Submitted:
04 June 2025
Posted:
05 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
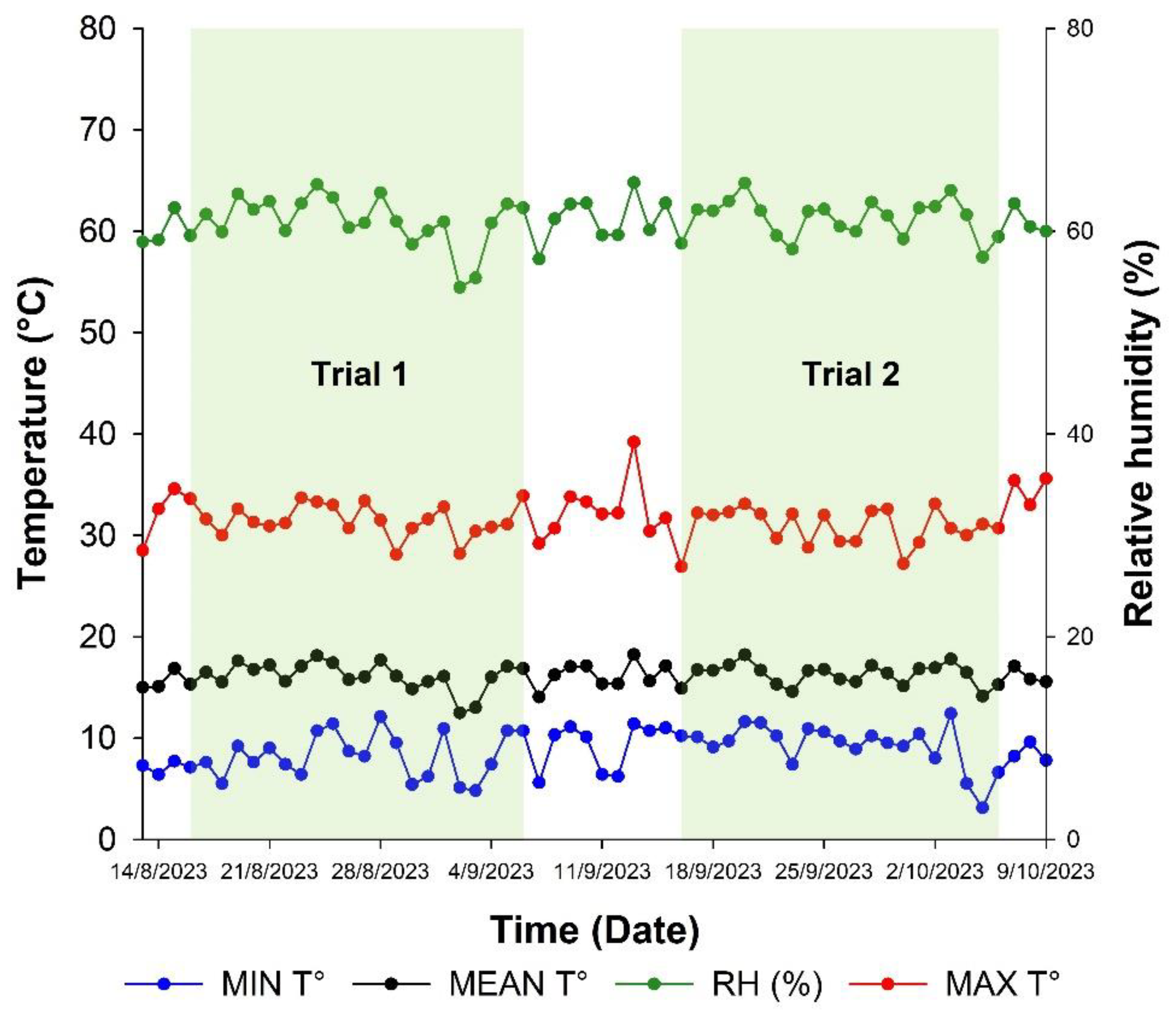
2.1. Location
2.1. Plant Material
2.3. Experimental Design and Field Establishment
2.4. Response Variables
2.5. Statistical Analysis
3. Results and Discussion
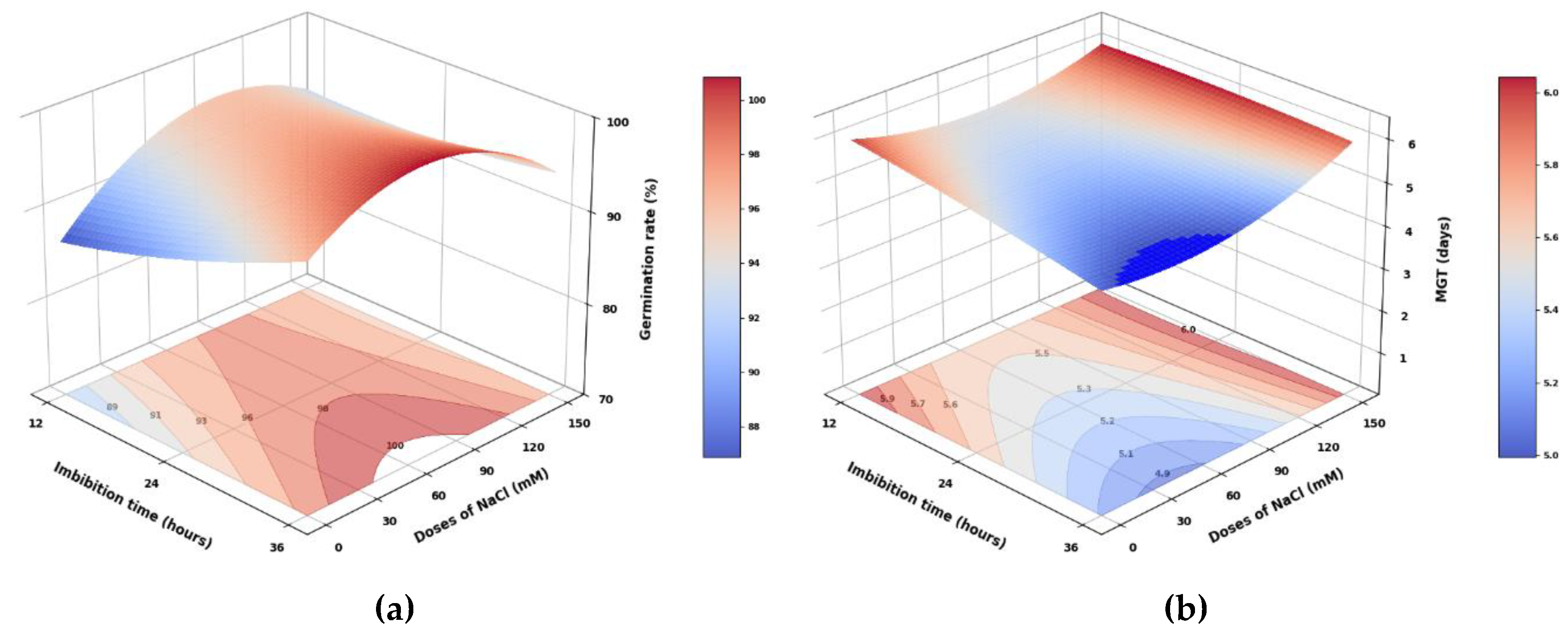
3.1. Effect of Imbibition Time and NaCl Doses on Germination Parameters in Pea Seeds
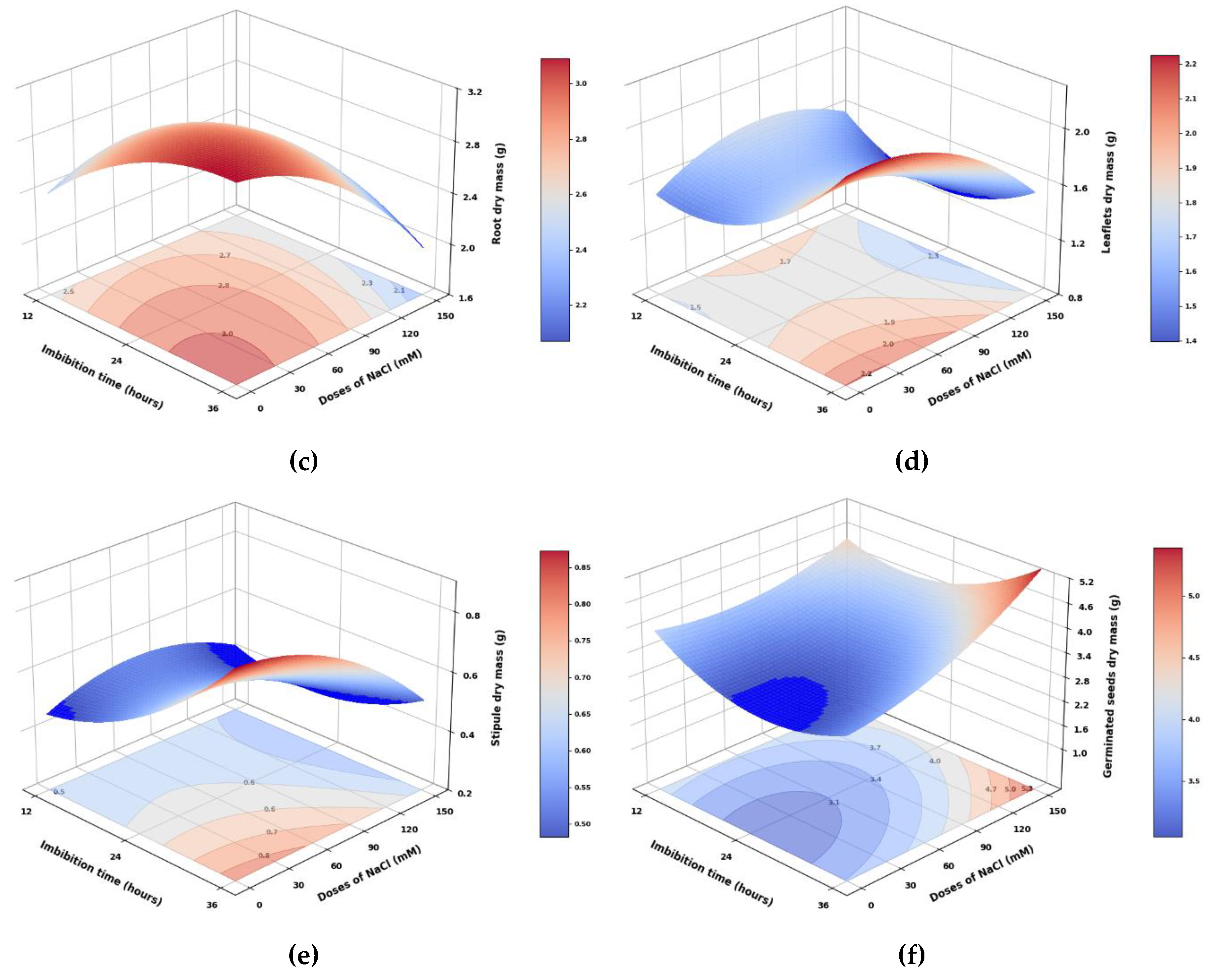
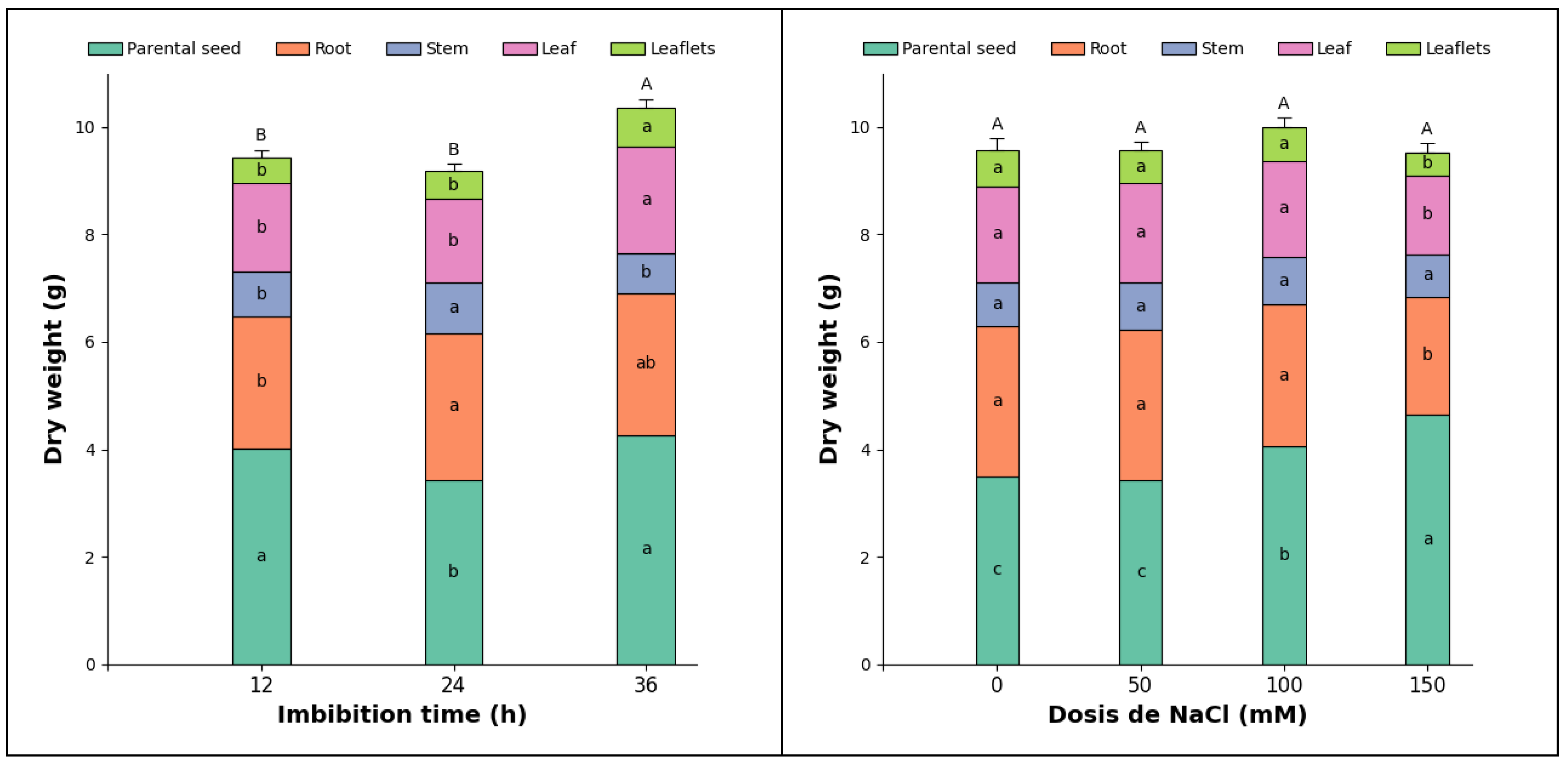
3.2. Effect of Imbibition Time and NaCl Doses on the Dry Mass Distribution of Different Organs in Pea Seedlings
3.3. Effect of Imbibition Time and NaCl Doses on the Leaf Area of Pea Seedlings
3.4. Effect of Imbibition Time and H2O2 Doses on Germination Parameters in Pea Seeds
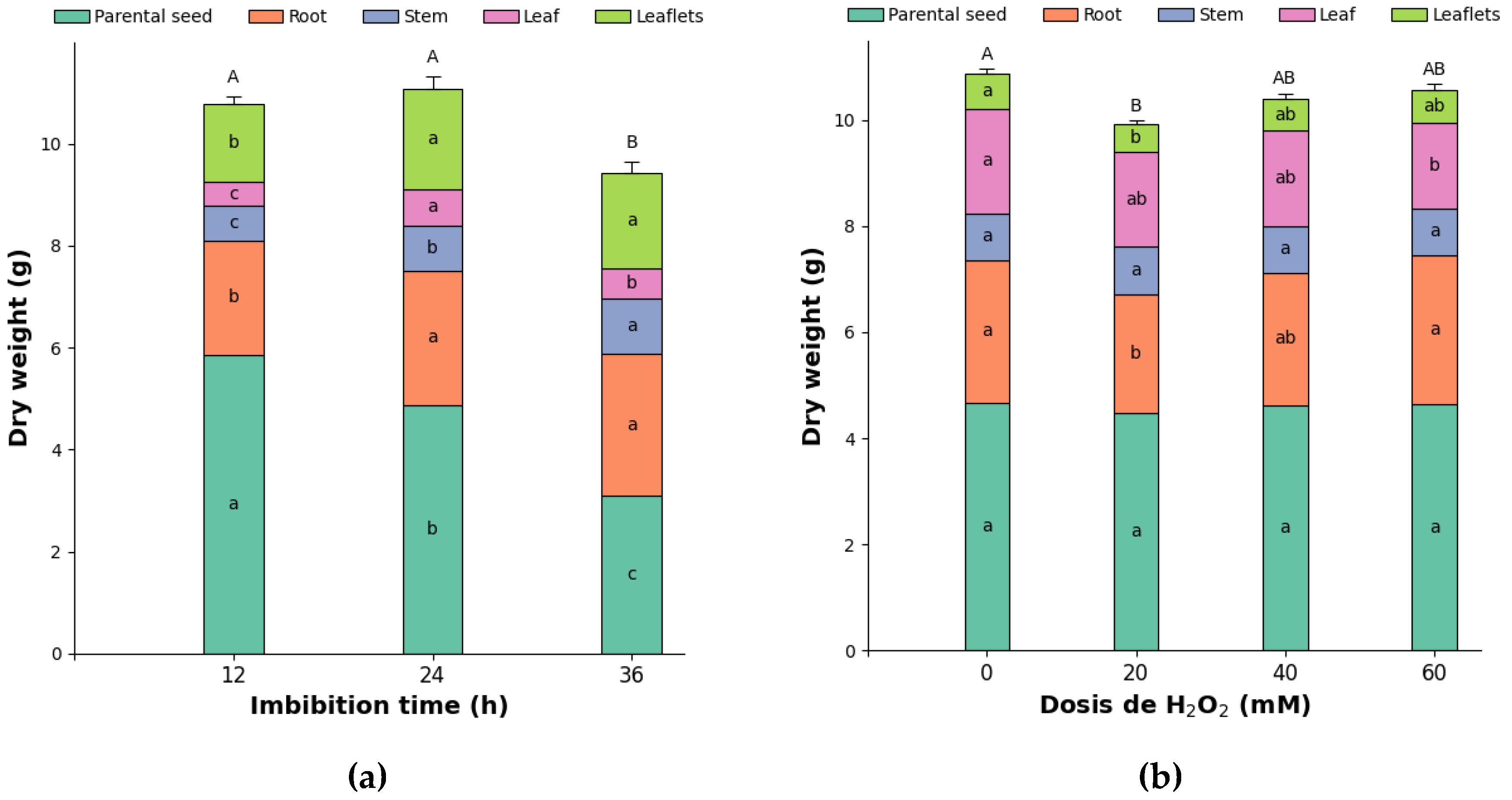
3.5. Effect of Imbibition Time and H2O2 Doses on the Dry Mass Distribution of Different Organs in Pea Seedlings
3.6. Effect of Imbibition Time and H2O2 Doses on the Leaf Area of Pea Seedlings
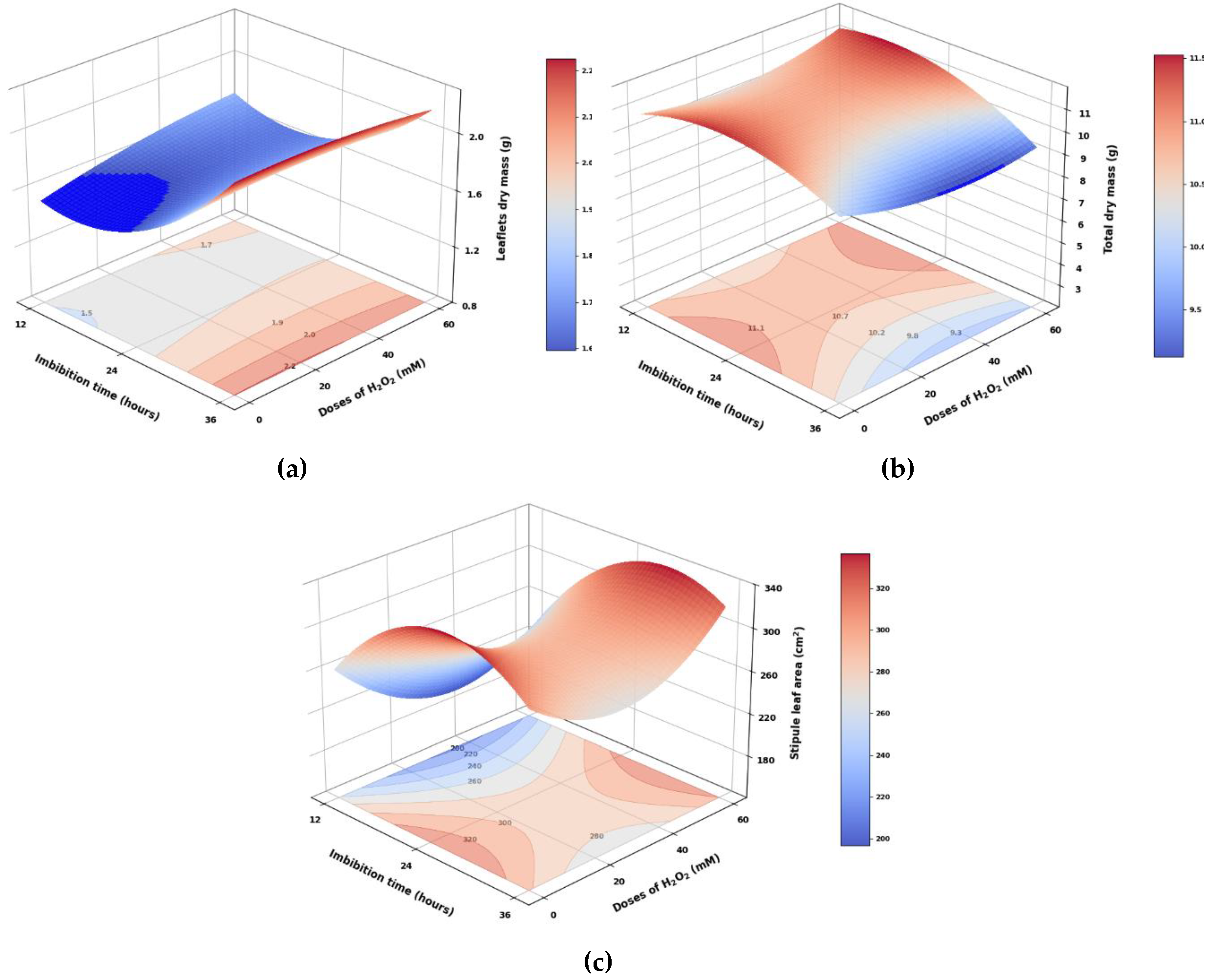
3.6. Analysis of the Response Surface Models Obtained for Pea Seed Germination Parameters and Seedling Biomass
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parihar, A.K.; Dixit, G.P.; Singh, U.; Singh, A.K.; Kumar, N.; Gupta, S. Potential of field pea as a nutritionally rich food legume crop. In Breeding for Enhanced Nutrition and Bio-Active Compounds in Food Legumes, 1st ed.; Gupta, D.S., Gupta, S., Kumar, J., Eds.; Springer Cham, Switzerland, 2021; pp. 47-82. [CrossRef]
- Checa, O.; Rodríguez, D.; Ruiz, M.; Muriel, J. La Arveja-Investigación y tecnología en el sur de Colombia, Editorial Universidad de Nariño, San Juan de Pasto, Colombia, 2022. http://sired.udenar.edu.co/id/eprint/7303.
- Agronet. Agricultural statistics: Area, production, yield and participation in pea cultivation. Available online: https://www.agronet.gov.co/Docu-ments/8-ARVEJA_2017.pdf (accessed on 11 02 2024) (In Spanish).
- Jaime-Guerrero, M.; Álvarez-Herrera, J. G.; Torres-Piña, D. S. Obtención de plántulas a partir de diferentes tratamientos pregerminativos en semillas de arveja. Entramado 2025, 21(41), 1-12. [CrossRef]
- Wagan, M.A.; Shar, H.A.; Miano, T.F.; Chandio, S.R.; Suthar, M.; Wagan, M.K.; Kumar, A.; Wagan, F.A.; Magsi, H.A. Influence of seed priming on germination of pea (Pisum sativum). Int. J. Agric. Stud. 2022, 1(1), 31-37. [CrossRef]
- Mahawar, M.K.; Samuel, D.V.K.; Sinha, J. P.; Jalgaonkar, K. Optimization of pea (Pisum sativum) seeds hydropriming by application of response surface methodology. Acta Physiol. Plant. 2016, 38, 1-13. [CrossRef]
- Khan, M.O.; Irfan, M.; Muhammad, A.; Ullah, I.; Nawaz, S.; Khalil, M.K.; Ahmad, M. A practical and economical strategy to mitigate salinity stress through seed priming. Front. Environ. Sci. 2022, 10, 991977. [CrossRef]
- Lal, S.K.; Kumar, S.; Sheri, V.; Mehta, S.; Varakumar, P.; Ram, B.; Borphukan, B.; James, D.; Fartyal, D.; Reddy, M.K. Seed priming: an emerging technology to impart abiotic stress tolerance in crop plants. In Advances in seed priming, 1st ed.; Rakshit, A., Singh, H.B., Eds.; Springer Singapore, Singapore, 2018; pp. 41-50. [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: mission possible?. Trends Plant Sci. 2016, 21(4), 329-340. [CrossRef]
- Kiran, K.R.; Deepika, V.B.; Swathy, P.S.; Prasad, K.; Kabekkodu, S.P.; Murali, T. S.; Satyamoorthy, K.; Muthusamy, A. ROS-dependent DNA damage and repair during germination of NaCl primed seeds. J. Photochem. Photobiol. B 2020, 213, 112050. [CrossRef]
- Pandolfi, C.; Mancuso, S.; Shabala, S. Physiology of acclimation to salinity stress in pea (Pisum sativum). Environ. Exp. Bot. 2012, 84, 44-51. [CrossRef]
- Senturk, B.; Sivritepe, O.H. NaCl priming alleviates the inhibiting effect of salinity during seeding growth of peas (Pisum sativum L.). Fresen. Environ. Bull. 2016, 25(10), 4202-4208. https://www.prt-parlar.de/download_list/?c=FEB_2016#.
- Ellouzi, H.; Oueslati, S.; Hessini, K.; Rabhi, M.; Abdelly, C. Seed-priming with H2O2 alleviates subsequent salt stress by preventing ROS production and amplifying antioxidant defense in cauliflower seeds and seedlings. Sci. Hortic. 2021, 288, 110360. [CrossRef]
- Moussa, H.R.; Mohamed, M.A.E.H. Role of nitric acid or H2O2 in antioxidant defense system of Pisum sativum L. under drought stress. Nat. Sci. 2011, 9, 211-216. https://www.sciencepub.net/nature/ns0905/30_5479ns0905_211_216.pdf.
- Cadena-Guerrero, M.M.; Yepes-Chamorro, D.B.; Romero, J.V. Estabilidad fenotípica de arveja (Pisum sativum L.) en la zona productora de Nariño, Colombia. Agron. Mesoam. 2021, 32, 841-853. [CrossRef]
- Checa, O.; Rodríguez, M.; Wu, X.; Blair, M. Introgression of the Afila gene into climbing garden pea (Pisum sativum L.). Agronomy 2020, 10(10), 1537. [CrossRef]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2(7), 1400033. [CrossRef]
- Pompelli, M.F.; Jarma-Orozco, A.; Rodríguez-Páez, L.A. Imbibition and germination of seeds with economic and ecological interest: Physical and biochemical factors involved. Sustainability 2023, 15(6), 5394. [CrossRef]
- Cao, Y.; Liang, L.; Cheng, B.; Dong, Y.; Wei, J.; Tian, X.; Peng, Y.; Li, Z. Pretreatment with NaCl promotes the seed germination of white clover by affecting endogenous phytohormones, metabolic regulation, and dehydrin-encoded genes expression under water stress. Int. J. Mol. Sci. 2018, 19(11), 3570. [CrossRef]
- Kyari, B.A.; Lawan, Z.A.; Waziri, M.S.; Ajiri, H.M.; Apagu, B.; Mari, H.; Ibrahim, M.A. Effect of imbibition time on hormonal changes of germinating Tamarindus indica and Prosopis juliflora. Indones. J. Agric. Res. 2022, 5(3), 219-230. [CrossRef]
- Saha, D.; Choyal, P.; Mishra, U.N.; Dey, P.; Bose, B.; Gupta, N.K.; Prathibha, M.D.; Mehta, B.K.; Kumar, P.; Pandey, S.; Chauhan, J.; Singhal, R.K. Drought stress responses and inducing tolerance by seed priming approach in plants. Plant Stress 2022, 4, 100066. [CrossRef]
- Naz, F.; Gul, H.; Hamayun, M.; Sayyed, A.; Khan, H.; Sherwani, S. Effect of NaCl stress on Pisum sativum germination and seedling growth with the influence of seed priming with potassium (KCL and KOH). Am.-Euras. J. Agric. Environ. Sci. 2014, 14(11), 1304-1311. [CrossRef]
- Souza, L.M.D.; Conceição, E.M.D.; Barbosa, M.R.; Palhares, L.; Santos, A.M.M.D.; Souza, R.A.D.; Houllou, L.M. Effect of seed priming with NaCl on the induction of salinity tolerance in Myracrodruon urundeuva Allemão in vitro. Ciênc. Florest. 2022, 32(4), 2199-2218. [CrossRef]
- Ehtaiwwesh, A.F.; Emsahel, M.J. Impact of salinity stress on germination and growth of pea (Pisum sativum L.) plants. Al-Mukhtar J. Sci. 2020, 35, 146-159. https://search.emarefa.net/detail/BIM-1324653.
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; Bhattacharya, S.; Jha, U.C.; Jespersen, D. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [CrossRef]
- Smolko, A.; Bauer, N.; Pavlović, I.; Pěnčík, A.; Novák, O.; Salopek-Sondi, B. Altered root growth, auxin metabolism and distribution in Arabidopsis thaliana exposed to salt and osmotic stress. Int. J. Mol. Sci. 2021, 22(15), 7993. [CrossRef]
- Colin, L.; Ruhnow, F.; Zhu, J.K.; Zhao, C.; Zhao, Y.; Persson, S. The cell biology of primary cell walls during salt stress. Plant cell 2023, 35(1), 201-217. [CrossRef]
- Jaime-Guerrero, M.; Álvarez-Herrera, J. G.; Camacho-Tamayo, J. Germinación y crecimiento de semillas de arveja var. ‘Santa Isabel’ sometidas a diferentes dosis de giberelinas. Rev. Investig. Agrar. Ambient. 2023, 14(2), 91-112. [CrossRef]
- Hernández, J. A. Seed science research: Global trends in seed biology and technology. Seeds 2022, 1(1), 1-4. [CrossRef]
- Hemalatha, G.; Renugadevi, J.; Eevera, T. Studies on seed priming with hydrogen peroxide for mitigating salt stress in rice. Int. J. Curr. Microbiol. App. Sci. 2017, 6(6), 691-695. [CrossRef]
- Hameed, A.; Hussain, S.; Nisar, F.; Rasheed, A.; Shah, S. Z. Seed priming as an effective technique for enhancing salinity tolerance in plants: mechanistic insights and prospects for saline agriculture with a special emphasis on halophytes. Seeds 2025, 4(1), 14. [CrossRef]
- Barba-Espín, G.; Hernández, J.A.; Diaz-Vivancos, P. Role of H2O2 in pea seed germination. Plant Signal. Behav. 2012, 7(2), 193-195. [CrossRef]
- Rodrigues, L.; Nogales, A.; Nunes, J.; Rodrigues, L.; Hansen, L.D.; Cardoso, H. Germination of Pisum sativum L. seeds is associated with the alternative respiratory pathway. Biology 2023, 12(10), 1318. [CrossRef]
- Liu, H.; Mu, Y.; Xuan, Y.; Wu, X.; Wang, W.; Zhang, H. Hydrogen peroxide signaling in the maintenance of plant root apical meristem activity. Antioxidants 2024, 13(5), 554. [CrossRef]
- Makhaye, G.; Mofokeng, M.M.; Tesfay, S.; Aremu, A.O.; Van Staden, J.; Amoo, S.O. Influence of plant biostimulant application on seed germination. In Biostimulants for crops from seed germination to plant development, 1st ed.; Gupta, S., Staden, J., Eds.; Academic Press, Cambridge, 2021; pp. 109-135. [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [CrossRef]
| Variable | Equation | Units |
| Germination rate | % | |
| Germination potential | % | |
| Mean germination time | Days to germination | |
| Germination rate index | Seeds germinated by day |
| Imbibition time (hours) | Doses of NaCl (mM) | GR (%) | GP (%) | MGT (days) | GRI (Number of seeds per day) |
| 12 | 0 | 82.92b | 34.17ab | 6.21a | 5.19d |
| 50 | 98.33a | 34.58ab | 5.30cd | 10.12ab | |
| 100 | 94.17ab | 35.00ab | 5.62abcd | 8.16abcd | |
| 150 | 93.75ab | 33.75ab | 6.04ab | 6.56cd | |
| 24 | 0 | 92.08ab | 37.92ab | 5.44bcd | 7.85abcd |
| 50 | 98.75a | 42.92a | 5.17d | 10.61a | |
| 100 | 96.25a | 32.50ab | 5.73abcd | 8.80abc | |
| 150 | 89.58ab | 24.58b | 6.01abc | 7.27bcd | |
| 36 | 0 | 97.50a | 41.67a | 5.04d | 9.73abc |
| 50 | 97.08a | 42.92a | 5.07d | 9.72abc | |
| 100 | 98.75a | 44.58a | 5.07d | 9.15abc | |
| 150 | 97.50a | 37.50ab | 5.91abc | 7.57abcd | |
| Factor | Level | ||||
| Imbibición time (hours) | 12 | 92.29b | 34.38b | 5.79a | 7.51b |
| 24 | 94.17ab | 34.48b | 5.59a | 8.63ab | |
| 36 | 97.71a | 41.67a | 5.27b | 9.04a | |
| Doses of NaCl (mM) | 0 | 90.83b | 37.92ab | 5.56b | 7.59bc |
| 50 | 98.06a | 40.14a | 5.18c | 10.15a | |
| 100 | 96.39a | 37.36ab | 5.47bc | 8.70ab | |
| 150 | 93.61ab | 31.94b | 5.98a | 7.13c | |
| Anova | Significance | ||||
| I | ** | ** | ** | ** | |
| D | ** | * | ** | ** | |
| I × D | * | ns | ** | * | |
| Imbibition time (hours) | Doses of NaCl (mM) |
SLA (cm2) |
LA (cm2) |
TLA (cm2) |
| 12 |
0 | 114.11cd | 257.77cd | 371.88de |
| 50 | 83.15d | 233.97d | 317.12de | |
| 100 | 123.12cd | 263.42cd | 386.54de | |
| 150 | 90.91d | 221.85d | 312.75de | |
| 24 |
0 | 183.19abc | 288.23cd | 471.41bcde |
| 50 | 146.80bcd | 290.03cd | 436.84cde | |
| 100 | 184.95abc | 310.33cd | 495.28bcd | |
| 150 | 87.08d | 199.38d | 286.47e | |
| 36 |
0 | 246.08a | 470.16a | 716.24a |
| 50 | 214.57ab | 447.60ab | 662.16ab | |
| 100 | 208.26ab | 392.93abc | 601.18abc | |
| 150 | 154.95bcd | 315.27bcd | 470.22bcde | |
| Factor | Level | |||
| Imbibición time (hours) | 12 | 102.82c | 244.25b | 347.07c |
| 24 | 150.51b | 271.99b | 422.50b | |
| 36 | 205.97a | 406.49a | 612.45a | |
| Doses of NaCl (mM) | 0 | 181.13a | 338.72a | 519.85a |
| 50 | 148.17a | 323.87a | 472.04a | |
| 100 | 172.11a | 322.22a | 494.34a | |
| 150 | 110.98b | 245.50b | 356.48b | |
| Anova | Significance | |||
| I | ** | ** | ** | |
| D | ** | ** | ** | |
| I × D | ns | ns | ns | |
| Imbibition time (hours) | Doses of H2O2 (mM) | GR (%) | GP (%) | MGT (days) | GRI (Number of seeds per day) |
| 12 | 0 | 95.42ab | 37.92a | 5.68ab | 8.31cd |
| 20 | 97.50ab | 37.08a | 5.81a | 8.30cd | |
| 40 | 92.92b | 41.25a | 5.70a | 8.06d | |
| 60 | 95.00ab | 40.42a | 5.75a | 8.14d | |
| 24 | 0 | 99.17a | 51.67a | 5.06c | 9.64ab |
| 20 | 96.25ab | 51.25a | 5.05c | 9.42ab | |
| 40 | 99.58a | 47.08a | 5.02c | 9.80a | |
| 60 | 97.92ab | 48.75a | 5.18bc | 9.30abc | |
| 36 | 0 | 98.75a | 42.08a | 5.66ab | 8.58bcd |
| 20 | 98.33ab | 47.08a | 5.57ab | 8.68bcd | |
| 40 | 97.08ab | 41.25a | 5.45abc | 8.84abcd | |
| 60 | 97.08ab | 40.00a | 5.44abc | 8.84abcd | |
| Factor | Level | ||||
| Imbibición time (hours) | 12 | 95.21b | 39.17b | 5.74a | 8.20c |
| 24 | 98.23a | 49.69a | 5.08c | 9.54a | |
| 36 | 97.81a | 42.60b | 5.53b | 8.74b | |
| Doses of H2O2 (mM) | 0 | 97.78a | 43.89a | 5.47a | 8.84a |
| 20 | 97.36a | 45.14a | 5.48a | 8.80a | |
| 40 | 96.53a | 43.19a | 5.39a | 8.90a | |
| 60 | 96.67a | 43.06a | 5.46a | 8.76a | |
| Anova | Significance | ||||
| I | ** | ** | ** | ** | |
| D | ns | ns | ns | ns | |
| I × D | ns | ns | ns | ns | |
| Imbibition time (hours) | Doses of H2O2 (mM) |
SLA (cm2) |
LA (cm2) |
TLA (cm2) |
| 12 | 0 | 247.15bce | 542.38ab | 789.53abcd |
| 50 | 215.29cde | 383.00b | 598.29cd | |
| 100 | 230.63bcde | 433.59b | 664.22bcd | |
| 150 | 200.01e | 329.52b | 529.53e | |
| 24 | 0 | 336.69ab | 545.54ab | 882.23abc |
| 50 | 283.85abcde | 432.84b | 716.69bcd | |
| 100 | 306.01abcde | 528.34ab | 834.35abc | |
| 150 | 319.75abc | 552.30ab | 872.05abc | |
| 36 | 0 | 310.93abcd | 510.02ab | 820.94abcd |
| 50 | 291.74abcde | 724.12a | 1015.86a | |
| 100 | 205.40de | 544.28ab | 749.68abcd | |
| 150 | 357.51a | 551.22ab | 908.73ab | |
| Factor | Level | |||
| Imbibición time (hours) | 12 | 223.27b | 422.12b | 645.39b |
| 24 | 311.57a | 514.75a | 826.33a | |
| 36 | 291.39a | 582.41a | 873.80a | |
| Doses of H2O2 (mM) | 0 | 298.25a | 532.65a | 830.90a |
| 20 | 263.63ab | 513.32a | 776.95a | |
| 40 | 247.35b | 502.07a | 749.42a | |
| 60 | 292.42ab | 477.68a | 770.10a | |
| Anova | Significance | |||
| I | ** | ** | ** | |
| D | * | ns | ns | |
| I × D | ** | ** | ** | |
| Estimated | GR | MGT | RDM | LDM | SDM | DMGS |
| a | 84.583333 | 6.292854 | 1.474960 | 1.982127 | 0.497687 | 6.332820 |
| b | 0.114583 | -0.020423 | 0.092714 | -0.060040 | -0.010087 | -0.259080 |
| c | 0.216667 | -0.015844 | 0.009269 | 0.008608 | 0.003287 | -0.011612 |
| d | 0.005787 | -0.000392 | -0.001329 | 0.001854 | 0.000573 | 0.004968 |
| e | -0.002222 | 0.000234 | -0.000288 | -0.000204 | -0.000095650 | 0.000415 |
| f | -0.001000 | 0.000088895 | -0.000042440 | -0.000038553 | -0.000015473 | 0.000065800 |
| r2 | 0.3036 | 0.5580 | 0.4111 | 0.5164 | 0.6027 | 0.5706 |
| Estimated | LDM | TDM | SLA |
| a | 0.445370 | 8.197823 | 78.391033 |
| b | 0.115916 | 0.298814 | 19.797850 |
| c | 0.001729 | -0.023079 | -4.055098 |
| d | -0.001893 | -0.006829 | -0.376688 |
| e | -0.000316 | -0.000886 | 0.037388 |
| f | 0.000006875 | 0.000700 | 0.049815 |
| r2 | 0.3469 | 0.4763 | 0.4126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





