1. Introduction
Corynebacterium pseudotuberculosis is the etiologic agent of caseous lymphadenitis (CLA), a chronic disease that primarily affects goats and sheep but can also infect cattle, horses, camelids, companion animals, and, rarely, humans exposed to infected material (e.g., farm workers and veterinarians) [
1]. It is a Gram-positive, pleomorphic, facultatively intracellular rod whose cell wall, rich in mycolic acids, confers resistance to lysosomal degradation and allows survival within macrophages [
2]. In addition to its intracellular lifestyle,
C. pseudotuberculosis produces enzymes capable of degrading extracellular matrix components and possesses iron-uptake systems that are essential for establishing chronic infections and abscess formation [
3].
Clinically, CLA is characterized by the formation of purulent, caseous abscesses in lymph nodes [
4,
5]. In goats, the most frequently affected sites are the pre-scapular, submandibular, and pre-femoral lymph nodes, whereas in sheep the disease typically presents as internal abscesses in mediastinal and bronchial lymph nodes [
4,
6]. In visceral presentations, affected organs include the lungs, liver, spleen, and kidneys, accompanied by systemic signs such as recurrent fever, lethargy, and weight loss in more advanced stages [
6].
Transmission occurs primarily by percutaneous routes, through skin injuries (shearing, castration, dehorning, branding, etc.) that allow the organism to penetrate the dermis [
7]. Indirect contamination arises from contact with contaminated facilities, equipment, or pastures, as well as consumption of infected water and feed [
7]. In confined systems, aerosolized dust can amplify the spread of infection [
8].
Definitive diagnosis of CLA is based on bacterial isolation from purulent exudate of abscesses, followed by biochemical identification and molecular confirmation by PCR targeting virulence and resistance genes present in C. pseudotuberculosis [
9]. For epidemiological surveillance and detection of subclinical animals, indirect ELISA serology is used, employing membrane antigens or proteins involved in iron metabolism to detect specific IgG antibodies [
10,
11]. This approach makes it possible to identify asymptomatic carriers and guide management and control strategies [
10].
In 2023, according to the Municipal Livestock Survey by the Brazilian Institute of Geography and Statistics (IBGE), the goat population in Brazil reached 12.9 million head, of which approximately 96 % are concentrated in the Northeast Region. Of this total, Bahia stood out as the leading state in goat raising, holding about 30.7 % of all goats in the country [
12]. In this area, CLA is endemic, and serological surveys in herds from Paraíba, Pernambuco, and Bahia reported seroprevalences ranging from 4.6% to 68.2% (
Table 1). Infection by
C. pseudotuberculosis causes substantial economic losses, including reduced weight gain, decreased milk production, condemnation and discard of hides and carcasses, and increased veterinary costs [
13].
By contrast, the state of Espírito Santo, is predominantly covered by the Atlantic Forest biome, and has no specific publications evaluating C. pseudotuberculosis seroprevalence in goat herds. Local environmental factors such as humid coastal climate and shared milking facilities, combined with management practices (lack of quarantine, low frequency of equipment disinfection), may favor pathogen survival in the environment and transmission among animals. Moreover, difficulty accessing diagnostic laboratories, especially in more remote municipalities, allows subclinical animals to persist and disseminate C. pseudotuberculosis without early detection.
This lack of regional data represents a critical gap in disease control: without information on prevalence and risk factors, it is impossible to design control strategies tailored to the goat production reality of Espírito Santo. Among these strategies are targeted vaccination programs, serological campaigns, and biosecurity protocols adapted to local conditions. Such measures include conducting ELISA tests to identify asymptomatic animals, adopting commercial vaccines that include membrane antigens and iron-uptake factors, and implementing good management practices, such as isolating animals with abscesses, properly discarding purulent material, and rigorously sanitizing milking equipment [
6,
14].
In this context, the present study aimed to (1) determine the seroprevalence of antibodies against C. pseudotuberculosis in goats from selected properties in Espírito Santo and (2) identify herd-level (herd size, infrastructure, CLA history, management practices) and individual-level (age, sex, reproductive status) risk factors associated with infection.
2. Materials and Methods
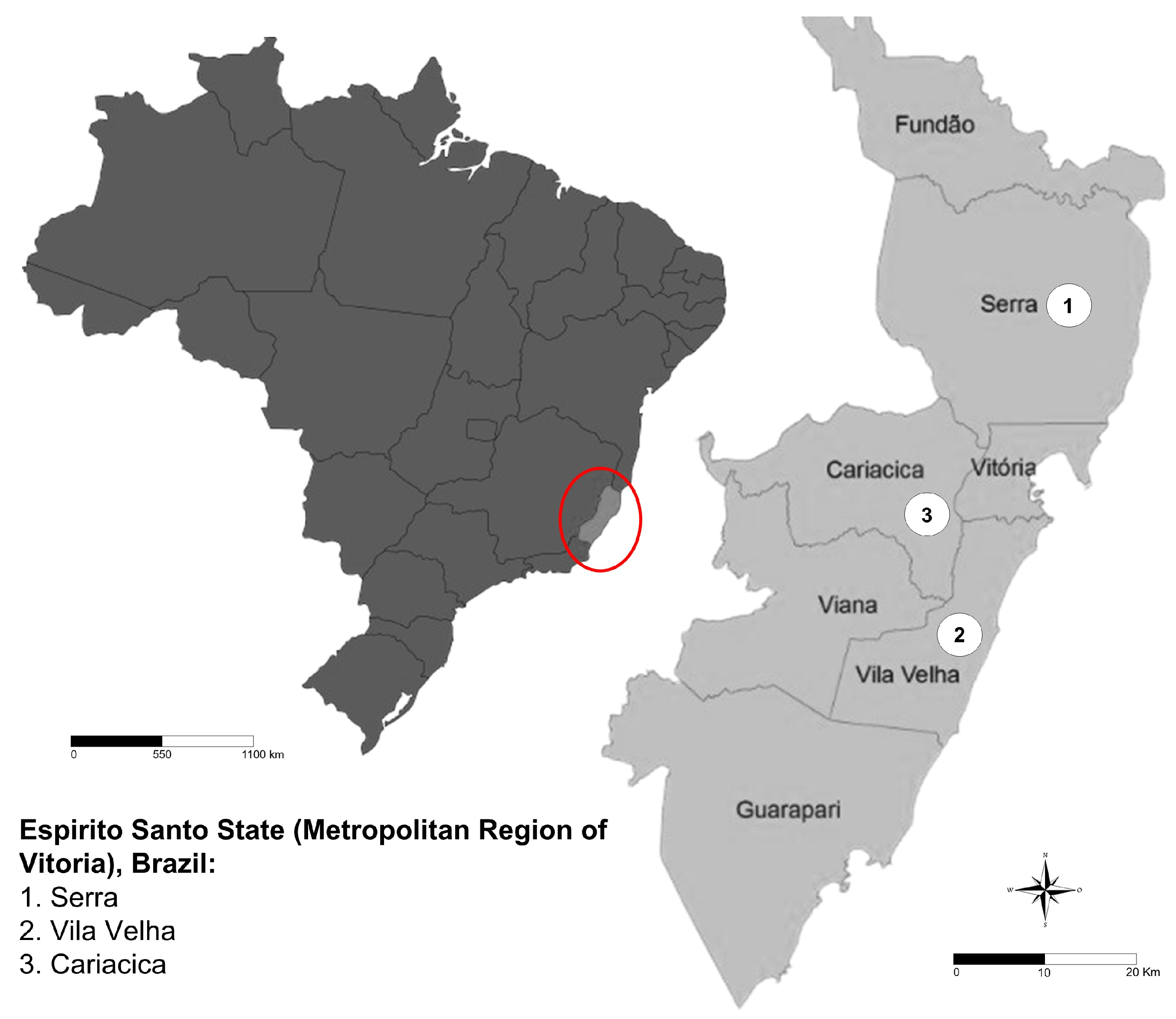
The state of Espírito Santo, located in the southeastern region of Brazil (
Figure 1), has a humid tropical climate, with average annual temperatures ranging from 22°C to 24°C. Annual precipitation exceeds 1,400 mm, mainly concentrated during the summer. The animals analyzed in this study were bred in farms from three cities in the state: Cariacica (20° 15’ 50” S 40° 25’ 12” W), Serra (20° 07’ 44” S 40° 18’ 28” W), and Vila Velha (20° 19’ 48” S 40° 17’ 31” W).
To identify the main production areas, properties recognized by the Association of Goat and Sheep Breeders of Espírito Santo were selected, forming a representative sample. The region includes 43 goat farms, totaling 1,618 animals. Sample size was calculated based on an expected prevalence of 10%, acceptable error of 0.05, and 95% confidence level, resulting in a minimum of 125 goats, as determined by Epi-Info software (Version 7.2).
Blood samples were collected from randomly selected animals on eight properties, with five milliliters obtained via jugular venipuncture. Sera were separated by centrifugation and stored at -20°C until serological analysis.
A structured questionnaire was administered to farm managers during blood sampling to capture both individual-level and herd-level variables. Individual animal data included sex (male/female), breed (pure/undefined), age, and health history (previous abscesses or other clinical signs). Property-level factors comprised facility conditions (building type, pen floor material), production system, herd composition (total number of goats and sheep), and land use.
A “semi-intensive system” was defined as one in which goats have access to controlled grazing for part of the day and receive supplemental feed; an “extensive system” refers to free-range grazing without supplementation; and an “intensive system” denotes full confinement in covered facilities. A “shelter” was defined as a covered enclosure used for overnight protection or during inclement weather. Flooring types were described as follows: “slatted floor” consists of wooden or concrete beams with gaps for drainage; “dirt floor” is compacted natural soil; and “concrete floor” is smooth, non-perforated concrete. Abscess presence was recorded via clinical examination by the investigator and producer report, with animals deemed positive if at least one palpable abscess or a producer-confirmed lesion within the preceding 30 days was noted. Veterinary assistance was categorized from owner interviews as “regular” (monthly to quarterly visits) or “sporadic” (responses only during disease outbreaks), encompassing any service type (vaccination, routine inspection, emergency treatment). Silage management was also owner-reported, with “absence of silage” when no silage was ever used. Animals were stratified by age into 0–12 months, 1–2 years, and > 2 years. To minimize information bias, the same researcher conducted all interviews.
The ELISA was performed as described by Barral et al. [
14], characterized by 96.9% sensitivity and 98.4% specificity, to detect specific antibodies against
C. pseudotuberculosis in goats. The previously expressed and purified PLD and CP40 recombinant proteins were used as antigens. Flat-bottom polystyrene microtiter plates (Costar) were sensitized with 100 µL of the recombinant protein solution diluted in carbonate-bicarbonate buffer (pH 9.6) and incubated for 16 hours at 4°C. After sensitization, the plates were washed twice with PBS-Tween 20 (PBST) and blocked with 200 µL of PBS containing 5% casein for 2 hours at 37°C. The plates were then rewashed, and the serum samples were diluted 1:400 in PBS containing 1% casein and added to the plates (100 µL per well). The plates were incubated for 1 hour at 37°C. After five washes with PBST, 100 µL of secondary antibody conjugated with horseradish peroxidase (Sigma-Aldrich) diluted in PBS 1% casein was added, followed by incubation for 45 minutes at 37°C. After five additional washes, the reaction was developed using O-phenylenediamine dihydrochloride (OPD) chromogen, citrate-phosphate buffer (pH 5.2) and H
2O
2 substrate for 10 minutes, and the reaction was stopped with 2N H₂SO₄. The optical density was read at 492 nm using a microplate reader (Bio-Rad). Positive and negative controls were also used. Samples were considered positive when the optical density exceeded the cut-off, calculated as mean plus three standard deviations of negative controls.
Data analysis was conducted using Epi-Info (Version 7.2) and SPSS (Version 28) softwares, considering a significance level of p < 0.05. The frequencies of positive results were determined for serological techniques and individual and collective variables presented in absolute and relative values.
To evaluate the risk factors for C. pseudotuberculosis infection, the response variable was defined based on ELISA test results, with associated factors analyzed using a random-effects logistic regression model. This model accounted for the dependence of observations from the same property while assuming independence between individual goats. Univariate analysis was conducted, and associations were quantified using odds ratios (OR) and 95% confidence intervals (CI 95%). Variables with p < 0.25 in the univariate analysis were included in the multivariate model. Variables exhibiting high multicollinearity were excluded from the logistic regression to ensure model stability. Models were built using forward selection, and the likelihood-ratio test determined the final model configuration.
3. Results
A total of 145 goat sera were included in this study. Regarding geographic distribution, 48.9 % (71/145) were from Cariacica, 33.1 % (48/145) from Vila Velha, and 18.6 % (27/145) from Serra. Regarding sex, 73.1 % (106/145) were female and 26.9 % (39/145) were male. The most represented age group was 40.0 % (58/145) for goats aged 1–2 years, followed by 14.5 % (21/145) for those 2–3 years and 8.9 % (13/145) for those over 3 years. For breed, 58.6 % (85/145) were of undefined breed and 41.4 % (60/145) were purebred.
The predominant production system was semi-intensive at 71.7 % (104/145), followed by extensive at 24.8 % (36/145) and intensive at 3.4 % (5/145). Regarding the presence of a shelter (a covered collective enclosure), 39.3 % (57/145) of goats were kept on properties with a shelter, whereas 60.7 % (88/145) had no such facility. As for flooring type, 37.2 % (54/145) of goats were housed on dirt floors, 35.9 % (52/145) on slatted floors, and 26.9 % (39/145) on concrete floors.
Among the 145 animals, 34.5% (50/145) tested positive for C. pseudotuberculosis-specific antibodies. Serra exhibited the highest positivity rate (42.3%), followed by Vila Velha (35.4%) and Cariacica (30.1%). Considering the ELISA sensitivity (96.9 %) and specificity (98.4 %), the adjusted (true) seroprevalence of C. pseudotuberculosis infection in the sampled goat population was estimated at 34.5 % (95 % CI: 27.0–42.0 %), indicating that test misclassification had minimal impact on the overall prevalence estimate.
Univariate analysis (p > 0.05) found no significant differences between cities. Regarding herd characteristics, females had more positive results (38.7%) than males (23.1%) (
Table 1), but no significant difference in seroprevalence was identified (p > 0.05).
Data suggest a significant difference in positivity by age (p < 0.05), with the highest prevalence in animals aged > 2 years (50%), indicating increased risk in older animals. Concerning breed, undefined breed goats had 40% positivity, with no statistical difference (p > 0.05). Extensive and semi-intensive systems predominated, with no correlation between management and breed.
Table 3 presents the results of both univariate and multivariate analyses of risk factors associated with seropositivity. In univariate analysis, age over two years (OR: 2.7; 95% CI: 1.1–5.2; p = < 0.05), presence of abscesses (OR: 6.3; 95% CI: 1.2–32.7; p = < 0.05), and veterinary assistance (OR: 0.2; 95% CI: 0.7–0.6; p = < 0.05) were associated with infection risk, the latter inversely. In logistic regression, only abscesses (OR: 4.7; 95% CI: 1–23.7; p = < 0.05) and absence of veterinary assistance (OR: 4.7; 95% CI: 1.6–13.6; p = < 0.05) remained significant risk factors.
4. Discussion
We found a 34.5% seroprevalence of
C. pseudotuberculosis in goats from Espírito Santo. This rate aligns with prior surveys in Brazil—ranging from 21% in Paraíba State [
16] to 81.8% in Minas Gerais State [
17] (Table 2)—confirming that CLA remains highly prevalent among small ruminants. Such high rates may result from inadequate management practices, such as lack of proper cleaning and disinfection of facilities, failure to isolate animals with abscesses, and improper disposal of caseous material into the environment. Moreover, high population densities on intensive farms favor rapid pathogen dissemination through direct contact and contaminated aerosols.
The significantly higher prevalence in Serra (42.3%) compared to other cities suggests that management practices and sanitary conditions may differ between localities. For example, farms in Serra may maintain higher stocking densities—more goats housed in the same space—which increases direct contact and facilitates transmission of
C. pseudotuberculosis. Furthermore, if pens and bedding are not cleaned regularly, caseous material from abscesses can accumulate in the environment, allowing the bacterium to survive longer. Inadequate ventilation in shelters can also create humid conditions that weaken the natural defenses of the animals and increase susceptibility to infection [
5]. El Khalfaoui et al. [
17] demonstrated that intensive systems characterized by overcrowding, insufficient airflow, and lack of abscess control are strongly associated with higher CLA risk.
Moreover, the markedly higher seroprevalence observed in goats aged two to three years (52.4 percent) further underscores the chronic nature of caseous lymphadenitis, since animals in this age bracket have simply had more time to encounter and harbor the pathogen [
18,
19]. Earlier investigations have likewise identified age as a critical risk factor: Guimarães et al. [
20] reported that older sheep in Minas Gerais exhibited the highest CLA rates, implying that prolonged exposure over successive seasons amplifies transmission within herds. Jeber et al. [
21] remind us that the incubation period for visible abscess formation can range from 25 to 140 days, meaning that younger animals may simply not have reached the stage at which lesions become detectable. Although seroprevalence in young goats was low, they remain at risk due to contact with seropositive mothers and older animals.
The study by Barbosa et al. [
17] similarly found that farms lacking routine veterinary oversight and having minimal infrastructure were associated with higher CLA rates, an effect that is exacerbated by animal age. Taken together, these findings indicate that, beyond intrinsic immunological maturation, the risk of CLA in older goats is amplified by prolonged environmental pressure, poor biosecurity practices, and the tendency of chronic carriers to spread infection during grazing and confinement.
The study highlighted that the presence of abscesses is one of the main risk factors for infection with
C. pseudotuberculosis and emphasizes the urgency of adopting stringent control measures at both the animal and herd levels. In practice, any goat showing a superficial or palpable abscess should be immediately removed from the flock to prevent direct contact with healthy animals. Abscesses must be properly drained or surgically opened, and the caseous material contained within needs to be disposed of safely (buried or incinerated) since de Farias et al. [
22] demonstrated that environmental contamination from improperly discarded abscess contents is a primary route of pathogen transmission. Despite this clear recommendation, many producers still neglect routine inspection of goats for early-stage abscesses or fail to isolate affected animals promptly, allowing the bacterium to persist in bedding, communal water sources, and on shared equipment.
Barnabé et al. [
16] reported that goats with skin lesions were 2.394 times more likely to present the disease when compared to those without visible lesions, which highlights how even small, unnoticed abrasions can develop into abscess sites and perpetuate disease spread. Beyond visible lesions, asymptomatic carriers play an equally important role in maintaining CLA within a herd. These animals harbor
C. pseudotuberculosis without showing clinical signs, shedding the bacterium into the barn air and onto shared surfaces through aerosols or secretions [
23]. As noted by Jeber et al. [
21], aerosolized spread from carrier individuals may seed new infections in susceptible goats, particularly in areas with poor ventilation or high stocking densities, where humidity and ammonia levels further impair local immunity. However, the caseous lesions associated with caseous lymphadenitis are characteristic but not exclusive, and they may be confused with lesions caused by other pyogenic organisms [
24]. Therefore, bacteriological isolation is essential for the definitive diagnosis of caseous lymphadenitis when considering symptomatic animals [
6,
8]; however, only ELISA can currently detect asymptomatic animals [
15].
Farms that receive regular veterinary care consistently have lower CLA rates because veterinarians can find and treat small abscesses early and put in place prevention plans (vaccinations, proper nutrition, and deworming) that lower the number of bacteria and stop its spread [
2,
17]. On the other hand, farms without routine veterinary visits often do not follow clear biosecurity rules, wait too long to treat sick animals, and handle disease based on personal experience instead of proven methods. This allows
C. pseudotuberculosis to survive in bedding, water troughs, and shared tools.
In Brazil, several challenges make veterinary care harder to access. In remote areas, there are few laboratories and it takes too long to send samples for testing, so many producers rely only on visible signs and miss hidden infections [
25,
26]. Although ELISA tests can find animals that carry the bacteria without showing symptoms, these tests are expensive and not widely available. At the same time, vaccines against CLA work differently under extensive grazing conditions, and many farmers decide they are not worth the cost and do not use them [
27].
The primary limitation of the study was the small sample size, since, due to the inability to contact all goat producers in the region, it was not possible to reach a substantial number of animals, and the selection of sampled farms based solely on those that agreed to participate, without a randomized approach. Nevertheless, because this research is pioneering in Espírito Santo, where no formal records of caseous lymphadenitis in goats exist to date, it fills an important local epidemiological gap. To mitigate potential biases, we collected detailed information on factors that could influence prevalence, such as species, age range, sanitary management, and vaccination status of the animals, and included these variables in multivariate statistical models (for example, logistic regression) to adjust for confounders that might distort the exposure–outcome association.
By including farms without an explicit clinical history of lymphadenitis, we avoided the bias of selecting only “high-risk” sites: a common issue in studies focused on large, affected herds. In addition, we selected farms of various sizes (small, medium, and large), management systems (conventional and extensive), and locations (peri-urban and rural areas) to ensure greater representativeness of regional conditions and thereby mitigate selection bias. Although we recognize the impossibility of complete probabilistic sampling, we adopted standardized protocols for data collection and analysis, conducted sensitivity analyses, and uniformized clinical and laboratory procedures to lend robustness to our conclusions. Thus, despite these limitations, we have solid evidence that any remaining bias did not undermine the primary conclusion: confirmation of C. pseudotuberculosis circulation among goats in Espírito Santo State, thereby laying the groundwork for broader epidemiological studies and stratified sampling in future rounds.
This study confirmed a 34.5% seroprevalence of C. pseudotuberculosis in Espírito Santo goats, showing the persistent challenge of controlling CLA in small ruminant populations. Clinically, high CLA rates translate into increased morbidity, reduced weight gain, lower milk and meat production, and a greater need for veterinary interventions. The association of abscess presence and limited veterinary oversight with higher infection rates indicates that strengthening herd health programs must become a priority to interrupt transmission.
As the first seroepidemiological investigation of CLA in Espírito Santo, our work fills a critical local data gap and establishes a baseline for targeted interventions. Offering subsidized or regionally coordinated ELISA screening will help identify asymptomatic carriers before they perpetuate infection, and evaluating vaccine efficacy under local field conditions is essential for developing cost-effective prevention strategies. By providing these insights, the study can guide future research on longitudinal impact, inform extension services, and support caprinoculture improvements, like promoting healthier herds, reducing economic losses, and enhancing the sustainability of goat farming in the region. Finally, future studies are needed to quantify the broader economic burden of CLA and to assess the long-term effectiveness of combined biosecurity and vaccination strategies in reducing disease prevalence.
Table 1.
Survey of Studies on Prevalence and Risk Factors for C. pseudotuberculosis Infection in Goats and Sheep in Brazil.
Table 1.
Survey of Studies on Prevalence and Risk Factors for C. pseudotuberculosis Infection in Goats and Sheep in Brazil.
| Region |
Species |
Method |
States |
Prevalence (Overall / by State) |
Risk Factors |
Authors |
| Northeast |
Goats & Sheep |
Clinical exam + culture |
Paraíba |
Overall: 68.2 % (N = 22) | Goats: 78.6 % (n = 14), Sheep: 50.0 % (n = 4). |
Herds where abscesses rupture naturally. |
[7] |
| |
Sheep |
Indirect ELISA |
Ceará, Paraíba, Piauí, RN, Sergipe |
Overall: 37.5 % (N = 866) |Ceará: 39.2 % (n = 158), Paraíba: 30.9 % (n = 83), Piauí: 42.2 % (n = 209), RN: 41.1 % (n = 227), Sergipe: 31.9 % (n = 189). |
Purebred sheep; acquisition of rams at fairs; separation of lambs from ewes; pond water for ewes; late disposal of infected animals |
[25] |
| |
Sheep |
Indirect ELISA |
Ceará, Paraíba, Piauí, RN, Sergipe |
Overall: 37.8 % (N = 996) | Ceará: 42.1 % (n = 547), Paraíba: 31.0 % (n = 281), Piauí: 40.0 % (n = 653), RN: 40.6 % (n = 564), Sergipe: 31.9 % (n = 593) |
Not reported |
[26] |
| |
Goats |
Ante-/post-mortem exam + culture |
Paraíba |
Overall: 21.4 % (N = 304). |
Cutaneous lesions/scars; female sex. |
[16] |
| |
Goats |
Indirect ELISA |
Ceará, Paraíba, Piauí, RN, Sergipe |
Overall: 30.5 % (N = 2 571) | Ceará: 24.0 % (n = 460), Paraíba: 29.0 % (n = 741), Piauí: 41.4 % (n = 396), RN: 33.0 % (n = 663), Sergipe: 22.5 % (n = 311) |
Age |
[13] |
| |
Goats |
Indirect ELISA |
Ceará, Paraíba, Piauí, RN, Sergipe |
Overall: 30.3 % (N = 2 744) | Ceará: 25.7 % (n = 435), Paraíba: 29.1 % (n = 741), Piauí: 35.9 % (n = 582), RN: 33.3 % (n = 675), Sergipe: 22.1 % (n = 311) |
No silage; no separation by sex or age; no breeder replacement; no abscess treatment before rupture. |
[22] |
| |
Goats |
Indirect ELISA + culture |
Rio Grande do Norte |
Overall: ELISA 11.3 % / Culture 2.0 % (N = 150) |
Not reported |
[28] |
| |
Sheep |
Indirect ELISA |
Ceará |
Overall: 34.1 % (N = 402) |
Not reported |
[29] |
| |
Goats |
Indirect ELISA |
Rio Grande do Norte |
Overall: 23.4 % (N = 385) |
Not reported |
[30] |
| |
Goats & Sheep |
Clinical exam, FNA, culture & PCR |
Maranhão |
Overall: 4.6 % (N = 56) |
Not reported |
[6] |
| |
Sheep |
Ante-/post-mortem exam + culture |
Amazonas |
Overall: 74.0 % (N = 51) |
Not reported |
[8] |
| Southeast |
Sheep |
Clinical exam, culture & PCR |
Minas Gerais |
Overall: 81.8 % (N = 22) |
Not reported |
[17] |
| |
Sheep |
Indirect ELISA |
Minas Gerais |
Overall: 70.9 % (N = 642) |
Age |
[20] |
| |
Sheep |
Culture + multiplex PCR |
São Paulo |
Overall: 34.0 % (culture); 58.0 % (PCR) (N = 202) |
Not reported |
[31] |
| |
Goats |
Indirect ELISA |
Minas Gerais |
Overall: 78.9 % (N = 676) |
Extensive production system; age |
[32] |
| Midwest |
Sheep |
Indirect ELISA |
Distrito Federal |
Overall: 44.0 % (N = 1 028) |
|
[33] |
| North |
Goats & Sheep |
Clinical exam, FNA & culture |
Amazonas |
Overall: 1.8 % (N = 562) |
Not reported |
[34] |
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization, L.V., B. F., and R.W.P.; methodology, L.V.. T.D.B. and R.W.P; validation, B.F. and R.W.P.; formal analysis, L.V., and T.DB.; investigation, L.V. and T.D.B.; resources, M.B., B. F. and R.W.P.; data curation, L.V.; writing—original draft preparation, L.V, M.B.; writing—review and editing, L.V, M.B. and L.S.; visualization, B.F. and R.W.P.; supervision, B.F. and R.W.P.; project administration, B.F.; funding acquisition, R.W.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Espírito Santo (FAPES) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ). LV is a PhD fellow from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). RP is a Technological Development fellow from CNPq (Proc. 310058/2022-8).
Institutional Review Board Statement
This project was approved by the Ethics Committee on the Use of Animals of Federal University of Espírito Santo (CEUA/UFES Number 15/2020).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank Vinicius Gumiero, from the Association of Goats and Sheep of the State of Espírito Santo (ACCOES), for his support in contacting goat breeders, and Francisca Soares, from the Laboratory of Immunology and Molecular Biology of the Universidade Federal da Bahia (UFBA), for technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest,
Abbreviations
The following abbreviations are used in this manuscript:
| CLA |
Caseous lymphadenitis |
| ELISA |
Enzyme-linked immunosorbent assay |
| PLD |
Phospholipase D |
| CP40 |
Caseous protease 40 |
| PBS |
Phosphate-buffered saline |
| PBST |
Phosphate-buffered saline with Tween 20 |
| OPD |
O-phenylenediamine dihydrochloride |
| H₂O₂ |
Hydrogen peroxide |
| OD₄₉₂ |
Optical density at 492 nm |
| OR |
Odds ratio |
| CI |
Confidence interval |
| Epi-Info |
Epidemiological Information Package |
| SPSS |
Statistical Package for the Social Sciences |
| RPM |
Revolutions per minute |
References
- Seyffert N, Silva RF, Jardin J, et al. (2014) Serological proteome analysis of Corynebacterium pseudotuberculosis isolated from different hosts reveals novel candidates for prophylactics to control caseous lymphadenitis. Vet Microbiol 174:255–260. [CrossRef]
- Ramos CP, Dorneles EMS, Haas DJ, et al. (2022) Molecular characterization of Corynebacterium pseudotuberculosis, C. silvaticum, and C. auriscanis by ERIC-PCR. Ciência Rural 52. [CrossRef]
- Domínguez MCR, de Oca Jiménez RM, Guerreo JAV (2021) Caseous lymphadenitis: virulence factors, pathogenesis and vaccines. Review. Rev Mex Cienc Pecu 12:1221–1249. [CrossRef]
- de Oliveira Zamprogna T, Ribeiro D, Azevedo VAC, et al. (2021) Bacteriological, cytological, and molecular investigation of Corynebacterium pseudotuberculosis, mycobacteria, and other bacteria in caseous lymphadenitis and healthy lymph nodes of slaughtered sheep. Brazilian Journal of Microbiology 52:431–438. [CrossRef]
- Khanamir R, Issa N, Abdulrahman R (2023) First study on molecular epidemiology of caseous lymphadenitis in slaughtered sheep and goats in Duhok Province, Iraq. Open Vet J 13:588. [CrossRef]
- Soares DM, Alves MS, Barros TM, et al. (2024) Isolation and molecular identification of Corynebacterium pseudotuberculosis in goats and sheeps with lymphadenitis. Ciência Animal 34:71–80.
- Andrade JSL, Azevedo SS, Teles JAA, et al. (2012) Ocorrência e fatores de risco associados à infecção por Corynebacterium pseudotuberculosis em caprinos e ovinos do semiárido paraibano. Pesquisa Veterinária Brasileira 32:116–120. [CrossRef]
- Souza MF, Carvalho AQ, Garino Jr F, Riet-Correa F (2011) Linfadenite caseosa em ovinos deslanados abatidos em um frigorífico da Paraíba. Pesquisa Veterinária Brasileira 31:224–230. [CrossRef]
- Baird GJ, Malone FE (2010) Control of caseous lymphadenitis in six sheep flocks using clinical examination and regular ELISA testing. Veterinary Record 166:358–362. [CrossRef]
- Oreiby AF (2015) Diagnosis of caseous lymphadenitis in sheep and goat. Small Ruminant Research 123:160–166. [CrossRef]
- Binns SH, Green LE, Bailey M (2007) Development and validation of an ELISA to detect antibodies to Corynebacterium pseudotuberculosis in ovine sera. Vet Microbiol 123:169–179. [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (2023) Produção Pecuária Municipal 2023. Prod Pec Munic 51:1–12.
- Farias AM, Alves JRA, Alves FSF, et al. (2018) Soroprevalência da infecção por Corynebacterium pseudotuberulosis em caprinos no Nordeste brasileiro utilizando técnica de imunoabsorção enzimática (ELISA-indireto). Pesquisa Veterinária Brasileira 38:1344–1350. [CrossRef]
- Rebouças MF, Loureiro D, Bastos BL, et al. (2013) Development of an indirect ELISA to detect Corynebacterium pseudotuberculosis specific antibodies in sheep employing T1 strain culture supernatant as antigen. Pesquisa Veterinária Brasileira 33:1296–1302. [CrossRef]
- Barral TD, Mariutti RB, Arni RK, et al. (2019) A panel of recombinant proteins for the serodiagnosis of caseous lymphadenitis in goats and sheep. Microb Biotechnol 12:1313–1323. [CrossRef]
- Barnabé NN da C, Silva JD da, Viana MP, et al. (2019) Characterization of caseous lymphadenitis in caprine animals slaughtered in a semi-arid region of Brazil. Semin Cienc Agrar 40:1867. [CrossRef]
- Barbosa V de M, Antunes ÚC, Silva NC, et al. (2012) Ocorrência de linfadenite caseosa em ovinos da raça santa inês com linfonodos superficiais reativos na região de Uberlândia, Minas Gerais. Sanidade Animal 69:109–115.
- El Khalfaoui N, El Amiri B, Cabaraux J-F, et al. (2024) Rearing Management and Its Impact on Caseous Lymphadenitis in Sheep. Animals 14:1504. [CrossRef]
- Loureiro D, Moura-Costa LF, Jordão RS, et al. (2017) Seroprevalence of antibodies against bacterial pathogens in sheep from Equatorial Guinea. Revue Scientifique et Technique de l’OIE 36:965–970. [CrossRef]
- Guimarães AS, Seyffert N, Bastos BL, et al. (2009) Caseous lymphadenitis in sheep flocks of the state of Minas Gerais, Brazil: Prevalence and management surveys. Small Ruminant Research 87:86–91. [CrossRef]
- Jeber ZKH, MohdJin Z, Jesse FF, et al. (2016) Influence of Corynebacterium pseudotuberculosis infection on level of acute phase proteins in goats. BMC Vet Res 12:48. [CrossRef]
- de Farias AEM, Alves JRA, Alves FSF, et al. (2019) Seroepidemiological characterization and risk factors associated with seroconversion to Corynebacterium pseudotuberculosis in goats from Northeastern Brazil. Trop Anim Health Prod 51:745–752. [CrossRef]
- Bastos BL, Loureiro D, Raynal JT, et al. (2013) Association between haptoglobin and IgM levels and the clinical progression of caseous lymphadenitis in sheep. BMC Vet Res 9:254. [CrossRef]
- Guedes MT, Souza BC, Sousa TJ, et al. (2015) Infecção por Corynebacterium pseudotuberculosis em equinos: aspectos microbiológicos, clínicos e preventivos. Pesquisa Veterinária Brasileira 35:701–708. [CrossRef]
- Alves JRA, de Farias AEM, Silva JD da, et al. (2020) Factors associated with the seroprevalence of caseous lymphadenitis in sheep from Northeastern Brazil. Prev Vet Med 182:105098. [CrossRef]
- Alves JRA, de Farias AEM, dos Anjos DM, et al. (2020) Seroepidemiological study of Caseous lymphadenitis in sheep from the Northeast region of Brazil using an indirect ELISA. Trop Anim Health Prod 52:1945–1952. [CrossRef]
- Moreira LS, Lopes N da R, Pereira VC, et al. (2022) The Association of Bacterin and Recombinant Proteins Induces a Humoral Response in Sheep against Caseous Lymphadenitis. Vaccines (Basel) 10. [CrossRef]
- Fernandes M da CR, Neto AAM, Lemos EIB, et al. (2023) Occurrence of caseous lymphadenitis in goats from a slaughterhouse in the Brazilian Northeast. Acta Veterinaria Brasilica 17:86–91. [CrossRef]
- Moura GHF, Lelis ICNG, Rocha CS da, et al. (2020) Seroprevalence of Corynebacterium pseudotuberculosis and Toxoplasma gondii in sheep in semi-arid region of Ceará state. Ciência Rural 50:1–8. [CrossRef]
- Maia Neto AA de, Fernandes M da CR, Farias HM de, et al. (2023) Soroprevalência da linfadenite caseosa em rebanhos de caprinos na zona rural de Mossoró-RN. In: Redin E (ed) Ciências Rurais em Foco - Volume 10. Editora Poisson, Belo Horizonte, pp 1–105.
- Nassar AF de C, Daniel GT, Ruiz R, et al. (2016) Diagnostic comparison of Corynebacterium pseudotuberculosis through microbiological culture and PCR in sheep samples. Arq Inst Biol (Sao Paulo) 82:. [CrossRef]
- Seyffert N, Guimarães AS, Pacheco LGC, et al. (2010) High seroprevalence of caseous lymphadenitis in Brazilian goat herds revealed by Corynebacterium pseudotuberculosis secreted proteins-based ELISA. Res Vet Sci 88:50–55. [CrossRef]
- Do Carmo FB, Guimarães AS, Pauletti RB, et al. (2012) Prevalência de anticorpos contra a linfadenite caseosa em criações comerciais de ovinos no Distrito Federal, Brasil. Arq Inst Biol 293–296.
- Caldas LFG de S, Ciríaco AL de S, Almeida KR de et al. (2021) Ocorrência e fatores de risco associados à infecção por Corynebacterium pseudotuberculosis na ovinocaprinocultura da região metropolitana de Manaus - AM. Rev Agrar Acad 4:15–23. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).