1. Introduction
Although immune checkpoint inhibitors (ICIs) alone or in combination with platinum-based chemotherapy have demonstrated survival benefits in patients with metastatic, non-oncogene addicted non-small cell lung cancer (NSCLC), disease may still ultimately progress. Before the advent of ICIs in the front-line setting, docetaxel (either alone or in combination with antiangiogenic drugs like nintedanib for adenocarcinoma histology or ramucirumab for all histologies) was considered the standard of care after disease progression on platinum-based chemotherapy. Other single-agent chemotherapies such as gemcitabine, vinorelbine or pemetrexed (for non-squamous histology, if not already included in first-line combinations) are often used in clinical practice as an alternative to docetaxel as second-line treatment or beyond. However, efficacy of single-agent chemotherapy in pre-treated patients remains disappointing. Moreover, data on single-agent chemotherapy primarily refer to patients pre-treated with platinum-based chemotherapy, while data for patients pre-treated with ICIs are extremely limited. Therefore, clinical research is actively evaluating innovative combinations compared to standard chemotherapy for patients whose disease progresses after ICIs +/- platinum-based chemotherapy.
In this study, we evaluated all randomized controlled trials (RCTs) that evaluated new combination treatments as second-line or beyond. Our analysis was aimed at assessing whether these combination treatments can confer a survival benefit in terms of overall survival (OS) compared with the standard-of-care.
Wang et al. [
1] have recently published a network meta-analysis on this topic in which all assessments of OS, typically reported in a Forest plot, were binary and therefore did not consider the timing at which events occurred and, accordingly, did not consider the follow-up duration of individual trials.
In the present study, we used a relatively new evidence-based method (the IPDfromKM method [
2]) to provide specific statistical support for the above conclusion given that the presence of a survival benefit with combination therapies is a matter of controversy. The IPDfromKM method operates by reconstructing individual patient data from the information published in clinical trials; in particular, an artificial intelligence (AI) software extracts as much information as possible from the graphs of the Kaplan-Meier curves thus generating these databases of reconstructed patients. This method has already found numerous successful applications, particularly in the fields of oncology [
3] and cardiology [
4].
2. Materials and methods
Literature search. We searched PubMed, Scopus and EMBASE to identify randomized controlled trials (RCTs) that met the eligibility criteria for the analysis. The final search was conducted on 10th of August 2024. The search term was constructed as follows: ["non-small cell lung cancer" OR "NSCLC" OR "non-small-cell lung cancer") AND ("advanced" OR "locally advanced" OR "metastatic") AND ("failure" OR "progression" OR "relapse" OR "after" OR "second-line therapy" OR "subsequent therapy” + filter “published in the last 5 years”]. The selection of articles was conducted in accordance with the PRISMA algorithm [
5].
Our inclusion criteria were represented by patients with non-oncogenic driven, unresectable locally advanced or metastatic NSCLC who experienced disease progression after immune checkpoint inhibitors and chemotherapy. The endpoint of our analysis was OS. We included all RCT that tested a combination treatment in the experimental group and used either docetaxel or a similar regimen of chemotherapy in the control group. Owing to the method of our analysis aimed at reconstructing individual patient data, another inclusion criterion was the availability of a Kaplan-Meier graph comparing the two above-mentioned treatment options based on the endpoint of OS.
Reconstruction of individual patient data from Kaplan-Meier curves and statistical analysis.
Our analysis, performed by application of the online version of the IPDfromKM method, included a first phase in which the graph of each Kaplan-Meier curve was digitized using Webplotdigitizer (version 4 online;
https://apps.automeris.io/ accessed 10 August 2024), and a second phase in which the AI algorithm reconstructed individual patient data (separately for each curve evaluated in the analysis) from the x-y coordinates deriving from the digitized KM curves [
2] (
https://www.trialdesign.org/one-page-shell.html#IPDfromKM, version 1.2.3.0, accessed 10 August 2024) . Once these databases of reconstructed patients had been created, indirect comparisons were made between the combination treatments and the standard of care, using the same statistical tests (e.g. Cox multiple regression model) as in studies based on “real” patients.
Design of the analysis. Regarding the design of our analysis, since five trials were selected as source of clinical material, we firstly selected OS as the endpoint of the statistical analysis and then performed an indirect comparison between the five arms pooled together of the studies treated with a combination treatment versus the five arms of the same studies treated with the standard of care (docetaxel or a similar regimen of chemotherapy). The hazard ratio (HR), with 95% confidence interval (CI), was the parameter for testing the superiority of combination treatments versus standard of care. Finally, the five control arms of the included trials were subjected to an assessment of cross-trial heterogeneity, which was based on Wald’s test and the likelihood ratio test. In the present investigation, we used three statistical packages ("survival," "survminer," "survRM2," and "readxl") of the R-platform (version 4.3.2).
3. Results
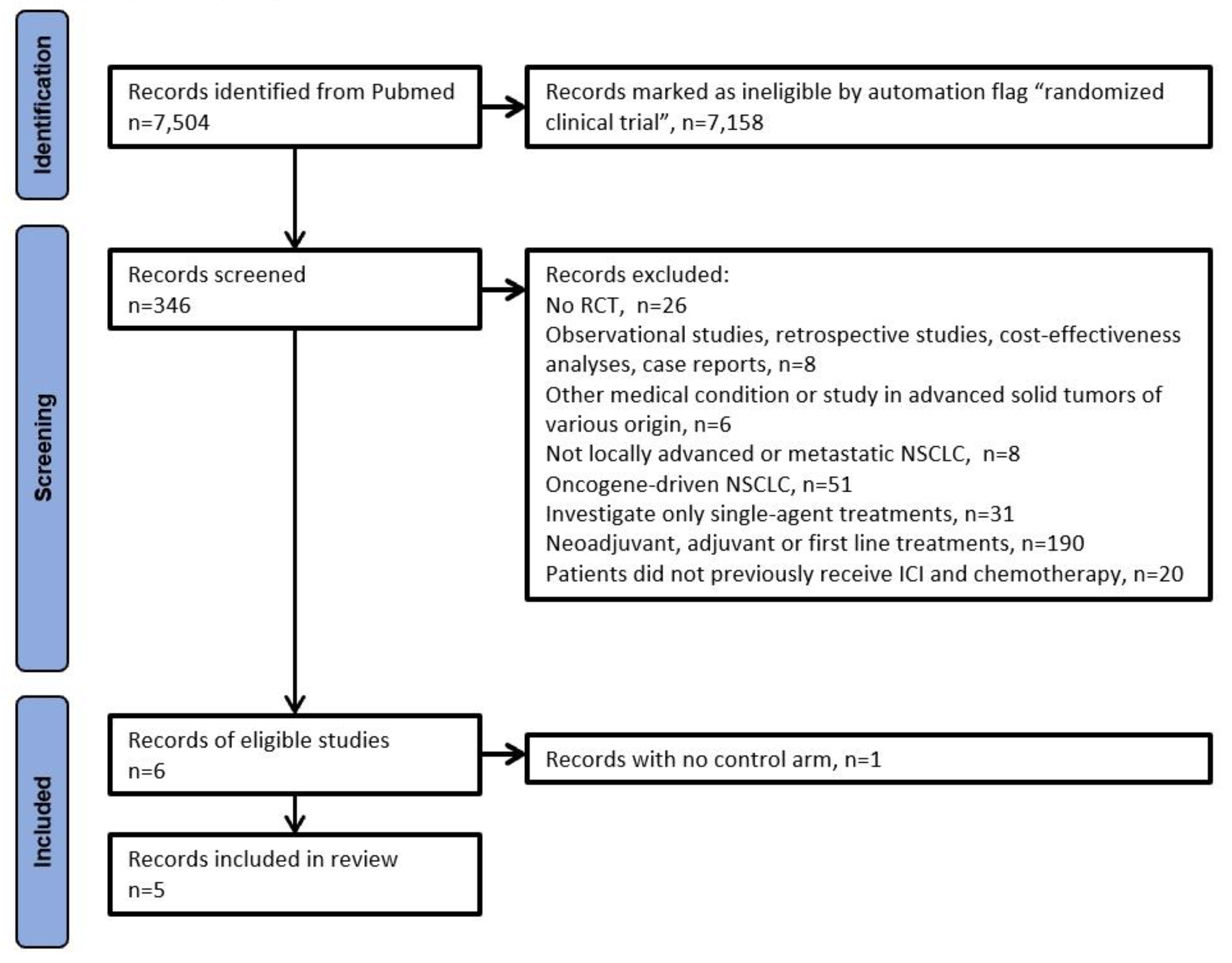
The PRISMA algorithm was used to select the trials eligible for our analysis. After selecting only RCTs according to the design of our analysis, 6 eligible were identified [
6,
7,
8,
9,
10,
11];
Figure 1 shows the PRISMA algorithm. We excluded the trials by Schoenfeld et al. [
11] owing to the absence of a control arm with standard chemotherapy; as a result, five RCTs were included in our analysis (
Table 1).
The combination regimens evaluated in these trials included pembrolizumab + chemotherapy, pembrolizumab + ramucirumab, sitravatinib + nivolumab, atezolizumab + cabozantinib, and canakinumab + docetaxel.
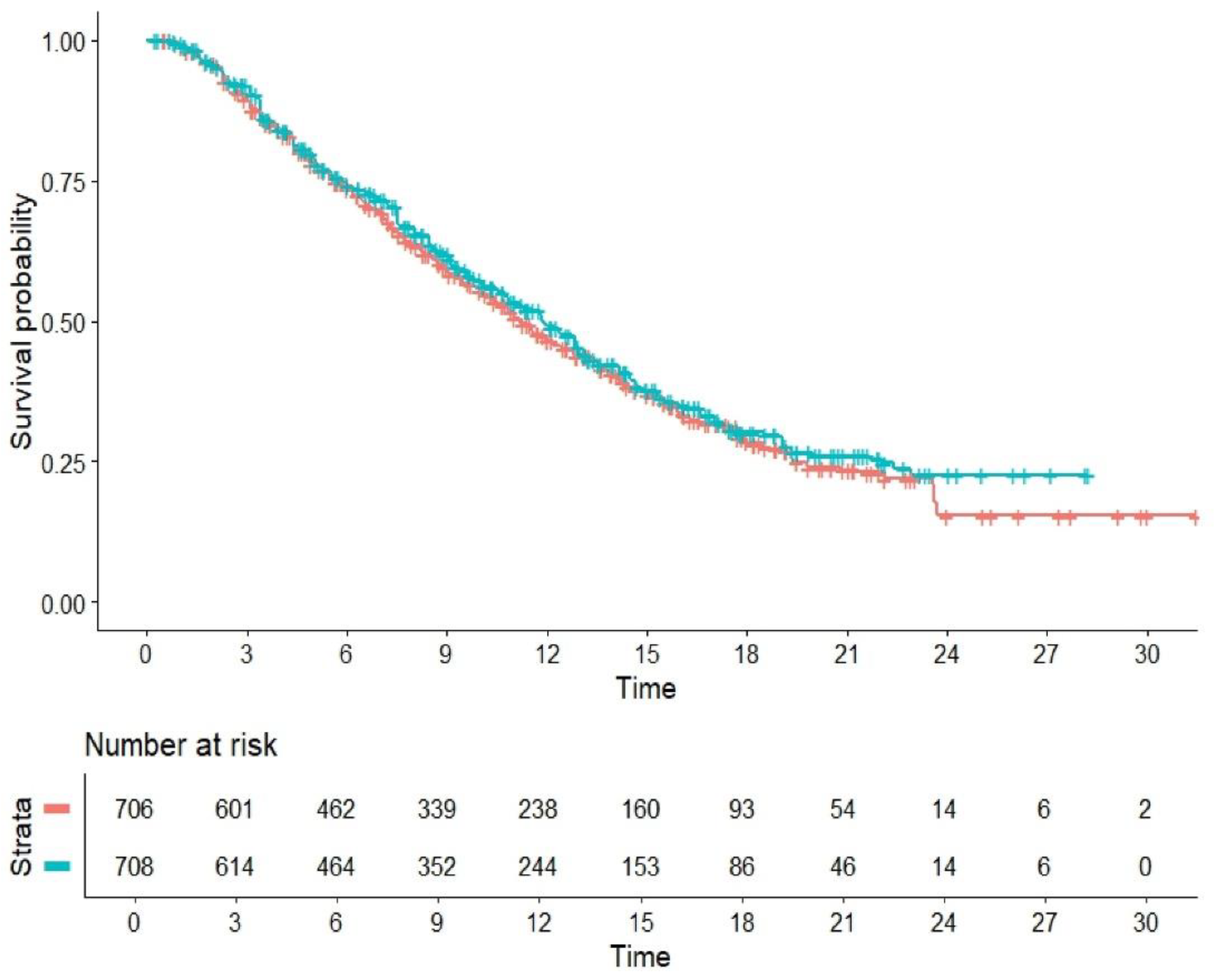
Figure 2 describes the results of our main analysis, which shows that the pooled curves of active and control arms are largely superimposed with one another. The HR for the comparison of combination treatments vs standard of care was HR=1.066 (95%CI, 0.9311 to 1.221; P=0.35). Among the five control arms (
Figure 3), the level of cross-trial heterogeneity was remarkably low (likelihood ratio test= 3.76 on 4 df; p=0.40; Wald test = 3.83 on 4 df, p=0.40).
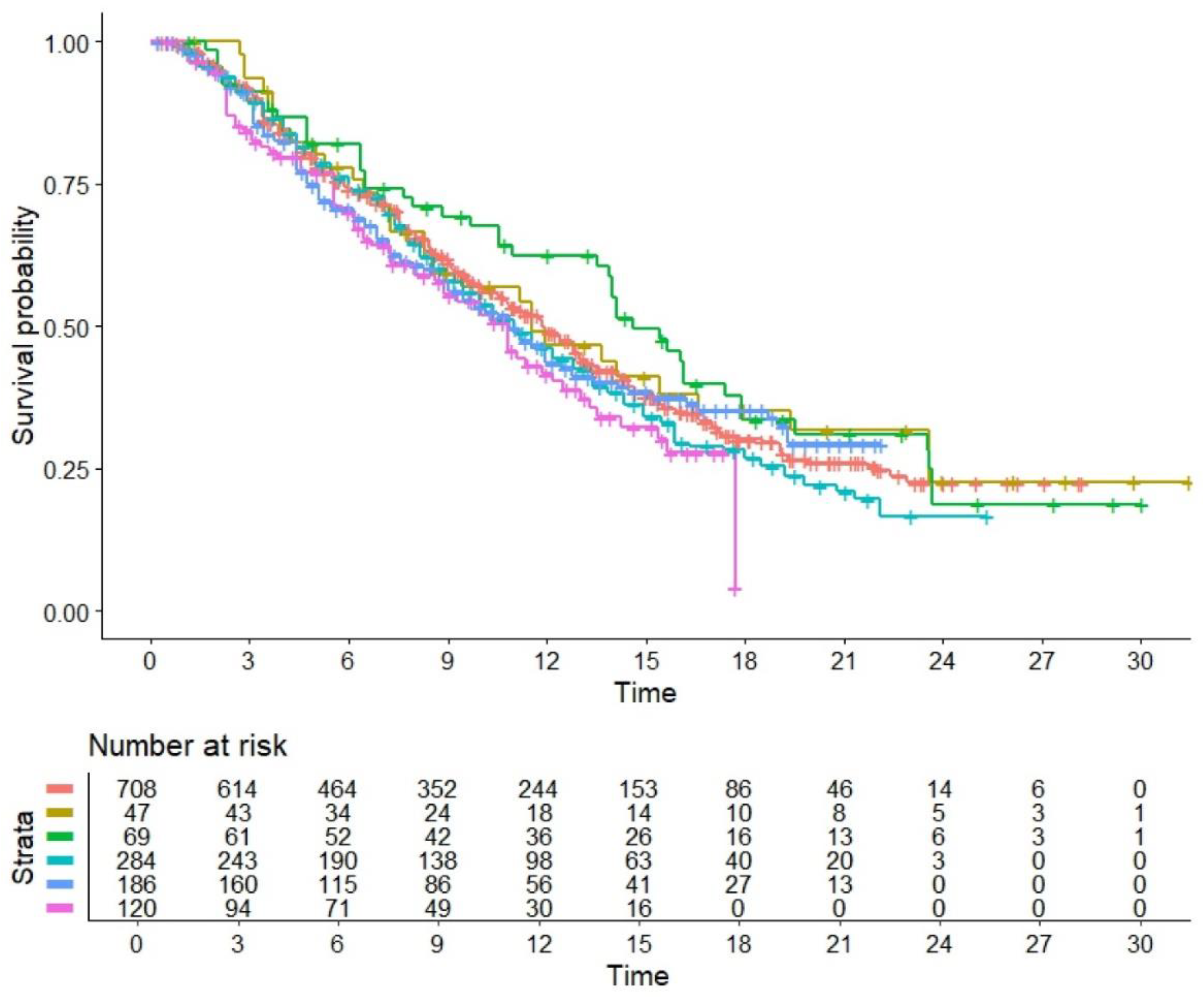
Finally,
Figure 4 compares the OS pattern between each of the 5 treatment groups with the 5 control arms pooled together. Among the 5 treatment groups, the arm of the trial by Paz-Ares et al. [
10] (canakinumab + docetaxel) showed the worse OS pattern (HR=1.33, 95%CI 1.03 - 1.71) while the study by Reckamp et.al. [
7] investigating ramucirumab + pembrolizumab demonstrated the best OS (HR=0.84, 95%CI 0.61 to 1.15); the other RCTs showed values of HR ranging from 0.91 to 1.11, but none of them reached the statistical nor the clinical significance compared to the standard of care. It should be noted that, unlike the other 4 trials, the trial by Borghaei et al. [
8] included exclusively patient with non-squamous histology; however, both
Figure 3 and
Figure 4 show that this difference had no impact on the pattern of OS.
4. Discussion
The availability of different treatment options for patients with NSCLC after failure of previous ICIs and chemotherapy offers a range of alternatives, that have been reviewed in the recent network meta-analysis published by Wang et al. [
1]. Given that the overall therapeutic framework of the numerous proposed treatments is complex, our analysis was intentionally kept as simple as possible and therefore addressed specifically a basic question: since docetaxel (or similar chemotherapy options) is currently considered the standard-of-care, is there any survival benefit in using a novel combination regimen under these circumstances? Our analysis was based only on RCTs that addressed this question: a total of 5 relevant RCTs were identified that tested 5 combinations of drugs for second-line or later treatment. After reconstruction of individual patient data, our pooled analysis based on 5 combination treatment arms and 5 control arms clearly demonstrated that the combination regimens do not determine any remarkable benefit in OS neither in terms of clinical relevance nor in terms of statistical significance. Our results substantially confirm those published in the meta-analysis by Wang et al. [
1], the only difference being that these authors reported that the antiangiogenic therapy combined with ICI [
7,12] demonstrated a benefit in OS compared to standard of care (HR= 0.69; 95%CI, 0.51 to 0.92); In our analysis we excluded the study by Ghiringhelli et al. [12] as it is a conference abstract, with a limited number of patients and it does not report results as KM curves. Still, in our hands, the other study included by Wang in this group [
7], investigating ramucirumab + pembrolizumab demonstrated the best HR for OS compared to the other combinations, although in a limited number of patients. In addition, the patient reconstruction approach does not use any of the statistical tests commonly used in meta-analysis studies, nor are forest plots the tool used to present survival results. We have presented the data using a KM summary graph, which makes the result of the analysis highly intuitive at first glance, while keeping the statistical analysis robust, using the Cox regression model.
The main limitation of the present analysis is the simplified nature of the basic questions that we investigated. On the one hand, the five included RCTs shared the advantage that they were all designed as direct comparison; on the other hand, the network meta-analysis by Wang et al [
1] examined a large number of outcomes, but most of the results on effectiveness were based on indirect comparisons, which represent a lower level of evidence.
In conclusion, the novel combination regimens investigated so far has not demonstrated survival benefit over standard of care for non-oncogene addicted NSCLC with progressive disease after ICIs and platinum-based chemotherapy, and the treatment of these patients remains an urgent unmet need.
Author Contributions
Conceptualization, Andrea Messori, Andrea Ossato, Lorenzo Gasperoni, Luna Del Bono, Alessandro Izzo and Vera Damuzzo; Data curation, Andrea Messori, Andrea Ossato and Vera Damuzzo; Formal analysis, Andrea Messori, Andrea Ossato and Vera Damuzzo; Investigation, Andrea Messori, Lorenzo Gasperoni, Luna Del Bono, Alessandro Izzo and Vera Damuzzo; Methodology, Andrea Messori, Andrea Ossato, Lorenzo Gasperoni, Luna Del Bono and Vera Damuzzo; Software, Andrea Messori, Andrea Ossato and Alessandro Izzo; Supervision, Andrea Ossato; Validation, Andrea Messori, Andrea Ossato, Alessandro Izzo and Vera Damuzzo; Visualization, Andrea Messori and Luna Del Bono; Writing – original draft, Andrea Messori, Andrea Ossato, Lorenzo Gasperoni and Vera Damuzzo; Writing – review & editing, Andrea Messori, Andrea Ossato, Lorenzo Gasperoni, Luna Del Bono and Alessandro Izzo.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The files saved in the format of IPDfromKM software are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
| CI |
confidence interval |
| HR |
hazard ratio |
| ICI |
immune checkpoint inhibitor |
| KM |
Kaplan-Meier |
| NSCLC |
non-small cell lung cancer |
| OS |
overall survival |
| RCT |
randomized controlled trial |
References
- Wang, K.; Fu, Z.; Sun, G.; Ran, Y.; Lv, N.; Wang, E.; Ding, H. Systemic treatment options for non-small cell lung cancer after failure of previous immune checkpoint inhibitors: a bayesian network meta-analysis based on randomized controlled trials. BMC Immunol. 2024, 25, 37–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2021, 21, 111–119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Messori, A.; Damuzzo, V.; Rivano, M.; Cancanelli, L.; Di Spazio, L.; Ossato, A.; Chiumente, M.; Mengato, D. Application of the IPDfromKM-Shiny Method to Compare the Efficacy of Novel Treatments Aimed at the Same Disease Condition: A Report of 14 Analyses. Cancers (Basel) 2023, 15, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Messori, A. Reconstruction of individual-patient data from the analysis of Kaplan-Meier curves: the use of this method has extended from oncology to cardiology – List of 28 studies in cardiology - (preprint). Open Science Framework, published March 2024. Available online: https://osf.io/qejus (accessed on 30 June 2024). [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Mohe, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef] [PubMed]
- Jung, H. A.; Park, S.; Choi, Y. L.; Lee, S. H.; Ahn, J. S.; Ahn, M. J.; Sun, J.M. Continuation of Pembrolizumab with Additional Chemotherapy after Progression with PD-1/PD-L1 Inhibitor Monotherapy in Patients with Advanced NSCLC: A Randomized, Placebo-Controlled Phase II Study. Clin. cancer research 2022, 28, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Reckamp, K.L.; Redman, M.W.; Dragnev, K.H.; et al. Phase II Randomized Study of Ramucirumab and Pembrolizumab Versus Standard of Care in Advanced Non-Small-Cell Lung Cancer Previously Treated With Immunotherapy-Lung-MAP S1800A. J. Clin. Oncol. 2022, 40, 2295–2306, published correction appears in J Clin Oncol. 2022 Sep 1;40(25):3002. doi: 10.1200/JCO.22.01464. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; de Marinis, F.; Dumoulin, D.; Reynolds, C.; Theelen, W. S. M. E.; Percent, I.; Gutierrez Calderon, V.; Johnson, M. L.; Madroszyk-Flandin, A.; Garon, E. B.; He, K.; Planchard, D.; Reck, M.; Popat, S.; Herbst, R. S.; Leal, T. A.; Shazer, R. L.; Yan, X.; Harrigan, R.; Peters, S.; et al. SAPPHIRE Investigators (2024). SAPPHIRE: phase III study of sitravatinib plus nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. Ann. Oncol. 2024, 35, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.; Pavlakis, N.; Kim, S.W.; et al. 60-CONTACT-01: efficacy and safety from a phase III study of atezolizumab (atezo) + cabozantinib (cabo) vs docetaxel (doc) monotherapy in patients (pts) with metastatic NSCLC (mNSCLC) previously treated with checkpoint inhibitors and chemotherapy. J. Thorac. Oncol. 2023, 18, S39–S40. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Goto, Y.; Wan-Teck Lim, D.; Halmos, B.; Chul Cho, B.; Cobo, M.; Luis González Larriba, J.; Zhou, C.; Demedts, I.; Atmaca, A.; Baka, S.; Mookerjee, B.; Portella, S.; Zhu, Z.; Wu, J.; Demanse, D.; Dharan, B.; Reck, M. Canakinumab in combination with docetaxel compared with docetaxel alone for the treatment of advanced non-small cell lung cancer following platinum-based doublet chemotherapy and immunotherapy (CANOPY-2): A multicenter, randomized, double-blind, phase 3 trial. Lung Cancer. 2024, 189, 107451. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, J.D.; Giobbie-Hurder, A.; Ranasinghe, S.; et al. Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2022, 23, 279–291. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).