Submitted:
05 January 2025
Posted:
06 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
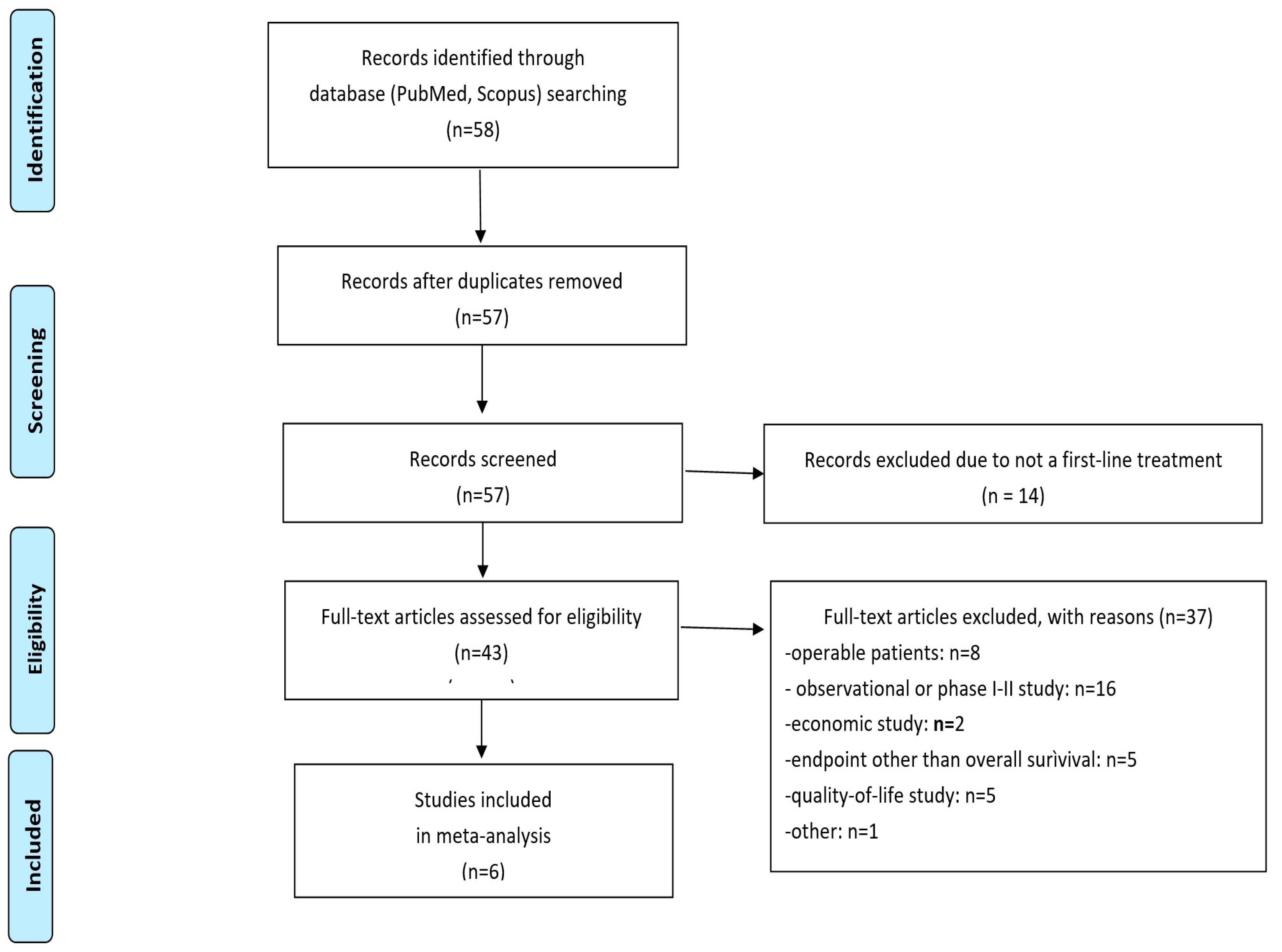
Study Design and Literature Search
Inclusion Criteria
Data Analysis
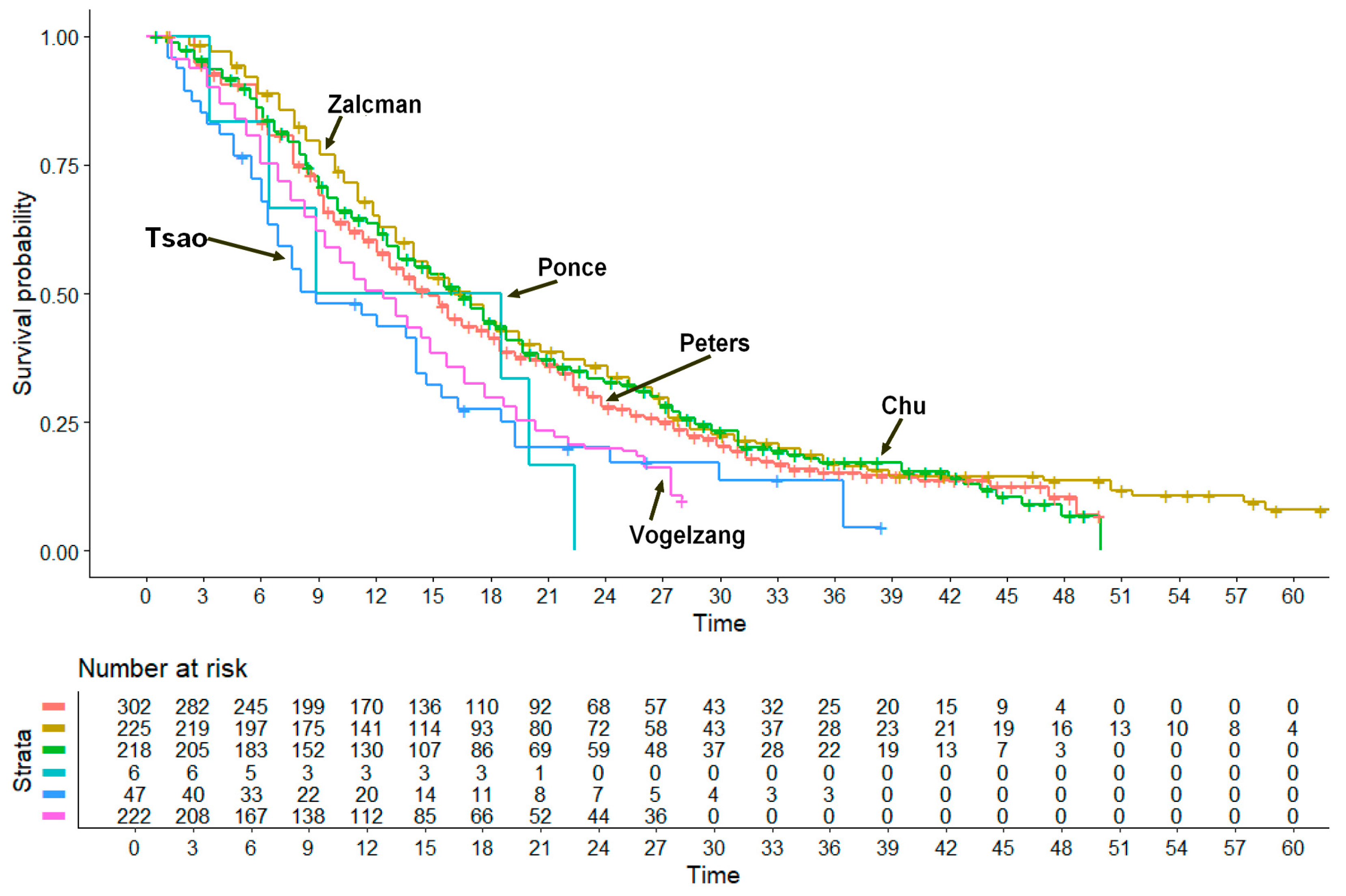
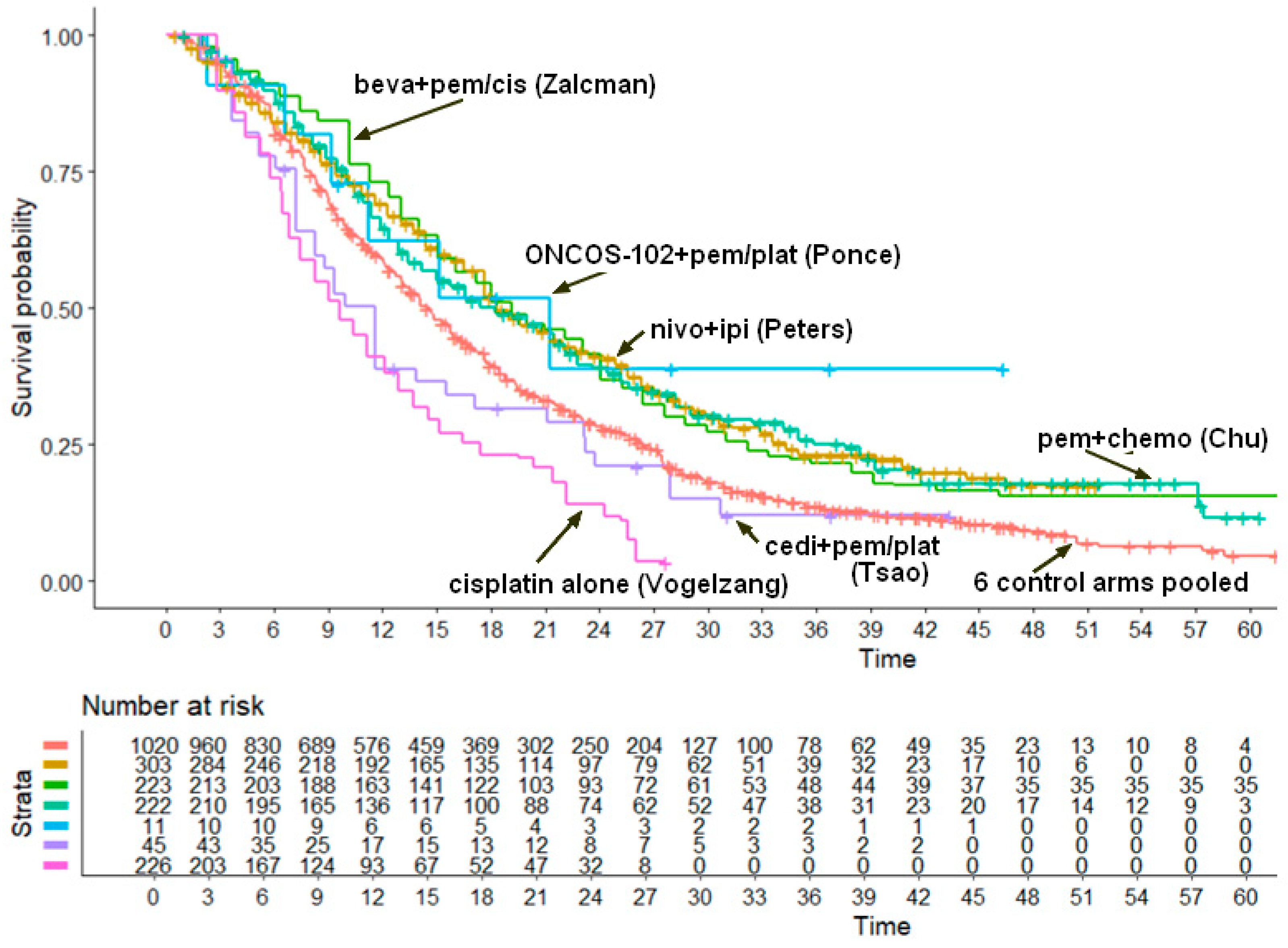
Reconstruction of Individual Patient Data from Kaplan-Meier Curves and Statistical Analysis
3. Results
- -
- nivolumab plus ipilimumab: 18.29 months (95% CI, 17.63 to 21.9);
- -
- bevacizumab plus pemetrexed plus cisplatin: 19.13 months (95% CI, 17.15 to 23.1);
- -
- chemotherapy plus pembrolizumab: 18.26 months (95% CI, 15.01 to 22.1);
- -
- ONCOS-102 plus pemetrexed plus cisplatin or carboplatin: 21.27 months (95% CI, 11.19 to not computable);
- -
- cediranib plus cisplatin-pemetrexed: 11.58 months (95% CI, 8.24 to 17.1);
- -
- cisplatin alone: 9.67 months (95% CI, 8.25 to 11.2).
4. Discussion
Author Contributions
Funding
Availability of Data and Materials
Acknowledgments
Ethical approval
Conflicts of Interest
References
- Schumann, S.O.; Kocher, G.; Minervini, F. Epidemiology, diagnosis and treatment of the malignant pleural mesothelioma, a narrative review of literature. J Thorac Dis. 2021, 13, 2510–2523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santos, C.; Dixe, M.D.A.; Sacadura-Leite, E.; Astoul, P.; Sousa-Uva, A. Asbestos Exposure and Malignant Pleural Mesothelioma: A Systematic Review of Literature. Port J Public Health 2023, 40, 188–202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scherpereel, A.; Astoul, P.; Baas, P.; Berghmans, T.; Clayson, H.; de Vuyst, P.; Dienemann, H.; Galateau-Salle, F.; Hennequin, C.; Hillerdal, G.; Le Péchoux, C.; Mutti, L.; Pairon, J.C.; Stahel, R.; van Houtte, P.; van Meerbeeck, J.; Waller, D.; f Weder, W.; European Respiratory Society/European Society of Thoracic Surgeons Task Force. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010, 35, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Amin, W.; Linkov, F.; Landsittel, D.P.; Silverstein, J.C.; Bashara, W.; Gaudioso, C.; Feldman, M.D.; Pass, H.I.; Melamed, J.; Friedberg, J.S.; Becich, M.J. Factors influencing malignant mesothelioma survival: a retrospective review of the National Mesothelioma Virtual Bank cohort. F1000Res 2018, 7, 1184. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.D.; Bayes, H.K.; Bardgett, J.; Wedderburn, S.; Kerr, K.M.; Currie, G.P. Survival from malignant mesothelioma: where are we now? J R Coll Physicians Edinb. 2015, 45, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Marchevsky, A.M.; Khoor, A.; Walts, A.E.; Nicholson, A.G.; Zhang, Y.Z.; Roggli, V.; Carney, J.; Roden, A.C.; Tazelaar, H.D.; Larsen, B.T.; LeStang, N.; Chirieac, L.R.; Klebe, S.; Tsao, M.S.; De Perrot, M.; Pierre, A.; Hwang, D.M.; Hung, Y.P.; Mino-Kenudson, M.; Travis, W.; Sauter, J.; Beasley, M.B.; Galateau-Sallé, F. Localized malignant mesothelioma, an unusual and poorly characterized neoplasm of serosal origin: best current evidence from the literature and the International Mesothelioma Panel. Mod Pathol. 2020, 33, 281–296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ricciardi, S.; Cardillo, G.; Zirafa, C.C.; Carleo, F.; Facciolo, F.; Fontanini, G.; Mutti, L.; Melfi, F. Surgery for malignant pleural mesothelioma: an international guidelines review. J Thorac Dis. 2018, 10 (Suppl. 2), S285–S292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pezzicoli, G.; Rizzo, M.; Perrone, M.; Minei, S.; Mutti, L.; Porta, C. A Glimpse in the Future of Malignant Mesothelioma Treatment. Front Pharmacol 2021, 12, 809337. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, E.; Kijima, T.; Kuribayashi, K.; Negi, Y.; Kanemura, S.; Mikami, K.; Doi, H.; Kitajima, K.; Nakano, T. First-line chemotherapy with pemetrexed plus cisplatin for malignant peritoneal mesothelioma. Expert Rev Anticancer Ther. 2017, 17, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; O'Brien, M.E.; Stahel, R.A.; Nackaerts, K.; Baas, P.; Karthaus, M.; Eberhardt, W.; Paz-Ares, L.; Sundstrom, S.; Liu, Y.; Ripoche, V.; Blatter, J.; Visseren-Grul, C.M.; Manegold, C. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaïve patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol. 2008, 3, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Baas, P.; Faivre-Finn, C.; Girard, N.; Nicholson, A.G.; Nowak, A.K.; Opitz, I.; Scherpereel, A.; Reck, M.; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2022, 33, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Scherpereel, A.; Cornelissen, R.; Oulkhouir, Y.; Greillier, L.; Kaplan, M.A.; et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate-743. Ann Oncol. Epub ahead of print. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Perrone, F.; Greillier, L.; Tu, W.; Piccirillo, M.C; Grosso, F.; et al. Pembrolizumab plus chemotherapy versus chemotherapy in untreated advanced pleural mesothelioma in Canada, Italy, and France: a phase 3, open-label, randomised controlled trial. Lancet 2023, 402, 2295–2306. [Google Scholar] [CrossRef]
- Ponce, S.; Cedrés, S.; Ricordel, C.; Isambert, N.; Viteri, S.; Herrera-Juarez, M.; et al. ONCOS-102 plus pemetrexed and platinum chemotherapy in malignant pleural mesothelioma: a randomized phase 2 study investigating clinical outcomes and the tumor microenvironment. J Immunother Cancer 2023, 11, e007552. [Google Scholar] [CrossRef]
- Tsao, A.S.; Miao, J.; Wistuba, II.; Vogelzang, N.J.; Heymach, J.V.; Fossella, F.V.; et al. Phase II Trial of Cediranib in Combination With Cisplatin and Pemetrexed in Chemotherapy-Naïve Patients With Unresectable Malignant Pleural Mesothelioma (SWOG S0905). J Clin Oncol. 2019, 37, 2537–2547. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; et al. Phase III Study of Pemetrexed in Combination With Cisplatin Versus Cisplatin Alone in Patients With Malignant Pleural Mesothelioma. J Clin Oncol. 2003, 41, 2125–2133. [Google Scholar] [CrossRef]
- Nowak, A.K.; Lesterhuis, W.J.; Kok, P.S.; Brown, C.; Hughes, B.G.; Karikios, D.J.; et al. Durvalumab with first-line chemotherapy in previously untreated malignant pleural mesothelioma (DREAM): a multicentre, single-arm, phase 2 trial with a safety run-in. Lancet Oncol. 2020, 21, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Messori, A.; Trippoli, S. Current treatments for inoperable mesothelioma: indirect comparisons based on individual patient data reconstructed retrospectively from 4 trials. J Chemother. 2023, 35, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021, 21, 111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Messori, A. Synthetizing Published Evidence on Survival by Reconstruction of Patient-Level Data and Generation of a Multi-Trial Kaplan-Meier Curve. Cureus 2021, 13, e19422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Messori, A. Reconstruction of individual-patient data from the analysis of Kaplan-Meier curves: this method is widely used in oncology and cardiology – List of 53 studies in oncology and 61 in cardiology (preprint). Open Science Framework 2024. url https://osf.io/zcf4r, accessed 10 November 2024. [Google Scholar] [CrossRef]
- Piragine, E.; Veneziano, S.; Trippoli, S.; Messori, A.; Calderone, V. Efficacy and Safety of Cardioband in Patients with Tricuspid Regurgitation: Systematic Review and Meta-Analysis of Single-Arm Trials and Observational Studies. J Clin Med. 2024, 13, 6393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cashman, K.D.; Ritz, C. Individual participant data (IPD)-level meta-analysis of randomised controlled trials among dark-skinned populations to estimate the dietary requirement for vitamin D. Syst Rev. 2019, 8, 128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wach, J.; Vychopen, M.; Güresir, A.; Guranda, A.; Nestler, U.; Güresir, E. A Long-Term Comparative Analysis of Endovascular Coiling and Clipping for Ruptured Cerebral Aneurysms: An Individual Patient-Level Meta-Analysis Assessing Rerupture Rates. J Clin Med. 2024, 13, 1778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zoupas, I.; Manaki, V.; Tasoudis, P.T.; Karela, N.R.; Avgerinos, D.V.; Mylonas, K.S. Totally Endoscopic Coronary Artery Bypass Graft: Systematic Review and Meta-Analysis of Reconstructed Patient-Level Data. Innovations (Phila) 2024, 19, 616–625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rogula, B.; Lozano-Ortega, G.; Johnston, K.M. A Method for Reconstructing Individual Patient Data From Kaplan-Meier Survival Curves That Incorporate Marked Censoring Times. MDM Policy Pract. 2022, 7, 23814683221077643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Mohe, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/, 2023.
- Correale, P.; Pentimalli, F.; Nardone, V.; Giordano, A.; Mutti, L. CONFIRM trial: what is the real efficacy of second-line immunotherapy in mesothelioma? Lancet Oncol. 2022, 23, e13. [Google Scholar] [CrossRef] [PubMed]
- Ossato, A.; Damuzzo, V.; Baldo, P.; Mengato, D.; Chiumente, M.; Messori, A. Immune checkpoint inhibitors as first line in advanced melanoma: Evaluating progression-free survival based on reconstructed individual patient data. Cancer Med. 2023, 12, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
| Study (First author, year and reference) |
Study design | Histology (number of patients) | Intervention vs. control | Follow-up (months) |
HR (95% CI)* | Total number of events/patients (n/N) |
|
|---|---|---|---|---|---|---|---|
| Treatment group | Control group |
||||||
| Peters et al. (2022) [12] | RCT | Epithelioid, n=455; non-epithelioid, n=150 |
Nivolumab plus ipilimumab vs. pemetrexed plus cisplatin |
54 | HR=0.73 (95% CI, 0.61 to 0.87) § | 212/303 | 234/302 |
| Zalcman et al. (2016) [13] | RCT | Epithelioid, n=179 (80%); non-epithelioid, n=44 (20%) |
Bevacizumab plus pemetrexed plus cisplatin vs. pemetrexed plus cisplatin |
80 | HR=0.77 (95% CI, 0.62 to 0.95) |
164/223 | 178/225 |
| Chu et al. (2023) [14] | RCT | Epithelioid, n=349 (79%); other, n=91 (21%) |
Chemotherapy† plus pembrolizumab vs. chemotherapy† | 60 | HR=0·79 (95% CI, 0.64 to 0.98) |
167/222 | 175/218 |
| Ponce et al. (2023) [15] | RCT §§ | Epithelioid, n=24 (77.4%); other, n=7 (22.6%) |
ONCOS-102** plus pemetrexed plus cisplatin/carboplatin vs. pemetrexed plus cisplatin/carboplatin | 33 | NR | 6/11 | 6/6 |
| Tsao et al. (2019) [16] | RCT | Epithelioid, n=69 (75%); other, n=23 (25%) |
Cediranib plus pemetrexed+cisplatin (with maintenance with cediranib) vs. placebo plus pemetrexed+cisplatin (with maintenance with placebo) | 40 |
HR=0.88 (80% CI, 0.65 to 1.17) |
39/45 | 41/47 |
| Vogelzang et al. (2003) [17] | RCT | Epithelioid, n=306 (68.3%); other, n=142 (31.7%) |
Pemetrexed plus cisplatin vs. cisplatin alone | 30 | HR=0.77Median OS, 12.1 vs. 9.3 months | NR/226 | NR/222 |
| *These values of HR are those reported by the authors in the original article. †The chemotherapy in this trial consisted in most cases of pemtrexed plus platinum. ** ONCOS-102 is an oncolytic adenovirus expressing granulocyte-macrophage colony-stimulating factor. § The paper by Peters et al. [12] did not explicitly report the number of deaths in the two patient groups; this information was therefore estimated from the number of deaths from 0 to 39 months previously reported in the article published by Baas et al. in 2021 [19] (200 and 219 deaths in the two groups, respectively) and by counting the deaths from 40 to 54 months (12 and 15 in the two groups, respectively) according to individual downward steps appearing in the Kaplan-Meier graph published by Peters et al. in 2022 [12]. §§ While the paper by Ponce et al. [15] included 20 patients in the treatment group vs. 6 in the control group, our analysis included only the subgroup of chemo-naïve patients who were 11 in the treatment group and 6 in the control group. Abbreviations: CI, confidence interval; HR, hazard ratio; RCT, randomized controlled trial; OS, overall survival; NR, not reported. | |||||||
| First author, year and reference | Treatment given to the experimental arm | Treatment given to the control arm | HR (with 95% CI) estimated from reconstrcucted patients |
|---|---|---|---|
| Peters et al. (2022) [12] | Nivolumab plus ipilimumab |
Pemetrexed plus cisplatin | HR=0.7149 (95% CI, 0.6139 to 0.8326; p<0.001) |
| Zalcman et al. (2016) [13] | Bevacizumab plus pemetrexed plus cisplatin |
Pemetrexed plus cisplatin | HR=0.7063 (95% CI, 0.6020 to 0.8288; p<0.001) |
| Chu et al. (2023) [14] | Chemotherapy plus pembrolizumab | Chemotherapy | HR=0.7297 (95% CI, 0.6170 to 0.8631; p<0.001) |
| Ponce et al. (2023) [15] | ONCO-102 plus pemetrexed plus cisplatin or carboplatin | Pemetrexed plus cisplatin | HR=0.5853 (95% CI, 0.2622 to 1.3067; p= 0.191165) |
| Tsao et al. (2019) [16] | Cediranib pluspemetrexed+cisplatin (with maintenance with cediranib) | Pemetrexed plus cisplatin | HR=1.2196 (95% CI, 0.8774 to 1.6953; p= 0.237475) § |
| Vogelzang et al. (2003) [17] | Cisplatin alone | Pemetrexed plus cisplatin | HR=1.7657 (95% CI, 1.5192 to 2.0523; p<0.001) §§ |
|
§ The reciprocal of this HR is 0.8199 (95% CI, 0.5899 to 1.1397). §§ The reciprocal of this HR is 0.5663 (95% CI, 0.483 to 0.6582). Abbreviations: CI, confidence interval; HR, hazard ratio. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





