Submitted:
12 June 2025
Posted:
16 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
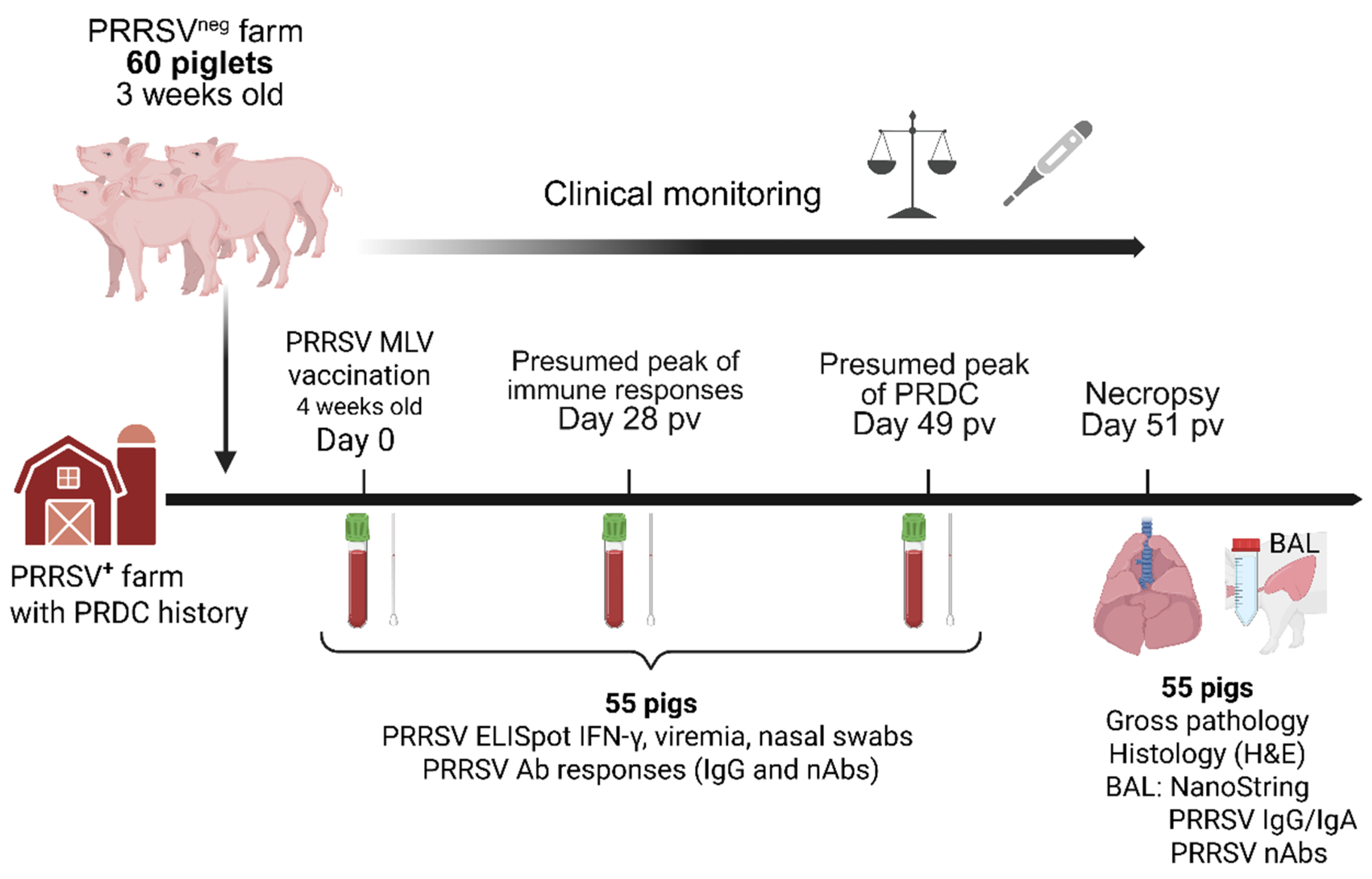
2.1. Study Design, Necropsy Procedure, Samples Collection and Processing
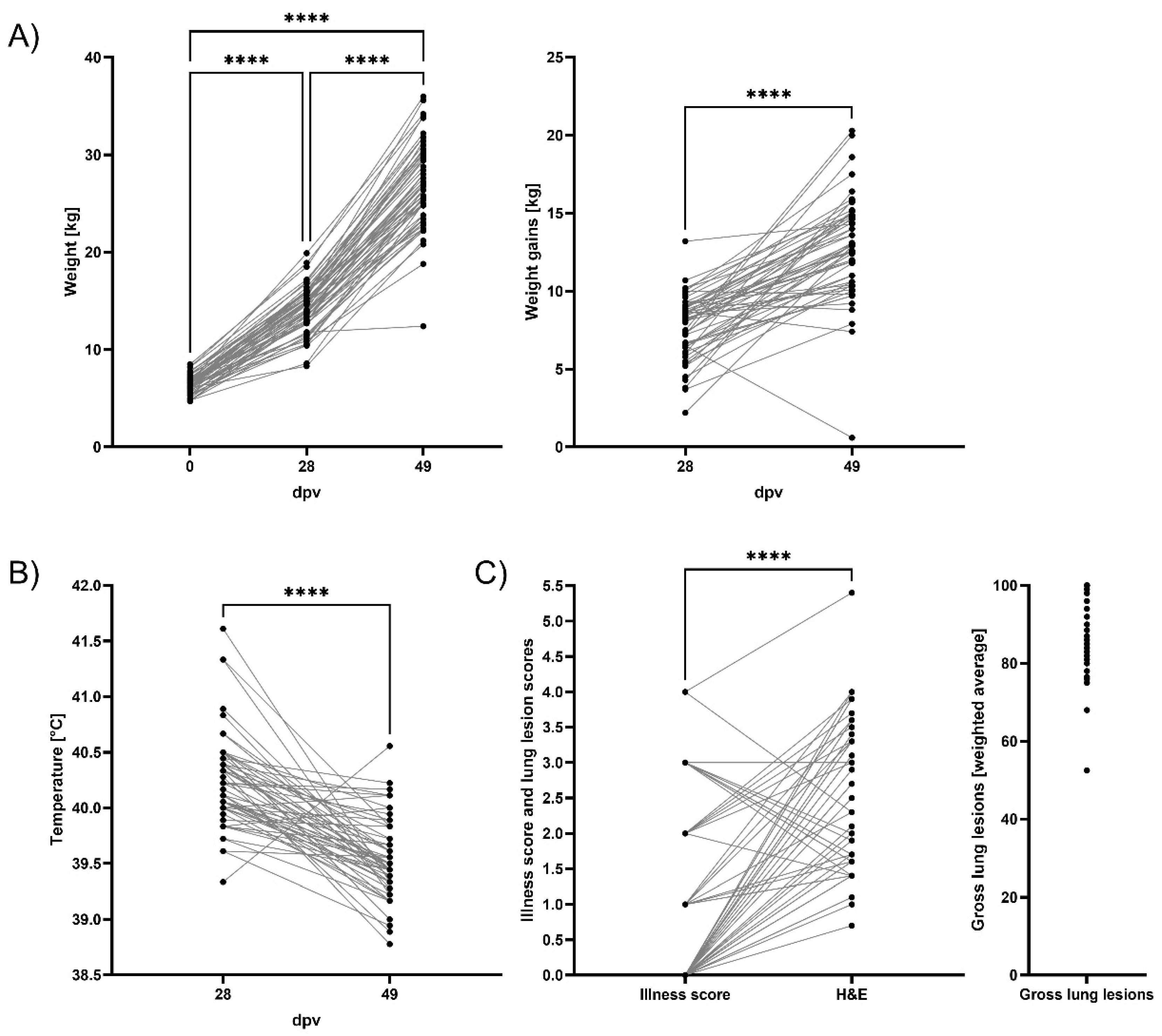
2.2. Clinical Score, Lung Gross Lesions and Histopathology Scoring
2.3. PRRSV−2 Isolation, Propagation, Titration
2.4. Viremia and Viral Shedding
2.5. Anti-PRRSV IgG and IgA Analyses
2.6. Neutralizing Antibodies Against PRRSV-2 Field Strain (NC20-1)
2.7. ELISpot IFN-γ Assay
2.8. NanoString Gene Expression Analysis
2.9. Statistical Analysis
3. Results
3.1. Weight Gains, Clinical Evaluation, and Lung Pathology
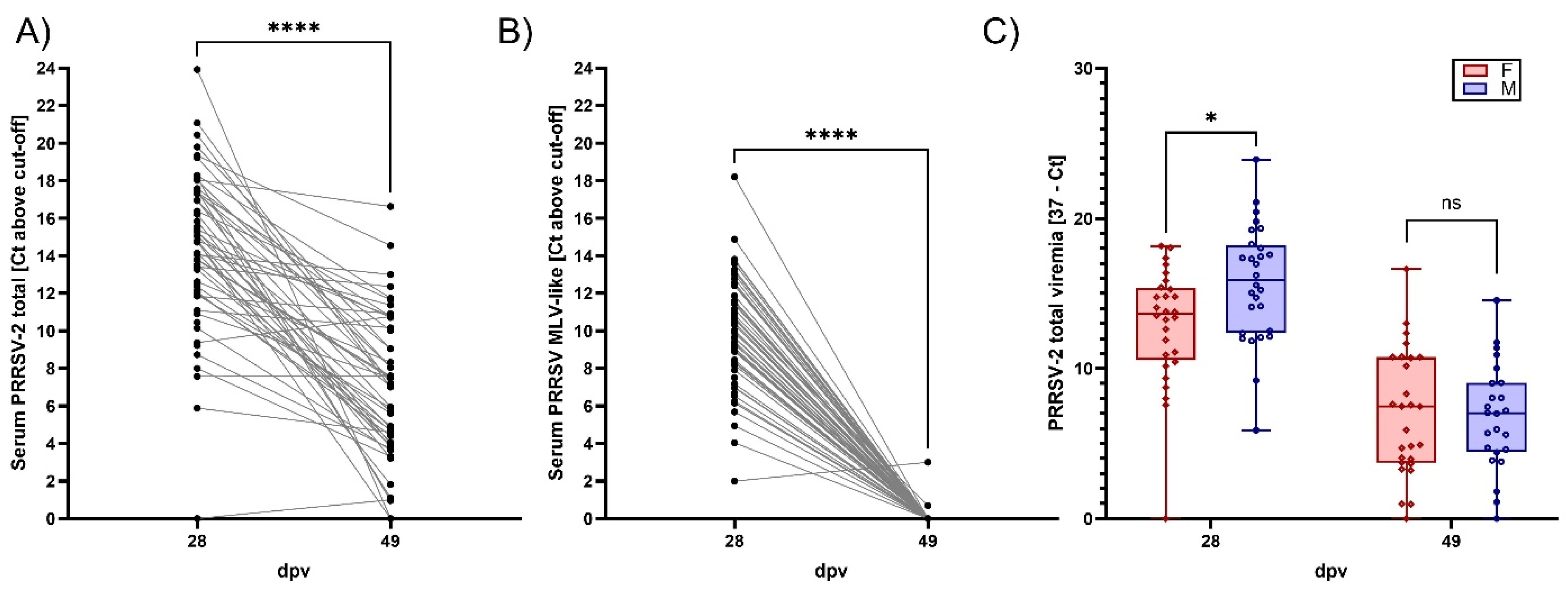
3.2. Viremia, BAL Viral Loads, and Viral Shedding of PRRSV-2
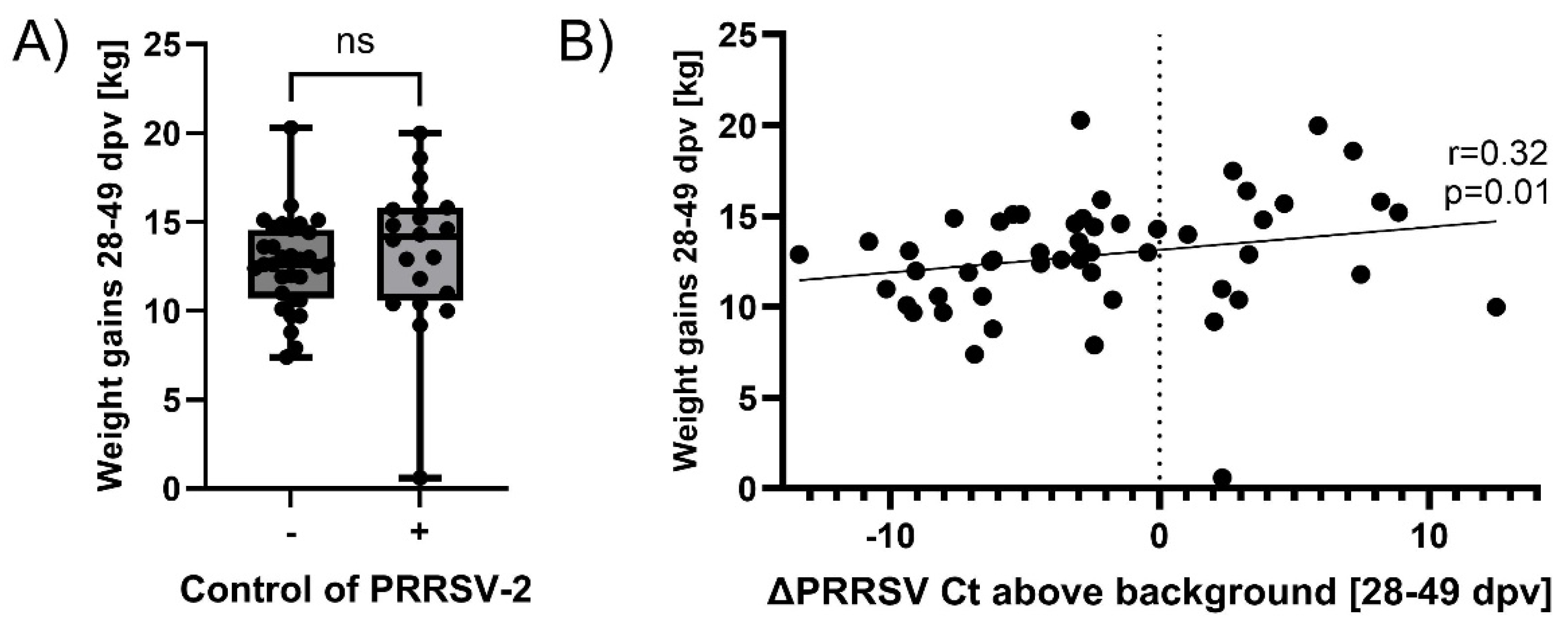
3.3. Controlling PRRSV-2 Field Strain Infection Improves Pig Weight Gains During PRDC
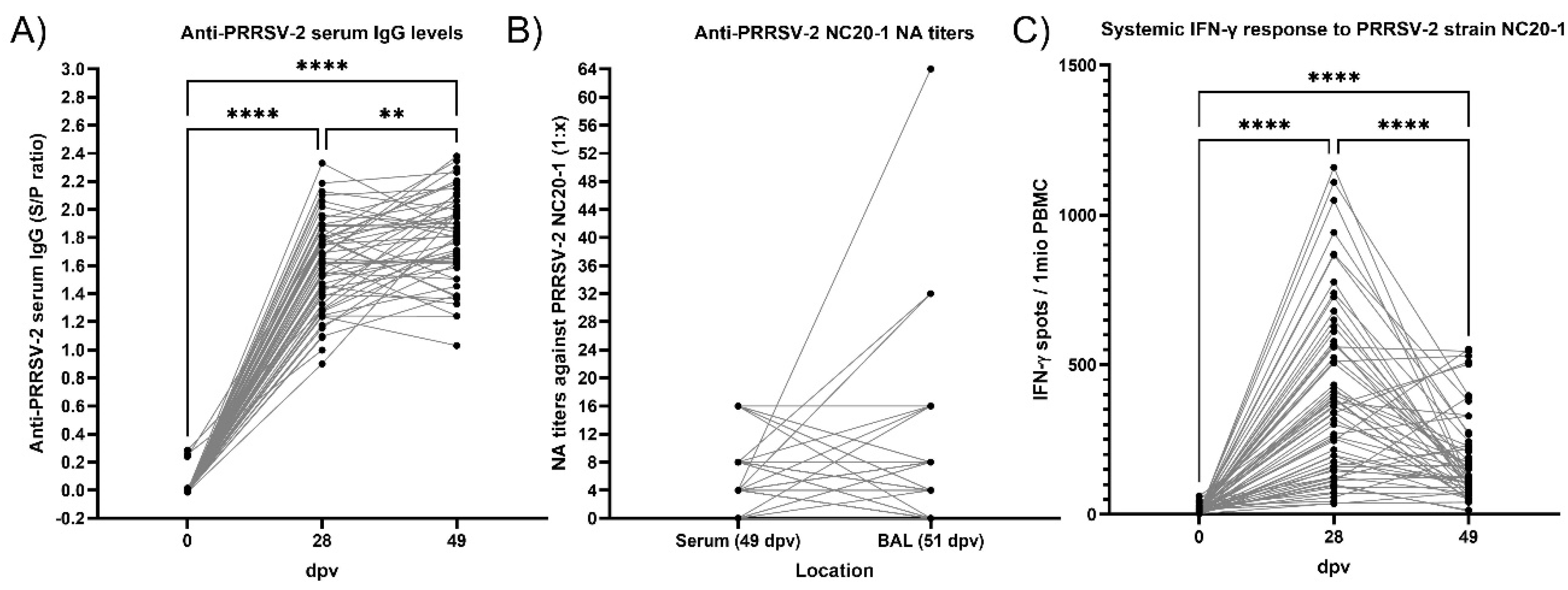
3.4. Antibody Response to PRRSV Vaccination and Exposure Before and During PRDC
3.5. Cellular Interferon-γ Response to PRRSV
3.6. Relations Between Anti-PRRSV Immune Parameters and PRRSV Levels
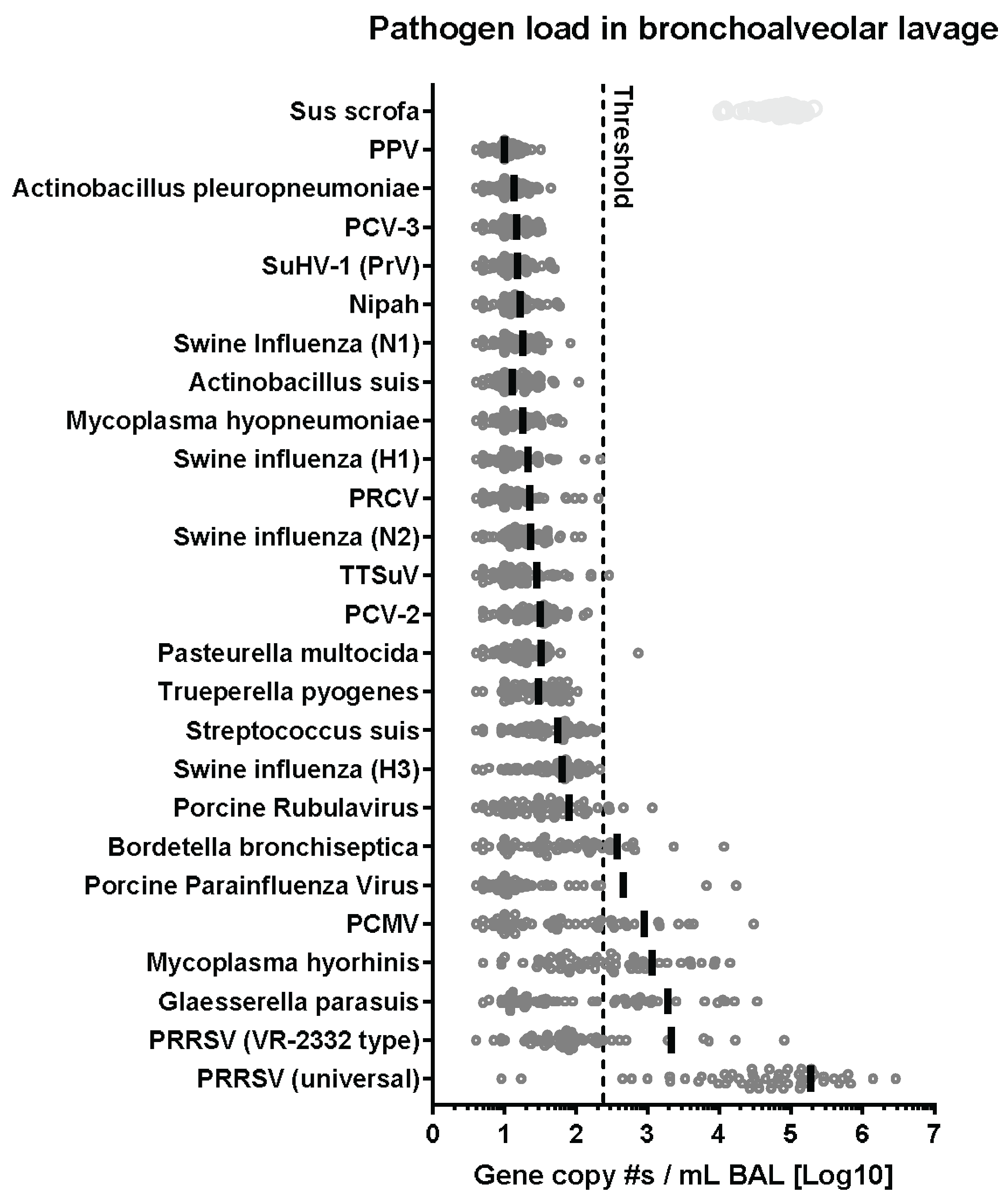
3.7. PRDC-Related Pathogens and Their Relationships with PRRSV
3.7.1. Overall Prevalence of 21 Respiratory Pathogens Involved in PRDC
3.7.2. Glaesserella (Haemophylus) Parasuis
3.7.3. Mycoplasma Hyorhinis
3.7.4. Porcine Cytomegalovirus
3.7.5. Porcine Parainfluenza Virus (PPIV)
3.7.6. Bordetella Bronchiseptica
3.7.7. Interaction of M. hyorhinis and B. bronchiseptica.
3.8. Influence of Pathogen Loads and PRRSV Immunity on PRDC Severity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brockmeier SL, H.P., Thacker EL. Porcine Respiratory Disease Complex. In Polymicrobial Diseases; Brogden KA, G.J., Ed.; ASM Press: Washington DC, 2002.
- Li, Z.J.; Wang, X.; Di, D.; Pan, R.Y.; Gao, Y.; Xiao, C.G.; Li, B.B.; Wei, J.C.; Liu, K.; Qiu, Y.F.; et al. Comparative analysis of the pulmonary microbiome in healthy and diseased pigs. Mol Genet Genomics 2021, 296, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Pirolo, M.; Espinosa-Gongora, C.; Bogaert, D.; Guardabassi, L. The porcine respiratory microbiome: recent insights and future challenges. Anim Microbiome 2021, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Boeters, M.; Garcia-Morante, B.; van Schaik, G.; Segalés, J.; Rushton, J.; Steeneveld, W. The economic impact of endemic respiratory disease in pigs and related interventions - a systematic review. Porcine Health Manag 2023, 9, 45. [Google Scholar] [CrossRef]
- Crisci, E.; Fraile, L.; Montoya, M. Cellular Innate Immunity against PRRSV and Swine Influenza Viruses. Vet Sci 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Saade, G.; Deblanc, C.; Bougon, J.; Marois-Crehan, C.; Fablet, C.; Auray, G.; Belloc, C.; Leblanc-Maridor, M.; Gagnon, C.A.; Zhu, J.Z.; et al. Coinfections and their molecular consequences in the porcine respiratory tract. Vet Res 2020, 51, 80. [Google Scholar] [CrossRef]
- Thacker, E.L. Immunology of the porcine respiratory disease complex. Vet Clin N Am-Food A 2001, 17, 551. [Google Scholar] [CrossRef]
- Choi, Y.K.; Goyal, S.M.; Joo, H.S. Retrospective analysis of etiologic agents associated with respiratory diseases in pigs. Can Vet J 2003, 44, 735–737. [Google Scholar]
- Opriessnig, T.; Gimenez-Lirola, L.G.; Halbur, P.G. Polymicrobial respiratory disease in pigs. Anim Health Res Rev 2011, 12, 133–148. [Google Scholar] [CrossRef]
- Burrai, G.P.; Hawko, S.; Dei Giudici, S.; Polinas, M.; Angioi, P.P.; Mura, L.; Alberti, A.; Hosri, C.; Hassoun, G.; Oggiano, A.; et al. The Synergic Role of Emerging and Endemic Swine Virus in the Porcine Respiratory Disease Complex: Pathological and Biomolecular Analysis. Vet Sci 2023, 10, 595. [Google Scholar] [CrossRef]
- Qin, S.; Ruan, W.; Yue, H.; Tang, C.; Zhou, K.; Zhang, B. Viral communities associated with porcine respiratory disease complex in intensive commercial farms in Sichuan province, China. Sci Rep 2018, 8, 13341. [Google Scholar] [CrossRef]
- Hansen, M.S.; Pors, S.E.; Jensen, H.E.; Bille-Hansen, V.; Bisgaard, M.; Flachs, E.M.; Nielsen, O.L. An investigation of the pathology and pathogens associated with porcine respiratory disease complex in Denmark. J Comp Pathol 2010, 143, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Petri, F.A.M.; Ferreira, G.C.; Arruda, L.P.; Malcher, C.S.; Storino, G.Y.; Almeida, H.M.S.; Sonalio, K.; Silva, D.G.D.; Oliveira, L.G. Associations between Pleurisy and the Main Bacterial Pathogens of the Porcine Respiratory Diseases Complex (PRDC). Animals 2023, 13, 1493. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, J.; Salogni, C.; Giovannini, S.; Vitale, N.; Boniotti, M.B.; Corradi, A.; Pozzi, P.; Pasquali, P.; Alborali, G.L. Association Between Infectious Agents and Lesions in Post-Weaned Piglets and Fattening Heavy Pigs With Porcine Respiratory Disease Complex (PRDC). Front Vet Sci 2020, 7, 636. [Google Scholar] [CrossRef] [PubMed]
- Fablet, C.; Marois-Crehan, C.; Simon, G.; Grasland, B.; Jestin, A.; Kobisch, M.; Madec, F.; Rose, N. Infectious agents associated with respiratory diseases in 125 farrow-to-finish pig herds: a cross-sectional study. Vet Microbiol 2012, 157, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.A.; Halbur, P.G.; Sorden, S.D. Three cases of porcine respiratory disease complex associated with porcine circovirus type 2 infection. J Swine Health Prod 2002, 10, 27–30. [Google Scholar] [CrossRef]
- D’Annunzio, G.; Ostanello, F.; Muscatello, L.V.; Orioles, M.; Jacumin, N.; Tommasini, N.; Leotti, G.; Luppi, A.; Mandrioli, L.; Sarli, G. Porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus alone or associated are frequent intralesional detected viruses in porcine respiratory disease complex cases in Northern Italy. Front Vet Sci 2023, 10, 1234779. [Google Scholar] [CrossRef]
- Renzhammer, R.; Auer, A.; Loncaric, I.; Entenfellner, A.; Dimmel, K.; Walk, K.; Rümenapf, T.; Spergser, J.; Ladinig, A. Retrospective Analysis of the Detection of Pathogens Associated with the Porcine Respiratory Disease Complex in Routine Diagnostic Samples from Austrian Swine Stocks. Vet Sci 2023, 10, 601. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, X.X.; He, D.X.; Ku, X.G.; Hong, B.; Zeng, W.; Zhang, H.F.; He, Q.G. Investigation and analysis of etiology associated with porcine respiratory disease complex in China from 2017 to 2021. Front Vet Sci 2022, 9, 960033. [Google Scholar] [CrossRef]
- Thakor, J.C.; Sahoo, M.; Karam Pal, S.; Rajendra, S.; Salauddin, Q.; Ajay, K.; Pradeep, K.; Sagar, P.; Rohit, S.; Nihar Ranjan, S. Porcine respiratory disease complex (PRDC) in Indian pigs: a slaughterhouse survey. Vet Ital 2023, 59, 23–38. [Google Scholar] [CrossRef]
- Eddicks, M.; Eddicks, L.; Stadler, J.; Hermanns, W.; Ritzmann, M. The porcine respiratory disease complex (PRDC) - a clinical review. Tieraerztl Prax G N 2021, 49, 120–132. [Google Scholar] [CrossRef]
- Chae, C. Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae. Vet J 2016, 212, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Anita, R.E.; Calderón-Rico, F.; Pérez-Duran, F.; Arenas-Arrocena, M.C.; Zamora-Avilés, A.G.; Franco-Correa, L.E.; Bravo-Patiño, A.; Hernández-Morales, I. Response of lymphocytes from pigs naturally infected with porcine respiratory disease complex at 3 different stages of development. Can J Vet Res 2023, 87, 110–119. [Google Scholar] [PubMed]
- Kick, A.R.; Amaral, A.F.; Cortes, L.M.; Fogle, J.E.; Crisci, E.; Almond, G.W.; Kaser, T. The T-Cell Response to Type 2 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). Viruses 2019, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Maisonnasse, P.; Bouguyon, E.; Piton, G.; Ezquerra, A.; Urien, C.; Deloizy, C.; Bourge, M.; Leplat, J.J.; Simon, G.; Chevalier, C.; et al. The respiratory DC/macrophage network at steady-state and upon influenza infection in the swine biomedical model. Mucosal Immunol 2016, 9, 835–849. [Google Scholar] [CrossRef]
- Pineiro, C.; Morales, J.; Dereu, A.; Wuyts, N.; Azlor, O.; Vizcaino, E.; Doncecchi, P. Individual Pig Care program improves productive performance and animal health in nursery-growing pigs. J Swine Health Prod 2014, 22, 296–299. [Google Scholar] [CrossRef]
- Halbur, P.G.; Paul, P.S.; Frey, M.L.; Landgraf, J.; Eernisse, K.; Meng, X.J.; Lum, M.A.; Andrews, J.J.; Rathje, J.A. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol 1995, 32, 648–660. [Google Scholar] [CrossRef]
- Opriessnig, T.; Madson, D.M.; Prickett, J.R.; Kuhar, D.; Lunney, J.K.; Elsener, J.; Halbur, P.G. Effect of porcine circovirus type 2 (PCV2) vaccination on porcine reproductive and respiratory syndrome virus (PRRSV) and PCV2 coinfection. Vet Microbiol 2008, 131, 103–114. [Google Scholar] [CrossRef]
- Frias-De-Diego, A.; Crisci, E. Use of Crystal Violet to Improve Visual Cytopathic Effect-based Reading for Viral Titration using TCID50 Assays. J Vis Exp 2022. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Rawal, G.; Krueger, K.M.; Yim-Im, W.; Li, G.; Gauger, P.C.; Almeida, M.N.; Aljets, E.K.; Zhang, J. Development, Evaluation, and Clinical Application of PRRSV-2 Vaccine-like Real-Time RT-PCR Assays. Viruses 2023, 15, 2240. [Google Scholar] [CrossRef]
- Spear, A.; Faaberg, K.S. Development of a genome copy specific RT-qPCR assay for divergent strains of type 2 porcine reproductive and respiratory syndrome virus. J Virol Methods 2015, 218, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.H.; Fang, Y.; Farwell, R.; Steffen-Bien, M.; Rowland, R.R.; Christopher-Hennings, J.; Nelson, E.A. A 10-kDa structural protein of porcine reproductive and respiratory syndrome virus encoded by ORF2b. Virology 2001, 287, 183–191. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Goodkind, T. The Sword of Truth; RosettaBooks, 2010. [Google Scholar]
- Collins, C.L.; Pluske, J.R.; Morrison, R.S.; McDonald, T.N.; Smits, R.J.; Henman, D.J.; Stensland, I.; Dunshea, F.R. Post-weaning and whole-of-life performance of pigs is determined by live weight at weaning and the complexity of the diet fed after weaning. Anim Nutr 2017, 3, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Moest, N.K.; Willard, N.C.; Shull, C.M.; McKilligan, D.; Ellis, M. Effect of Piglet Weaning Weight on Wean-to-Finish Growth Performance and Ultrasound Carcass Measures. J Anim Sci 2023, 101, skad341.010. [Google Scholar] [CrossRef]
- Wagner, J.; Kneucker, A.; Liebler-Tenorio, E.; Fachinger, V.; Glaser, M.; Pesch, S.; Murtaugh, M.P.; Reinhold, P. Respiratory function and pulmonary lesions in pigs infected with porcine reproductive and respiratory syndrome virus. Vet J 2011, 187, 310–319. [Google Scholar] [CrossRef]
- Pessoa, J.; da Costa, M.R.; Manzanilla, E.G.; Norton, T.; McAloon, C.; Boyle, L. Managing respiratory disease in finisher pigs: Combining quantitative assessments of clinical signs and the prevalence of lung lesions at slaughter. Prev Vet Med 2021, 186, 105208. [Google Scholar] [CrossRef]
- Kick, A.R.; Amaral, A.F.; Frias-De-Diego, A.; Cortes, L.M.; Fogle, J.E.; Crisci, E.; Almond, G.W.; Käser, T. The Local and Systemic Humoral Immune Response Against Homologous and Heterologous Strains of the Type 2 Porcine Reproductive and Respiratory Syndrome Virus. Frontiers in Immunology 2021, 12. [Google Scholar] [CrossRef]
- Proctor, J.; Wolf, I.; Brodsky, D.; Cortes, L.M.; Frias-De-Diego, A.; Almond, G.W.; Crisci, E.; Negrão Watanabe, T.T.; Hammer, J.M.; Käser, T. Heterologous vaccine immunogenicity, efficacy, and immune correlates of protection of a modified-live virus porcine reproductive and respiratory syndrome virus vaccine. Frontiers in Microbiology 2022, 13, 977796. [Google Scholar] [CrossRef]
- Kick, A.R.; Wolfe, Z.C.; Amaral, A.F.; Cortes, L.M.; Almond, G.W.; Crisci, E.; Gauger, P.C.; Pittman, J.; Käser, T. Maternal Autogenous Inactivated Virus Vaccination Boosts Immunity to PRRSV in Piglets. Vaccines 2021, 9, 106. [Google Scholar] [CrossRef]
- Liu, J.-K.; Wei, C.-H.; Yang, X.-Y.; Dai, A.-L.; Li, X.-H. Multiplex PCR for the simultaneous detection of porcine reproductive and respiratory syndrome virus, classical swine fever virus, and porcine circovirus in pigs. Molecular and Cellular Probes 2013, 27, 149–152. [Google Scholar] [CrossRef]
- Giammarioli, M.; Pellegrini, C.; Casciari, C.; De Mia, G.M. Development of a novel hot-start multiplex PCR for simultaneous detection of classical swine fever virus, African swine fever virus, porcine circovirus type 2, porcine reproductive and respiratory syndrome virus and porcine parvovirus. Vet Res Commun 2008, 32, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Rao, P.; Jiang, Y.; Opriessnig, T.; Yang, Z. A sensitive multiplex real-time PCR panel for rapid diagnosis of viruses associated with porcine respiratory and reproductive disorders. Mol Cell Probes 2014, 28, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, H.; Zhao, X.; Ni, J.; Gan, S.; Dong, W.; Du, J.; Zhou, X.; Wang, X.; Song, H. Detection and differentiation of seven porcine respiratory pathogens using a multiplex ligation-dependent probe amplification assay. The Veterinary Journal 2024, 305, 106124. [Google Scholar] [CrossRef] [PubMed]
- Lung, O.; Ohene-Adjei, S.; Buchanan, C.; Joseph, T.; King, R.; Erickson, A.; Detmer, S.; Ambagala, A. Multiplex PCR and Microarray for Detection of Swine Respiratory Pathogens. Transbound Emerg Dis 2017, 64, 834–848. [Google Scholar] [CrossRef]
- Goto, Y.; Fukunari, K.; Tada, S.; Ichimura, S.; Chiba, Y.; Suzuki, T. A multiplex real-time RT-PCR system to simultaneously diagnose 16 pathogens associated with swine respiratory disease. J Appl Microbiol 2023, 134. [Google Scholar] [CrossRef]
- Resende, T.P.; Marshall Lund, L.; Rossow, S.; Vannucci, F.A. Next-Generation Sequencing Coupled With in situ Hybridization: A Novel Diagnostic Platform to Investigate Swine Emerging Pathogens and New Variants of Endemic Viruses. Front Vet Sci 2019. [Google Scholar] [CrossRef]
- Han, X.; Xia, Z. Application of Host-Depleted Nanopore Metagenomic Sequencing in the Clinical Detection of Pathogens in Pigs and Cats. Animals 2023, 13, 3838. [Google Scholar] [CrossRef]
- Neujahr, A.C.; Loy, D.S.; Loy, J.D.; Brodersen, B.W.; Fernando, S.C. Rapid detection of high consequence and emerging viral pathogens in pigs. Front Vet Sci 2024, 11. [Google Scholar] [CrossRef]
- Vereecke, N.; Zwickl, S.; Gumbert, S.; Graaf, A.; Harder, T.; Ritzmann, M.; Lillie-Jaschniski, K.; Theuns, S.; Stadler, J. Viral and Bacterial Profiles in Endemic Influenza A Virus Infected Swine Herds Using Nanopore Metagenomic Sequencing on Tracheobronchial Swabs. Microbiol Spectr 2023, 11, e0009823. [Google Scholar] [CrossRef]
- Martín-Valls, G.E.; Li, Y.; Díaz, I.; Cano, E.; Sosa-Portugal, S.; Mateu, E. Diversity of respiratory viruses present in nasal swabs under influenza suspicion in respiratory disease cases of weaned pigs. Front Vet Sci 2022, 9, 1014475. [Google Scholar] [CrossRef]
- Watt, R.G.; Plowrigiit, W.; Sabo, A.; Edington, N. A Sensitive Cell Culture System for the Virus of Porcine Inclusion Body Rhinitis (Cytomegalic Inclusion Disease). Research in Veterinary Science 1973, 14, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Palzer, A.; Haedke, K.; Heinritzi, K.; Zoels, S.; Ladinig, A.; Ritzmann, M. Associations among Haemophilus parasuis, Mycoplasma hyorhinis, and porcine reproductive and respiratory syndrome virus infections in pigs with polyserositis. Can Vet J 2015, 56, 285–287. [Google Scholar] [PubMed]
- Yu, J.; Wu, J.; Zhang, Y.; Guo, L.; Cong, X.; Du, Y.; Li, J.; Sun, W.; Shi, J.; Peng, J.; et al. Concurrent highly pathogenic porcine reproductive and respiratory syndrome virus infection accelerates Haemophilus parasuis infection in conventional pigs. Vet Microbiol 2012, 158, 316–321. [Google Scholar] [CrossRef]
- Guan, Z.; Pang, L.; Ouyang, Y.; Jiang, Y.; Zhang, J.; Qiu, Y.; Li, Z.; Li, B.; Liu, K.; Shao, D.; et al. Secondary Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus (HP-PRRSV2) Infection Augments Inflammatory Responses, Clinical Outcomes, and Pathogen Load in Glaesserella-parasuis-Infected Piglets. Vet Sci 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Solano, G.I.; Segalés, J.; Collins, J.E.; Molitor, T.W.; Pijoan, C. Porcine reproductive and respiratory syndrome virus (PRRSv) interaction with Haemophilus parasuis. Vet Microbiol 1997, 55, 247–257. [Google Scholar] [CrossRef]
- Segalés, J.; Domingo, M.; Solano, G.I.; Pijoan, C. Porcine reproductive and respiratory syndrome virus and Haemophilus parasuis antigen distribution in dually infected pigs. Vet Microbiol 1999, 64, 287–297. [Google Scholar] [CrossRef]
- Kawashima, K.; Yamada, S.; Kobayashi, H.; Narita, M. Detection of porcine reproductive and respiratory syndrome virus and Mycoplasma hyorhinis antigens in pulmonary lesions of pigs suffering from respiratory distress. J Comp Pathol 1996, 114, 315–323. [Google Scholar] [CrossRef]
- Lee, J.-A.; Oh, Y.-R.; Hwang, M.-A.; Lee, J.-B.; Park, S.-Y.; Song, C.-S.; Choi, I.-S.; Lee, S.-W. Mycoplasma hyorhinis is a potential pathogen of porcine respiratory disease complex that aggravates pneumonia caused by porcine reproductive and respiratory syndrome virus. Vet Immunol Immunop 2016, 177, 48–51. [Google Scholar] [CrossRef]
- Welch, M.; Krueger, K.; Zhang, J.; Pineyro, P.; Patterson, A.; Gauger, P. Pathogenesis of an experimental coinfection of porcine parainfluenza virus 1 and influenza A virus in commercial nursery swine. Vet Microbiol 2023, 285, 109850. [Google Scholar] [CrossRef]
- Wozniak, A.; Cybulski, P.; Denes, L.; Balka, G.; Stadejek, T. Detection of Porcine Respirovirus 1 (PRV1) in Poland: Incidence of Co-Infections with Influenza A Virus (IAV) and Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in Herds with a Respiratory Disease. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Sozzi, E.; Leo, G.; Bertasio, C.; Alborali, G.L.; Salogni, C.; Tonni, M.; Formenti, N.; Lelli, D.; Moreno, A.; Trogu, T.; et al. Presence and Characterisation of Porcine Respirovirus 1 (PRV1) in Northern Italy. Pathogens 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Brockmeier, S.L.; Palmer, M.V.; Bolin, S.R. Effects of intranasal inoculation of porcine reproductive and respiratory syndrome virus, Bordetella bronchiseptica, or a combination of both organisms in pigs. Am J Vet Res 2000, 61, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Gois, M.; Kuksa, F.; Sisák, F. Experimental infection of gnotobiotic piglets with Mycoplasma hyorhinis and Bordetella bronchiseptica. Zentralbl Veterinarmed B 1977, 24, 89–96. [Google Scholar] [CrossRef] [PubMed]
| Pig # | PRRSV-2 M (general) | Glaes. Parasuis |
Myco. Hyorhinis |
Porcine Cytomegalovirus (PCMV) |
Parainfluenza virus | Swine influenza (general) |
PRRSV-1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
NanoString Normalized Reads |
ISU Ct |
NanoString Normalized Reads |
ISU Ct |
NanoString Normalized Reads |
ISU Ct |
NanoString Normalized Reads |
ISU Ct |
NanoString Normalized Reads |
ISU Ct |
NanoString Normalized Reads |
ISU Ct |
ISU Ct |
|
| 12 | 78491 | 30.3 | 494 | 29.9 | 733 | 27.8 | 64 | 27.2 | 16 | ≥38 | 111 | ≥38 | ≥37 |
| 18 | 44172 | 30.1 | 814 | 30.1 | 838 | 31.3 | 473 | 30.9 | 17204 | 24.1 | 74 | ≥38 | ≥37 |
| 22 | 20863 | 28.6 | 9550 | 26.7 | 8795 | 30.4 | 18 | ≥35 | 6601 | 24.5 | 108 | ≥38 | ≥37 |
| 28 | 523774 | 27.6 | 48 | ≥35 | 4038 | 30.7 | 24 | ≥35 | 24 | ≥38 | 48 | ≥38 | ≥37 |
| 36 | 162850 | 26.6 | 70 | ≥35 | 60 | 34.7 | 4320 | 26.8 | 40 | 33.1 | 120 | ≥38 | ≥37 |
|
47 Neg |
17 | ≥37 | 9 | ≥35 | 9 | 32.9 | 4 | ≥35 | 4 | ≥38 | 4 | ≥38 | ≥37 |
| 48 | 9615 | 30 | 5 | ≥35 | 5 | ≥35 | 9 | 30.0 | 5 | ≥38 | 5 | ≥38 | ≥37 |
| Pig # | PRRSV-2 Ct D28 |
PRRSV-2 Ct D49 |
MLV-like Ct D28 |
Ct MLV- Ct PRRSV-2 D28 | Pig # | PRRSV-2 Ct D28 |
PRRSV-2 Ct D49 |
MLV-like Ct D28 |
Ct MLV- Ct PRRSV-2 D28 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 22.20 | 26.20 | 23.84 | 1.63 | 29 | 27.63 | 26.23 | 29.09 | 1.46 |
| 2 | 24.93 | 32.59 | 26.39 | 1.46 | 31 | 17.77 | 28.95 | 26.15 | 8.38 |
| 3 | 20.79 | 33.21 | 30.47 | 9.68 | 32 | 21.16 | 33.78 | 27.09 | 5.92 |
| 4 | 29.00 | 33.69 | 30.85 | 1.86 | 33 | 20.04 | 31.05 | 24.22 | 4.19 |
| 5 | 24.86 | N/A | 26.06 | 1.20 | 34 | 19.63 | 26.23 | 24.45 | 4.82 |
| 6 | 26.09 | 29.51 | 27.38 | 1.29 | 35 | 22.90 | 25.62 | 27.69 | 4.79 |
| 7 | 21.72 | 32.17 | 23.41 | 1.68 | 36 | 18.95 | 20.37 | 27.35 | 8.40 |
| 8 | 18.97 | 28.95 | 26.57 | 7.60 | 37 | 23.48 | 32.08 | 25.39 | 1.91 |
| 9 | 21.95 | 31.30 | 23.19 | 1.24 | 38 | 13.08 | ≥37 | 25.56 | 12.49 |
| 10 | 24.38 | 28.67 | 25.84 | 1.46 | 39 | 25.91 | 26.83 | 28.01 | 2.10 |
| 11 | 25.15 | 26.08 | 26.67 | 1.52 | 40 | 22.85 | 33.12 | 30.03 | 7.17 |
| 12 | 23.23 | 29.52 | 25.55 | 2.32 | 41 | 23.20 | 29.42 | 24.59 | 1.39 |
| 13 | ≥37 | 29.35 | ≥37 | 0.00 | 42 | 21.75 | 29.95 | 28.70 | 6.95 |
| 14 | 22.27 | 35.19 | 27.92 | 5.65 | 43 | 19.69 | 27.95 | 26.30 | 6.61 |
| 15 | 26.55 | N/A | 27.74 | 1.19 | 44 | 23.73 | 32.31 | 25.44 | 1.71 |
| 16 | 24.50 | N/A | 25.86 | 1.36 | 45 | 19.63 | 29.55 | 30.00 | 10.36 |
| 17 | 17.66 | 22.45 | 18.80 | 1.14 | 46 | 26.85 | 33.35 | 28.07 | 1.23 |
| 18 | 22.94 | 24.00 | 25.13 | 2.19 | 47 | 22.21 | ≥37 | 24.51 | 2.30 |
| 19 | 18.85 | 36.04 | 26.98 | 8.13 | 48 | 19.42 | 26.98 | 23.99 | 4.57 |
| 20 | 24.65 | 31.40 | 27.71 | 3.06 | 49 | 31.11 | 32.39 | 32.06 | 0.95 |
| 21 | 18.72 | 25.26 | 27.59 | 8.88 | 50 | 23.59 | 24.64 | 28.83 | 5.24 |
| 22 | 28.27 | 33.27 | 29.82 | 1.54 | 51 | 27.78 | N/A | 29.49 | 1.71 |
| 23 | 20.06 | 33.03 | 28.65 | 8.59 | 52 | 22.23 | 32.94 | 23.73 | 1.49 |
| 24 | 16.56 | 29.99 | 25.59 | 9.03 | 53 | 15.92 | 29.81 | 31.31 | 15.39 |
| 25 | 19.54 | 35.90 | 28.10 | 8.56 | 54 | 20.63 | 25.34 | 22.12 | 1.49 |
| 26 | 25.09 | 31.10 | 26.56 | 1.47 | 55 | 25.00 | 32.28 | 32.96 | 7.96 |
| 27 | 21.44 | N/A | 30.31 | 8.87 | 56 | 21.60 | 26.29 | 23.24 | 1.64 |
| 28 | 29.43 | 29.39 | 30.76 | 1.33 | 59 | 17.20 | 27.97 | 28.55 | 11.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





