Submitted:
16 June 2025
Posted:
18 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
Moringa Genus
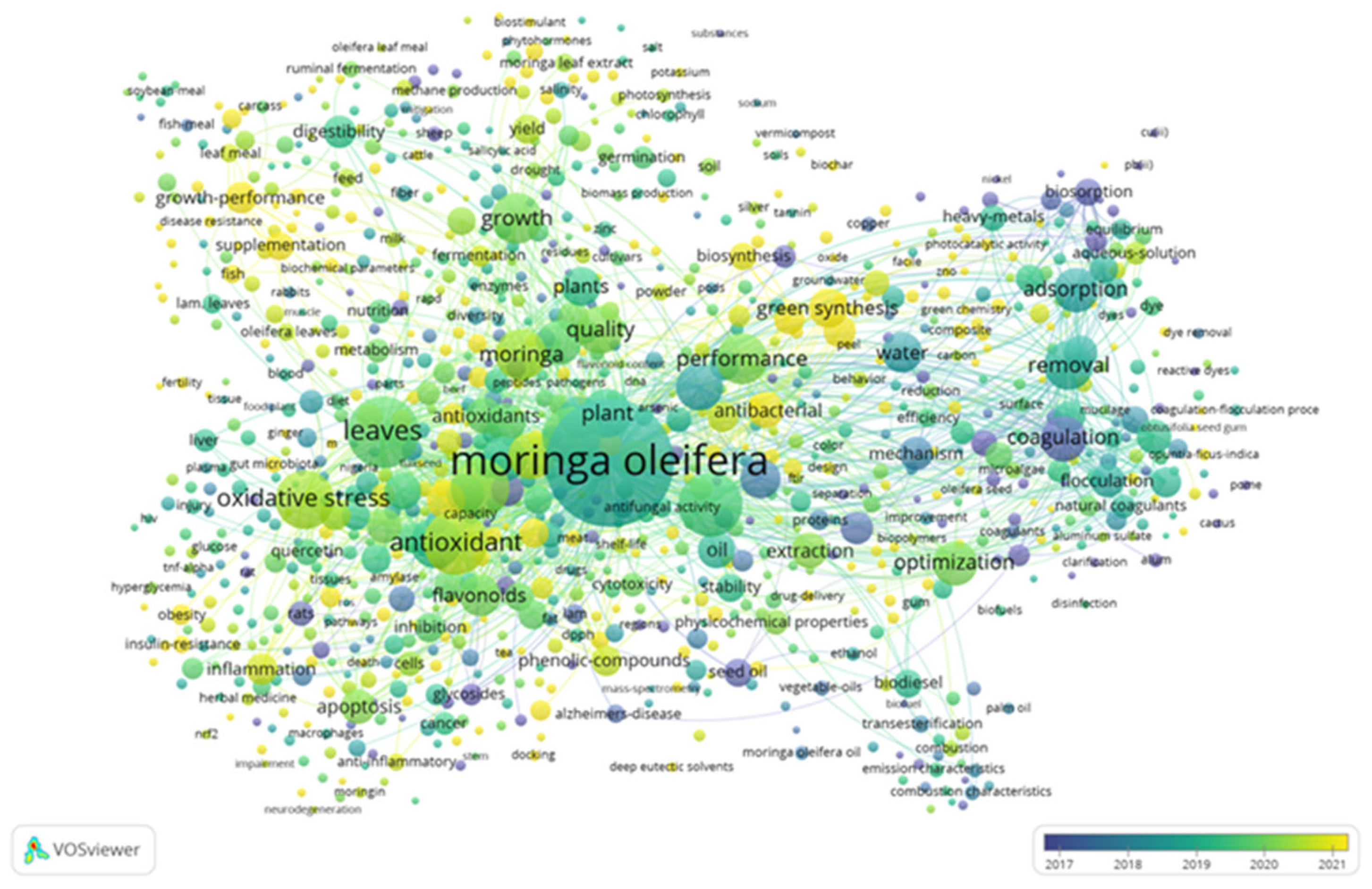
2. Current Status of Moringa oleifera in Global Literature Over Time
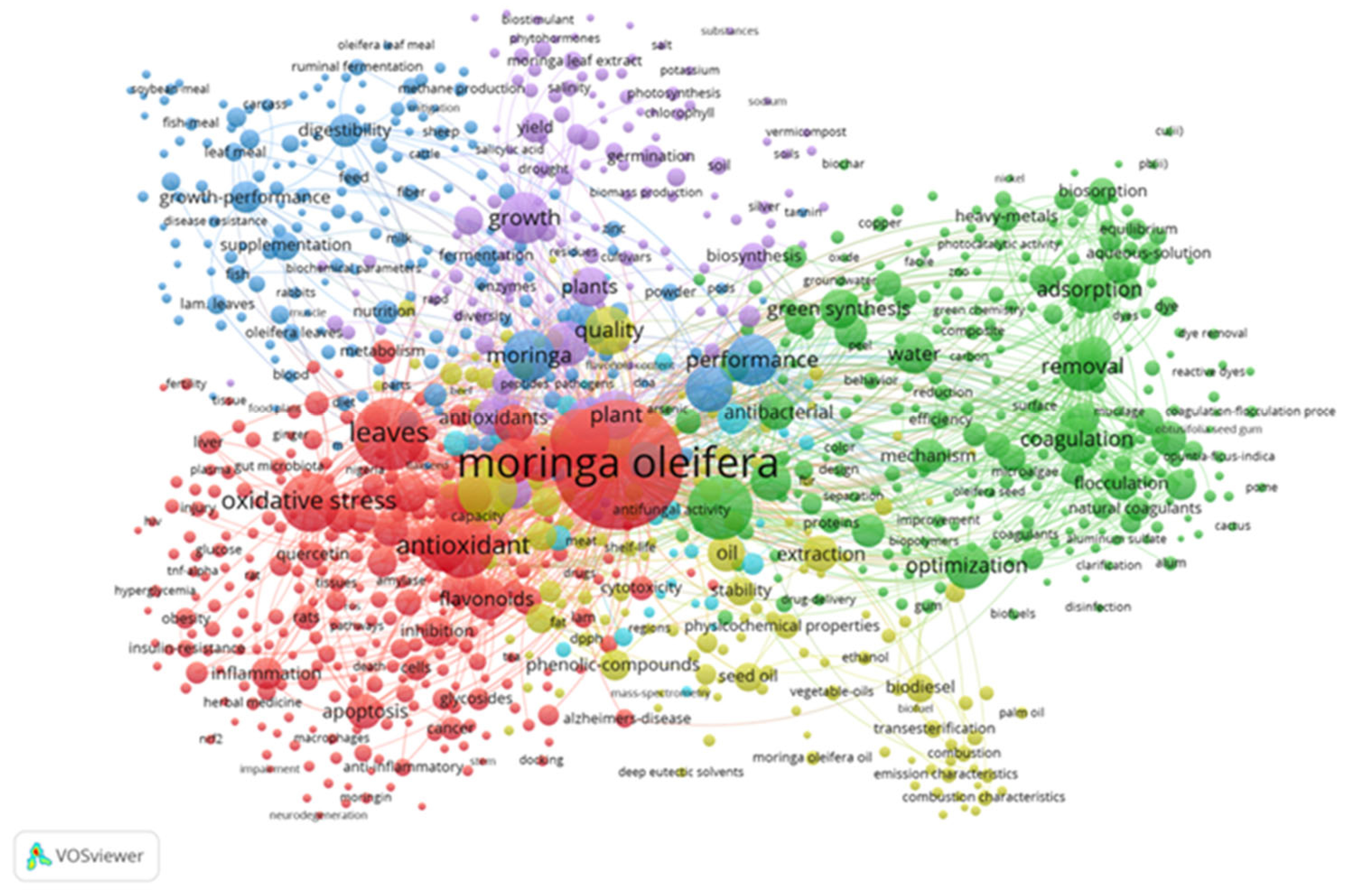
| Color clusters | Description | Keywords | Focus |
| Red Cluster | Health and Biomedical Applications | antioxidant, oxidative stress, apoptosis, cancer, inflammation, flavonoids, quercetin | Pharmacological and therapeutic effects of Moringa oleifera, particularly in managing oxidative stress, inflammation, and diseases like cancer and Alzheimer’s. |
| Green Cluster | Environmental and Water Treatment Applications | adsorption, coagulation, removal, biosorption, flocculation, heavy metals, optimization | Use of Moringa oleifera as a natural coagulant or biosorbent for water purification and environmental cleanup. |
| Blue Cluster | Animal Nutrition and Feed | growth-performance, digestibility, fermentation, supplementation, metabolism, sheep, goats | Application of Moringa oleifera in livestock nutrition, enhancing animal growth, digestion, and health. |
| Purple Cluster | Agricultural and Plant-Based Research | germination, photosynthesis, biomass, biosynthesis, yield, phytohormones, quality | Moringa oleifera in plant growth, productivity, and sustainable agriculture. |
| Yellow Cluster: | Oil Extraction and Biofuel | seed oil, biodiesel, transesterification, extraction, stability | Industrial and biochemical extraction of oil from Moringa oleifera seeds for use in biodiesel and bio-based industries. |
3. Current Application of Moringa in White Biotechnology
3.1. Biofuels and Bioenergy Industries
3.2. Bioprocess Applied to the Extraction of Moringa Phytochemicals
3.3. Food Industry
3.4. Textile Industry
4. Current Application of Moringa in Green Biotechnology
4.1. Agriculture Industry
4.2. Green Nano-Industry
4.3. Water Treatment Industry
5. Current Application of Moringa in Red Biotechnology
5.1. Pharmaceutical Industry
5.2. Biomedical Industry
5.3. Cosmetic Industry
6. Current Application of Moringa in Blue Biotechnology
7. Current Application of Moringa in Yellow Biotechnology
8. Current Application of Moringa in Brown Biotechnology
9. Current Application of Moringa in Violet Biotechnology
10. Current Application of Gold Biotechnology in Moringa Research
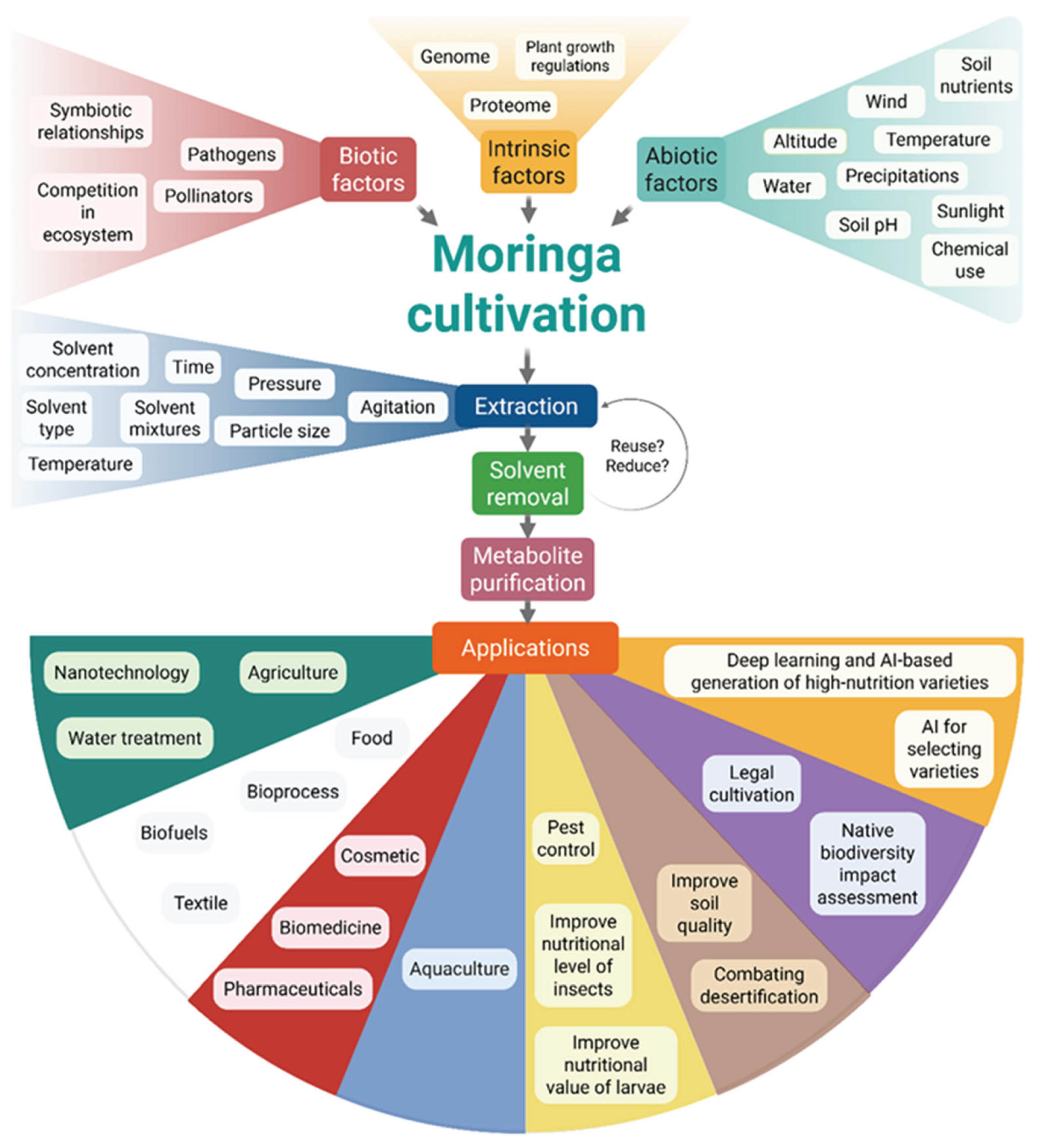
11. Biotechnological Challenges
12. Future Directions for Unlocking the MO Potential
12.1. Agrigenomics and Breeding
12.2. Standardization for Clinical Use
12.3. Green Nanotechnology
12.4. Policy and Sustainability
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Billiones, R. Biotechnology – diverse as the colours of the rainbow. Med Writ. 2023, 32, 6–7. [Google Scholar] [CrossRef]
- El Bilali, H. , et al., Research on Moringa (Moringa oleifera Lam.) in Africa. Plants (Basel), 2024. 13(12).
- Razis, A.F.A.; Ibrahim, M.D.; Kntayya, S.B. Health Benefits of Moringa oleifera. Asian Pac. J. Cancer Prev. 2014, 15, 8571–8576. [Google Scholar] [CrossRef]
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef]
- Singh, A.K.; Rana, H.K.; Tshabalala, T.; Kumar, R.; Gupta, A.; Ndhlala, A.R.; Pandey, A.K. Phytochemical, nutraceutical and pharmacological attributes of a functional crop Moringa oleifera Lam: An overview. South Afr. J. Bot. 2020, 129, 209–220. [Google Scholar] [CrossRef]
- Boopathi, N.M.; Abubakar, B.Y. Botanical Descriptions of Moringa spp. Compendium of Plant Genomes, 2021.
- Hamada, F.A.; Sabah, S.S.; Mahdy, E.M.; El-Raouf, H.S.A.; El-Taher, A.M.; El-Leel, O.F.; Althobaiti, A.T.; Ghareeb, M.A.; Randhir, R.; Randhir, T.O. Genetic, phytochemical and morphological identification and genetic diversity of selected Moringa species. Sci. Rep. 2024, 14, 1–18. [Google Scholar] [CrossRef]
- El-Haddad, A.E.; El-Deeb, E.M.; Koheil, M.A.; El-Khalik, S.M.A.; Hefnawy, H.M.E. Nitrogenous phytoconstituents of genus Moringa: spectrophotometrical and pharmacological characteristics. Med. Chem. Res. 2019, 28, 1591–1600. [Google Scholar] [CrossRef]
- Hamada, F.A.; Sabah, S.S.; Mahdy, E.M.; El-Raouf, H.S.A.; El-Taher, A.M.; El-Leel, O.F.; Althobaiti, A.T.; Ghareeb, M.A.; Randhir, R.; Randhir, T.O. Genetic, phytochemical and morphological identification and genetic diversity of selected Moringa species. Sci. Rep. 2024, 14, 1–18. [Google Scholar] [CrossRef]
- Ojeda-López, J.; Marczuk-Rojas, J.P.; Polushkina, O.A.; Purucker, D.; Salinas, M.; Carretero-Paulet, L. Evolutionary analysis of the Moringa oleifera genome reveals a recent burst of plastid to nucleus gene duplications. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zeng, Y.; Zhang, J.; Yang, C.; Yan, L.; Wang, X.; Shi, C.; Xie, J.; Dai, T.; Peng, L.; et al. High quality reference genome of drumstick tree (Moringa oleifera Lam.), a potential perennial crop. Sci. China Life Sci. 2015, 58, 627–638. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, H.; Liu, M.; Liao, X.; Sahu, S.K.; Fu, Y.; Song, B.; Cheng, S.; Kariba, R.; Muthemba, S.; et al. The draft genomes of five agriculturally important African orphan crops. GigaScience 2019, 8, 152. [Google Scholar] [CrossRef]
- Tena, G. Sequencing forgotten crops. Nat. Plants 2019, 5, 5–5. [Google Scholar] [CrossRef] [PubMed]
- Neale, D.B.; Martínez-García, P.J.; De La Torre, A.R.; Montanari, S.; Wei, X.-X. Novel Insights into Tree Biology and Genome Evolution as Revealed Through Genomics. Annu. Rev. Plant Biol. 2017, 68, 457–483. [Google Scholar] [CrossRef] [PubMed]
- Michael, T.P.; VanBuren, R. Building near-complete plant genomes. Curr. Opin. Plant Biol. 2020, 54, 26–33. [Google Scholar] [CrossRef]
- Alavilli, H.; Poli, Y.; Verma, K.S.; Kumar, V.; Gupta, S.; Chaudhary, V.; Jyoti, A.; Sahi, S.V.; Kothari, S.L.; Jain, A. Miracle Tree Moringa oleifera: Status of the Genetic Diversity, Breeding, In Vitro Propagation, and a Cogent Source of Commercial Functional Food and Non-Food Products. Plants 2022, 11, 3132. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.K.; Moustafa, A.F.; Aboud, A.A.; Halim, K.S.A. Effective utilization of Moringa seeds waste as a new green environmental adsorbent for removal of industrial toxic dyes. J. Mater. Res. Technol. 2019, 8, 1798–1808. [Google Scholar] [CrossRef]
- Gharsallah, K.; Rezig, L.; Msaada, K.; Chalh, A.; Soltani, T. Chemical composition and profile characterization of Moringa oleifera seed oil. South Afr. J. Bot. 2021, 137, 475–482. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Montesano, D.; Lucini, L. The potential of Moringa oleifera in food formulation: a promising source of functional compounds with health-promoting properties. Curr. Opin. Food Sci. 2021, 42, 257–269. [Google Scholar] [CrossRef]
- Aleman-Ramirez, J. , et al., The role of Moringa oleifera in the development of alternative biofuels, under the concept of an integral one-tree biorefinery: A minireview. Biofuels, Bioproducts and Biorefining, 2025.
- Fakayode, O.A.; Ajav, E.A. Process optimization of mechanical oil expression from Moringa (Moringa oleifera) seeds. Ind. Crop. Prod. 2016, 90, 142–151. [Google Scholar] [CrossRef]
- Oladipo, B.; Betiku, E. Process optimization of solvent extraction of seed oil from Moringa oleifera: An appraisal of quantitative and qualitative process variables on oil quality using D-optimal design. Biocatal. Agric. Biotechnol. 2019, 20. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Y.; Yang, R.; Liu, X.; Yang, Q.; Qin, X. The application of ultrasound and microwave to increase oil extraction from Moringa oleifera seeds. Ind. Crop. Prod. 2018, 120, 1–10. [Google Scholar] [CrossRef]
- Rajesh, Y.; Khan, N.M.; Shaikh, A.R.; Mane, V.S.; Daware, G.; Dabhade, G. Investigation of geranium oil extraction performance by using soxhlet extraction. Mater. Today: Proc. 2022, 72, 2610–2617. [Google Scholar] [CrossRef]
- Nuchdang, S.; Phruetthinan, N.; Paleeleam, P.; Domrongpokkaphan, V.; Chuetor, S.; Chirathivat, P.; Phalakornkule, C. Soxhlet, microwave-assisted, and room temperature liquid extraction of oil and bioactive compounds from palm kernel cake using isopropanol as solvent. Ind. Crop. Prod. 2022, 176. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Kotoka, F. Kinetics and thermodynamic studies on Moringa oleifera oil extraction for biodiesel production via transesterification. Biofuels 2019, 13, 341–349. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical fluid extraction and characterisation of Moringa oleifera leaves oil. Sep. Purif. Technol. 2013, 118, 497–502. [Google Scholar] [CrossRef]
- Díaz, Y.; Tabio, D.; Rondón, M.; Piloto-Rodríguez, R.; Fernández, E. Phenomenological model for the prediction of Moringa oleifera extracted oil using a laboratory Soxhlet apparatus. Grasas y Aceites 2021, 72, e422–e422. [Google Scholar] [CrossRef]
- Ojewumi, M. , et al., Optimization of Oil from Moringa oleifera seed using Soxhlet Extraction method. The Korean Journal of Food & Health Convergence, 2019. 5(5): p. 11-25.
- Garcia-Fayos, B.; Arnal, J.; Sancho, M.; Rodrigo, I. Moringa oleifera for drinking water treatment: influence of the solvent and method used in oil-extraction on the coagulant efficiency of the seed extract. Desalination Water Treat. 2016, 57, 23397–23404. [Google Scholar] [CrossRef]
- Nebolisa, N.M.; Umeyor, C.E.; Ekpunobi, U.E.; Umeyor, I.C.; Okoye, F.B. Profiling the effects of microwave-assisted and soxhlet extraction techniques on the physicochemical attributes of Moringa oleifera seed oil and proteins. Oil Crop. Sci. 2023, 8, 16–26. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Gaweł-Bęben, K.; Rutka, A.; Blicharska, E.; Tatarczak-Michalewska, M.; Kulik-Siarek, K.; Kukula-Koch, W.; Malinowska, M.A.; Szopa, A. Moringa oleifera (drumstick tree)—nutraceutical, cosmetological and medicinal importance: a review. Front. Pharmacol. 2024, 15, 1288382. [Google Scholar] [CrossRef]
- Sukarni, S.; Anis, S.; Aminullah, A.Y.; Assidiq, M.A.; Mufti, N.; Abdullah, T.A.T.; Johari, A.; Hadi, M.; Sanjaya, E.; Wibawa, A.; et al. Moringa oleifera Seeds Potential as Biofuel via Thermal Conversion Method Based on Morphological and Chemical Content Evaluation.CONFERENCE NAME, LOCATION OF CONFERENCE, COUNTRYDATE OF CONFERENCE; p. 01016.
- Abdullah, N.H.; Osman, M.E. Second Generation Biofuel Production from Moringa oleifera Pod Husks Utilizing Cellulases of A New Decaying Fungus; Cladosporium halotolerans MDP OP903200. Egypt. J. Bot. 2023, 64, 341–357. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; De-Montijo-Prieto, S.; Áznar-Ramos, M.J.; Martín-García, B.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Guerra-Hernández, E.J.; García-Villanova, B.; Verardo, V.; Gómez-Caravaca, A.M. Integrated biotechnological process based on submerged fermentation and sonotrode extraction as a valuable strategy to obtain phenolic enriched extracts from moringa leaves. Food Res. Int. 2024, 201, 115602. [Google Scholar] [CrossRef]
- Gunalan, S.; Thangaiah, A.; Rathnasamy, V.K.; Janaki, J.G.; Thiyagarajan, A.; Kuppusamy, S.; Arunachalam, L. Microwave-assisted extraction of biomolecules from moringa (Moringa oleifera Lam.) leaves var. PKM 1: A optimization study by response surface methodology (RSM). Kuwait J. Sci. 2023, 50, 339–344. [Google Scholar] [CrossRef]
- Gunalan, S.; Thangaiah, A.; Janaki, J.G.; Thiyagarajan, A.; Kuppusamy, S.; Arunachalam, L.; Nikalje, G. Optimization of Microwave-Assisted Extraction Method for Increased Extraction Yield and Total Phenol Content from Moringa Leaves (Moringa oleifera Lam.) var. PKM 1. Adv. Agric. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Simon, S.; K, S.; Joseph, J.; George, D. Optimization of extraction parameters of bioactive components from Moringa oleifera leaves using Taguchi method. Biomass- Convers. Biorefinery 2022, 13, 11973–11982. [Google Scholar] [CrossRef]
- Kessler, J.C.; Martins, I.M.; Manrique, Y.A.; Rodrigues, A.E.; Barreiro, M.F.; Dias, M.M. Advancements in conventional and supercritical CO2 extraction of Moringa oleifera bioactives for cosmetic applications: A review. J. Supercrit. Fluids 2024, 214. [Google Scholar] [CrossRef]
- Saidu, A.; Abdulrahman, A.; Imam, Z. Effect of processing methods on the proximate and phytochemical constituents of Moringa Oleifera (Lamarck, 1785) leaves. Sci. World J. 2023, 18, 272–275. [Google Scholar] [CrossRef]
- Stanley, O.O.; Emmanuel, K.A.; Jenyo-Oni, A. Phytochemical Screening of Moringa oleifera Leaf Extracts under Different Solvents. Int. J. Aquac. Fish. Sci. 2024, 10, 066–072. [Google Scholar] [CrossRef]
- Gandji, K.; Salako, V.K.; Fandohan, A.B.; Assogbadjo, A.E.; Kakaï, R.L.G. Factors Determining the Use and Cultivation of Moringa oleifera Lam. in the Republic of Benin. Econ. Bot. 2018, 72, 332–345. [Google Scholar] [CrossRef]
- Farooq, F.; Rashid, N.; Ibrar, D.; Hasnain, Z.; Ullah, R.; Nawaz, M.; Irshad, S.; Basra, S.M.A.; Alwahibi, M.S.; Elshikh, M.S.; et al. Impact of varying levels of soil salinity on emergence, growth and biochemical attributes of four Moringa oleifera landraces. PLOS ONE 2022, 17, e0263978. [Google Scholar] [CrossRef]
- Aslam, M.F.; Basra, S.M.A.; Hafeez, M.B.; Khan, S.; Irshad, S.; Iqbal, S.; Saqqid, M.S.; Akram, M.Z. Inorganic fertilization improves quality and biomass of Moringa oleifera L. Agrofor. Syst. 2019, 94, 975–983. [Google Scholar] [CrossRef]
- Atteya, A.K.G.; Albalawi, A.N.; El-Serafy, R.S.; Albalawi, K.N.; Bayomy, H.M.; Genaidy, E.A.E. Response of Moringa oleifera Seeds and Fixed Oil Production to Vermicompost and NPK Fertilizers under Calcareous Soil Conditions. Plants 2021, 10, 1998. [Google Scholar] [CrossRef]
- Santos, R.S.; Neto, J.V.E.; Bonfim, B.R.S.; Difante, G.S.; Bezerra, J.D.V.; Lista, F.N.; Gurgel, A.L.C.; Bezerra, M.G.S. Growth and Biomass Production of Moringa Cultivated in Semiarid Region as Responses to Row Spacing and Cuts. Trop. Anim. Sci. J. 2021, 44, 183–187. [Google Scholar] [CrossRef]
- Trigo, C.; Castelló, M.L.; Ortolá, M.D.; García-Mares, F.J.; Soriano, M.D. Moringa oleifera: An Unknown Crop in Developed Countries with Great Potential for Industry and Adapted to Climate Change. Foods 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Gebrezihar, T.A.; Desta, Z.; Hagos, H. Assessing Factors Affecting Moringa Production at North-western Zone of Tigray, Ethiopia. Agric. Sci. 2020, 2. [Google Scholar] [CrossRef]
- Kumssa, D.B.; Joy, E.J.M.; Young, S.D.; Odee, D.W.; Ander, E.L.; Magare, C.; Gitu, J.; Broadley, M.R.; Glover-Amengor, M. Challenges and opportunities for Moringa growers in southern Ethiopia and Kenya. PLOS ONE 2017, 12, e0187651. [Google Scholar] [CrossRef]
- Orisawayi, A.O.; Koziol, K.; Hao, S.; Tiwari, S.; Rahatekar, S.S. Development of hybrid electrospun alginate-pulverized moringa composites. RSC Adv. 2024, 14, 8502–8512. [Google Scholar] [CrossRef]
- Beluci, N.d.C.L.; Homem, N.C.; Amorim, M.T.S.P.; Bergamasco, R.; Vieira, A.M.S. Biopolymer extracted from Moringa oleifera Lam. in conjunction with graphene oxide to modify membrane surfaces. Environ. Technol. 2019, 41, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Chitra, R.; Krishna, M.V.; Selvasekarapandian, S. Study on novel biopolymer electrolyte Moringa oleifera gum with ammonium nitrate. Polym. Bull. 2021, 79, 3555–3572. [Google Scholar] [CrossRef]
- Shivanna, S.K.; Naik, N.L.; Nataraj, B.H.; Rao, P.S. Moringa marvel: navigating therapeutic insights and safety features for future functional foods. J. Food Meas. Charact. 2024, 18, 4940–4971. [Google Scholar] [CrossRef]
- Oyeyinka, A.T.; Oyeyinka, S.A. Moringa oleifera as a food fortificant: Recent trends and prospects. J. Saudi Soc. Agric. Sci. 2018, 17, 127–136. [Google Scholar] [CrossRef]
- Cao, J.; Shi, T.; Wang, H.; Zhu, F.; Wang, J.; Wang, Y.; Cao, F.; Su, E. Moringa oleifera leaf protein: Extraction, characteristics and applications. J. Food Compos. Anal. 2023, 119. [Google Scholar] [CrossRef]
- Kumar, M. , et al., Moringa oleifera Lam. seed proteins: Extraction, preparation of protein hydrolysates, bioactivities, functional food properties, and industrial application. Food Hydrocolloids, 2022.
- Kashyap, P. , et al., Recent Advances in Drumstick (Moringa oleifera) Leaves Bioactive Compounds: Composition, Health Benefits, Bioaccessibility, and Dietary Applications. Antioxidants, 2022. 11.
- Falowo, A.B.; Mukumbo, F.E.; Idamokoro, E.M.; Lorenzo, J.M.; Afolayan, A.J.; Muchenje, V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: A review. Food Res. Int. 2018, 106, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Pompa, S.; Torres-Castillo, J.; Castro-López, C.; Rojas, R.; Sánchez-Alejo, E.; Ngangyo-Heya, M.; Martínez-Ávila, G. Moringa plants: Bioactive compounds and promising applications in food products. Food Res. Int. 2018, 111, 438–450. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Montesano, D.; Lucini, L. The potential of Moringa oleifera in food formulation: a promising source of functional compounds with health-promoting properties. Curr. Opin. Food Sci. 2021, 42, 257–269. [Google Scholar] [CrossRef]
- Gharsallah, K. , et al., Moringa oleifera: Processing, phytochemical composition, and industrial application. South African Journal of Botany, 2023.
- Sharma, K. , et al., Moringa (Moringa oleifera Lam.) polysaccharides: Extraction, characterization, bioactivities, and industrial application. International journal of biological macromolecules, 2022.
- Ajagun-Ogunleye, M.O.; Ebuehi, O.A.T. Evaluation of the anti-aging and antioxidant action of Ananas sativa and Moringa oleifera in a fruit fly model organism. J. Food Biochem. 2020, 44, e13426. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, F.; Berteotti, C.; Schirinzi, V.; Poli, C.; Arrighi, R.; Leone, A. Moringa oleifera and Blood Pressure: Evidence and Potential Mechanisms. Nutrients 2025, 17, 1258. [Google Scholar] [CrossRef]
- Gull, T.; Nouman, W.; Olson, M.E. Industrial applications, toxicological impact and marketing trends of Moringa oleifera food products, a review. South Afr. J. Bot. 2024, 176, 141–157. [Google Scholar] [CrossRef]
- Kurtulbaş, E.; Albarri, R.; Torun, M.; Şahin, S. Encapsulation of Moringa oleifera leaf extract in chitosan-coated alginate microbeads produced by ionic gelation. Food Biosci. 2022, 50. [Google Scholar] [CrossRef]
- Louisa, M.; Patintingan, C.G.H.; Wardhani, B.W.K. Moringa Oleifera Lam. in Cardiometabolic Disorders: A Systematic Review of Recent Studies and Possible Mechanism of Actions. Front. Pharmacol. 2022, 13, 792794. [Google Scholar] [CrossRef]
- Agunbiade, O.J.; Famutimi, O.G.; Kadiri, F.A.; Kolapo, O.A.; Adewale, I.O. Studies on peroxidase from Moringa oleifera Lam leaves. Heliyon 2021, 7, e06032. [Google Scholar] [CrossRef]
- Agunbiade, O.J.; Adewale, I.O. Studies on latent and soluble polyphenol oxidase from Moringa oleifera Lam. leaves. Biocatal. Agric. Biotechnol. 2022, 45. [Google Scholar] [CrossRef]
- Barzan, G.; Sacco, A.; Giovannozzi, A.M.; Portesi, C.; Schiavone, C.; Salafranca, J.; Wrona, M.; Nerín, C.; Rossi, A.M. Development of innovative antioxidant food packaging systems based on natural extracts from food industry waste and Moringa oleifera leaves. Food Chem. 2023, 432, 137088. [Google Scholar] [CrossRef]
- Dubeni, Z.B.; Buwa-Komoreng, L.V.; Mthi, S. The Potential Application of Moringa oleifera Extracts as Natural Preservatives of Chicken Meat. Pharmacogn. Mag. 2024. [Google Scholar] [CrossRef]
- Hemapriya, G., R. Abinaya, and S. Kumar, Textile Effluent Treatment Using Moringa Oleifera. International journal of innovative research and development, 2015. 4.
- Vilaseca, M.; López-Grimau, V.; Gutiérrez-Bouzán, C. Valorization of Waste Obtained from Oil Extraction in Moringa Oleifera Seeds: Coagulation of Reactive Dyes in Textile Effluents. Materials 2014, 7, 6569–6584. [Google Scholar] [CrossRef]
- Worku, G.D.; Abate, S.N. Efficiency comparison of natural coagulants (Cactus pads and Moringa seeds) for treating textile wastewater (in the case of Kombolcha textile industry). Heliyon 2025, 11, e42379. [Google Scholar] [CrossRef]
- Temesgen, S.; Endale, M.; Barega, M.; Habte, M.; Ahmed, S. "Extraction and Application of Moringa Oleifera Seed Kernel Starch for Warp Yarn Sizing in Textile Industry". Trends Text. Eng. Fash. Technol. 2019, 5, 1–5. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef]
- Arif, Y.; Bajguz, A.; Hayat, S. Moringa oleifera Extract as a Natural Plant Biostimulant. J. Plant Growth Regul. 2022, 42, 1291–1306. [Google Scholar] [CrossRef]
- Oberoi, H.K.; Manchanda, P.; Kumar, A.; Umakanth, A.V.; Dhakad, A.K.; Kaur, M.; Kaur, H. Moringa Leaf Extract (MLE) Seed Priming Provides Early Seedling Protection to Biofuel Crop: Sweet Sorghum—Against Salinity. Sugar Tech 2024, 26, 835–850. [Google Scholar] [CrossRef]
- Banerjee, M.; Rajeswari, V.D. Green synthesis of selenium nanoparticles using leaf extract of Moringa oleifera, their biological applications, and effects on the growth of Phaseolus vulgaris-: Agricultural synthetic biotechnology for sustainable nutrition. Biocatal. Agric. Biotechnol. 2023, 55. [Google Scholar] [CrossRef]
- Irshad, S.; Matloob, A.; Ghaffar, A.; Hussain, M.B.; Tahir, M.H.N. Agronomic and biochemical aspects of moringa dried leaf extract mediated growth and yield improvements in soybean. New Zealand J. Crop. Hortic. Sci. 2024, 1–20. [Google Scholar] [CrossRef]
- Alhudhaibi, A.M. , et al., Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress. Green Processing and Synthesis, 2024. 13(1): p. 20240012.
- Ngcobo, B.L., I. Bertling, and S. Mbuyisa, Evaluating the efficacy of Moringa oleifera leaf extracts prepared using different solvents on growth, yield and quality of tomatoes and peppers. Journal of Horticulture and Postharvest Research, 2024. 7(4): p. 389-406.
- Rajani, S.K.; Meena, R.K.; Mishra, P.; Patni, V. Moringa oleifera Lam.: An Updated Review on Micropropagation and Pharmacological Properties. Micropropagation of Medicinal Plants, 2024: p. 171-198.
- Muniandi, S.K.; Ariff, F.F.M.; Pisar, M.M.; Harun, S.T.; Abdullah, M.Z.; Abdullah, F.; Hashim, S.N.A.M.; Bahari, S.N.S.; Saffie, N. Crop Improvement of Moringa oleifera L. through Genotype Screening for the Development of Clonal Propagation Techniques of High-Yielding Clones in Malaysia. Biology 2024, 13, 785. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Faroda, P.; Ameta, K.; Sharma, A.; Gupta, A.K. In vitro morphogenesis and micro-morpho-anatomical developments in Moringa concanensis Nimmo.: An endemic tree of Indian sub-continent. Curr. Plant Biol. 2024, 39. [Google Scholar] [CrossRef]
- Choudhary, R.; Kumari, A.; Kachhwaha, S.; Kothari, S.; Jain, R. Moringa oleifera: Biosynthesis strategies for enhanced metabolites and role in green nanoparticle synthesis. South Afr. J. Bot. 2024, 170, 271–287. [Google Scholar] [CrossRef]
- Nagime, P.V.; Singh, S.; Chidrawar, V.R.; Rajput, A.; Syukri, D.M.; Marwan, N.T.; Shafi, S. Moringa oleifera: A plethora of bioactive reservoirs with tremendous opportunity for green synthesis of silver nanoparticles enabled with multifaceted applications. Nano-Structures Nano-Objects 2024, 40. [Google Scholar] [CrossRef]
- Banerjee, M.; Rajeswari, V.D. Green synthesis of selenium nanoparticles using leaf extract of Moringa oleifera, their biological applications, and effects on the growth of Phaseolus vulgaris-: Agricultural synthetic biotechnology for sustainable nutrition. Biocatal. Agric. Biotechnol. 2023, 55. [Google Scholar] [CrossRef]
- Katata-Seru, L.; Moremedi, T.; Aremu, O.S.; Bahadur, I. Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: Removal of nitrate from water and antibacterial activity against Escherichia coli. J. Mol. Liq. 2018, 256, 296–304. [Google Scholar] [CrossRef]
- Mohammed, G.M.; Hawar, S.N.; Ali, S. Green Biosynthesis of Silver Nanoparticles from Moringa oleifera Leaves and Its Antimicrobial and Cytotoxicity Activities. Int. J. Biomater. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Wahyudi, S.; Rizoputra, I.; Panatarani, C.; Faizal, F.; Bahtiar, A. Green Synthesis of Carbon Nanodots (CNDs) Moderated by Flavonoid Extracts from Moringa oleifera Leaves and Co-Doped Sulfur/Nitrogen (NS – CNDs – Fla) and Their Potential for Heavy Metals Sensing Application. J. Fluoresc. 2024, 1–13. [Google Scholar] [CrossRef]
- Srivastava, N.; Singh, R.; Ahmad, I.; Suliman, M.; Tripathi, S.C.; Rai, A.K.; Gupta, V.K. Lignocellulosic Moringa oleifera bark enabled biofabrication of MgO nanocatalyst: Application in developing temperature tolerance fungal cellulase cocktail. Ind. Crop. Prod. 2023, 207. [Google Scholar] [CrossRef]
- Simões, A.R.; de Souza, A.T.; Meurer, E.C.; Scaliante, M.H.N.O.; Cortelo, T.H. Moringa oleifera: technological innovations and sustainable therapeutic potencials. 2025, 22, e13594–e13594. [CrossRef]
- Ahizi, A.E.; Njoku, C.N.; Onyelucheya, O.E.; Anusi, M.O.; Okonkwo, I.J.; Okoye, P.U.; Igwegbe, C.A. Optimization of Moringa oleifera cationic protein/zeolite adsorbent blend for synthetic turbid water treatment. Sustain. Water Resour. Manag. 2022, 9, 1–12. [Google Scholar] [CrossRef]
- Panigrahi, C.; Kamal, S.; Qin, J.; Ziemann, S.; Rahman, E.; House, M.; Dutcher, C.; Xiong, B. Removal of Pristine and UV-Weathered Microplastics from Water: Moringa oleifera Seed Protein as a Natural Coagulant. Environ. Eng. Sci. 2024, 41, 477–489. [Google Scholar] [CrossRef]
- Pareek, A. , et al., Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. International Journal of Molecular Sciences, 2023. 24.
- Arora, S. and S. Arora, Nutritional significance and therapeutic potential of Moringa oleifera: The wonder plant. Journal of food biochemistry, 2021.
- Abdelazim, A.M.; Afifi, M.; Abu-Alghayth, M.H.; Alkadri, D.H.; Dhull, S.B. Moringa oleifera: Recent Insights for Its Biochemical and Medicinal Applications. J. Food Biochem. 2024, 2024, 1–21. [Google Scholar] [CrossRef]
- Jikah, A.N.; Edo, G.I. Moringa oleifera: a valuable insight into recent advances in medicinal uses and pharmacological activities. J. Sci. Food Agric. 2023, 103, 7343–7361. [Google Scholar] [CrossRef] [PubMed]
- Barman, A.; Kotal, A.; Das, M. Synthesis of metal based nano particles from Moringa Olifera and its biomedical applications: A review. Inorg. Chem. Commun. 2023, 158. [Google Scholar] [CrossRef]
- Rani, N.Z.A.; Kumolosasi, E.; Jasamai, M.; Jamal, J.A.; Lam, K.W.; Husain, K. In vitro anti-allergic activity of Moringa oleifera Lam. extracts and their isolated compounds. BMC Complement. Altern. Med. 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Sun, M.C.; Ruhomally, Z.B.; Boojhawon, R.; Neergheen-Bhujun, V.S. Consumption of Moringa oleifera Lam Leaves Lowers Postprandial Blood Pressure. J. Am. Coll. Nutr. 2019, 39, 54–62. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Aftab, K.; Shaheen, F.; Gilani, A.-U. Hypotensive Constituents from the Pods ofMoringa oleifera. Planta Medica 1998, 64, 225–228. [Google Scholar] [CrossRef]
- Ma, K. , et al., Antihypertensive activity of the ACE–renin inhibitory peptide derived from Moringa oleifera protein. Food & Function, 2021. 12(19): p. 8994-9006.
- Oboh, G.; Oluokun, O.O.; Oyeleye, S.I.; Ogunsuyi, O.B. Moringa seed-supplemented diets modulate ACE activity but not its gene expression in L-NAME-induced hypertensive rats. Biomarkers 2022, 27, 684–693. [Google Scholar] [CrossRef]
- Silveira, F.D.; Gomes, F.I.F.; Val, D.R.D.; Freitas, H.C.; de Assis, E.L.; de Almeida, D.K.C.; Braz, H.L.B.; Barbosa, F.G.; Mafezoli, J.; da Silva, M.R.; et al. Biological and Molecular Docking Evaluation of a Benzylisothiocyanate Semisynthetic Derivative From Moringa oleifera in a Pre-clinical Study of Temporomandibular Joint Pain. Front. Neurosci. 2022, 16, 742239. [Google Scholar] [CrossRef]
- Afrin, S.; Hossain, A.; Begum, S. Effects of Moringa oleifera on working memory: an experimental study with memory-impaired Wistar rats tested in radial arm maze. BMC Res. Notes 2022, 15, 314. [Google Scholar] [CrossRef]
- Bin-Meferij, M.M.; El-Kott, A.F. The radioprotective effects of Moringa oleifera against mobile phone electromagnetic radiation-induced infertility in rats. International Journal of Clinical and Experimental Medicine 2015, 8, 12487–12497. [Google Scholar] [PubMed]
- Sinha, M.; Das, D.K.; Bhattacharjee, S.; Majumdar, S.; Dey, S. Leaf Extract ofMoringa oleiferaPrevents Ionizing Radiation-Induced Oxidative Stress in Mice. J. Med. Food 2011, 14, 1167–1172. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Wu, H.; Tang, S.; Zhang, Y.; Yang, B.; Yang, H.; Huang, L. Therapeutic effect of Moringa oleifera leaves on constipation mice based on pharmacodynamics and serum metabonomics. J. Ethnopharmacol. 2022, 282, 114644. [Google Scholar] [CrossRef]
- Cáceres, A.; Saravia, A.; Rizzo, S.; Zabala, L.; De Leon, E.; Nave, F. Pharmacologie properties of Moringa oleifera. 2: Screening for antispasmodic, antiinflammatory and diuretic activity. J. Ethnopharmacol. 1992, 36, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Fekadu, N.; Basha, H.; Meresa, A.; Degu, S.; Girma, B.; Geleta, B. Diuretic activity of the aqueous crude extract and hot tea infusion of Moringa stenopetala (Baker f.) Cufod. leaves in rats. J. Exp. Pharmacol. 9. [CrossRef]
- Buabeid, M.A. , et al., Anti-inflammatory and anti-angiogenic aattributes of Moringa oleifera Lam. And its nanoclay-based pectin-sericin films. Frontiers in Pharmacology, 2022. 13: p. 890938.
- Omodanisi, E.I., Y. G. Aboua, and O.O. Oguntibeju, Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of Moringa oleifera in diabetes-induced nephrotoxic male Wistar rats. Molecules, 2017. 22(4): p. 439.
- Kumar, R.; Varghese, S.; Ramamurthy, S.; Varadarajan, S.; Balaji, T.M.; Karthick, B.P.; Thiagarajan, K. Assessing the In Vitro Antioxidant and Anti-inflammatory Activity of Moringa oleifera Crude Extract. J. Contemp. Dent. Pr. 2022, 23, 437–442. [Google Scholar] [CrossRef]
- Sayed, A.M.E. , et al., UPLC-MS/MS and GC-MS based metabolites profiling of Moringa oleifera seed with its anti-Helicobacter pylori and anti-inflammatory activities. Natural Product Research, 2022. 36(24): p. 6433-6438.
- Wang, F.; Bao, Y.; Zhang, C.; Zhan, L.; Khan, W.; Siddiqua, S.; Ahmad, S.; Capanoglu, E.; Skalicka-Woźniak, K.; Zou, L.; et al. Bioactive components and anti-diabetic properties of Moringa oleifera Lam. Crit. Rev. Food Sci. Nutr. 2021, 62, 3873–3897. [Google Scholar] [CrossRef] [PubMed]
- Patriota, L.L.d.S.; Ramos, D.d.B.M.; dos Santos, A.C.L.A.; Silva, Y.A.; e Silva, M.G.; Torres, D.J.L.; Procópio, T.F.; de Oliveira, A.M.; Coelho, L.C.B.B.; Pontual, E.V.; et al. Antitumor activity of Moringa oleifera (drumstick tree) flower trypsin inhibitor (MoFTI) in sarcoma 180-bearing mice. Food Chem. Toxicol. 2020, 145, 111691. [Google Scholar] [CrossRef]
- Promkum, C.; Kupradinun, P.; Tuntipopipat, S.; Butryee, C. Nutritive Evaluation and Effect of Moringa oleifera pod on Clastogenic Potential in the Mouse. 2010, 11, 627–632.
- S. , S.; Shenoy, K.B. Septilin: A versatile anticlastogenic, antigenotoxic, antioxidant and histoprotective herbo-mineral formulation on cisplatin-induced toxicity in mice. Mutat. Res. Toxicol. Environ. Mutagen. 2022, 874-875. [Google Scholar] [CrossRef]
- Abo-Elsoud, R.A.E.A. , et al., Moringa oleifera alcoholic extract protected stomach from bisphenol A–induced gastric ulcer in rats via its anti-oxidant and anti-inflammatory activities. Environmental Science and Pollution Research, 2022. 29(45): p. 68830-68841.
- Dalhoumi, W.; Guesmi, F.; Bouzidi, A.; Akermi, S.; Hfaiedh, N.; Saidi, I. Therapeutic strategies of Moringa oleifera Lam. (Moringaceae) for stomach and forestomach ulceration induced by HCl/EtOH in rat model. Saudi J. Biol. Sci. 2022, 29, 103284. [Google Scholar] [CrossRef]
- Tian, H.; Wen, Z.; Liu, Z.; Guo, Y.; Liu, G.; Sun, B. Comprehensive analysis of microbiome, metabolome and transcriptome revealed the mechanisms of Moringa oleifera polysaccharide on preventing ulcerative colitis. Int. J. Biol. Macromol. 2022, 222, 573–586. [Google Scholar] [CrossRef]
- Adeoye, A.O.; Falode, J.A.; Jeje, T.O.; Agbetuyi-Tayo, P.T.; Giwa, S.M.; Tijani, Y.O.; Akinola, D.E. Modulatory Potential of Citrus sinensis and Moringa oleifera Extracts and Epiphytes on Rat Liver Mitochondrial Permeability Transition Pore. Curr. Cancer Drug Targets 2022, 19, 1–1. [Google Scholar] [CrossRef] [PubMed]
- Alkhudhayri, D.A.; Osman, M.A.; Alshammari, G.M.; Al Maiman, S.A.; Yahya, M.A. Moringa peregrina leaf extracts produce anti-obesity, hypoglycemic, anti-hyperlipidemic, and hepatoprotective effects on high-fat diet fed rats. Saudi J. Biol. Sci. 2021, 28, 3333–3342. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Sun, C.; Li, R.; Li, W.; Ge, Z.; Adu-Frimpong, M.; Xu, X.; Yu, J. Amelioration action of gastrodigenin rhamno-pyranoside from Moringa seeds on non-alcoholic fatty liver disease. Food Chem. 2022, 379, 132087. [Google Scholar] [CrossRef]
- Chuang, P.; Lee, C.; Chou, J.; Murugan, M.; Shieh, B.; Chen, H. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour. Technol. 2007, 98, 232–236. [Google Scholar] [CrossRef]
- Donli, P.; Dauda, H. Evaluation of aqueous Moringa seed extract as a seed treatment biofungicide for groundnuts. Pest Manag. Sci. 2003, 59, 1060–1062. [Google Scholar] [CrossRef]
- Ghasi, S.; Nwobodo, E.; Ofili, J. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed wistar rats. J. Ethnopharmacol. 2000, 69, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Balaraman, R.; Amin, A.; Bafna, P.; Gulati, O. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol. 2003, 86, 191–195. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agroclimatic Origins of Drumstick Tree (Moringa oleifera Lam.) Leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Patriota, L.L.d.S.; Santos, D.K.D.D.N.; Barros, B.R.d.S.; Aguiar, L.M.d.S.; Silva, Y.A.; dos Santos, A.C.L.A.; e Silva, M.G.; Coelho, L.C.B.B.; Paiva, P.M.G.; Pontual, E.V.; et al. Evaluation of the In Vivo Acute Toxicity and In Vitro Hemolytic and Immunomodulatory Activities of the Moringa oleifera Flower Trypsin Inhibitor (MoFTI). Protein Pept. Lett. 2021, 28, 665–674. [Google Scholar] [CrossRef]
- Anudeep, S.; Prasanna, V.K.; Adya, S.M.; Radha, C. Characterization of soluble dietary fiber from Moringa oleifera seeds and its immunomodulatory effects. Int. J. Biol. Macromol. 2016, 91, 656–662. [Google Scholar] [CrossRef]
- Coriolano, M.C.; Brito, J.d.S.; Patriota, L.L.d.S.; Soares, A.K.d.A.; de Lorena, V.M.; Paiva, P.M.; Napoleao, T.H.; Coelho, L.C.; de Melo, C.M. Immunomodulatory Effects of the Water-soluble Lectin from Moringa oleifera Seeds (WSMoL) on Human Peripheral Blood Mononuclear Cells (PBMC). Protein Pept. Lett. 2018, 25, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Viera, G.H.F.; Mourão, J.A.; Ângelo, Â.M.; Costa, R.A.; Vieira, R.H.S.d.F. Antibacterial effect (in vitro) of Moringa oleifera and Annona muricata against Gram positive and Gram negative bacteria. Rev. do Inst. de Med. Trop. de Sao Paulo 2010, 52, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Redha, A.A.; Perna, S.; Riva, A.; Petrangolini, G.; Peroni, G.; Nichetti, M.; Iannello, G.; Naso, M.; Faliva, M.A.; Rondanelli, M. Novel insights on anti-obesity potential of the miracle tree, Moringa oleifera: A systematic review. J. Funct. Foods 2021, 84. [Google Scholar] [CrossRef]
- Adarthaiya, S. and A. Sehgal, Moringa oleifera Lam. as a potential plant for alleviation of the metabolic syndrome—A narrative review based on in vivo and clinical studies. Phytotherapy Research, 2023. 38: p. 755-775.
- Nova, E.; Redondo-Useros, N.; Martínez-García, R.M.; Gómez-Martínez, S.; Díaz-Prieto, L.E.; Marcos, A. Potential of Moringa oleifera to Improve Glucose Control for the Prevention of Diabetes and Related Metabolic Alterations: A Systematic Review of Animal and Human Studies. Nutrients 2020, 12, 2050. [Google Scholar] [CrossRef]
- David, S.; Dapar, M.P.; Jimam, N.S. Clinical antihypertensive efficacy and safety of Moringa oleifera Lam. (Moringaceae) leaf: a systematic review. J. Pharm. Bioresour. 2025, 22, 1–12. [Google Scholar] [CrossRef]
- Louisa, M.; Patintingan, C.G.H.; Wardhani, B.W.K. Moringa Oleifera Lam. in Cardiometabolic Disorders: A Systematic Review of Recent Studies and Possible Mechanism of Actions. Front. Pharmacol. 2022, 13, 792794. [Google Scholar] [CrossRef]
- Gambo, A.; Moodley, I.; Babashani, M.; Babalola, T.K.; Gqaleni, N.; Monera-Penduka, T.G. A double-blind, randomized controlled trial to examine the effect of Moringa oleifera leaf powder supplementation on the immune status and anthropometric parameters of adult HIV patients on antiretroviral therapy in a resource-limited setting. PLOS ONE 2021, 16, e0261935. [Google Scholar] [CrossRef]
- Fungtammasan, S.; Phupong, V.; Ho, J.J. The effect of Moringa oleifera capsule in increasing breastmilk volume in early postpartum patients: A double-blind, randomized controlled trial. PLOS ONE 2021, 16, e0248950. [Google Scholar] [CrossRef]
- Anumula, L.; Ramesh, S.; Chinni, S.K.; Punamalli, P.; Kolaparthi, V.S.K.; A, L. Clinical Assessment of Moringa oleifera as a Natural Crosslinker for Enhanced Dentin Bond Durability: A Randomized Controlled Trial. Cureus 2023, 15, e46304. [Google Scholar] [CrossRef]
- Tshabalala, T.; Ncube, B.; Madala, N.E.; Nyakudya, T.T.; Moyo, H.P.; Sibanda, M.; Ndhlala, A.R. Scribbling the Cat: A Case of the “Miracle” Plant, Moringa oleifera. Plants 2019, 8, 510. [Google Scholar] [CrossRef]
- Villegas-Vazquez, E.Y.; Gómez-Cansino, R.; Marcelino-Pérez, G.; Jiménez-López, D.; Quintas-Granados, L.I. Unveiling the Miracle Tree: Therapeutic Potential of Moringa oleifera in Chronic Disease Management and Beyond. Biomedicines 2025, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Badwaik, H.R.; Al Hoque, A.; Kumari, L.; Sakure, K.; Baghel, M.; Giri, T.K. Moringa gum and its modified form as a potential green polymer used in biomedical field. Carbohydr. Polym. 2020, 249, 116893. [Google Scholar] [CrossRef] [PubMed]
- Bessalah, S. , et al., Antibacterial, Anti-Biofilm, and Anti-Inflammatory Properties of Gelatin–Chitosan–Moringa-Biopolymer-Based Wound Dressings towards Staphylococcus aureus and Escherichia coli. Pharmaceuticals, 2024. 17.
- Kumar, R.; Singh, B. Functional network copolymeric hydrogels derived from moringa gum: Physiochemical, drug delivery and biomedical properties. Int. J. Biol. Macromol. 2024, 275, 133352. [Google Scholar] [CrossRef]
- Kamel, S. , et al., Wound Dressings Based on Sodium Alginate–Polyvinyl Alcohol–Moringa oleifera Extracts. Pharmaceutics, 2023. 15.
- Gheorghita, R.; Filip, R.; Lupaescu, A.-V.; Iavorschi, M.; Anchidin-Norocel, L.; Gutt, G. Innovative Materials with Possible Applications in the Wound Dressings Field: Alginate-Based Films with Moringa oleifera Extract. Gels 2023, 9, 560. [Google Scholar] [CrossRef]
- Sharma, S.; Bal, T. Evaluation of a green synthesized biopolymer polymethyl methacrylate grafted Moringa gum amphiphilic graft copolymer (MOG-g-PMMA) with polymeric-surfactant like properties for biopharmaceutical applications. Polym. Bull. 2024, 81, 17017–17047. [Google Scholar] [CrossRef]
- Banik, S.; Biswas, S.; Karmakar, S. Extraction, purification, and activity of protease from the leaves of Moringa oleifera. F1000Research 2018, 7, 1151. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.C.; Martins, I.M.; Manrique, Y.A.; Rodrigues, A.E.; Barreiro, M.F.; Dias, M.M. Advancements in conventional and supercritical CO2 extraction of Moringa oleifera bioactives for cosmetic applications: A review. J. Supercrit. Fluids 2024, 214. [Google Scholar] [CrossRef]
- El-Sharkawy, R.M.; El-Hadary, A.E.; Essawy, H.S.; El-Sayed, A.S.A. Rutin of Moringa oleifera as a potential inhibitor to Agaricus bisporus tyrosinase as revealed from the molecular dynamics of inhibition. Sci. Rep. 2024, 14, 1–16. [Google Scholar] [CrossRef]
- Abidin, Z. , et al., Moringa oleifera Leaves’ Extract Enhances Nonspecific Immune Responses, Resistance against Vibrio alginolyticus, and Growth in Whiteleg Shrimp (Penaeus vannamei). Animals: An Open Access Journal from MDPI, 2021. 12.
- Shan, T.C.; Al Matar, M.; Makky, E.A.; Ali, E.N. The use of Moringa oleifera seed as a natural coagulant for wastewater treatment and heavy metals removal. Appl. Water Sci. 2016, 7, 1369–1376. [Google Scholar] [CrossRef]
- Villaseñor-Basulto, D.L.; Astudillo-Sánchez, P.D.; del Real-Olvera, J.; Bandala, E.R. Wastewater treatment using Moringa oleifera Lam seeds: A review. J. Water Process. Eng. 2018, 23, 151–164. [Google Scholar] [CrossRef]
- Salem, A. El-Salam, and R. Abdel, Moringa plant powders as repellent effect against the stored products insects. 2020.
- Sanusi, L.; Ibrahim, N.D. Comparative efficacy of moringa, neem and lemon grass leaf powders in the control of bean beetle (Callosobruchus maculatus Fab.) infesting cowpea (Vigna unguiculata L. Walp). J. Agric. Environ. 2024, 20, 211–225. [Google Scholar] [CrossRef]
- Mohammed, A.L.; Iddriss, M. EFFECT OF MORINGA (MORINGA OLEIFERA) LEAF POWDER, NEEM (AZADIRACHTA INDICA) LEAF POWDER, AND CAMPHOR ON WEEVIL (CALLOSOBRUCHUS MACULATUS F.) IN STORED COWPEA (VIGNA UNGUICULATA (L.) WALP) SEEDS. Spring 2023, 55, 257–269. [Google Scholar] [CrossRef]
- Ria, E.R.; Hidayat, E.; Muliani, Y.; Komariah, A.; Abdullah, R.; Masnenah, E.; Kantikowati, E. Moringa Leaf Powder as Environmentally Friendly Repellent Agent for Controlling the Warehouse Insect Pest for Black Soybean Grain. J. Agrosci 2024, 1, 235–245. [Google Scholar] [CrossRef]
- Eseabasi, R. and U. Ime O, Effect of Moringa oleifera Leaf Powder and Seed Oil on Insect Pests of Stored Maize and Cowpea. International Journal of Life Science and Agriculture Research, 2024.
- Santos, N. , et al., Insecticidal Activity of Lectin Preparations from Moringa oleifera Lam. (Moringaceae) Seeds Against Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Plants, 2025. 14.
- Damilola, A.M. and M.F.O. Temitope, Assessment of Moringa oleifera as Bio-Pesticide against Podagrica spp on the growth and yield of Okra (Abelmoschus esculentus L. Moench). Journal of Horticulture, 2020. 7: p. 1-11.
- Kotsou, K.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Rumbos, C.I.; Athanassiou, C.G.; Lalas, S.I. Enhancing the Nutritional Profile of Tenebrio molitor Using the Leaves of Moringa oleifera. Foods 2023, 12, 2612. [Google Scholar] [CrossRef]
- Mirhashemi, M.S.; Mohseni, S.; Hasanzadeh, M.; Pishvaee, M.S. Moringa oleifera biomass-to-biodiesel supply chain design: An opportunity to combat desertification in Iran. J. Clean. Prod. 2018, 203, 313–327. [Google Scholar] [CrossRef]
- Al-Khalifah, N. and A. Shanavaskhan, Moringa oleifera Lam., a promising crop species for arid conditions of Saudi Arabia and Moringa peregrina (Forssk.) Fiori, a native wild species for crop improvement. 2017: p. 159-170.
- Bilali, H.E. , et al., Research on Moringa (Moringa oleifera Lam.) in Africa. Plants, 2024. 13.
- Vaknin, Y.; Mishal, A. The potential of the tropical “miracle tree” Moringa oleifera and its desert relative Moringa peregrina as edible seed-oil and protein crops under Mediterranean conditions. Sci. Hortic. 2017, 225, 431–437. [Google Scholar] [CrossRef]
- Bayomy, H.M.; Alamri, E.S.; Alharbi, B.M.; Almasoudi, S.E.; Ozaybi, N.A.; Mohammed, G.M.; Genaidy, E.A.; Atteya, A.K.G. Oil Yield and Bioactive Compounds of Moringa oleifera Trees Grown Under Saline Conditions. Plants 2025, 14, 509. [Google Scholar] [CrossRef]
- Ricardo, A. , Seed characteristics, oil content and fatty acid composition of moringa (Moringa oleifera Lam.) seeds from three arid land locations in Ecuador. Industrial Crops and Products, 2019.
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef]
- Mashamaite, C.; Mothapo, P.; Albien, A.; Pieterse, P.; Phiri, E. A SUSPECT under the National Environmental Management Biodiversity Act (NEM:BA) Moringa oleifera's ecological and social costs and benefits. South Afr. J. Bot. 2020, 129, 249–254. [Google Scholar] [CrossRef]
- Ayoub, M.; Hussain, S.; Khan, A.; Zahid, M.; Wahid, J.A.; Zhifang, L.; Rehman, R. A Predictive Machine Learning and Deep Learning Approach on Agriculture Datasets for New Moringa Oleifera Varieties Prediction. Pak. J. Eng. Technol. 2022, 5. [Google Scholar] [CrossRef]
- Ndayakunze, A.; Steyn, J.M.; du Plooy, C.P.; Araya, N.A. Measurement and modelling of Moringa transpiration for improved irrigation management. Agric. Water Manag. 2024, 305. [Google Scholar] [CrossRef]
| M. peregrina | M. stenopetala | M. oleifera | Ref | |
| Total of phenolic compounds |
200.12 mg/100 g | 243.00 mg/100 g | 241.05 mg/100 g | [9] |
| Total of flavonoids | 7 mg/100 g | 3.05 mg/100 g | 6.06 mg/100 g | [9] |
| Antioxidant activity | 1066.39 mg/100 g | 1226.75 mg/100 g | 745.64 mg/100 g | [9] |
| Study title | NCT Number | Locations | Study status | Sex | Age | Phases | Study type | Conditions | Summary |
| Effect of Moringa oleifera mouthwash | NCT05191069 | Islamabad Capital Territory, Pakistan | Unknown* | All | Child, adult | NA | Interventional | Orthodontic appliance complication | This study evaluates the effectiveness of MO mouthwash in enhancing oral hygiene during orthodontic treatment. It examines its role in preventing gingivitis, periodontitis, plaque formation, enamel demineralization, tooth discoloration, and reducing bacterial load in plaque. |
| Moringa oleifera on bone density | NCT03026660 | Boone, North Carolina, United States | Completed | Female | Adult, older adult | NA | Interventional | Osteoporosis, osteopenia, postmenopausal osteoporosis | This study aims to evaluate the effects of daily 1000 mg MO supplementation over 12 weeks on bone structure and function in postmenopausal women. |
| Moringa oleifera- antiretroviral pharmacokinetic drug interaction | NCT01410058 | Harare, Zimbabwe | Completed | All | Adult, older adult | - | Observational | HIV | The use of MO Lam leaf powder at its traditional dosage did not significantly affect the steady-state pharmacokinetics of nevirapine. |
| Moringa supplementation for improved milk output | NCT05333939 | Lexington, Kentucky, United States | Completed | All | Child, adult, older adult | NA | Interventional | Breastfeeding | The study gathers data on whether daily 4g MO supplementation for four weeks enhances breast milk quantity, quality, and infant health versus placebo. Moringa is expected to boost milk output and the proportion of the infant's intake from the mother. |
| Effect of Moringa oleifera infusion on health | NCT04314258 | Moka, Please Select, Mauritius | Unknown* | All | Adult, older adult | NA | Interventional | Metabolic syndrome | This study explores the effects of MO leaf tea on health markers in hyperglycemic individuals (fasting blood glucose ≥ 5.5 mmol/L). Objectives include assessing impacts on blood glucose, lipid profiles, and antioxidant levels, comparing healthy and hyperglycemic individuals. |
| Effect of Moringa oleifera leaves on glycemic control of women with type 2 diabetes | NCT06517602 | Tindouf, Algeria | Completed | Female | Adult, older adult | NA | Interventional | Type 2 diabetes | This clinical trial assessed if daily MO leaf powder supplementation, alongside oral hypoglycemic therapy, improved glycemic control in Sahrawi women with type 2 diabetes. Researchers measured changes in glycosylated hemoglobin, fasting blood glucose, and clinical, metabolic, and body composition parameters at the study's start and end. |
| Remineralization efficacy of Moringa oleifera varnish vs MI varnish in initial carious lesions over 6 months follow up: a randomized controlled clinical trial | NCT06905379 | Cairo, Egypt | Not yet recruiting | All | Adult | NA | Interventional | White spot lesions [initial caries] on smooth surface of toot | This clinical trial assessed the remineralization efficacy of MO varnish versus MI Varnish (CPP-ACP) on incipient carious lesions. Participants aged 25–35 with at least one active white spot lesion (WSL) and good oral hygiene provided informed consent |
| Effects of Moringa oleifera on hsCRP and Hgba1c level of patients in Hospital ng Maynila medical center diabetic clinic | NCT02308683 | Location not provided | Completed | All | Adult, older adult | Phase1 | Interventional | Diabetes | This cohort study investigates the effects of MO leaf supplementation on inflammation and glycemic control in patients with type 2 diabetes. The study focuses on high-sensitivity C-reactive protein (hsCRP) as a key inflammatory marker, along with HbA1c. clinical outcomes. |
| Effect of Moringa leaf extract on disease activity in rheumatoid arthritis patients | NCT05665985 | Surakarta, Central Java, Indonesia | Completed | Female | Adult | Phase 1, phase 2 | Interventional | Rheumatoid arthritis | This study evaluates the effects of MO extract on rheumatoid arthritis activity. Patients received MO in a 30-day treatment regimen to assess changes in disease activity during the intervention. |
| Effect of aerobic training and Moringa oleifera on dyslipidemia and cardiac endurance | NCT04164771 | Location not provided | Unknown* | Male | Adult | NA | Interventional | Dyslipidemias | Moringa leaves are highly effective against various diseases, particularly diabetes, blood pressure issues, dyslipidemia, and cancer. |
| Effect of Moringa oleifera on metformin plasma level in type 2 diabetes mellitus patients | NCT03189407 | Location not provided | Completed | All | Adult, older adult | NA | Interventional | Type 2 diabetes mellitus | This study evaluated the effects of a seven-day, twice-daily hot water infusion of dried MO leaves on the plasma concentrations of Metformin in type 2 diabetes patients already on Metformin for at least three years months. |
| Moringa oleifera (drumstick leaves) for improving haemoglobin, vitamin a status and underweight among adolescent girls in rural Bangladesh: a quasi-experimental study | NCT04156321 | Dhaka, Bangladesh | Unknown* | Female | Child | Phase 3 | Interventional | Assess the impact of Moringa leaves on serum heamoglobin and vitamin A level among the adolescent girls | NA |
| Anticariogenic effect of Moringa oleifera mouthwash compared to chlorhexidine mouthwash | NCT04575948 | Location not provided | Not yet recruiting | All | Adult | Phase 2, phase 3 | Interventional | Plaque, dental, antimicrobial, mouthwash, cytotoxicity | Part I: This in-vitro study aims to formulate a nontoxic mouthwash from MO leaves extract, which has antimicrobial activity, for use in Part II. Additionally, the mouthwash's stability and efficacy will be evaluated. Part II: This randomized controlled trial assesses the antibacterial, antiplaque, and anticariogenic effects of MO mouthwash versus chlorhexidine mouthwash. |
| Effects of Moringa oleifera leaves on glycemia, lipemia and inflammatory profile in prediabetic patients | NCT04734132 | Madrid, Spain | Completed | All | Adult, older adult | NA | Interventional | Prediabetes | This proposal studies the efficacy of MO in controlling glycaemia in prediabetic subjects. A 3-month dietary intervention with MO dry leaf capsules will be compared to a placebo. |
| Nutritional impact of Moringa oleifera leaf supplementation in mothers and children | NCT04587271 | Kisumu, Kenya | Completed | All | Child, adult, older adult | NA | Interventional | Malnutrition, wasting, and growth failure | The primary outcomes were infant growth and maternal milk production, while secondary outcomes included maternal and infant vitamin A and iron status and changes in their intestinal health. |
| Effects of Allium sativum and Moringa oleifera extract on dental enamel | NCT05744752 | Karachi, Sindh, Pakistan | Unknown* | Male | Child | NA | Interventional | Lead poisoning | The objective is to compare the protective effects of Allium sativum (AS) and MO on dental enamel defects from lead and to determine their benefits in remineralizing dental enamel. |
| Effect of Moringa oleifera leaf on hemoglobin levels in anemia | NCT05737862 | Bandung, West Java, Indonesia | Completed | Female | Child, adult | Phase 3 | Interventional | Anemia of pregnancy | This study aimed to compare hemoglobin levels in pregnant women between the treatment group, which received Moringa leaf capsules and iron tablets, and the control group, which received only iron tablets. |
| Evaluation of Artemisia annua and Moringa | NCT03366922 | Mbarara, SouthWestern, Uganda | Completed | All | Adult, older adult | NA | Interventional | HIV infections | Determine the effect of A. annua L. and MO leaf powder on CD4 cell count and immunological indices in HIV patients receiving Highly Active Antiretroviral Therapy. |
| Anti-plaque and anti-gingivitis effects of Moringa plant extract and fluoride toothpastes | NCT05390099 | Giza, Egypt | Unknown* | All | Child | NA | Interventional | Oral disease | This study assesses and compares the anti-plaque and anti-gingivitis effects of Moringa plant extract and fluoride toothpastes in Egyptian children. |
| Effect of Moringa oleifera capsule in increasing breast milk volume in early postpartum patients | NCT04487613 | Bangkok, Thailand | Completed | Female | Adult, older adult | Phase 4 | Interventional | Postpartum women | This study aims to assess how MO leaf capsules influence breast milk production. |
| Effects of Moringa oleifera leaf powders on hematological profiles in pregnant women with iron deficiency anemia | NCT06875947 | Cianjur, West Java, Indonesia | Not yet recruiting | Female | Adult | Phase 4 | Interventional | Iron deficiency anemia of pregnancy, pregnancy complications, inflammation, Moringa oleifera, cytokines (IL-1, IL-6), hepcidin | This study investigates micronized Moringa leaf powders as a natural supplement to enhance hemoglobin levels in pregnant women with iron deficiency anemia. Participants will undergo regular blood tests to assess hemoglobin levels, iron status markers (hepcidin, TIBC), and inflammatory cytokines (IL-1, IL-6). The study also evaluates the safety of Moringa supplements, focusing on liver and kidney functions. |
| Impact of dried Moringa oleifera leaves in enhancing hemoglobin status | NCT03514472 | Location not provided | Completed | Female | Child, adult | NA | Interventional | Anemia, iron deficiency | This research project targets nutritional deficiencies, particularly iron deficiency anemia, in reproductive-aged females from underprivileged groups. Anemia can result in stillbirths, preterm deliveries, and low birth weight, potentially leading to cognitive disabilities, emphasizing the need for priority treatment. |
| Effect of Moringa leaf capsules on glycemic control of type 2 diabetic patients | NCT06125873 | Islamabad, Federal, Pakistan | Enrolling by invitation | All | Adult, older adult | Phase 2 | Interventional | Diabetes mellitus type 2 | A clinical trial will involve 50 patients randomly divided into two groups to compare glycemic control in Type 2 Diabetes Mellitus using MO capsules. |
| Evaluation of Moringa oleifera leaf extract versus sodium hypochlorite in pulpectomy of nonvital primary molars | NCT06948526 | El-Manial, Giza, Egypt | Not yet recruiting | All | Child | NA | Interventional | Nonvital primary molars | This trial compares the success of MO leaf extract and sodium hypochlorite as intracanal irrigants in pulpectomy of nonvital primary molars in children aged 3 to 7. It evaluates clinical parameters (pain, swelling, mobility) and radiographic healing (periapical changes, root resorption) over 12 months. |
| Antifungal potential of Moringa oleifera against otomycosis | NCT04768829 | Minya, Egypt | Completed | All | Adult | Early phase 1 | Interventional | Otomycosis | One group of patients with otomycosis received Nystatin ear drops, while the other received Moringa ear drops. An otolaryngologist performs an endoscopic examination, and their swabs will be analyzed using ELISA assays. |
| A study to explore the effect of Moringa oleifera (E-HS-01) on flow mediated dilatation and hemodynamics | NCT05002881 | Mumbai, Maharashtra, India | Unknown* | Male | Adult | NA | Interventional | Endothelial function | This study evaluates how MO affects vascular endothelial function, investigating its vasodilation potential by analyzing flow-mediated dilation (FMD) and blood flow velocity (BFV) in healthy males. |
| Effect of Moringa oleifera leaf extract on postoperative pain and bacterial reduction in mandibular premolars | NCT05348824 | Location not provided | Unknown* | All | Adult | Phase 2, phase 3 | Interventional | Necrotic pulp | This study clinically compares post-operative pain intensity and bacterial reduction with MO leaf extract solution versus 2.5% NaOCl in asymptomatic necrotic mandibular premolars treated in a single visit. |
| The cardiovascular and renal effects of Moringa oleifera extracts and Stevia rebaudiana Bertoni in patients with type II diabetes mellitus | NCT04254029 | Yaounde, Cameroon | Completed | All | Adult, older adult | Phase 4 | Interventional | Benefits of capsules of M. oleifera and Stevia rebaudiana Bertoni in patients with type 2 diabetes mellitus before and after 45 days of add-on therapy | This study aimed to evaluate MO and stevia's cardiovascular and renal benefits in type 2 diabetes patients over 8 weeks. |
| Antidiabetic potiential of Moringa and Dom extract | NCT05898750 | Minya, Egypt | Completed | All | Adult | Early phase 1 | Interventional | Diabetes | The antidiabetic properties of Hyphaene thebaica fruits and MO leaves will be studied in type 2 diabetic patients consuming tea from both for six weeks. Their fasting blood glucose levels will be monitored daily, alongside other biomarkers such as insulin concentration, lipid profile, liver enzymes, c-peptide, and glycated hemoglobin. |
| Moringa; delivering nutrition and economic value to the people of Malawi | NCT04092517 | Aberdeen, United Kingdom | Completed | All | Adult, older adult | NA | Interventional | Malnourishment | This study compares Moringa as a substitute in supplementary foods to evaluate nutrient bioavailability, bioactives, and the plant's activities. It assesses Moringa's potential as an economically viable crop to support a resilient food supply chain in Malawi, ensuring access to essential nutrients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





