Submitted:
24 December 2024
Posted:
25 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Synthesis of the Cordierite Filler
- (1)
- Kaolin - SiO2 =53.87 %, Al2O3 = 28.24%, Fe2O3 = 1.48%, CaO = 0.64 %, Na2O = 0.03 %, and K2O = 0.05 %;
- (2)
- Alumina - SiO2 = 0.15 %, Al2O3 = 95.12 %, MgO = 0.01 %, Fe2O3 = 0.1%, CaO = 0.17 %, Na2O = 0.04 %, and K2O = 0.02 %;
- (3)
- Talc - SiO2 = 60.97 %, Al2O3 = 1.68 %, MgO = 28.91 %, Fe2O3 = 2.23 %, CaO = 2.95 %, Na2O = 0.87 %, and K2O = 0.91 %.
2.2. Instrumental Methods for Characterization of the Synthetized Cordierite Filler
2.3. Preparation and Mix-Design of the Protective Coating
2.4. Instrumental Methods for Characterization of the Protective Coating
3. Results and Discussion
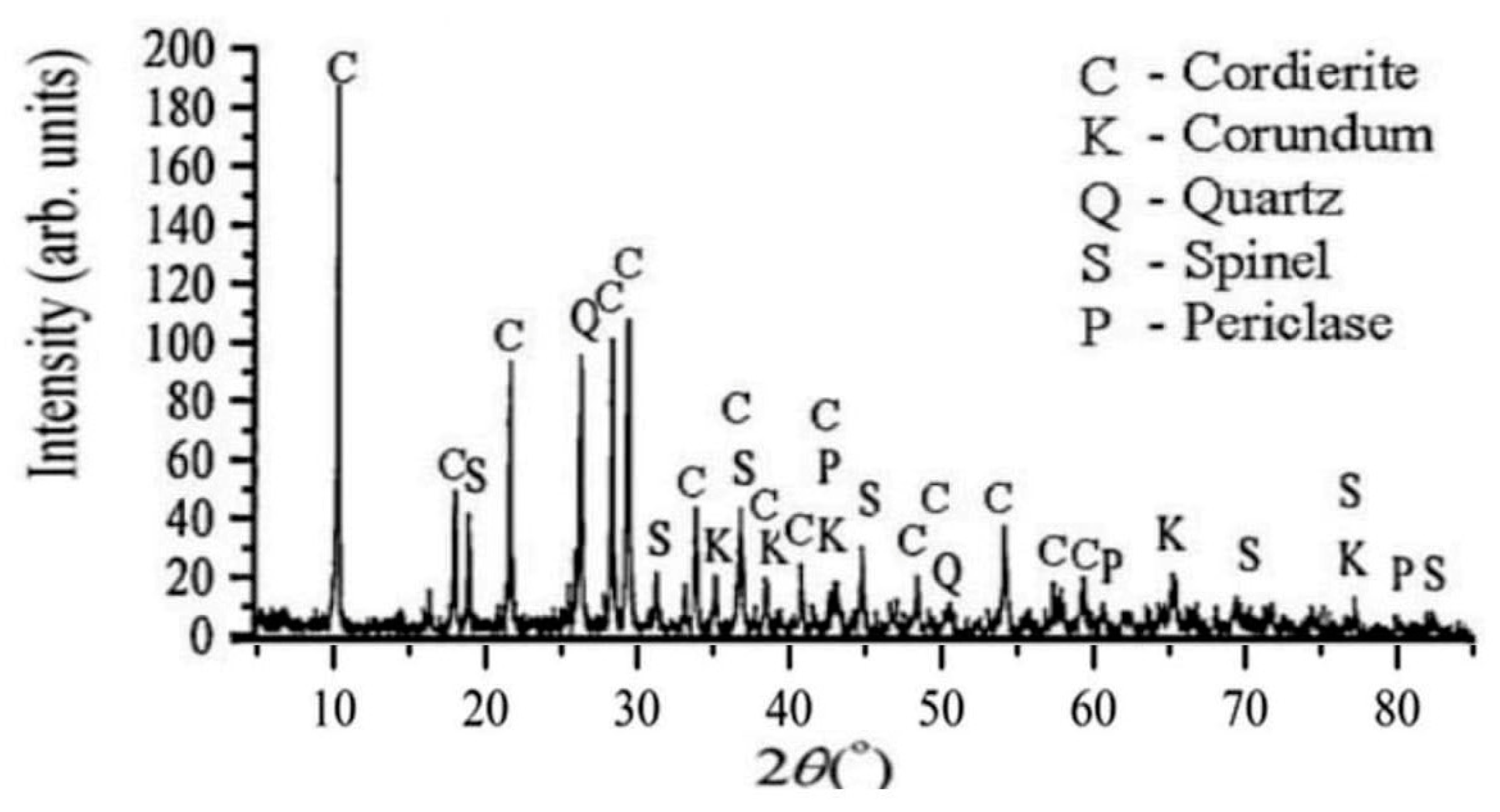
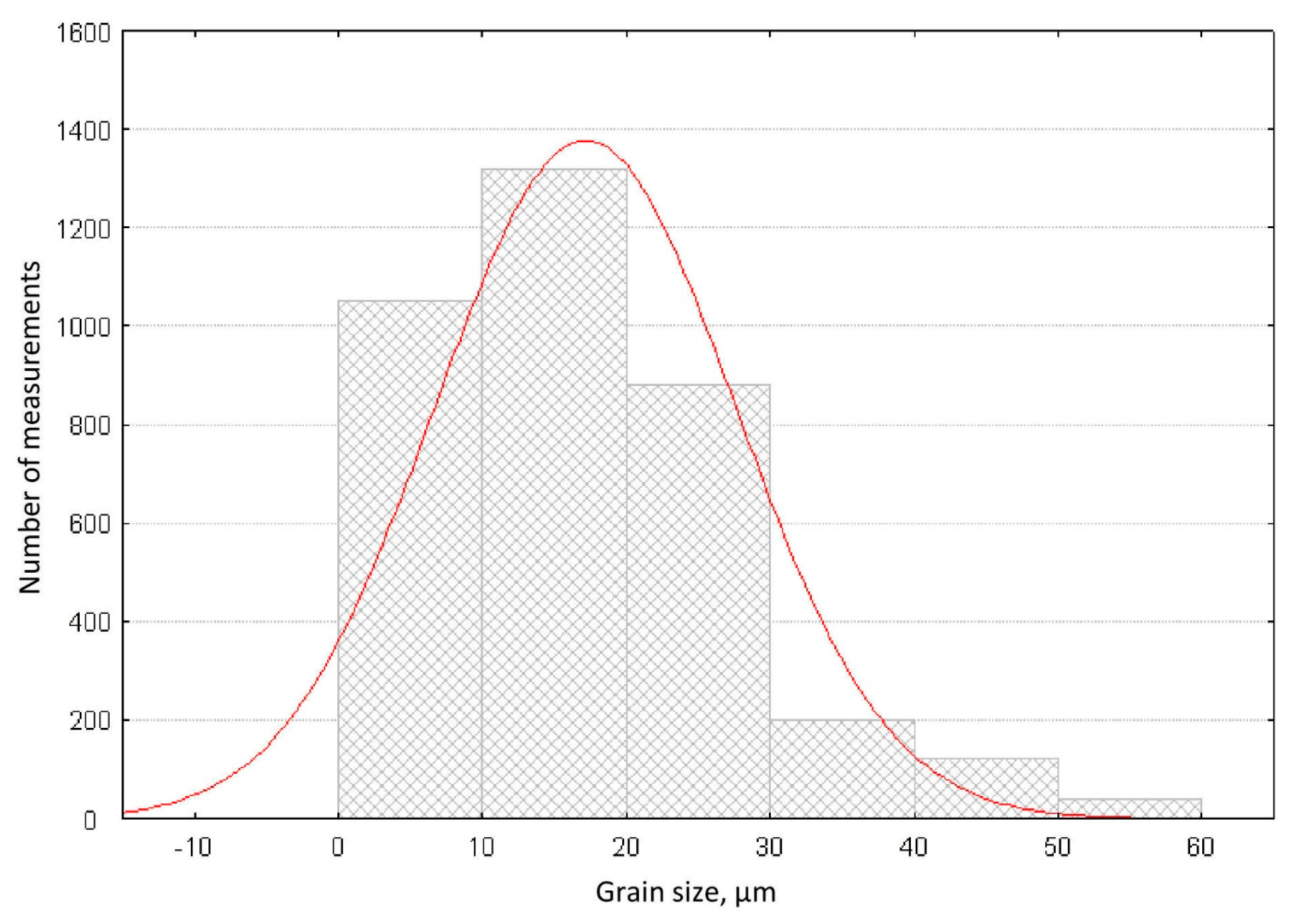
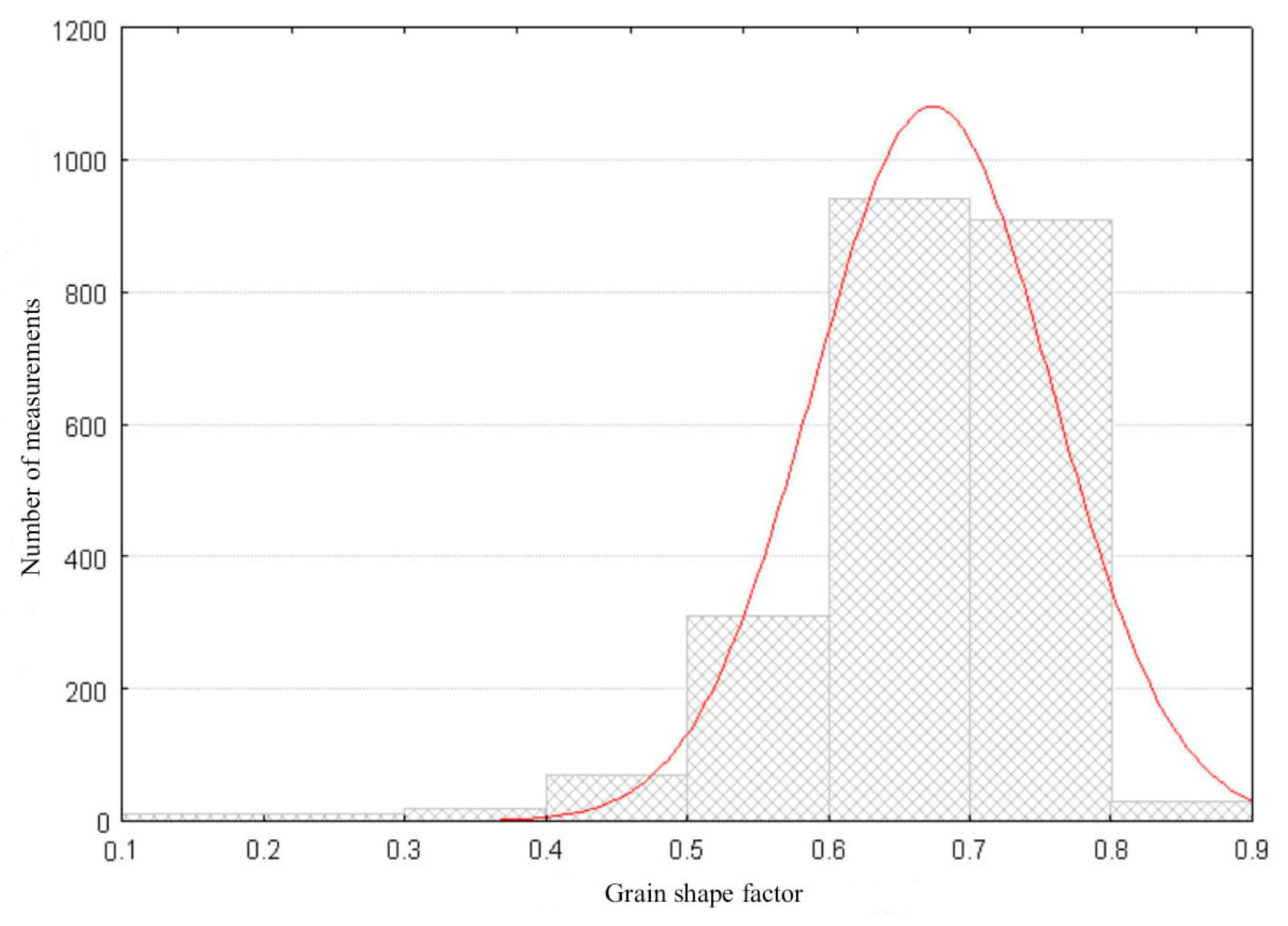
3.1. Properties of the Synthetized Cordierite Filler
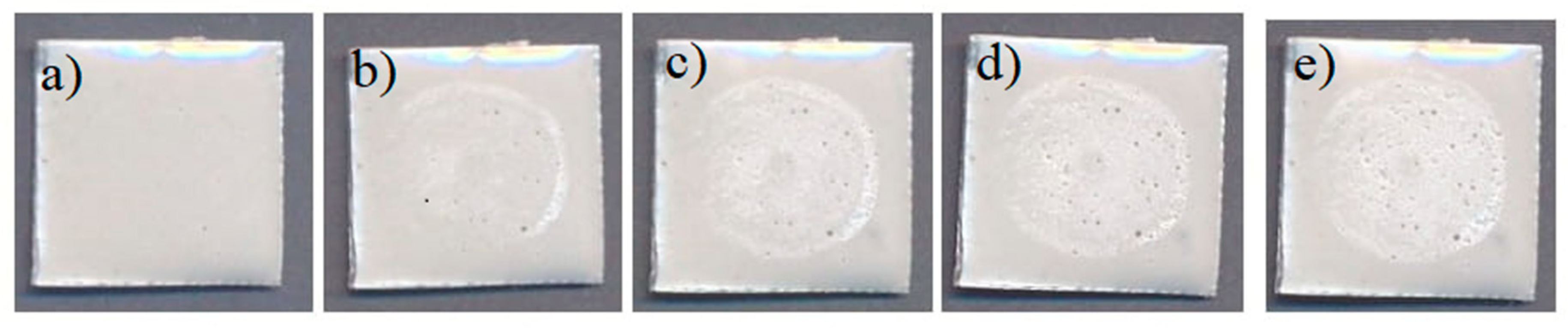
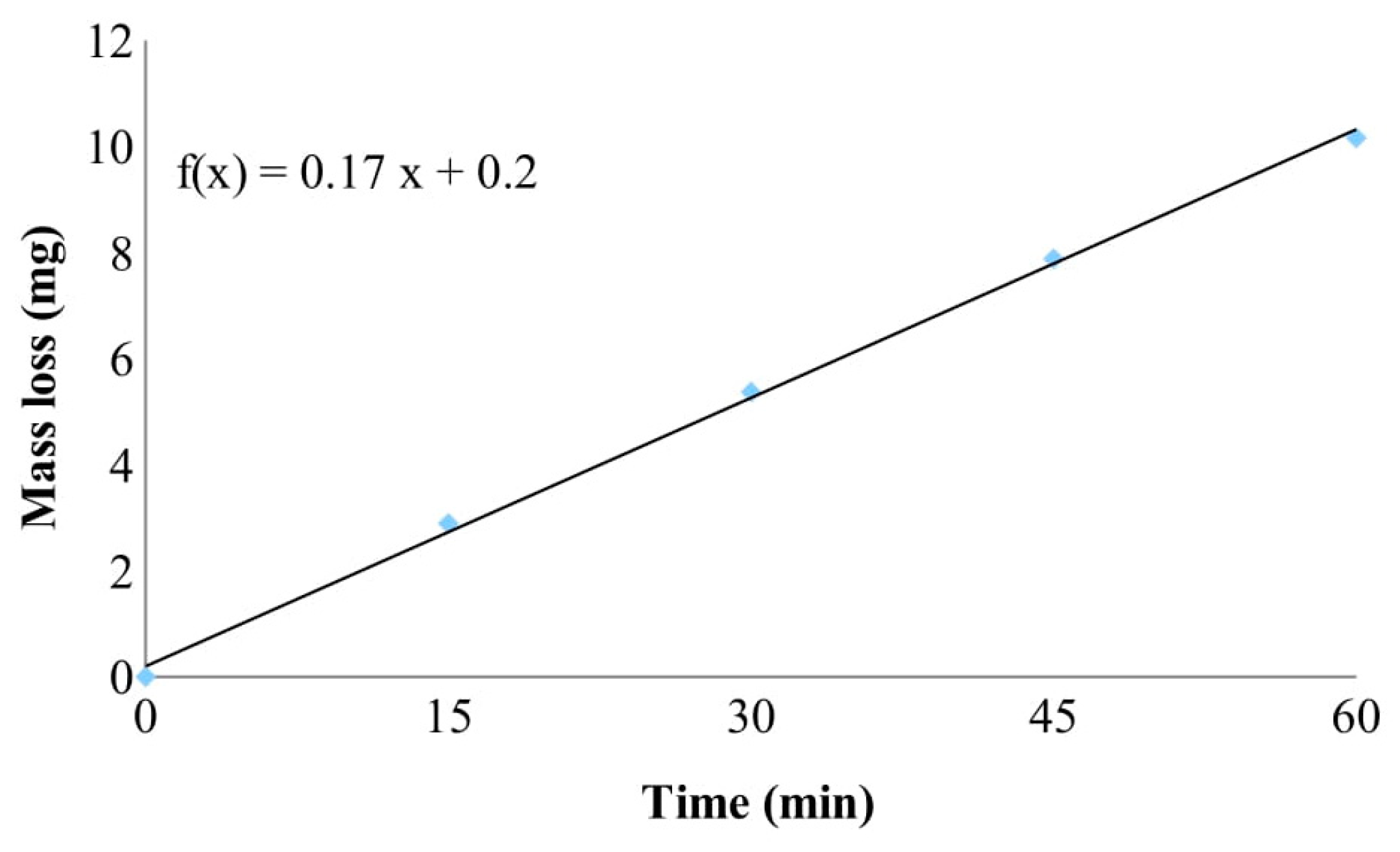
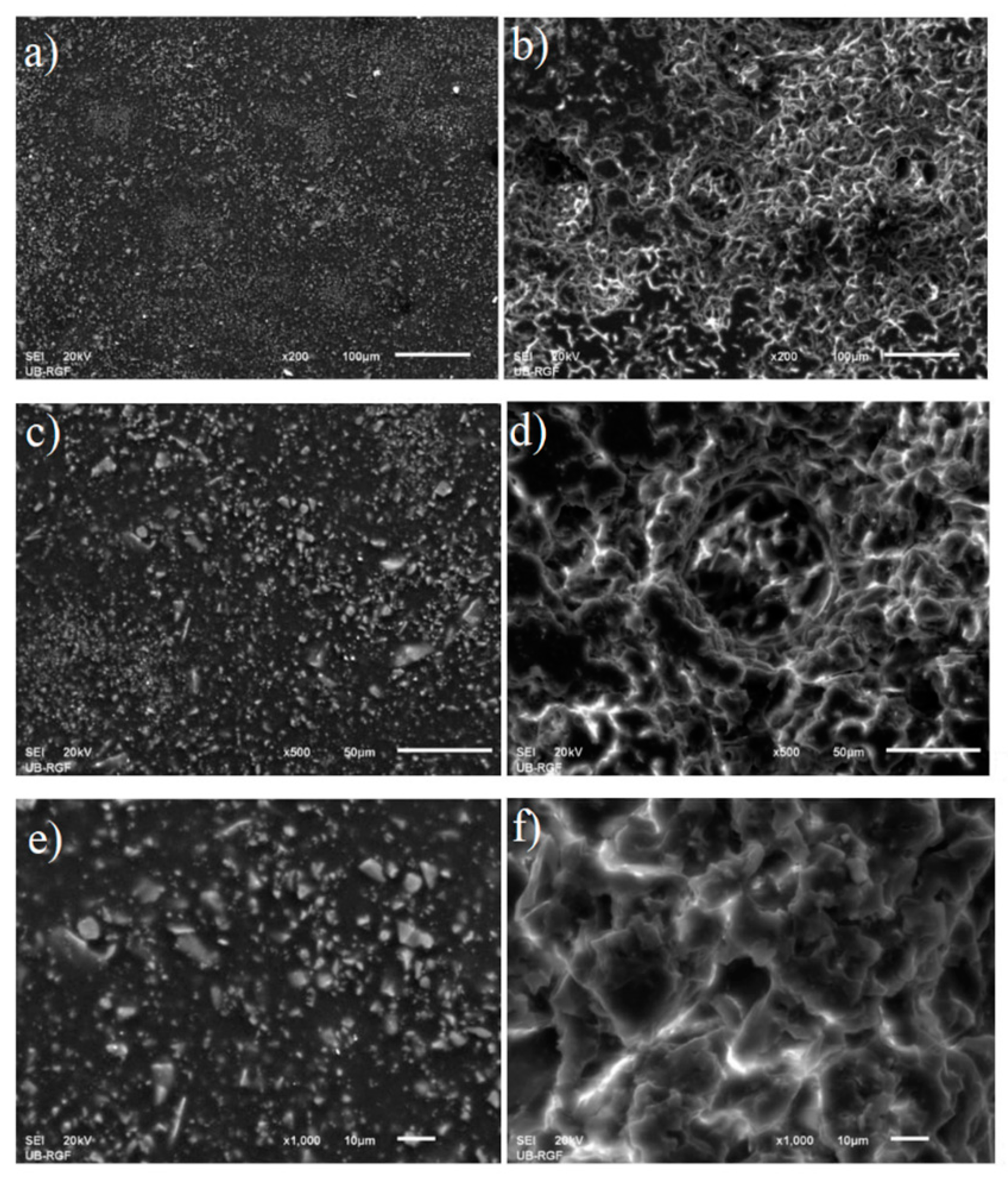
3.2. Properties of Protective Coating: Superficial Damage Formation and Development
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Almusallam, A.; Khan, F.; Dulaijan, S.; Al-Amoudi, O. Effectiveness of surface coatings in improving concrete durability. Cem. Concr. Compos. 2003, 25, 473–481. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. 2016, 132, 578–590. [CrossRef]
- Korjakins, A.; Kara, P.; Toropovs, N. Improving Quality of High Performance Concrete by Cavitation Treatment of the Raw Materials. Procedia Eng. 2013, 57, 597–604. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Chen, C.; Chen, J.; Zhou, J.; Deng, J. Study on the influence mechanism of material damage on the cavitation erosion properties of hydraulic concrete. Constr. Build. Mater. 2023, 400. [Google Scholar] [CrossRef]
- Park, D. Carbonation of concrete in relation to CO2 permeability and degradation of coatings. Constr. Build. Mater. 2008, 22, 2260–2268. [Google Scholar] [CrossRef]
- Aguiar, J.B.; Júnior, C. Carbonation of surface protected concrete. Constr. Build. Mater. 2013, 49, 478–483. [Google Scholar] [CrossRef]
- Shariatmadar, M.; Gholamhosseini, P.; Abdorrezaee, Z.; Ghorbanzadeh, S.; Feizollahi, S.; Hosseini, F.; Shahraki, F.A.; Mahdavian, M. Leveraging polyaniline grafted micaceous iron oxide as a dual active-barrier pigment for anti-corrosion polymer coatings. Surf. Coatings Technol. 2024, 479. [Google Scholar] [CrossRef]
- Wang, H.; Feng, P.; Lv, Y.; Geng, Z.; Liu, Q.; Liu, X. A comparative study on UV degradation of organic coatings for concrete: Structure, adhesion, and protection performance. Prog. Org. Coatings 2020, 149, 105892. [Google Scholar] [CrossRef]
- Selvaraj, R.; Selvaraj, M.; Iyer, S. Studies on the evaluation of the performance of organic coatings used for the prevention of corrosion of steel rebars in concrete structures. Prog. Org. Coatings 2009, 64, 454–459. [Google Scholar] [CrossRef]
- Qu, H.; Feng, M.; Li, M.; Tian, D.; Zhang, Y.; Chen, X.; Li, G. Enhancing the carbonation and chloride resistance of concrete by nano-modified eco-friendly water-based organic coatings. Mater. Today Commun. 2023, 37. [Google Scholar] [CrossRef]
- Pavlovic, M.; Dojcinovic, M.; Nikolic, J.; Terzic, A.; Pavicevic, V.; Drmanic, S.; Kurtanovic, E. Application of waste raw materials as a reinforcement for protective coatings based on pyrophyllite. Chem. Ind. Chem. Eng. Q. 2024, 29–29. [Google Scholar] [CrossRef]
- Gu, S.; Shi, H.; Li, J.; Xu, H.; Udoh, I.I.; Liu, F.; Han, E.-H. Self-diagnosing and active protective dual-functional water-borne polyurethane coating based on smart mesoporous containers. Prog. Org. Coatings 2023, 183. [Google Scholar] [CrossRef]
- Sun, F.-C.; Fu, J.-H.; Peng, Y.-X.; Jiao, X.-M.; Liu, H.; Du, F.-P.; Zhang, Y.-F. Dual-functional intumescent fire-retardant/self-healing water-based plywood coatings. 154, 1061; 87. [Google Scholar] [CrossRef]
- S. A. T. Nejad, S. S. A. T. Nejad, S. Amanian, E. Alibakhshi, M. Hajisoltani, S. A. Haddadi, M. Arjmand, B. Ramezanzadeh, M. Mahdavian. Enhancing epoxy-silicone coating's protection performance: Harnessing the power of sulfur-doped graphene oxide. Prog. Org. Coat. 2024. [Google Scholar] [CrossRef]
- K. Bobzin, L. K. Bobzin, L. Zhao, H. Heinemann, E. Burbaum, S. Li. Effect of heat treatment on the structure, fracture toughness and oxidation behavior of a silicon coating by atmospheric plasma spraying. Surf. Coat. Technol. 2023. [Google Scholar] [CrossRef]
- Qi, C.; Dam-Johansen, K.; Weinell, C.E.; Wu, H. Synthesis of micro-structured zinc particles by thermal evaporation and their application in zinc containing coatings for steel corrosion protection. Prog. Org. Coatings 2023, 187. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, D.; Li, Y.; Jackson, E.; Fang, Y.; Zhang, Y.; Xie, N.; Shi, X. Investigation into the Synergistic Effect of Nano-sized Materials on the Anti-corrosion Properties of a Waterborne Epoxy Coating. Int. J. Electrochem. Sci. 2016, 11, 6023–6042. [Google Scholar] [CrossRef]
- Soleimani, M.; Bagheri, E.; Mosaddegh, P.; Rabiee, T.; Fakhar, A.; Sadeghi, M. Stable waterborne epoxy emulsions and the effect of silica nanoparticles on their coatings properties. Prog. Org. Coatings 2021, 156, 106250. [Google Scholar] [CrossRef]
- Yao, H.; Li, L.; Li, W.; Qi, D.; Fu, W.; Wang, N. Application of nanomaterials in waterborne coatings: A review. Resour. Chem. Mater. 2022, 1, 184–200. [Google Scholar] [CrossRef]
- Semmler, C.; Gyoktepeliler-Akin, E.; Killinger, A. Plasma sprayed ceramic coatings for the thermal protection of carbon fiber reinforced plastics (CFRP): Thermal and mechanical properties of YSZ, aluminum titanate, cordierite and mullite coatings. Surf. Coatings Technol. 2023, 462. [Google Scholar] [CrossRef]
- M. Ferraris, M. M. Ferraris, M. Salvo, F. Smeacetto. Cordierite-mullite coating for SiC/SiC composites. J. Euro. Ceram. Soc. 2002. [Google Scholar] [CrossRef]
- Wei, X. Shi, D. Cui, Z. Wei, S. Hong. Effect of 3.5 % NaCl solution with different Na2S concentrations on ultrasonic cavitation erosion behaviors of HVOF sprayed WC-Ni coatings. Ultrason. Sonochem. 2023. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Si, C.; Tian, Y.; Xu, S. Microstructure development and cavitation erosion resistance enhancement of additive manufactured Hastelloy C276 alloy coating on martensitic stainless–steel via directed energy deposition. Opt. Laser Technol. 2023, 171. [Google Scholar] [CrossRef]
- Kumar, P.; Singal, S.; Gohil, P.P. A technical review on combined effect of cavitation and silt erosion on Francis turbine. Renew. Sustain. Energy Rev. 2023, 190. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Chen, C.; Chen, J.; Zhou, J.; Deng, J. Study on the influence mechanism of material damage on the cavitation erosion properties of hydraulic concrete. Constr. Build. Mater. 2023, 400. [Google Scholar] [CrossRef]
- Dojčinović, M.; Cvetković, R.P.; Sedmak, A.; Popović, O.; Cvetković, I.; Radu, D. Effect of Shielding Gas Arc Welding Process on Cavitation Resistance of Welded Joints of AlMg4.5Mn Alloy. Materials 2023, 16, 4781. [Google Scholar] [CrossRef] [PubMed]
- B. Kljajević: Software program OZARIA 2.5 for determining the size and shape of grains of refractory fillers, VAGA Lab, Belgrade, Serbia (2000).
- Pavlovic, M.; Andric, L.; Radulovic, D.; Drmanic, S.; Djordjevic, N.; Petrov, M. Influence of mechanical activation of a cordierite -based filler on sedimentation stability of lost foam refractory coatings. Sci. Sinter. 2019, 51, 15–25. [Google Scholar] [CrossRef]
- M. Pavlović, M. M. Pavlović, M. Dojčinović: Kavitaciona oštećenja refrakcionih materijala, Akademska misao, Belgrade, Serbia, 2020, p. 7466. [Google Scholar]
- ASTM G32-16 Red Standard Test Method for Cavitation Erosion Using Vibratory Apparatus (Standard + Redline PDF Bundle), https://webstore.ansi.org/standards/astm/astmg3216red (accessed 15.01. 2024.
- M. Hauer, F. M. Hauer, F. Gärtner, S. Krebs, T. Klassen, M. Watanabe, S. Kuroda, W.Kro¨mmer, K. Henkel. Process selection for the Fabrication of cavitation erosion-Resistant bronze Coating by Thermal and Kinetic Spraying in Maritime Applications. J. Therm. Spray Tech. 30 (2021) 1310. [CrossRef]
- Geiger, C.A.; Rager, H.; Czank, M. Cordierite III: the site occupation and concentration of Fe3+. Contrib. Miner. Pet. 2000, 140, 344–352. [Google Scholar] [CrossRef]
- Götze, J.; Pan, Y.; Müller, A. Mineralogy and mineral chemistry of quartz: A review. Miner. Mag. 2021, 85, 639–664. [Google Scholar] [CrossRef]
- Goudar, S. P.; Shah, S. S.; Shirali, G. S. Echocardiography of Coarctation of the Aorta, Aortic Arch Hypoplasia, and Arch Interruption: Strategies for Evaluation of the Aortic Arch. Cardiol Young 2016, 26(8), 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- F.P. Glasser. Cordierite. In Concise Encyclopedia of Advanced Ceramic Materials, Editor(s): R.J. Brook, Pergamon Press, Oxford, UK, 1991, p. 9780; -92. [CrossRef]
- J. Juan, K. J. Juan, K. Zubillaga. Roundness in quartz grains from inland and coastal dune sands, Altar Desert, Sonora, Mexico. Bol. Soc. Geol. Mex. 2009. [Google Scholar] [CrossRef]
- Ma. del Carmen Gutiérrez-Castorena, W. R. Ma. del Carmen Gutiérrez-Castorena, W. R. Effland. 21 - Pedogenic and Biogenic Siliceous Features. In Interpretation of Micromorphological Features of Soils and Regoliths in Editor(s): G. Stoops, V. Marcelino, F. Mees, Elsevier, Amsterdam, Netherlands, 2010, p. 9780. [Google Scholar] [CrossRef]
- Arai, S.; Tamura, A.; Miura, M.; Morishita, T. Origin of spinel-hosted mineral inclusions in mantle peridotite from Setogawa in the Circum-Izu Massif Serpentine Belt, central Japan: Implications for the chromitite genesis. Ore Geol. Rev. 2021, 140, 104422. [Google Scholar] [CrossRef]
- Hossain, Z.; Fabricius, I.L.; Christensen, H.F. Elastic and nonelastic deformation of greensand. Lead. Edge 2009, 28, 86–88. [Google Scholar] [CrossRef]
- Santamarina, J.C. and Cho, G. Csoil. Behavior: The Role of Particle Shape. Conference on Advances in Geotechnical Engineering, London, 29-31 March 2004, 604-617.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




