Submitted:
29 May 2025
Posted:
30 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
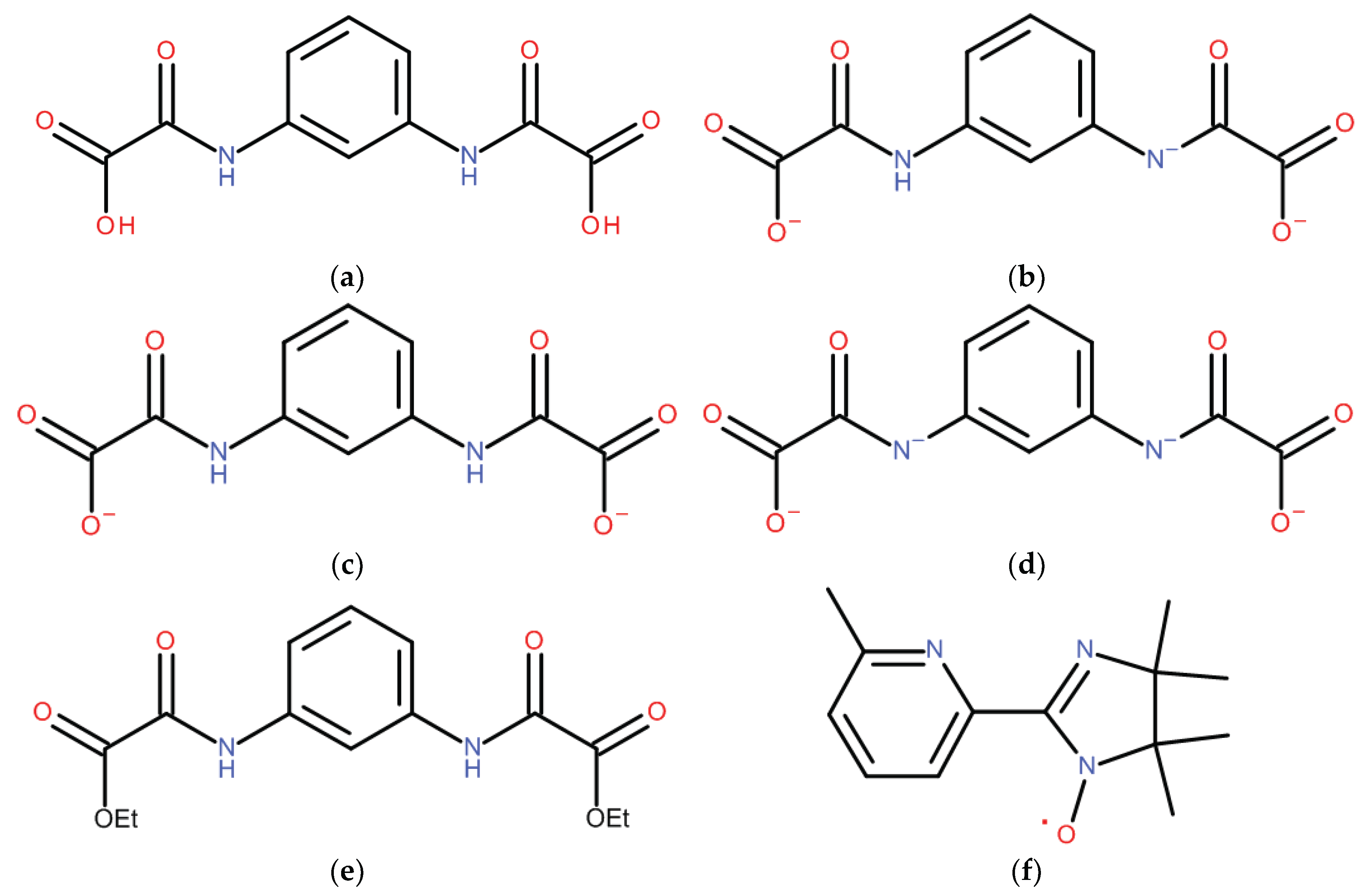
2.1. Synthesis and Characterization
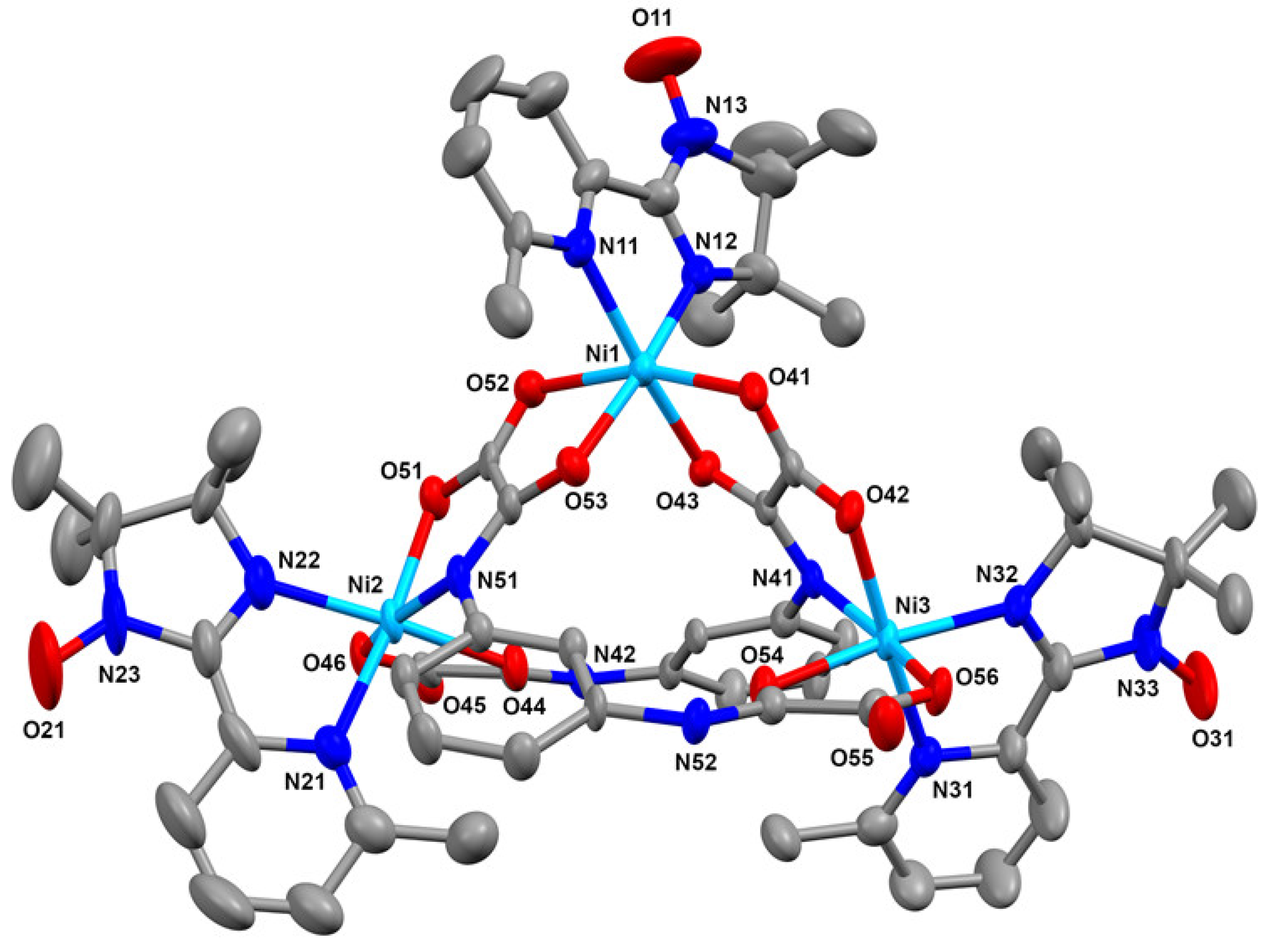
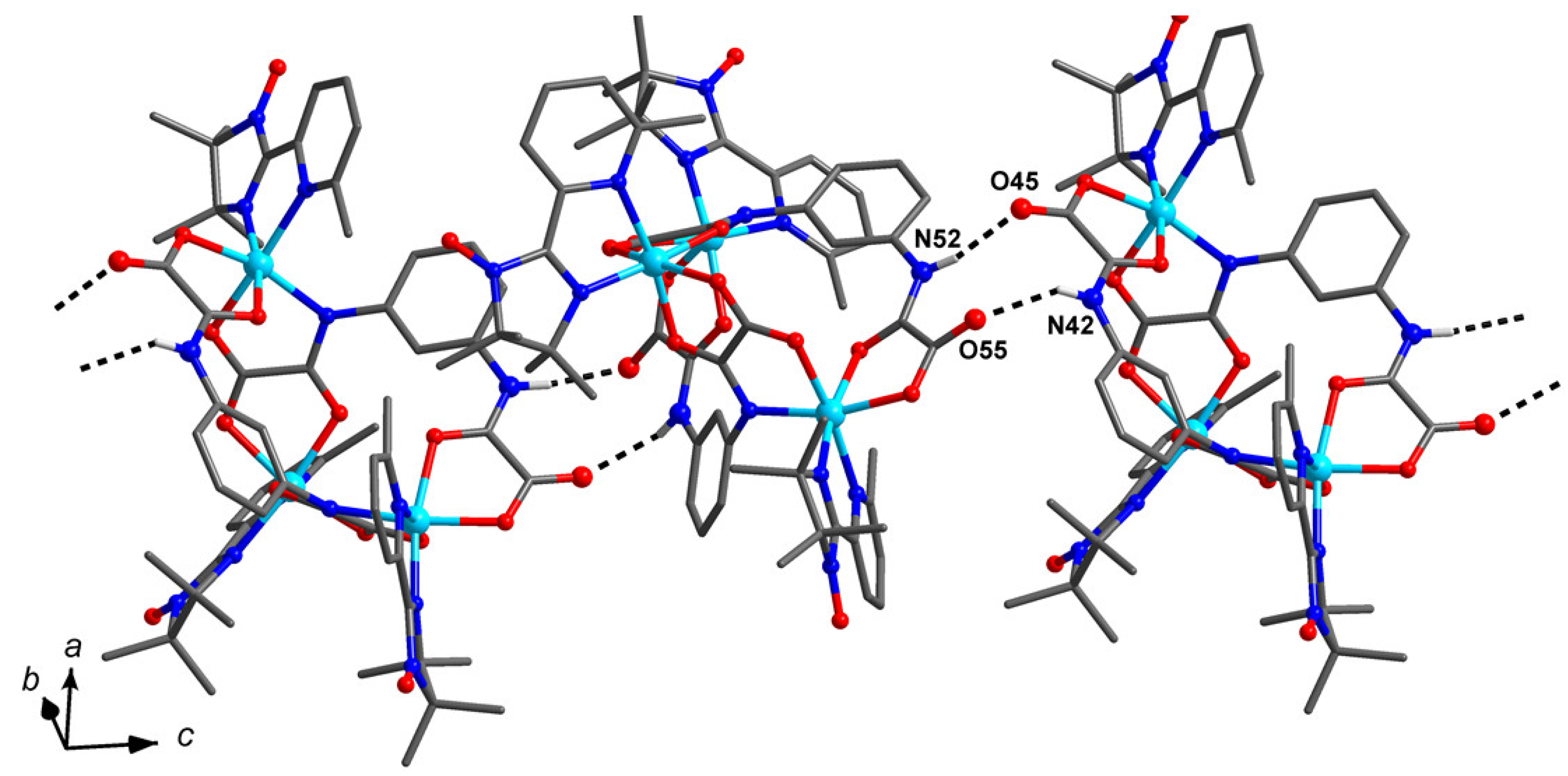
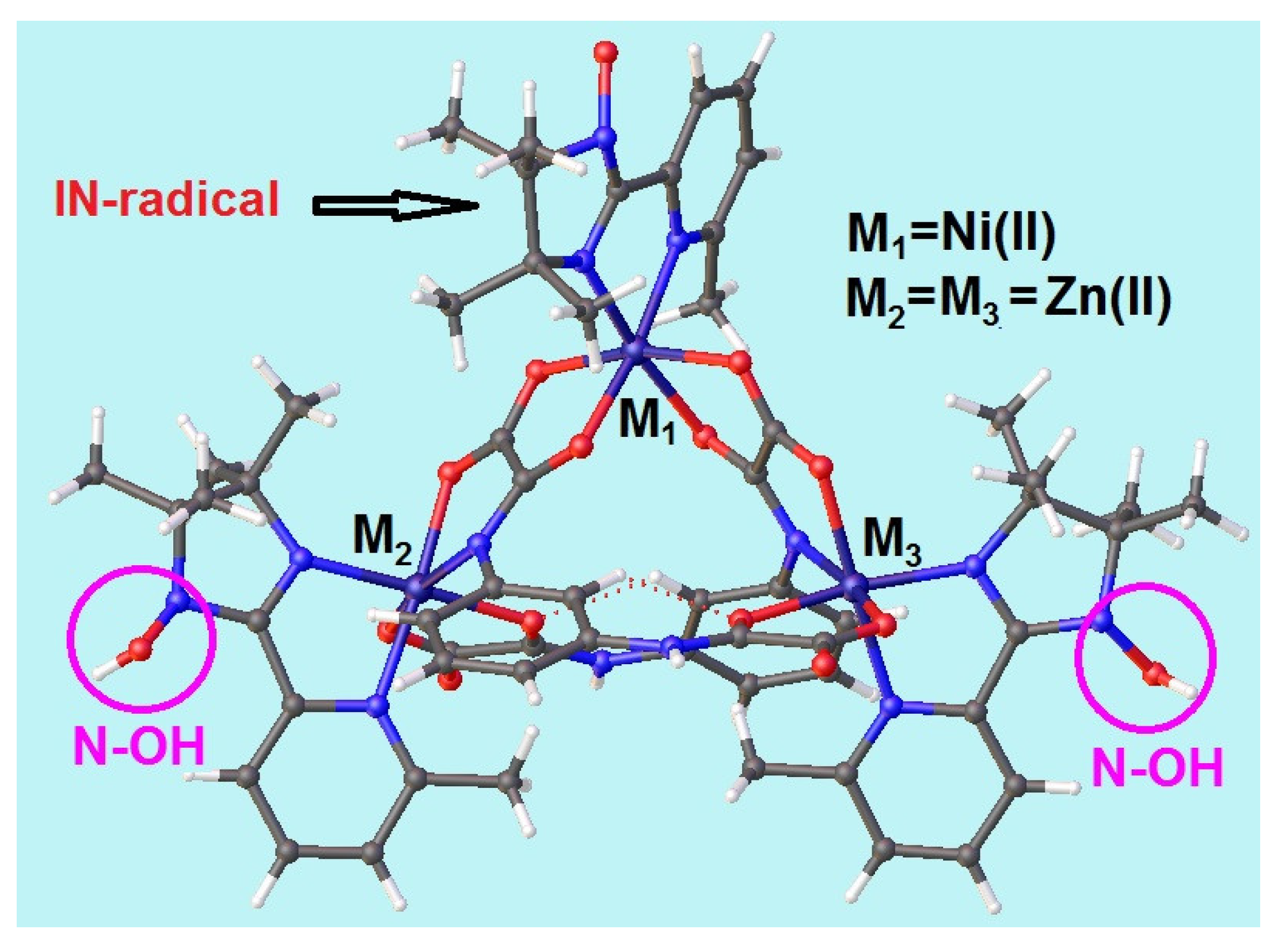
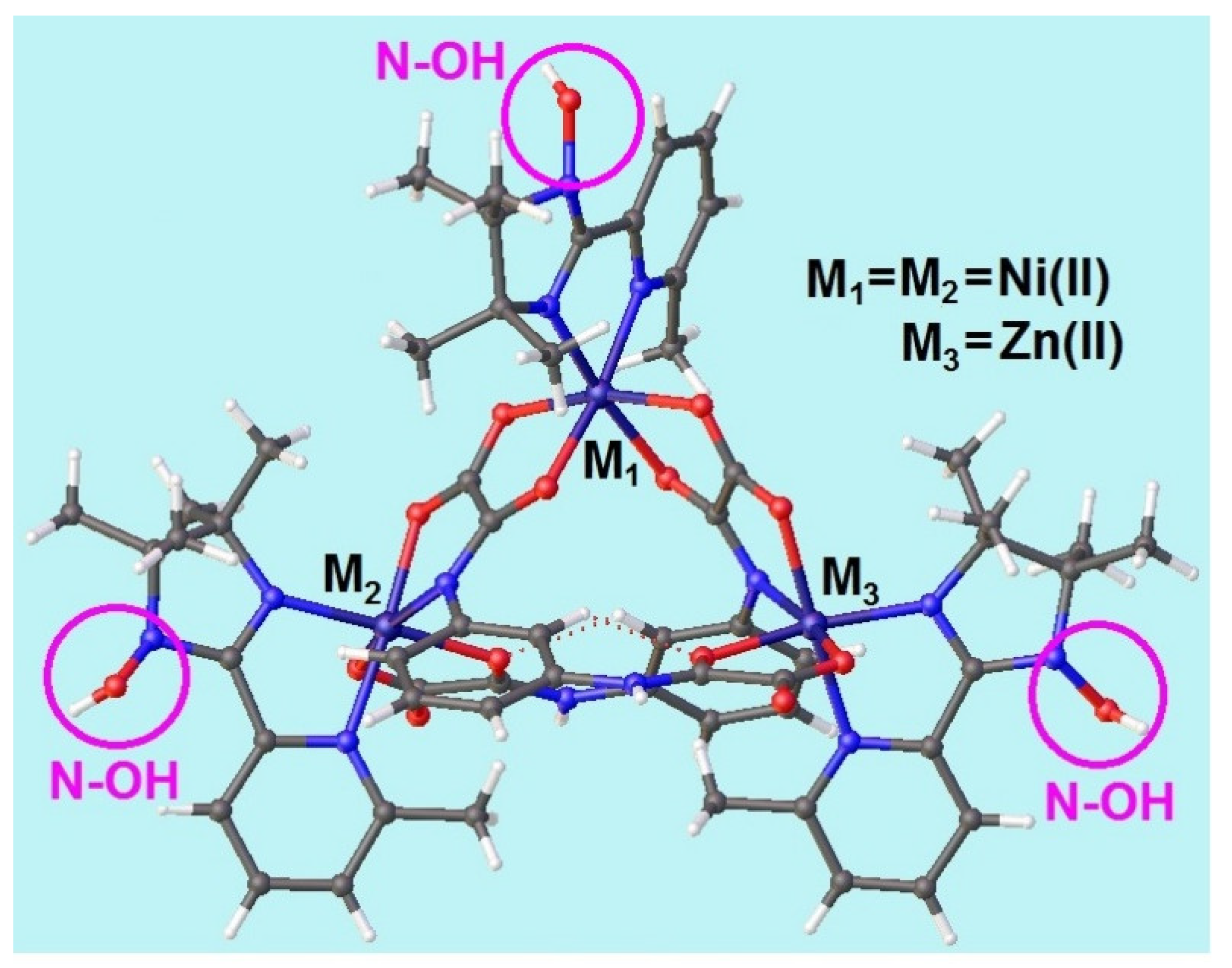
2.2. Crystal and Molecular Structure
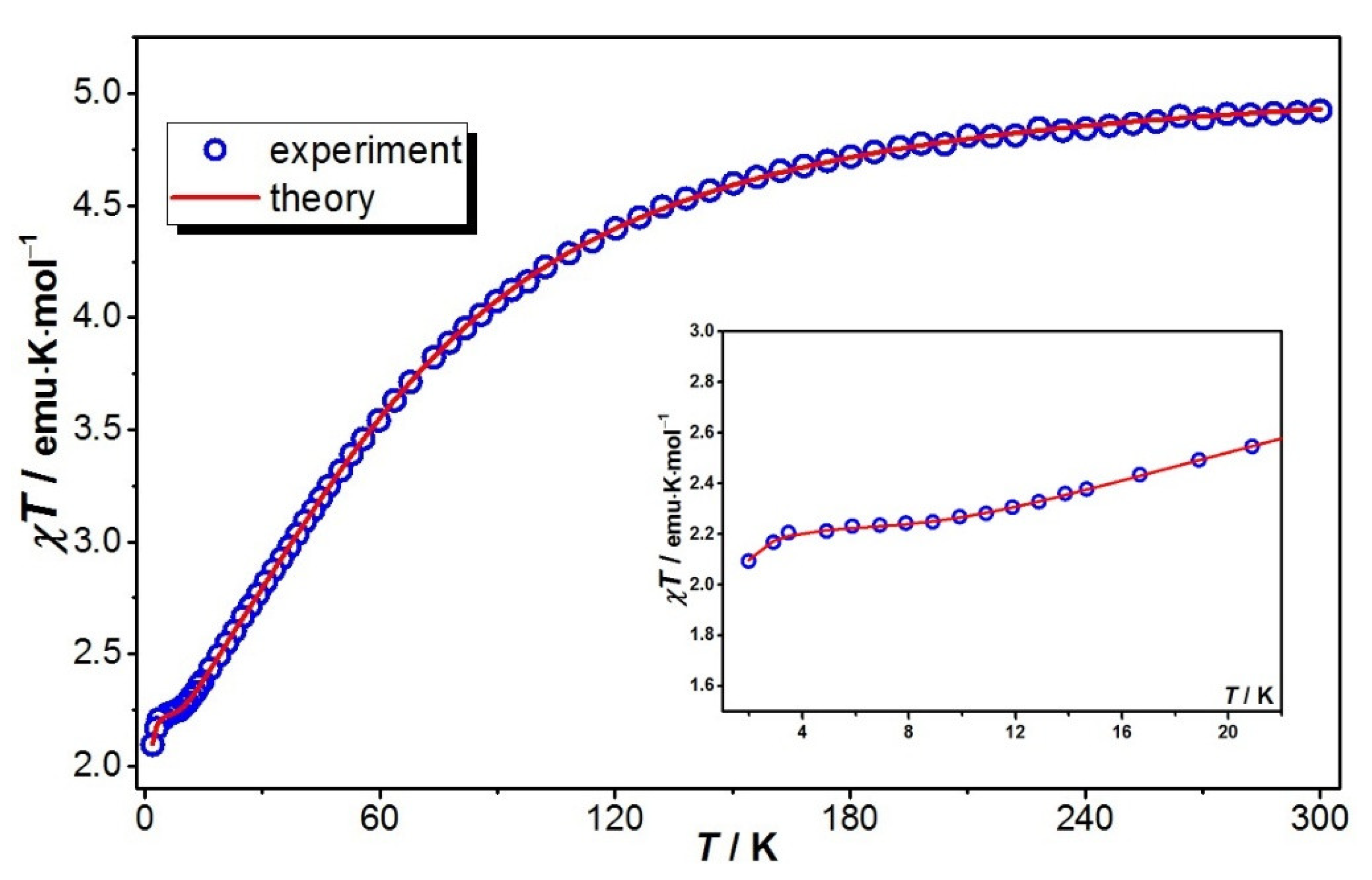
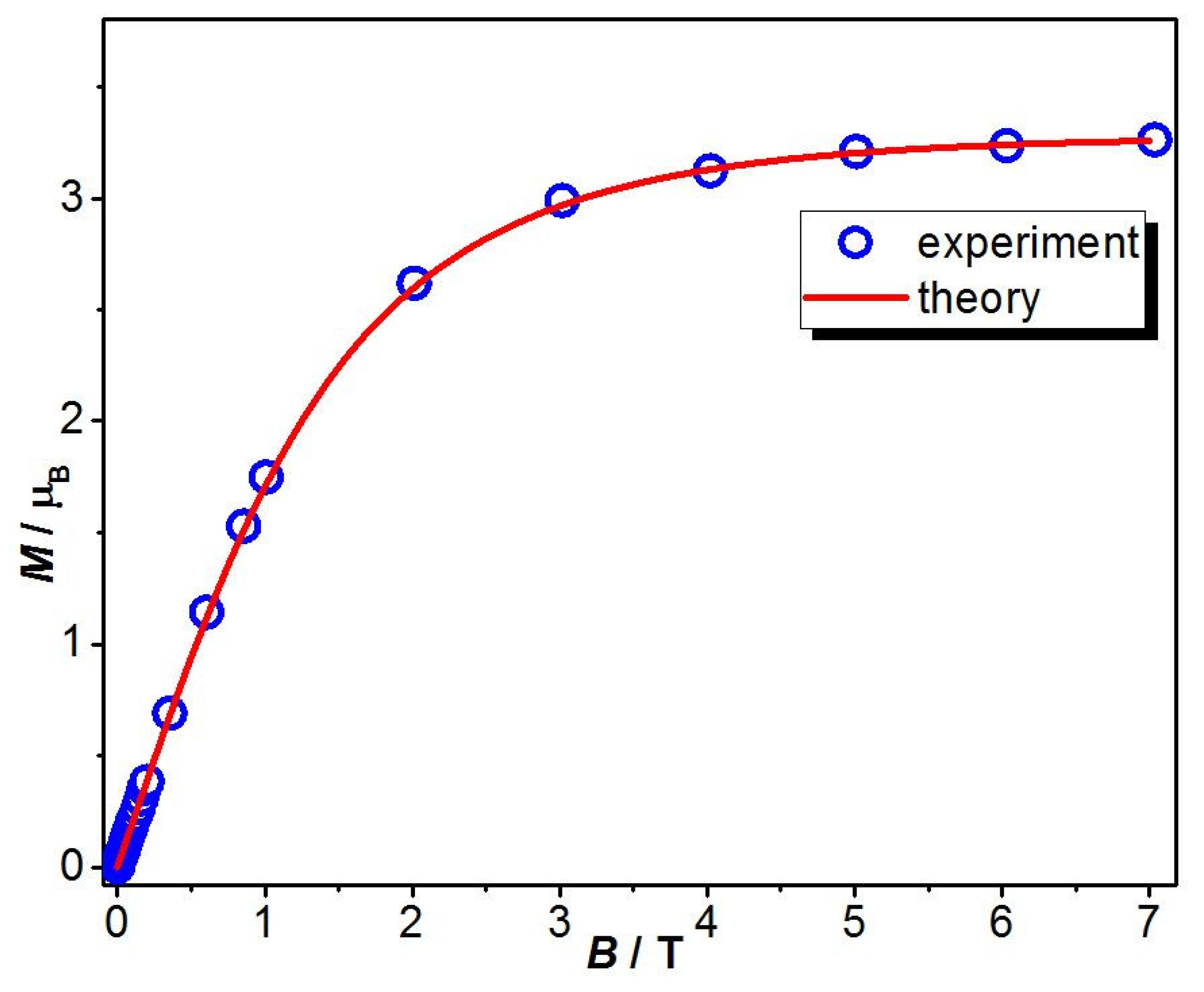
2.3. Magnetic Studies
2.3.1. Cryomagnetic Measurements
2.3. Magnetic Behavior Modeling and Theoretical Calculations
2.3.1. Theoretical Model and Exhaustive Parameter Set Required for Magnetic Data Simulation
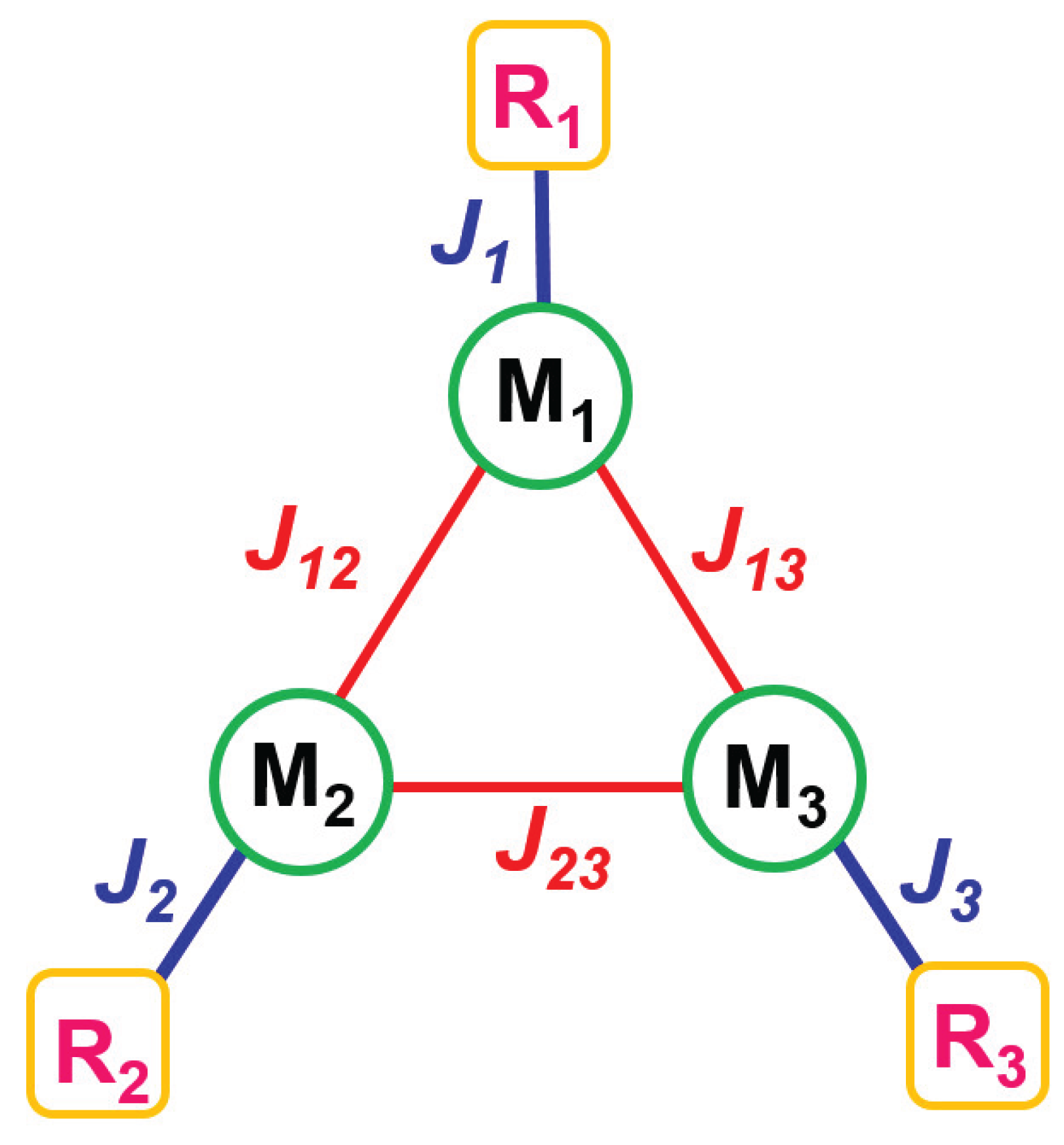
3. Materials and Methods
3.1. Instrumental and Physical Measurements
3.2. Theoretical Calculations
3.3. Preparations
3.3.1. Synthesis of [Ni3(L3−)2(IN)3]∙5CH3OH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhatt, V.; Ram, S. The Role of Ligands, Polytopic Ligands and Metal Organic Ligands (Mols) in Coordination Chemistry. Chem. Sci. Rev. Lett. 2015, 4, 414–428.
- Ruiz, R.; Faus, J.; Lloret, F.; Julve, M.; Journaux, Y. Coordination Chemistry of N,N′-Bis(Coordinating Group Substituted)Oxamides: A Rational Design of Nuclearity Tailored Polynuclear Complexes. Coord. Chem. Rev. 1999, 193–195, 1069–1117. [CrossRef]
- Pardo, E.; Ruiz-García, R.; Cano, J.; Ottenwaelder, X.; Lescouëzec, R.; Journaux, Y.; Lloret, F.; Julve, M. Ligand Design for Multidimensional Magnetic Materials: A Metallosupramolecular Perspective. Dalt. Trans. 2008, 2780. [CrossRef]
- Dul, M.-C.; Pardo, E.; Lescouëzec, R.; Journaux, Y.; Ferrando-Soria, J.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Cangussu, D.; et al. Supramolecular Coordination Chemistry of Aromatic Polyoxalamide Ligands: A Metallosupramolecular Approach toward Functional Magnetic Materials. Coord. Chem. Rev. 2010, 254, 2281–2296. [CrossRef]
- Journaux, Y.; Ferrando-Soria, J.; Pardo, E.; Ruiz-Garcia, R.; Julve, M.; Lloret, F.; Cano, J.; Li, Y.; Lisnard, L.; Yu, P.; et al. Design of Magnetic Coordination Polymers Built from Polyoxalamide Ligands: A Thirty Year Story. Eur. J. Inorg. Chem. 2018, 2018, 228–247. [CrossRef]
- Mariano, L. dos S.; Rosa, I.M.L.; De Campos, N.R.; Doriguetto, A.C.; Dias, D.F.; do Pim, W.D.; Valdo, A.K.S.M.; Martins, F.T.; Ribeiro, M.A.; De Paula, E.E.B.; et al. Polymorphic Derivatives of NiII and CoII Mesocates with 3D Networks and “Brick and Mortar” Structures: Preparation, Structural Characterization, and Cryomagnetic Investigation of New Single-Molecule Magnets. Cryst. Growth Des. 2020, 20, 2462–2476. [CrossRef]
- Simões, T.R.G.; do Pim, W.D.; Silva, I.F.; Oliveira, W.X.C.; Pinheiro, C.B.; Pereira, C.L.M.; Lloret, F.; Julve, M.; Stumpf, H.O. Solvent-Driven Dimensionality Control in Molecular Systems Containing CuII, 2,2′-Bipyridine and an Oxamato-Based Ligand. CrystEngComm 2013, 15, 10165. [CrossRef]
- Lisnard, L.; Chamoreau, L.-M.; Li, Y.; Journaux, Y. Solvothermal Synthesis of Oxamate-Based Helicate: Temperature Dependence of the Hydrogen Bond Structuring in the Solid. Cryst. Growth Des. 2012, 12, 4955–4962. [CrossRef]
- de Campos, N.R.; Simosono, C.A.; Landre Rosa, I.M.; da Silva, R.M.R.; Doriguetto, A.C.; do Pim, W.D.; Gomes Simões, T.R.; Valdo, A.K.S.M.; Martins, F.T.; Sarmiento, C. V.; et al. Building-up Host–Guest Helicate Motifs and Chains: A Magneto-Structural Study of New Field-Induced Cobalt-Based Single-Ion Magnets. Dalt. Trans. 2021, 50, 10707–10728. [CrossRef]
- Francescon, J.E.; de J Pfau, S.C.; Borth, K.W.; Marinho, M.V.; Pedroso, E.F.; Giese, S.O.K.; Pereira, C.L.M.; Miorim, A.J.F.; Maciel, G.M.; Hughes, D.L.; et al. Isomorphic Oxamate Derivatives Triple Mesocates: From the Synthesis to Antibacterial Activities. J. Mol. Struct. 2025, 1328, 141272. [CrossRef]
- Fernández, I.; Ruiz, R.; Faus, J.; Julve, M.; Lloret, F.; Cano, J.; Ottenwaelder, X.; Journaux, Y.; Muñoz, M.C. Ferromagnetic Coupling through Spin Polarization in a Dinuclear Copper(II) Metallacyclophane. Angew. Chemie Int. Ed. 2001, 40, 3039–3042. [CrossRef]
- Pardo, E.; Morales-Osorio, I.; Julve, M.; Lloret, F.; Cano, J.; Ruiz-García, R.; Pasán, J.; Ruiz-Pérez, C.; Ottenwaelder, X.; Journaux, Y. Magnetic Anisotropy of a High-Spin Octanuclear Nickel(II) Complex with a Meso -Helicate Core. Inorg. Chem. 2004, 43, 7594–7596. [CrossRef]
- Pardo, E.; Ruiz-García, R.; Lloret, F.; Julve, M.; Cano, J.; Pasán, J.; Ruiz-Pérez, C.; Filali, Y.; Chamoreau, L.-M.; Journaux, Y. Molecular-Programmed Self-Assembly of Homo- and Heterometallic Penta- and Hexanuclear Coordination Compounds: Synthesis, Crystal Structures, and Magnetic Properties of Ladder-Type Cu II 2 M II x (M = Cu, Ni; x = 3, 4) Oxamato Complexes with Cu II 2 Metall. Inorg. Chem. 2007, 46, 4504–4514. [CrossRef]
- Cangussu, D.; Pardo, E.; Dul, M.-C.; Lescouëzec, R.; Herson, P.; Journaux, Y.; Pedroso, E.F.; Pereira, C.L.M.; Stumpf, H.O.; Carmen Muñoz, M.; et al. Rational Design of a New Class of Heterobimetallic Molecule-Based Magnets: Synthesis, Crystal Structures, and Magnetic Properties of Oxamato-Bridged (M′=LiI and MnII; M=NiII and CoII) Open-Frameworks with a Three-Dimensional Honeycomb Architecture. Inorganica Chim. Acta 2008, 361, 3394–3402. [CrossRef]
- Pardo, E.; Cangussu, D.; Lescouëzec, R.; Journaux, Y.; Pasán, J.; Delgado, F.S.; Ruiz-Pérez, C.; Ruiz-García, R.; Cano, J.; Julve, M.; et al. Molecular-Programmed Self-Assembly of Homo- and Heterometallic Tetranuclear Coordination Compounds: Synthesis, Crystal Structures, and Magnetic Properties of Rack-Type Cu II 2 M II 2 Complexes (M = Cu and Ni) with Tetranucleating Phenylenedioxamato Bridgi. Inorg. Chem. 2009, 48, 4661–4673. [CrossRef]
- Dul, M.-C.; Ottenwaelder, X.; Pardo, E.; Lescouëzec, R.; Journaux, Y.; Chamoreau, L.-M.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F. Ferromagnetic Coupling by Spin Polarization in a Trinuclear Copper(II) Metallacyclophane with a Triangular Cage-Like Structure. Inorg. Chem. 2009, 48, 5244–5249. [CrossRef]
- Pardo, E.; Ferrando-Soria, J.; Dul, M.; Lescouëzec, R.; Journaux, Y.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Cañadillas-Delgado, L.; et al. Oligo- m -phenyleneoxalamide Copper(II) Mesocates as Electro-Switchable Ferromagnetic Metal–Organic Wires. Chem. – A Eur. J. 2010, 16, 12838–12851. [CrossRef]
- Dul, M.-C.; Lescouëzec, R.; Chamoreau, L.-M.; Journaux, Y.; Carrasco, R.; Castellano, M.; Ruiz-García, R.; Cano, J.; Lloret, F.; Julve, M.; et al. Self-Assembly, Metal Binding Ability, and Magnetic Properties of Dinickel(Ii) and Dicobalt(Ii) Triple Mesocates. CrystEngComm 2012, 14, 5639. [CrossRef]
- Oliveira, W.X.C.; Ribeiro, M.A.; Pinheiro, C.B.; da Costa, M.M.; Fontes, A.P.S.; Nunes, W.C.; Cangussu, D.; Julve, M.; Stumpf, H.O.; Pereira, C.L.M. Palladium(II)–Copper(II) Assembling with Bis(2-Pyridylcarbonyl)Amidate and Bis(Oxamate) Type Ligands. Cryst. Growth Des. 2015, 15, 1325–1335. [CrossRef]
- T. da Cunha, T.; X. C. Oliveira, W.; F. Pedroso, E.; Lloret, F.; Julve, M.; L. M. Pereira, C. Heterobimetallic One-Dimensional Coordination Polymers MICuII (M = Li and K) Based on Ferromagnetically Coupled Di- and Tetracopper(II) Metallacyclophanes. Magnetochemistry 2018, 4, 38. [CrossRef]
- Wojnar, M.K.; Laorenza, D.W.; Schaller, R.D.; Freedman, D.E. Nickel(II) Metal Complexes as Optically Addressable Qubit Candidates. J. Am. Chem. Soc. 2020, 142, 14826–14830. [CrossRef]
- Tlemsani, I.; Lambert, F.; Suaud, N.; Herrero, C.; Guillot, R.; Barra, A.-L.; Gambarelli, S.; Mallah, T. Assessing the Robustness of the Clock Transition in a Mononuclear S = 1 Ni(II) Complex Spin Qubit. J. Am. Chem. Soc. 2025, 147, 4685–4688. [CrossRef]
- Yamamoto, Y.; Suzuki, T.; Kaizaki, S. Syntheses, Structures, Magnetic, and Spectroscopic Properties of Cobalt(Ii), Nickel(Ii) and Zinc(Ii) Complexes Containing 2-(6-Methyl)Pyridyl-Substituted Nitronyl and Imino Nitroxide†. J. Chem. Soc. Dalt. Trans. 2001, 2943–2950. [CrossRef]
- Higashikawa, H.; Inoue, K.; Maryunina, K.Y.; Romanenko, G. V.; Bogomyakov, A.S.; Kuznetsova, O. V.; Fursova, E.Y.; Ovcharenko, V.I. Synthesis, Structure and Magnetic Properties of the Pentanuclear Complex [Fe2(CN)12Ni3(L)6]·27H2O, Where L Is Nitronyl Nitroxide. J. Struct. Chem. 2009, 50, 1155–1158. [CrossRef]
- Kuznetsova, О. V.; Romanenko, G. V.; Letyagin, G.A.; Bogomyakov, A.S. Binuclear Co(II), Ni(II), and Cu(II) Hexafluoroacetylacetonates with Spin-Labeled Nitrophenols. J. Struct. Chem. 2023, 64, 1470–1481. [CrossRef]
- Ovcharenko, V.; Kuznetsova, O.; Fursova, E.; Letyagin, G.; Romanenko, G.; Bogomyakov, A.; Zueva, E. Simultaneous Introduction of Two Nitroxides in the Reaction: A New Approach to the Synthesis of Heterospin Complexes. Inorg. Chem. 2017, 56, 14567–14576. [CrossRef]
- Luneau, D.; Rey, P.; Laugier, J.; Belorizky, E.; Cogne, A. Ferromagnetic Behavior of Nickel(II)-Imino Nitroxide Derivatives. Inorg. Chem. 1992, 31, 3578–3584. [CrossRef]
- Vostrikova, K.E.; Luneau, D.; Wernsdorfer, W.; Rey, P.; Verdaguer, M. A S = 7 Ground Spin-State Cluster Built from Three Shells of Different Spin Carriers Ferromagnetically Coupled, Transition-Metal Ions and Nitroxide Free Radicals. J. Am. Chem. Soc. 2000, 122, 718–719. [CrossRef]
- Tsukahara, Y.; Kamatani, T.; Iino, A.; Suzuki, T.; Kaizaki, S. Synthesis, Magnetic Properties, and Electronic Spectra of Bis(β-Diketonato)Chromium(III) and Nickel(II) Complexes with a Chelated Imino Nitroxide Radical: X-Ray Structures of [Cr(AcaMe)2(IM2py)]PF6 and [Ni(Acac)2(IM2py)]. Inorg. Chem. 2002, 41, 4363–4370. [CrossRef]
- Babailov, S.P.; Peresypkina, E. V.; Journaux, Y.; Vostrikova, K.E. Nickel(II) Complex of a Biradical: Structure, Magnetic Properties, High NMR Temperature Sensitivity and Moderately Fast Molecular Dynamics. Sensors Actuators B Chem. 2017, 239, 405–412. [CrossRef]
- Hayakawa, K.; Shiomi, D.; Ise, T.; Sato, K.; Takui, T. Stable Iminonitroxide Biradical in the Triplet Ground State. Chem. Lett. 2004, 33, 1494–1495. [CrossRef]
- Zhi-Liang, L.; Li-Cun, L.; Dai-Zheng, L.; Zong-Hui, J.; Shi-Ping, Y.; Zhi-Liang, L.; Li-Cun, L.; Dai-Zheng, L.; Zong-Hui, J.; Shi-Ping, Y. Exchange Interaction of a Novel Heterospin Polynuclear Complex Containing Transition Metals and Imino Nitroxide Radicals: { [(CuL)Nid (IM-2Py) 2 ] (C10 4) 2 } 2 ·2H 2 O. Chinese J. Chem. 2003, 21, 133–138. [CrossRef]
- Oshio, H.; Yamamoto, M.; Ito, T.; Kawauchi, H.; Koga, N.; Ikoma, T.; Tero-Kubota, S. Experimental and Theoretical Studies on Ferromagnetically Coupled Metal Complexes with Imino Nitroxides. Inorg. Chem. 2001, 40, 5518–5525. [CrossRef]
- Petrov, P.A.; Romanenko, G. V.; Shvedenkov, Y.G.; Ikorskii, V.N.; Ovcharenko, V.I.; Reznikov, V.A.; Sagdeev, R.Z. Complexes of Cu II, Ni II, and Co II with 2-Cyano-2-(1-Oxyl-4,4,5,5-Tetramethyl-4,5-Dihydro-1H-Imidazol-2-Yl)-1-R-Ethylenolates. Russ. Chem. Bull. 2004, 53, 99–108. [CrossRef]
- Yamamoto, Y.; Suzuki, T.; Kaizaki, S. Crystal Structures, Magnetic and Spectroscopic Properties of Manganese(II), Cobalt(II), Nickel(II) and Zinc(II) Dichloro Complexes Bearing Two 2-Pyridyl-Substituted Imino Nitroxides †. J. Chem. Soc. Dalt. Trans. 2001, 1566–1572. [CrossRef]
- Kuznetsova, O. V.; Romanenko, G. V.; Bogomyakov, A.S.; Ovcharenko, V.I. Synthesis, Structure, and Magnetic Properties of Heterospin Polymers MI [MII(Hfac)L2]. Russ. J. Coord. Chem. 2020, 46, 521–527. [CrossRef]
- Spek, A.L. CheckCIF Validation ALERTS: What They Mean and How to Respond. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1–11. [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [CrossRef]
- Shoji, M.; Koizumi, K.; Kitagawa, Y.; Kawakami, T.; Yamanaka, S.; Okumura, M.; Yamaguchi, K. A General Algorithm for Calculation of Heisenberg Exchange Integrals J in Multispin Systems. Chem. Phys. Lett. 2006, 432, 343–347. [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152. [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A Powerful New Program for the Analysis of Anisotropic Monomeric and Exchange-coupled Polynuclear d - and f -block Complexes. J. Comput. Chem. 2013, 34, 1164–1175. [CrossRef]
- Wright, J.B.; Hall, C.M.; Johnson, H.G. N,N’-(Phenylene)Dioxamic Acids and Their Esters as Antiallergy Agents. J. Med. Chem. 1978, 21, 930–935. [CrossRef]
- Blay, G.; Fernández, I.; Pedro, J.R.; Ruiz-García, R.; Muñoz, M.C.; Cano, J.; Carrasco, R. A Hydrogen-Bonded Supramolecular Meso-Helix. European J. Org. Chem. 2003, 2003, 1627–1630. [CrossRef]
| DFT level1 | J1 | J2 | J3 | J12 | J13 |
|---|---|---|---|---|---|
| B3LYP | 157.4 | 179.5 | 146.1 | −26.6 | −26.3 |
| TPSSh | 209.6 | 231.4 | 188.4 | −37.5 | −37.3 |
| cam-B3LYP | 116.3 | 137.9 | 112.3 | −17.8 | −17.4 |
| LC-BLYP | 131.4 | 155.0 | 125.6 | −25.7 | −25.6 |
| wB97m-v | 98.2 | 116.5 | 95.9 | −14.6 | −14.4 |
| CAS(n,m) roots(Q,D) |
E(Q)–E(D) CASSCF |
J1 CASSCF |
E(Q)–E(D) NEVPT2 |
J1 NEVPT2 |
|---|---|---|---|---|
| 13,9)/(2, 2) | -95.9 | 32.0 | -108.6 | 36.2 |
| CAS(n,m) roots(Q,T,S) |
E (Singlet) | E (Triplet) | E(Quintet) | J12 |
|---|---|---|---|---|
| cas (10,9)/(2,2,2)) | 0 | 3.5 | 10.6 | −1.75 |
| NEVPT2 | 0 | 7.7 | 21.3 | −3.85 |
| SH para-meter | Method | ||||||
| cas(8,5)1 | NEVPT22 | B3LYP3 | TPSSh3 | cam-B3LYP3 | LC-BLYP3 | wB97m-v3 | |
| g | 2.29 | 2.22 | 2.17 | 2.12 | 2.18 | 2.15 | 2.14 |
| D | 7.43 | 4.92 | 2.49 | 1.83 | 2.01 | 8.07 | 3.20 |
| E/D | 0.069 | 0.087 | 0.328 | 0.183 | 0.297 | 0.243 | 0.162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





