1. Introduction
1.1. Indoor Residual Spraying (IRS) of Insecticides
Disease vector control mainly relies on two key interventions: insecticide-treated nets (ITNs) and indoor residual spraying (IRS). While ITN products predominately rely on the pyrethroid class of insecticides, IRS products employ a range of insecticidal classes, with alternative modes of action, which are effective against pyrethroid-resistant mosquitoes. IRS, however, is a comparatively expensive vector control intervention, with cost modelling of IRS campaigns suggesting that product costs comprise ~33% of the total costs [
1]. New active ingredients (a.i.’s) in development are expected to be more expensive, potentially increasing product costs and, consequently, IRS campaign costs. IRS encounters significant challenges that affect product efficacy and cost-effectiveness, such as the varied surfaces on which a.i.’s are sprayed, absorption into those surfaces, suboptimal presentation of the insecticide on the surface, and the effect of surface pH on the insecticide's longevity. Insecticides can also have significantly different physical-chemical properties, complicating standardising IRS formulations. Therefore, any technologies that can reduce the amount of insecticide required while maintaining comparative mortality, or simplify formulation development could significantly reduce product costs and impact IRS cost-effectiveness.
1.2. The Physical State of Insecticides and Biological Efficacy
In general, research focuses on the correlation between the total insecticide concentration of a product and its bioefficacy. However, the physical state of an insecticide, and how that may change over time, can play a significant role in understanding how insecticidal products perform in the lab and field.
The physical state of an insecticide can either be crystalline (solid with aligned molecular lattice bonds) or amorphous (solid with no orderly aligned lattice bonds). Several studies have shown that different insecticide crystal structures (polymorphs) can affect insecticidal activity, leading to higher insect mortality if the most efficacious polymorph is controlled and used [
2,
3,
4,
5,
6,
7]. When insecticides are in a crystalline form, the pickup by a target organism (i.e., a mosquito) is influenced by several factors, including crystal size and shape. However, in an amorphous form, it is believed that the insecticide material exhibits diffusion within the carrier substrate (including on the surface), potentially offering a larger surface area for pickup. So, insecticides presented in the amorphous state could be more efficacious than the crystalline state. Therefore, understanding which physical state of an insecticide gives the best efficacy, and optimising formulations to maintain long-term stability are important factors to consider when developing an insecticidal product.
1.3. Silica as a Carrier of Insecticide
Silica has been used as a carrier for insecticides for decades [
8]. It can offer the advantage of solid diffusion [
9], a slow delivery mechanism that can reduce an insecticide's environmental toxicity while increasing its bioefficacy against a target organism. However, the insecticide’s physical state and its presentation within or on a silica carrier are poorly understood and not controlled. It is possible that by altering the parameters of the silica carrier, the crystallinity of an insecticide could be controlled by that carrier to hold it in an amorphous physical state. This is one of several ways X-tec silica could enhance the bioefficacy of insecticides compared to standard IRS (
Figure 1).
The critical crystallisation size of an insecticide refers to the minimum size at which insecticide particles can form stable crystals without redissolving. The critical crystallisation size can be understood by using mathematical and computer modelling [
10,
11]. These models can be applied to insecticides to provide a theoretical size at which the molecule can be maintained in an amorphous solid form.
To test this, a bespoke silica carrier (‘X-tec silica’) was developed with pores of specific diameter and volume, to the specifications of the critical crystallisation size of an insecticide. To prove the concept of bespoke silica particles, clothianidin was chosen as an example insecticide and X-tec silica was loaded with clothianidin. Clothianidin-loaded X-tec silica was sprayed at different application rates to see if the insecticide content of the IRS formulation could be reduced while maintaining its residual biological activity. If similar or improved bioefficacy is achieved by reduced concentrations, or if the spray's durability (longevity) is improved by the X-tec silica, this could result in cost savings compared to standard IRS formulations.
2. Materials and Methods
2.1. Molecular Computer Modelling of Silica Particles
Molecular computer modelling was used to simulate silica pores to determine the critical crystallisation size of clothianidin. The system used for the computer modelling is a parallel Linux cluster (Barkla, High Performance Computing resource, University of Liverpool, UK).
Preliminary analysis was conducted to demonstrate the capabilities of molecular computer modelling and understand how a functionalised silica surface can interact with water and functional groups. A molecular dynamic simulation was used to build an amorphous silica surface of 1 nm thick slab in a 9 x 9 nm cell (
Figure 2a). The surface was functionalised in the software by annealing and adding hydroxyl groups in a specific concentration. Then a 6 nm pore was built to represent a real pore system (
Figure 2b). The surface was further functionalised by adding C18 chain groups to reduce the hydrophilicity (
Figure 2c). The mean maximum distance of the C18 from the silica surface was 1.4 nm, reducing the pore diameter to 3 nm. This amorphous silica surface model (
Figure 2) was then used to illustrate the effect of absorbing clothianidin to the amorphous silica surface.
2.2. X-tec Silica Manufacture
The X-tec silica was manufactured using a proprietary process to the specifications defined by the modelling analysis. The X-tec silica particles were then loaded with 10% clothianidin to produce the formulated product (hereafter referred to as ‘10% X-tec silica’).
2.3. Material Characterisation of 10% X-tec Silica
Material characterisation was conducted to measure the specifications of 10% X-tec silica to validate the manufacturing process and computer modelling using the following methods:
2.4. Carbon, Hydrogen, and Nitrogen (CHN) Analysis
CHN analysis was carried out to confirm the loading of X-tec silica. Carbon, hydrogen, nitrogen, and sulphur were analysed using a ThermoFisher CHNS elemental analyser. Dried and powdered samples were combusted in a tin sample crucible with a vanadium pentoxide catalyst in an oxygen environment. The resulting gas mixture containing N2, CO2, H2O, and SO2 flowed into the chromatographic column and was detected with a thermal conductivity detector (TCD).
2.5. Nitrogen Porosimetry
Nitrogen porosimetry was used to measure the surface area, pore diameter, and pore volume of the unloaded and 10% loaded silica particles using nitrogen as the filling material. Apparent surface areas were measured by nitrogen adsorption at 77.3 K using a Micromeritics ASAP 2020 volumetric adsorption analyser. Powder samples were degassed offline at 393 K for 12 h under a dynamic vacuum (10−5 bar). Before the adsorption test, the inert gas was removed using a high vacuum provided by the turbo molecular drag pump. The specific surface areas were evaluated using the BET model. Pore size distributions of the silica were obtained by fitting the nonlocal density functional theory to the adsorption data.
2.6. Particle Size Measurement
To analyse the average particle size of the 10% X-tec silica, a powder multisizer technique (Beckman Coulter Multisizer 3) in volume mode was used, by detecting changes in electrical impedance as particles pass through a small aperture.
2.7. Scanning Electron Microscopy/Energy Dispersive X-Ray Spectroscopy (SEM/EDS)
SEM/EDS was used as a quality control technique to assess if the particles were damaged during manufacturing or formulation, and as a qualitative technique to detect clothianidin on the X-tec silica. A powdered 10% X-tec silica sample was applied on a carbon tape and coated with Chromium. Images of the sample's surface were taken using a SEM (Tescan FIB SEM S8000G). During visual investigation of the surface, EDS (Oxford EDS system) was used for elemental analysis to confirm the origin of the features observed. Chlorine (Cl) and sulphur (S) were used to identify clothianidin (C6N5H8SO2Cl).
2.8. Powder X-Ray Diffraction (PXRD)
PXRD was carried out to confirm that the X-tec silica could maintain the amorphous physical state of clothianidin at a 10% loading. PXRD patterns were recorded on a Bruker D8 Advance diffractometer with Cu Kα radiation and a voltage of 40 kV. Data were collected in the 2θ range of 2–40° with steps of 0.02°.
2.9. Entomological Testing: Mosquito Rearing
The Anopheles gambiae s.s. Kisumu strain is reared and maintained in the Liverpool Insect Testing Establishment (LITE) insectaries at the Liverpool School of Tropical Medicine (LSTM), under controlled environmental conditions and a standardised blood feeding procedure (Williams et al., 2019). The strain was first colonised in Kenya in 1975 and sourced from the Malaria Research and Reference Reagent Resource Centre. Adult mosquitoes are kept in 30 x 30 cm mesh cages at 27 ± 1°C and 75% RH ± 5% with ad libitum access to a 10% glucose solution on cotton wool. The mosquitoes used in these experiments were non-blood fed 2-5-day-old adult females.
2.10. Cone Bioassays
10% X-tec silica was mixed with water containing 2% v/v methanol and sprayed on glazed and unglazed tiles using an auto-load precision spray potter tower (Burkard Manufacturing Co Ltd, Rickmansworth, United Kingdom) at three different application rates (30, 60 and 90 mg a.i./m2). After application tiles were kept for one week before testing to allow drying time. Tiles were tested at 1 week and 8 months post-spray application. Tiles were stored after spray application and between tests at 30±2°C and 80±10% RH.
The bioefficacy of the sprayed tiles was determined using a standard WHO cone bioassay [
13]. Two to four tiles from each treatment group were tested at a time exposing 10 ± 2 mosquitoes per tile for 30 minutes. Mosquitoes exposed to untreated tiles and unformulated X-tec silica tiles were used as controls. Knock-down was assessed at 60 minutes post-exposure, and mosquito mortality was recorded at 24, 48, 72, 96 and 120 hours post-exposure. All tests were conducted at 27 ± 2 °C and 75 ± 10% relative humidity.
3. Results
3.1. Molecular Computer Modelling of Silica Particles
The molecular computer modelling suggested that the optimum specifications of X-tec silica particles to be used as carriers for clothianidin were 5 µm spheres manufactured by reaction-limited kinetics with 7-8 nm pores, a surface area of 200 m
2/g, 2.0 silanols nm
2, and a minimal of vicinal or geminal silanols (
Figure 3).
3.2. X-tec Silica Manufacture
The manufactured silica were an average of size of 5 µm spheres with 7 nm pores and 170 m2/g surface area. The 10% X-tec silica had an average particle size of 5.64µm.
3.3. Material Characterisation of 10% X-tec Silica
CHN Analysis
The CHN analysis was carried out to confirm that the loading of X-Tec silica was successful and at the expected loading rate. The expected (calculated) value was 2.4% carbon, with the measured value of 3.9% carbon. The proximity of the estimated and measured values indicates that the silica was loaded with the target AI concentration.
3.4. Nitrogen Porosimetry
Compared to the unloaded X-tec silica, the 10% X-tec silica showed significant reductions in specific surface area (16% reduction) and mean pore volume (14% reduction) (
Table 1). The mean pore diameter did not change after formulation.
3.5. SEM/EDS
SEM analysis found traces of clothianidin around the 10% X-tec silica. This was confirmed by detecting Cl and S (stoichiometrically equal as it is on clothianidin) from the EDS (
Figure 4). The silica particles maintained their shape without being damaged or altered during the manufacturing and formulation.
3.6. PXRD Analysis
The PXRD analysis was conducted to confirm that the X-Tec silica can maintain the amorphous physical state of clothianidin. The PXRD patterns showed no peaks, confirming that the sample contains an amorphous silica powder with no diffracting crystalline component (a representative example is shown in
Figure 5).
3.7. Entomological Testing: Cone Bioassays
One-week post-application, on glazed tiles, all three application rates (30, 60 and 90 mg a.i./m
2) of the 10% X-tec silica resulted in >80% mosquito mortality within 24 hours (
Figure 6, additional
Table 1). Replicates were pooled following exposure, so for this dataset error bars could not be calculated. On unglazed tiles (
Figure 7, Additional Table 2), efficacy was lower; however, still surpassed >80% mortality for the two higher concentrations (by 24 hours for 90 mg a.i./m2, N = 41, and 48 hours for 60 mg a.i./m
2, N = 40). Mortality was <60% for the 30 mg a.i./m
2 application rate at the final time point (120 hours, N = 40). At 8 months post-spray application, on glazed tiles (
Figure 8, additional Table 3), 100% mortality was reached within 24-hour hours at 60 (N = 20) and 90 (N = 21) mg a.i./m
2 application rates, and within 48-hours at 30 mg a.i./m
2 (N = 20). On unglazed tiles (
Figure 9, additional Table 4), 96-hour mortality was not recorded, however, 100% mortality was reached within 72 hours (90 mg a.i./m
2, N = 18) and 120 hours (60 mg a.i./m
2, N = 19). For the lowest concentration (30 mg a.i./m
2) mortality only reached 25% (N = 20).
4. Discussion
The results, highlighted by the PXRD and SEM/EDS data, suggest that the 10% X-tec silica particles were successfully manufactured to control the crystallinity of the clothianidin and hold it in an amorphous physical state. The average particle size and SEM images show that the silica remained undamaged during the formulation process, with the CHN data indicating that the expected loading of clothianidin was successfully formulated in the silica batch. The surface area, measured by porosimetry, was significantly reduced in the 10% X-tec silica compared to the unformulated silica, indicating that the pores are filled up by the amorphous clothianidin. This study did not conduct a comparison on the efficacy of amorphous versus crystalline clothianidin, so cannot determine which state is more efficacious. However, the ability to control the physical state of an insecticide could have far-reaching implications in cases where an insecticide is known to be more efficacious in a particular state. Particularly, if cost-modelling shows that the costs of the bespoke silica is offset by the reduced active ingredient needed to achieve the same, or improved efficacy.
The 10% X-tec silica demonstrated high bioefficacy against the insecticide-susceptible Kisumu mosquito strain, with most dose/substrate combinations achieving 100% mortality within 120-hours at 8 months post-spray application. The current clothianidin-based IRS product on the market specifies a target dose of 300 mg a.i./m2. Therefore, the demonstration of 100% efficacy at substantially reduced doses (60 & 90 mg a.i./m2) is positive. Even at a 10-fold reduced dose (30 mg a.i./m2) mortality was observed, although its efficacy was surface dependent (1-week post-spray application: 100% glazed tiles, 58% unglazed tiles; 8-months post-spray application; 100% glazed tiles, 25% unglazed tiles). Although the X-tec silica is assumed to reduce the absorption of the insecticide, the reduction in efficacy on the unglazed tiles at the lowest dose suggests the effect of capillary action directly affects the bioavailability of the compound It is likely that at the lower dose, less active ingredient is available for mosquito pickup, causing reduced mortality. However, it is critical to understand that the presentation of any insecticide, in any physical state, will be impacted by the substrate on which it is sprayed. Nevertheless, the efficacy seen at these lower doses suggests silica could be used to improve the cost-effectiveness of IRS applications, and a cost-benefit analysis should be conducted. Further testing should consider investigating the efficacy of the formulated silica on pyrethroid-resistant mosquito strains and exploring the efficacy of formulated silica with alternative active ingredients.
5. Conclusions
This study investigated the potential of bespoke X-tec silica particles as a unique carrier for insecticides. Full material characterisation confirmed the Xtec silica particles could successfully control the physical state of clothianidin and maintain bioefficacy at up to 8 months post-spray.
Author Contributions
Conception: PM, SR. Chemistry consultation: PM. Bioassays: JS, AG. Data Analysis: SH, PM, JS, AG, KS. Chemical analysis: SH, PM, KS. Interpreting the data: SH, SR, PM, DN, NL. Drafting the manuscript: SH, NL, DN. Editing the manuscript: SH, NL SR, PM, KS, JS, AG, DN.
Funding
This study was funded by the Bill and Melinda Gates Foundation (grant no. INV-058567)
https://www.gatesfoundation.org/ through the Innovative Vector Control Consortium. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files], or by contacting the corresponding author.
Acknowledgments
The authors would like to thank Richard Adey from IVCC for their contributions in the project. The Materials Innovation Factory at the University of Liverpool for their facilities for the materials characterization. The members of Liverpool Insect Testing Establishment (LITE) team at LSTM for rearing all mosquitoes.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Irish SR, Nimmo D, Bharmel J, Tripet F, Müller P, Manrique-Saide P, et al. A review of selective indoor residual spraying for malaria control. Malar J. 2024, 23, 1–12. [Google Scholar]
- Yang J, Erriah B, Hu CT, Reiter E, Zhu X, López-Mejías V, et al. A deltamethrin crystal polymorph for more effective malaria control. Proc Natl Acad Sci U S A. 2020, 117, 26633–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu X, Hu CT, Erriah B, Vogt-Maranto L, Yang J, Yang Y, et al. Imidacloprid Crystal Polymorphs for Disease Vector Control and Pollinator Protection. J Am Chem Soc. 2021, 143, 17144–52. [Google Scholar] [CrossRef] [PubMed]
- Yang J, Hu CT, Zhu X, Zhu Q, Ward MD, Kahr B. DDT Polymorphism and the Lethality of Crystal Forms. Angew Chem Int Ed Engl. 2017, 56, 10165–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu X, Hu CT, Yang J, Joyce LA, Qiu M, Ward MD, et al. Manipulating Solid Forms of Contact Insecticides for Infectious Disease Prevention. J Am Chem Soc. 2019, 141, 16858–64. [Google Scholar] [CrossRef] [PubMed]
- Niu X, Yang R, Zhang H, Yang J. Crystal engineering in the development of improved pesticide products. Advanced Agrochem. 2022, 1, 39–60. [Google Scholar] [CrossRef]
- Carson J, Erriah B, Herodotou S, Shtukenberg AG, Smith L, Ryazanskaya S, et al. Overcoming insecticide resistance in Anopheles mosquitoes by using faster-acting solid forms of deltamethrin. Malar J. 2023, 22, 1–8. [Google Scholar]
- Shawir M, le Patourel GNJ, Moustafa FI. Amorphous silica as an additive to dust formulations of insecticides for stored grain pest control. J Stored Prod Res. 1988, 24, 123–30. [Google Scholar] [CrossRef]
- Guo Y, Ren L, Li X, Wang Z, Zhang Y, Zhang S, et al. Bio-based clothianidin-loaded solid dispersion using composite carriers to improve efficacy and reduce environmental toxicity. Pest Manag Sci. 2021, 77, 5246–54. [Google Scholar] [CrossRef] [PubMed]
- Nanev, CN. Critical size of crystals growing under diffusion conditions for loss of polyhedral stability. J Cryst Growth. 1994, 140, 381–7. [Google Scholar] [CrossRef]
- Zhang S, Han J, Gao Y, Guo B, Reiter G, Xu J. Determination of the Critical Size of Secondary Nuclei on the Lateral Growth Front of Lamellar Polymer Crystals. Macromolecules. 2019, 52, 7439–47. [Google Scholar] [CrossRef]
- Williams J, Flood L, Praulins G, Ingham VA, Morgan J, Lees RS, et al. Characterisation of Anopheles strains used for laboratory screening of new vector control products. Parasit Vectors. 2019, 12, 522. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for laboratory and field-testing of long-lasting insecticidal nets. WHO/HTM/NTD/WHOPES/20131. Geneva: World Health Organization; 2013.
Figure 1.
Diagrammatic illustration of the potential benefits of using X-tec silica particles (bottom) to enhance insecticide bioefficacy compared to standard IRS (top); (A) Improved pickup: The insecticide is loaded onto the X-tec silica, which can be easily picked up by the mosquito tarsi (leg) compared to standard IRS. (B) Physical state: The X-tec silica maintains the insecticide in its amorphous state, the more efficacious form for most insecticides. In standard IRS it is hard to maintain the amorphous state on the surface, so, the insecticide can be amorphous or crystalline. (C): Reduced absorption: Many standard IRS formulations have challenges with absorption into porous surfaces (such as mud and wood) which reduces the surface concentration of the insecticide available to be picked up by the mosquito. X-tec silica should protect the insecticide eliminating this issue. (D): Increased surface area: Rough, irregular surfaces can affect pickup by reducing surface area contact with the mosquito tarsi. The size of the X-tec silica particles can be adjusted to enhance pickup and reduce loss into the substrate surface.
Figure 1.
Diagrammatic illustration of the potential benefits of using X-tec silica particles (bottom) to enhance insecticide bioefficacy compared to standard IRS (top); (A) Improved pickup: The insecticide is loaded onto the X-tec silica, which can be easily picked up by the mosquito tarsi (leg) compared to standard IRS. (B) Physical state: The X-tec silica maintains the insecticide in its amorphous state, the more efficacious form for most insecticides. In standard IRS it is hard to maintain the amorphous state on the surface, so, the insecticide can be amorphous or crystalline. (C): Reduced absorption: Many standard IRS formulations have challenges with absorption into porous surfaces (such as mud and wood) which reduces the surface concentration of the insecticide available to be picked up by the mosquito. X-tec silica should protect the insecticide eliminating this issue. (D): Increased surface area: Rough, irregular surfaces can affect pickup by reducing surface area contact with the mosquito tarsi. The size of the X-tec silica particles can be adjusted to enhance pickup and reduce loss into the substrate surface.
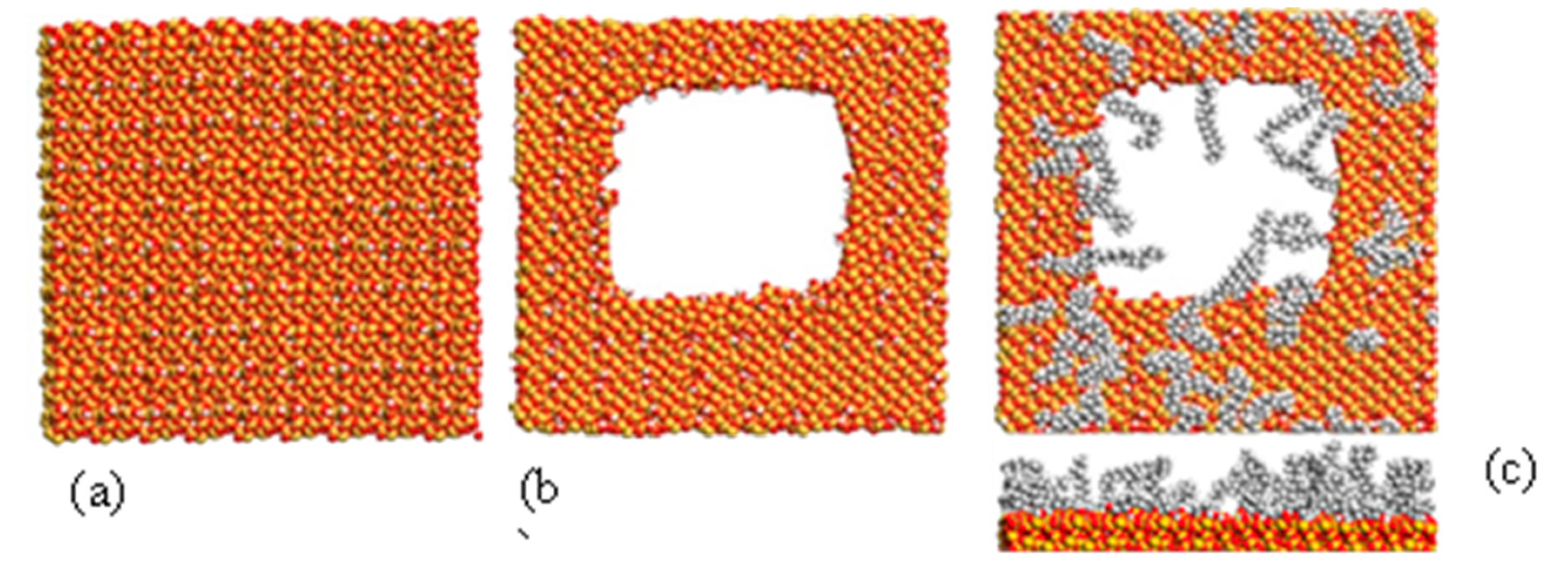
Figure 2.
Molecular computer modelling of amorphous silica surface: a) initial non-porous surface, b) surface with 6 nm pore, c) C18 functionalised surface of top and side view.
Figure 2.
Molecular computer modelling of amorphous silica surface: a) initial non-porous surface, b) surface with 6 nm pore, c) C18 functionalised surface of top and side view.
Figure 3.
Computer modelling of amorphous silica surface with a 6 nm pore having clothianidin mixed to its surface; (left) clothianidin molecules, (middle) amorphous silica surface mixed with clothianidin, and (right) the resulted silica surface with attached clothianidin molecules.
Figure 3.
Computer modelling of amorphous silica surface with a 6 nm pore having clothianidin mixed to its surface; (left) clothianidin molecules, (middle) amorphous silica surface mixed with clothianidin, and (right) the resulted silica surface with attached clothianidin molecules.
Figure 4.
SEM/EDS analysis of the 10% X-tec silica.
Figure 4.
SEM/EDS analysis of the 10% X-tec silica.
Figure 5.
A representative pattern of formulated X-tec silica. The PXRD pattern shows X-tec 5µm silica formulated with 2wt.% Clothianidin (orange), the pre-formulated blank X-tec silica (blue), and the reference PXRD pattern of crystalline clothianidin TGAI clothianidin (red). The PXRD pattern will show peaks when the material is crystalline as X-rays will be reflected from the same angle when the lattice planes are in parallel to each other (aligned), whereas, if the material is amorphous the planes will have random alignment and hence the X-rays will be scatter into different angles; therefore, no peaks will be recorded on the PXRD pattern.
Figure 5.
A representative pattern of formulated X-tec silica. The PXRD pattern shows X-tec 5µm silica formulated with 2wt.% Clothianidin (orange), the pre-formulated blank X-tec silica (blue), and the reference PXRD pattern of crystalline clothianidin TGAI clothianidin (red). The PXRD pattern will show peaks when the material is crystalline as X-rays will be reflected from the same angle when the lattice planes are in parallel to each other (aligned), whereas, if the material is amorphous the planes will have random alignment and hence the X-rays will be scatter into different angles; therefore, no peaks will be recorded on the PXRD pattern.
Figure 6.
1-week post-spray application: 60-minute knockdown (KD) and 24–120-hour mortality of An. gambiae s.s. Kisumu exposed in cone bioassays to glazed tiles sprayed with 10% X-tec silica at three application rates (30, 60 and 90 mg a.i./m2), unformulated silica (NIRS-SB-0 Xtec silica), and an untreated control tile. Replicates were pooled therefore no error bars are shown.
Figure 6.
1-week post-spray application: 60-minute knockdown (KD) and 24–120-hour mortality of An. gambiae s.s. Kisumu exposed in cone bioassays to glazed tiles sprayed with 10% X-tec silica at three application rates (30, 60 and 90 mg a.i./m2), unformulated silica (NIRS-SB-0 Xtec silica), and an untreated control tile. Replicates were pooled therefore no error bars are shown.
Figure 7.
1-week post-spray application: 60-minute knockdown (KD) and 24–120-hour mortality of An. gambiae s.s. Kisumu exposed in a cone bioassays to unglazed tiles sprayed with 10% X-tec silica at three application rates (30, 60 and 90 mg a.i./m2), unformulated silica (NIRS-SB-0 Xtec silica), and an untreated control tile. Error bars display standard deviation of the sample.
Figure 7.
1-week post-spray application: 60-minute knockdown (KD) and 24–120-hour mortality of An. gambiae s.s. Kisumu exposed in a cone bioassays to unglazed tiles sprayed with 10% X-tec silica at three application rates (30, 60 and 90 mg a.i./m2), unformulated silica (NIRS-SB-0 Xtec silica), and an untreated control tile. Error bars display standard deviation of the sample.
Figure 8.
8-month post-spray application: 60-minute knockdown (KD) and 24–120-hour mortality of An. gambiae s.s. Kisumu exposed in a cone bioassays to glazed tiles sprayed with 10% X-tec silica at three application rates (30, 60 and 90 mg a.i./m2), unformulated silica (NIRS-SB-0 Xtec silica), and an untreated control tile. Error bars display standard deviation of the sample.
Figure 8.
8-month post-spray application: 60-minute knockdown (KD) and 24–120-hour mortality of An. gambiae s.s. Kisumu exposed in a cone bioassays to glazed tiles sprayed with 10% X-tec silica at three application rates (30, 60 and 90 mg a.i./m2), unformulated silica (NIRS-SB-0 Xtec silica), and an untreated control tile. Error bars display standard deviation of the sample.
Figure 9.
8-month post-spray application: 60-minute knockdown (KD) and 24–120-hour mortality (96-hour mortality was not recorded) of An. gambiae s.s. Kisumu exposed in a cone bioassays to unglazed tiles sprayed with 10% X-tec silica at three application rates (30, 60 and 90 mg a.i./m2), unformulated silica (NIRS-SB-0 Xtec silica), and an untreated control tile. Error bars display standard deviation of the sample.
Figure 9.
8-month post-spray application: 60-minute knockdown (KD) and 24–120-hour mortality (96-hour mortality was not recorded) of An. gambiae s.s. Kisumu exposed in a cone bioassays to unglazed tiles sprayed with 10% X-tec silica at three application rates (30, 60 and 90 mg a.i./m2), unformulated silica (NIRS-SB-0 Xtec silica), and an untreated control tile. Error bars display standard deviation of the sample.
Table 1.
Summary of nitrogen porosimetry data detailing specific surface area (SSA), mean pore volume (MPV) and mean pore diameter (MPD) for unloaded and 10% clothianidin-loaded X-TEC silica.
Table 1.
Summary of nitrogen porosimetry data detailing specific surface area (SSA), mean pore volume (MPV) and mean pore diameter (MPD) for unloaded and 10% clothianidin-loaded X-TEC silica.
| Name |
SSA (m2/g ) |
MPV (cm3/g) |
MPD (nm) |
| X-TEC silica (unloaded) |
167 |
0.42 |
7.3 |
| X-TEC silica (10% Clothianidin) |
140 |
0.36 |
6.9 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).