Submitted:
24 June 2025
Posted:
25 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
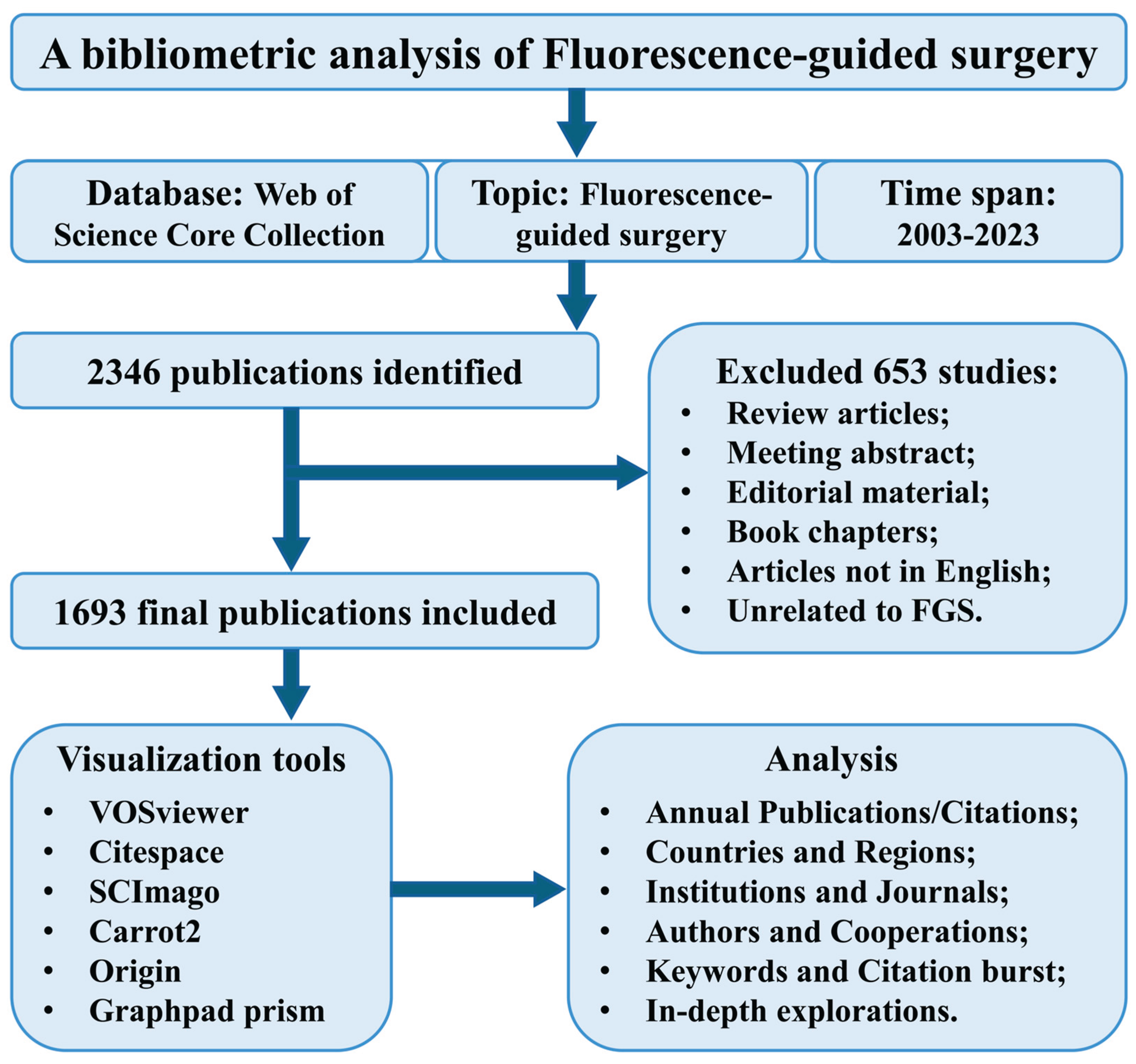
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis
3. Results
3.1. Global Publication Trends and Inter-Country Collaboration
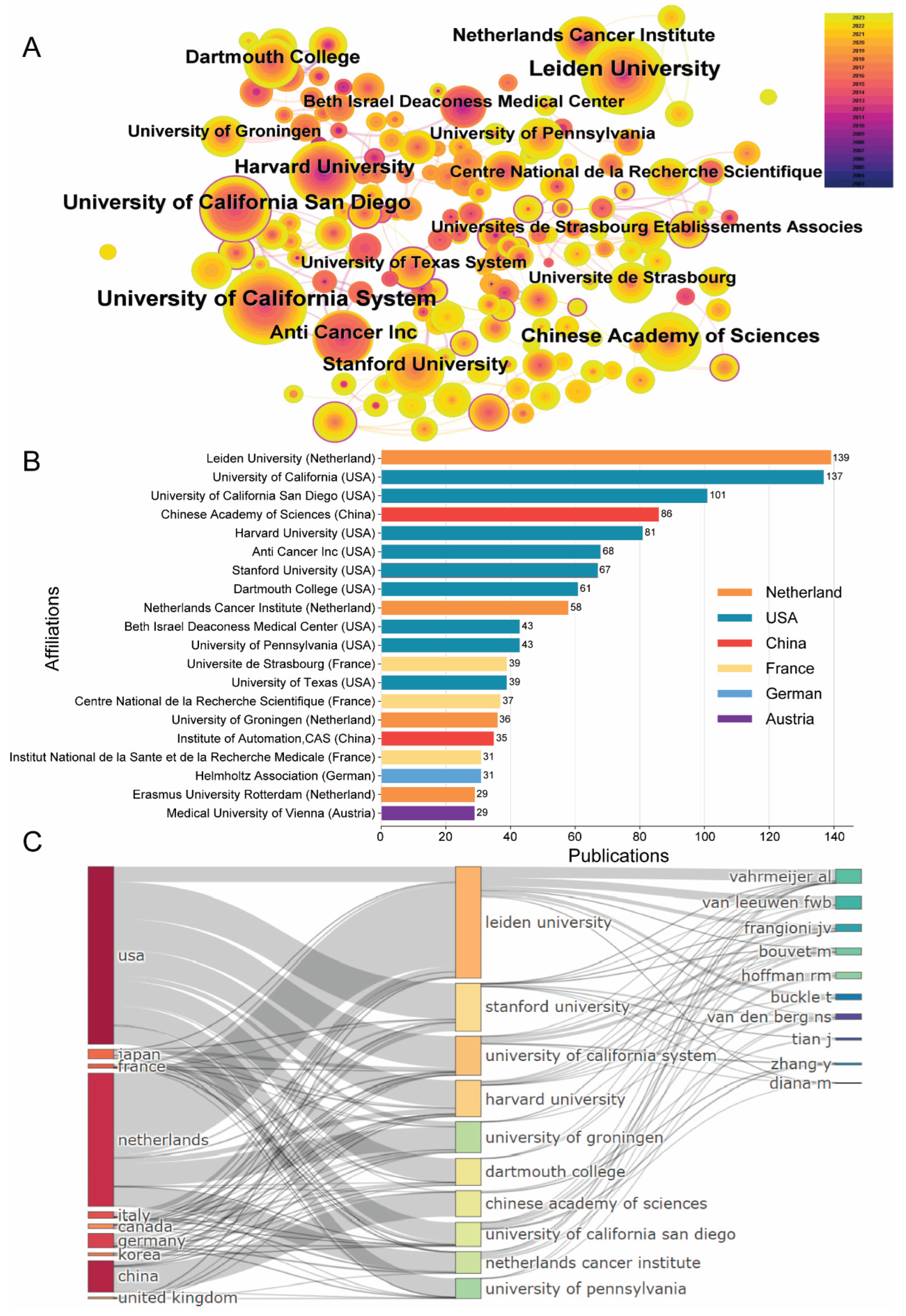
3.2. Most Related Institutions of FGS Research
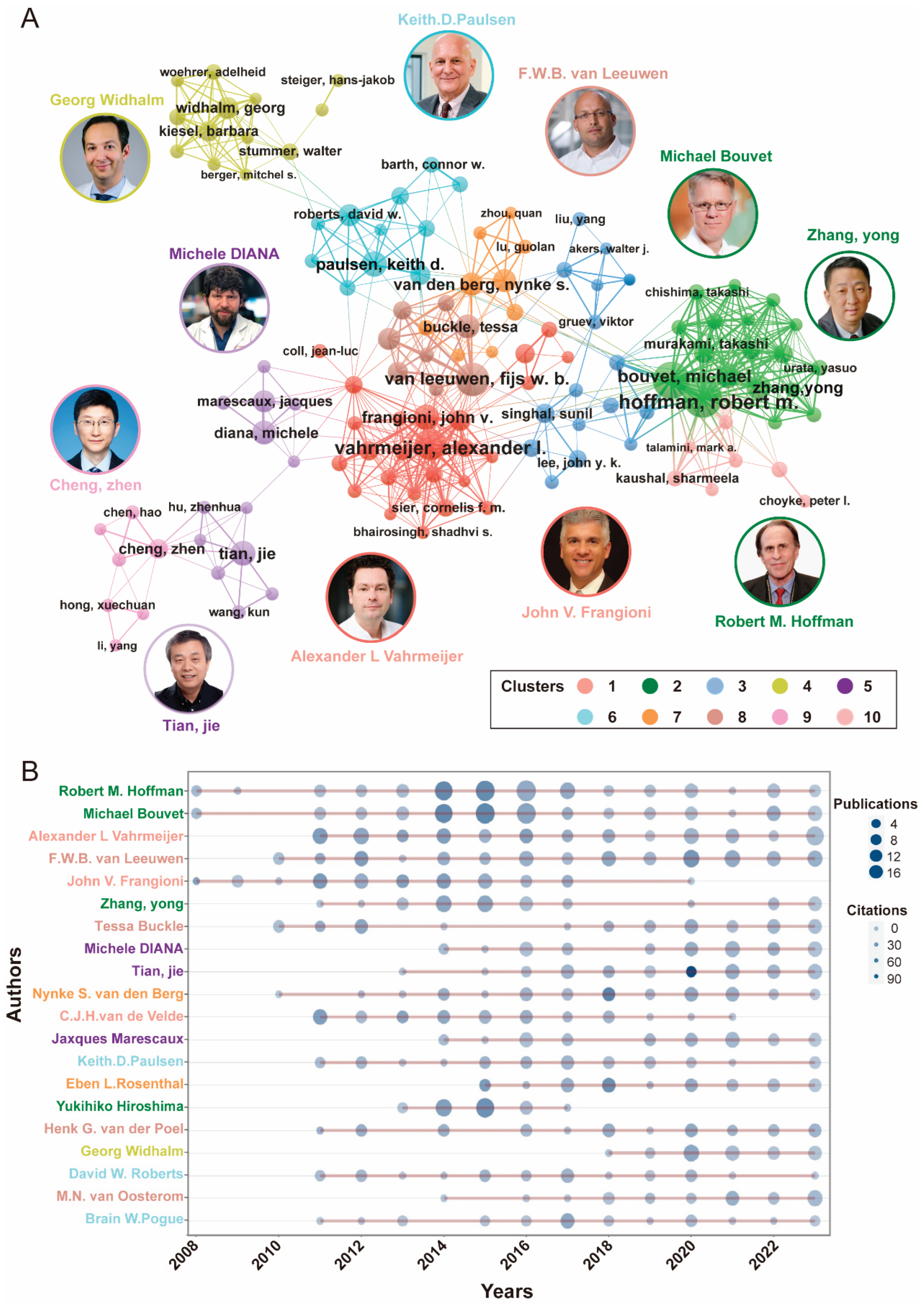
3.3. Most Contributing Authors and Collaborative Networks
| Rank | Authors | Country | Articles | AF1 | TC2 | H-index |
|---|---|---|---|---|---|---|
| 1 | Robert M. Hoffman | USA | 83 | 9.97 | 2377 | 30 |
| 2 | Michael Bouvet | USA | 79 | 8.42 | 2310 | 30 |
| 3 | Alexander L Vahrmeijer | Netherlands | 68 | 6.30 | 2578 | 31 |
| 4 | F.W.B van Leeuwen | Netherlands | 68 | 8.17 | 2326 | 29 |
| 5 | John V. Frangioni | USA | 40 | 5.46 | 2843 | 30 |
| 6 | Zhang, Yong | Singapore | 37 | 4.15 | 970 | 19 |
| 7 | Michele Diana | France | 36 | 4.03 | 623 | 14 |
| 8 | Tessa Buckle | Netherlands | 35 | 4.31 | 1267 | 17 |
| 9 | Tian, Jie | China | 34 | 4.16 | 1169 | 16 |
| 10 | Nynke S. van den Berg | Netherlands | 34 | 3.27 | 1402 | 20 |
| 11 | C.J.H. van de Velde | Netherlands | 32 | 3.18 | 1948 | 26 |
| 12 | Jaxques Marescaux | France | 31 | 3.64 | 563 | 13 |
| 13 | Keith. D. Paulsen | USA | 30 | 3.58 | 972 | 18 |
| 14 | Eben L. Rosenthal | USA | 30 | 2.95 | 1218 | 16 |
| 15 | Yukihiro Hiroshima | Japan | 29 | 2.78 | 1067 | 21 |
| 16 | Henk G. van der Poel | Netherlands | 29 | 3.38 | 1321 | 18 |
| 17 | Georg Widhalm | Austria | 26 | 2.05 | 263 | 8 |
| 18 | David W. Roberts | USA | 25 | 3.37 | 952 | 18 |
| 19 | M.N. van Oosterom | Netherlands | 25 | 2.82 | 344 | 13 |
| 20 | Brain W. Pogue | USA | 24 | 3.17 | 552 | 15 |
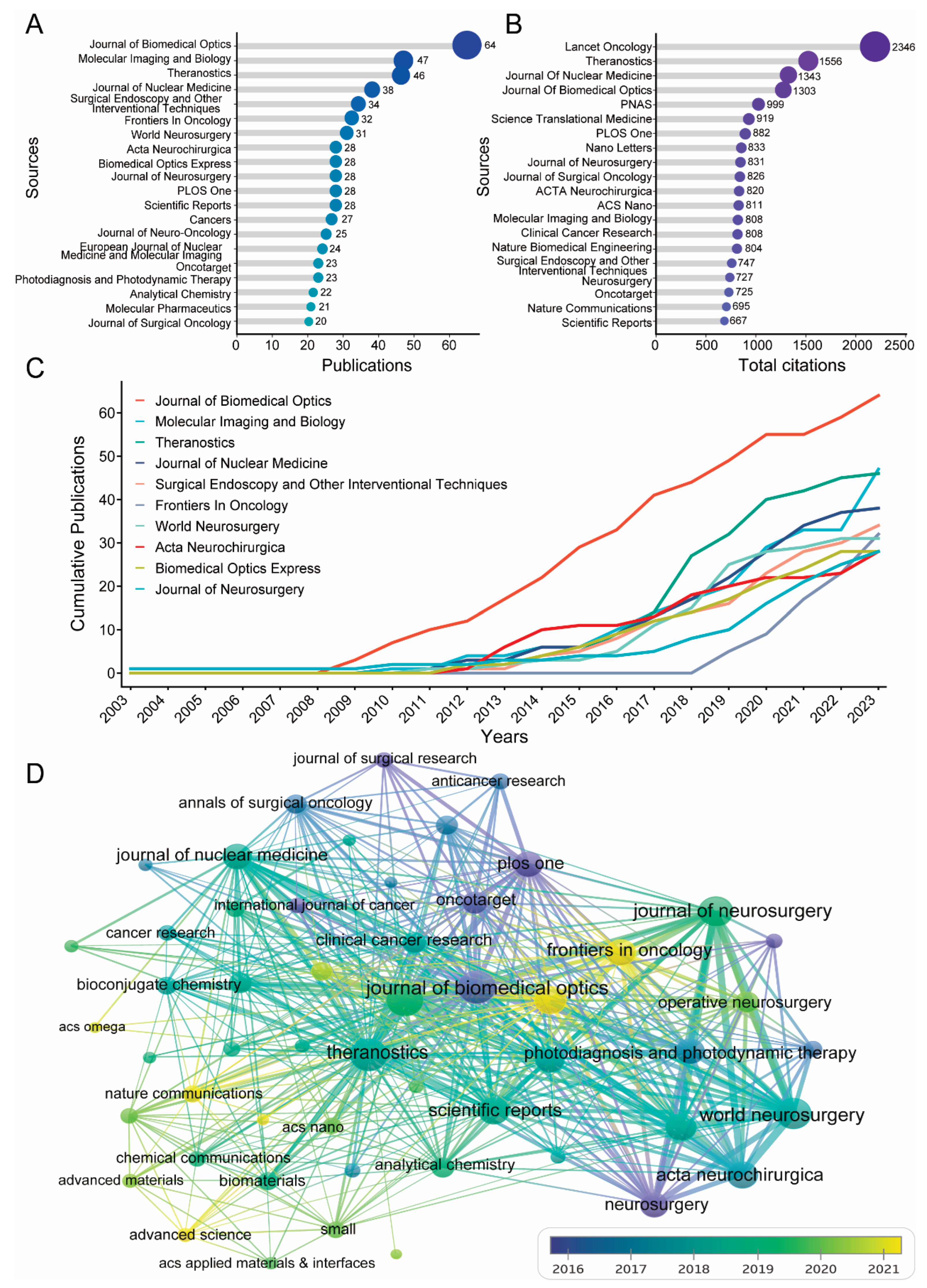
| Rank | Sources | Articles | TC1 | AAC2 |
|---|---|---|---|---|
| 1 | Journal of Biomedical Optics | 64 | 1303 | 20.36 |
| 2 | Molecular Imaging and Biology | 46 | 808 | 17.57 |
| 3 | Theranostics | 46 | 1556 | 33.83 |
| 4 | Journal of Nuclear Medicine | 38 | 1343 | 35.34 |
| 5 | Surgical Endoscopy and Other Interventional Techniques | 34 | 747 | 21.97 |
| 6 | Frontiers In Oncology | 32 | 197 | 6.16 |
| 7 | World Neurosurgery | 31 | 566 | 18.26 |
| 8 | Acta Neurochirurgica | 28 | 820 | 29.29 |
| 9 | Biomedical Optics Express | 28 | 411 | 14.68 |
| 10 | Journal of Neurosurgery | 28 | 831 | 29.68 |
| 11 | PLOS One | 28 | 882 | 31.50 |
| 12 | Scientific Reports | 28 | 667 | 23.82 |
| 13 | Cancers | 27 | 177 | 6.56 |
| 14 | Journal of Neuro-Oncology | 25 | 423 | 16.92 |
| 15 | European Journal of Nuclear Medicine and Molecular Imaging | 24 | 506 | 21.08 |
| 16 | Oncotarget | 23 | 725 | 31.52 |
| 17 | Analytical Chemistry | 22 | 623 | 28.32 |
| 18 | Photodiagnosis and Photodynamic Therapy | 22 | 310 | 14.09 |
| 19 | Journal of Surgical Oncology | 20 | 826 | 41.30 |
| 20 | Molecular Pharmaceutics | 20 | 407 | 20.35 |
3.4. Analysis of the Most Relevant Journals and Papers in the FGS Research Field
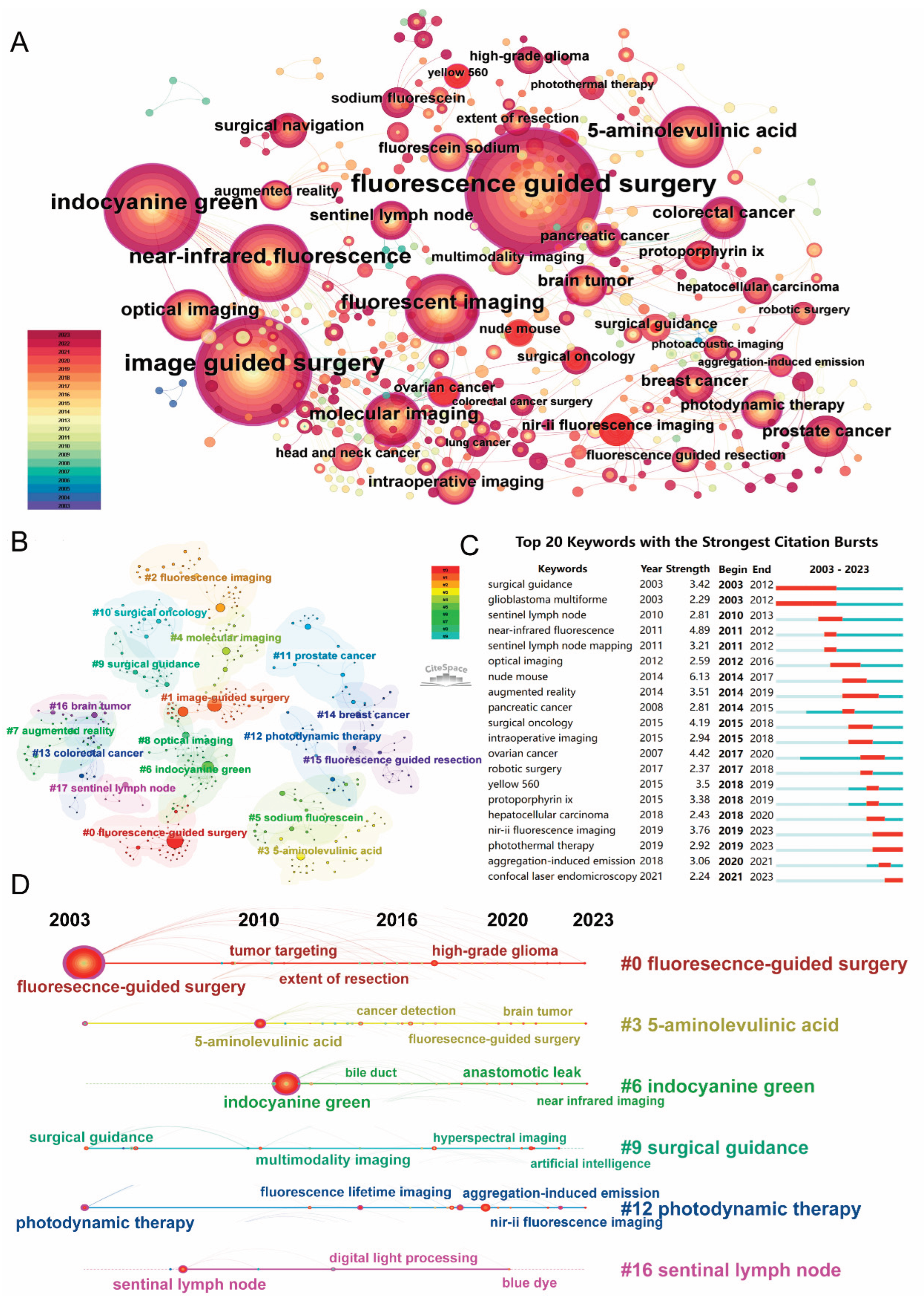
3.5. Research Hotspots Analyzing Through Keywords and Bursts Analyses from 2003 to 2023
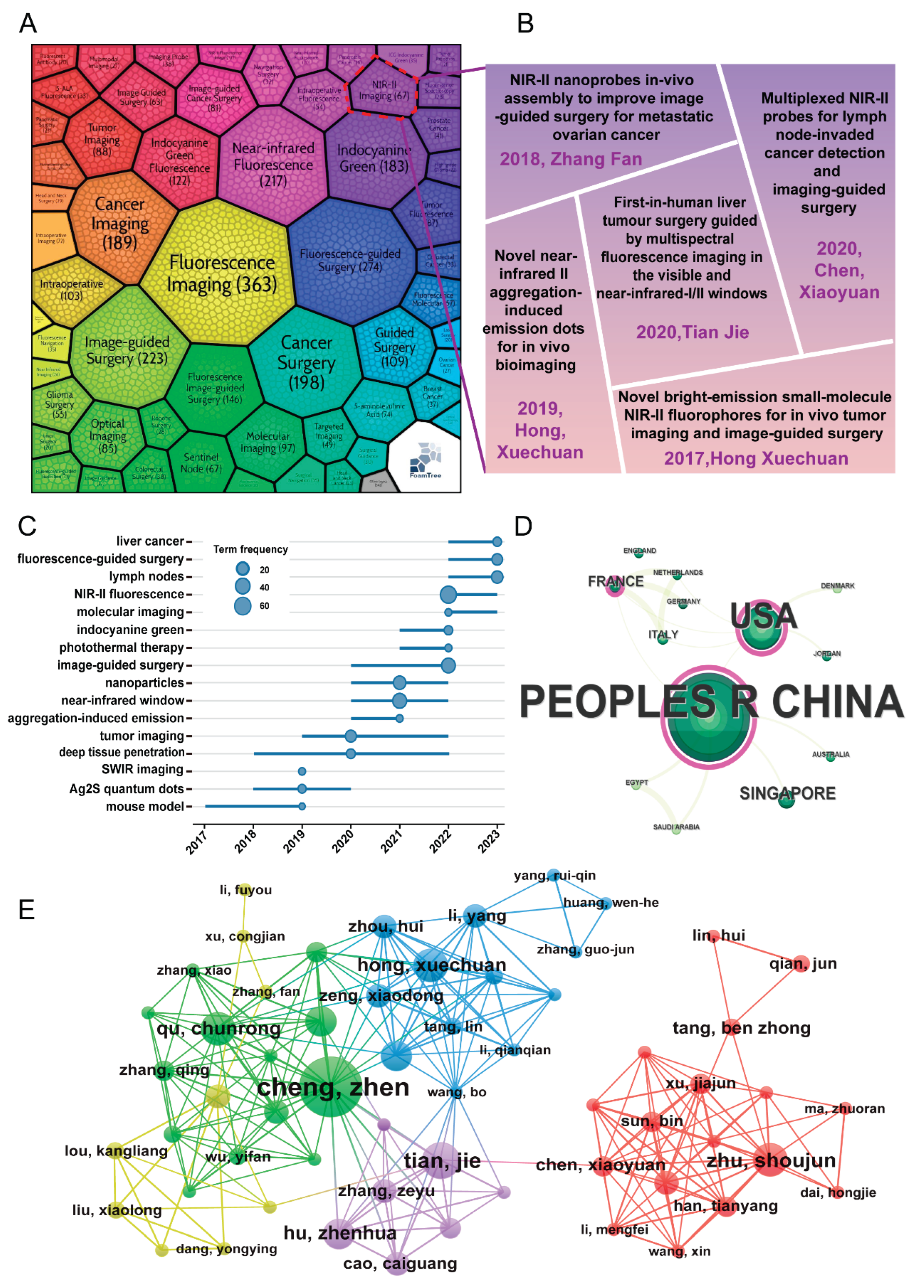
3.6. In-Depth Exploration of NIR-II Imaging in FGS Research Field
4. Discussion
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Ethics approval and Consent to participate
Consent for publication
Declaration of Competing Interest
References
- Mieog, J.S.D.; Achterberg, F.B.; Zlitni, A.; Hutteman, M.; Burggraaf, J.; Swijnenburg, R.J.; Gioux, S.; Vahrmeijer, A.L. Fundamentals and developments in fluorescence-guided cancer surgery. Nat Rev Clin Oncol 2022, 19, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol 2013, 10, 507–518. [Google Scholar] [CrossRef]
- Liu, J.T.C.; Sanai, N. Trends and Challenges for the Clinical Adoption of Fluorescence-Guided Surgery. J Nucl Med 2019, 60, 756–757. [Google Scholar] [CrossRef]
- Stewart, H.L.; Birch, D.J.S. Fluorescence Guided Surgery. Methods Appl Fluoresc 2021, 9. [Google Scholar] [CrossRef]
- Moore, G.E. Fluorescein as an Agent in the Differentiation of Normal and Malignant Tissues. Science 1947, 106, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.E.; Peyton, W.T.; et al. The clinical use of fluorescein in neurosurgery; the localization of brain tumors. J Neurosurg 1948, 5, 392–398. [Google Scholar] [CrossRef]
- Egloff-Juras, C.; Bezdetnaya, L.; Dolivet, G.; Lassalle, H.P. NIR fluorescence-guided tumor surgery: new strategies for the use of indocyanine green. Int J Nanomedicine 2019, 14, 7823–7838. [Google Scholar] [CrossRef]
- Porcu, E.P.; Salis, A.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Indocyanine green delivery systems for tumour detection and treatments. Biotechnol Adv 2016, 34, 768–789. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Du, Y.; Ye, J.; Kou, D.; Qiu, J.; Wang, J.; Tian, J.; Chen, X. Intraoperative imaging-guided cancer surgery: from current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics 2014, 4, 1072–1084. [Google Scholar] [CrossRef]
- Solidoro, R.; Centonze, A.; Miciaccia, M.; Baldelli, O.M.; Armenise, D.; Ferorelli, S.; Perrone, M.G.; Scilimati, A. Fluorescent imaging probes for in vivo ovarian cancer targeted detection and surgery. Med Res Rev 2024. [Google Scholar] [CrossRef]
- Wang, X.; Teh, C.S.C.; Ishizawa, T.; Aoki, T.; Cavallucci, D.; Lee, S.Y.; Panganiban, K.M.; Perini, M.V.; Shah, S.R.; Wang, H.; et al. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann Surg 2021, 274, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Broggi, M.; Schebesch, K.M.; Hohne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin Cancer Res 2018, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Pu, T.; Liu, Y.; Pei, Y.; Peng, J.; Wang, Z.; Du, M.; Liu, Q.; Zhong, F.; Zhang, M.; Li, F.; et al. NIR-II Fluorescence Imaging for the Detection and Resection of Cancerous Foci and Lymph Nodes in Early-Stage Orthotopic and Advanced-Stage Metastatic Ovarian Cancer Models. ACS Appl Mater Interfaces 2023, 15, 32226–32239. [Google Scholar] [CrossRef]
- Yang, R.Q.; Lou, K.L.; Wang, P.Y.; Gao, Y.Y.; Zhang, Y.Q.; Chen, M.; Huang, W.H.; Zhang, G.J. Surgical Navigation for Malignancies Guided by Near-Infrared-II Fluorescence Imaging. Small Methods 2021, 5, e2001066. [Google Scholar] [CrossRef]
- Feng, Z.; Tang, T.; Wu, T.; Yu, X.; Zhang, Y.; Wang, M.; Zheng, J.; Ying, Y.; Chen, S.; Zhou, J.; et al. Perfecting and extending the near-infrared imaging window. Light Sci Appl 2021, 10, 197. [Google Scholar] [CrossRef]
- Li, S.; Cheng, D.; He, L.; Yuan, L. Recent Progresses in NIR-I/II Fluorescence Imaging for Surgical Navigation. Front Bioeng Biotechnol 2021, 9, 768698. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, Z.; Luo, Y.; Kwok, R.T.K.; Zhao, Z.; Tang, B.Z. Recent advances of aggregation-induced emission materials for fluorescence image-guided surgery. Biomaterials 2022, 288, 121709. [Google Scholar] [CrossRef]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Qu, Z.; Shen, J.; Li, Q.; Xu, F.; Wang, F.; Zhang, X.; Fan, C. Near-IR emissive rare-earth nanoparticles for guided surgery. Theranostics 2020, 10, 2631–2644. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, Y.; Zheng, H.; Zhang, L.; Zhao, H.; Sang, X.; Xu, Y.; Lu, X. Evolutionary patterns and research frontiers in neoadjuvant immunotherapy: a bibliometric analysis. Int J Surg 2023, 109, 2774–2783. [Google Scholar] [CrossRef]
- Chen, C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A 2004, 101 Suppl 1, 5303–5310. [Google Scholar] [CrossRef]
- van der Vorst, J.R.; Schaafsma, B.E.; Hutteman, M.; Verbeek, F.P.; Liefers, G.J.; Hartgrink, H.H.; Smit, V.T.; Lowik, C.W.; van de Velde, C.J.; Frangioni, J.V.; et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013, 119, 3411–3418. [Google Scholar] [CrossRef] [PubMed]
- Ruscio, J. Taking Advantage of Citation Measures of Scholarly Impact: Hip Hip h Index! Perspect Psychol Sci 2016, 11, 905–908. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tosevska, A.; Klager, E.; Eibensteiner, F.; Laxar, D.; Stoyanov, J.; Glisic, M.; Zeiner, S.; Kulnik, S.T.; Crutzen, R.; et al. Virtual and Augmented Reality Applications in Medicine: Analysis of the Scientific Literature. J Med Internet Res 2021, 23, e25499. [Google Scholar] [CrossRef]
- Shinoda, J.; Yano, H.; Yoshimura, S.; Okumura, A.; Kaku, Y.; Iwama, T.; Sakai, N. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Technical note. J Neurosurg 2003, 99, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; Group, A.L.-G.S. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006, 7, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat Biomed Eng 2020, 4, 259–271. [Google Scholar] [CrossRef]
- Tan, L.; Wang, X.; Yuan, K.; Yin, T.; Du, R.; Shen, L.; Zhu, Z.; Yu, S.; Zhang, H.; Wang, G. Structural and temporal dynamics analysis on drug-eluting stents: History, research hotspots and emerging trends. Bioact Mater 2023, 23, 170–186. [Google Scholar] [CrossRef]
- Kleinberg, J. Bursty and hierarchical structure in streams. Data Min Knowl Disc 2003, 7, 373–397. [Google Scholar] [CrossRef]
- Nezhat, C.H.; Odunsi, T. Intelligent light and florescence-guided surgery augmenting the surgeon's visual perception. Fertil Steril 2020, 114, 980. [Google Scholar] [CrossRef]
- Hoffman, R.M. Application of GFP imaging in cancer. Lab Invest 2015, 95, 432–452. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, L.; Jiang, P.; Moossa, A.R.; Penman, S.; Hoffman, R.M. Dual-color fluorescence imaging distinguishes tumor cells from induced host angiogenic vessels and stromal cells. Proc Natl Acad Sci U S A 2003, 100, 14259–14262. [Google Scholar] [CrossRef] [PubMed]
- Sier, C.F.M.; Vahrmeijer, A.L. NIR Fluorescence Imaging of Colon Cancer With cRGD-ZW800-1-Response. Clin Cancer Res 2021, 27, 4938. [Google Scholar] [CrossRef]
- de Valk, K.S.; Handgraaf, H.J.; Deken, M.M.; Sibinga Mulder, B.G.; Valentijn, A.R.; Terwisscha van Scheltinga, A.G.; Kuil, J.; van Esdonk, M.J.; Vuijk, J.; Bevers, R.F.; et al. A zwitterionic near-infrared fluorophore for real-time ureter identification during laparoscopic abdominopelvic surgery. Nat Commun 2019, 10, 3118. [Google Scholar] [CrossRef]
- Fox, I.J.; Wood, E.H. Indocyanine green: physical and physiologic properties. Proc Staff Meet Mayo Clin 1960, 35, 732–744. [Google Scholar]
- Liberale, G.; Bohlok, A.; Bormans, A.; Bouazza, F.; Galdon, M.G.; El Nakadi, I.; Bourgeois, P.; Donckier, V. Indocyanine green fluorescence imaging for sentinel lymph node detection in colorectal cancer: A systematic review. Eur J Surg Oncol 2018, 44, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Leiloglou, M.; Kedrzycki, M.S.; Chalau, V.; Chiarini, N.; Thiruchelvam, P.T.R.; Hadjiminas, D.J.; Hogben, K.R.; Rashid, F.; Ramakrishnan, R.; Darzi, A.W.; et al. Indocyanine green fluorescence image processing techniques for breast cancer macroscopic demarcation. Sci Rep 2022, 12, 8607. [Google Scholar] [CrossRef]
- Alfano, M.S.; Molfino, S.; Benedicenti, S.; Molteni, B.; Porsio, P.; Arici, E.; Gheza, F.; Botticini, M.; Portolani, N.; Baiocchi, G.L. Intraoperative ICG-based imaging of liver neoplasms: a simple yet powerful tool. Preliminary results. Surg Endosc 2019, 33, 126–134. [Google Scholar] [CrossRef]
- Desmettre, T.; Devoisselle, J.M.; Mordon, S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv Ophthalmol 2000, 45, 15–27. [Google Scholar] [CrossRef]
- Okubo, K.; Uenosono, Y.; Arigami, T.; Matsushita, D.; Yanagita, S.; Kijima, T.; Amatatsu, M.; Ishigami, S.; Maemura, K.; Natsugoe, S. Quantitative assessment of fluorescence intensity of ICG in sentinel nodes in early gastric cancer. Gastric Cancer 2018, 21, 776–781. [Google Scholar] [CrossRef]
- Giordano, L.; Familiari, M.; Galli, A.; Howardson, B.; Bussi, M. Trimming of Facial Artery Myomucosal Flap (FAMM) using Indocyanine Green Fluorescence Video-Angiography: Operative Nuances. Ann Surg Oncol 2022, 29, 8361. [Google Scholar] [CrossRef] [PubMed]
- de Valk, K.S.; Deken, M.M.; Schaap, D.P.; Meijer, R.P.; Boogerd, L.S.; Hoogstins, C.E.; van der Valk, M.J.; Kamerling, I.M.; Bhairosingh, S.S.; Framery, B.; et al. Dose-Finding Study of a CEA-Targeting Agent, SGM-101, for Intraoperative Fluorescence Imaging of Colorectal Cancer. Ann Surg Oncol 2021, 28, 1832–1844. [Google Scholar] [CrossRef]
- Nishio, N.; van den Berg, N.S.; van Keulen, S.; Martin, B.A.; Fakurnejad, S.; Zhou, Q.; Lu, G.; Chirita, S.U.; Kaplan, M.J.; Divi, V.; et al. Optimal Dosing Strategy for Fluorescence-Guided Surgery with Panitumumab-IRDye800CW in Head and Neck Cancer. Mol Imaging Biol 2020, 22, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Ma, H.; Zhu, S.; Lau, J.; Ma, R.; Liu, Y.; Lin, L.; Chandra, S.; Wang, S.; Zhu, X.; et al. Multiplexed NIR-II Probes for Lymph Node-Invaded Cancer Detection and Imaging-Guided Surgery. Adv Mater 2020, 32, e1907365. [Google Scholar] [CrossRef]
- Sar, D.; Ostadhossein, F.; Moitra, P.; Alafeef, M.; Pan, D. Small Molecule NIR-II Dyes for Switchable Photoluminescence via Host -Guest Complexation and Supramolecular Assembly with Carbon Dots. Adv Sci (Weinh) 2022, 9, e2202414. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Zhao, L.; Wang, Z.G.; Liu, S.L.; Pang, D.W. Near-Infrared-II Quantum Dots for In Vivo Imaging and Cancer Therapy. Small 2022, 18, e2104567. [Google Scholar] [CrossRef]
- Guo, B.; Feng, Z.; Hu, D.; Xu, S.; Middha, E.; Pan, Y.; Liu, C.; Zheng, H.; Qian, J.; Sheng, Z.; et al. Precise Deciphering of Brain Vasculatures and Microscopic Tumors with Dual NIR-II Fluorescence and Photoacoustic Imaging. Adv Mater 2019, 31, e1902504. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Goh, C.C.; Ng, L.G.; Liu, B. NIR-II-Excited Intravital Two-Photon Microscopy Distinguishes Deep Cerebral and Tumor Vasculatures with an Ultrabright NIR-I AIE Luminogen. Adv Mater 2019, 31, e1904447. [Google Scholar] [CrossRef]
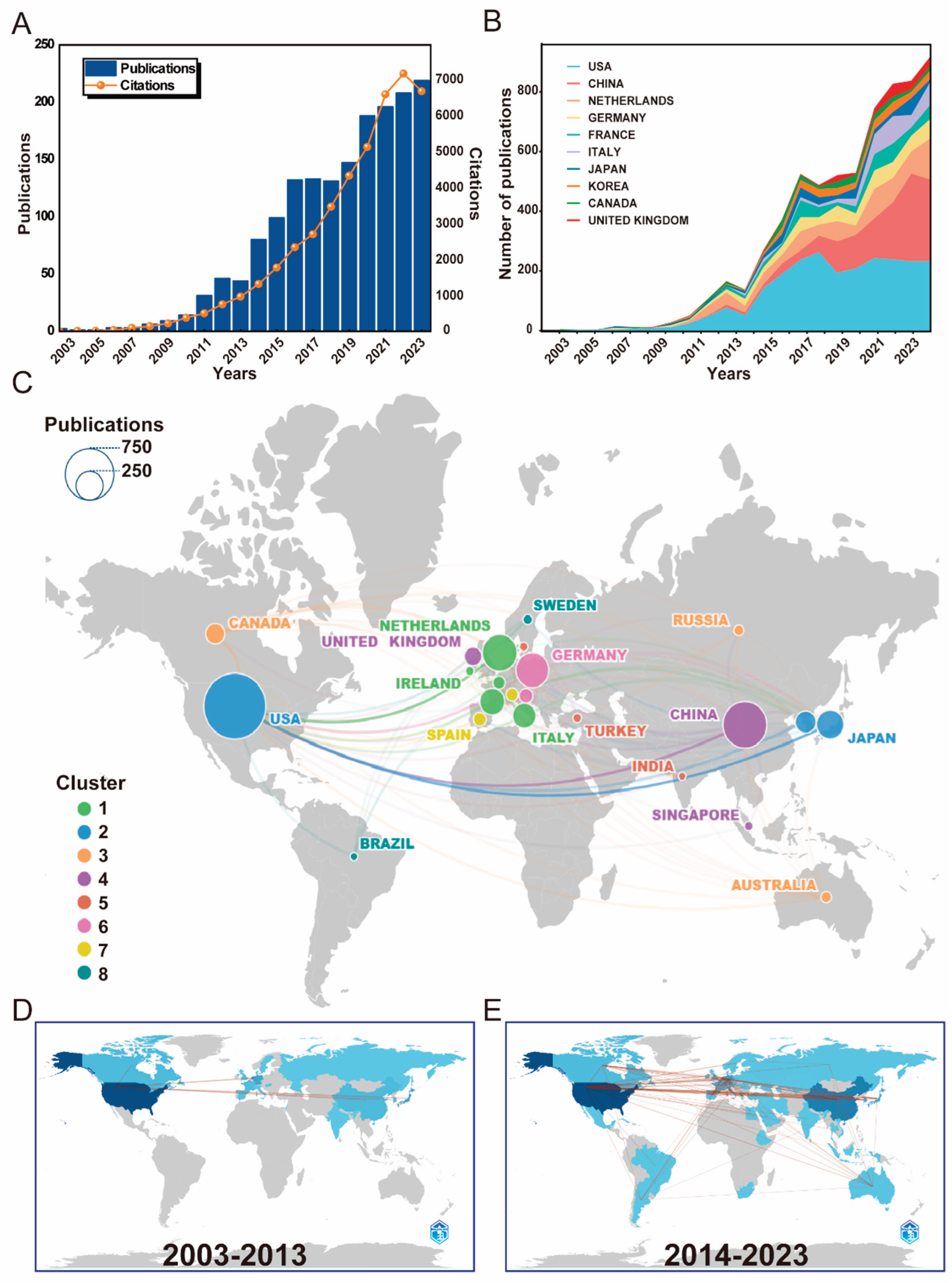
| Rank | Country | Articles | Freq | MCP Ratio1 | Total Citations |
Average article citations |
|---|---|---|---|---|---|---|
| 1 | USA | 586 | 0.34389671 | 0.34300341 | 17986 | 30.60 |
| 2 | China | 313 | 0.18368545 | 0.16613419 | 7793 | 24.80 |
| 3 | Netherlands | 176 | 0.10328638 | 0.55113636 | 5855 | 33.30 |
| 4 | Germany | 122 | 0.07159624 | 0.2704918 | 4719 | 41.40 |
| 5 | France | 79 | 0.0463615 | 0.36708861 | 1354 | 18.30 |
| 6 | Italy | 66 | 0.03873239 | 0.27272727 | 1286 | 19.20 |
| 7 | Japan | 58 | 0.03403756 | 0.12068966 | 1511 | 26.10 |
| 8 | Korea | 48 | 0.02816901 | 0.27083333 | 788 | 16.40 |
| 9 | United Kingdom | 35 | 0.02053991 | 0.2 | 483 | 13.80 |
| 10 | Canada | 34 | 0.01995305 | 0.38235294 | 778 | 22.90 |
| 11 | Austria | 26 | 0.01525822 | 0.61538462 | 215 | 8.30 |
| 12 | Spain | 19 | 0.01115023 | 0.15789474 | 393 | 26.20 |
| 13 | Belgium | 14 | 0.00821596 | 0.42857143 | 274 | 19.60 |
| 14 | Denmark | 13 | 0.00762911 | 0.07692308 | 225 | 17.30 |
| 15 | Ireland | 13 | 0.00762911 | 0.23076923 | 136 | 10.50 |
| 16 | Switzerland | 12 | 0.00704225 | 0.33333333 | 252 | 21.00 |
| 17 | RUSSIA | 10 | 0.00586854 | 0.2 | 133 | 13.30 |
| 18 | Sweden | 8 | 0.00469484 | 0 | 100 | 12.50 |
| 19 | Turkey | 7 | 0.00410798 | 0.28571429 | 101 | 14.40 |
| 20 | Australia | 6 | 0.00352113 | 0.66666667 | 31 | 5.20 |
| Rank | Year | Source | First Author | Paper Title | TC1 | TC per Year | Normalized TC |
|---|---|---|---|---|---|---|---|
| 1 | 2006 | Lancet Oncol | Walter Stumme | Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial | 2344 | 123.37 | 2.13 |
| 2 | 2006 | Nano Lett | Weibo Cai | Peptide-Labeled Near-Infrared Quantum Dots for Imaging Tumor Vasculature in Living Subjects | 807 | 42.47 | 0.73 |
| 3 | 2020 | Nat Biomed Eng | Zhenhua Hu | First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows | 527 | 105.40 | 21.46 |
| 4 | 2014 | Sci Transl Med | Evan Phillips | Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe | 527 | 47.91 | 10.70 |
| 5 | 2010 | PNAS | Emilia S Olson | Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases | 462 | 30.80 | 5.44 |
| 6 | 2018 | Nat Commun | Peiyuan Wang | NIR-II nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer | 319 | 45.57 | 9.74 |
| 7 | 2015 | Clin Cancer Res | Eben L Rosenthal | Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer | 317 | 31.70 | 8.14 |
| 8 | 2011 | Eur Urol | Henk G van der Poel | Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: clinical proof of concept of an integrated functional imaging approach using a multimodal tracer | 257 | 18.36 | 4.23 |
| 9 | 2009 | J Surg Oncol | Kunihito Gotoh | A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation | 241 | 15.06 | 3.30 |
| 10 | 2013 | Cancer | Joost R van der Vorst | Near-infrared fluorescence-guided resection of colorectal liver metastases | 224 | 18.67 | 4.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





