Submitted:
01 July 2025
Posted:
01 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
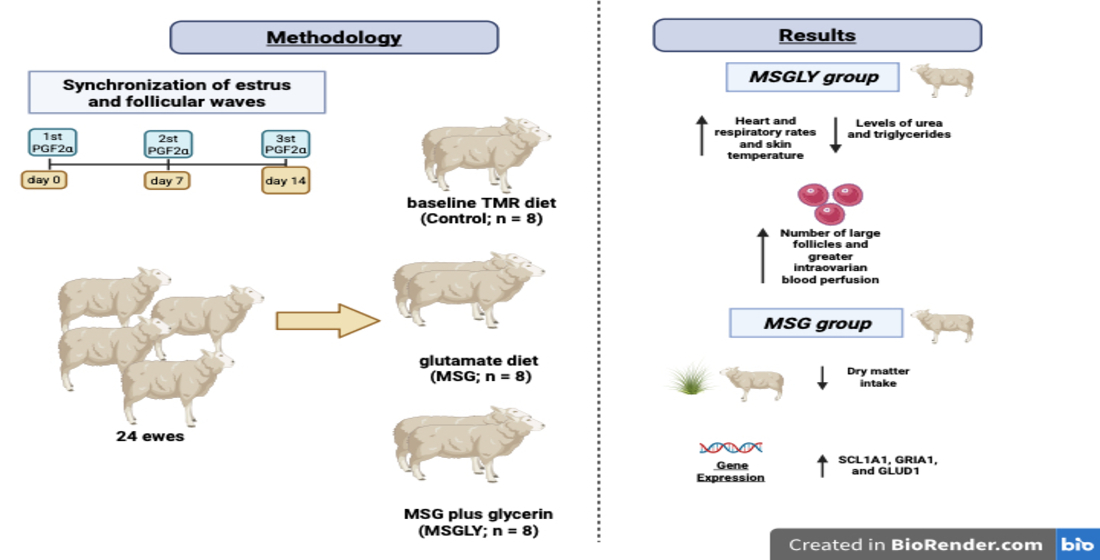
2. Materials and Methods
2.1. Ethic Statements and Location Facility
2.2. Animals, Feeding and Housing Management, and Pre-Experimental Conditions
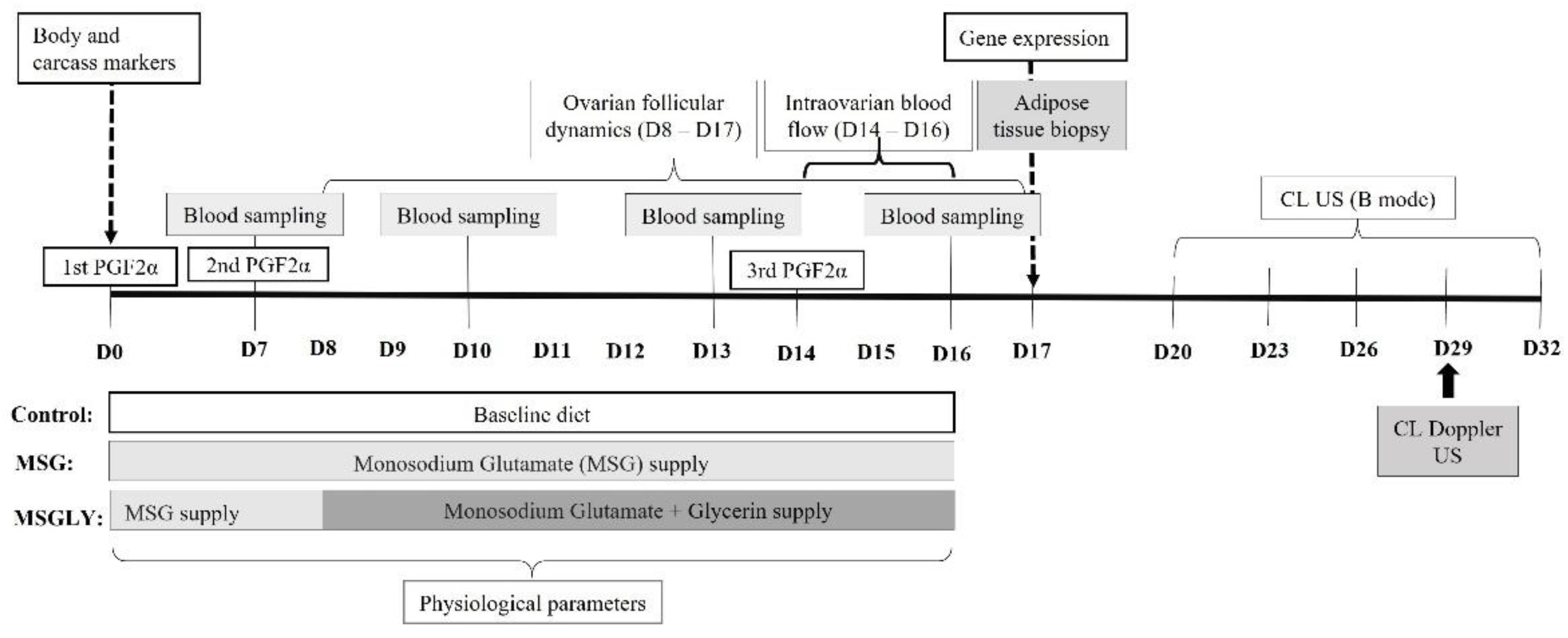
2.3. Experimental Design
2.4. Assessment of Ovarian Function Outcomes
2.5. Physiological Effort During the Period of Dietary Supplementation
2.6. Blood Sampling and Metabolite Assay
2.7. Adipose Tissue Sample Collection
2.8. RNA Isolation and Reverse Transcription Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) of Glutamate and Energy Genetic Markers
2.9. Data Statistics and Analysis
3. Results
3.1. Feed Intake
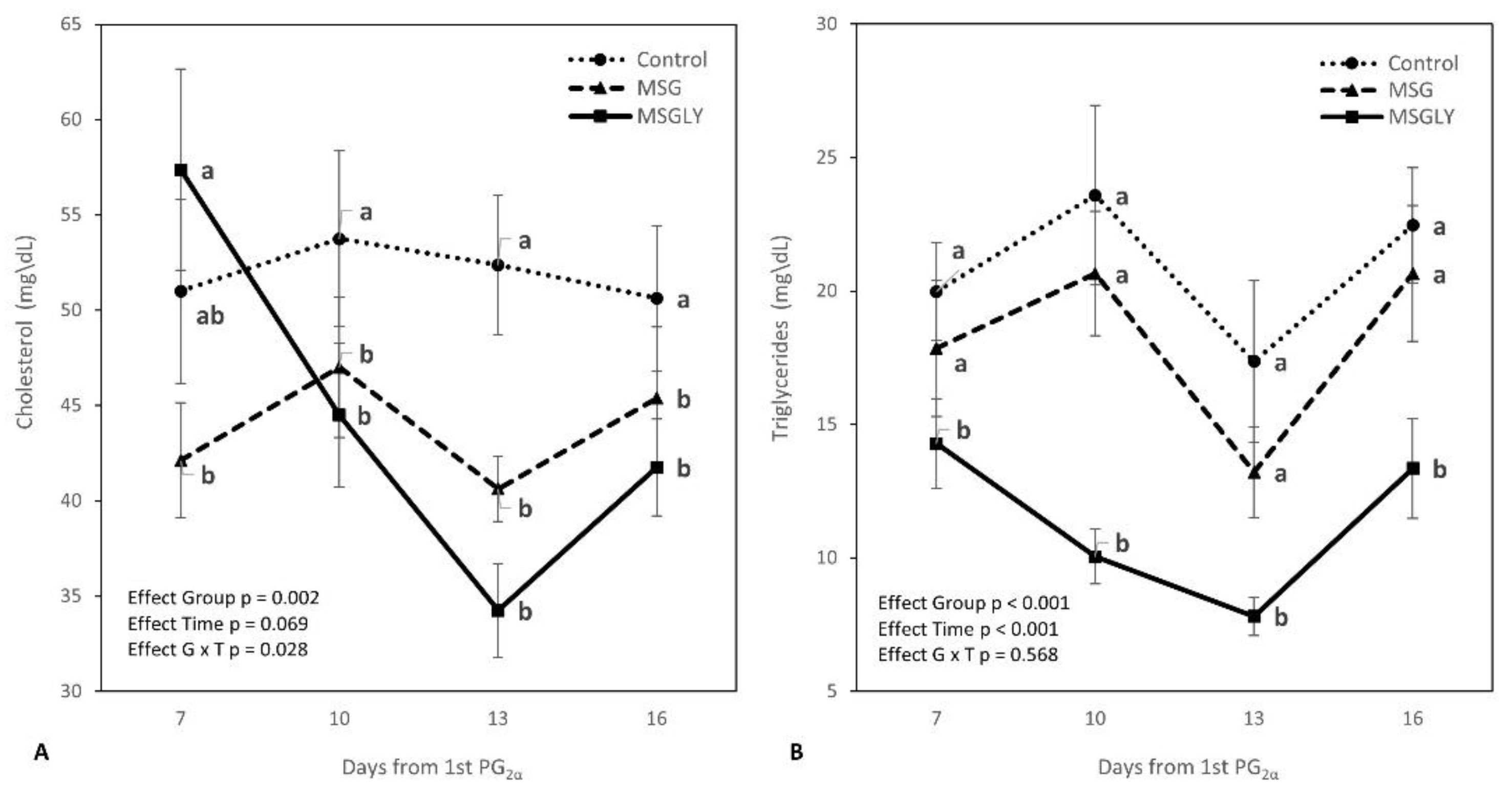
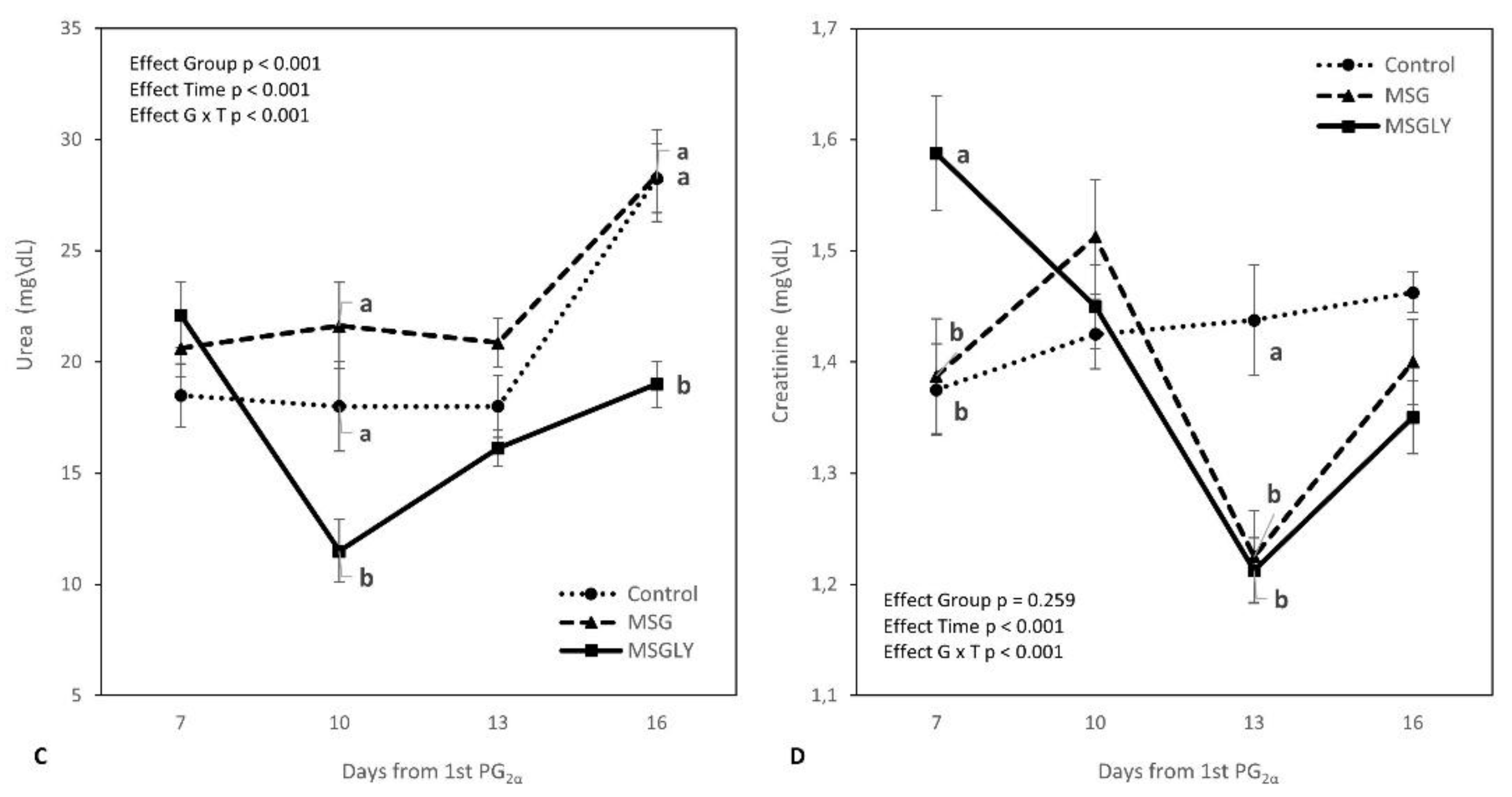
3.2. Physiological and Metabolic Efforts
3.3. Ovarian Function Outcomes
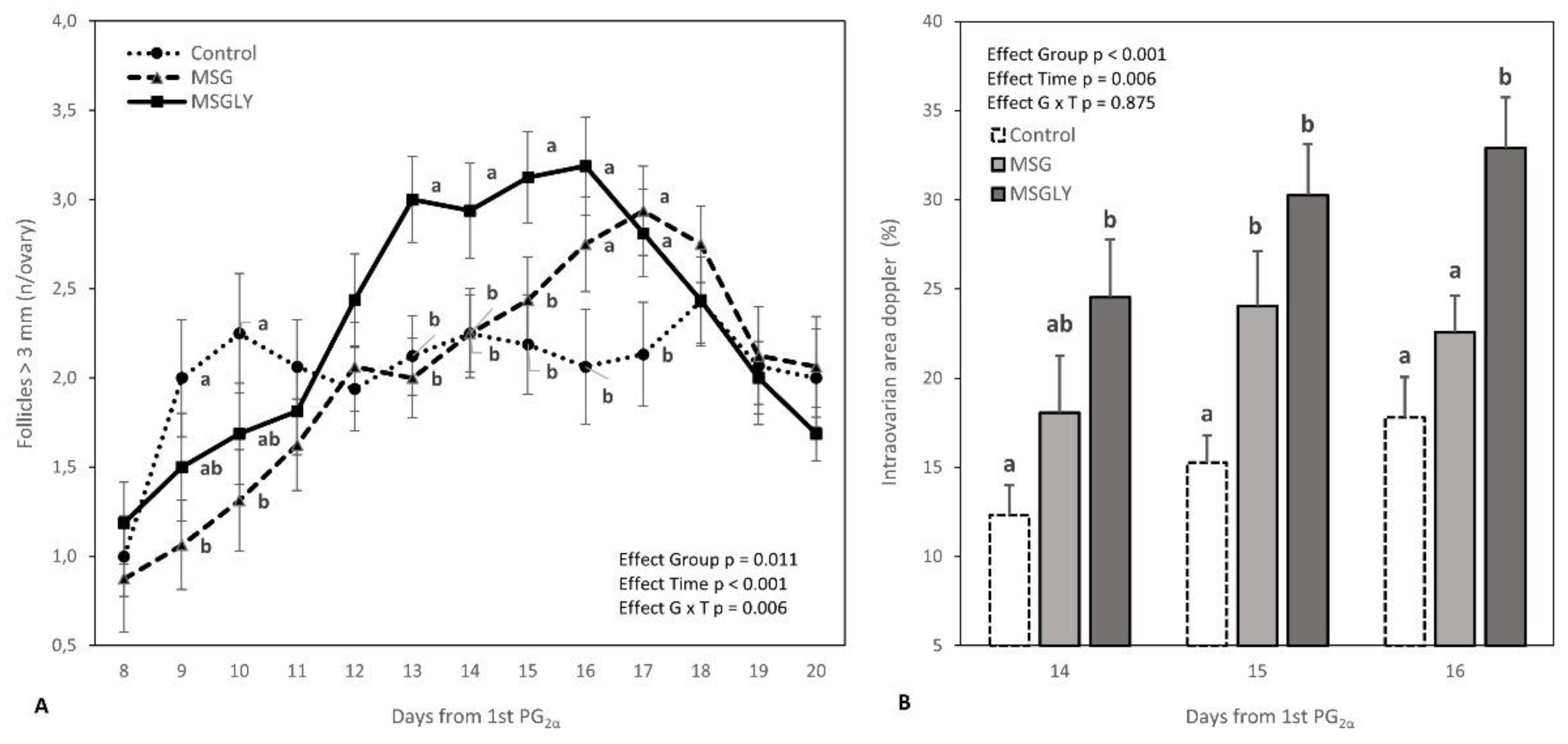
3.3.1. Follicular Turnover Before Ovulation Induction
3.3.2. Follicular Dynamics and Intraovarian Blood Perfusion After Ovulation Induction
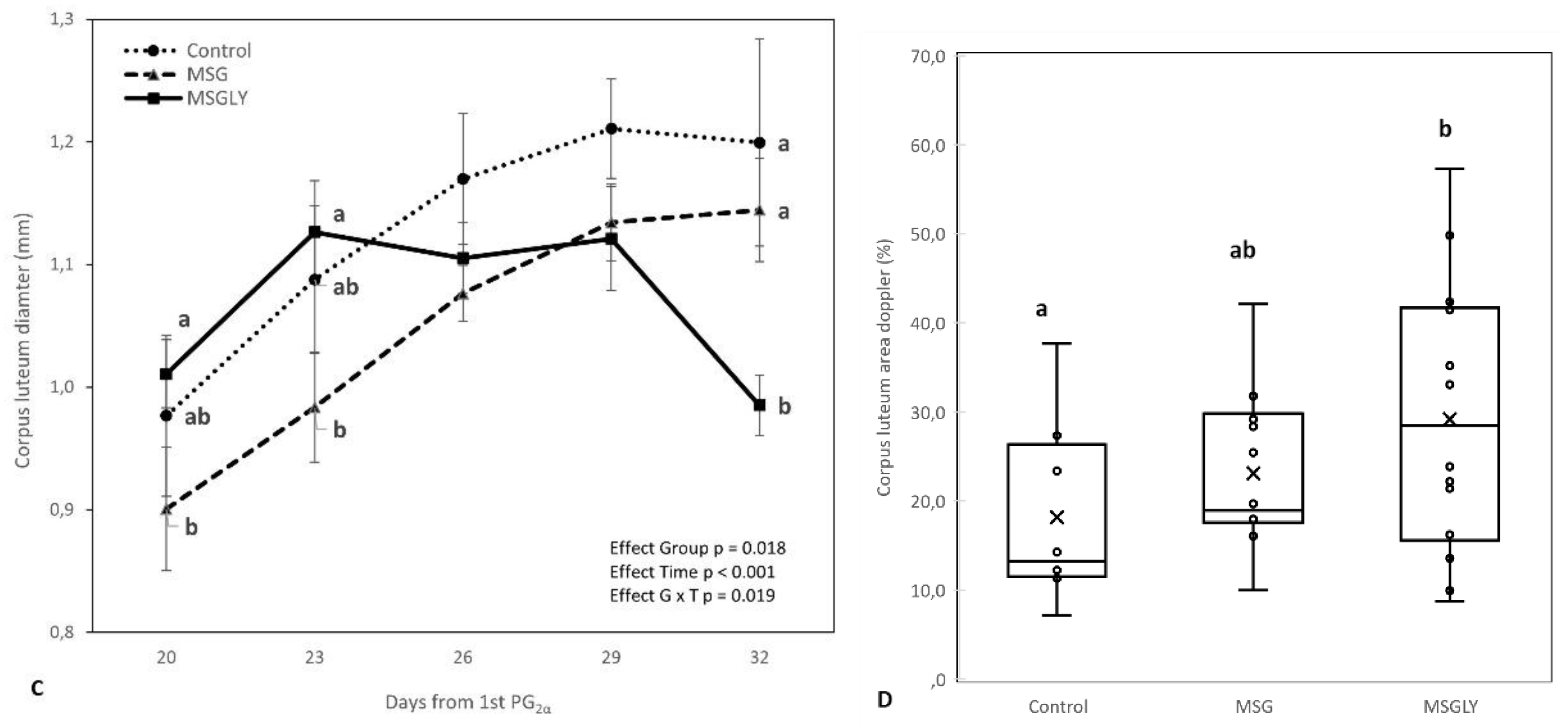
3.3.3. Corpus Luteum Growth, Luteal Blood Perfusion Area, and Ovulatory Rate
3.4. Expression of Gene Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Bazer, F.W.; Johnson, G.A.; Satterfield, M.C.; Washburn, S.E. Metabolism and Nutrition of L-Glutamate and L-Glutamine in Ruminants. Animals 2024, 14, 1788. [Google Scholar] [CrossRef] [PubMed]
- Meza-Herrera, C.A.; Vergara-Hernández, H.P.; Paleta-Ochoa, A.; Álvarez-Ruíz, A.R.; Véliz-Deras, F.G; Arellano-Rodríguez, G.; Rosales-Nieto, C.A.; Macías-Cruz, U.; Rodríguez-Martínez, R.; Carrillo, E. Glutamate supplementation reactivates ovarian function while increasing serum insulin and triiodothyronine concentrations in yearling Criollo x Saanen-Alpine goats during the anestrous season. Animals 2020, 10, 234. [Google Scholar] [CrossRef]
- Conde, A.J.H.; Alves, J.P.M.; Fernandes, C.C.L.; Silva, M.R.L.; Cavalcanti, C.M.; Bezerra, A.F.; Rondina, D. Effect of one or two fixed glutamate doses on follicular development, ovarian-intraovarian blood flow, ovulatory rate, and corpus luteum quality in goats with a low body condition score. Anim. Reprod. 2023, 20, e20220117. [Google Scholar] [CrossRef] [PubMed]
- 4. Luna-Garcia, L.A.; Meza-Herrera, C.A.; Perez-Marin, C.C.; De Santiago-Miramontes, A.; Flores-Salas, J.M.; Corona, R.; Marin-Tinoco, R.I. Targeted glutamate supply boosts insulin concentrations, ovarian activity, and ovulation rate in yearling goats during the anestrous season. Biology 2023, 12, 7, 1041. [CrossRef]
- Gilbreath, K.R.; Bazer, F.W.; Satterfield, M.C.; Wu, G. Amino acid nutrition and reproductive performance in ruminants. Adv Exp Med Biol. 2021, 1285, 43–61. [Google Scholar] [CrossRef]
- Soares, A.C.S.; Alves, J.P.M.; Fernandes, C.C.L.; Silva, M.R.L.; Conde, A.J.H.; Teixeira, D.Í.A.; Rondina, D. Use of monosodium-glutamate as a novel dietary supplement strategy for ovarian stimulation in goats. Animal Reproduction 2023, 20, e20230094. [Google Scholar] [CrossRef]
- Hernández-Marín, J.A.; Ángel-Sahagún, C.A.; Rojas-García, A.R.; Cigarroa-Vázquez, F.A.; Maki-Díaz, G.; Cadena-Villegas, S. Response of a metabolic restorative and monosodium glutamate on pregnancy rate in sheep. Agricultural Ecosystems and Resources 2024, 11, SPE4. [Google Scholar]
- Tabassum, S.; Ahmad, S.; Madiha, S.; Shahzad, S.; Batool, Z.; Sadir, S.; Haider, S. Free L-glutamate-induced modulation in oxidative and neurochemical profile contributes to enhancement in locomotor and memory performance in male rats. Scientific reports 2020, 10, 1, 11206. [CrossRef]
- Glanowska, K.M.; Moenter, S.M. Endocannabinoids and prostaglandins both contribute to GnRH neuron-GABAergic afferent local feedback circuits. Journal of Neurophysiology 2011, 106, 6, 3073–3081. [CrossRef]
- McCoard, S.A.; Pacheco, D. The significance of N-carbamoylglutamate in ruminant production. J Animal Sci Biotechnol 2023, 14, 48. [Google Scholar] [CrossRef]
- Reyes-Aguirre, L.I.; Ferraro, S.; Quintero, H.; Sánchez-Serrano, S.L.; Gómez-Montalvo, A.; Lamas, M. Glutamate-induced epigenetic and morphological changes allow rat Müller cell dedifferentiation but not further acquisition of a photoreceptor phenotype. Neuroscience 2013, 254, 347–360. [Google Scholar] [CrossRef]
- ANP. Agência Nacional do Petróleo, Gás Natural e Biocombustíveis. 2024.
- Rodrigues, F.V.; Silva, C.M.G.; Lima, I.M.T.; Silva, A.M.; Fernandes, C.C.L.; Rondina D. Effect of oral drenching of glycerin as a source of pre-mating energetic supplementation on reproductive response in goats. Animal Reproduction 2018, 12, 4,890–898.
- Sotgiu, F.D.; Porcu, C.; Pasciu, V.; Dattena, M.; Gallus, M.; Argiolas, G.; Berlinguer, F.; Molle, G. Towards a sustainable reproduction management of dairy sheep: glycerol-based formulations as alternative to ecg in milked ewes mated at the end of anoestrus period. Animals, 2021, 11, 4, 922. [CrossRef]
- Andrade, M.A.M.M.; Alves, J.P.M.; Galvão, I.T.O.M.; Cavalcanti, C.M.; Silva, M.R.L.; Conde, A.J.H.; Bezerra, A.F.; Fernandes, C.C.L.; Teixeira, D.I.A.; Rondina, D. Glycerin supplementation strategies for three or seven days affects oxidative stress, follicle dynamics and ovulatory response in Morada Nova sheep. Animal Reproduction 2022, 19, e20200025. [Google Scholar] [CrossRef] [PubMed]
- Luna-García, L.A.; Meza-Herrera, C.A.; Pérez-Marín, C.C.; Corona, R.; Luna-Orozco, J.R.; Véliz-Deras, F.G.; Gutierrez-Guzman, U.N. Goats as valuable animal model to test the targeted glutamate supplementation upon antral follicle number, ovulation rate, and LH-Pulsatility. Biology 2022, 11, 7, 1015. [CrossRef]
- Lass, G.; Li, X.F.; Voliotis, M.; Wall, E.; de Burgh, R.A.; Ivanova, D.; McIntyre C.; Lin, X.Hua.; Colledge, W.H.;. Lightman, S.L; Tsaneva-Atanasova, K.; O’Byrne, K.T. GnRH pulse generator frequency is modulated by kisspeptin and GABA-glutamate interactions in the posterodorsal medial amygdala in female mice. Journal of Neuroendocrinology 2022, 34, 11, e13207. [CrossRef]
- Moore, A.M.; Novak, A.G.; Lehman, M.N. KNDy neurons of the hypothalamus and their role in GnRH pulse generation: an update. Endocrinology 2024, 165, 2, bqad194. [CrossRef]
- Evans, M.C.; Hill, J.W.; Anderson, G.M. Role of insulin in the neuroendocrine control of reproduction. Journal of neuroendocrinology 2021, 33, 4, e12930. [CrossRef]
- de Carvalho Papa, P.; Vargas, A.M.; da Silva, J.L.T.; Nunes, M.T.; Machado, U.F. GLUT4 protein is differently modulated during development of obesity in monosodium glutamate-treated mice. Life Sciences 2002, 71,16, 1917–1928. [CrossRef]
- Sugimoto, M.; Sasaki, S.; Watanabe, T.; Nishimura, S.; Ideta, A.; Yamazaki, M.; Matsuda, K.; Yuzaki, M.; Sakimura, K.; Aoyagi, Y.; Sugimoto, Y. Ionotropic glutamate receptor AMPA1 is associated with ovulation rate. PLoS ONE 2010, 5, e13817. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Liu, Y.; Cao, G.; Di, R.; Wang, J.; Chu, M. Polymorphism and expression of GLUD1 in relation to reproductive performance in Jining Grey goats. Archives Animal Breeding 2023, 66, 4, 411–419. [CrossRef]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007.
- Heinrichs, J; Kononoff, P. The Penn state particle separator. Penn State Extension, University Park, PA. DSE 2013, 186, 1–8.
- Wang, W.; Patra, A.K.; Puchala, R.; Ribeiro, L.; Gipson, T.A.; Goetsch, A.L. Effects of dietary inclusion of tannin-rich sericea lespedeza hay on relationships among linear body measurements, body condition score, body mass indexes, and performance of growing alpine doelings and katahdin ewe lambs. Animals 2022, 12, 3183. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.C.L.; Aguiar, L.H.; Calderón, C.E.M.; Silva, A.M.; Alves, J.P.M.; Rossetto, R.; Bertolini, L.R.; Bertolini, M.; Rondina, D. Nutritional impact on gene expression and competence of oocytes used to support embryo development and livebirth by cloning procedures in goats. Animal Reproduction Science 2018, 188, 1–12. [Google Scholar] [CrossRef]
- Viñoles, C.; Paganoni, B.; Glover, K.M.M.; Milton, J.T.B.; Blache, D.; Blackberry, M.A.; Martin, G.B. The use of a’first-wave’model to study the effect of nutrition on ovarian follicular dynamics and ovulation rate. Reproduction 2010, 140, 865. [Google Scholar] [CrossRef]
- Mach, N.; Bach, A.; Devant, MEffects of crude glycerin supplementation on performance and meat quality of Holstein bulls fed high-concentrate diets. Journal of Animal Science 2009, 87, 2, 632–638. [CrossRef]
- Oliveira, M.E.; Feliciano, M.A.; D’Amato, C.C.; Oliveira, L.G.; Bicudo, S.D.; Fonseca, J.F.; Bartlewski, P.M. Correlations between ovarian follicular blood flow and superovulatory responses in ewes. Anim. Reprod. Sci. 2014, 144, 30–37. [Google Scholar] [CrossRef]
- Balaro, M.F.A.; Santos, A.S.; Moura, L.F.G.M.; Fonseca, J.F.; Brandão, F.Z. Luteal dynamic and functionality assessment in dairy goats by luteal blood flow, luteal biometry, and hormonal assay Theriogenology 2017, 95,118–26. [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25,4, 402–408. [CrossRef]
- da Silva, C.P.; Fernandes, C.C.L.; Alves, J.P.M.; de Oliveira, F.B.B.; Silva, A.M.; de Souza, F.C., Cavalcante, C.M.; Conde A.J.H.; Coutinho A.R.; Rondina, D. Effect of Short-Term Glycerin Supplementation on Follicle Dynamics and Pregnancy Rate in Goats. Ruminants, 2023, 3, 4, 445–456. [CrossRef]
- Lima, A.M.; Cruz, G.R.B.; Costa, R.G.; Ribeiro, N.L.; Beltrão Filho, E.M.; Sousa, S.; Santos, D.G. Physical-chemical and microbiological quality of milk and cheese of goats fed with bidestilated glycerin. Food Science and Technology 2021, 41, 25–33. [Google Scholar] [CrossRef]
- Mo, D.; Zeng, Z.H.; Sui, X.; Li, R.; Yang, Y.H. Role of glucose metabolism and signaling pathways at different stages of ovarian folliculogenesis. Reproductive and Developmental Medicine 2024, 8, 02, 111–120. [CrossRef]
- Takahashi, H.; Yokoi, N.; Seino, S. Glutamate as intracellular and extracellular signals in pancreatic islet functions. Proceedings of the Japan Academy, Series B 2019, 95, 6, 246–260. [CrossRef]
- Calderón-Leyva, G.; Meza-Herrera, C.A.; Rodriguez-Martinez, R.; Angel-García, O.; Rivas-Muñoz, R.; Delgado-Bermejo, J.V.; Véliz-Deras, F.G. Effect of glutamate and/or testosterone administration on appetitive and consummatory sexual behaviors in pubertal rams and their influence on the reproductive performance of nulliparous anovulatory ewes. Journal of Veterinary Behavior 2019, 30, 96–102. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Satterfield, M.C.; Gilbreath, K.R.; Posey, E.A.; Sun, Y. Nutrition and metabolism of L-arginine in ruminants. In: Wu, G. (eds) Recent advances in animal nutrition and metabolism. Advances in experimental medicine and biology 2022, 1354.
- Ruiz de Chávez, J.A.; Guzmán, A.; Zamora-Gutiérrez, D.; Mendoza, G.D.; Melgoza, L.M.; Montes, S.; Rosales-Torres, A.M. Supplementation with rumen-protected L-arginine-HCl increased fertility in sheep with synchronized estrus. Tropical animal health and production 2015, 47, 1067–1073. [Google Scholar] [CrossRef]
- Bulbarela-Garcia, G.; Pro-Martinez, A.; Becerril-Pérez, C.M.; Diaz-Rivera, P.; Rosendo-Ponce, A.; Gallegos-Sanchez, J. Effect of L-arginine and fish oil on the reproductive performance of hair sheep synchronization with a progestagen. Agrociencia 2009, 43, 3, 371–377.
- Hussein, H.A.; Hassaneen, A.S.A.; Ali, M.E.; Sindi, R.A.; Ashour, A.M.; Fahmy, S.M.; Swelum, A.A.; Ahmed, A.E. The impact of rumen-protected l-arginine oral supplementation on libido, semen quality, reproductive organ biometry, and serum biochemical parameters of rams. Frontiers in Veterinary Science 2022, 9, 899434. [Google Scholar] [CrossRef]
- Kaminski, S.L.; Redmer, D.A.; Bass, C.S.; Keisler, D.H.; Carlson, L.S.; Vonnahme, K.A.; Dorsam S.T.; Grazul-Bilska, A.T. The effects of diet and arginine treatment on serum metabolites and selected hormones during the estrous cycle in sheep. Theriogenology 2015, 83, 5, 808–816. Theriogenology, 2015; 5, 808–816. [CrossRef] [PubMed]
- Inagaki, N.; Kuromi, H.; Gonoi, T.; Okamoto, Y.; Ishida, H.; Seino, Y.; Seino, S. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. The FASEB journal 1995, 9, 8, 686–691. [CrossRef]
- Dupont, J.; Scaramuzzi, R.J. Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle. Biochemical Journal 2016, 473, 11, 1483–1501. [CrossRef] [PubMed]
- Clasadonte, J.; Sharif, A.; Baroncini, M.; Prevot, V. Gliotransmission by prostaglandin E2: a prerequisite for GnRH neuronal function? Frontiers in endocrinology 2011, 2, 91. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, C.M.; Silva, M.R.L.; Conde, A.J.H; Bezerra, A.F.; Alves, J.P.M.; Fernandes, C.C.L.; Teixeira, D.Í.A.; Rêgo, A.C., Rondina, D. Effect of periconception high fat diets on maternal ovarian function, foetal and placentome growth, and vascular umbilical development in goats. Reprod Domest Anim. 2022, 57, 12, 1481–1492. [CrossRef]
- Habibizad, J.; Riasi, A.; Kohram, H.; Rahmani, H.R. Effect of long-term or short-term supplementation of high energy or high energy-protein diets on ovarian follicles and blood metabolites and hormones in ewes. Small Ruminant Research 2015, 132, 37–43. [Google Scholar] [CrossRef]
- Kumawat, B.L.; Kumar, P.; Mahla, A.S.; Kumar, A.; Kumar, A.; Singh, R.; Kumar, A. A novel action of insulin sensitizing drug as a potential promotor of preovulatory follicles, ovulation rate and prolificacy in sheep. Veterinary Research Communications 2024, 48, 2, 849–863. [CrossRef]
- Kayode, O.T.; Rotimi, D.E.; Olaolu, T.D.; Adeyemi, O.S. Ketogenic diet improves and restores redox status and biochemical indices in monosodium glutamate-induced rat testicular toxicity. Biomedicine & Pharmacotherapy 2020, 127, 110227. [CrossRef]
- Brown, A.J.; Coates, H.W.; Sharpe, L.J. Cholesterol synthesis. In Biochemistry of lipids, lipoproteins and membranes. Elsevier 2021, 317–355.
- De Felice, B.; Santillo, M.; Serù, R.; Damiano, S.; Matrone, G.; Wilson, R.R.; Mondola, P. Modulation of 3-hydroxy-3-methylglutaryl-CoA reductase gene expression by CuZn superoxide dismutase in human fibroblasts and HepG2 cells. Gene expression, 2018, 12, 1, 29. [CrossRef]
- Kohan, A.B.; Yang, Q.; Xu, M.; Lee, D.; Tso, P. Monosodium glutamate inhibits the lymphatic transport of lipids in the rat. American Journal of Physiology-Gastrointestinal and Liver Physiology 2016, 311, 4, G648–G654. [CrossRef]
- Sukmak, M.; Kyaw, T.S.; Nahok, K.; Sharma, A.; Silsirivanit, A.; Lert-Itthiporn, W.; Cha’on, U. Urinary metabolic profile and its predictive indexes after MSG consumption in rat. Plos one 2024, 19, 9, e0309728. [CrossRef]
- Kassab, R.B.; Theyab, A.; Al-Ghamdy, A.O.; Algahtani, M., Mufti, A.H.; Alsharif, K.F.; Elmasry, H. A. Protocatechuic acid abrogates oxidative insults, inflammation, and apoptosis in liver and kidney associated with monosodium glutamate intoxication in rats. Environmental Science and Pollution Research 2022, 1–14. [CrossRef]
- Batchu, P.; Terrill, T.H.; Kouakou, B.; Estrada-Reyes, Z.M.; Kannan, G. Plasma metabolomic profiles as affected by diet and stress in Spanish goats. Scientific Reports 2021, 11, 1, 12607. [CrossRef]
- Kupczyński, R.; Szumny, A.; Wujcikowska, K.; Pachura, N. Metabolism, ketosis treatment and milk production after using glycerol in dairy cows: A review. Animals 2020, 10, 8, 1379. [CrossRef]
- Michaelis, E.K.; Wang, X.; Pal, R.; Bao, X.; Hascup, K.N.; Wang, Y.; Gerhardt, G.A. Neuronal Glud1 (glutamate dehydrogenase 1) over-expressing mice: increased glutamate formation and synaptic release, loss of synaptic activity, and adaptive changes in genomic expression. Neurochemistry international 2011, 59(4), 473–481. [CrossRef]
- Liang, D.; Xue, Z.; Xue, J.; Xie, D.; Xiong, K.; Zhou, H.; Chen, Y.H. Sinoatrial node pacemaker cells share dominant biological properties with glutamatergic neurons. Protein & Cell 2021, 12, 7, 545–556. [CrossRef]
- Olson, A.L. Regulation of GLUT4 and insulin-dependent glucose flux. International Scholarly Research Notices 2012, 1, 856987. [Google Scholar] [CrossRef]
- Ripoli, C.; Spinelli, M.; Natale, F.; Fusco, S.; Grassi, C. Glucose overload inhibits glutamatergic synaptic transmission: A novel role for creb-mediated regulation of synaptotagmins 2 and 4. Frontiers in cell and developmental biology 2020, 8, 810. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sawa, R.; Wake, I.; Morimoto, A.; Okimura, Y. Glucose-mediated inactivation of AMP-activated protein kinase reduces the levels of L-type amino acid transporter 1 mRNA in C2C12 cells. Nutrition Research 2017, 47, 13–20. [Google Scholar] [CrossRef]
- Hernández-Melchor, D.; Ramírez-Martínez, L.; Cid, L.; Palafox-Gómez, C.; López-Bayghen, E.; Ortega, A. EAAT1-dependent slc1a3 Transcriptional Control depends on the Substrate Translocation Process. ASN neuro 2022, 14, 17590914221116574. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.M.; Phang, Y.X.; Liu, Z.; Lewis, S.A.; Aljohani, A.; McGahee, A.; Ntambi, J.M. Hepatic oleate regulates insulin-like growth factor-binding protein 1 partially through the mTORC1-FGF21 axis during high-carbohydrate feeding. International Journal of Molecular Sciences 2022, 23, 23, 14671. [CrossRef]
- Chiang, V.S.C.; Park, J.H. (2020). Glutamate in male and female sexual behavior: Receptors, transporters, and steroid independence. Frontiers in behavioral neuroscience 2020, 14, 589882. [CrossRef]
- Barth, C.; Villringer, A. y Sacher, J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience 2015, 9, 37. [CrossRef]
- Porter, D.T.; Goodman, R.L.; Hileman, S.M.; Lehman, M.N. Evidence that synaptic plasticity of glutamatergic inputs onto KNDy neurones during the ovine follicular phase is dependent on increasing levels of oestradiol. Journal of Neuroendocrinology 2021, 33, 12945. [Google Scholar] [CrossRef]
- Conde, A.J.H.; Fernandes, C.C.L.; Alves, J.P.M.; Cavalcanti, C.M.; Bezerra, A.F.; Silva, M.R.L.; Ferreira A.C.A.; Figuereido, J.R.; Rondina, D.). Efficacy of transient nutritional supplementation with an independent action stimuli pathway to support oocyte quality retrieved via ovum pick-up in the early postpartum period of lactating anovulatory goats. Theriogenology 2025, 117507. [CrossRef]
- Olson, A.L. “Regulated of GLUT4 transcription and gene expression,” Current Medicinal Chemistry. Immunology, Endocrine & Metabolic Agents 2005, 5, 219–225. [Google Scholar] [CrossRef]
- Mizera, J.; Pomierny, B.; Sadakierska-Chudy, A.; Bystrowska, B.; Pomierny-Chamiolo, L. Disruption of glutamate homeostasis in the brain of rat offspring induced by prenatal and early postnatal exposure to maternal high-sugar diet. Nutrients 2022, 14, 11, 2184. [CrossRef]
- López-Gatius, F.; Garcia-Ispierto, I. Clinical overview of luteal deficiency in dairy cattle. Animals 2022, 12, 15, 1871. [CrossRef] [PubMed]
- Mlyczyńska, E.; Kieżun, M.; Kurowska, P.; Dawid, M.; Pich, K.; Respekta, N.; Rak, A. New aspects of corpus luteum regulation in physiological and pathological conditions: involvement of adipokines and neuropeptides. Cells 2022, 11, 6, 957. [CrossRef] [PubMed]
- Kurowska, P.; Mlyczyńska, E.; Dupont, J.; Rak, A. Novel insights on the corpus luteum function: Role of vaspin on porcine luteal cell angiogenesis, proliferation and apoptosis by activation of GRP78 receptor and MAP3/1 kinase pathways. International Journal of Molecular Sciences 2020, 21, 18, 6823. [CrossRef]
- Xu, X.; Wang, R.; Pei, L.; Wang, Q.; Liu, C. Glucose Transport by Follicle-Stimulating Hormone Is Mediated Through the Akt/FOXO1 Pathway in Ovine Granulosa Cells. Vet Med Sci. 2025, 11, e70294. [Google Scholar] [CrossRef]
- Adermark, L.; Gutierrez, S.; Lagström, O.; Hammarlund, M.; Licheri, V.; Johansson, M.E. Weight gain and neuroadaptations elicited by high fat diet depend on fatty acid composition. Psychoneuroendocrinology 2021, 126, 105143. [Google Scholar] [CrossRef]
- Hu, Q.X.; Klatt, G.M.; Gudmundsrud, R.; Ottestad-Hansen, S.; Verbruggen, L.; Massie, A.; Zhou, Y. Semi-quantitative distribution of excitatory amino acid (glutamate) transporters 1–3 (EAAT1-3) and the cystine-glutamate exchanger (xCT) in the adult murine spinal cord. Neurochemistry International 2020, 140, 104811, ISSN 0197-0186. [CrossRef]
- Meira, A.N.; Moreira, G.C.M.; Coutinho, L.L.; Mourão, G.B.; Azevedo, H.C.; Muniz, E.N.; Machado, A.L.; Sousa, L.P.; Pedrosa, V.B.; Pinto, L.F.B. Carcass and commercial cut yield of Santa Ines sheep affected by polymorphisms of the LEP gene. Small Ruminant Research 2018, 166, 21-128. [CrossRef]
- Szczepkowska, A.; Harazin, A.; Barna, L.; Deli, M.A.; Skipor, J. Identification of Reference Genes for Circadian Studies on Brain Microvessels and Choroid Plexus Samples Isolated from Rats. Biomolecules 2021, 11, 8, 1227. [CrossRef]
| Gene | Length | Direction | Primer (5′to 3′) | Gene Bank accession no. | References |
|---|---|---|---|---|---|
| GLUT4 | 167 | Forward | 5′ATCTTTGGCTTCGTGGCCTT | >XM_027974995.3 (Ovis aries) | [72] |
| Reverse | 3′TCCGCCACATACTGGAAACC | ||||
| GRIA1 | 121 | Forward | 5′CTGAACGAGCAGGGGCTTTT | >XM_042250658.2 (Ovis aries) | [73] |
| Reverse | 3′CCACATTGCTGAGGCTGAGA | ||||
| GLUD1 | 196 | Forward | 5′TTGAATGCTGGGGGAGTGAC | >NM_001278567.1 (Ovis aries) | [22] |
| Reverse | 3′CTTGGAACTCTGCTGTGGGT | ||||
| SLC1A1 | 183 | Forward | 5′AGCAACACTGCCTGTCACTT | >XM_004004350.5 (Ovis aries) | [74] |
| Reverse | 3′ATGATCTGCCCAACGCTCAA | ||||
| SLC1A3 | 107 | Forward | 5′TGTTCTCAGAGCCACCACGA | >XM_042233857.2 (Ovis aries) | [74] |
| Reverse | 3′CAGCTCGCATCCCCATCTTT | ||||
| LEPTIN | 189 | Forward | 5′GTGGACCCCTGTACCGATTC | >XM_027968780.2 (Ovis aries) | [75] |
| Reverse | 3′GCCCAGGGATGAAGTCCAAA | ||||
| RPS18 | 174 | Forward | 5′AGTTCCAGCACATCTTGCGA | >XM_004018745.5 (Ovis aries) | [76] |
| Reverse | 3′GTTCCACCTCGTCCTCAGTG |
| Stages | Temperature (°C) | Time | |
|---|---|---|---|
| Holding phase | 95 °C | 10 min. | |
| Denaturation phase | 95 °C | 15 seg. | 40 cycles |
| Annealing phase | 60 °C | 1 min. | |
| Extension phase | 95 °C | 15 seg. | |
| Melting curve phase | 60 °C | 1 min | |
| 95 °C | 15 seg |
| Parameters | Group | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | MSG | MSGLY | SEM | Group | Time | DR | G vs. T | G vs DR | |
| Body and carcass markers* | |||||||||
| BMI | 8.6 | 9.0 | 8.7 | 0.205 | 0.186 | - | - | - | - |
| SLFT, mm | 4.1 | 5.1 | 4.2 | 0.366 | 0.350 | - | - | - | - |
| KFT, mm | 2.2 | 2.3 | 2.5 | 0.103 | 0.682 | - | - | - | - |
| LD, mm | 17.4 | 19.4 | 17.9 | 0.884 | 0.122 | - | - | - | - |
| Feed intake | |||||||||
| DMI, g/MW | 68.3a | 61.2b | 67.2a | 0.973 | 0.001 | < 0.001 | - | 0.136 | - |
| DMI, % BW | 2.7a | 2.4b | 2.7a | 0.042 | < 0.001 | < 0.001 | - | 0.192 | - |
| Physiological effort | |||||||||
| Rectal temperature, ºC | 38.1a | 38.3b | 38.3b | 0.020 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.806 |
| Surface temperature, ºC | 34.0a | 34.0a | 34.6b | 0.050 | < 0.001 | < 0.001 | < 0.001 | 0.451 | 0.007 |
| Heart rate, beats/min | 70.3a | 70.6a | 72.5b | 0.426 | 0.024 | 0.039 | < 0.001 | 0.002 | 0.016 |
| Respiratory rate, breaths/min | 32.1a | 37.5b | 40.1c | 0.667 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.103 |
| Metabolic effort | |||||||||
| Glucose, mg/dL | 62.3 | 60.9 | 60.6 | 0.513 | 0.363 | 0.590 | - | 0.288 | - |
| Total Protein, mg/dL | 6.4 | 6.1 | 6.2 | 0.058 | 0.083 | 0.854 | - | 0.052 | - |
| Parameters | Group | p Value | |||||
|---|---|---|---|---|---|---|---|
| Control | MSG | MSGLY | SEM | Group | Time | G vs. T | |
| Follicles traits before ovulation induction* | |||||||
| Follicles, < 3 mm, n\ovary | 2.7 | 2.8 | 2.6 | 0.076 | 0.400 | 0.184 | 0.340 |
| Follicles ≥ 3 mm, n\ovary | 1.9a | 1.5b | 1.9a | 0.068 | 0.005 | < 0.001 | 0.154 |
| Follicle ≥ 6 mm, n\ovary | 0.1a | 0.1a | 0.2b | 0.018 | 0.011 | 0.065 | 0.229 |
| Total follicles, n\ovary | 4.6 | 4.3 | 4.5 | 0.068 | 0.115 | < 0.001 | 0.034 |
| Largest follicle size, mm | 4.5ab | 4.2a | 4.7b | 0.086 | 0.029 | < 0.001 | 0.943 |
| Ovarian response after ovulation induction** | |||||||
| Follicles, < 3 mm, n\ovary | 1.8ab | 2.0b | 1.5a | 0.090 | 0.040 | 0.410 | 0.783 |
| Follicles ≥ 3 mm, n\ovary | 2.2a | 2.5a | 3.1b | 0.092 | < 0.001 | 0.686 | 0.770 |
| Follicle ≥ 6 mm, n\ovary | 0.3 | 0.4 | 0.3 | 0.040 | 0.699 | 0.011 | 0.978 |
| Total follicles, n\ovary | 4.0a | 4.5b | 4.6b | 0.084 | 0.010 | 0.842 | 0.756 |
| Largest follicle size, mm | 5.6 | 5.7 | 5.4 | 0.108 | 0.590 | 0.410 | 0.936 |
| Multiple CL rate, % (n/n)*** | 25 (2/8) | 63 (5/8) | 63 (5/8) | - | - | - | - |
| nº of CL, n/ewe | 1.1a | 1.8b | 1.8b | 0.098 | 0.033 | - | - |
| Parameters | Group | p Value | |||
|---|---|---|---|---|---|
| Control | MSG | MSGLY | SEM | Group | |
| Glutamate markers | |||||
| SCL1A1 | 0.032a | 0.274b | 0.060a | 0.047 | 0.029 |
| SCL1A3 | 0.111 | 0.248 | 0.257 | 0.050 | 0.457 |
| GRIA1 | 0.171a | 0.387b | 0.125a | 0.047 | 0.023 |
| GLUD1 | 0.093a | 0.409b | 0.194a | 0.057 | 0.025 |
| Glucose and energy regulation | |||||
| GLUT4 | 0.166 | 0.355 | 0.108 | 0.060 | 0.237 |
| LEP | 0.143 | 0.313 | 0.242 | 0.064 | 0.555 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





