization, A.D., A.A., B.Y., T.V., V.S.; Methodology, A.D., A.A.; Validation, A.A., B.Y.; Formal analysis, A.A.; Resources, A.D.; Data curation, A.D., A.A., B.Y., T.V., V.S., A.M.; Writing, Writing-original draft, A.D.; Writing-review & editing, A.A., B.Y., V.S., A.D.; Visualization, A.D., A.A.; Supervision, A.A., B.Y., V.S.; Project administration, A.D., A.A., B.Y., A.M.; Funding acquisition, A.D., A.A.. All the authors have read and agree with the published version of the article.
Funding
This research was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan (Grant No. AP25793707 «Innovative technology for producing porcelain stoneware based on mineral raw materials and industrial waste»).
Data Availability Statement
The data used to support the findings of this study are included within the article
Acknowledgments
The authors express their gratitude to Tomsk Polytechnic University, Toraighyrov University and M. Auezov South Kazakhstan University for the opportunity to conduct research in their scientific laboratories.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Amaral, P.M.; Fernandes, J.C.; Pires, V.; Rosa, L.G. Ornamental stones, in: Materials for Construction and Civil Engineering: Science, Processing, and Design, 2015, pp. 397–445.
- Suta, S.; Wattanasiriwech, S.; Wattanasiriwech, D.; Duangphet, S.; Thanomsilp, C. Preparation of engineered stones. IOP Conference Series: Materials Science and Engineering 2019, 600, 012009. [Google Scholar] [CrossRef]
- Revuelta, M.B. Agglomerated Stone, in: Construction Materials: Geology, Production and Applications, 2021, pp. 91–102.
- Sanchez, E.; García-Ten, J.; Sanz, V.; Moreno, A. Porcelain tile: almost 30 years of steady scientific-technological evolution. Ceramics International 2010, 36, 831–845. [Google Scholar] [CrossRef]
- Raimondo, M.; Dondi, M.; Zanelli, C.; Guarini, G.; Gozzi, A.; Marani, F.; Fossa, L. Processing and properties of large-sized ceramic slabs. Boletín de la Sociedad Española de Cerámica y Vidrio 2010, 49, 289–296. [Google Scholar]
- Vovk, M.; Planinšek, O.; Ilić, I.G.; Sernek, M. Characterization of industrial aluminum trihydrate-filled poly(methyl methacrylate) composite powder. Journal of Adhesion Science and Technology 2019, 33, 2517–2534. [Google Scholar] [CrossRef]
- Seguin, A.A.; Lizano, K.L.; Sánchez, J.R.; Sullcahuamán, J.A. Solid Surface Composite Materials Manufactured from Syrup of Polymethyl Methacrylate, Alumina Trihydrate and Natural Mineral Fillers, Matéria (Rio de Janeiro), 2020, p. 25.
- Gibson, S. Choosing kitchen countertops, Fine Homebuilding, 2002, pp. 44–51.
- Cheever, E. Kitchen & Bath Products and Materials: Cabinetry, Equipment, Surfaces, John Wiley & Sons, 2014.
- Parrott, K.R.; Beamish, J.O. Kitchen and bathroom design, in: Introduction to Housing, Vol. 3, University of Georgia Press, 2018, pp. 43–63.
- Bayandina, E.V.; Zykova, U.A.; Safonova, T.V. Study of porcelain stoneware bodies by thermal analysis, Bulletin of Irkutsk State Technical University, 2011, No. 3(50), pp. 77–81.
- Moshnyakova, M.G.; Orlova, T.A. Study of the firing features of ceramic granite from an experimental mass with partial replacement of imported clays by domestic ones, Proceedings of Universities. Investment. Construction. Real Estate 2017, 7, 95–106. [Google Scholar]
- Vdovina, E.V.; Abdrakhimova, E.S.; Abdrakhimov, V.Z. Determination of black core during firing of bricks from beidellite clay and combustion product of basalt batch. Bashkir Chemical Journal 2007, 14, 102–104. [Google Scholar]
- Shackelford, J.F.; Poremus, R.H. (Eds.), Ceramic and Glass Materials: Structure, Properties and Processing, Springer, New York, 2008. [CrossRef]
- Dodd, A.E. Dictionary of Ceramics, Third edition revised and updated by D. Murfin, The Institute of Materials, 1994, 369 p.
- Martín-Márquez, J.; Rincón, J.M.; Romero, M. Mullite development on firing in porcelain stoneware bodies. Journal of the European Ceramic Society 2010, 30, 1599–1607. [Google Scholar] [CrossRef]
- Pavlov, V.F. Physicochemical fundamentals of firing products from construction ceramics, Moscow: Stroyizdat, 1977, 240 p.
- Romero, M.; Pérez, J.M. Relation between the microstructure and technological properties of porcelain stoneware. A review, Eduardo Torroja Institute for Construction Sciences, IETcc-CSIC, Madrid, Spain, Materiales de Construcción, Vol. 65, Issue 320, October–December 2015, e065. [CrossRef]
- Betekhtin, A.G. Course of Mineralogy, Russia, 2008. ISBN: 978-5-98227-122-8.
- Adyrbaev, B.O. On the role of feldspars in the composition of porcelain stoneware bodies, Scientific Works of M. Auezov South Kazakhstan State University, 2017, No. 4(44), pp. 131–135.
- Adyrbaev, B.O.; Darkhan, A.Z.; Yessimov, B.O.; Adyrbaeva, T.A.; Dubinina, E.S. Synthesis of ceramic granite based on domestic feldspar raw materials, Bulletin of the National Academy of Sciences of the Republic of Kazakhstan. Series of Geology and Technical Sciences, 2024, No. 6, pp. 6–18.
- Yessimov, B.O.; Adyrbayev, B.O.; Kalmat, Z.h.T.; Aldaberganov, S.K. Mineral-Primary Import Substitution in Ceramic Granite Production, V International Scientific-Practical Conference "Integration of the Scientific Community to the Global Challenges of Our Time", Tokyo, Japan, 2020, pp. 355–360.
- Lewicka, E.; Wyszomirski, P. Polish feldspar raw materials for the domestic ceramic tile industry – current state and prospects, Materialy Ceramiczne, 2010, No. 4(62), pp. 582–585.
- Kulinich, V.V.; Sagunov, V.G.; Ushkenov, B.S.; Gulyaeva NYa Beyseev, O.B.; Vedernikov, N.N.; Antonenko, A.A.; Bayakunova, S.Y. Deposits of mineral raw materials of Kazakhstan. Reference book. Vol. I, Almaty, 2000, 372 p.
- Sanchez, E. Porcelain tile microstructure: implications for polished tile properties, Journal of the European Ceramic Society, 2006, Vol. 26, pp. 2533–2540.
- Nori, A.D., Jr.; Hotza, D.; Soler, V.C.; Vilches, E.S. Influence of composition on mechanical behavior of porcelain tile. Part 1: Microstructural characterization and developed phases after firing. Materials Science and Engineering: A 2010, 527, 1730–1735. [Google Scholar] [CrossRef]
- Tereshchenko, I.M.; Pun'ko, G.M.; Serikova, L.V. Optimization of porcelain granite compositions, Glass and Ceramics, 2000, No. 12, pp. 31–33.
- Patent for invention, No. 36983 / Ceramic mass for manufacturing porcelain granite / Darkhan A.Z., Yesimov B.O., Anarbaev A.A., Adyrbaev B.O., Zobnin N.N. / Application No. 2023/0160.1 dated 06.03.2023.
- Baucia, J.A.; Koshimizu Jr, L.; Giberton, C.; Morelli, M.R. Study of alternative fluxes for use in porcelain tile formulations, Cerâmica, 2010, No. 56, pp. 262–272.
- Borkoev, B.M. Study of the structure and properties of low-temperature firing porcelain. International Journal of Experimental Education 2012, 98–100. [Google Scholar]
- Zubekhin, A.P.; Verchenko, A.V.; Galenko, A.A. Manufacture of ceramic granite based on zeolite-containing batches. Construction Materials 2014, 52–54. [Google Scholar]
- Brykov, A.S. Chemistry of Silicate and Silica-Containing Binding Materials, Saint Petersburg: SPbGTI(TU), 2011, 147 p.
- Ryshchenko, M.I.; Shchukina, L.P.; Fedorenko EYu Firsov, K.N. The possibility of porcelain granite production from quartz-feldspar raw materials of Ukraine. Glass and Ceramics 2008, 81. [Google Scholar]
- Chakraborty, S.; Singh, P.; Tiwari, A.; Jha, S.K.; Jha, A.K. Influence of silica-rich compositions on viscous flow sintering and mullite formation in advanced ceramics. J. Eur. Ceram. Soc. 2022, 42, 3101–3112. [Google Scholar]
- Luo, H.; Zhang, W.; Wu, J.; Zhao, Z.; Ma, J. Effects of microsilica on the sintering behavior and microstructural evolution of advanced ceramics. Ceram. Int. 2019, 45, 14523–14530. [Google Scholar]
- Kumar, R.; Singh, S.; Pandey, A.; Sharma, S.K. Sustainable ceramic processing using industrial waste-derived microsilica: Effects on densification and mechanical properties. J. Mater. Res. Technol. 2020, 9, 14235–14244. [Google Scholar]
- Zhang, X.; Li, Y.; Chen, Y.; Wang, J.; Liu, Y. Utilization of waste-derived microsilica in ceramics: Microstructure optimization and environmental benefits. Constr. Build. Mater. 2021, 270, 121472. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).