Submitted:
03 June 2025
Posted:
03 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
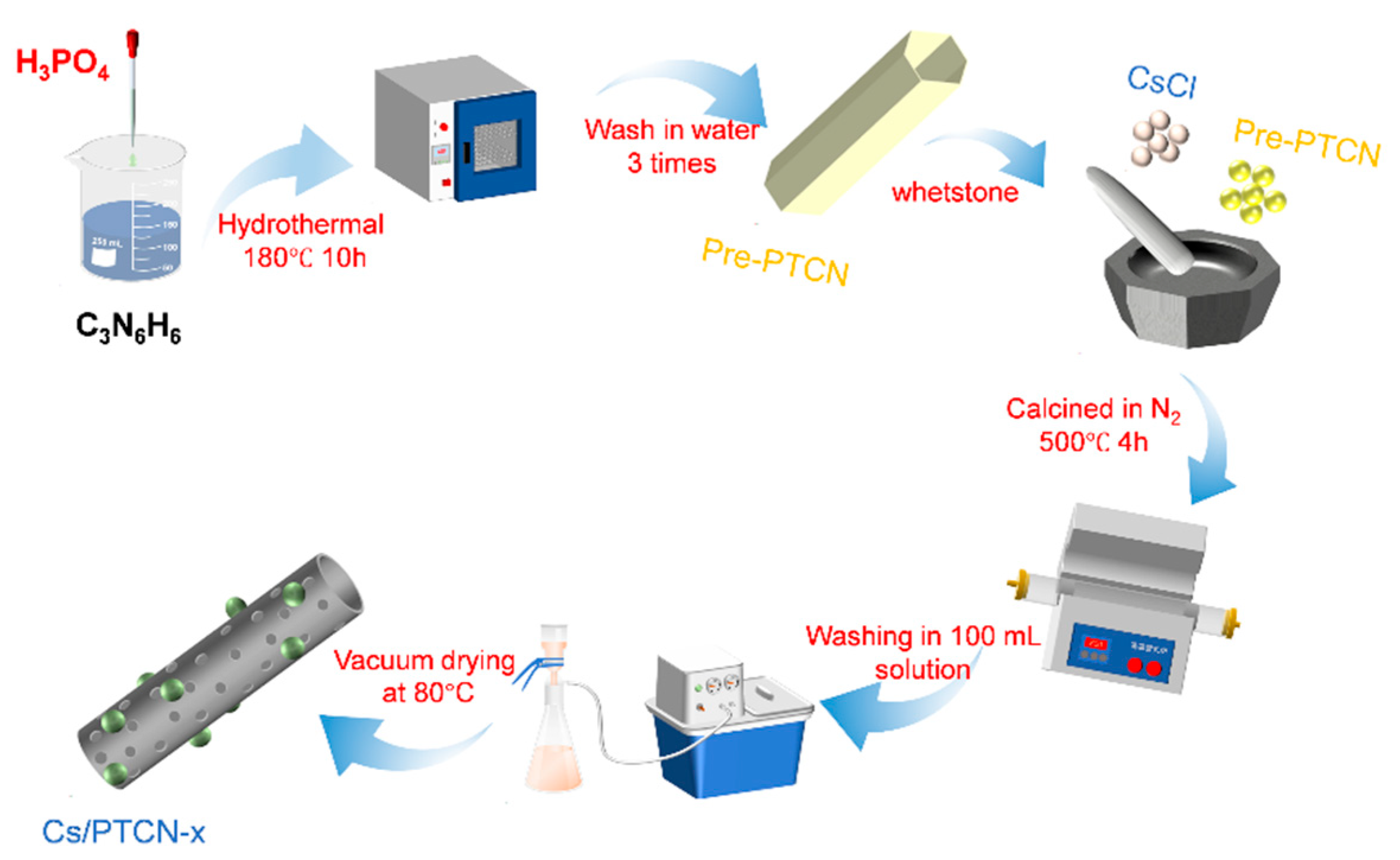
2.1. Synthesis of P-Doped Tubular Carbon Nitride
2.2. Synthesis of Cs-Doped Carbon Nitride
2.3. Synthesis of P-Cs Co-Doped Tubular Carbon Nitride
3. Results and Discussion
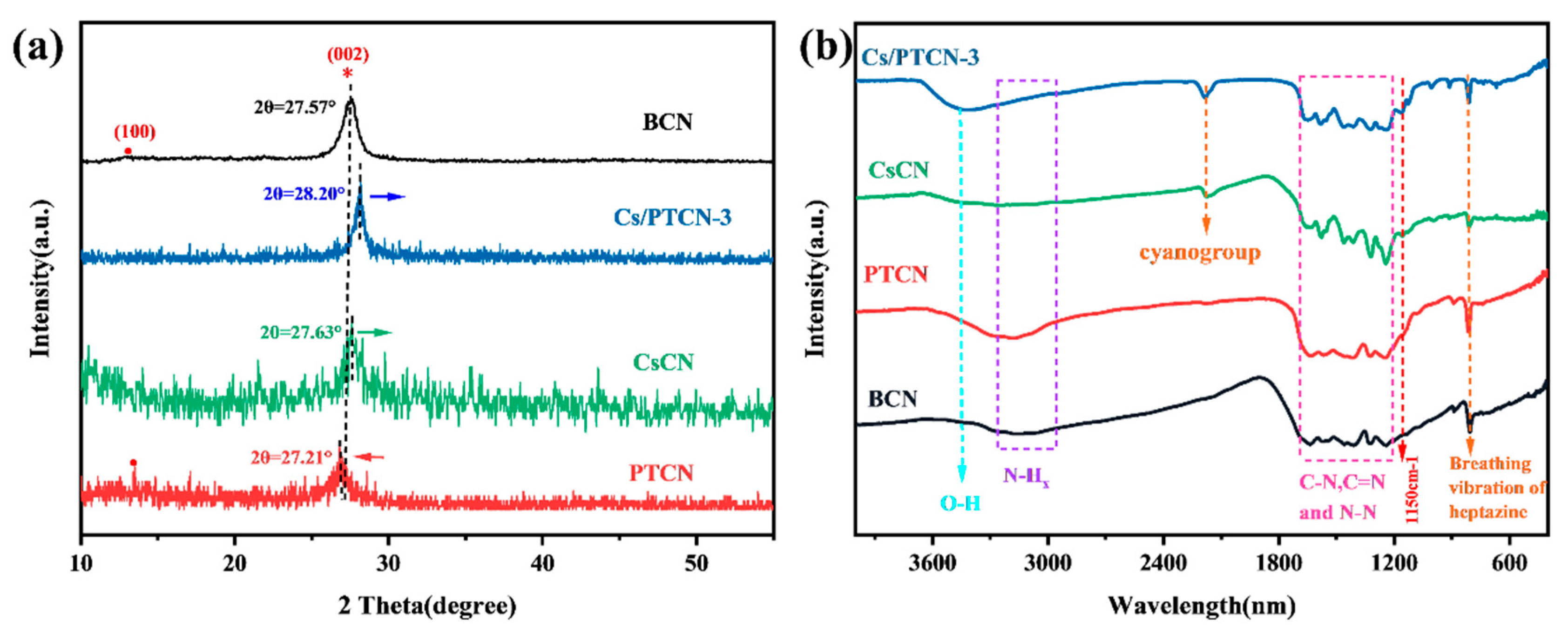
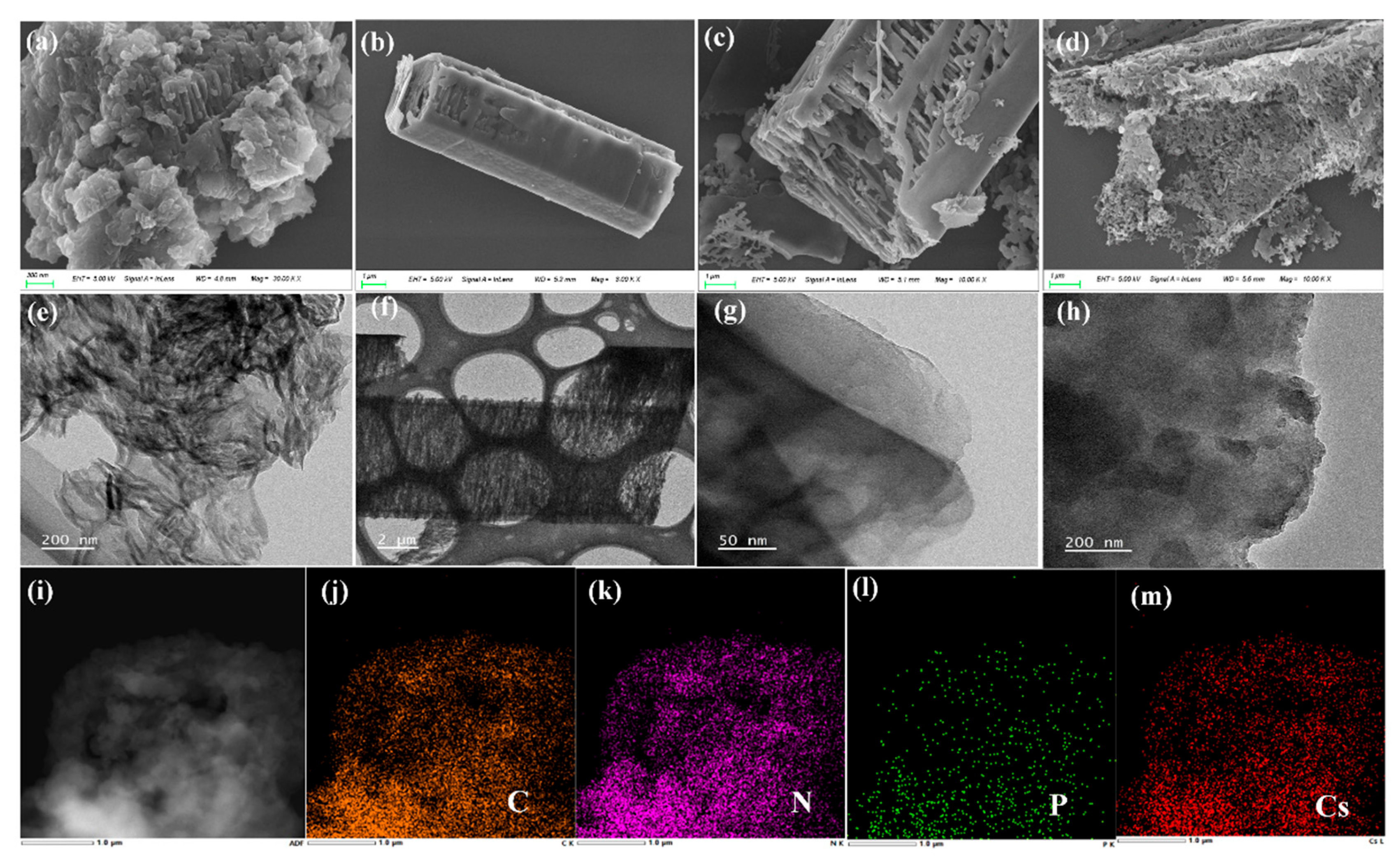
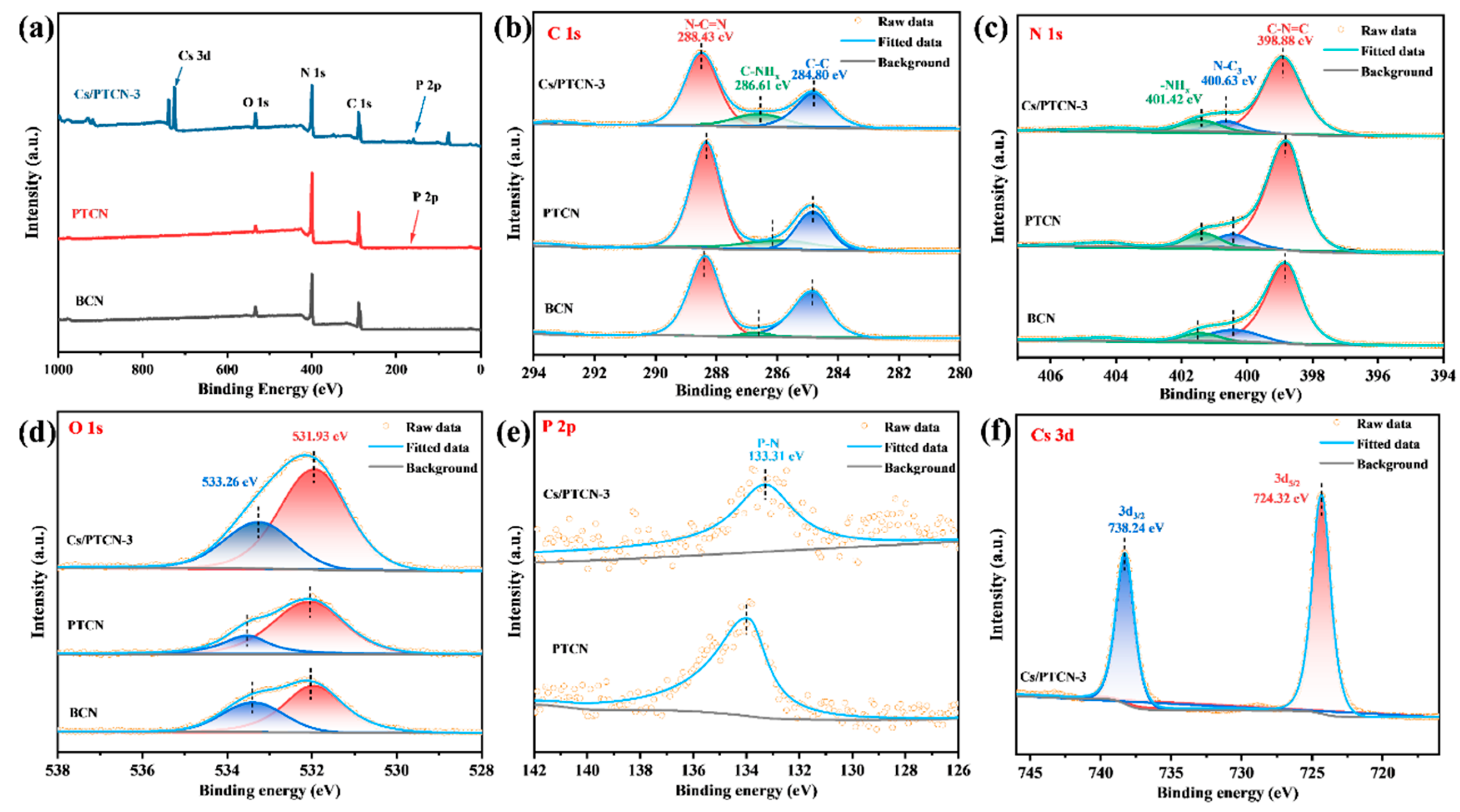
3.1. Characterization of Material Structure
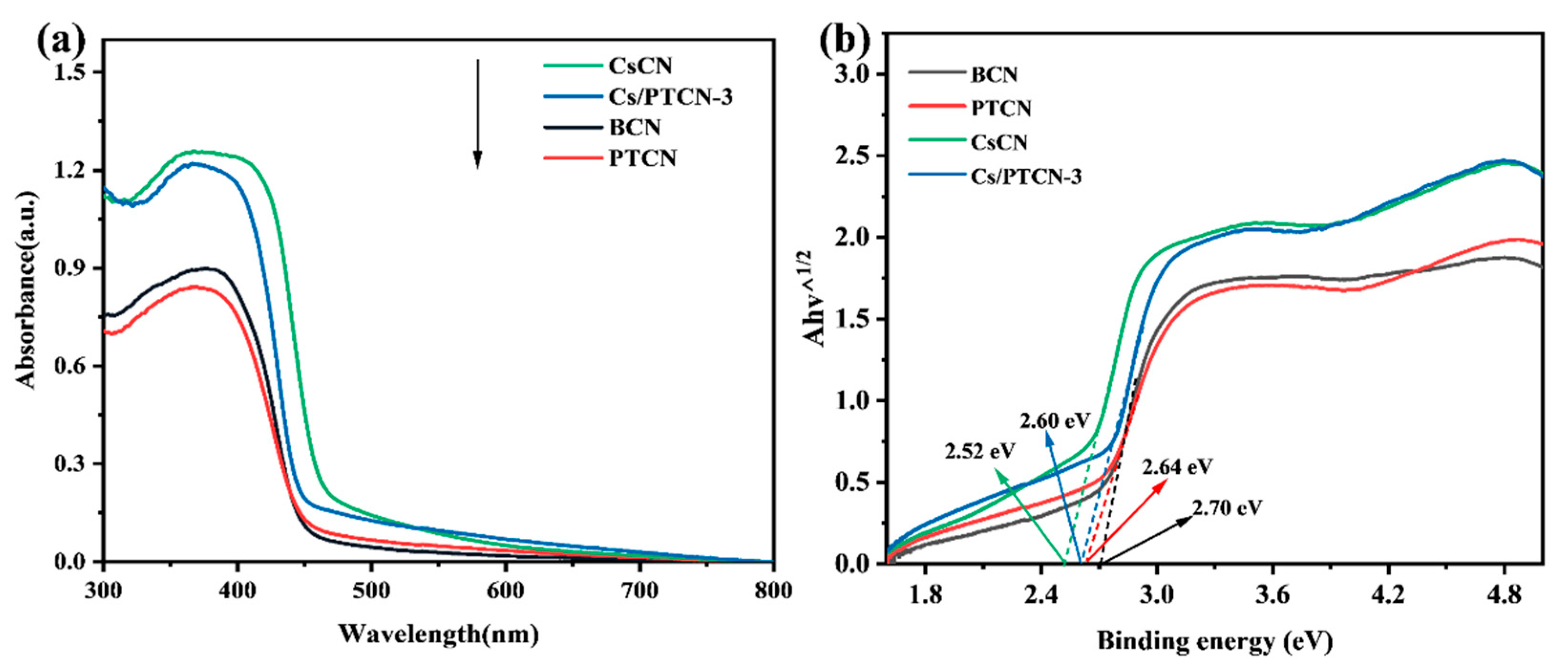
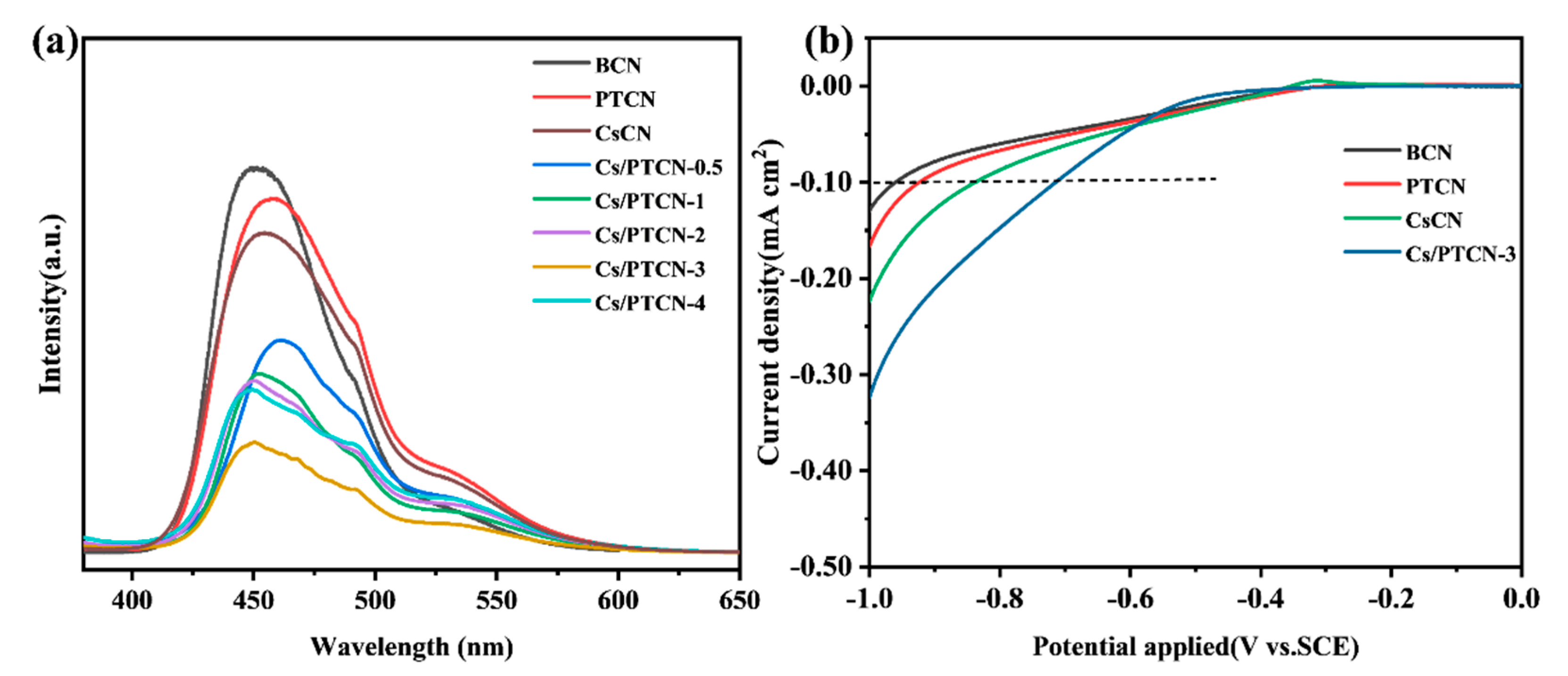
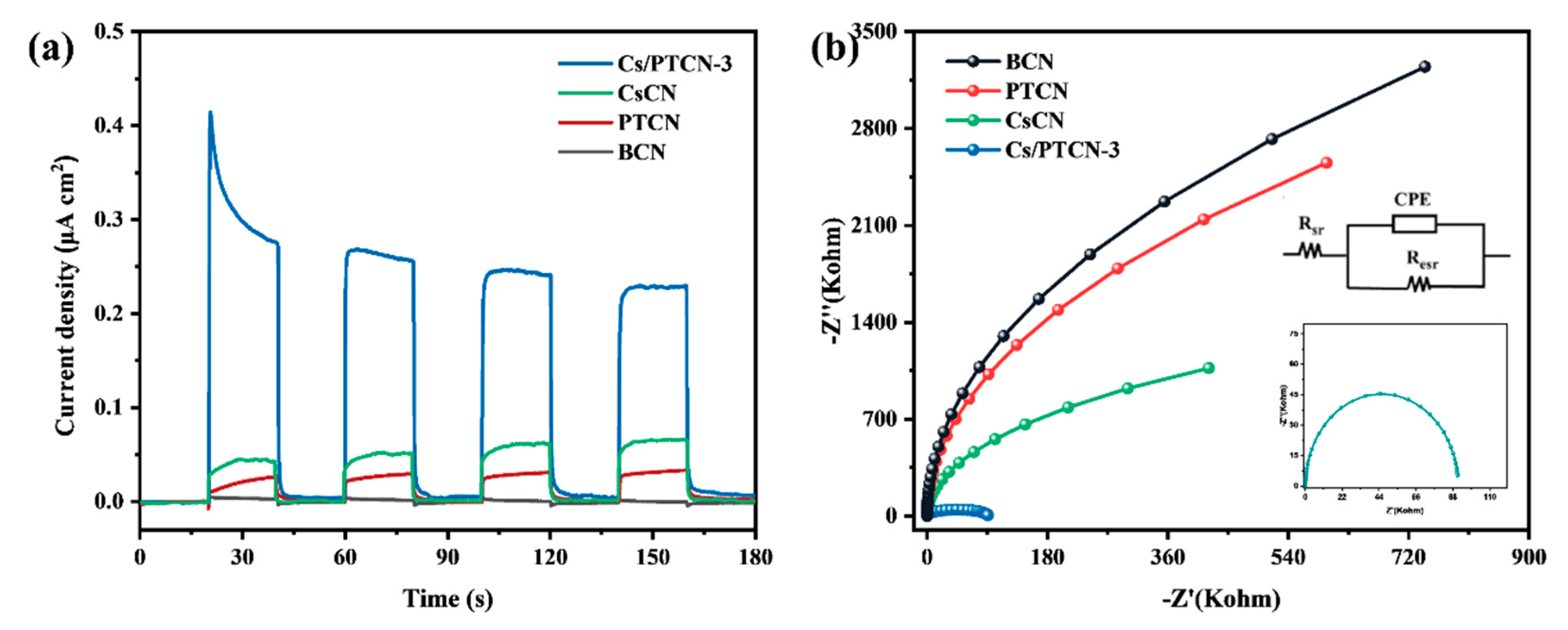
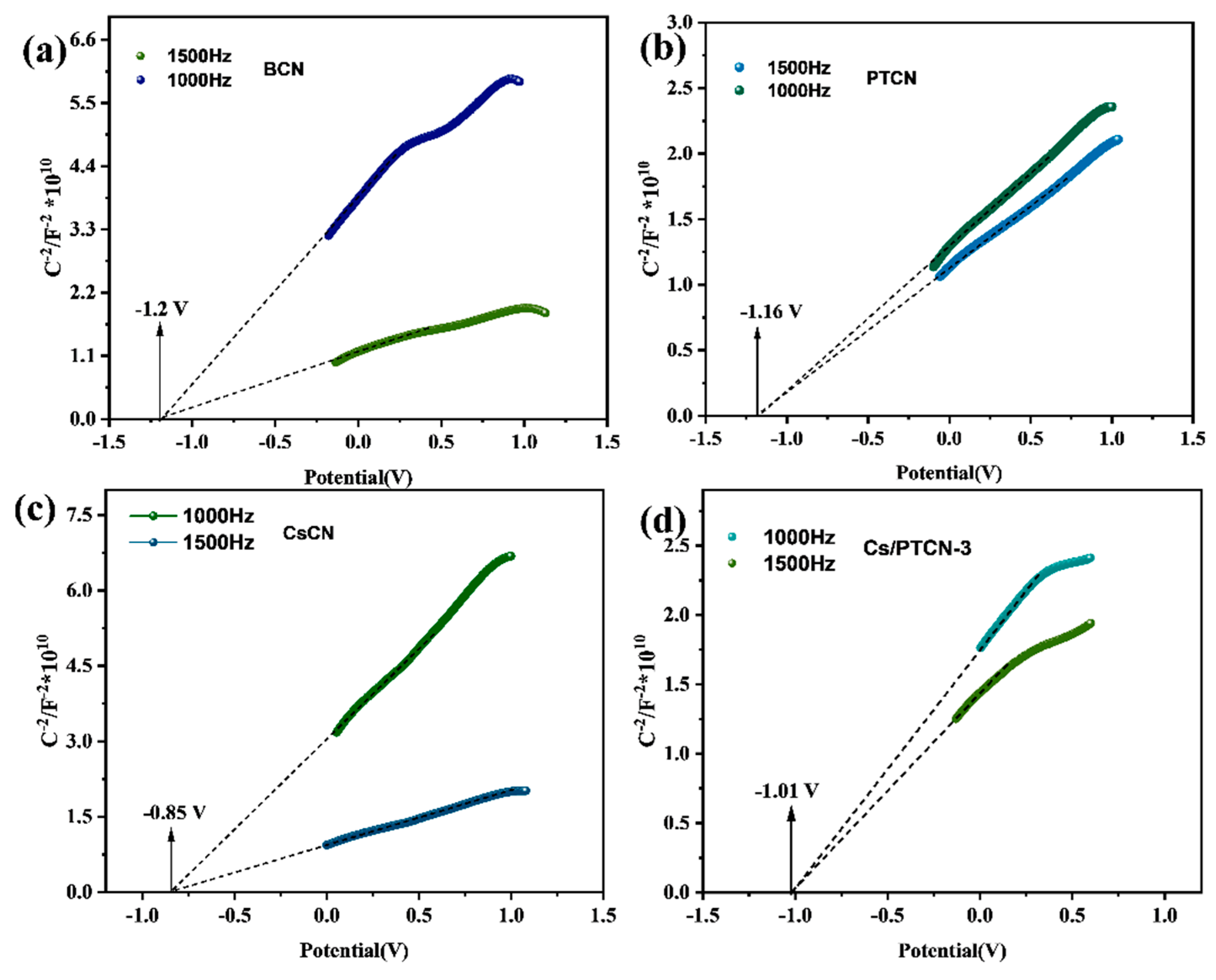
3.2. Optical Properties and Charge Carrier Behavior
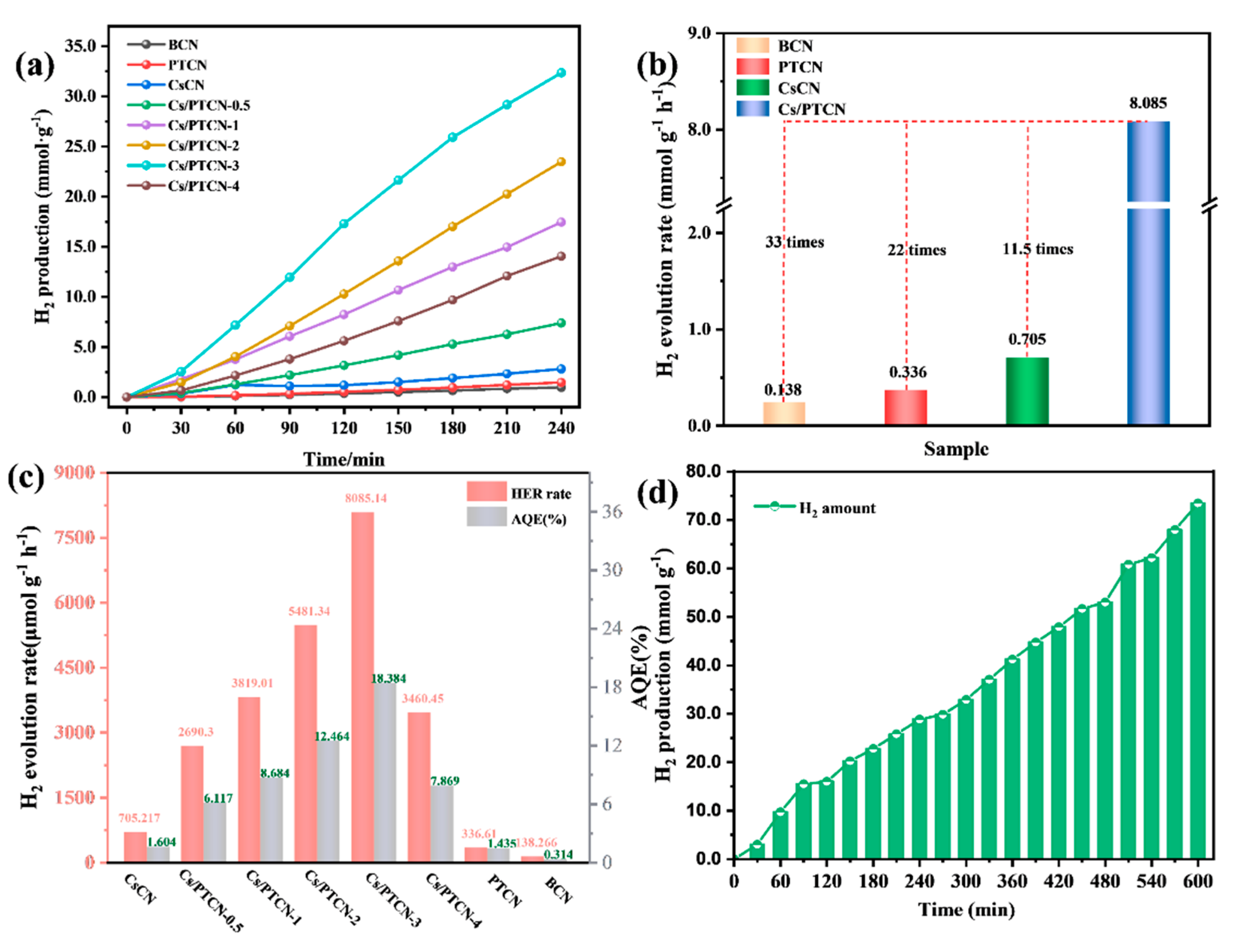
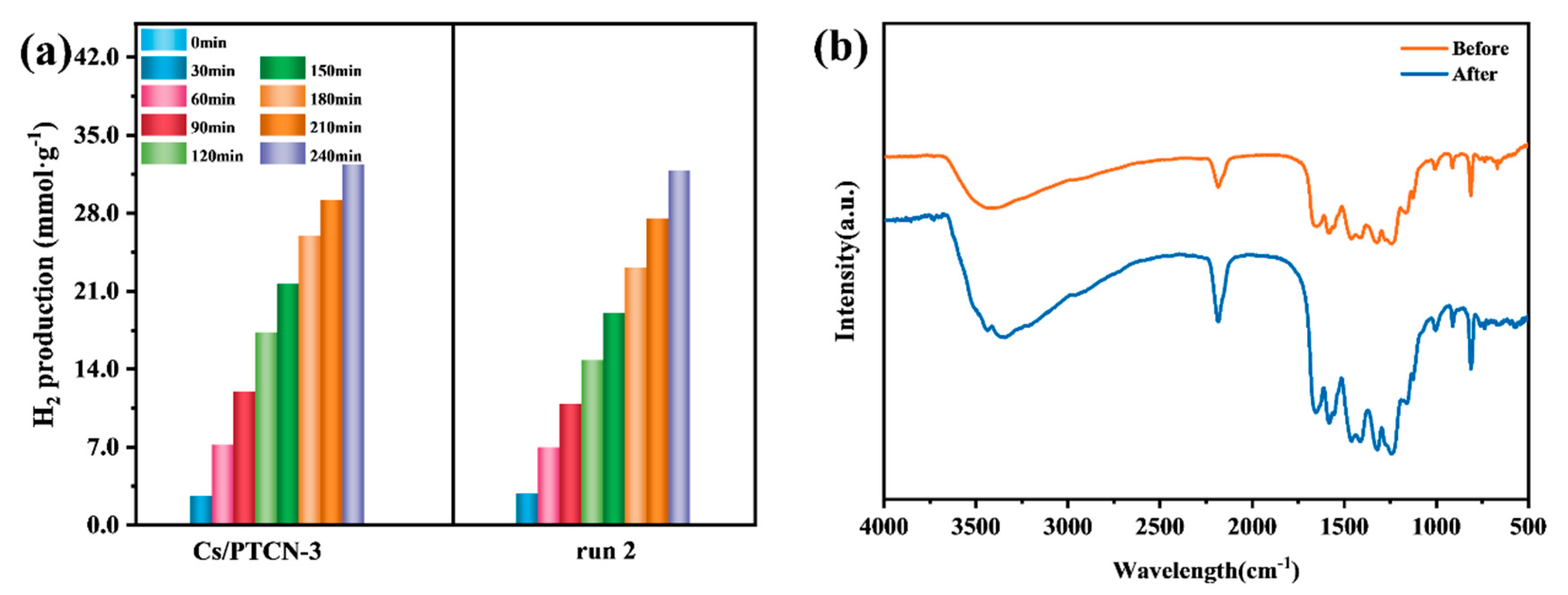
3.3. Evaluation of Photocatalytic Hydrogen Evolution Performance
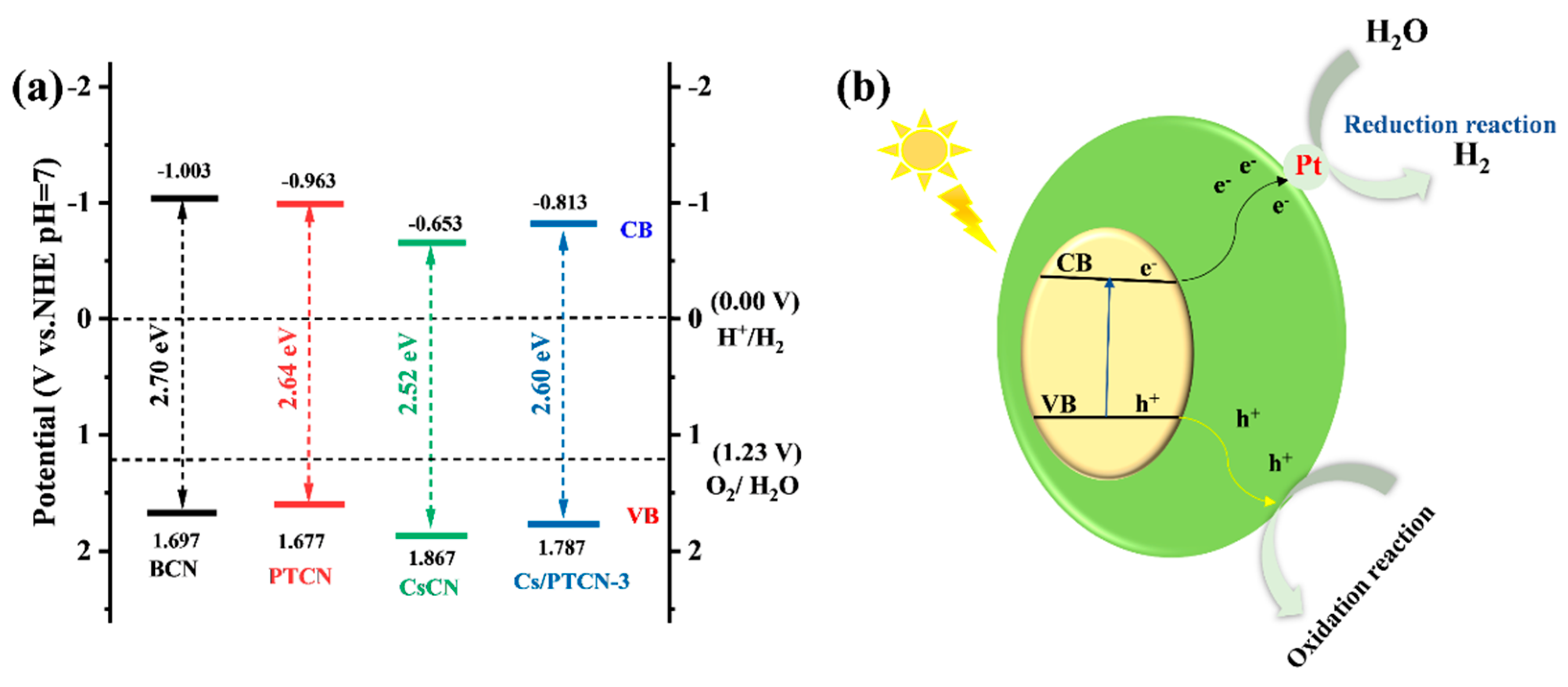
3.4. Proposed Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Gunawan, D.; Zhang, J. J.; Li, Q. Y.; et al. Materials Advances in Photocatalytic Solar Hydrogen Production: Integrating Systems and Economics for a Sustainable Future. Advanced Materials 2024, 36, 2404618. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Ahmed Navid, I.; Ma, Y. J.; et al. Solar-to-hydrogen Wfficiency of More Than 9% in Photocatalytic Water Splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Islam, M.; Gupta, A. ZnO nanowire-decorated 3D printed pyrolytic carbon for solar light-driven photocatalytic degradation of wastewater contaminants. Advanced Composites and Hybrid Materials 2025, 8, 109. [Google Scholar] [CrossRef]
- Lan, R. M.; Hu, Z. F.; Liu, H. R.; et al. Passivating Lattice Oxygen in ZnO Nanocrystals to Reduce its Interactions with the Key Intermediates for a Selective Photocatalytic Methane Oxidation to Methanol. Angewandte Chemie International Edition 2025, 64, e202425186. [Google Scholar] [CrossRef]
- Wu, Y. H.; Yan, Y. Q.; Deng, Y. X.; et al. Rational construction of S-scheme CdS quantum dots/In2O3 hollow nanotubes heterojunction for enhanced photocatalytic H2 evolution. Chinese Journal of Catalysis 2025, 70, 333–340. [Google Scholar] [CrossRef]
- Sun, R. J.; Zhu, Z. J.; Tian, N.; et al. Hydrogen Bonds and In situ Photoinduced Metallic Bi0/Ni0 Accelerating Z-Scheme Charge Transfer of BiOBr@NiFe-LDH for Highly Efficient Photocatalysis. Angewandte Chemie International Edition 2024, 63, e202408862. [Google Scholar] [CrossRef]
- Chen, Y. H.; Zhang, L.; Chen, S.; et al. Synthesis of Heteromorphic Bi2WO6 Films With an Interpenetrate 1D/2D Network Structure for Efficient and Stable Photocatalytic Degradation of VOCs. Advanced Materials 2024, 36, 2407400. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Z. P.; Shi, Y.; et al. Donor–Acceptor Type Carbon Nitride Photocatalysts in Photocatalysis: Current Understanding, Applications and Challenges. Small 2025, 21, 2409903. [Google Scholar] [CrossRef]
- Pan, Y. L.; Zhang, Q.; Huang, G. Z.; et al. Efficient photocatalytic H2 evolution over tubular mesoporous carbon nitride with N-vacancy by microwave-assisted synthesis. Science China Chemistry 2025, 68, 866–873. [Google Scholar] [CrossRef]
- Wan, S. P.; Ou, M.; Wang, Y. N.; et al. Protonic acid-assisted universal synthesis of defect abundant multifunction carbon nitride semiconductor for highly-efficient visible light photocatalytic applications. Applied Catalysis B: Environmental 2019, 258, 118011. [Google Scholar] [CrossRef]
- Bi, L. B.; Shen, J. M.; Yan, P. W.; et al. Efficient photodegradation of micropollutants by tubular carbon nitride: The role of nitrogen defects. Journal of Cleaner Production 2023, 419. [Google Scholar] [CrossRef]
- Yue, J. P.; Yang, H. P.; Zhou, L.; et al. H2O2 photosynthesis on doubly heteroatom (K and Na) intercalated carbon nitride: Collaboration and division of boosting in-layer and inter-layer carrier transfer. Chemical Engineering Journal 2025, 505, 159434. [Google Scholar] [CrossRef]
- Palani, G.; Apsari, R.; Hanafiah, M. M.; et al. Metal-Doped Graphitic Carbon Nitride Nanomaterials for Photocatalytic Environmental Applications—A Review. Nanomaterials 2022, 12, 1754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Y.; Chen, C. C.; Liu, F. T.; et al. Elucidating the tandem synergistic roles of Cs-O dual sites confined in carbon nitride toward selective photoreduction H2O2 production coupled with xylose oxidation. Chemical Engineering Journal 2025, 509, 161204. [Google Scholar] [CrossRef]
- Tahereh, M. S.; Hossein, F.; Byeong Kyu, L.; et al. Caesium sites coordinated in Boron-doped porous and wrinkled graphitic carbon nitride nanosheets for efficient charge carrier separation and Transfer: Photocatalytic H2 and H2O2 production. Chemical Engineering Journal 2021, 423, 130067. [Google Scholar] [CrossRef]
- Ran, J. R.; Ma, T. Y.; Gao, G. P.; et al. Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production. Energy & Environmental Science 2015, 8, 3708–3717. [Google Scholar] [CrossRef]
- Liu, Q.; Li, H.; Zhang, H.; et al. The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride. Chinese Chemical Letters 2022, 33, 4756–4760. [Google Scholar] [CrossRef]
- Tong, H. J.; Odutola, J.; Song, J. S.; et al. Boosting the Quantum Efficiency of Ionic Carbon Nitrides in Photocatalytic H2O2 Evolution via Controllable n → π* Electronic Transition Activation. Advanced Materials 2024, 36, 2412753. [Google Scholar] [CrossRef]
- Qin, J. N.; Barrio, J.; Peng, G. M.; et al. Direct growth of uniform carbon nitride layers with extended optical absorption towards efficient water-splitting photoanodes. Nature Communications 2020, 11, 4701. [Google Scholar] [CrossRef]
- You, Z. Y.; Li, G.; Wang, C. H.; et al. The synergistic effect of potassium ions and nitrogen defects on carbon nitride for enhanced photocatalytic hydrogen evolution. International Journal of Hydrogen Energy 2023, 48, 15934–15943. [Google Scholar] [CrossRef]
- Dong, J. T.; Zhao, J. Z.; Yan, X. W.; et al. Construction of carbonized polymer dots/potassium doped carbon nitride nanosheets Van der Waals heterojunction by ball milling method for facilitating photocatalytic CO2 reduction performance in pure water. Applied Catalysis B: Environment and Energy 2024, 351, 123993. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, M. m.; Liu, J.; et al. Anchoring Ag Atom on Carbon Vacancy Enriched Carbon Nitride to Synergistically Promote CO2 Photoredution with Water. Advanced Functional Materials 2024, 35, 2413232. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, Y. x.; Jia, Z. t.; et al. Boron and Oxygen Dual-Doped Carbon Nitride Nanotubes with Frustrated Lewis Pairs for Efficient Electrocatalytic Ammonia Synthesis. Small Methods 2024, 2401672. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z. G.; Zhang, X.; Jiang, Z. X.; et al. Phosphorus and sulphur co-doping of g-C3N4 nanotubes with tunable architectures for superior photocatalytic H2 evolution. International Journal of Hydrogen Energy 2019, 44, 20042–20055. [Google Scholar] [CrossRef]
- Jang, D.; Lee, S.; Kwon, N. H.; et al. Preparation of carbon nitride nanotubes with P-doping and their photocatalytic properties for hydrogen evolution. Carbon 2023, 208, 290–302. [Google Scholar] [CrossRef]
- Adnan, M.; Lee, W.; Irshad, Z.; et al. Managing Interfacial Defects and Charge-Carriers Dynamics by a Cesium-Doped SnO2 for Air Stable Perovskite Solar Cells. Small 2024, 20, 2402268. [Google Scholar] [CrossRef]
- Wu, B. G.; Jiang, B. J.; Guo, C. L.; et al. Mild-Condition Photocatalytic Reforming of Methanol-Water by a Hierarchical, Asymmetry Carbon Nitride. Angewandte Chemie International Edition 2024, 64, 6525–6534. [Google Scholar] [CrossRef]
- Ma, M. M.; Li, J. Z.; Zhu, X. G.; et al. Enhancing multifunctional photocatalysis with acetate-assisted cesium doping and unlocking the potential of Z-scheme solar water splitting. Carbon Energy 2023, 6, e447. [Google Scholar] [CrossRef]
- Fang, H. X.; Guo, H.; Niu, C. G.; et al. Hollow tubular graphitic carbon nitride catalyst with adjustable nitrogen vacancy: Enhanced optical absorption and carrier separation for improving photocatalytic activity. Chemical Engineering Journal 2020, 402, 126185. [Google Scholar] [CrossRef]
- Du, J.; Li, S. M.; Du, Z. G.; et al. Boron/oxygen-codoped graphitic carbon nitride nanomesh for efficient photocatalytic hydrogen evolution. Chemical Engineering Journal 2021, 407, 127114. [Google Scholar] [CrossRef]
- Ali, A.; Tsuyoshi, M.; Sagiri, W.; et al. Determination of enantiomeric excess of carboxylates by fluorescent macrocyclic sensors. Chemical Science 2016, 7, 2016–2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




