Introduction
Lung cancer is one of the most common and deadly malignant tumors, with high morbidity and mortality rates [
1,
2]. The pathogenesis of lung cancer is highly complex and diverse, influenced by various factors, among which the immune microenvironment and immune evasion have become key areas of research and challenges [
3]. Programmed cell death protein 1 (PD-1) and its natural ligand PD-L1 are the most studied immune checkpoints [
4,
5]. It has been confirmed that PD-L1/PD-1 plays a critical role in the occurrence and development of lung cancer. Immune therapy drugs targeting PD-L1 and PD-1 have been widely applied in the treatment of lung cancer, especially with groundbreaking results in the treatment of advanced-stage lung cancer patients, showing great potential [
6,
7]. However, unfortunately, not all lung cancer patients respond well to immune checkpoint inhibitors targeting PD-L1/PD-1, and resistance to PD-L1/PD-1 inhibitors significantly limit their clinical application and therapeutic efficacy [
8]. Both primary and acquired resistance are closely related to intrinsic and extrinsic tumor mechanisms and have become the biggest clinical obstacles for achieving long-term survival in patients with advanced or metastatic lung cancer [
9,
10].
Longchai Jiangxue Fang (LC) is a traditional Chinese medicine compound that has shown potential and effectiveness in the treatment of myeloproliferative neoplasms (MPN). MPN is a group of rare blood cancers, including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). Longchai Jiangxue Fang may exert its therapeutic effects by modulating metabolism and influencing relevant molecular targets [
11]. One study explored the molecular targets and mechanisms of Longchai Jiangxue Fang in treating MPN, based on genomic and proteomic analysis. This suggests that researchers are investigating how Longchai Jiangxue Fang affects the disease progression of MPN at the molecular level [
11]. Additionally, a clinical study has been registered with the China Clinical Trial Registry, aiming to evaluate the clinical efficacy of Longchai Jiangxue Granules in treating MPN and identify the biological characteristics of the effective patient population.
Materials and Methods
Cells and Culture
Human lung cancer cell lines A549 and H1299, mouse lung cancer cell line LLC were purchased from the National Infrastructure of Cell Line Resource (Shanghai, China). All cells were incubated in RPMI-1640 medium with 10% fetal bovine serum (FBS, Hyclone, USA) and 1% penicillin/streptomycin (Gibco, USA) at 37°C in a humidified atmosphere. T cells were isolated from peripheral blood using T cell isolation kit (StemCell, China).
Cell viability and Proliferation Detection
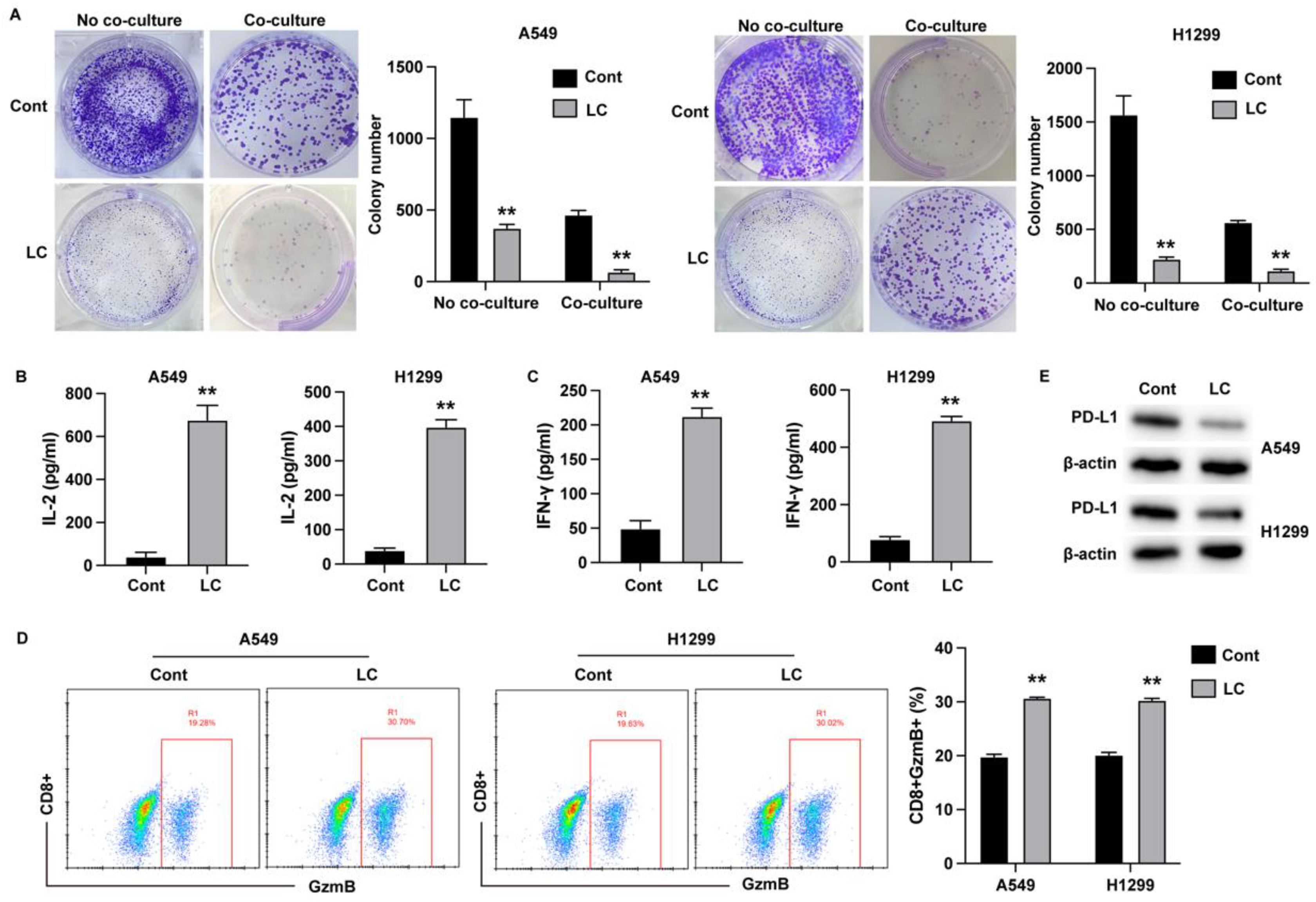
Cell viability was assessed using the Cell Counting Kit-8 (CCK-8, SolarBio, China) according to the manufacturer’s instructions. Lung cancer cells were seeded into a 96-well plate at a density of 5,000 cells per well and incubated at 37°C for the specified time points. After incubation, CCK-8 reagent was added and the cells were further incubated for 2 hours. The absorbance at 450 nm was measured using a microplate reader. For the colony formation assay, cells were seeded into a 6-well plate and incubated for 10 days. Visible colonies were stained with crystal violet for 20 minutes, and images were captured using a digital camera.
Western Blotting Assay
Cell pellets were lysed using RIPA buffer, and the protein concentration was determined using the BCA protein assay kit. Protein samples containing 40 μg of protein were separated by SDS-PAGE and transferred onto PVDF membranes, which were then blocked with 5% skim milk for 2 hours. The membranes were incubated overnight at 4°C with primary antibodies targeting PD-L1, AKT, pAKT, PI3K, pPI3K, and β-actin. After washing, the membranes were incubated with HRP-conjugated secondary antibodies and developed using ECL reagent for visualization
Quantitative Real Time PCR (qPCR)
Total RNA of cancer cells was extracted using Trizol reagent (Qiagen, USA). The RNA (1 µg) was then reverse transcribed into cDNA using a Reverse Transcription kit (Qiagen, USA). PCR amplification was performed using SYBR Green qPCR mix (Thermo, USA).
T Cell-Cancer Cell Co-Culture System
Human peripheral blood samples were collected from donors, and peripheral blood mononuclear cells (PBMCs) were isolated using the Ficoll method. For T cell activation, PBMCs were incubated with Dynabeads Human T-Activator CD3 and CD28 (Gibco, USA) according to the manufacturer’s protocol for 3 days. For co-culture experiments, T cells and lung cancer cells were placed in the upper and lower chambers of a Transwell system, respectively, at a T cell to cancer cell ratio of 10:1, and cultured for 3 days. T cell activation was assessed by incubating the cells with anti-CD8, and anti- GzmB antibodies, followed by analysis using a flow cytometer (BD Bioscience).
Enzyme-Linked Immunosorbent Assay (ELISA)
The culture medium was collected and centrifuged at 3500 rpm for 15 minutes to obtain the serum samples. The levels of inflammatory factors were measured by ELISA using commercial kits (Elabscience, USA) following the manufacturer’s instructions.
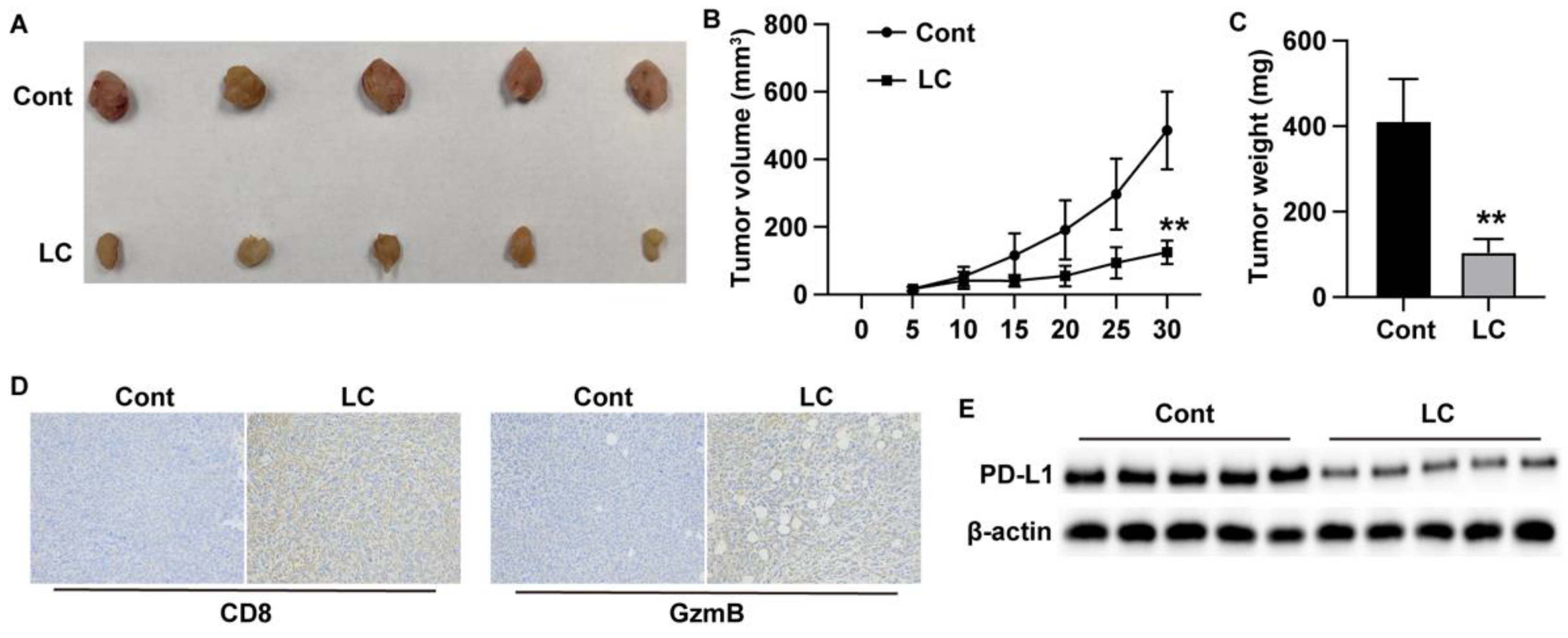
Xenograft Model
The animal experiments were approved by the Ethics Committee of Kunshan Hospital of Traditional Chinese Medicine. Male C57BL/6 mice, aged 4 to 6 weeks, were purchased from Vital River Laboratory (China). LLC cells (5 × 10⁶ cells per site) were suspended in 50 µl saline and injected into the fat pad on the back of the mice. Tumor size was measured and calculated every three days. After 30 days of feeding, the tumors were harvested and analyzed through western blot and histological staining.
IHC staining
Paraffin-embedded tissue sections were deparaffinized and blocked with goat serum. After incubation with primary antibodies against CD8 and GzmB, the sections were incubated with HRP-conjugated secondary antibodies and developed using DAB for visualization. Images were captured using a light microscope (Leica, Germany).
Network Pharmacology Analysis
The target proteins of LC were predicted using the CTD database (
https://ctdbase.org/). Relevant information on LC was retrieved from GeneCard (
https://www.genecards.org), DisGENET database (
https://www.disgenet.org), and the GEO database by searching the keyword. Duplicate and false-positive genes were excluded to identify disease targets closely associated with lung cancer disease processes. A "Compound-Target" network was constructed to link drug targets with disease targets using Cytoscape 3.7.2 software. The "NetworkAnalyzer" function was used for network topology analysis. Protein-protein interaction (PPI) analysis was performed using the STRING database (
https://cn.string-db.org), and the results were visualized in Cytoscape 3.7.2 software. Additionally, KEGG signaling pathway enrichment was conducted using the DAVID database (
https://david.ncifcrf.gov/).
Statistics
All statistical analyses were carried out using SPSS and GraphPad Prism 7.0 software. Comparisons between experimental groups were performed using two-sided Student’s t-test and one-way ANOVA followed by Dunnett’s multiple comparison test. A p-value of < 0.05 was considered statistically significant.
Results
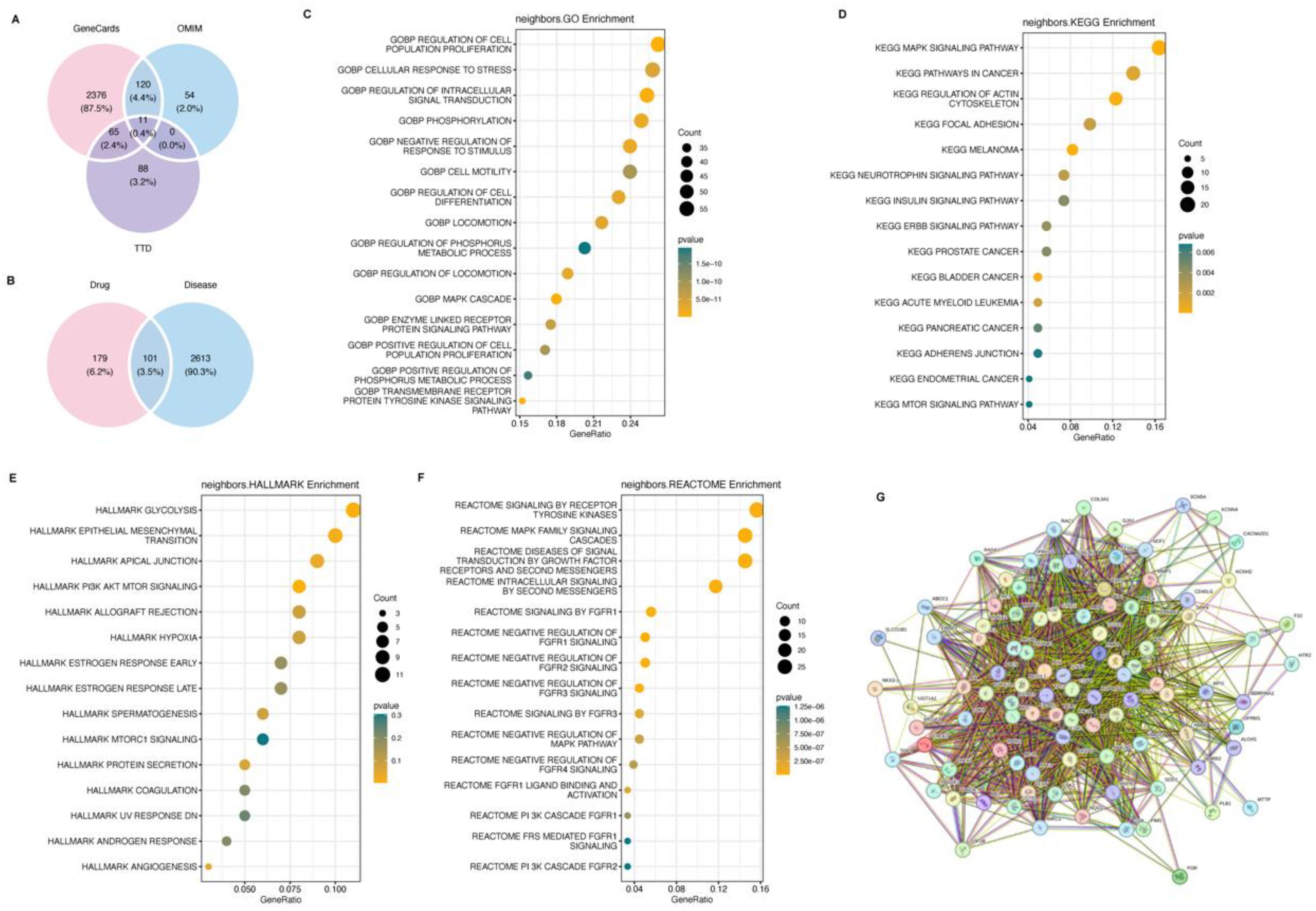
Network Pharmacology Analysis of Potential Targeting Signal Pathway of Longchai Jiangxue Prescription in Lung Cancer
Using Genecard, TTD and OMIM, 2572, 164 and 185 disease targets were obtained, respectively (
Figure 3A), among which 101 were correlated with lung cancer-related targets (
Figure 3B). We further displayed the GO, KEGG pathways and halllmarks that altered in lung cancer cells under LC treatment, which revealed PI3K-AKT as the most abundant signaling pathway (
Figure 3C-G).
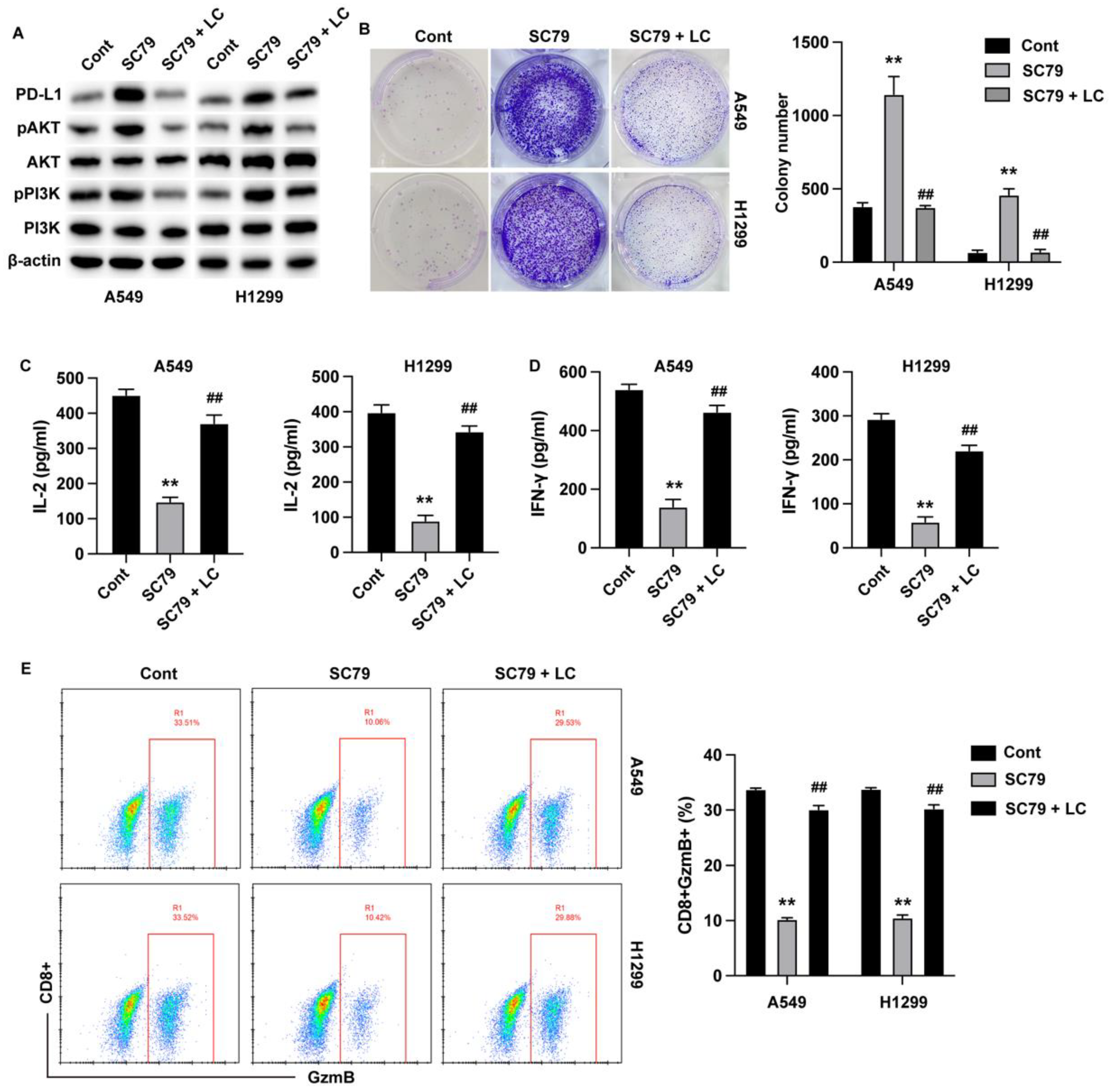
Longchai Jiangxue Prescription Regulates the Killing Effect of T Cells on Lung Cancer Cells Through AKT Signaling Pathway
To validate the regulatory role of AKT signaling in LC-treated lung cancer immune evasion, we treated lung cancer cells with Akt activator SC79 and LC in the co-culture model. Cellular experiments confirmed that the phosphorylation of PI3K and AKT was repressed by LC treatment in both A549 cells and H1299 cells (
Figure 4A). Activation of AKT signaling boosted proliferation of lung cancer cells (
Figure 4B), repressed the production of immune cytokines (
Figure 4C and D) and inhibited the activation of cytotoxic T cells (
Figure 4E), whereas treatment with LC abolished these effects.
Discussion
Immunotherapy for lung cancer has made significant progress in recent years, particularly with the application of immune checkpoint inhibitors (such as PD-1/PD-L1 inhibitors) in patients with advanced non-small cell lung cancer (NSCLC). However, despite the breakthrough efficacy demonstrated in some patients, it still faces many clinical challenges and issues. Approximately half of the patients do not respond to immunotherapy, and some may develop resistance even after an initial response. Tumor cells evade immune system surveillance through various mechanisms, including activation of immune checkpoints, recruitment of immune-suppressive cells, and changes in the tumor microenvironment. Therefore, immunotherapy for lung cancer faces numerous challenges in clinical application. Exploring the efficient therapeutic reagent and strategy is a critical issue for lung cancer therapy.
Traditional Chinese medicine has gained extensive research and application in cancer treatment. Many Chinese herbal formulations have been found to possess anti-cancer properties and can exert therapeutic effects through various mechanisms. For example, Xiaochaihu Decoction can regulate the immune system and enhance anti-tumor immune responses. Studies have shown that herbs such as Bupleurum (Chaihu) and Scutellaria (Huangqin) can enhance the body’s immune response by modulating the activity of T cells, natural killer (NK) cells, and macrophages [
12,
13]. Shenling Baizhu Powder is widely used to treat symptoms associated with cancer-related weakness, fatigue, and loss of appetite, particularly in patients with gastrointestinal cancers (such as gastric and colorectal cancer). By enhancing the body’s immune function, it increases the ability to recognize and eliminate tumor cells [
14,
15]. Huangqi Decoction is commonly used to boost the immune system in cancer patients, especially when used in combination with chemotherapy, to reduce side effects and improve therapeutic efficacy [
16,
17]. Astragalus (Huangqi) enhances the body’s immune response to inhibit tumor cell growth and spread. The active components in Astragalus, such as Astragalus polysaccharides, can increase the activity of T cells, B cells, and NK cells, thereby improving the body’s anti-tumor capabilities [
18]. In this study, we found that Longchai Jiangxue formulation exhibited notable anti-growth and immune activation effects to lung cancer cells both in vitro and in vivo.
As reported by previous study, UPLC/Q-TOF-MS/MS, systems pharmacology, and molecular biology analysis revealed the active ingredients and mechanisms of Longchai Jiangxue Fang in treating polycythemia vera (PV). In this study, 84 compounds were identified, and 143 potential targets related to the disease and drug were predicted. Key genes such as MTOR, HIF1A, JAK2, VEGFA, STAT3, AKT1, TERT, and MAPK1 appeared in the protein-protein interaction (PPI) network, indicating that these genes may be involved in the treatment of PV [
11]. These studies suggest that Longchai Jiangxue Fang may exert therapeutic effects by influencing multiple molecular targets and signaling pathways, especially in the treatment of MPN-related tumors. However, further clinical research and scientific validation are needed to clarify its effectiveness and mechanisms in tumor treatment. Consistently, our current work demonstrated that Longchai Jiangxue Fang treatment promoted the T cell activation and toxicity towards lung cancer cells and suppressed the expression of PD-L1 in lung cancer cells. Further network pharmacology analysis demonstrated that AKT signaling is a promising signaling pathway that involved in the immunotherapy of Longchai Jiangxue Fang against lung cancer cells. We further utilized AKT signaling activator in combination with Longchai Jiangxue Fang, and verified that Longchai Jiangxue Fang treatment notably abolished the lung cancer growth that activated by AKT.
In conclusion, our findings provided novel evidence for the anti-tumor function of Longchai Jiangxue Fang, and further mechanical study indicated that Longchai Jiangxue Fang possibly repress lung cancer cell growth via targeting the PD-L1 expression and Akt signaling.
References
- Chen P, Liu Y, Wen Y, et al., Non-small cell lung cancer in China, Cancer Commun (Lond)[J], 2022, 42 (10): 937-970. [CrossRef]
- Cho J W, Hong M H, Ha S J, et al., Genome-wide identification of differentially methylated promoters and enhancers associated with response to anti-PD-1 therapy in non-small cell lung cancer, Exp Mol Med[J], 2020, 52 (9): 1550-1563. [CrossRef]
- Lin M, Huang Z, Chen Y, et al., Lung cancer patients with chronic obstructive pulmonary disease benefit from anti-PD-1/PD-L1 therapy, Front Immunol[J], 2022, 13: 1038715. [CrossRef]
- Han Y, Liu D, Li L, PD-1/PD-L1 pathway: current researches in cancer, Am J Cancer Res[J], 2020, 10 (3): 727-742.
- Pauken K E, Torchia J A, Chaudhri A, et al., Emerging concepts in PD-1 checkpoint biology, Semin Immunol[J], 2021, 52: 101480. [CrossRef]
- Huang C, Ren S, Chen Y, et al., PD-L1 methylation restricts PD-L1/PD-1 interactions to control cancer immune surveillance, Sci Adv[J], 2023, 9 (21): eade4186. [CrossRef]
- Reck M, Remon J, Hellmann M D, First-Line Immunotherapy for Non-Small-Cell Lung Cancer, J Clin Oncol[J], 2022, 40 (6): 586-597. [CrossRef]
- Rosner S, Reuss J E, Forde P M, PD-1 Blockade in Early-Stage Lung Cancer, Annu Rev Med[J], 2019, 70: 425-435. [CrossRef]
- Liu C, Zheng S, Wang Z, et al., KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer, Cancer Commun (Lond)[J], 2022, 42 (9): 828-847. [CrossRef]
- Rizvi N A, Hellmann M D, Snyder A, et al., Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer, Science[J], 2015, 348 (6230): 124-128. [CrossRef]
- Ming J, Liu W, Wu H, et al., The active ingredients and mechanisms of Longchai Jiangxue Formula in treating PV, based on UPLC/Q-TOF-MS/MS, systematic pharmacology, and molecular biology validation, Biomed Pharmacother[J], 2021, 140: 111767. [CrossRef]
- Li J, Xie M, Gan Y, [Effect of Xiaochaihu decoction and different herbal formulation of component on inhibiting H22 liver cancer in mice and enhancing immune function], Zhongguo Zhong Yao Za Zhi[J], 2008, 33 (9): 1039-1044.
- Zeng Y, Xiao S, Yang L, et al., Systematic analysis of the mechanism of Xiaochaihu decoction in hepatitis B treatment via network pharmacology and molecular docking, Comput Biol Med[J], 2021, 138: 104894. [CrossRef]
- Deng X, Zhang C, Yang Y, et al., Shenling Baizhu Decoction (SLBZD) may play a synergistic role of tirelizumab in the treatment of colorectal cancer by influencing the imbalance of colon flora and Tumor microenvironment, J Cancer[J], 2024, 15 (1): 30-40. [CrossRef]
- Ming W, Yun Z, Clinical value of modified Shenling Baizhu powder in treating targeted therapy-induced diarrhea in non-small cell lung cancer, J Tradit Chin Med[J], 2024, 44 (5): 1000-1005. [CrossRef]
- Li L, Lu Y, Liu Y, et al., Network Pharmacology Analysis of Huangqi Jianzhong Tang Targets in Gastric Cancer, Front Pharmacol[J], 2022, 13: 882147. [CrossRef]
- Zheng W, Wu J, Peng Y, et al., Tumor-Associated Neutrophils in Colorectal Cancer Development, Progression and Immunotherapy, Cancers (Basel)[J], 2022, 14 (19). [CrossRef]
- Bian Y, Wang G, Zhou J, et al., Astragalus membranaceus (Huangqi) and Rhizoma curcumae (Ezhu) decoction suppresses colorectal cancer via downregulation of Wnt5/β-Catenin signal, Chin Med[J], 2022, 17 (1): 11. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).