Submitted:
29 June 2025
Posted:
30 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Methods
2.1. Materials and the Coating Process
2.1.1. 7N01 Substrates
2.1.2. Preparation of Silane Solution with Cerium Nitrate and Nano Zeolite Particles
2.1.3. Preparation of the Composite Silane Film and Top-Coated Epoxy Coating
2.2. Characterization
3. Results and Discussion
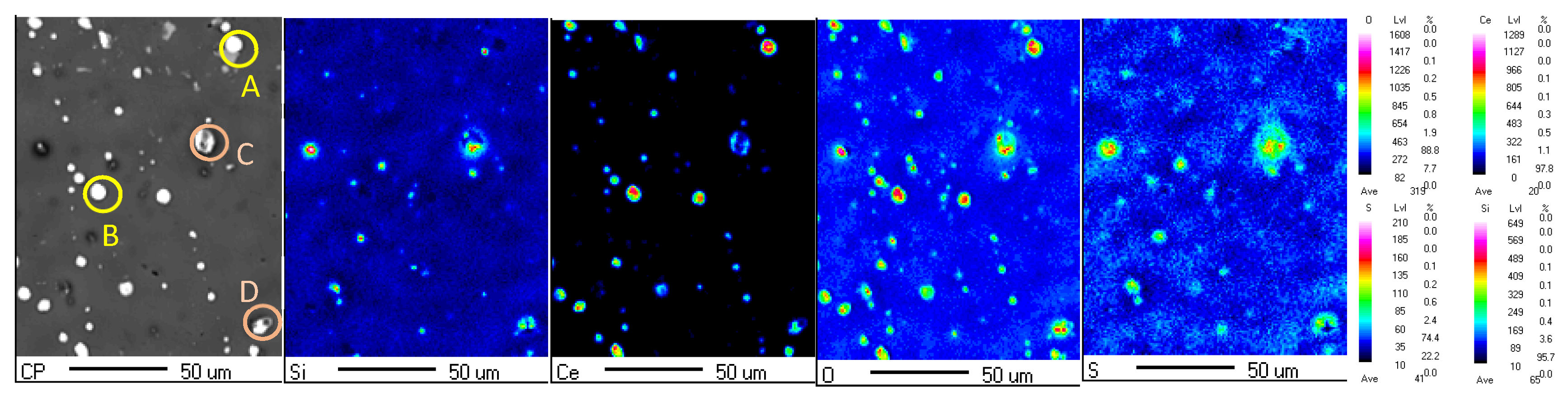
3.1. Effect of Zeolite Particles on the Surface Pretreated Substrates
3.2. Corrosion Performance of the Epoxy Coated Substrates
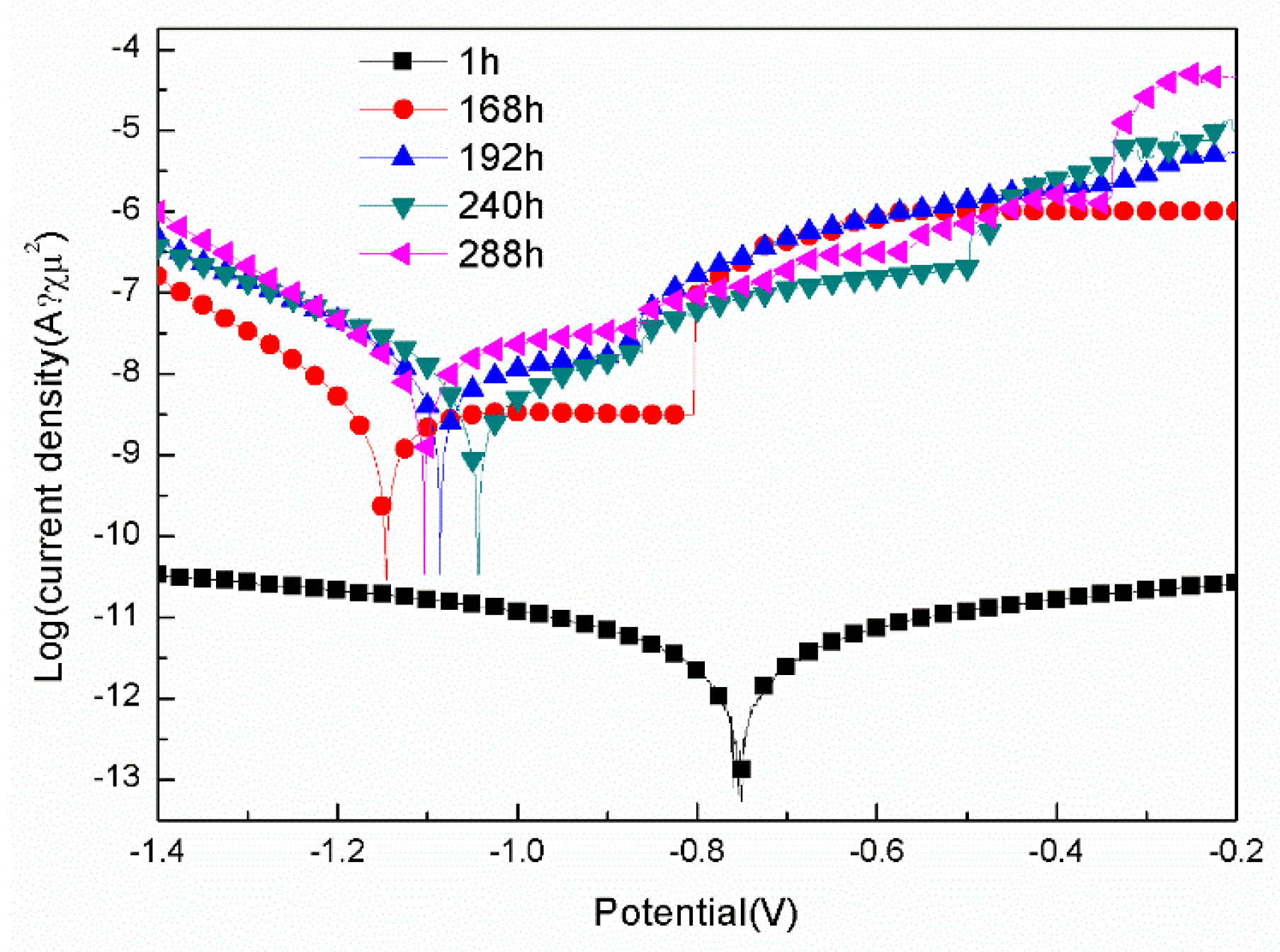
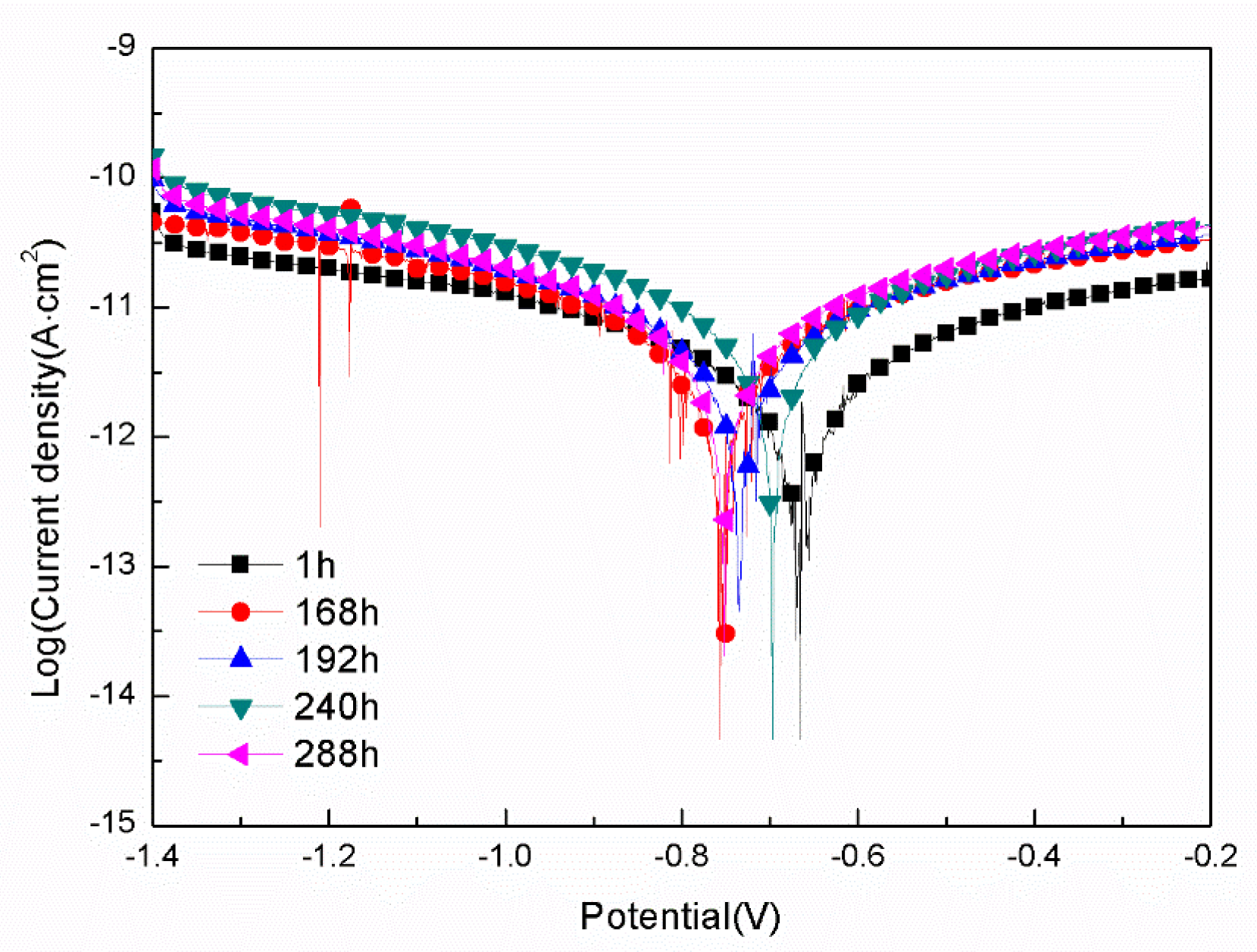
3.2.1. Polarization Tests
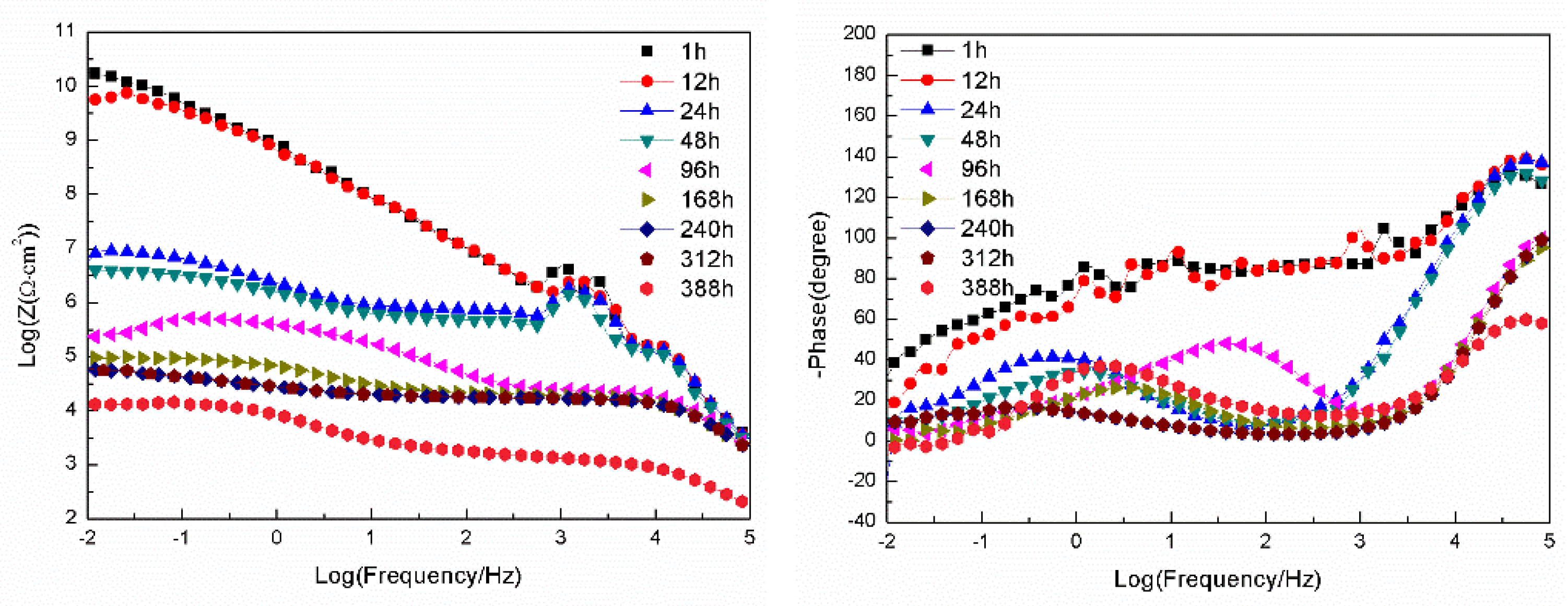
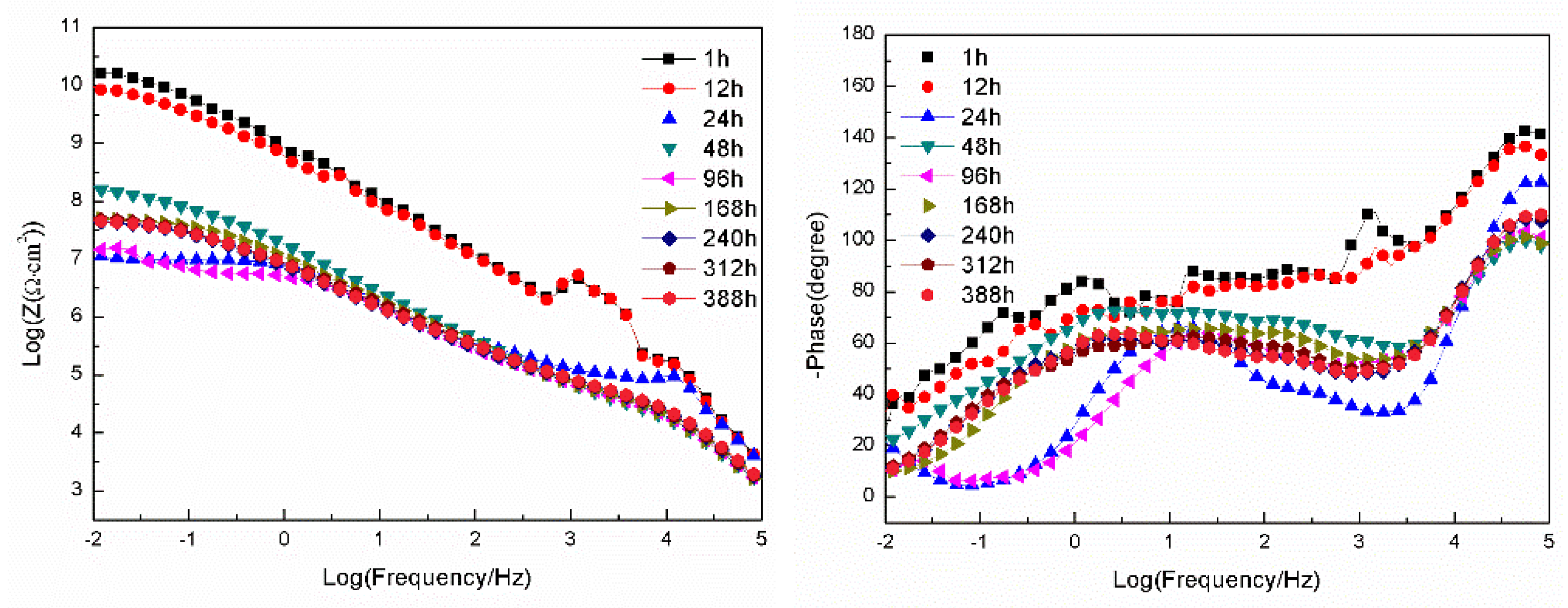
3.2.2. EIS Tests
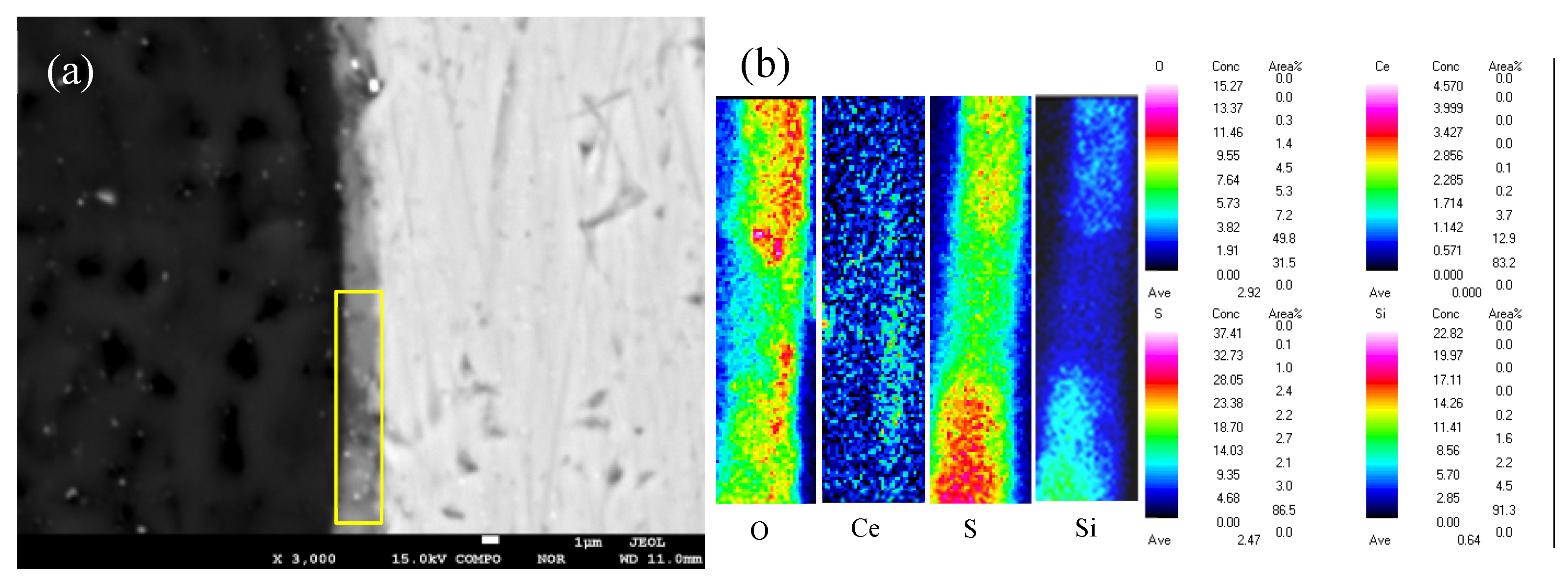
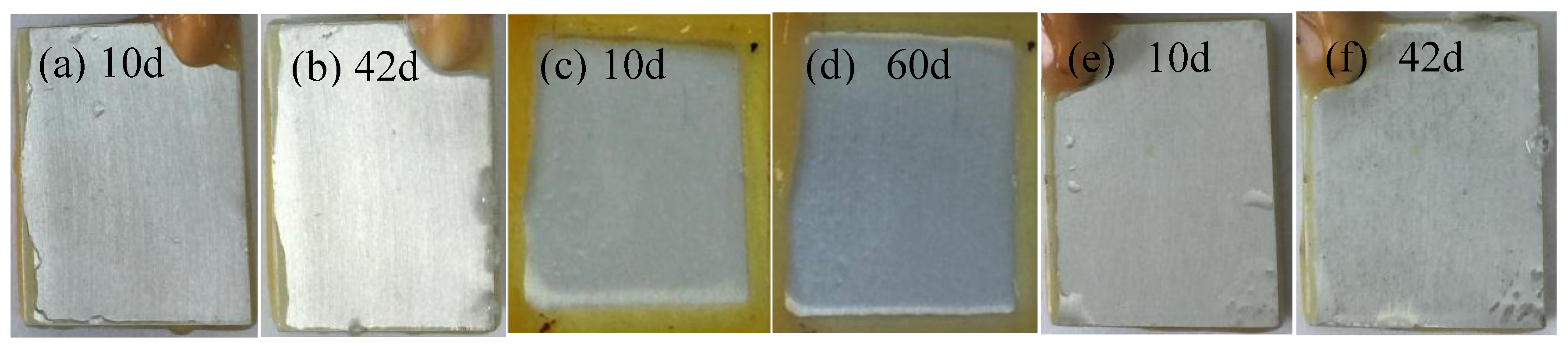
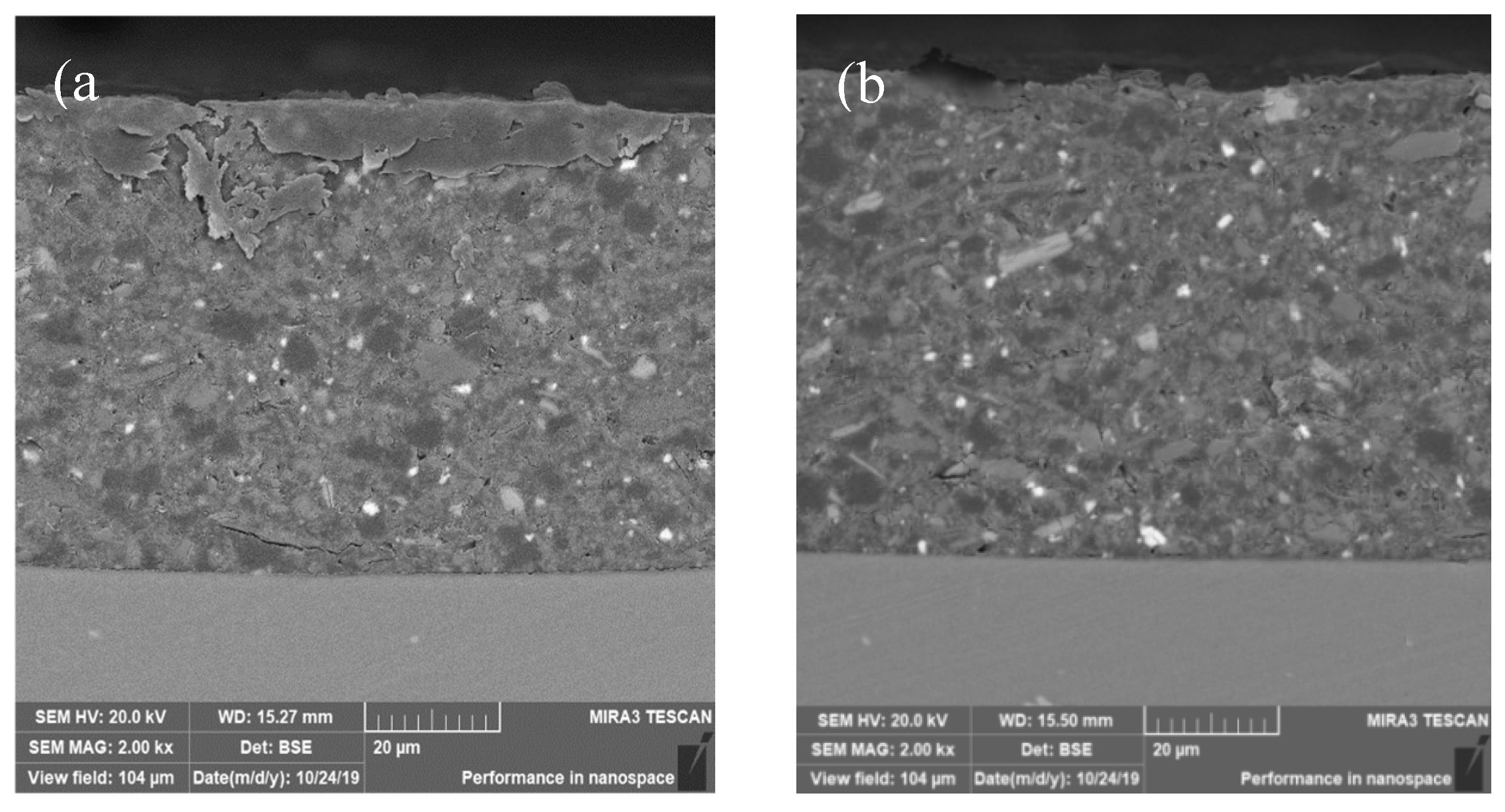
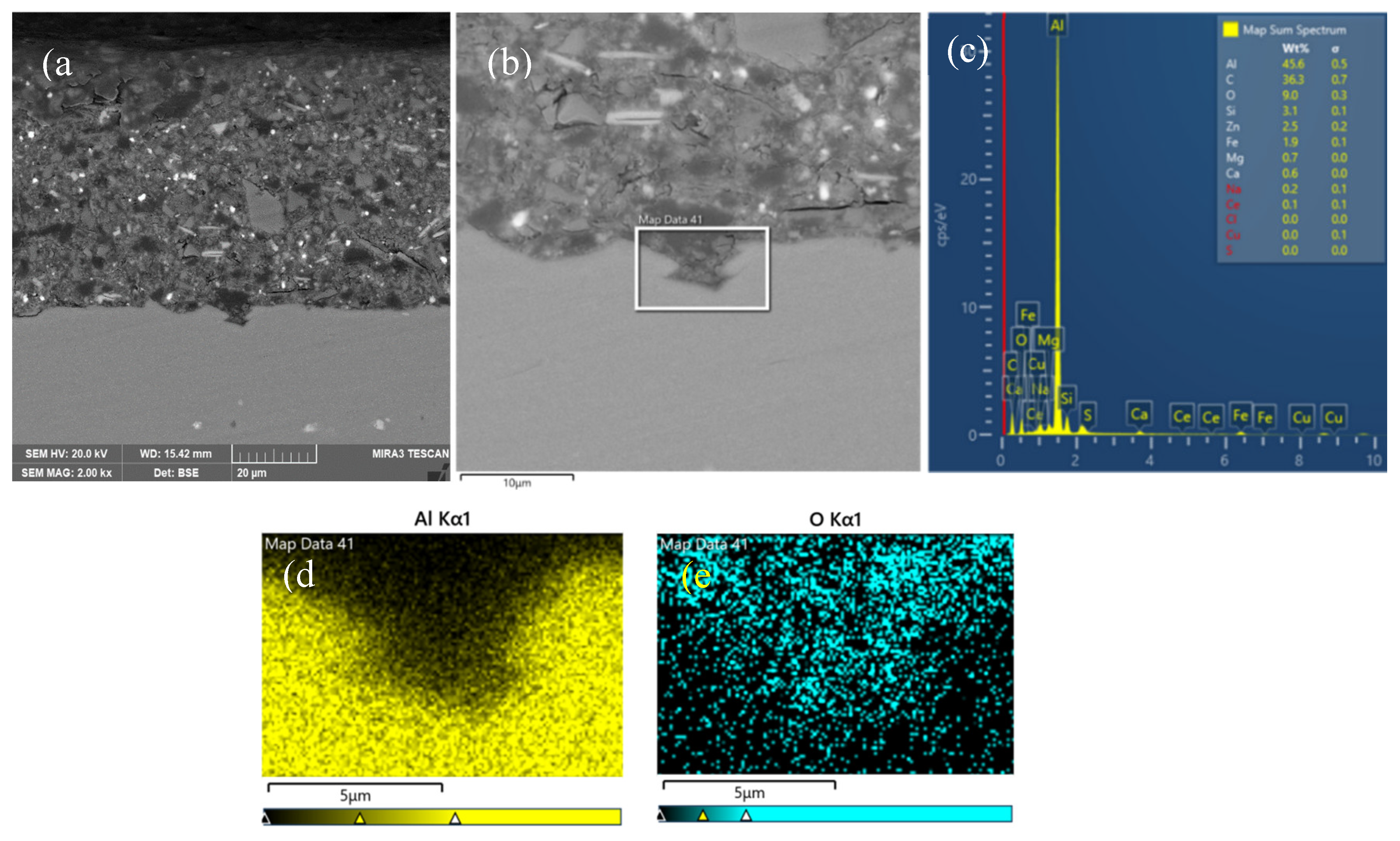
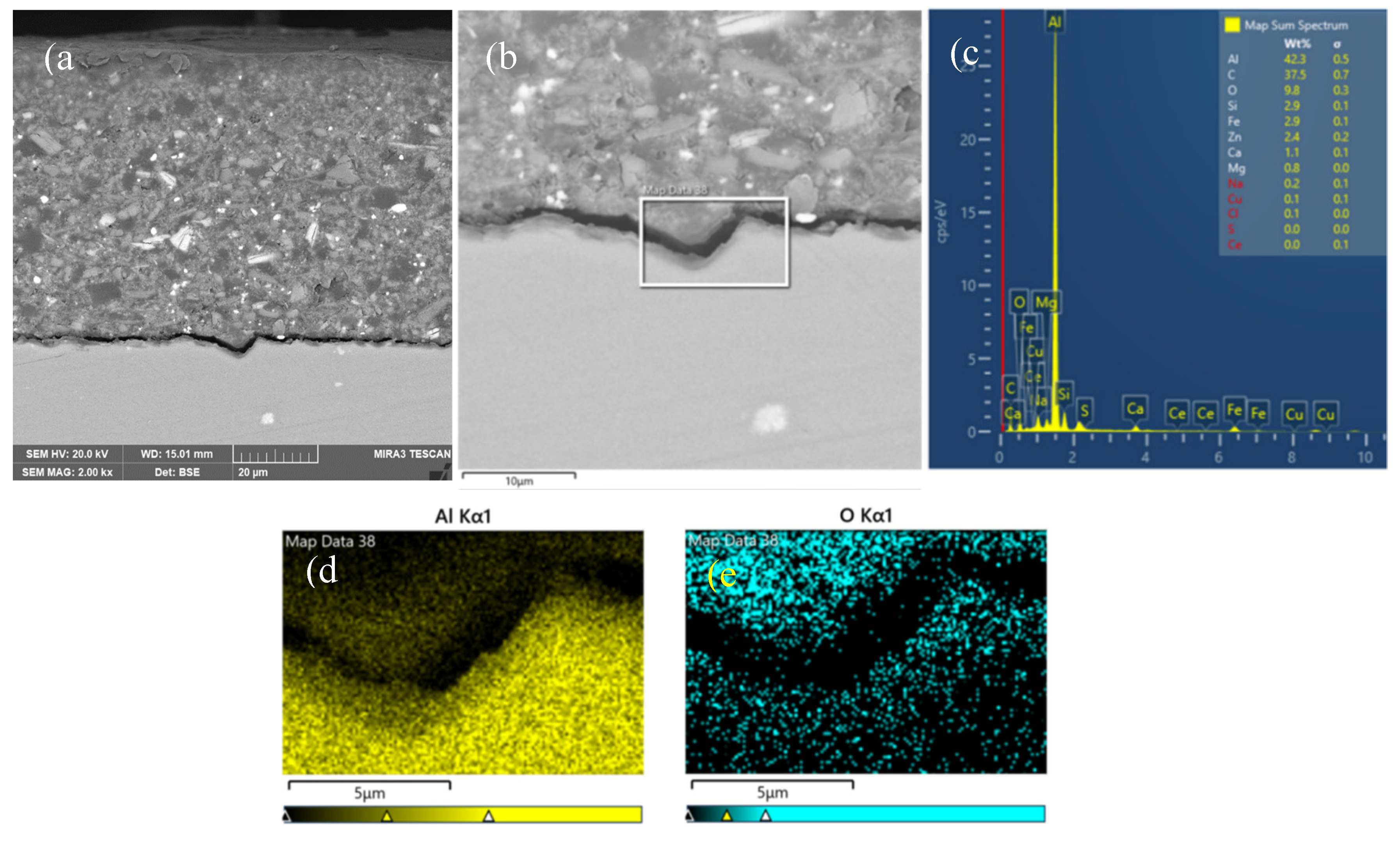
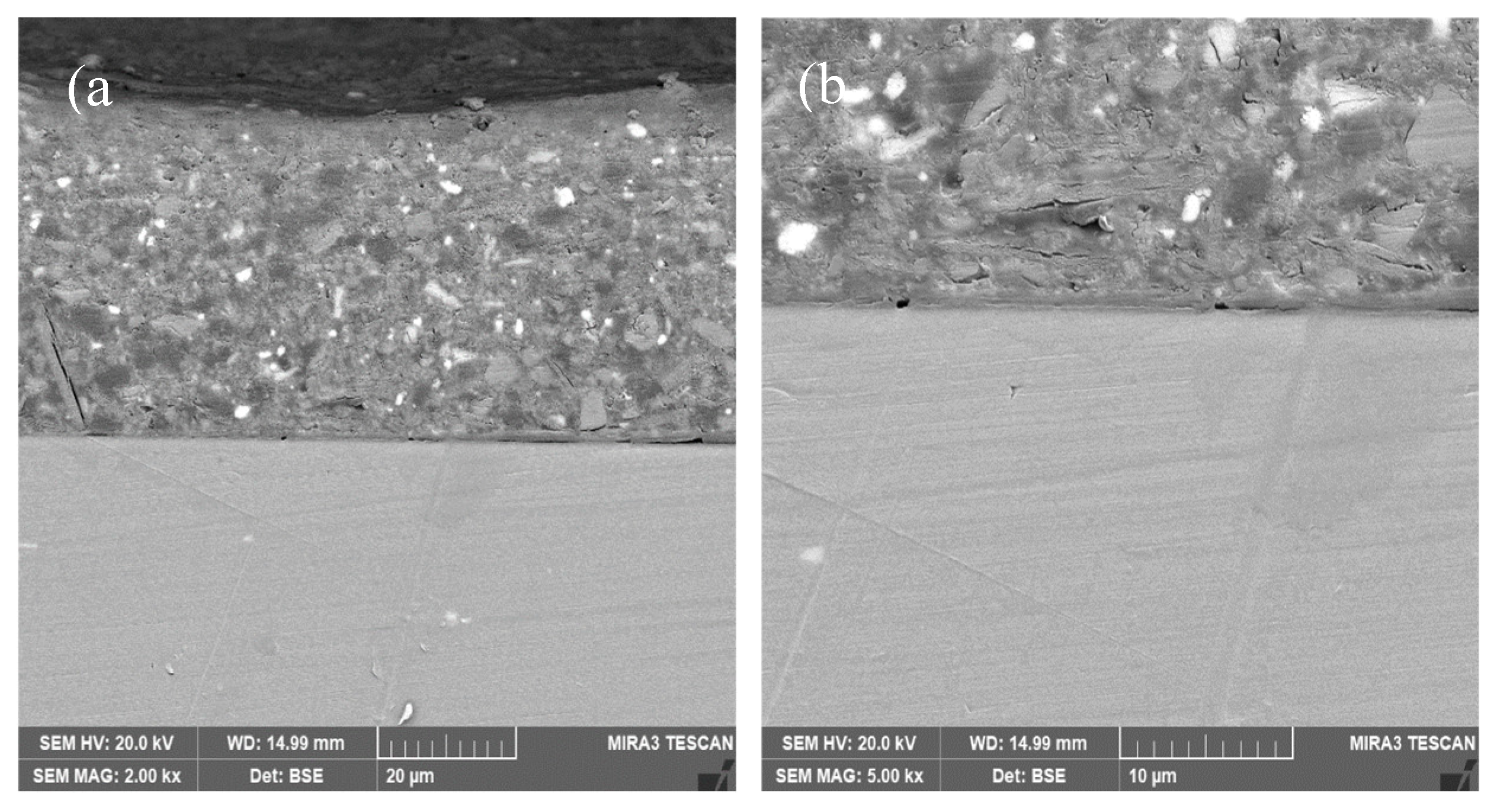
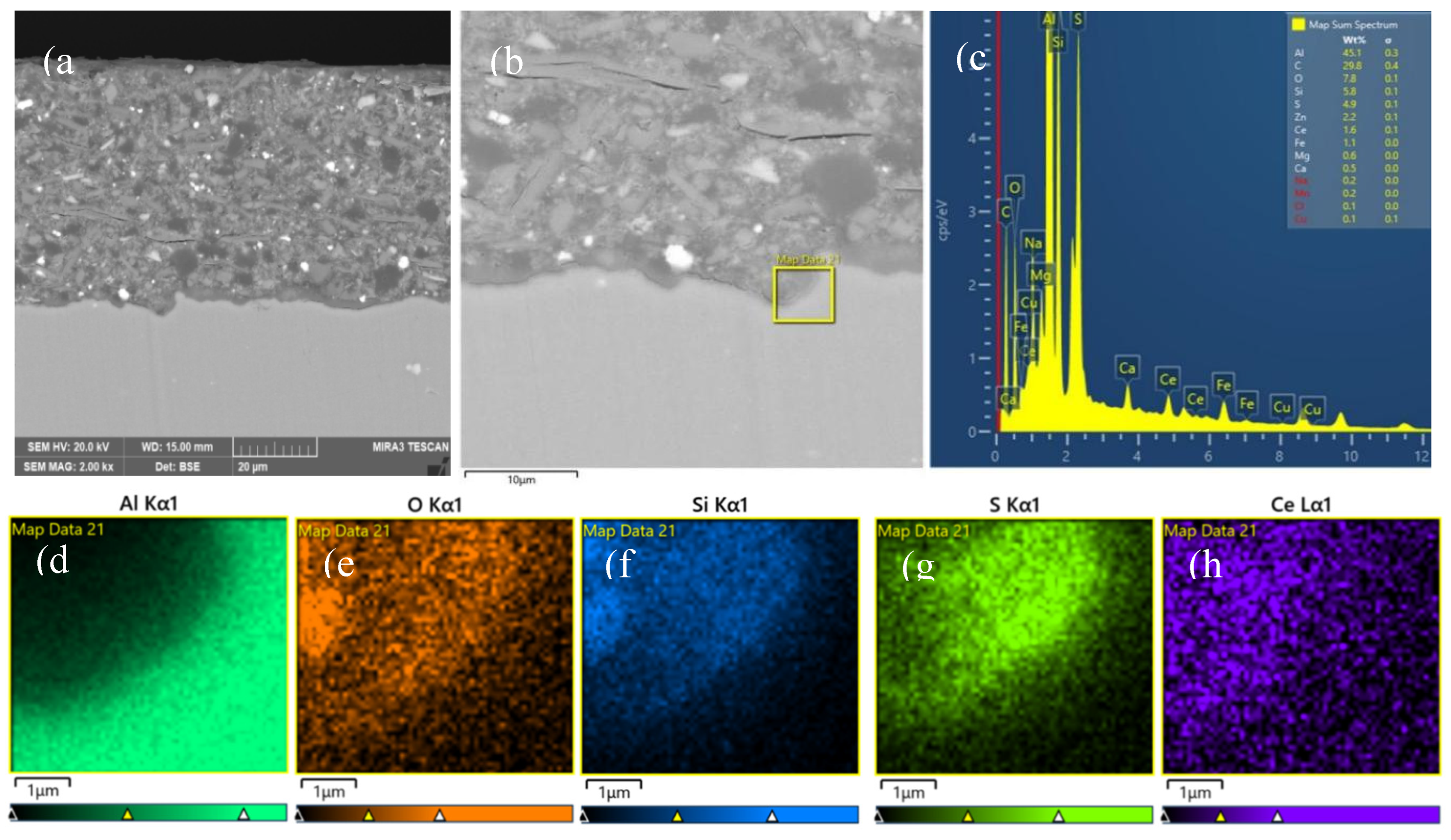
3.3. Interfacial Microstructure Between Epoxy Coating and Al Substrate After Salt Spray Test
4. Conclusions
- (1)
- For the samples with epoxy coatings on the blank 7N01 substrates, 288 h immersion in 3.5 wt% NaCl solution causes Icorr increases from 10-12 A/cm2 to 10-8 A/cm2, while for the samples with epoxy coatings on the 7N01 substrates pretreated by 5 % BTESPT doped with 5×10-3 M cerium nitrate and 0.5 g/l zeolite particles 288 h immersion causes only a little increase of Icorr from 10-13 A/cm2 to 10-12 A/cm2.
- (2)
- For the samples with epoxy coatings on the blank 7N01 substrates, 388 h immersion causes the impedance values at 10-2 Hz declines rapidly from 1010 Ω·cm2 to 1.26×104 Ω·cm2. While for the 7N01 substrates pretreated by 5 % BTESPT doped with 5×10-3 M cerium nitrate and 0.5 g/l zeolite particles the impedance value at 10-2 Hz remains above 107 Ω·cm2, more than two to three orders of magnitude higher than the former.
- (3)
- The composite silane film greatly lessens the interfacial region corrosion between the coating and the substrate. For the samples with epoxy coatings on the blank 7N01 substrates, salt spray test of 30 and 60 days causes the interfacial region from flat into apparent irregularity, and appearance of crack due to severe corrosion activity. While for the substrate pretreated by the composite silane film it endures only slight corrosion.
- (4)
- The thickness of the composite silane film by 5 % BTESPT doped with 5×10-3 M cerium nitrate and 0.5 g/l zeolite particles is about 2.1 μm. Cerium ions are located in the silane film and zeolite particles. The high barrier effect of the composite silane film, and cerium ions releasing from the silane network and the zeolite particles provide active protection to the substrate surface beneath the epoxy coating.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, B.; Wang, X.; Chen, H.; Hu, J.; Huang, C.; Gou, G. Influence of heat treatment on the strength and fracture toughness of 7N01 aluminum alloy. J. Alloys Compd. 2016, 678, 160–166. [Google Scholar] [CrossRef]
- Wang, S.; Luo, B.; Bai, Z.; He, C.; Jiang, G. Effect of pre-ageing on nucleating of GP zones and precipitation, strength and stress corrosion properties of 7N01 alloy. J. Alloys Compd. 2024, 980, 173681. [Google Scholar] [CrossRef]
- Qin, C.; Gou, G.Q.; Che, X.L.; Chen, H.; Chen, J.; Li, P.; Gao, W. Effect of composition on tensile properties and fracture toughness of Al-Zn-Mg alloy (A7N01S-T5) used in high speed trains. Mater. Des. 2016, 91, 278–285. [Google Scholar] [CrossRef]
- van den Brand, J.; Van Gils, S.; Beentjes, P.C.J.; Terryn, H.; Sivel, V.; de Wit, J.H.W. Improving the adhesion between epoxy coatings and aluminium substrates. Prog. Org. Coat. 2004, 51, 339–350. [Google Scholar] [CrossRef]
- van den Brand, J.; Van Gils, S.; Terryna, H.; Sivel, V.G.M.; de Wit, J.H.W. Changes in epoxy-coated aluminium due to exposure to water. Prog. Org. Coat. 2004, 51, 351–364. [Google Scholar] [CrossRef]
- Anwar, S.; Li, X. A review of high-quality epoxy resins for corrosion-resistant applications. J. Coat. Technol. Res. 2024, 21, 461–480. [Google Scholar] [CrossRef]
- Pessoa, M.O.; Freitas, B.R.; de O, J.; Santos, S.L.B.S.; da Silva, B.P.; Capelossi, V.R.; Cotting, F. Anticorrosion and adhesion performance of a monolayer and double layer silane-epoxy coating systems applied on carbon steel. Surf. Coat. Technol. 2024, 485, 130909. [Google Scholar] [CrossRef]
- Hong, S.G.; Cave, N.G.; Boerio, F.J. Modification of epoxy/metal interphases by adsorbed contaminants. J. Adhesion 1992, 36, 265–279. [Google Scholar] [CrossRef]
- Boerio, F.J.; Hong, P.P. Non-destructive characterization of epoxy-dicyandiamide interphases using surface-enhanced Raman scattering. Mater. Sci. Eng. A 1990, 126, 245–252. [Google Scholar] [CrossRef]
- van Ooij, W.J.; Sabata, A.; Appelhans, A.D. Application of surface analysis techniques to the study of paint/metal interfaces related to adhesion and corrosion performance. Surf. Interface Anal. 1991, 17, 403–420. [Google Scholar] [CrossRef]
- Nakamae, K.; Airu, X.; Asaoka, S. Localization of the curing agent at an epoxy resin/oxidized aluminium interface. Int. J. Adhes. Adhes. 1995, 15, 15–20. [Google Scholar] [CrossRef]
- Van Ooij, W.J.; Zhu, D.; Stacy, M.; Seth, A.; Mugada, T.; Gandhi, J.; Puomi, P. Corrosion Protection Properties of Organofunctional Silanes - An Overview. Tsinghua Sci. Technol. 2005, 10, 639–664. [Google Scholar] [CrossRef]
- Zhu, D.; van Ooij, W.J. Enhanced corrosion resistance of AA 2024-T3 and hot-dip galvanized steel using a mixture of bis-[triethoxysilylpropyl]tetrasulfide and bis-[trimethoxysilylpropyl]amine, Electrochim. Acta 2004, 49, 1113–1125. [Google Scholar] [CrossRef]
- Zhu, D.; van Ooij, W.J. Corrosion protection of AA 2024-T3 by bis-[3-(triethoxysilyl)propyl]tetrasulfide in sodium chloride solution. Part 2: mechanism for corrosion protection. Corros. Sci. 2003, 45, 2177–2197. [Google Scholar] [CrossRef]
- Zhu, D.; van Ooij, W.J. Corrosion protection of AA 2024-T3 by bis-[3-(triethoxysilyl)propyl]tetrasulfide in neutral sodium chloride solution. Part 1: corrosion of AA 2024-T3. Corros. Sci. 2003, 45, 2163–2175. [Google Scholar]
- van Ooij, W.J.; Zhu, D.Q. Electrochemical impedance spectroscopy of bis-[triethoxysilypropyl]tetrasulfide on Al 2024-T3 substrates. Corrosion 2001, 57, 413–427. [Google Scholar] [CrossRef]
- Song, J.; van Ooij, W.J. Bonding and corrosion protection mechanisms of γ-APS and BTSE silane films on aluminum substrates. J. Adhes. Sci. Technol. 2003, 17, 2191–2221. [Google Scholar] [CrossRef]
- Pluddeman, E.P. Silane Coupling Agents, 2nd ed.; Plenum Press: New York, 1990. [Google Scholar]
- Montemor, M.F.; Rosqvist, A.; Fagerholm, H.; Ferreira, M.G.S. The early corrosion behaviour of hot dip galvanised steel pre-treated with bis-1,2-(triethoxysilyl)ethane. Prog. Org. Coat. 2004, 51, 188–194. [Google Scholar] [CrossRef]
- Cabral, A.M.; Duarte, R.G.; Montemor, M.F.; Zheludkevich, M.L.; Ferreira, M.G.S. Analytical characterisation and corrosion behaviour of bis-[triethoxysilylpropyl]tetrasulphide pre-treated AA2024-T3. Corros. Sci. 2005, 47, 869–881. [Google Scholar] [CrossRef]
- Trabelsi, W.; Triki, E.; Dhouibi, L.; Ferreira, M.G.S.; Zheludkevich, M.L.; Montemor, M.F. The use of pre-treatments based on doped silane solutions for improved corrosion resistance of galvanised steel substrates. Surf. Coat. Technol. 2006, 200, 4240–4250. [Google Scholar] [CrossRef]
- Cabral, A.M.; Duarte, R.G.; Montemor, M.F.; Ferreira, M.G.S. A comparative study on the corrosion resistance of AA2024-T3 substrates pre-treated with different silane solutions- Composition of the films formed. Prog. Org. Coat. 2005, 54, 322–331. [Google Scholar] [CrossRef]
- Fedel, M.; Olivier, M.-G.; Poelman, M.; Deflorian, F.; Rossi, S.; Druart, M.-E. Corrosion protection properties of silane pre-treated powder coated galvanized steel. Prog. Org. Coat. 2009, 66, 118–128. [Google Scholar] [CrossRef]
- Romanoa, A.; Fedel, M.; Deflorian, F.; Olivier, M. Silane sol-gel film as pretreatment for improvement of barrier properties and filiform corrosion resistance of 6016 aluminium alloy covered by cataphoretic coating. Prog. Org. Coat. 2011, 72, 695–702. [Google Scholar] [CrossRef]
- Chen, M.A.; Xie, X.; Zhang, X.M. Interactions of BTESPT silane and maleic anhydride grafted polypropylene with epoxy and application to improve adhesive durability between epoxy and aluminium sheet. Prog. Org. Coat. 2009, 66, 40–51. [Google Scholar] [CrossRef]
- Palanivel, V.; Huang, Y.; Van Ooij, W.J. Effects of addition of corrosion inhibitors to silane films on the performance of AA2024-T3 in a 0.5 M NaCl solution. Prog. Org. Coat. 2005, 53, 153–168. [Google Scholar] [CrossRef]
- Naderi, R.; Fedel, M.; Deflorian, F.; Poelman, M.; Olivier, M. Synergistic effect of clay nanoparticles and cerium component on the corrosion behavior of eco-friendly silane sol-gel layer applied on pure aluminum. Surf. Coat. Technol. 2013, 224, 93–100. [Google Scholar] [CrossRef]
- Tian, Z.; Shi, H.; Liu, F.; Xu, S.; Han, E.H. Inhibiting effect of 8-hydroxyquinoline on the corrosion of silane-based sol-gel coatings on AA 2024-T3. Prog. Org. Coat. 2015, 82, 81–90. [Google Scholar] [CrossRef]
- Alinejad, S.; Naderi, R.; Mahdavian, M. The effect of zinc cation on the anticorrosion behavior of an eco-friendly silane sol-gel coating applied on mild steel. Prog. Org. Coat. 2016, 101, 142–148. [Google Scholar] [CrossRef]
- Trabelsi, W.; Cecilio, P.; Ferreira, M.G.S.; Montemor, M.F. Electrochemical assessment of the self-healing properties of Ce-doped silane solutions for the pre-treatment of galvanised steel substrates. Prog. Org. Coat. 2005, 54, 276–284. [Google Scholar] [CrossRef]
- Nikpour, S.; Naderi, R.; Mahdavai, M. Synergistic effect of mentha longifolia and zinc cations in silane primer coating to improve protection properties of the subsequent epoxy coating. Prog. Org. Coat. 2019, 127, 55–69. [Google Scholar] [CrossRef]
- Bahrami, M.; Borhani, G.H.; Bakhshi, S.R.; Ghasemi, A. Optimization of effective processing parameters of hybrid anti-corrosion Si/Zr sol-gel coatings doped with cerium salt for aluminum alloy 6061. J. Sol-Gel Sci. Technol. 2017, 81, 921–933. [Google Scholar] [CrossRef]
- Cabral, A.M.; Trabelsi, W.; Serra, R.; Montemor, M.F.; Zheludkevich, M.L.; Ferreira, M.G.S. The corrosion resistance of hot dip galvanised steel and AA2024-T3 pre-treated with bis-[triethoxysilylpropyl] tetrasulfide solutions doped with Ce(NO3)3. Corros. Sci. 2006, 48, 3740–3758. [Google Scholar] [CrossRef]
- Shi, H.; Liu, F.; Han, E. Corrosion behaviour of sol-gel coatings doped with cerium salts on 2024-T3 aluminum alloy. Mater. Chem. Phys. 2010, 124, 291–297. [Google Scholar] [CrossRef]
- Dias, S.A.S.; Marques, A.; Lamaka, S.V.; Simoes, A.; Diamantino, T.C.; Ferreira, M.G.S. The role of Ce(III)-enriched zeolites on the corrosion protection of AA2024-T3. Electrochim. Acta 2013, 112, 549–556. [Google Scholar] [CrossRef]
- Dias, S.A.S.; Lamaka, S.V.; Nogueira, C.A.; Diamantino, T.C.; Ferreira, M.G.S. Sol-gel coatings modified with zeolite fillers for active corrosion protection of AA2024. Corros. Sci. 2012, 62, 153–162. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Yasakau, K.A.; Salvado, I.M.M.; Ferreira, M.G.S. Nanostructured sol-gel coatings doped with cerium nitrate as pre-treatments for AA2024-T3 Corrosion protection performance. Electrochim. Acta 2005, 51, 208–217. [Google Scholar] [CrossRef]
- Tiringer, U.; Milošev, I.; Durán, A.; Castro, Y. Hybrid sol-gel coatings based on GPTMS/TEOS containing colloidal SiO2 and cerium nitrate for increasing corrosion protection of aluminium alloy 7075-T6. J. Sol-Gel Sci. Technol. 2018, 85, 546–557. [Google Scholar] [CrossRef]
- Kawai, T.; Tsutsumi, K. Reactivity of silanol groups on zeolite surfaces. Colloid Polym. Sci. 1998, 276, 992–998. [Google Scholar] [CrossRef]
- Peng, S.; Sun, L.; Lin, H.Q.; Deng, Y.L.; Chen, M.A. Preparation and corrosion performance of silane/Ce films on 7N01 aluminum alloy, High Performance Structural Materials, Y. Han (ed.), Springer Nature Singapore Pte Ltd. 2018, pp: 255-267.
- Calabrese, L.; Bonaccorsi, L.; Proverbio, E. Corrosion protection of aluminum 6061 in NaCl solution by silane-zeolite composite coatings. J. Coat. Technol. 2012, 9, 597–607. [Google Scholar] [CrossRef]
- Montemor, M.F.; Ferreira, M.G.S. Analytical characterization of silane films modified with cerium activated nanoparticles and its relation with the corrosion protection of galvanised steel substrates. Prog. Org. Coat. 2008, 63, 330–337. [Google Scholar] [CrossRef]
- Palanivel, V.; Zhu, D.; van Ooij, W.J. Nanoparticle-Filled Silane Films as Chromate Replacements for Aluminum Alloys. Prog. Org. Coat. 2003, 47, 384–392. [Google Scholar] [CrossRef]
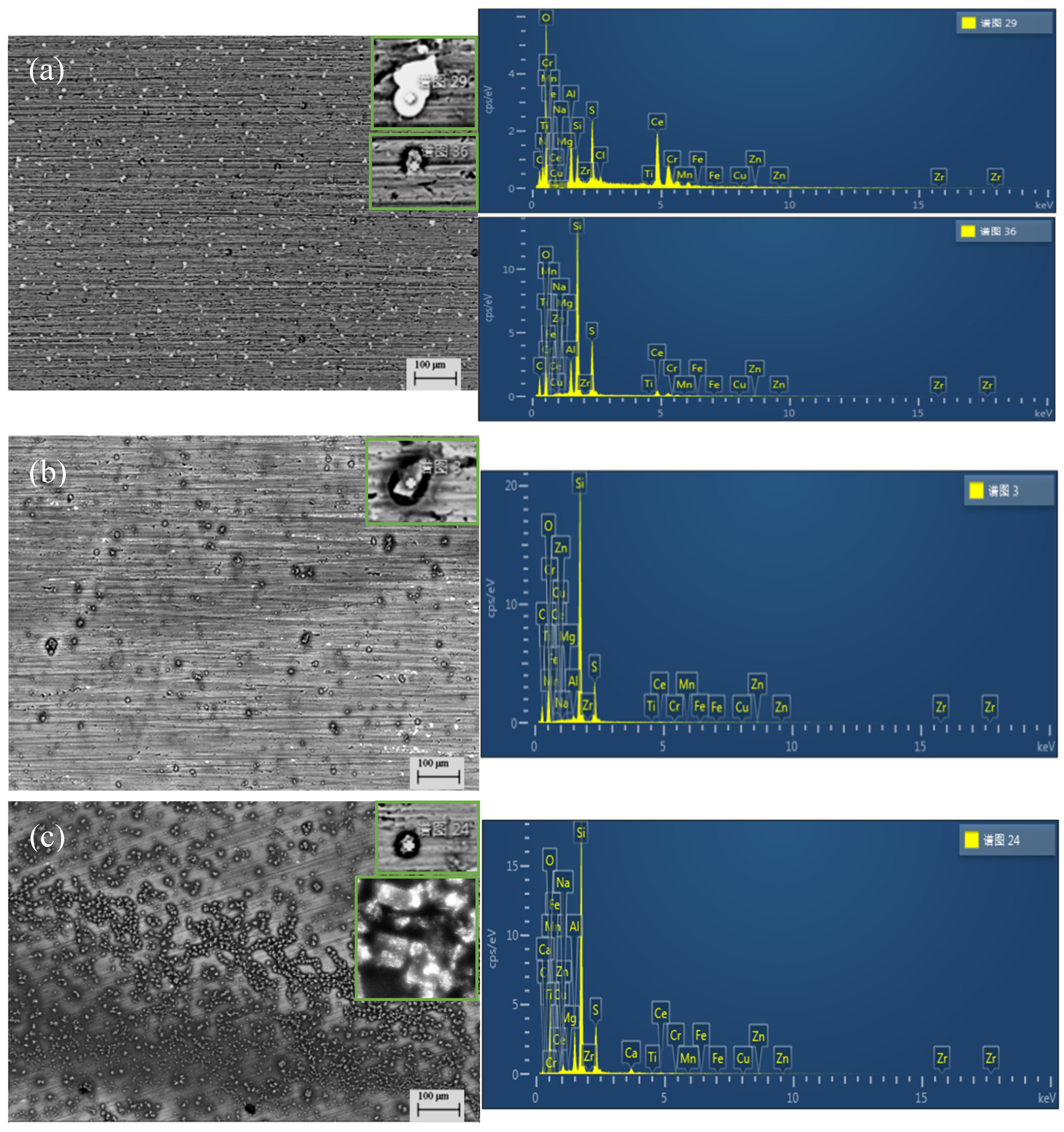
| point | C | O | Al | Si | S | Ce | Zn | Mg | Cu | Mn | Zr |
| 29 | 17.56 | 37.33 | 5.87 | 2.69 | 5.83 | 28.79 | 1.04 | 0 | 0.15 | 0 | 0 |
| 36 | 33.31 | 26.81 | 4.06 | 21.08 | 8.79 | 5.12 | 0.43 | 0.08 | 0.01 | 0.11 | 0 |
| 3 | 30.53 | 34.73 | 0.47 | 26.55 | 6.41 | 0.51 | 0.31 | 0.01 | 0.02 | 0 | 0.10 |
| 24 | 19.38 | 38.76 | 4.26 | 27.32 | 7.44 | 0.59 | 0.13 | 0.12 | 0.07 | 0 | 0.08 |
| Immersion time (h) | Ecorr(V/SCE) | Icorr (A/cm2) | Pitting potential(V) |
| 1 | -0.75 | 1.20×10-12 | None |
| 168 | -1.15 | 2.83×10-9 | -0.80 |
| 192 | -1.09 | 1.12×10-8 | -0.87 |
| 240 | -1.04 | 5.58×10-9 | -0.87 |
| 288 | -1.10 | 1.43×10-8 | -0.88 |
| Immersion time (h) | Ecorr(V/SCE) | Icorr (A/cm2) | Pitting potential(V) |
| 1 | -0.67 | 7.02×10-13 | None |
| 168 | -0.76 | 2.78×10-12 | None |
| 192 | -0.73 | 3.34×10-12 | None |
| 240 | -0.70 | 4.18×10-12 | None |
| 288 | -0.75 | 3.60×10-12 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




