1. Introduction
Since the NSABP B-06 study showed an acceptable recurrence rate for breast conservation therapy compared to mastectomy [1], surgeons have been striving to improve the process to provide the best possible outcomes and improve the patient experience. Improvements in screening mammograms since that time have allowed us to detect malignancy at smaller sizes and earlier stages often when these lesions are non-palpable. This has led to the development of multiple techniques to localize non-palpable lesions intraoperatively, hoping to decrease the rates of positive margins and the need for re-excision and improve the overall patient experience. Several methods have been employed in the past. Historically, this was done with wire-guided localization (WGL), which required placement on the day of the procedure by radiology and had the potential for dislodgement [2] and the uncomfortable effect of requiring patients to have a wire protruding through their skin until surgery. Wireless localization methods have recently been popularized, including initially with radioactive seeds. However, this required the introduction of numerous policies and resources in systems that chose to adopt this method given the radioactive nature of the device placed [3,4]. More recently, non-radioactive localization devices have been popularized, and they use various technologies to help with intraoperative localization, including radar, radiofrequency (R), and magnetic technologies [5,6]. The benefit of these includes the ability to be placed days to months pre-operatively, which helps improve the operating room (OR) day's efficiency and removes the externally visible component of the wire to improve the patient experience. Previous studies have shown that positive margin rates for these devices are comparable to wire localization [4]. Several studies have also demonstrated an improvement in the cost-effectiveness of these methods, given the reduction in OR delays and improvements in re-excision rates [7,8].
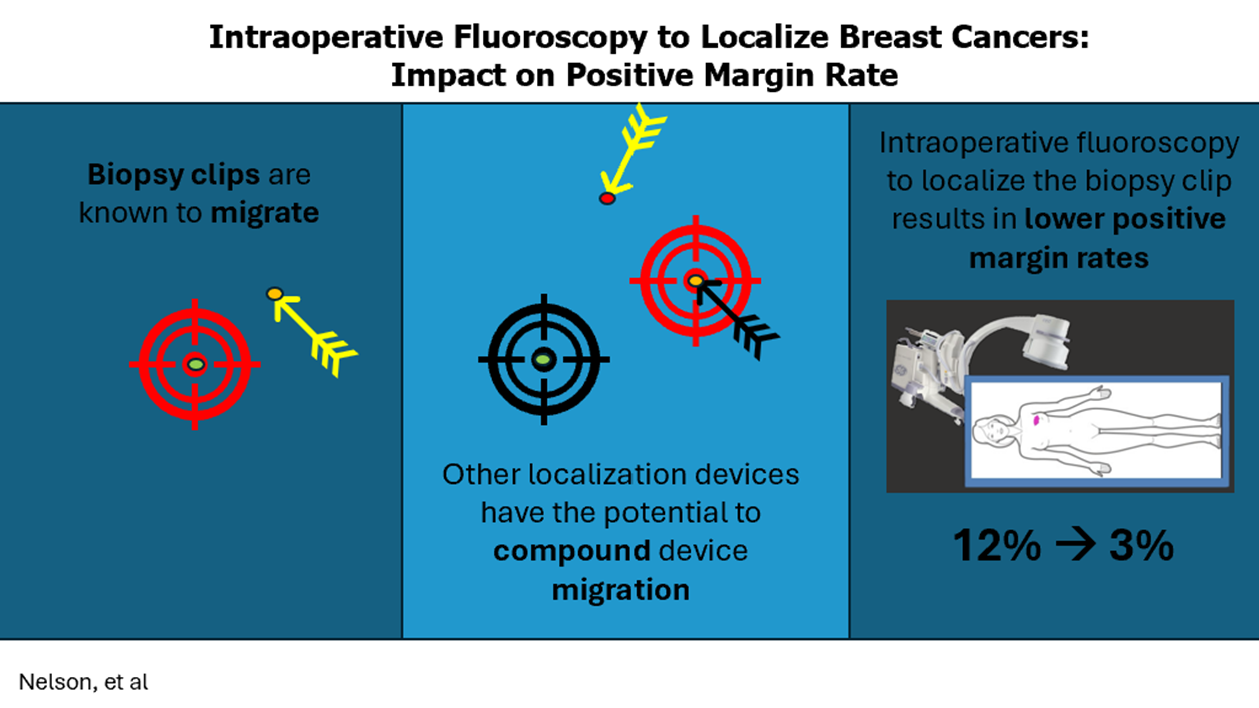
We questioned whether a technology widely available, inexpensive, and used by virtually every general surgeon during their training/practice, such as fluoroscopy, could be used for the same aim. Previously, we found that Fluoroscopic Intraoperative Neoplasia Detection (FIND) was a feasible method of intraoperative localization and had improved margin rates compared to traditional wire localization [9]. However, up to this point no data had compared this method to non-wire-based localization technologies.
One downfall of all devices-based localization methods is the potential for device migration. It is well known that clips placed following core needle biopsy (CNB) have the potential for migration. Kass et al. showed that the clip placement following CNB migrates 13.5 mm +/- 1.6 mm, SEM (95% CI = 10.3 mm to 16.7 mm).10 Often, after a biopsy of small non-palpable lesions, there is no residual imaging evidence of the lesion, and localization devices are placed based on the location of the original biopsy clip. If the lesions are then localized with another device that also has the potential for migration this could effectively compound the migrated distance from the area being localized to the site of the actual tumor bed. In our study, we evaluated whether fluoroscopic intraoperative neoplasia detection (FIND) could help improve positive margin rates by eliminating the second localization and removing an added opportunity for device migration. We also wanted to investigate whether distance from the localization device in patients who underwent second localization procedures correlated with a positive margin rate.
2. Materials and Methods
Patients:
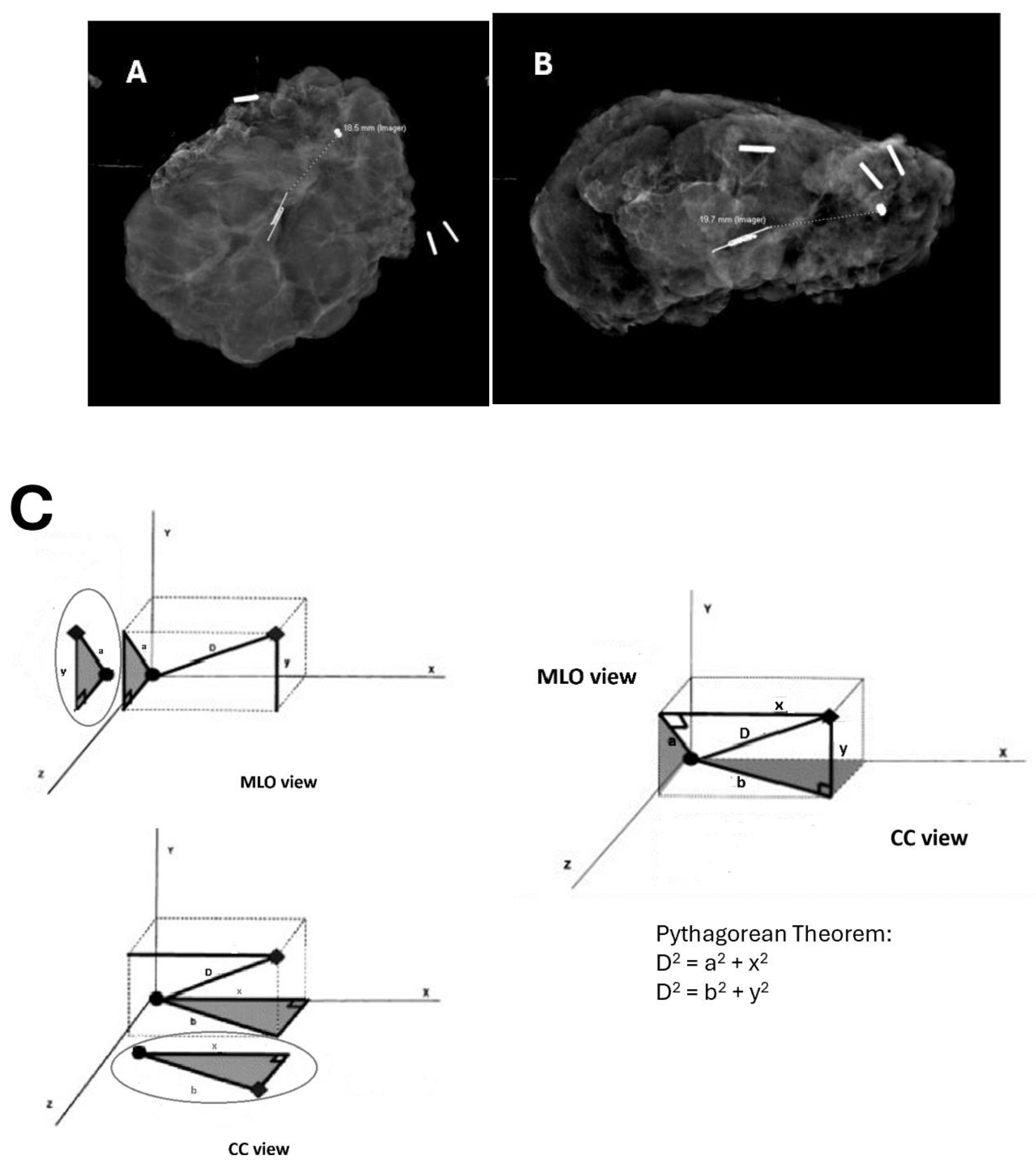
This is a retrospective review of patients from a single institution. After IRB approval (#19-0185) patients were identified by querying billing software to identify a list of patients who had undergone procedures using the CPT codes 19281-19288, 19301, 19302, 19120, 19125, 19290, 19291, 76000, 76098, 76942, 77001, 77002,77021,77031,77032 between Sept 1, 2016, and August 21, 2023. A list of patients was identified. Charts were reviewed, and patients between the ages of 18 and 99 diagnosed with non-palpable malignancy were identified. These were further reviewed to determine how their tumors were localized intraoperatively, whether by radiofrequency/radar localization or FIND. Their charts were reviewed, and information such as patient age, BMI, smoking status, presence of comorbidities, initial core biopsy, and surgical pathology, including tumor characteristics, size and margin status, and imaging, were reviewed. Imaging included an initial mammogram, ultrasound (US), and MRI if performed along with localization films and intraoperative specimen mammograms. Distance between the radiofrequency and radar localization devices and the initial biopsy clip was measured in 2 dimensions on either the specimen mammogram or the localization films (if only one view was obtained of the specimen mammogram).
The Pythagorean theorem was used to calculate the distance in space travel from the initial biopsy clip to the localization device based on these measurements, and this distance was recorded (
Figure 1). We also reviewed operative results, including operative notes, operative times, fluoroscopy dosing, whether plastic surgery was involved, and whether and for what indication re-operation was performed. Data was recorded and obtained through the REDCap database.
FIND procedure:
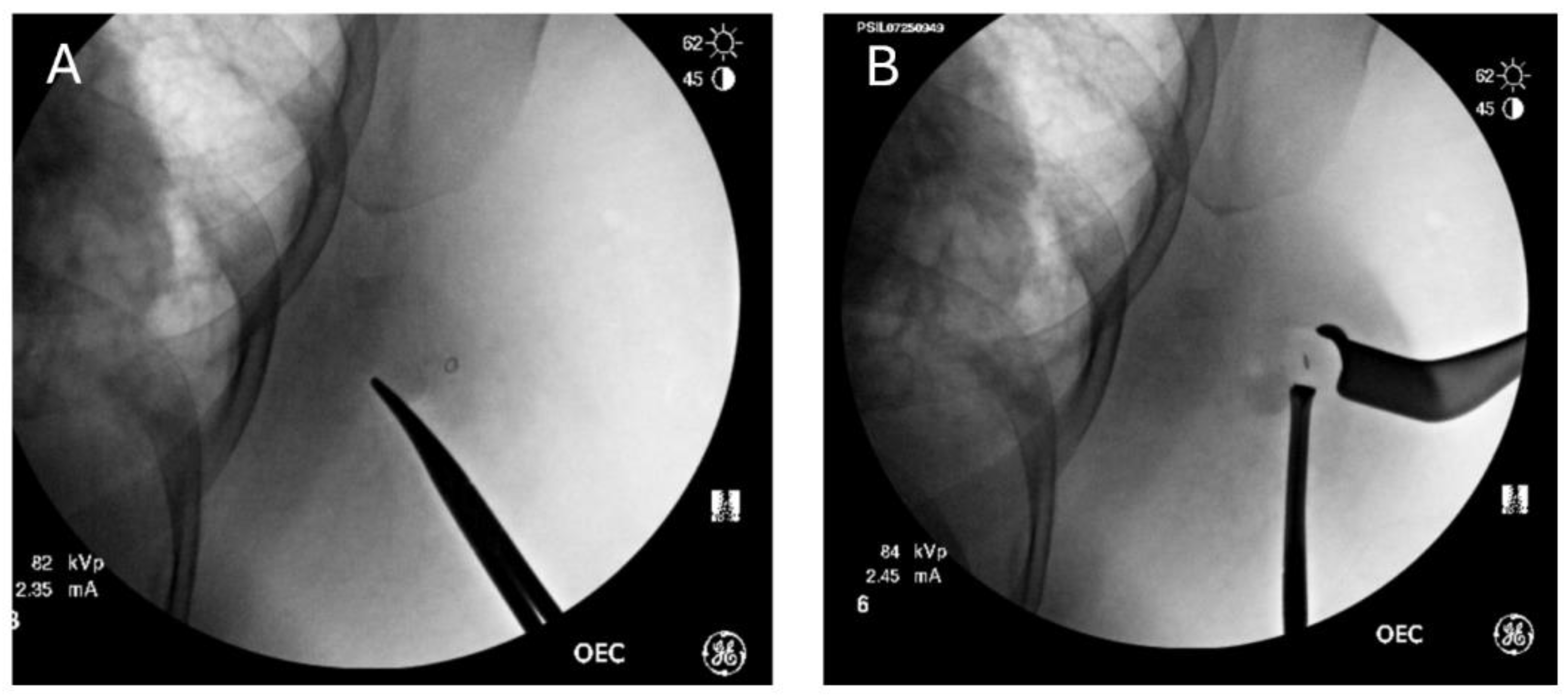
Coordination with our institution’s radiology department was performed before our study to ensure that radio-opaque clips had been placed at the time of the initial breast biopsy. Intraoperatively, care is taken to ensure that metal bars from the OR bed or lead wires do not obscure the intended field of vision. The C-arm fluoroscope is then used on low-dose spine settings to detect and localize the clip within the breast. Pulsed images are used to minimize overall fluoroscopy time. Depth is determined by applying external pressure with a closed hemostat at varying depths from anterior to posterior. The depth of the clip correlates to the depth where the clip is noted to move when external pressure is applied to the skin. Dissection is performed with real-time feedback and a direct correlation between the C-arm images and what is visualized in the breast until the lesion containing the biopsy clip is removed (
Figure 2). This can be confirmed immediately by taking a post-removal image showing that the clip is no longer within the breast.
Radiofrequency/Radar localization:
Our institution used both the Hologic LOCalizerTM RFID and SAVI SCOUT® Radar devices during this trial. Breast radiology placed these in standard fashion, using either ultrasound or tomosynthesis-guided localization pre-operatively. Post-placement films confirmed placement, and distances from the placement device to the initial biopsy clips were noted.
Outcomes:
Our primary outcome was the final negative margin rate. This was defined as no tumor on ink for malignant lesions and >2mm margins for DCIS as defined by the margin consensus statements from the Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline published in 2014 and 2016 respectively [11,12]. Secondary outcomes included re-operation rate, post-operative complications, and OR time.
Statistical Analysis:
Chi-square was used to calculate the two-tailed p-value between the groups with respect to positive margin rate, neoadjuvant chemotherapy rates between the groups, whether shave margins were performed, and whether plastic surgery was involved. Fischer’s Exact Test was used to determine the difference between the FIND and R groups with respect to lesion pathology, lesion laterality, and number of lesions in the specimen. Student’s T-test was used to evaluate the age differences, number of positive nodes, tumor size, OR time, and distance from the R device to the biopsy clip.
3. Results
Patients undergoing partial mastectomy for non-palpable malignancy from the dates of Sept 1, 2016 to August 2023. We identified 161 patients who underwent localization using FIND during that time and 55 patients who underwent radiofrequency or radar localizations (R). Six patients with malignant tumors localized using FIND had positive margins (3.7%). In contrast, seven patients with tumors localized using radiofrequency or radar had positive margins (12.7%) (p-value = 0.01). We also identified 8 out of 119 patients (6.7%) in the FIND group who underwent re-operation, six for positive margins, and two for SLN biopsy after they were found to have invasive ductal carcinoma on surgical pathology when initial core needle biopsy had identified only DCIS. In the R group, seven out of 49 patients (14.3%) underwent re-operation. Six underwent re-operation for positive margins, and 1 underwent SLN biopsy after she was found to have invasive disease on final pathology when core needle biopsy had only shown DCIS. One patient with < 2mm margin for DCIS elected not to undergo re-excision.
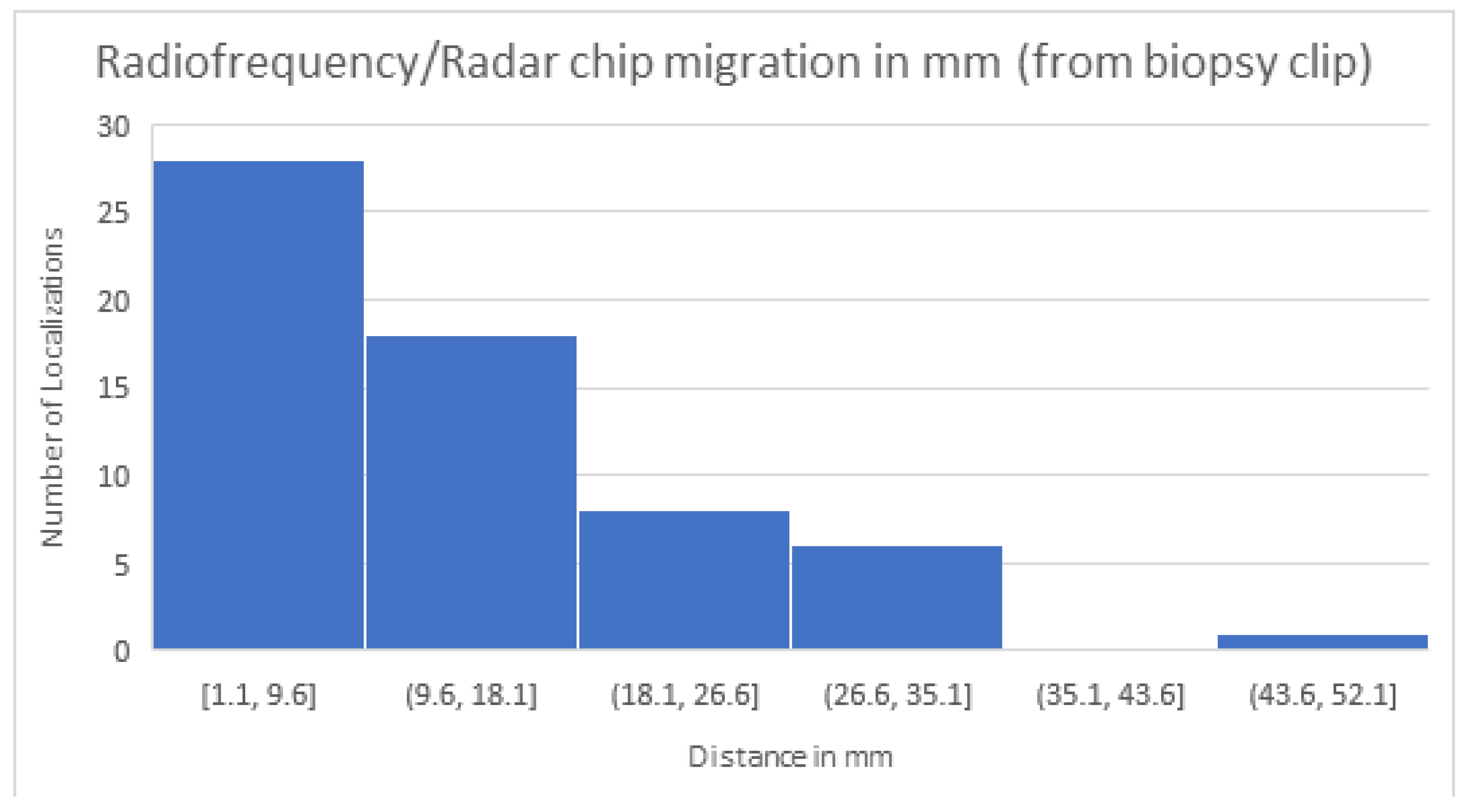
We then compared the distances from the R device to the biopsy clip in patients with positive and negative margins. The average distance from the localization device to the biopsy clip for patients with positive margins was 19.2 mm (SD 10.3). For patients with negative margins, the average distance from the localization device to the biopsy clip was 12.5 mm (SD 12.5). One patient was noted to have clip migration at the time of localization, and these accounted for the distance of 44.4 mm in the negative margin group and were not used to localize the lesion. (
Figure 3)
Patient and lesion characteristics:
There was no statistically significant difference in patient age or tumor characteristics between those who were localized with FIND versus R. Mean age was 63.4 (SD 12.1) in the FIND group and 59.9 (SD 15.6) in the R group. There was no statistically significant difference in the type of tumor localized in the FIND versus R groups with 23 (14.3%) and 14 (25.5%) being pure DCIS which was not statistically significant (p = 0.06); 89 (55.3%) and 33 (60.0%) being IDC with or without DCIS; and 7 (4.4%) and 6 (10.9%) being invasive lobular carcinoma. Tumor size was similar between the groups, with the average size in mm in the FIND group at 11.76mm (SD 9.58) and 13.8 (SD 13.6) in the R group (p = 0.33). Clinical node positivity was similar between groups, with a number of positive nodes averaging 0.4 (SD 1.2) in the FIND group versus 0.5 (SD 1.6) in the R group (p = 0.52) (
Table 1).
Treatment characteristics:
There was a significant difference in the number of patients who had undergone neoadjuvant chemotherapy, with 11 (22.5%) having received NAC in the R group versus 16 (10.1%) in the FIND group (p-value 0.02). There was no difference in the number of lesions within the specimen between the groups. Only one lesion was identified within the specimen in 96.27% of the FIND group and 90.91% of the R group. Three patients in the FIND group (1.86%) and one patient in the R group (1.82%) had three lesions identified within the specimen. Two fellowship-trained breast surgeons performed the surgery. The localization method was performed per the surgeon's preference, with one surgeon performing almost exclusively FIND and the other performing a combination of FIND and R localizations. Of the surgeons who performed the FIND procedure, there was no inter-surgeon difference in the rate of positive margins for the FIND procedure. There was no difference in the use of shave margins between the groups, with 85.1% (137 of 161) of the patients in the FIND group and 85.5% (47 of 55) of the patients in the R group having shave margins performed. There was a statistically significant difference in the OR time in minutes, with the R group having a mean OR time of 128.4 (SD 64.33) minutes and the FIND group having a mean OR time of 150.1 (SD 85.77) minutes (p = 0.05) (
Table 2).
Primary Outcome:
Final pathology identified final positive margins in six of the FIND group, with a positive margin rate of 3.7%. In the R group, seven patients had final positive margins or a 12.7% positive margin rate, which was statistically significant (p=0.02). Due to the small number of patients with final positive margins, multivariate analysis could not be performed.
Secondary Outcomes:
Re-operation rates were found to be eight in the FIND group (4.9%) versus seven in the R group (12.7%) (p-value = 0.05). In the FIND group 2, patients had invasive cancers on their initial surgical pathology after core needle biopsy showed DCIS, and they returned to the OR for sentinel lymph node biopsy. In the R group, one patient who had DCIS with a single margin of less than 2mm elected not to return to the OR after multidisciplinary discussions. Another patient who initially had DCIS on core needle biopsy but invasive cancer on surgical pathology returned to the OR for SLN biopsy. For the patients in the R group, we wanted to evaluate whether the distance between the R localization device and the initial biopsy clip changed the positive margin rate. Overall, the range for device migration was between 1.1mm and 44.4mm. The patient with a 44.4mm migration was one in whom the initial biopsy clip was known to be displaced from the initial lesion. This patient had negative final margins. For patients with positive margins localized by R, the average distance from the biopsy clip to the localization device was 19.2 mm, with a standard deviation of 10.3. For patients localized with R with final negative margins, the distance between the initial biopsy clip and the R localization device was 12.5 mm, with an SD of 9.6. While there was a trend towards a larger distance from the biopsy clip to the R device in patients with positive margins, this was not statistically significant. The large SD was secondary to the patient with a known biopsy clip displacement with a considerable distance between the biopsy clip and device but with negative margins. In this case, the intraoperative localization was done based on the R device location, not the clip location.
4. Discussion
The positive margin rate in the R group were similar to historical rates for needle localization at 12 percent, which is significantly greater than the positive margin rate in the FIND group at only three percent. The lower positive margin rate also led to a lower re-operation rate using the FIND procedure at 6% versus 14% for R device localization, which was statistically significant. Patients in the R group with positive margins tended to have a larger distance between the R localization device and the initial biopsy clip than those with negative margins. This highlights the compounding issue that a second device localization may have the potential to increase the distance from the instrument used for localization and the true tumor bed, which plays a role in the rate of positive margins. The only tumor or operative differences that were statistically significant between the groups were the trend for more neoadjuvant therapy use in the R group and longer OR times by an average of 22 minutes in the FIND group. There were no statistically significant differences between the two groups in other factors traditionally attributed to the possibility of a higher positive margin rate, such as pure DCIS, invasive lobular carcinoma, or use of shave margins.
As screening techniques have improved, the majority of newly diagnosed breast cancers are non-palpable. Various techniques have been employed to localize these tumors. Wire localization was the gold standard in years past, but this is now being replaced by other methods performed before surgery, improving OR time utilization and coordination between surgery and radiology. However, this still requires patients to undergo an additional procedure before surgery, one in which they are awake and still have the associated discomfort and cost involved in doing this. Fluoroscopy is available in virtually every system, and every general surgeon with or without additional training in breast utilizes fluoroscopy during training and in practice; thus, this technique should be easily adaptable to any system especially rural or low-resource settings. Hayes et al. found that R localization techniques reduced operating room start delays from averaging 40 minutes with WGL to 11 minutes with R localization, which results in overall cost savings to the system [7]. FIND should have no delays as it does not require a pre-operative localization procedure and thus improves OR efficiency, overall cost, need for re-excision and patient experience. Another advantage of using the FIND procedure over radar or radiofrequency localization is that there is no limitation on tumor depth, as several studies have shown with R devices, which are difficult to localize at depths greater than 4.5cm. Previous studies have also shown issues with potential device damage due to electrocautery, causing certain radar devices to short out and stop working, which can lead to difficulties in localization [13,14]. There is no such issue with FIND as the target being localized does not send out a signal and cannot be deactivated by cautery.
Limitations
The limitation of this study is that it was a single-institution retrospective study. While there was no inter-surgeon difference in positive margin rates for the FIND procedure, it only evaluated the results from two clinically active surgeons with a practice dedicated 100% to breast surgery. Results may vary for surgeons who do not dedicate this practice volume to breast surgery. As with every new technique, a learning curve is associated with using the FIND procedure. Previous studies have indicated that the learning curve for non-wire localized technologies is around 5-7 cases [15]. We do not believe that the learning curve for FIND localization is any different, although the exact number of cases to complete the learning curve has yet to be studied. Another limitation is that institutional practices may differ in their use of clips as some are more radiolucent (bar, ring or coil shape) than others (ribbon).
Implications:
Ideally, once more surgeons become comfortable using this technique, we would like to evaluate the performance of the FIND technique compared to R localization techniques in a prospective randomized multi-institutional study. For now, we believe this technique could quickly and easily be adopted into the repertoire of a general or a dedicated breast surgeon, even in a low-resource rural setting. This technique allows for improvement in positive margin rate and the need for re-excision. It also has the potential to decrease the cost of the system, improve efficiency for the surgeon, and improve the overall experience for the patient.
5. Conclusions
FIND is a novel method of intraoperative localization that obviates the need for pre-operative procedures to localize tumors. Intraoperative fluoroscopy to localize the initial biopsy clip decreases the potential for compounding errors due to increased distances from the localization device to the tumor bed. This also makes for a very time and cost-efficient method to localize tumors as there should be no intraoperative delays while awaiting device placement, and it requires no purchasing of additional localization systems or devices and relies only on the standard C-arm fluoroscopy that is already available in virtually every operating room system.
Author Contributions
Conceptualization: Nelson, Weiser, Klimberg; Data Curation: Nelson, Den, Weiser; Formal Analysis: Nelson, Biai, Klimberg; Methodology: Nelson, Weiser, Biai, Klimberg; Supervision: Nelson, Biai, Klimberg; Writing: Original draft – Nelson, Den, Klimberg; Writing – review and editing: Nelson, Den, Posleman, Robinson, Silva, Klimberg; Investigation: Robinson, Posleman, Silva, Klimberg.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of The University of Texas Medical Branch (#19-0185).
Informed Consent Statement
Patient consent was waived due to the fact that this was a retrospective review of previously performed procedures and was deemed exempt by IRB
Data Availability Statement
Data is available in a privately maintained institutional database through RedCaps.
Acknowledgments
During the preparation of this work the author(s) used no generative AI or AI-assisted technologies. Data from the first 79 patients with non-palpable malignancies localized using FIND was reported by Weiser et al in the Journal of the American College of Surgeons, Volume 236(4), April 2023. This data is referenced in our paper. We have added an additional 82 patients localized using FIND and 55 using Radar and Radiofrequency methods which were not included with the previous data.
Conflicts of Interest
The authors declare no conflicts of interest
Abbreviations
The following abbreviations are used in this manuscript:
| FIND |
Fluoroscopic Intraoperative Neoplasia Detection |
| R |
Radar & Radiofrequency |
| DCIS |
Ductal Carcinoma In Situ |
| OR |
Operating Room |
References
- Fisher, B., Anderson, S., Fisher, E. R., Redmond, C., et al. Significance of ipsilateral breast tumour recurrence after lumpectomy. The Lancet 1991; 338(8763). [CrossRef]
- Meloni, G. B., Becchere, M. P., Soro, D., Profili, S., & Canalis, G. C. (1995). [Localization of non-palpable lesions of the breast using a metallic guide. Potential complications]. La Radiologia Medica, 89(5).
- Law, W., Look Hong, N., Ravi, A., Day, L., Somani, Y., Wright, F. C., Nofech-Mozes, S., Tran, W. T., & Curpen, B. (2021). Budget Impact Analysis of Preoperative Radioactive Seed Localization. Annals of Surgical Oncology, 28(3). [CrossRef]
- Srour, M. K., Kim, S., Amersi, F., Giuliano, A. E., & Chung, A. (2021). Comparison of Multiple Wire, Radioactive Seed, and Savi Scout® Radar Localizations for Management of Surgical Breast Disease. Annals of Surgical Oncology, 28(4). [CrossRef]
- Dave, R. V., Barrett, E., Morgan, J., Chandarana, M., Elgammal, S., Barnes, N., Sami, A., Masudi, T., Down, S., Holcombe, C., Potter, S., Somasundaram, S. K., Mylvaganam, S., Maxwell, A., & Harvey, J. (2022). Wire-and magnetic-seed-guided localization of impalpable breast lesions: IBRA-NET localisation study. British Journal of Surgery, 109(3). [CrossRef]
- Tayeh, S., Wazir, U., & Mokbel, K. (2021). The evolving role of radiofrequency guided localisation in breast surgery: A systematic review. In Cancers (Vol. 13, Issue 19). [CrossRef]
- M., H., E., B., & H., W. (2017). SCOUT RADAR localization improves breast surgery operating room start times compared with wire localization. Annals of Surgical Oncology, 24(2).
- Wright, C. M., Moorin, R. E., Saunders, C., Marinovich, M. L., Taylor, D. B., Bourke, A. G., Westcott, E. J., Chong, C. Y. L., Liang, R., Hughes, R. L., & Elder, E. (2021). Cost-effectiveness of radioguided occult lesion localization using 125I seeds versus hookwire localization before breast-conserving surgery for non-palpable breast cancer. British Journal of Surgery, 108(7). [CrossRef]
- Weiser, R., Manno, G. C., Cass, S. H., Chen, L., Kuo, Y. F., He, J., Robinson, A. S., Posleman Monetto, F., Silva, H. C., & Klimberg, V. S. (2023). Fluoroscopic Intraoperative Breast Neoplasm and Node Detection. Journal of the American College of Surgeons, 236(4). [CrossRef]
- Kass, R., Kumar, G., Klimberg, V. S., Kass, L., Henry-Tillman, R., Johnson, A., Colvert, M., Lane, S., Harshfield, D., Korourian, S., Parrish, R., & Mancino, A. (2002). Clip migration in stereotactic biopsy. American Journal of Surgery, 184(4). [CrossRef]
- Moran, M. S., Schnitt, S. J., Giuliano, A. E., Harris, J. R., Khan, S. A., Horton, J., Klimberg, S., Chavez-Macgregor, M., Freedman, G., Houssami, N., Johnson, P. L., & Morrow, M. (2014). Society of surgical oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages i and II invasive breast cancer. Annals of Surgical Oncology, 21(3). [CrossRef]
- Morrow, M., Van Zee, K. J., Solin, L. J., Houssami, N., Chavez-MacGregor, M., Harris, J. R., Horton, J., Hwang, S., Johnson, P. L., Marinovich, M. L., Schnitt, S. J., Wapnir, I., & Moran, M. S. (2016). Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Journal of Clinical Oncology, 34(33). [CrossRef]
- Falcon, S., Weinfurtner, R. J., Mooney, B., & Niell, B. L. (2018). SAVI SCOUT® localization of breast lesions as a practical alternative to wires: Outcomes and suggestions for trouble-shooting. Clinical Imaging, 52, 280–286. [CrossRef]
- Woo, S. C., Wong, T., Chau, C. M., Fung, W. Y., Chan, R. L. S., Yung, A. W. T., & Ma, J. K. F. (2022). Radar Localisation of Non-palpable Breast Lesions in a Chinese Population: a Pilot Study. Hong Kong Journal of Radiology, 25(3). [CrossRef]
- Christenhusz, A., den Dekker, B. M., van Dalen, T., Jongen, L., van der Schaaf, M. C., Alic, L., ten Haken, B., Pijnappel, R. M., & Dassen, A. E. (2023). Radiofrequency localization of nonpalpable breast cancer in a multicentre prospective cohort study: feasibility, clinical acceptability, and safety. Breast Cancer Research and Treatment, 201(1). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).