Submitted:
09 June 2025
Posted:
10 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Production of Biochar
2.2. Characterizations
3. Results
3.1. Particle Size Distribution of Biochar
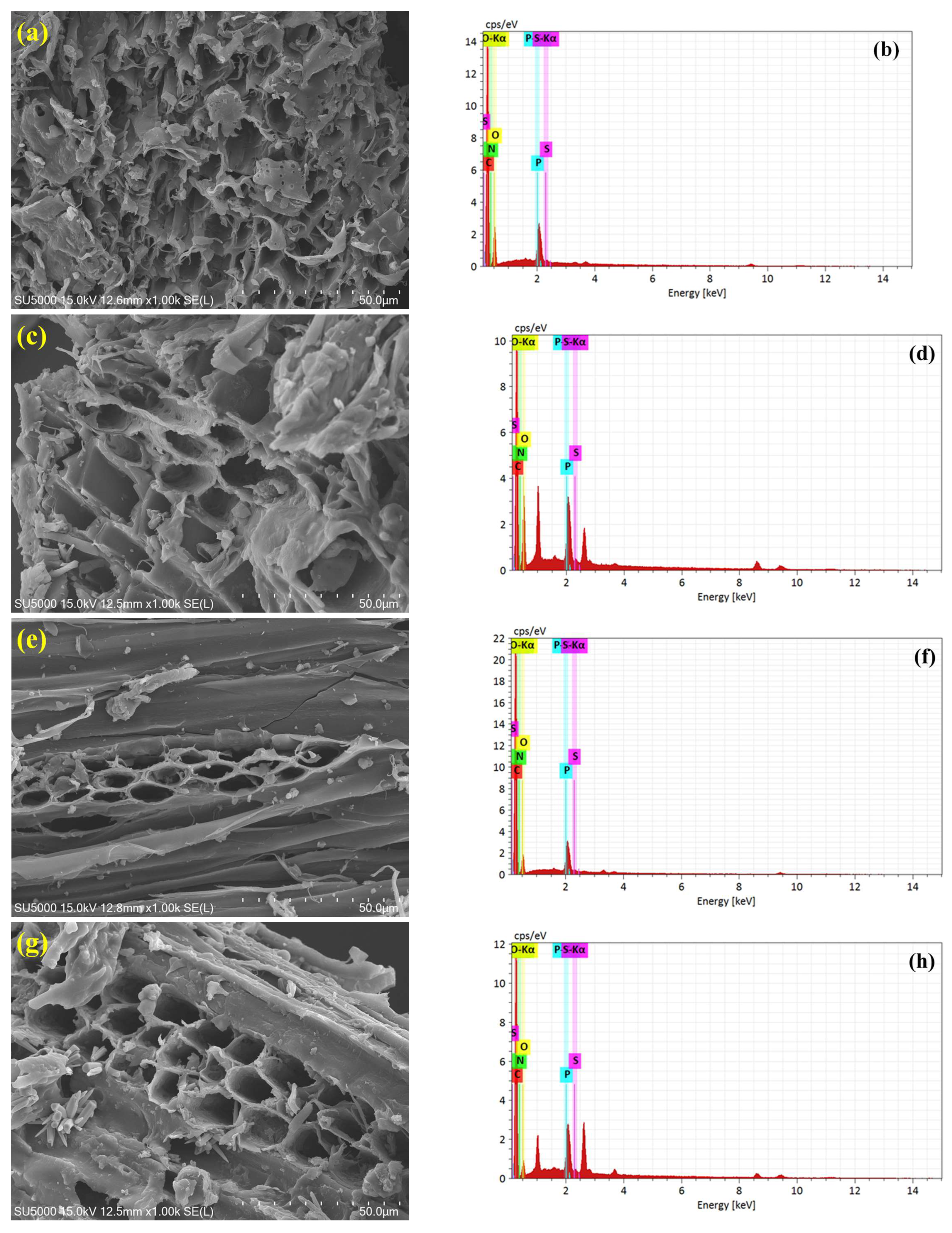
3.2. Scanning Electron Microscopy and Energy-Dispersive X-Ray Spectroscopy
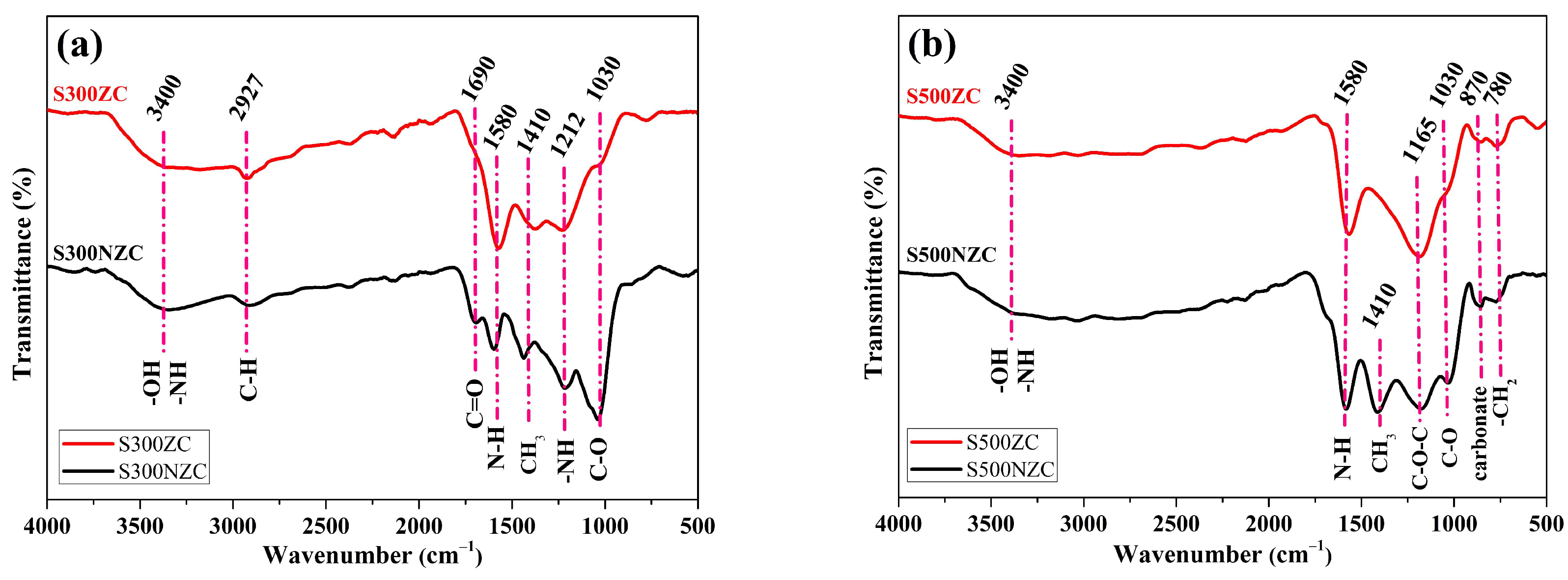
3.3. FTIR Spectroscopy
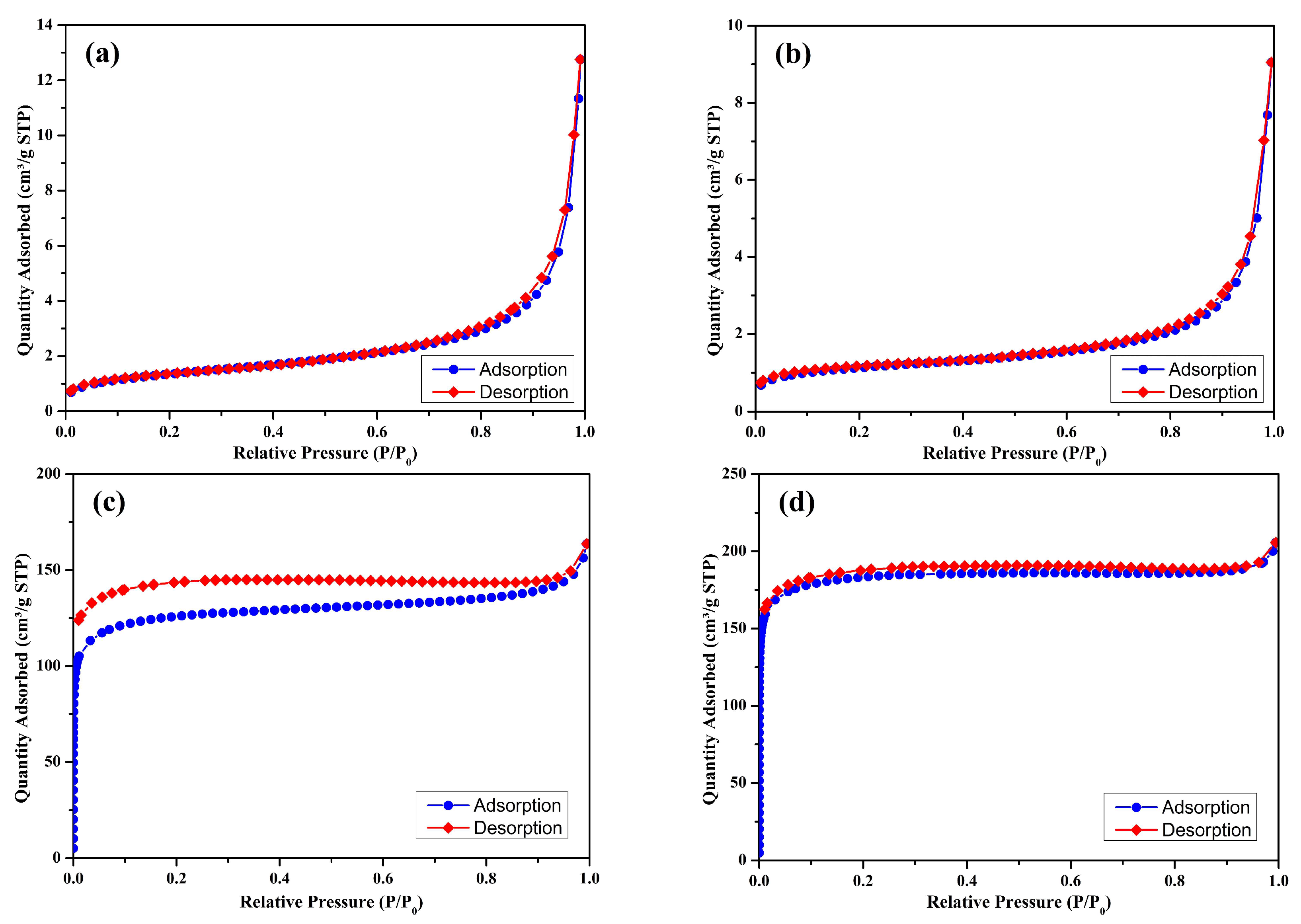
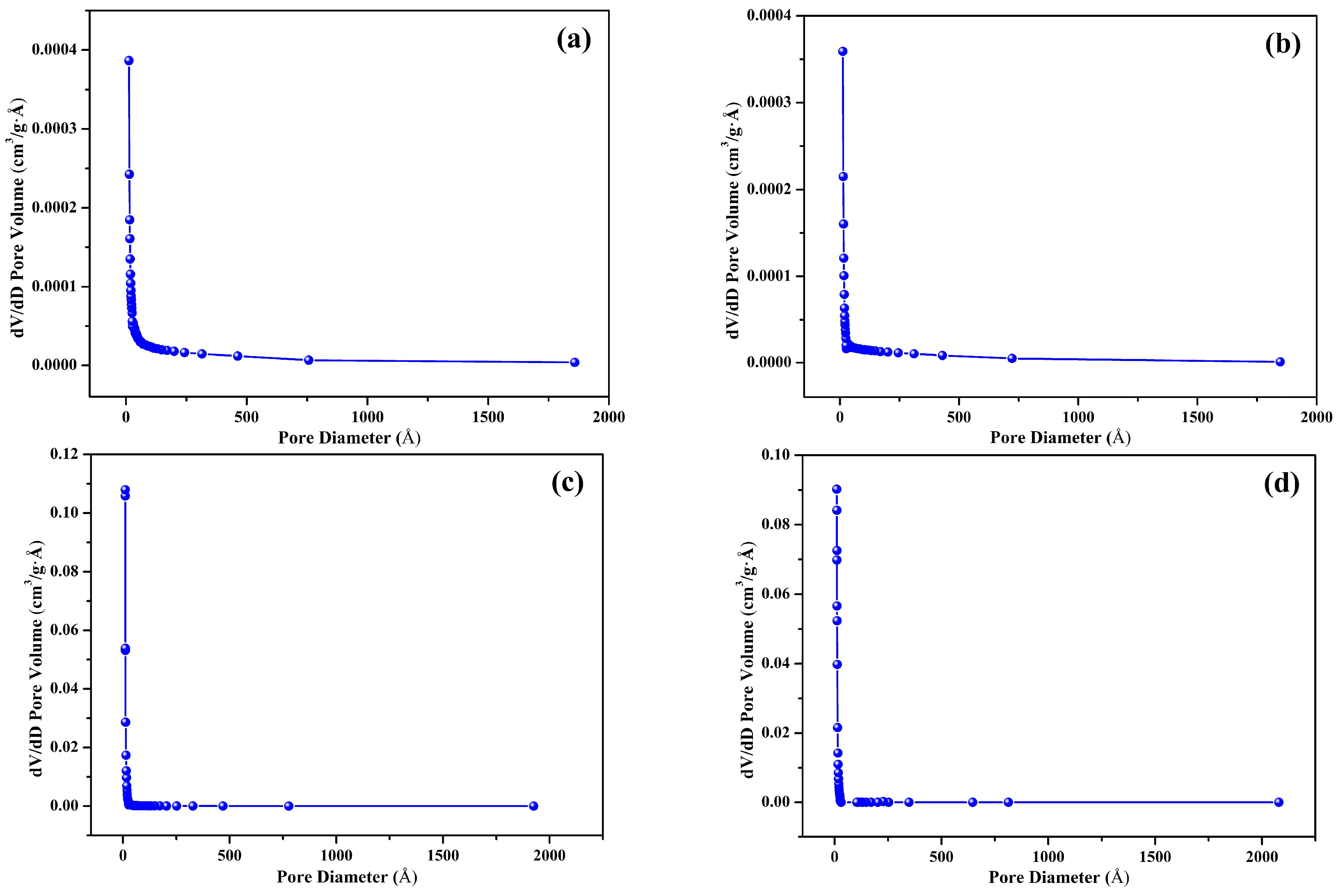
3.4. Surface Area and Pore Structure
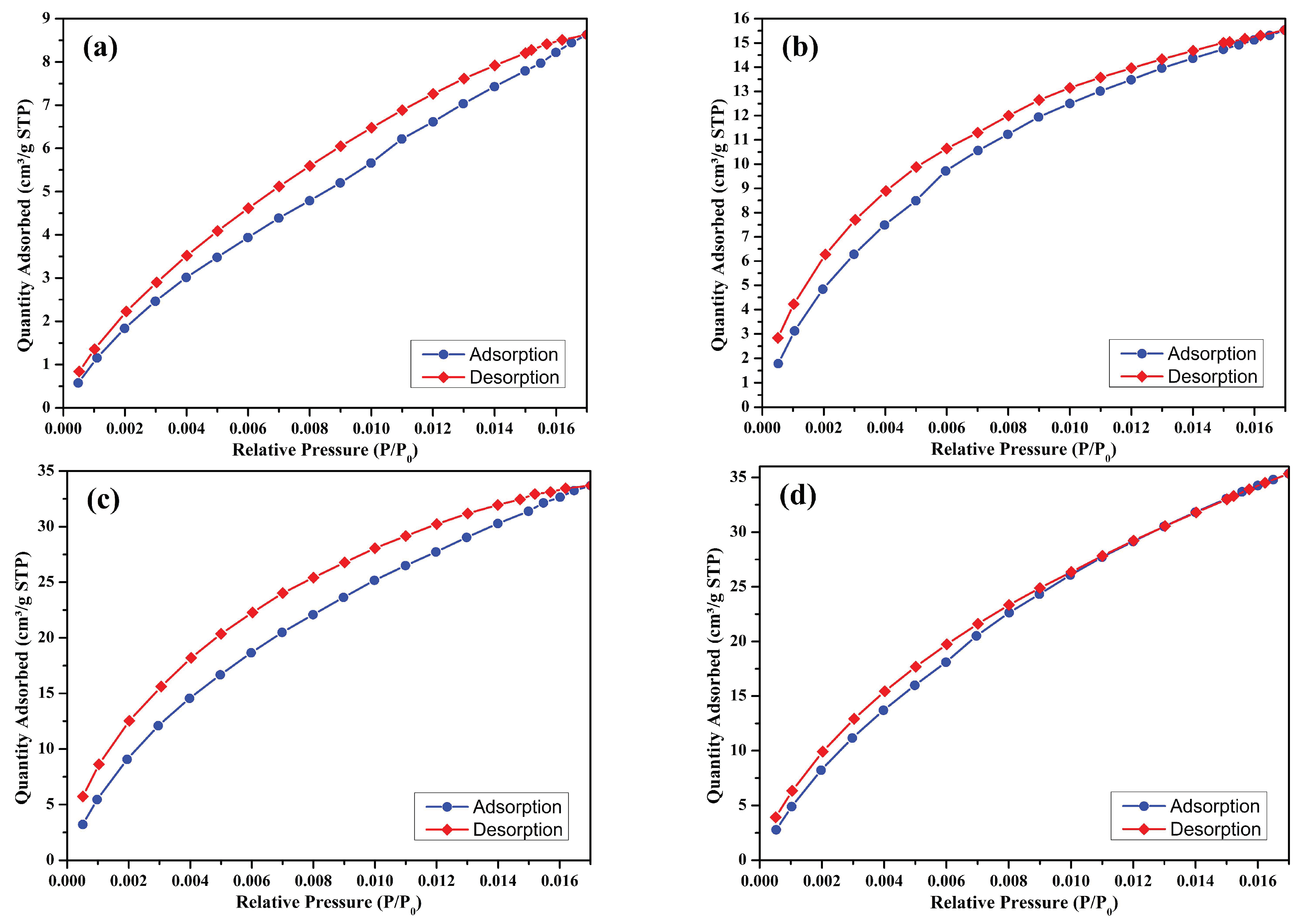
3.5. CO2 Adsorption Characteristics
4. Conclusions
- ZnCl2 activation significantly enhances microporosity and surface area of sawdust-derived biochars, especially at elevated pyrolysis temperatures, making them more effective for gas adsorption applications.
- Pyrolysis temperature plays a critical role in pore development. Samples prepared at 500 °C exhibit higher surface areas and more developed microporous structures than those produced at 300 °C.
- The BET surface area increased from 4.12 m2/g (S500NZC) to 717.60 m2/g (S500ZC) after ZnCl2 activation at 500 °C, demonstrating the powerful effect of chemical activation on pore architecture.
- Average pore diameter reduced significantly upon activation, with S500ZC achieving a narrow average pore size of 14.13 Å, ideal for CO2 adsorption due to its compatibility with the kinetic diameter of CO2 molecules (~3.3 Å).
- CO2 adsorption capacity followed the trend S300NZC < S300ZC < S500NZC < S500ZC, correlating well with micropore area and BET surface area, emphasizing the dominant role of microporosity in CO2 physisorption.
- Maximum CO2 adsorption capacity of 1.58 mmol/g (35.34 cm3/g STP) was achieved by the S500ZC sample, demonstrating the synergy between high pyrolysis temperature and ZnCl2 activation.
- Overall, ZnCl2-activated sawdust biochar at 500 °C emerges as a sustainable, scalable, and efficient material for CO2 capture, offering an excellent balance of high surface area, narrow pore distribution, and eco-friendly synthesis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amer, N. M.; Lahijani, P.; Mohammadi, M.; Mohamed, A. R. , Modification of biomass-derived biochar: A practical approach towards development of sustainable CO2 adsorbent. Biomass Conversion and Biorefinery 2024, 14, 7401–7448. [Google Scholar] [CrossRef]
- Yang, S.; Yang, D.; Shi, W.; Deng, C.; Chen, C.; Feng, S. , Global evaluation of carbon neutrality and peak carbon dioxide emissions: Current challenges and future outlook. Environmental Science and Pollution Research 2023, 30, 81725–81744. [Google Scholar] [CrossRef] [PubMed]
- Jones, M. W.; Peters, G. P.; Gasser, T.; Andrew, R. M.; Schwingshackl, C.; Gütschow, J.; Houghton, R. A.; Friedlingstein, P.; Pongratz, J.; Le Quéré, C. , National contributions to climate change due to historical emissions of carbon dioxide, methane, and nitrous oxide since 1850. Scientific Data 2023, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.; Habiba, U. E.; Khan, W.; Shah, A.; Rahim, S.; De los Rios-Escalante, P. R.; Farooqi, Z.-U.-R.; Ali, L.; Shafiq, M. , Climate change due to increasing concentration of carbon dioxide and its impacts on environment in 21st century; a mini review. Journal of King Saud University-Science 2023, 35, 102693. [Google Scholar] [CrossRef]
- Alli, Y. A.; Bamisaye, A.; Bamidele, M. O.; Etafo, N. O.; CHKIRIDA, S.; Lawal, A.; Hammed, V. O.; Akinfenwa, A. S.; Hanson, E.; Nwakile, C. , Transforming waste to wealth: Harnessing carbon dioxide for sustainable solutions. Results in Surfaces and Interfaces 2024, 100321. [Google Scholar] [CrossRef]
- Nunes, L. J. , The rising threat of atmospheric CO2: a review on the causes, impacts, and mitigation strategies. Environments 2023, 10, 66. [Google Scholar] [CrossRef]
- Hanson, E.; Nwakile, C.; Hammed, V. O. , Carbon Capture, Utilization, and Storage (CCUS) Technologies: Evaluating the Effectiveness of Advanced CCUS Solutions for Reducing CO2 Emissions. Results in Surfaces and Interfaces 2024, 100381. [Google Scholar] [CrossRef]
- Wilkes, M. D.; Mukherjee, S.; Brown, S. Compression system power requirements for various CO2 sources and transportation options. In Computer Aided Chemical Engineering, Elsevier: 2021; Vol. 50, pp 1439-1444.
- Oyenekan, B.; Huang, S.; Lindsay, I. Application of post combustion CO2 capture to natural gas liquefaction plants, Proceedings of the 2nd Annual Gas Processing Symposium, 2010; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Nouri, E.; Raouf, F.; Jamali Alyani, S.; Kardan, A.; Mataei Moghaddam, A. , Carbon dioxide capture and utilization in post-combustion: a review. Environmental Science and Pollution Research 2025, 1–32. [Google Scholar] [CrossRef]
- Soo, X. Y. D.; Lee, J. J. C.; Wu, W.-Y.; Tao, L.; Wang, C.; Zhu, Q.; Bu, J. , Advancements in CO2 capture by absorption and adsorption: A comprehensive review. Journal of CO2 Utilization 2024, 81, 102727. [Google Scholar] [CrossRef]
- Zentou, H.; Hoque, B.; Abdalla, M. A.; Saber, A. F.; Abdelaziz, O. Y.; Aliyu, M.; Alkhedhair, A. M.; Alabduly, A. J.; Abdelnaby, M. M. Recent advances and challenges in solid sorbents for CO2 capture. Carbon Capture Science & Technology 2025, 100386. [Google Scholar]
- Kwon, C. W.; Tae, S.; Mandal, S. Comparative Analysis of CO2 Adsorption Performance of Bamboo and Orange Peel Biochars. Molecules 2025, 30, 1607. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Ishak, S.; Adnin, R. J.; Lee, D.-E.; Park, T. , An approach to utilize date seeds biochar as waste material for thermal energy storage applications. Journal of Energy Storage 2023, 68, 107739. [Google Scholar] [CrossRef]
- Mandal, S.; Ishak, S.; Lee, D.-E.; Park, T. , Optimization of eco-friendly Pinus resinosa biochar-dodecanoic acid phase change composite for the cleaner environment. Journal of Energy Storage 2022, 55, 105414. [Google Scholar] [CrossRef]
- Al Masud, M. A.; Shin, W. S.; Sarker, A.; Septian, A.; Das, K.; Deepo, D. M.; Iqbal, M. A.; Islam, A. R. M. T.; Malafaia, G. , A critical review of sustainable application of biochar for green remediation: Research uncertainty and future directions. Science of The Total Environment 2023, 904, 166813. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. , Biochar stability in soil: meta-analysis of decomposition and priming effects. Gcb Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Afshar, M.; Mofatteh, S. , Biochar for a sustainable future: Environmentally friendly production and diverse applications. Results in Engineering 2024, 102433. [Google Scholar] [CrossRef]
- Mandal, S.; Ishak, S.; Ariffin, M. A. M.; Lee, D.-E.; Park, T. , Effect of pore structure on the thermal stability of shape-stabilized phase change materials. journal of materials research and technology 2023, 25, 465–479. [Google Scholar] [CrossRef]
- Mandal, S.; Ishak, S.; Lee, D.-E.; Park, T. , Shape-stabilized orange peel/myristic acid phase change materials for efficient thermal energy storage application. Energy Reports 2022, 8, 9618–9628. [Google Scholar] [CrossRef]
- Ishak, S.; Mandal, S.; Lgaz, H.; Atinafu, D. G.; Mohammad Harmay, N. S.; Lee, H.-S.; Abdul Shukor Lim, N.; Abdullah, M. M. A. B.; Yang, H.-M. , Microscopic molecular insights of different carbon chain fatty acids on shape-stabilized phase change composite. Journal of Thermal Analysis and Calorimetry 2024, 149, 9203–9221. [Google Scholar] [CrossRef]
- Qiao, Y.; Wu, C. , Nitrogen enriched biochar used as CO2 adsorbents: a brief review. Carbon Capture Science & Technology 2022, 2, 100018. [Google Scholar]
- Zhang, C.; Ji, Y.; Li, C.; Zhang, Y.; Sun, S.; Xu, Y.; Jiang, L.; Wu, C. , The application of biochar for CO2 capture: influence of biochar preparation and CO2 capture reactors. Industrial & Engineering Chemistry Research 2023, 62, 17168–17181. [Google Scholar]
- Dong, K.; Zhai, Z.; Guo, A. , Effects of pore parameters and functional groups in coal on CO2/CH4 adsorption. ACS omega 2021, 6, 32395–32407. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Jung, J.; Chen, D.; Leong, K.; Song, S.; Li, F.; Mohan, B. C.; Yao, Z.; Prabhakar, A. K.; Lin, X. H. , Biochar industry to circular economy. Science of the Total Environment 2021, 757, 143820. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hu, J.; Xiong, H.; Xiao, Y. , Application and properties of microporous carbons activated by ZnCl2: adsorption behavior and activation mechanism. ACS omega 2020, 5, 9398–9407. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Ross, A. B.; Mariner, T.; Hammerton, J.; Fitzsimmons, M. , Hydrochars produced by hydrothermal carbonisation of seaweed, coconut shell and oak: effect of processing temperature on physicochemical adsorbent characteristics. SN Applied Sciences 2022, 4, 203. [Google Scholar] [CrossRef]
- Liu, C.; Zhi, Y.; Yu, Q.; Tian, L.; Demir, M.; Colak, S. G.; Farghaly, A. A.; Wang, L.; Hu, X. , Sulfur-enriched nanoporous carbon: a novel approach to CO2 adsorption. ACS Applied Nano Materials 2024, 7, 5434–5441. [Google Scholar] [CrossRef]
- Majumdar, A.; Tran, K. D.; Malhotra, D.; Tran, D. T.; Kim, N. H.; Lee, J. H. , Heteroatom-Doped Carbon Allotropes in Water-Splitting Application. Heteroatom-Doped Carbon Allotropes: Progress in Synthesis, Characterization, and Applications 2024, 177–222. [Google Scholar]
- Kopitha, K.; Elakneswaran, Y.; Kitagaki, R.; Saito, R.; Tsujino, M.; Nishida, A.; Senboku, H.; Hiroyoshi, N. , N-methyldiethanolamine (MDEA) as an effective CO2 absorbent for direct air capture (DAC) in cement-based materials. Chemical Engineering Journal 2023, 475, 146067. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, J.; Zhang, X.; Feng, Y.; Zhang, H.; Zhang, S.; Chen, H. , Enhance SO2 adsorption performance of biochar modified by CO2 activation and amine impregnation. Fuel 2018, 224, 138–146. [Google Scholar] [CrossRef]
- Shi, J.; Xing, D.; Lia, J. , FTIR studies of the changes in wood chemistry from wood forming tissue under inclined treatment. Energy Procedia 2012, 16, 758–762. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, Y.; Tsang, S. C. E. , Nitrogen-enriched carbonaceous materials with hierarchical micro-mesopore structures for efficient CO2 capture. Chemical Engineering Journal 2012, 185, 374–379. [Google Scholar] [CrossRef]
- Mukome, F. N.; Zhang, X.; Silva, L. C.; Six, J.; Parikh, S. J. , Use of chemical and physical characteristics to investigate trends in biochar feedstocks. Journal of agricultural and food chemistry 2013, 61, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Dai, Q.; You, Z. , Chemo-physical analysis and molecular dynamics (MD) simulation of moisture susceptibility of nano hydrated lime modified asphalt mixtures. Construction and Building Materials 2015, 101, 536–547. [Google Scholar] [CrossRef]
- Thongsamer, T.; Vinitnantharat, S.; Pinisakul, A.; Werner, D. , Chitosan impregnation of coconut husk biochar pellets improves their nutrient removal from eutrophic surface water. Sustainable Environment Research 2022, 32, 39. [Google Scholar] [CrossRef]
- Hadjiivanov, K. I.; Panayotov, D. A.; Mihaylov, M. Y.; Ivanova, E. Z.; Chakarova, K. K.; Andonova, S. M.; Drenchev, N. L. , Power of infrared and Raman spectroscopies to characterize metal-organic frameworks and investigate their interaction with guest molecules. Chemical Reviews 2020, 121, 1286–1424. [Google Scholar] [CrossRef]
- He, F.; Zhou, L.; Fang, M.; Sui, C.; Li, W.; Yang, L.; Li, M.; He, X. , Fabrication and simulation analysis of flexible polymethylsilsesquioxane (PMSQ) aerogels by using dimethyl sulfoxide (DMSO) as solvent. Materials & Design 2019, 173, 107777. [Google Scholar]
- Siipola, V.; Tamminen, T.; Källi, A.; Lahti, R.; Romar, H.; Rasa, K.; Keskinen, R.; Hyväluoma, J.; Hannula, M.; Wikberg, H. , Effects of biomass type, carbonization process, and activation method on the properties of bio-based activated carbons. 2018.
- Grube, M.; Shvirksts, K.; Denina, I.; Ruklisa, M.; Semjonovs, P. , Fourier-transform infrared spectroscopic analyses of cellulose from different bacterial cultivations using microspectroscopy and a high-throughput screening device. Vibrational Spectroscopy 2016, 84, 53–57. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Srinivasan, R.; Kan, E. , Facile and economical functionalized hay biochar with dairy effluent for adsorption of tetracycline. ACS omega 2020, 5, 16521–16529. [Google Scholar] [CrossRef]
- Volkov, D. S.; Rogova, O. B.; Proskurnin, M. A. , Temperature dependences of IR spectra of humic substances of brown coal. Agronomy 2021, 11, 1822. [Google Scholar] [CrossRef]
- Slavov, D.; Tomaszewska, E.; Grobelny, J.; Drenchev, N.; Karashanova, D.; Peshev, Z.; Bliznakova, I. , FTIR spectroscopy revealed nonplanar conformers, chain order, and packaging density in diOctadecylamine-and Octadecylamine-passivated gold nanoparticles. Journal of Molecular Structure 2024, 1314, 138827. [Google Scholar] [CrossRef]
- Sethupathi, S.; Zhang, M.; Rajapaksha, A. U.; Lee, S. R.; Mohamad Nor, N.; Mohamed, A. R.; Al-Wabel, M.; Lee, S. S.; Ok, Y. S. , Biochars as potential adsorbers of CH4, CO2 and H2S. Sustainability 2017, 9, 121. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, H.; Shao, J.; Chen, Y.; Feng, Y.; Wang, X.; Chen, H. , Effects of hydrofluoric acid pre-deashing of rice husk on physicochemical properties and CO2 adsorption performance of nitrogen-enriched biochar. Energy 2015, 91, 903–910. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, H.; Feng, Y.; Chen, Y.; Wang, X.; Chen, H. , Nitrogen enriched biochar modified by high temperature CO2–ammonia treatment: characterization and adsorption of CO2. Chemical Engineering Journal 2014, 257, 20–27. [Google Scholar] [CrossRef]
- Singh, G.; Kim, I. Y.; Lakhi, K. S.; Joseph, S.; Srivastava, P.; Naidu, R.; Vinu, A. , Heteroatom functionalized activated porous biocarbons and their excellent performance for CO 2 capture at high pressure. Journal of Materials Chemistry A 2017, 5, 21196–21204. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Mohamed, A. R. , Metal incorporated biochar as a potential adsorbent for high capacity CO2 capture at ambient condition. Journal of CO2 Utilization 2018, 26, 281–293. [Google Scholar] [CrossRef]
- Shahkarami, S.; Dalai, A. K.; Soltan, J. , Enhanced CO2 adsorption using MgO-impregnated activated carbon: impact of preparation techniques. Industrial & Engineering Chemistry Research 2016, 55, 5955–5964. [Google Scholar]
- Ello, A. S.; de Souza, L. K.; Trokourey, A.; Jaroniec, M. , Development of microporous carbons for CO2 capture by KOH activation of African palm shells. Journal of CO2 Utilization 2013, 2, 35–38. [Google Scholar] [CrossRef]
- Manyà, J. J.; González, B.; Azuara, M.; Arner, G. , Ultra-microporous adsorbents prepared from vine shoots-derived biochar with high CO2 uptake and CO2/N2 selectivity. Chemical Engineering Journal 2018, 345, 631–639. [Google Scholar] [CrossRef]
- Shahkarami, S.; Azargohar, R.; Dalai, A. K.; Soltan, J. , Breakthrough CO2 adsorption in bio-based activated carbons. Journal of environmental sciences 2015, 34, 68–76. [Google Scholar] [CrossRef]
- Thote, J. A.; Iyer, K. S.; Chatti, R.; Labhsetwar, N. K.; Biniwale, R. B.; Rayalu, S. S. , In situ nitrogen enriched carbon for carbon dioxide capture. Carbon 2010, 48, 396–402. [Google Scholar] [CrossRef]
- Boonpoke, A.; Chiarakorn, S.; Laosiripojana, N.; Towprayoon, S.; Chidthaisong, A. , Synthesis of activated carbon and MCM-41 from bagasse and rice husk and their carbon dioxide adsorption capacity. J. Sustain. Energy Environ 2011, 2, 77–81. [Google Scholar]
| Parameter | S300NZC | S300ZC | S500NZC | S500ZC | |
|---|---|---|---|---|---|
| Transmittance (Red) (%) | 88.4 | 81.1 | 60.8 | 58.6 | |
| Transmittance (Blue) (%) | 88.7 | 82.1 | 59.5 | 57.8 | |
| Median size (μm) | 11.489 | 8.444 | 5.704 | 5.749 | |
| Mean size (μm) | 17.334 | 9.461 | 11.494 | 8.052 | |
| Diameter on cumulative(μm) | 10 (%) | 4.387 | 4.393 | 1.616 | 2.208 |
| 50 (%) | 11.489 | 8.444 | 5.704 | 5.749 | |
| 90 (%) | 33.330 | 15.299 | 25.913 | 14.589 | |
| Biochar ID | Element | C | N | O | P | S |
|---|---|---|---|---|---|---|
| S300NZC | Mass (%) | 60.1 | 12.7 | 23.8 | 3.2 | 0.2 |
| S300ZC | Mass (%) | 53.9 | 10.7 | 30.6 | 4.4 | 0.4 |
| S500NZC | Mass (%) | 65.7 | 15.8 | 15.6 | 2.8 | 0.1 |
| S500ZC | Mass (%) | 66.7 | 15.8 | 12.8 | 4.3 | 0.4 |
| Biochar ID | BET Surface Area(m²/g) | Micropore Area(m²/g) | BJH Pore diameter(Å) |
|---|---|---|---|
| S300NZC | 4.9574 | 0.2089 | 90.172 |
| S300ZC | 4.1229 | 1.3304 | 86.059 |
| S500NZC | 458.1404 | 365.0903 | 15.578 |
| S500ZC | 717.5997 | 616.6000 | 14.134 |
| Biochar ID | CO2 adsorbed volume |
|---|---|
| S300NZC | 8.691 |
| S300ZC | 15.5306 |
| S500NZC | 33.696 |
| S500ZC | 35.3396 |
| No | Feedstock | Activation | Pyrolysis at (℃) | Post surface treatment | Surface area (m2/g) | CO2 intake at 25 ℃ (mmol/g) | Surface features | Ref. |
|---|---|---|---|---|---|---|---|---|
| Korean oak | - | 400 | - | 0.597 | - | [44] | ||
| Soybean stover | - | 700 | - | - | 0.707 | - | [44] | |
| Japanese oak | - | 500 | - | - | 0.379 | - | [44] | |
| Rice husk | HF | 830 | N2 & ammonia at 600 ℃ | 451.02 | 1.8 | 5.03 wt.% N | [45] | |
| Cotton stalk | KOH | 600 | N2 & ammonia at 700 ℃ | 297 | 1.1 | Amine groups | [46] | |
| Arundo donax | Chitosan/ZnCl2 | 500 | - | 1863 | 2.1 | 3.91 wt.% N | [47] | |
| Walnut shell | Mg(NO3)2 · 6H2O | 900 | Heating in N2 at 500 ˚C | 292 | 1.9 | Amine groups | [48] | |
| Whitewood | Mg(NO3)2 · 6H2O | 500 | Steam activation | 615 | 1.1 | 5.41 wt.% N | [49] | |
| Africa palm shells | KOH | 600 | - | 365 | 1.9 | Ultra micropores | [50] | |
| Vine shoots | None | 600 | In CO2 for 3h at 800 ˚C | 767 | 1.58 | - | [51] | |
| Vine shoots | KOH: H2O (5:1) | 600 | Heating at 700 °C for 1h | 1439 | 1.98 | Presence of N | [51] | |
| Whitewood | Steam | 500 | - | 840 | 1.34 | Presence of N | [52] | |
| Whitewood | CO2 | 500 | - | 820 | 1.43 | Presence of N | [52] | |
| Whitewood | KOH | 500 | - | 1400 | 1.77 | Micro porosity | [52] | |
| Bamboo stem | None | 500 | - | 9.72 | 1.01 | Micro porosity | [13] | |
| Orange peel | None | 500 | - | 51.63 | 0.63 | Presence of amine functional groups | [13] | |
| Soybean | ZnCl2 | 600 | CO2 Physical activation | 811 | 0.93 | Presence of N | [53] | |
| Bagasse | ZnCl2 | 500 | - | 923 | 1.74 | - | [54] | |
| Rice husk | ZnCl2 | 500 | - | 927 | 1.29 | - | [54] | |
| Sawdust | None | 300 | - | 4.95 | 0.39 | Presence of N | Present study | |
| Sawdust | None | 500 | - | 4.12 | 0.69 | Presence of N | Present study | |
| Sawdust | ZnCl2 | 300 | - | 458.14 | 1.50 | Presence of O-functional groups | Present study | |
| Sawdust | ZnCl2 | 500 | - | 717.60 | 1.58 | Presence of O-functional groups | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





