The fast-paced evolution of the Internet and the rise in social media users have dramatically altered how consumers interact with the pharmaceutical market. Digital platforms have become vital for seeking health information, with over 40% of pharmaceutical consumers now looking for drug-related info online (Leventhal, 2025; Zheng et al., 2023). Interestingly, research from AlMuammar et al. (2021) indicates that 78% of patients are doing their research on medications online before they even visit a doctor, which really highlights our growing reliance on digital sources. However, even with this shift, pharmaceutical companies are still falling behind other industries in adopting digital advertising strategies. This is primarily due to strict regulations, ethical concerns, and a lack of in-depth research on digital promotion within the sector (Dremova & Solomka, 2022; Bharskar & Siddheshwar, 2020).
The retail and tech sectors have started to adopt marketing strategies that harness artificial intelligence for advertising, personalize content interactively, and analyze data in more sophisticated manners. Meanwhile, the pharmaceutical industry is contending with strict government regulations that impact its infrastructure (Morgan & Zane, 2022). In many countries, advertising towards a Direct to Consumer audience (DTC) is highly regulated, making it difficult for pharmaceutical companies to market their products through social media platforms, influencer ads, or direct advertisements online (Willis & Delbaere, 2021).
Gupta et al. (2024) observed that 77% of companies in the pharmaceutical industry recognize the importance of digital advertising. However, only 45% actually utilize these channels, largely due to restrictive legislation. In addition, there’s been a decline in consumer interaction, primarily due to the medical, legal, and regulatory review (MLR) processes that delay the approval of marketing materials (Davies, 2024). A mismatch between advertising strategies and consumer demands often leads to wasted resources, lost sales, and a lack of marketing awareness among consumers.
Although digital digital offers significant potential, there's a surprising lack of research in the pharmaceutical sector. Traditionally, advertising efforts from pharmaceutical companies have been geared towards physicians, medical conferences, and printed advertisements. However, patient-focused strategies are now embracing modern technologies like AI chatbots, telemedicine, personalized virtual calls, and other digital marketing methods (Miozza et al., 2024). According to recent findings from Kassem et al. (2022), using AI in pharmaceutical marketing can increase consumer engagement by an impressive 63%, while also helping to maintain regulatory compliance. Furthermore, AI-based predictive analytics and automated content generation have been effective in advertising drugs within the confines of the law (Aritra & Indu, 2025).
Regulatory organizations, such as the Food and Drug Administration (FDA), have strict advertising regulations that require full disclosure and prohibit misleading information (Perrone, 2024). In addition, there is some debate about the unscrupulous advertising of some medicines, especially with social media influencers peddling weight loss drugs (Davis, 2024). Meeting advertising guidelines and data protection legislation like GDPR is, according to Williamson (2025), still one of the pharmacy industry’s most difficult problems in trying to market to consumers on the internet.
To navigate regulatory constraints in pharmaceutical advertising, companies are increasingly employing digital strategies such as AI-generated promotional content, algorithm-driven message personalization, and coordinated cross-platform campaigns spanning websites, social media, and mobile apps (Platforce, 2023). These strategies aim not only to ensure compliance but also to enhance consumer engagement through interactive and value-driven content. Recent studies highlight that formats such as quizzes, expert-led videos, blogs, and virtual consultations are particularly effective in fostering trust and improving brand recognition for OTC medications by creating personalized, immersive experiences (Zafar et al., 2025). Moreover, the adoption of advanced customer relationship management (CRM) platforms enables real-time, data-driven communication between healthcare providers and consumers, further supporting targeted messaging and advertising effectiveness.
Despite the growing adoption of digital tools, there remains a significant research gap concerning which digital advertising strategies are both effective and compliant when promoting OTC pharmaceutical products (Dremova et al., 2021; Nguyen & Patel, 2023). This study builds on the latest developments in AI-driven, interactive, and personalized communication formats to explore a key question: How can pharmaceutical companies leverage targeted digital advertising techniques—like personalized recommendations, gamification, educational content, and AI-enabled chatbots—to enhance consumer awareness of OTC drugs while ensuring compliance with regulatory standards? To explore this, the study applies a mixed-methods approach grounded in the Technology Acceptance Model (TAM) and Theory of Planned Behavior (TPB), combining qualitative interviews and a large-scale survey. The findings aim to advance both theoretical understanding and practical application of digital advertising in one of the most regulated consumer markets.
2. Theoretical Foundation and Literature Review
2.1. Regulatory Constraints in Pharmaceutical Advertising
Pharmaceutical advertising operates under strict regulatory scrutiny, especially concerning prescription (Rx) drugs, which are often prohibited from being advertised directly to consumers in many countries. Even in the case of over-the-counter (OTC) products, content must avoid misleading claims and adhere to medical accuracy. Regulatory bodies emphasize the importance of evidence-based messaging, transparent sourcing, and ethical presentation. These constraints limit the use of traditional persuasive techniques, prompting companies to adopt alternative, compliant strategies such as educational content, peer-driven endorsements, and platform-specific targeting.Building on this regulatory context, the following sections explore digital advertising strategies that have shown potential to increase awareness in ways that remain compliant with these restrictions.
2.2. Content-Driven Strategies for Consumer Awareness
The impact of digital technologies on advertising in the pharmaceutical industry has been profound, especially in the over-the-counter (OTC) sector. Research shows that content marketing, personalized ads, and digital tools play a key role in boosting awareness about the product following ad exposure (Willis & Delbaere, Verma et al., 2024; Anis & Hassali, 2022; Enyinda et al., 2018). These strategies are not merely informational—they aim to create psychological resonance by engaging consumers cognitively, emotionally, and behaviorally through immersive brand experiences. Such engagement mechanisms are especially critical in highly regulated industries like pharmaceuticals, where advertising must simultaneously foster consumer trust and comply with stringent ethical and legal standards (Srivastava et al., 2023).
Social media has emerged as a critical channel for OTC pharmaceutical advertising, offering opportunities for direct and interactive engagement with consumers. Research by Sattora et al. (2024) indicates that platforms like TikTok and Instagram achieve 50% greater engagement with medical content than traditional advertising methods. In line with this, Willis and Delbaere (2021) show that micro-influencers play a key role in building brand trust, as consumers are more likely to view recommendations from relatable figures as credible, rather than those coming from conventional corporate messaging.
Supporting these trends, research by Syrkiewicz-Świtała et al. (2016) found that collaborations between pharmaceutical brands and medical experts on social media can enhance audience trust. Similarly, Chen and Wang (2021) discovered that endorsements from influencers and healthcare professionals can increase the likelihood of product purchases. This underscores the power of peer-driven advertising strategies where trust is mediated by perceived authenticity and social validation. Consistent with broader literature on advertising engagement, this evidence supports the role of social influence, descriptive norms, and message credibility in shaping consumer attitudes and behavioral responses.
Content marketing has become a central component of digital pharmaceutical advertising, encompassing formats such as educational articles, science-based blog posts, infographics, and video content. Content marketing replaces traditional advertising by concentrating on education and evidence-based information. When direct promotions are limited, companies can provide valuable content that educates consumers about health conditions, treatment options, and product categories—without making any promotional claims. Furthermore, Studies indicate that consumers are more likely to trust brands that offer scientifically validated medical information, particularly when presented in engaging visual formats (Singh et al., 2023; Ali et al., 2022; Sample et al., 2019). Similarly, Ali et al. (2022) found that endorsements from expert physicians combined with the use of infographics can enhance trust in pharmaceutical companies, which aligns with broader studies highlighting the effectiveness of visual content in marketing (Sample et al., 2019). Moreover, Mohamed and Shoufan (2024) discovered that educational videos on platforms like YouTube engage audiences more quickly than text-based content, a finding that echoes trends in digital marketing literature. This trend is in line with research on customer engagement, highlighting the importance of educational strategies. Similar to pro-social or environmental campaigns, pharmaceutical advertising needs to move beyond passive awareness to actively influence behavior, guiding consumers from initial exposure to informed decision-making and purchase intention.
2.3. Personalized Strategies for Raising Awareness
Given the evolving regulatory landscape, pharmaceutical advertisers must balance innovation with compliance, ensuring that their digital communication strategies align with both legal standards and consumer expectations (Mor et al., 2024).In this context, personalized advertising has emerged as a particularly effective approach for enhancing awareness of OTC products. Leveraging machine learning algorithms, firms can analyze consumer search behavior, browsing history, and engagement patterns to deliver highly tailored advertising messages (Aritra & Indu, 2025; Tomar & Pandey, 2024). This data-driven approach has been proven to significantly enhance message relevance and improve conversion outcomes (Aritra & Indu, 2025; Tomar & Pandey, 2024). According to authors, Personalized ads—whether they come from programmatic display or targeted email campaigns—tend to outperform traditional mass advertising formats in click-through and engagement rates, showcasing the strategic value of personalization in pharmaceutical advertising. Moreover, personalized advertising enables pharmaceutical brands to tailor messages based on user behavior and preferences without explicitly promoting drug usage. By focusing on informative content and contextual relevance rather than persuasive claims, these strategies help navigate regulatory boundaries while still engaging consumers.
A major breakthrough in pharmaceutical advertising is the introduction of AI-powered chatbots. These tools automate user interactions, provide personalized recommendations, and enhance customer service in the pharmaceutical sector. Rammal et al. (2024) highlight that AI-driven platforms help reduce the workload for pharmacists by offering round-the-clock customer support, enabling pharmaceutical companies to manage a higher volume of inquiries more efficiently. Furthermore, research by Clark & Bailey (2024) indicates that users in the pharmaceutical market favor receiving information from AI chatbots, as long as the answers are well-structured, evidence-based, and derived from medical data. In a similar vein, Zomorodi et al. (2024) discovered that AI recommendation systems boost the accuracy of product suggestions, enabling pharmaceutical brands to customize information to meet individual consumer needs in real time. Further backing these insights, Laymouna et al. (2024) showed that AI-based conversational agents can increase user engagement, especially in telemedicine and pharmaceutical e-commerce environments. Together, these insights suggest that well-designed chatbots can serve not only as informational tools, but as persuasive advertising agents capable of fostering trust, guiding decision-making, and personalizing the consumer journey in compliance with regulatory standards. Pharmaceutical companies can use chatbots to provide product information in a more interactive and user-focused manner, which helps lower the risk of unsolicited promotions. By embedding regulatory filters and responding to user-initiated queries, chatbots enhance product awareness while staying within the boundaries of legal communication.
Beyond conversational tools like chatbots, AI now plays a strategic role in pharmaceutical advertising through predictive analytics and marketing automation. According to a study by Dixon et al. (2024), these technologies can anticipate consumer needs by analyzing their browsing patterns and past purchases, resulting in more accurate and timely promotional messaging. This level of behavioral insight supports hyper-personalized targeting and allows for content delivery that aligns closely with individual preferences and health-related interests. Additionally, Verma et al. (2024) found that pharmaceutical companies that embrace AI-driven marketing automation see a rise in consumer engagement, lustrating how data-informed advertising strategies can enhance relevance, responsiveness, and overall communication effectiveness.
2.4. Omnichannel Strategies for Raising Awareness
In contemporary pharmaceutical advertising, the integration of AI technologies with omnichannel strategies has become essential for delivering a cohesive and personalized customer experience across digital platforms. As noted by Paiola et al. (2023), consumers expect a seamless transition between social media, manufacturer websites, and online pharmacies. This significantly emphasizes the importance of consistent messaging and a strong brand presence at every digital touchpoint. Kaiponen (2021) found that pharmaceutical companies that adopt omnichannel marketing strategies see a 40% increase in conversion rates, as customers are more likely to engage with brands that offer a cohesive and interconnected experience. Similarly, Gao et al. (2019) observed that companies that integrate all their customer interaction channels—such as email, social media, mobile apps, and e-commerce—often achieve higher customer retention and brand loyalty, emphasizing the advertising value of interconnected communication channels. Omnichannel strategies empower pharmaceutical brands to maintain a consistent yet unobtrusive presence across various digital channels. Instead of falling back on repetitive or overly aggressive messaging, these approaches concentrate on delivering relevant content in formats that suit the context—be it through social media, mobile apps, emails, or their own websites.
2.5. Theoretical Framework: TAM and TPB in Pharmaceutical Advertising
The perception of pharmaceutical information by consumers in digital environments is influenced by several behavioral and technological factors. This research is anchored in two prominent theoretical models: the Technology Acceptance Model (TAM) (Davis, 1989) and the Theory of Planned Behavior (TPB) (Ajzen, 1991). Both have effectively explained how individuals engage with digital platforms, particularly in health-related situations.
TAM focuses on how perceived usefulness and ease of use influence acceptance of digital platforms. In pharmaceutical advertising, studies have shown that user-friendly medical websites, which feature clear layouts and intuitive navigation, help build trust and encourage users to return (Vega et al., 2011; Kim, 2014). Personalized content, especially when it comes from AI-driven recommendations, has been shown to enhance how reliable consumers find it and increase their confidence (Hassan et al., 2025). Websites that provide interactive tools, use clear language, and organize their content well can significantly improve how satisfied consumers feel with digital health information (Zhang & Kim, 2022).
In parallel, TPB emphasizes that attitudes, subjective norms, and perceived behavioral control influence behavioral intentions. In the context of pharmaceutical advertising, endorsements from doctors, health experts, bloggers, and peers can have a significant impact on how much trust consumers place in a product and their decision-making processes (Rollins et al., 2020). Social validation, particularly through user-generated content and online community engagement, strengthens credibility and facilitates behavioral alignment (Yu et al., 2024).
Additionally, affective engagement has emerged as a critical factor in digital advertising effectiveness. Triggers such as storytelling, humor, and personal testimonials can significantly enhance brand recall, trust, and the overall effectiveness of advertising campaigns (Bhargavi, 2024; Vrtana & Krizanova, 2023). Narrative-based content has been found to generate stronger connections than purely rational or scientific messaging (Kang et al., 2020).
Together, the Technology Acceptance Model (TAM) and the Theory of Planned Behavior (TPB) provide a comprehensive framework for understanding the impact of digital advertising strategies on consumer behavior in the pharmaceutical sector. This study uses these models to investigate how various tools—such as chatbots, educational content, gamification, and emotionally resonant narratives—can effectively raise awareness of OTC medications while adhering to regulatory requirements.
2.6. Hypotheses Development
Building on this theoretical framework, we craft hypotheses to delve into how consumers respond to essential digital advertising tools—such as chatbots, educational content, gamification, and social influence—specifically in relation to OTC drug awareness. These hypotheses are anchored in the Technology Acceptance Model (TAM) and the Theory of Planned Behavior (TPB), aiming to clarify how digital strategies can boost perceived usefulness, accessibility, and credibility. This section presents these hypotheses in detail.
According to TAM, consumers’ intention to engage with digital platforms is largely driven by their perceptions of usefulness and ease of use (Davis, 1989). In the context of pharmaceutical advertising, tools such as AI-powered chatbots or structured educational interfaces are more likely to attract consumer engagement when they are perceived as beneficial, intuitive, and easy to navigate. This engagement supports deeper information processing, longer attention spans, and increased awareness of OTC medications. Previous research confirms that usability and informativeness in digital health platforms enhance user trust and content recall (Rahimi et al., 2018). Therefore, we hypothesize that greater perceived usefulness and ease of access to digital marketing tools will lead to increased consumer engagement with OTC drug information (H1).
According to TAM, perceived usefulness is a key predictor of whether users adopt and engage with digital tools. By using personalized promotional messages and recommendation systems, companies can enhance this perceived usefulness, delivering content that resonates with individual needs and interests. This relevance boosts cognitive engagement, leading to better attention and retention of messages. In the context of marketing over-the-counter (OTC) drugs, personalization can therefore boost awareness by encouraging users to actively engage with information that addresses their specific concerns or preferences. Accordingly, we hypothesize that greater perceived usefulness of digital marketing tools causally increases consumer awareness of OTC drugs (H2).
Gamification and interactivity promote positive attitudes toward digital content by making it more engaging and enjoyable—key drivers of behavioral intention in TPB. Such formats ignite intrinsic motivation and encourage participation voluntarily, which leads to a greater interest in the message. Additionally, viral content amplifies visibility and social relevance, motivating consumers to seek out more information about products (Willis & Delbaere, 2021). Thereby, it is hypothesized that the use of gamification, interactive mechanics (e.g., surveys, challenges, quizzes), and viral content leads to higher levels of consumer interest in OTC advertising (H3).
In the TPB framework, subjective norms refer to the perceived expectations of important others, which significantly shape individual behavioral intentions. When it comes to health decisions, endorsements from trusted figures, such as doctors or medical influencers serve as social proof, boosting the credibility of the message and easing any perceived risks. Research has demonstrated that expert opinions can enhance the acceptance of information, particularly in areas where consumers may lack technical expertise (Bhutada & Rollins, 2015). Thus, expert and peer recommendations are expected to strengthen the perceived credibility of OTC advertising. Following this, we hypothesized that Endorsements from experts, physicians, and bloggers positively influence consumers’ perceptions of the credibility of OTC advertising messages (H4).
According to the Theory of Planned Behavior (TPB), perceived behavioral control refers to how much belief a person has in their capability to execute a specific behavior. When consumers find the process of accessing health information to be easy, trustworthy, and manageable, they tend to weave that information into their decision-making and evaluations more effectively. This includes comparing products, assessing active ingredients, or verifying safety claims before purchase. Tools such as chatbots or online expert resources reduce perceived effort and uncertainty, thereby increasing the likelihood of using that information in concrete decisions (Thapa et al., 2020; Jia et al., 2021). Subsequently, it was hypothesized that the more easily and reliably users perceive access to pharmaceutical information (e.g., via chatbots, online consultations, or expert articles), the more likely they are to incorporate this information into their medication selection decisions (H5).
Educational content supports brand credibility by providing transparent, expert-backed information that consumers perceive as trustworthy. In the context of pharmaceuticals, where knowledge gaps are common, professionally curated content—such as blogs or expert Q&As—reduces uncertainty and builds confidence in the source. Studies show that trusted information environments increase both brand trust and message effectiveness (Purcarea, 2019). Accordingly, it is hypothesized that exposure to educational content enhances consumer trust in pharmaceutical brands (H6).
Humor in advertising activates emotional responses that enhance cognitive processing, leading to stronger memory encoding and message recall. While memorability itself is not equivalent to brand awareness, it functions as a cognitive gateway: a memorable message is more likely to be retrieved, recognized, and associated with a specific brand over time. This process reinforces awareness by increasing the likelihood that consumers will recall the brand in future decision-making contexts (Krishnan & Chakravarti, 2003; Eisend, 2021). Within the TPB framework, humor also fosters positive attitudes, which can further strengthen attention and message receptivity—key precursors to awareness-related outcomes. This is why we hypothesized that humorous content in promotional messages contributes to increased brand awareness of OTC medications (H7).
While chatbots can effectively share information and simulate conversations, they often struggle to establish trust in health-related contexts due to their lack of human presence and perceived expertise. In sensitive fields like pharmaceuticals, consumers tend to trust sources that are verified by experts or involve human mediation. Studies indicate that while users may find chatbot communication useful and accessible, they often perceive it as less credible, particularly when it comes to medical or safety-related information (Seitz et al., 2022; Liu et al., 2022). Thus, it is hypothesized that chatbot-based communication has a limited effect on trust formation in the context of OTC drug marketing (H8).
Within the Theory of Planned Behavior (TPB), descriptive norms—defined as individuals’ perceptions of what others commonly do—are central in shaping attention, attitudes, and behavioral intentions (Ajzen, 1991). In digital pharmaceutical advertising, socially shareable formats such as influencer collaborations, viral challenges, or flash mobs amplify perceived peer behavior and social validation. By observing others’ engagement, consumers are more likely to consider the content relevant and credible (Ahmed et al., 2024). These formats also enhance the frequency of exposure and boost cognitive engagement, leading to a greater memorability of the message. While product recall refers to the ability to remember specific information, it also serves as a vital precursor to awareness. It enables consumers to recognize, identify, and associate a brand or product when making decisions (Varnali & Gorgulu, 2018). Therefore, content that is widely seen and shared boosts overall awareness by increasing both mental availability and social relevance. Accordingly, we hypothesized that shareable content—like influencer collaborations, challenges, and flash mobs—improves consumer awareness of OTC medications (H9).
3. Methodology
3.1. Data Collection
To enable effective digital communication and raise consumer awareness of OTC pharmaceutical products, it is essential to consider the preferences of the target audience with respect to communication channels, content formats, and message types. In a highly competitive and regulated market, a nuanced understanding of these preferences at the awareness stage can inform more targeted and adaptive advertising strategies.
To explore these aspects, qualitative data were collected through semi-structured interviews, striking a balance between asking standardized questions and allowing for deeper discussions on any unexpected topics that come up during the conversation. . The study included two segments: end consumers and employees of marketing departments of major pharmaceutical companies. End consumers were defined by demographic, psychographic, geographic, and behavioral criteria: residents of million-plus cities aged 18 and over, active internet users, health-conscious. Interviews were conducted online, transcribed, and analyzed thematically.
We conducted 10 semi-structured interviews, which aligns with established qualitative research practices (Vasileiou et al., 2018; DeJonckheere & Vaughn, 2019). Prior literature suggests that a sample size of 5 to 15 participants is appropriate for exploratory research aiming to generate primary consumer insights, rather than statistical generalizability. This approach enabled us to examine in depth the attitudes, preferences, and challenges consumers experience in relation to digital pharmaceutical marketing. All participants provided informed consent, and the data were anonymized to ensure confidentiality.
In addition, this sample size was adequate to reach data saturation, the stage when no additional patterns are detected, and therefore, confirm useful conclusions could be drawn (Guest et al., 2005). To ensure data reliability, triangulation was conducted by systematically comparing interview themes with survey results to identify overlaps and contradictions. For example, consumer preferences expressed in interviews were cross-validated with trends observed in quantitative responses. Additionally, secondary data from academic and industry sources were used to support or refine emerging categories. Member-checking was performed by sending each participant a summary of their responses along with the researcher’s initial interpretations. Participants were asked to confirm accuracy or suggest corrections. Their feedback led to minor refinements in wording and thematic emphasis, ensuring that the final analysis reflected their intended meaning.
The criteria for forming an interview sample among employees were - experience as junior managers in the marketing department from 1 year in large companies with 1000+ employees, age from 25-45 years old, which corresponds with research practice. The age range of 25–45 was selected to reflect a segment of marketing professionals who are typically active in operational roles and most likely to engage directly with evolving digital tools. While this focused sampling strategy increases the relevance and depth of insight within the target group, it may limit the generalizability of findings to marketers in smaller firms, senior-level strategists, or professionals outside the specified age range. These limitations are acknowledged and considered when interpreting the results.
For the quantitative phase, we surveyed 384 respondents through convenience sampling, carefully balancing the sample across age and gender to enhance demographic diversity. A sample size of 384 is statistically sound for large populations, allowing for a 95% confidence level and a margin of error of approximately ±5%, which is a standard in social science research (Krejcie & Morgan, 1970). While not fully randomized, this approach allowed us to capture a broad range of consumer perspectives relevant to digital pharmaceutical marketing. Similar to most online surveys, the results might be influenced by self-selection bias and may not apply to populations with limited digital access. Our questionnaire will include a blend of Likert scale questions and open-ended questions, giving us a richer perspective on consumer motivations and the hurdles they encounter. To reduce the risk of self-selection bias, we distributed the survey across multiple channels (e.g., social media, email lists, professional platforms) and clearly stated that participation was open to a wide audience regardless of prior interest in pharmaceutical marketing. Additionally, no incentives were provided, which helped avoid overrepresentation of highly motivated respondents.
3.2. Ethical Considerations
All participants were adults who voluntarily agreed to take part in the study after being informed about its purpose, the voluntary nature of participation, and their right to withdraw at any time without penalty. Informed consent was obtained in writing prior to data collection. To protect participants’ privacy, all responses were anonymized and any identifying information was excluded from the analysis and final manuscript. The study posed minimal risk to participants and did not involve any vulnerable populations or sensitive topics.
3.3. Data Analysis
The hypotheses were tested using Spearman’s rank-order correlation at a significance level of 0.05. This method was selected because the data collected from the survey included ordinal variables, such as ranked preferences for communication channels (e.g., chatbots, expert articles, influencer content) and Likert-scale responses related to drug awareness and attitudes toward digital advertising approaches. Spearman’s correlation is appropriate for examining monotonic relationships between such variables without assuming a normal distribution or linearity (Coleman et al., 2022). Given that many of the questionnaire items captured subjective perceptions and preferences measured on ordinal or non-normally distributed scales, Spearman’s rho was more suitable than Pearson’s correlation, which requires interval-level data and normality. This choice aligns with research practices in consumer behavior and health marketing contexts where perception-based and rank-order variables are common (Rahimi et al., 2018; Rollins et al., 2020; Bhargavi, 2024; Vrtana & Krizanova, 2023).
After that, Structural Equation Modeling (SEM) was applied to analyze the direct, indirect, and mediated effects, giving a more comprehensive understanding of digital marketing influences. For the SEM model, three latent constructs were created - Digital Marketing Features, Awareness Drivers, and Consumer Awareness of OTC Medications. The latent variable Digital Marketing Influence was composed of six indicators: gamification, viral content, personalized marketing, humor, and social endorsements. Due to its theoretical connection to trust, consumer education was treated as an independent predictor. These components were chosen from existing literature in digital health communication and adapted into health communication instruments through surveys with Likert scales as frameworks (Willis & Delbaere, 2021; Krishnan & Chakravarti, 2003; Eisend, 2021). All items were operationalized using Likert-scale items such as “I pay more attention to OTC ads when they include challenges or interactive features,” and adapted from validated instruments. Where necessary, new items were developed, and a panel of academic and industry experts (n = 4) was assembled to review these items to ensure content validity.
The model also included observed mediators (Awareness Drivers)—trust, perceived usefulness, and ease of access to information—which together represented the core psychological mechanisms through which digital content exposure leads to greater awareness. Trust and usefulness were measured using items from the Technology Acceptance Model (TAM) and prior studies on digital health credibility (Kim et al.,2024; Purcarea, 2019). Ease of access was evaluated through consumer perceptions of clarity, speed, and reliability of online information.
The third construct, Consumer Awareness, evaluated the extent to which respondents recognized or recalled OTC products and reported an increased awareness of options due to digital marketing exposure. Items focused on brand recognition, message recall, and the intention to use this information in purchase decisions.
All measurement items were pre-tested in a pilot study (n = 30) to assess clarity and internal consistency. Confirmatory factor analysis confirmed acceptable factor loadings (λ > 0.60) and internal reliability (Cronbach’s α > 0.70).
In addition to the confirmatory factor analysis used in the SEM model, we also performed an exploratory factor analysis (EFA) to identify the key latent dimensions that shape consumer perceptions of OTC drug advertising. This analysis aims to uncover the main factors that influence the perception of OTC drug advertising. The factor names were based on existing research in the field of digital marketing. The sample size of 384 respondents meets the minimum requirements for factor analysis (Bartlett et al., 2001). Utilizing this method will provide valuable insights into the factors that should be prioritized when executing marketing campaigns to enhance awareness in the digital landscape, allowing companies to focus on what really matters, rather than on minor details.
4. Results
4.1. Correlation Analysis
Correlation analysis revealed significant relationships between consumer preferences and digital advertising approaches tools. Spearman correlation coefficients (r) were evaluated in terms of their strength and directionality, with values above 0.3 considered as moderate and above 0.5 as strong.
The results align with the core ideas of the Technology Acceptance Model (TAM) and the Theory of Planned Behavior (TPB), illustrating how digital advertising affects consumer awareness and trust in over-the-counter (OTC) drug information. As it was shown in
Table 1, it was found that consumers who enjoy leaving reviews often face challenges in locating trustworthy information (r = 0.449, p < 0.001), pointing to a notable gap in reliable online resources. This highlights the vital role of expert content and user reviews in fostering trust. Additionally, the positive association between users’ difficulty in finding credible information and their interest in chatbot tools (r = 0.398, p < 0.001) suggests that AI assistants may be perceived as helpful in streamlining the information-gathering process and enhancing user engagement. While this does not establish causality, the observed correlation supports the relevance of chatbot interaction in relation to hypotheses H1, H5, and H8.
Perception of information usefulness was also found to significantly influence preference for personalized marketing (r = 0.355, p < 0.001), highlighting the need for custom content strategies to increase drug awareness, as confirmed by H2. Increased attention to information credibility correlates with interest in online consultations with physicians (r = 0.329, p < 0.001), demonstrating the importance of authoritative sources in digital pharmaceutical promotion (H4, H5). Finally, preference for data visualization is associated with overcoming the complexity of information perception (r = 0.325, p < 0.001), highlighting the importance of infographics and interactive content in educational digital marketing strategies (H6).
These results confirm that digital marketing tools not only facilitate access to information about medicines, but also shape behavioral patterns to increase audience trust and awareness.
4.2. SEM Analysis Results
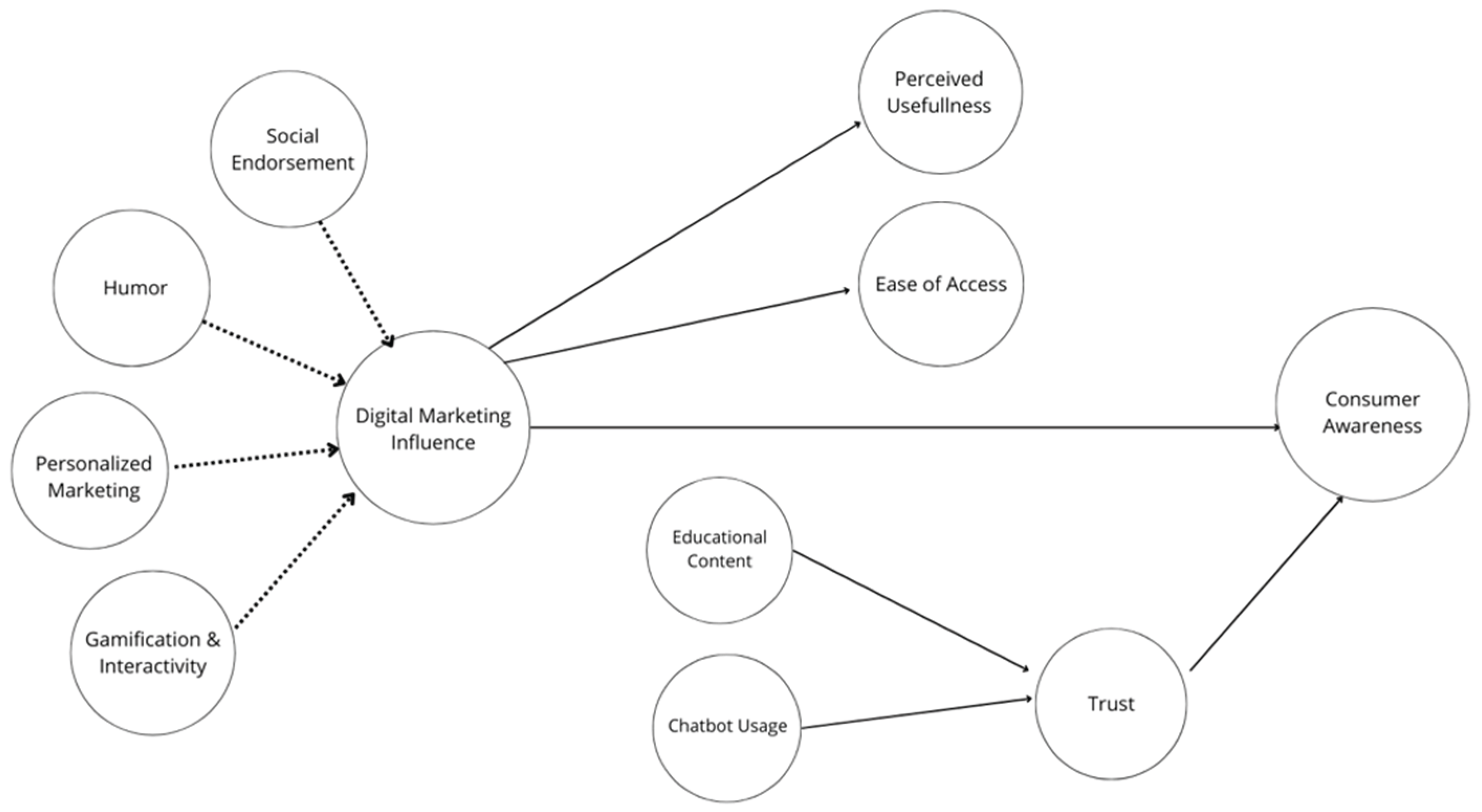
According to the SEM analysis, Digital Marketing Influence is a strong predictor of Consumer Awareness (β = 0.52, p < 0.001), as shown in
Table 2. This result indicates that elements such as gamification, viral content, and personalized marketing can significantly improve brand recall and recognition. Moreover, Awareness Drivers played a crucial mediating role (β = 0.34, p < 0.001), suggesting that perceived usefulness, easy access to information, and trust are important mechanisms through which digital advertising strategies affect awareness formation (see Fig.1). While Trust had a weaker direct effect on awareness (β = 0.21, p = 0.018), it remains a significant factor, especially as it is strongly influenced by Educational Content (β = 0.45, p < 0.001). This supports the notion that credible, expert-driven information enhances the perceived reliability of pharmaceutical messages.
Mediation analysis was formally conducted within the SEM framework using the bootstrapping method with 5,000 resamples to test the significance of indirect effects. The findings indicated that trust served as a partial mediator in the relationship between educational content and consumer awareness (β = 0.15, p = 0.027). This suggests that exposure to high-quality educational materials can enhance awareness, at least in part by increasing brand trust. Additionally, the Awareness Drivers construct - which includes trust, usefulness, and ease of access - served as a significant mediator between digital marketing influence and consumer awareness (β = 0.34, p < 0.001). All mediation effects were evaluated using bias-corrected confidence intervals and were found to be statistically significant. Moderation effects were not considered in this model, as the theoretical framework was focused exclusively on the mediating mechanisms that drive awareness formation.
To evaluate how well the model fits, we looked at several fit indices that are commonly discussed in SEM literature. These included the Comparative Fit Index (CFI), Tucker–Lewis Index (TLI), Root Mean Square Error of Approximation (RMSEA), and Standardized Root Mean Square Residual (SRMR). The results showed a good fit, with values of CFI = 0.961, TLI = 0.948, RMSEA = 0.045, and SRMR = 0.038. All these figures meet or even surpass the standard thresholds for a good model fit (CFI and TLI should be at least 0.90, RMSEA should be 0.06 or lower, and SRMR should be 0.08 or lower). This suggests that the model we proposed is a strong representation of the data we observed (Hu & Bentler, 1999).
The correlation analysis revealed a significant relationship between trust, perceived usefulness, and consumer awareness. However, the SEM results indicated that the awareness drivers—which include trust, perceived usefulness, and how easily information can be accessed—act as a key mediator between digital advertising features and consumer awareness. Within this construct, trust played a more limited role, whereas usefulness and accessibility had stronger effects.
These findings are consistent with the theoretical assumptions of both the Technology Acceptance Model (TAM) and the Theory of Planned Behavior (TPB). According to TAM, perceived usefulness and ease of use are essential factors that influence user behavior regarding technology adoption (Davis, 1989). In this study, we discovered that these constructs—illustrated through the awareness drivers—significantly mediate the relationship between digital advertising strategies and awareness. This reinforces TAM’s idea that when technology is functional and easy to use, it enhances user engagement with content and facilitates deeper information processing.
From the TPB perspective (Ajzen, 1985), attitudes, subjective norms, and perceived behavioral control shape behavioral intentions. Trust, in this context, aligns with the attitudinal component. Its limited mediating effect suggests that instrumental beliefs (e.g., about the usefulness and ease of accessing information) may be more influential in forming awareness than purely affective evaluations. Furthermore, the influence of viral content and expert or influencer endorsements in the model reflects the TPB component of subjective norms, which shape behavior through perceived social expectations and credibility signals.
Figure 1.
Structural Equation Model. Note: All paths are standardized estimates. p < .05, p < .01, p < .001.*.
Figure 1.
Structural Equation Model. Note: All paths are standardized estimates. p < .05, p < .01, p < .001.*.
4.3. Factor Analysis Results
To identify the key variables affecting consumer perception of pharmaceutical information, factor analysis was conducted using the principal component method with varimax rotation. The value of the Kaiser-Meyer-Olkin (KMO) criterion was 0.82 and Bartlett's test of sphericity was significant (p < 0.001), confirming the suitability of the data for factor analysis.
A total of five main factors were identified, explaining 67% of the total variance. As shown in
Table 3, the first factor, which focuses on clarity and completeness of information (λ = 2.2370), stresses how vital it is to present content in a clear, concise manner, supported by visuals like infographics and explanatory videos. The second factor, relevance of search results (λ = 1.7780), suggests that when content is relevant to what users are looking for, it greatly enhances their satisfaction and eases their cognitive load. The third factor—interactive engagement (λ = 1.5770)—highlights how tools like chatbots, educational quizzes, and gamification can boost user interaction with the information. The fourth factor, source credibility (λ = 1.3510), points to the importance of having direct access to expert opinions and medical research, which helps build trust in the information. Finally, the fifth factor—attractiveness of presentation (λ = 0.7340)—shows that using modern visual formats, such as animated videos and social media stories, makes content about medicines more memorable.
The findings reveal important insights into how consumers perceive pharmaceutical information and highlight factors that could influence their satisfaction with digital sources. The key factors identified—like the clarity and completeness of the information, the relevance of search results, interactive engagement, the credibility of sources, and the attractiveness of the presentation—are all linked to significant concepts from the Technology Acceptance Model (TAM) and the Theory of Planned Behavior (TPB). These results suggest that educational content, interactive features, and expert sources can play a vital role in building trust and engagement with the audience. However, to confirm these hypotheses, we need to conduct more thorough statistical analyses, including regression analysis and structural modeling techniques.
This option effectively emphasizes the descriptive role of factor analysis and correctly points out the need for further testing to validate the hypotheses.
5. Discussion
5.1. Theoretical Contributions
This study contributes to advertising theory by extending the Technology Acceptance Model (TAM) and the Theory of Planned Behavior (TPB) into the context of highly regulated pharmaceutical marketing. While earlier literature has confirmed the predictive strength of TAM and TPB in general digital settings, our research highlights how regulatory constraints can influence the dynamics of these models.
Moreover, the study brings to light a crucial distinction between two kinds of factors that drive awareness: instrumental drivers, such as perceived usefulness and ease of access, and social-normative cues, like endorsements from influencers and viral content. This distinction sharpens our understanding of how the components of the Theory of Planned Behavior (TPB) can be applied in scenarios that require regulatory compliance. By empirically validating these mechanisms using Structural Equation Modeling (SEM) and factor analysis, the research provides solid evidence that the Technology Acceptance Model (TAM) and TPB framework can be effectively expanded to include perceived informational credibility and digital trust in industries that are heavily regulated. This extension is particularly relevant in healthcare, where consumers' behavioral responses are more sensitive to perceived risk and message authority than in conventional consumer markets.
SEM results confirmed that Digital Marketing Influence is a strong direct predictor of consumer awareness (β = 0.52, p < 0.001), validating hypotheses H2, H3, H7, and H9. While earlier works (e.g., Willis & Delbaere, 2021; Krishnan & Chakravarti, 2003; Eisend, 2021) highlighted the role of personalization, gamification, or viral campaigns individually, this study extends that work by demonstrating their combined effect on awareness. Particularly, humor and emotionally resonant content—underexplored in prior pharmaceutical marketing research—proved to be significant predictors of brand recall and attention.
Further, both perceived usefulness (β = 0.34) and ease of access (β = 0.33) significantly mediated the effect of digital tools on awareness, confirming H1 and H5 and aligning with TAM (Davis, 1989). These findings reflect that the more consumers perceive tools (e.g., chatbots, recommendation engines) as helpful and intuitive, the more likely they are to process and retain pharmaceutical information—supporting studies by Verma et al. (2024) and Vega et al., (2011).
Interestingly, trust, while a weaker direct predictor of awareness (β = 0.21), played an important mediating role in the relationship between educational content and awareness (β = 0.15), supporting H6. This finding reinforces TPB’s construct of attitude toward the behavior (Ajzen, 1985), showing that expert-driven communication boosts perceived message credibility. Our model confirms that educational content (β = 0.45) is a core antecedent of trust, echoing insights from Purcarea et al. (2019), but goes further by statistically validating the indirect pathway to awareness.
Unlike Davies et al. (2024), who focused on the constraints of pharmaceutical marketing, our study presents strategies for overcoming these limitations through educational content and expert endorsements. This is consistent with the findings of Rollins et et al. (2022), who argue that evidence-based communication can reduce consumer skepticism. However, we go further by showing that educational content is most effective when embedded within a broader strategy that includes personalization and interactivity, building on the insights of Willis & Delbaere et al. (2021) regarding the limited impact of static content in driving consumer behavior.
Notably, chatbot interaction showed a positive, albeit limited, impact on trust (β = 0.19), confirming H8. This supports earlier work by Rammal et al. (2022) and Clark & Bailey (2015), who emphasized the role of digital tools in pharmaceutical promotion. However, we extend this knowledge by showing that chatbots, while improving informational access, have a limited effect on trust formation, echoing concerns raised by Seitz et al. (2022) and Liu et al. (2022) regarding the preference for expert-verified content over AI-generated responses.
A further contribution of this study lies in the use of exploratory factor analysis to structure consumer perception. We identified five crucial dimensions: clarity, relevance, interactivity, source credibility, and presentation aesthetics. Furthermore, our factor analysis reinforces the arguments made by Singh et al. (2023) and Vega et al. (2011), who highlight the critical roles of credibility, clarity, and interactivity in effective digital health communication. We build on these findings by demonstrating that a multimodal marketing approach—blending text, visuals, and interactive elements—enhances information retention and increases consumer awareness.
In addition to the quantitative findings, qualitative interviews reinforced and enriched the results by revealing that consumers consistently valued expert-backed information, ease of access, and engaging formats. Many participants emphasized that trust in pharmaceutical brands increases when educational content is paired with personalization and clear digital interfaces, which supports the mediating role of trust and accessibility observed in the SEM results.
5.2. Practical Implications
For pharmaceutical marketers, this study provides actionable guidance on how to design AI-driven campaigns that comply with regulatory frameworks while effectively building consumer awareness. The findings suggest that personalized and interactive content—such as chatbots, quizzes, and gamified messaging—can increase engagement, but only when paired with credible, expert-backed educational material that reinforces trust.
To raise awareness of OTC drugs through digital advertising, it is essential to focus on personalized, AI-driven content that adapts to user behavior, ensuring that interactions are both relevant and timely. Retargeting strategies can help re-engage users who have previously shown interest in specific medications, while personalized email campaigns can cater to those seeking preventative care or exploring treatments, effectively enhancing awareness for each group. Building trust plays a pivotal role in this process, and AI-powered chatbots, supported by scientific references, along with interactive FAQ sections, can provide real-time expert responses. Additionally, implementing a certification system to verify OTC drug information will further bolster credibility.
To boost engagement, integrating gamification and interactive content such as quizzes, challenges, and gamified learning experiences makes education enjoyable and memorable. Offering loyalty points for engagement and utilizing interactive symptom checkers can guide users to the right OTC solutions, further raising awareness. Influencer and community engagement are equally crucial in building trust and awareness. By partnering with micro-influencers, healthcare experts, and wellness bloggers, brands can create authentic content that resonates with audiences. Developing community-driven content hubs for sharing stories and recommendations, along with emphasizing social proof marketing, strengthens brand credibility and fosters trust. Lastly, ensuring regulatory compliance is vital for maintaining credibility and awareness. Automated systems can help verify that marketing materials adhere to FDA, GDPR, and local regulations, while giving consumers control over their data privacy settings and adopting localized marketing strategies will ensure compliance across different regions, ultimately enhancing brand awareness in diverse markets.
6. Conclusions
The results indicate that awareness is influenced by more than just a single factor; it is shaped by a whole ecosystem of elements. Digital advertising approaches, such as gamification, personalized messaging, and humor have been shown to greatly enhance brand recognition and make content more memorable. Additionally, educational content and expert endorsements are vital for building trust, which ultimately contributes to awareness. These findings serve to validate the existing theoretical models while also extending them by uncovering the crucial roles of trust, usefulness, and accessibility in the context of regulated health communication.
However, several methodological limitations must be acknowledged. First, Structural Equation Modeling (SEM), while useful for examining complex relationships, assumes linearity and requires large samples for stable estimates. The latent variables were operationalized using self-reported Likert items, which are subject to response bias and may not fully capture the depth of constructs like trust or emotional engagement. Moreover, mediation effects, though tested via bootstrapping, do not imply causality and may be influenced by omitted variables not accounted for in the model (e.g., prior brand familiarity or individual health status). Furthermore, correlation analysis revealed statistically significant associations, but such relationships remain non-directional. This limits any claim about the causal pathways between digital marketing preferences and awareness-related behaviors. Third, exploratory factor analysis, while useful for identifying key dimensions of consumer satisfaction, is inherently interpretative. The naming and grouping of factors were based on theoretical judgment and factor loadings, which, while statistically valid, may vary with different samples or cultural contexts.
The findings have some limitations regarding their generalizability. The quantitative data were collected using convenience sampling, and the qualitative interviews were conducted with a small, demographically specific group of junior marketing professionals. Therefore, we shouldn't interpret the results as being representative of the entire population of healthcare consumers or marketing practitioners. Additionally, this study was limited to one national context and didn't account for cultural, regulatory, or linguistic differences that could affect how consumers understand pharmaceutical information.
Future research should definitely focus on these limitations by employing longitudinal or experimental designs to evaluate the long-term effects of digital marketing on awareness and behavior. It is vital to include a more diverse and representative sample that spans various ages, health literacy levels, and cultural backgrounds. Additionally, integrating other conceptual variables such as risk perception, regulatory skepticism, or digital fatigue could provide valuable insights. On the theoretical level, future studies could refine the TAM–TPB integration by testing new constructs such as perceived transparency, regulatory alignment, or message overload—all of which may moderate or mediate awareness formation in pharmaceutical contexts.
Appendix A: Ethics
This research was conducted independently and without affiliation to any academic or research institution. As such, formal review and approval by an Institutional Review Board (IRB) or equivalent ethics committee was not obtained. Nonetheless, the study adhered to the ethical standards outlined in the Belmont Report and the Declaration of Helsinki.
1. **Respect for Persons**
Participants were fully informed about the nature and goals of the study, the voluntary nature of participation, and their right to withdraw at any time without penalty. Informed consent was obtained prior to participation. Written consent was secured for the survey portion, and verbal consent was documented for interviews.
2. **Beneficence**
The study involved minimal risk. The questions posed during both the survey and interviews did not include sensitive or invasive topics. Steps were taken to ensure anonymity and data confidentiality. All identifying information was excluded from transcripts, notes, and analyses.
3. **Justice**
Participants were recruited based on their relevance to the research questions. No vulnerable populations were targeted or included. The goal of the research is to contribute to advertising practices that are more responsive to consumer perspectives, which benefits both industry and the public.
No online behavioral data, network traffic, passwords, or social media data were collected. All data were gathered directly from consenting adults and stored securely.
References
- Ahmed, S., Islam, T., & Ghaffar, A. (2024). Shaping Brand Loyalty through Social Media Influencers: The Mediating Role of Follower Engagement and Social Attractiveness. SAGE Open, 14(2). [CrossRef]
- Ajzen, I. (1991). The theory of planned behavior. Organizational Behavior and Human Decision Processes, 50(2), 179–211. [CrossRef]
- Ali, K. E., Naser, A. Y., Al-Rousan, R., Alwafi, H., AbuAlhommos, A. K., Alsairafi, Z. K., Salawati, E. M., Samannodi, M., & Dairi, M. S. (2022). The attitude and acceptability towards medical promotional tools and their influence on physicians’ prescribing practices in Jordan and Iraq: a cross-sectional study. BMC Health Services Research, 22(1). [CrossRef]
- AlMuammar, S. A., Noorsaeed, A. S., Alafif, R. A., Kamal, Y. F., & Daghistani, G. M. (2021). The use of internet and social media for health information and its consequences among the population in Saudi Arabia. Cureus. [CrossRef]
- Anis, M. S., & Hassali, M. A. (2022). Pharmaceutical Digital Marketing of Non-prescription Drugs: A Systematic Scoping review. Research Journal of Pharmacy and Technology, 941–946. [CrossRef]
- Aritra, S., & Indu, S. (2025). Harnessing the power of artificial intelligence in pharmaceuticals: Current trends and future prospects. Intelligent Pharmacy. [CrossRef]
- Ajzen, I. (1991). The theory of planned behavior. Organizational Behavior and Human Decision Processes, 50(2), 179–211. [CrossRef]
- Bartlett, I., II, Kotrlik, J. W., Higgins, C. C., Ball State University, & Louisiana State University. (2001). Organizational research: Determining appropriate sample size in survey research. In Information Technology, Learning, and Performance Journal (Vol. 19, Issue 1, pp. 43–44). https://www.opalco.com/wp-content/uploads/2014/10/Reading-Sample-Size1.pdf.
- Bhargavi, L. M. (2024). THE ROLE OF EMOTIONAL APPEAL IN MODERN ADVERTISING: A STUDY ON ROLE OF E-ADVERTISEMENT ON CONSUMER BUYING BEHAVIOUR. ShodhKosh Journal of Visual and Performing Arts, 5(1). [CrossRef]
- Bharskar, G. R., & Siddheshwar, S. (2020). DIGITAL MARKETING IN PHARMACEUTICAL SECTOR. INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCE AND HEALTH CARE, 2(10). [CrossRef]
- Chen, J., & Wang, Y. (2021). Social media use for health purposes: Systematic review. Journal of Medical Internet Research, 23(5), e17917. [CrossRef]
- Clark, M., & Bailey, S. (2024, January 1). Chatbots in Health care: Connecting patients to information. NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK602381/.
- Coleman, J. (2022). Spearman rank order correlation. In The SAGE encyclopedia of research design (2 ed., Vol. 4, pp. 1568-1570). SAGE Publications, Inc. [CrossRef]
- Davies, J. (2024, October 17). 8 Digital Marketing Challenges in Pharma - phamax Digital. phamax Digital. https://phamax-digital.ch/academy/digital-marketing-challenges/.
- Davis, F. D. (1989). Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Quarterly, 13(3), 319. [CrossRef]
- Davis, N. (2024, December 26). ‘Wild west’: experts concerned by illegal promotion of weight-loss jabs in UK. The Guardian. https://www.theguardian.com/society/2024/dec/26/experts-concern-promotions-weight-loss-jabs-uk.
- DeJonckheere, M., & Vaughn, L. M. (2019). Semistructured interviewing in primary care research: a balance of relationship and rigour. Family Medicine and Community Health, 7(2), e000057. [CrossRef]
- Dixon, D., Sattar, H., Moros, N., Kesireddy, S. R., Ahsan, H., Lakkimsetti, M., Fatima, M., Doshi, D., Sadhu, K., & Hassan, M. J. (2024). Unveiling the Influence of AI Predictive analytics on patient Outcomes: A Comprehensive Narrative review. Cureus. [CrossRef]
- Dremova, N. B., & Solomka, S. V. (2022). WORLD PHARMACEUTICAL MARKET: TRENDWATCHING. Laboratornaya I Klinicheskaya Meditsina Farmatsiya, 3, 56–68. [CrossRef]
- Eisend, M. (2021). The influence of humor in advertising: Explaining the effects of humor in two-sided messsages. Psychology and Marketing, 39(5), 962–973. [CrossRef]
- Enyinda, C. I., Ogbuehi, A. O., & Mbah, C. H. (2018). Building pharmaceutical relationship marketing and social media impact. International Journal of Pharmaceutical and Healthcare Marketing, 12(2), 198–230. [CrossRef]
- Gao, L., Melero, I., & Sese, F. J. (2019). Multichannel integration along the customer journey: a systematic review and research agenda. Service Industries Journal, 40(15–16), 1087–1118. [CrossRef]
- Guest, G., Bunce, A., & Johnson, L. (2005). How many interviews are enough? Field Methods, 18(1), 59–82. [CrossRef]
- Gupta, R., Ramachandran, R., & Ross, J. S. (2024). Tackling the excesses of pharmaceutical marketing and promotion. BMJ, e076797. [CrossRef]
- Hassan, N., Abdelraouf, M., & El-Shihy, D. (2025). The moderating role of personalized recommendations in the trust–satisfaction–loyalty relationship: an empirical study of AI-driven e-commerce. Future Business Journal, 11(1). [CrossRef]
- Jia, X., Pang, Y., & Liu, L. S. (2021). Online Health Information Seeking Behavior: A Systematic Review. Healthcare, 9(12), 1740. [CrossRef]
- Kaiponen, T. (2021). Omnichannel marketing in a pharmaceutical company – how to optimally reach the customers in the COVID-19 era? Theseus. https://urn.fi/URN:NBN:fi:amk-2021111520318.
- Kassem, N. R. G., Mbata, N. a. O., Usuemerai, N. P. A., & Ogbewele, N. E. G. (2022). Leveraging data analytics for optimizing pharmacy marketing strategies: Enhancing patient engagement and medication adherence. World Journal of Advanced Research and Reviews, 16(1), 1184–1195. [CrossRef]
- Kim, Y. (2014). Trust in health information websites: A systematic literature review on the antecedents of trust. Health Informatics Journal, 22(2), 355–369. [CrossRef]
- Kang, J., Hong, S., & Hubbard, G. T. (2020). The role of storytelling in advertising: Consumer emotion, narrative engagement level, and word-of-mouth intention. Journal of Consumer Behaviour, 19(1), 47–56. [CrossRef]
- Krejcie, R. V., & Morgan, D. W. (1970). Determining sample size for research activities. Educational and Psychological Measurement, 30(3), 607–610. [CrossRef]
- Krishnan, H. S., & Chakravarti, D. (2003). A process analysis of the effects of humorous advertising executions on brand claims memory. Journal of Consumer Psychology, 13(3), 230–245. [CrossRef]
- Laymouna, M., Ma, Y., Lessard, D., Schuster, T., Engler, K., & Lebouché, B. (2024). Roles, users, benefits, and Limitations of Chatbots in health care: Rapid review. Journal of Medical Internet Research, 26, e56930. [CrossRef]
- Leventhal, R. (2025, April 4). The 2025 Healthcare Consumer: Online information can turn consumers into patients—and customers. EMARKETER. Retrieved from: https://www.emarketer.com/content/online-information-turn-consumers-patients-and-customers. Accessed 23 February, 2025.
- Liu, Y., Yan, W., Hu, B., Li, Z., & Lai, Y. L. (2022). Effects of personalization and source expertise on users’ health beliefs and usage intention toward health chatbots: Evidence from an online experiment. Digital Health, 8, 205520762211297. [CrossRef]
- Miozza, M., Brunetta, F., & Appio, F. P. (2024). Digital transformation of the Pharmaceutical Industry: A future research agenda for management studies. Technological Forecasting and Social Change, 207, 123580. [CrossRef]
- Mor, J., Kaur, T., Menkes, D. B., Peter, E., & Grundy, Q. (2024). Pharmaceutical industry promotional activities on social media: a scoping review. Journal of Pharmaceutical Health Services Research, 15(4). [CrossRef]
- Morgan, C., & Zane, D. M. (2022). Practitioner perspectives on key challenges in pharmaceutical marketing and future research opportunities. Journal of Public Policy & Marketing, 41(4), 368–382. [CrossRef]
- Paiola, M., Khvatova, T., Schiavone, F., & Ferraris, A. (2023). How do omnichannel strategies contribute to value-based healthcare? An orchestra-based analysis. Journal of Business Research, 167, 114175. [CrossRef]
- Perrone, M. (2024, November 14). New FDA rules for TV drug ads: Simpler language and no distractions | AP News. AP News. https://apnews.com/article/drug-ads-fda-risks-side-effects-influencers-80bbe076f4ed743ebde3923dd28be004.
- Purcarea, P. E. V. L. (2019). The impact of marketing strategies in healthcare systems. Journal of Medicine and Life, 12(2), 93–96. [CrossRef]
- Rahimi, B., Nadri, H., Afshar, H. L., & Timpka, T. (2018). A Systematic review of the technology acceptance model in health Informatics. Applied Clinical Informatics, 09(03), 604–634. [CrossRef]
- Rammal, D. S., Alomar, M., & Palaian, S. (2024). AI-Driven pharmacy practice: Unleashing the revolutionary potential in medication management, pharmacy workflow, and patient care. Pharmacy Practice, 22(2), 1–11.
- Rollins, B., Huh, J., Bhutada, N., & Perri, M. (2020). Effects of endorser type and testimonials in direct-to-consumer prescription drug advertising (DTCA). International Journal of Pharmaceutical and Healthcare Marketing, 15(1), 1–17. [CrossRef]
- Sample, K. L., Hagtvedt, H., & Brasel, S. A. (2019b). Components of visual perception in marketing contexts: a conceptual framework and review. Journal of the Academy of Marketing Science, 48(3), 405–421. [CrossRef]
- Seitz, L., Bekmeier-Feuerhahn, S., & Gohil, K. (2022). Can we trust a chatbot like a physician? A qualitative study on understanding the emergence of trust toward diagnostic chatbots. International Journal of Human-Computer Studies, 165, 102848. [CrossRef]
- Singh, Y., Eisenberg, M. D., & Sood, N. (2023). Factors associated with public trust in pharmaceutical manufacturers. JAMA Network Open, 6(3), e233002. [CrossRef]
- Srivastava, R., Gupta, P., Kumar, H., & Tuli, N. (2023). Digital customer engagement: A systematic literature review and research agenda. Australian Journal of Management, 031289622311770. [CrossRef]
- Syrkiewicz-Świtała, M., Romaniuk, P., & Ptak, E. (2016c). Perspectives for the use of social media in e-Pharmamarketing. Frontiers in Pharmacology, 7. [CrossRef]
- Thapa, D. K., Visentin, D. C., Kornhaber, R., West, S., & Cleary, M. (2020). The influence of online health information on health decisions: A systematic review. Patient Education and Counseling, 104(4), 770–784. [CrossRef]
- Tomar, G., & Pandey, L. (2024). Influence of personalized advertising on consumer engagement and conversion rates. International Journal of Research in Engineering and Management, 7(3), 62–66. [CrossRef]
- Varnali, K., & Gorgulu, V. (2018). Determinants of brand recall in social networking sites. In IGI Global eBooks (pp. 454–476). [CrossRef]
- Vasileiou, K., Barnett, J., Thorpe, S., & Young, T. (2018). Characterising and justifying sample size sufficiency in interview-based studies: systematic analysis of qualitative health research over a 15-year period. BMC Medical Research Methodology, 18(1). [CrossRef]
- Vega, L. C., DeHart, T., & Montague, E. (2011). Trust between patients and health websites: a review of the literature and derived outcomes from empirical studies. Health and Technology, 1(2–4), 71–80. [CrossRef]
- Verma, S., Tiwari, R. K., & Singh, L. (2024b). Integrating technology and trust: Trailblazing role of AI in reframing pharmaceutical digital outreach. Intelligent Pharmacy, 2(3), 435–440. [CrossRef]
- Vrtana, D., & Krizanova, A. (2023). The power of emotional advertising appeals: examining their influence on consumer purchasing behavior and Brand–Customer relationship. Sustainability, 15(18), 13337. [CrossRef]
- Williamson, K. (2025, January 9). Pharmaceutical marketing in the Digital Age: Strategies and challenges. https://www.pharmafocuseurope.com/articles/strategies-and-challenges-in-pharma-marketing?utm_source=chatgpt.com.
- Willis, E., & Delbaere, M. (2021). Patient Influencers: the next frontier in Direct-to-Consumer pharmaceutical marketing. Journal of Medical Internet Research, 24(3), e29422. [CrossRef]
- Yu, X., Wang, H., & Chen, Z. (2024). The role of User-Generated Content in the Sustainable Development of Online Healthcare Communities: Exploring the moderating influence of signals. Sustainability, 16(9), 3739. [CrossRef]
- Zafar, N. H., Siddiqui, N. F. A., & Haroon, N. Z. (2025). Digital and social determinants of consumer decision making in OTC Pharmaceuticals. the Critical Review of Social Sciences Studies, 3(1), 742–760. [CrossRef]
- Zhang, Y., & Kim, Y. (2022). Consumers’ Evaluation of Web-Based Health Information Quality: Meta-analysis. Journal of Medical Internet Research, 24(4), e36463. [CrossRef]
- Zheng, Y., Liu, J., Tang, P. K., Hu, H., & Ung, C. O. L. (2023). A systematic review of self-medication practice during the COVID-19 pandemic: implications for pharmacy practice in supporting public health measures. Frontiers in Public Health, 11. [CrossRef]
- Zomorodi, M., Ghodsollahee, I., Martin, J. H., Talley, N. J., Salari, V., Pławiak, P., Rahimi, K., & Acharya, U. R. (2024). RECOMED: A comprehensive pharmaceutical recommendation system. Artificial Intelligence in Medicine, 157, 102981. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).