Submitted:
28 May 2025
Posted:
29 May 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- Indication for elective AVR with a bioprosthesis (via full sternotomy, mini-sternotomy, or right anterior mini-thoracotomy)
- Age > 65 years
- Severe AS with New York Heart Association (NYHA) class ≥ II symptoms
- Critical aortic stenosis on preoperative echocardiography: aortic valve area ≤ 1.0 cm² (or indexed area < 0.6 cm²/m²), mean gradient > 40 mmHg, peak velocity > 4 m/s, or Doppler velocity index < 0.25
- Sinotubular junction to annulus diameter ratio < 1.3
- Aortic root dimensions suitable for a Perceval valve (annulus 19–27 mm)
- Signed informed consent.
- Indication for elective AVR with a bioprosthesis (via full sternotomy, mini-sternotomy, or right anterior mini-thoracotomy)
- Age > 65 years
- Severe AS with NYHA class ≥ II symptoms
- Critical aortic stenosis on preoperative echocardiography: aortic valve area ≤ 1.0 cm² (or indexed area < 0.6 cm²/m²), mean gradient > 40 mmHg, peak velocity > 4 m/s, or Doppler velocity index < 0.25
- Sinotubular junction to annulus diameter ratio < 1.3
- Aortic root dimensions suitable for a Perceval valve (annulus 19–27 mm)
- Signed informed consent.
2.1. Operative Technique
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Aortic Cross-Clamp |
| AS | Aortic Stenosis |
| AVR | Aortic Valve Replacement |
| AVR + CABG | Aortic Valve Replacement plus Coronary Artery Bypass Grafting |
| BMI | Body Mass Index |
| BSA | Body Surface Area |
| CABG | Coronary Artery Bypass Grafting |
| CPB | Cardiopulmonary Bypass |
| CT or MDCT | (Multi-Detector) Computed Tomography |
| DVI | Doppler Velocity Index |
| EF / LVEF | Ejection Fraction / Left Ventricular Ejection Fraction |
| ICU | Intensive Care Unit |
| LVEF | Left Ventricular Ejection Fraction |
| NYHA | New York Heart Association |
| SD | Standard Deviation |
| STS | Society of Thoracic Surgeons |
| SU-AVR | Sutureless Aortic Valve Replacement |
| TEE | Transesophageal Echocardiography |
| TTE | Transthoracic Echocardiography |
| Vmax | Peak Aortic Jet Velocity |
| ACC | Aortic Cross-Clamp |
References
- Carabello, B.A.; Paulus, W.J. Aortic stenosis. Lancet 2009, 373, 956-966.
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O'Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014, 63, e57-185. [CrossRef]
- Khot, U.N.; Novaro, G.M.; Popović, Z.B.; Mills, R.M.; Thomas, J.D.; Tuzcu, E.M.; Hammer, D.; Nissen, S.E.; Francis, G.S. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med 2003, 348, 1756-1763. [CrossRef]
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; De Bonis, M.; Evangelista, A.; et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012, 42, S1-44. [CrossRef]
- Edwards, W.S.; Smith, L. Aortic valve replacement with a subcoronary ball valve. Surg Forum 1958, 9, 309-313.
- Berretta, P.; Andreas, M.; Meuris, B.; Langenaeken, T.; Solinas, M.; Concistrè, G.; Kappert, U.; Arzt, S.; Santarpino, G.; Nicoletti, A.; et al. Sutureless and rapid deployment versus sutured aortic valve replacement: a propensity-matched comparison from the Sutureless and Rapid Deployment International Registry. Eur J Cardiothorac Surg 2022, 62. [CrossRef]
- Sá, M.P.; Jabagi, H.; Dokollari, A.; Awad, A.K.; Van den Eynde, J.; Malin, J.H.; Sicouri, S.; Torregrossa, G.; Ruhparwar, A.; Weymann, A.; et al. Early and late outcomes of surgical aortic valve replacement with sutureless and rapid-deployment valves versus transcatheter aortic valve implantation: Meta-analysis with reconstructed time-to-event data of matched studies. Catheter Cardiovasc Interv 2022, 99, 1886-1896. [CrossRef]
- Powell, R.; Pelletier, M.P.; Chu, M.W.A.; Bouchard, D.; Melvin, K.N.; Adams, C. The Perceval Sutureless Aortic Valve: Review of Outcomes, Complications, and Future Direction. Innovations (Phila) 2017, 12, 155-173. [CrossRef]
- Phan, K.; Tsai, Y.C.; Niranjan, N.; Bouchard, D.; Carrel, T.P.; Dapunt, O.E.; Eichstaedt, H.C.; Fischlein, T.; Gersak, B.; Glauber, M.; et al. Sutureless aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015, 4, 100-111. [CrossRef]
- Shrestha, M.; Folliguet, T.; Meuris, B.; Dibie, A.; Bara, C.; Herregods, M.C.; Khaladj, N.; Hagl, C.; Flameng, W.; Laborde, F.; et al. Sutureless Perceval S aortic valve replacement: a multicenter, prospective pilot trial. J Heart Valve Dis 2009, 18, 698-702.
- Müller, H.; Szalkiewicz, P.; Benedikt, P.; Ratschiller, T.; Schachner, B.; Schröckenstein, S.; Zierer, A. Single-center real-world data and technical considerations from 100 consecutive patients treated with the Perceval aortic bioprosthesis. Frontiers in Cardiovascular Medicine 2024, Volume 11 - 2024. [CrossRef]
- Lamberigts, M.; Szecel, D.; Rega, F.; Verbrugghe, P.; Dubois, C.; Meuris, B. Sutureless aortic valves in isolated and combined procedures: Thirteen years of experience in 784 patients. The Journal of Thoracic and Cardiovascular Surgery 2024, 167, 1724-1732.e1721. [CrossRef]
- Englberger, L.; Carrel, T.P.; Doss, M.; Sadowski, J.; Bartus, K.; Eckstein, F.F.; Asch, F.M.; Martens, S. Clinical performance of a sutureless aortic bioprosthesis: five-year results of the 3f Enable long-term follow-up study. J Thorac Cardiovasc Surg 2014, 148, 1681-1687. [CrossRef]
- Nguyen, A.; Fortin, W.; Mazine, A.; Bouchard, D.; Carrier, M.; El Hamamsy, I.; Lamarche, Y.; Demers, P. Sutureless aortic valve replacement in patients who have bicuspid aortic valve. The Journal of Thoracic and Cardiovascular Surgery 2015, 150, 851-857.
- Flameng, W.; Herregods, M.C.; Hermans, H.; Van der Mieren, G.; Vercalsteren, M.; Poortmans, G.; Van Hemelrijck, J.; Meuris, B. Effect of sutureless implantation of the Perceval S aortic valve bioprosthesis on intraoperative and early postoperative outcomes. J Thorac Cardiovasc Surg 2011, 142, 1453-1457. [CrossRef]
- Sian, K.; Li, S.; Selvakumar, D.; Mejia, R. Early results of the Sorin® Perceval S sutureless valve: systematic review and meta-analysis. Journal of Thoracic Disease 2017, 9, 711-724.
- Niinami, H.; Sawa, Y.; Shimokawa, T.; Domoto, S.; Nakamura, Y.; Sakaguchi, T.; Ito, T.; Toda, K.; Amano, A.; Gersak, B. 1-year outcomes of patients implanted with the Perceval sutureless valve: the Japanese post-marketing surveillance study. Heart Vessels 2023, 38, 949-956. [CrossRef]
- Schizas, N.; Samiotis, I.; Nazou, G.; Iliopoulos, D.C.; Anagnostopoulos, I.; Kousta, M.; Papaioannou, N.; Argiriou, M.; Dedeilias, P. Perceval-S over time. Clinical outcomes after ten years of usage. Journal of Cardiothoracic Surgery 2024, 19, 192. [CrossRef]
- Concistrè, G.; Santarpino, G.; Pfeiffer, S.; Farneti, P.; Miceli, A.; Chiaramonti, F.; Solinas, M.; Glauber, M.; Fischlein, T. Two alternative sutureless strategies for aortic valve replacement: a two-center experience. Innovations (Phila) 2013, 8, 253-257. [CrossRef]
- Martens, S.; Sadowski, J.; Eckstein, F.S.; Bartus, K.; Kapelak, B.; Sievers, H.H.; Schlensak, C.; Carrel, T. Clinical experience with the ATS 3f Enable® Sutureless Bioprosthesis. Eur J Cardiothorac Surg 2011, 40, 749-755. [CrossRef]
- Miceli, A.; Gilmanov, D.; Murzi, M.; Marchi, F.; Ferrarini, M.; Cerillo, A.G.; Quaini, E.; Solinas, M.; Berti, S.; Glauber, M. Minimally invasive aortic valve replacement with a sutureless valve through a right anterior mini-thoracotomy versus transcatheter aortic valve implantation in high-risk patients. Eur J Cardiothorac Surg 2016, 49, 960-965. [CrossRef]
- Shrestha, M.; Maeding, I.; Höffler, K.; Koigeldiyev, N.; Marsch, G.; Siemeni, T.; Fleissner, F.; Haverich, A. Aortic valve replacement in geriatric patients with small aortic roots: are sutureless valves the future? Interact Cardiovasc Thorac Surg 2013, 17, 778-782; discussion 782. [CrossRef]
- Cerillo, A.G.; Amoretti, F.; Mariani, M.; Cigala, E.; Murzi, M.; Gasbarri, T.; Solinas, M.; Chiappino, D. Increased Gradients After Aortic Valve Replacement With the Perceval Valve: The Role of Oversizing. Ann Thorac Surg 2018, 106, 121-128. [CrossRef]
- Laborde, F.; Fischlein, T.; Hakim-Meibodi, K.; Misfeld, M.; Carrel, T.; Zembala, M.; Madonna, F.; Meuris, B.; Haverich, A.; Shrestha, M. Clinical and haemodynamic outcomes in 658 patients receiving the Perceval sutureless aortic valve: early results from a prospective European multicentre study (the Cavalier Trial)†. Eur J Cardiothorac Surg 2016, 49, 978-986. [CrossRef]
- Erfe, J.M.; Malaisrie, S.C.; Andrei, A.C.; Pham, D.T.; Churyla, A.; Kruse, J.; Piotter, C.; Xu, Y.; McCarthy, P.M. Outcomes of Sutureless/Rapid Deployment Valves Compared to Traditional Bioprosthetic Aortic Valves. Ann Thorac Surg 2021, 111, 1884-1891. [CrossRef]
- Berretta, P.; Andreas, M.; Carrel, T.P.; Solinas, M.; Teoh, K.; Fischlein, T.; Santarpino, G.; Folliguet, T.; Villa, E.; Meuris, B.; et al. Minimally invasive aortic valve replacement with sutureless and rapid deployment valves: a report from an international registry (Sutureless and Rapid Deployment International Registry)†. Eur J Cardiothorac Surg 2019, 56, 793-799. [CrossRef]
- Folliguet, T.A.; Laborde, F.; Zannis, K.; Ghorayeb, G.; Haverich, A.; Shrestha, M. Sutureless perceval aortic valve replacement: results of two European centers. Ann Thorac Surg 2012, 93, 1483-1488. [CrossRef]
- Santarpino, G.; Pfeiffer, S.; Concistrè, G.; Fischlein, T. A supra-annular malposition of the Perceval S sutureless aortic valve: the 'χ-movement' removal technique and subsequent reimplantation. Interact Cardiovasc Thorac Surg 2012, 15, 280-281. [CrossRef]
- Gilmanov, D.; Miceli, A.; Ferrarini, M.; Farneti, P.; Murzi, M.; Solinas, M.; Glauber, M. Aortic valve replacement through right anterior minithoracotomy: can sutureless technology improve clinical outcomes? Ann Thorac Surg 2014, 98, 1585-1592. [CrossRef]
- Glauber, M.; Di Bacco, L.; Cuenca, J.; Di Bartolomeo, R.; Baghai, M.; Zakova, D.; Fischlein, T.; Troise, G.; Viganò, G.; Solinas, M. Minimally Invasive Aortic Valve Replacement with Sutureless Valves: Results From an International Prospective Registry. Innovations (Phila) 2020, 15, 120-130. [CrossRef]
- Moscarelli, M.; Santarpino, G.; Athanasiou, T.; Mastroroberto, P.; Fattouch, K.; Nasso, G.; Speziale, G. A pooled analysis of pacemaker implantation after Perceval sutureless aortic valve replacement. Interact Cardiovasc Thorac Surg 2021, 33, 501-509. [CrossRef]
- Fischlein, T.; Folliguet, T.; Meuris, B.; Shrestha, M.L.; Roselli, E.E.; McGlothlin, A.; Kappert, U.; Pfeiffer, S.; Corbi, P.; Lorusso, R. Sutureless versus conventional bioprostheses for aortic valve replacement in severe symptomatic aortic valve stenosis. J Thorac Cardiovasc Surg 2021, 161, 920-932. [CrossRef]
- Lorusso, R.; Folliguet, T.; Shrestha, M.; Meuris, B.; Kappetein, A.P.; Roselli, E.; Klersy, C.; Nozza, M.; Verhees, L.; Larracas, C.; et al. Sutureless versus Stented Bioprostheses for Aortic Valve Replacement: The Randomized PERSIST-AVR Study Design. Thorac Cardiovasc Surg 2020, 68, 114-123. [CrossRef]
- Szecel, D.; Eurlings, R.; Rega, F.; Verbrugghe, P.; Meuris, B. Perceval Sutureless Aortic Valve Implantation: Midterm Outcomes. Ann Thorac Surg 2021, 111, 1331-1337. [CrossRef]
- Sievers, H.H.; Schmidtke, C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007, 133, 1226-1233. [CrossRef]
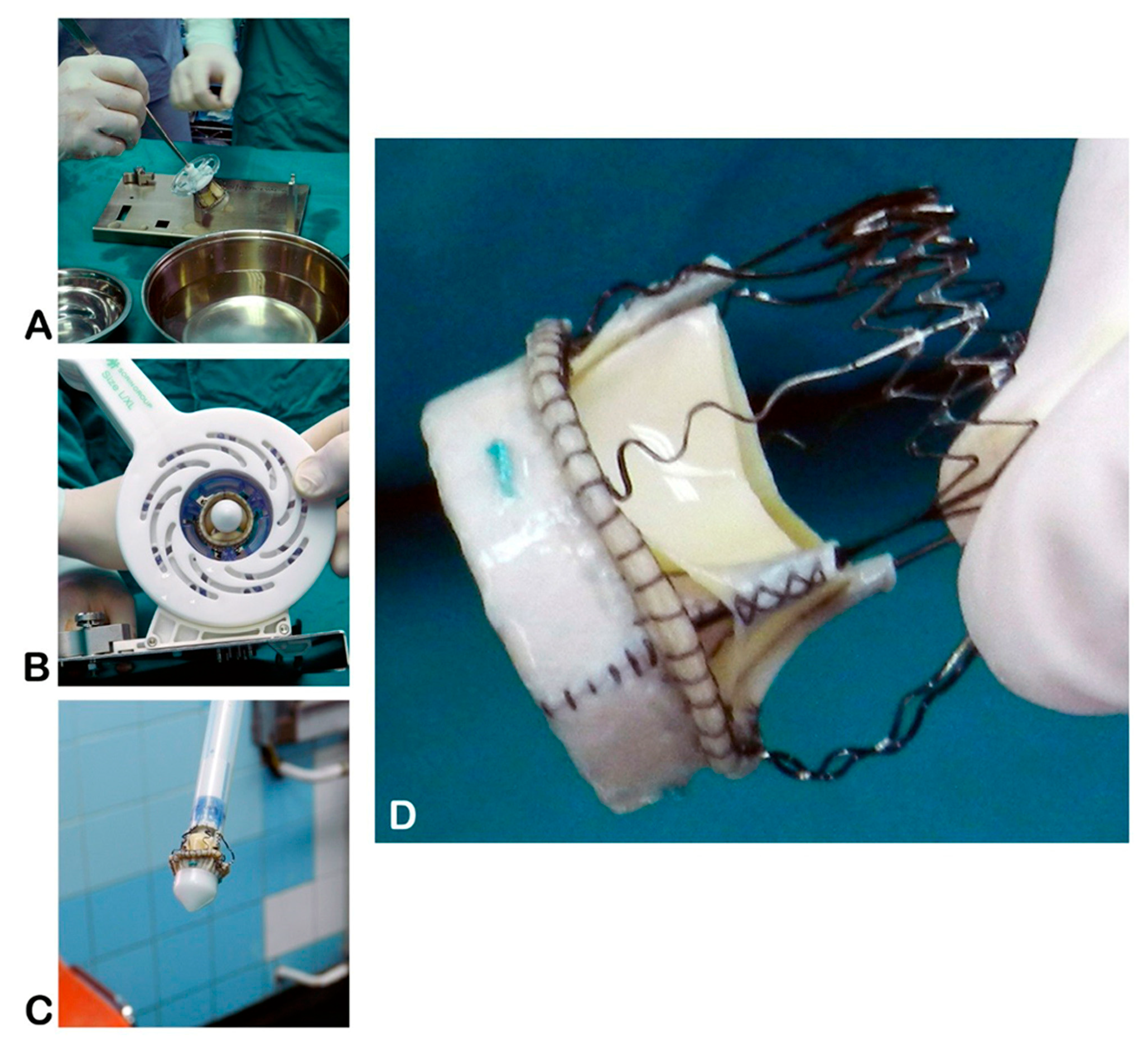
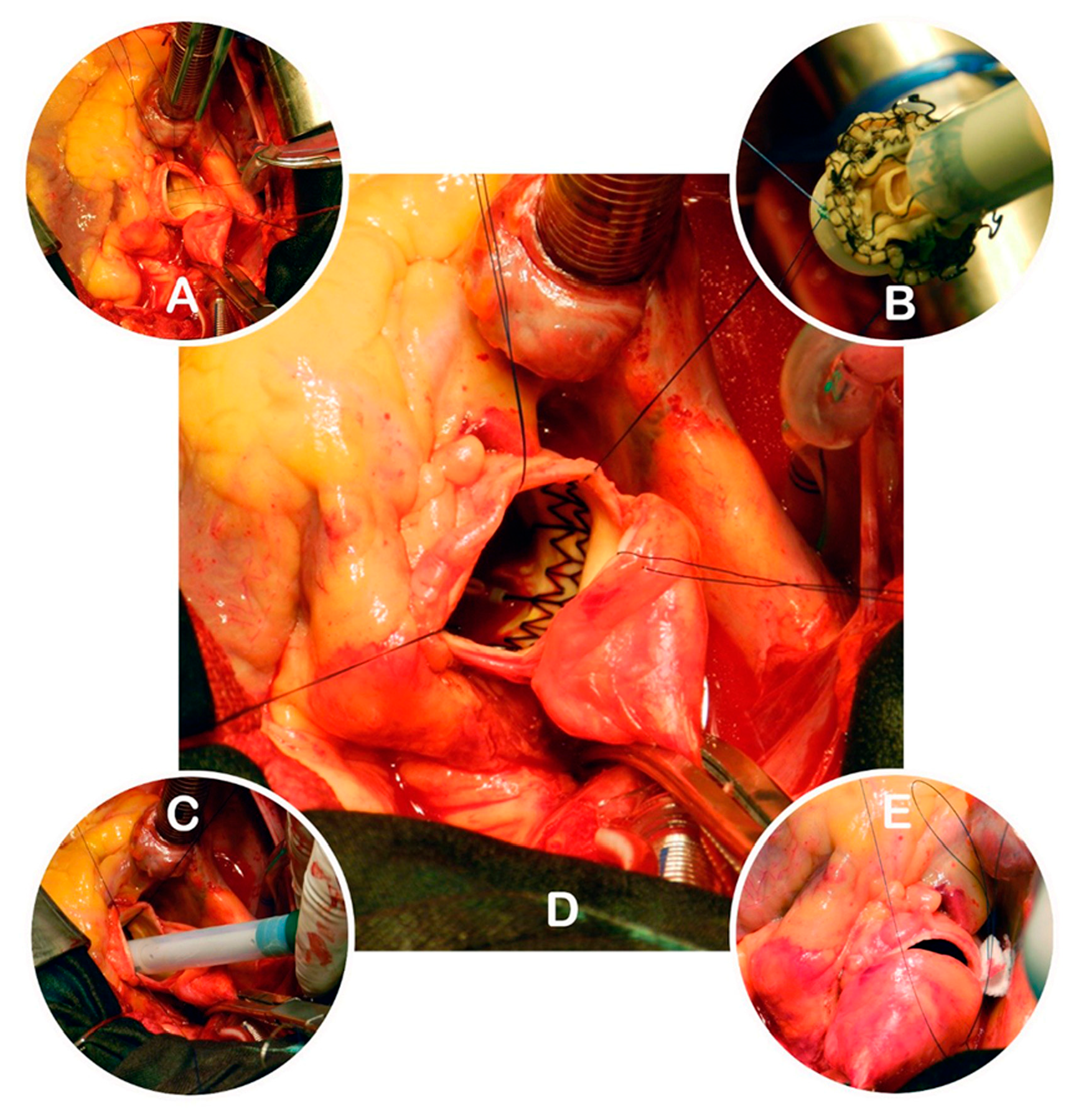
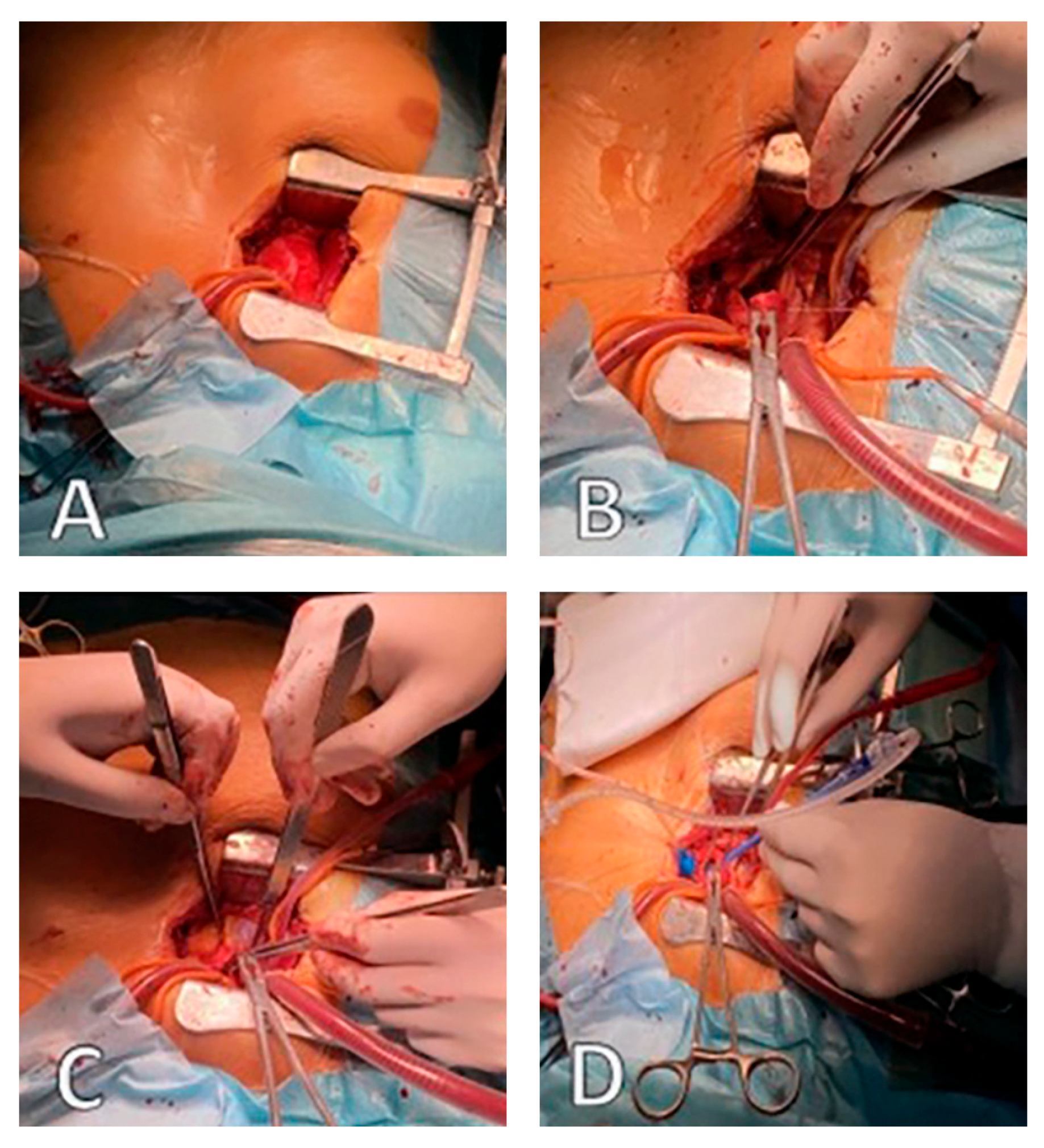
| Characteristic | Sutureless (N = 74) | Stented (N = 159) | p-value |
|---|---|---|---|
| Age (years) | 72.61 ± 7.21 | 72.67 ± 7.19 | > 0.05 |
| Female, n (%) | 34 (45.9%) | 69 (43.4%) | > 0.05 |
| Male, n (%) | 40 (54.1%) | 90 (56.6%) | > 0.05 |
| Weight (kg) | 77.92 ± 8.40 | 75.82 ± 7.60 | > 0.05 |
| Height (cm) | 165.60 ± 14.50 | 166.86 ± 15.22 | > 0.05 |
| BMI (kg/m²) | 26.6 ± 4.67 | 28.3 ± 11.53 | > 0.05 |
| BSA (m²) | 1.81 ± 0.14 | 1.92 ± 0.19 | > 0.05 |
| Procedure: AVR (isolated) | 61 (82%) | 127 (80%) | > 0.05 |
| Procedure: AVR + CABG | 13 (18%) | 32 (20%) | > 0.05 |
| Peak transvalvular gradient (mmHg) | 76 ± 26 | 74 ± 23 | > 0.05 |
| Mean transvalvular gradient (mmHg) | 52 ± 17 | 52 ± 17 | > 0.05 |
| Aortic valve area (cm²) | 0.61 ± 0.15 | 0.90 ± 0.24 | < 0.05 |
| Bicuspid aortic valve, n (%) | 2 (2.7%) | 8 (5.0%) | > 0.05 |
| NYHA class II | 47 (63.5%) | 89 (56.0%) | > 0.05 |
| NYHA class III | 27 (36.5%) | 70 (44.0%) | > 0.05 |
| Coronary artery disease, n (%) | 16 (21.6%) | 33 (20.7%) | > 0.05 |
| Hypertension, n (%) | 70 (94%) | 122 (76%) | < 0.05 |
| Diabetes mellitus, n (%) | 33 (44%) | 64 (40%) | > 0.05 |
| Chronic lung disease, n (%) | 14 (19%) | 32 (20%) | > 0.05 |
| Neurological disease, n (%) | 9 (12%) | 20 (12.5%) | > 0.05 |
| Renal impairment, n (%) | 14 (19%) | 22 (14%) | > 0.05 |
| Peripheral vascular disease, n (%) | 11 (15%) | 16 (10%) | > 0.05 |
| Dyslipidemia, n (%) | 49 (66%) | 63 (40%) | < 0.05 |
| Current/previous smoking, n (%) | 50 (67.6%) | 102 (64.1%) | > 0.05 |
| LVEF < 30%, n (%) | 3 (4%) | 5 (3%) | > 0.05 |
| LVEF 30–50%, n (%) | 16 (22%) | 28 (18%) | > 0.05 |
| LVEF > 50%, n (%) | 55 (74%) | 126 (79%) | > 0.05 |
| Euro SCORE-II | 1.95 ± 0.84 | 1.76 ± 0.94 | > 0.05 |
| STS score | 1.80 ± 0.74 | 1.54 ± 0.64 | > 0.05 |
| Characteristic | Sutureless (N = 74) | Stented (N = 159) | p-value |
|---|---|---|---|
| CPB time (min) | |||
| AVR (isolated) | 83.8 ± 20.6 (n = 61) | 82.7 ± 21.8 (n = 127) | > 0.05 |
| AVR + CABG | 120.3 ± 38.2 (n = 13) | 101.4 ± 36.5 (n = 32) | < 0.05 |
| Full sternotomy | 96.4 ± 44.5 (n = 51) | 97.6 ± 42.8 (n = 137) | > 0.05 |
| Upper mini sternotomy | 88.4 ± 21.4 (n = 17) | 89.2 ± 43.6 (n = 22) | > 0.05 |
| Right anterior thoracotomy | 94.0 ± 9.2 (n = 6) | — (n = 0) | — |
| ACC time (min) | |||
| AVR (isolated) | 54.5 ± 14.6 (n = 61) | 56.8 ± 11.6 (n = 127) | > 0.05 |
| AVR + CABG | 92.1 ± 29.3 (n = 13) | 104.5 ± 29.6 (n = 32) | < 0.05 |
| Full sternotomy | 65.8 ± 27.6 (n = 51) | 67.6 ± 22.8 (n = 137) | > 0.05 |
| Upper mini sternotomy | 53.6 ± 15.8 (n = 17) | 55.4 ± 13.7 (n = 22) | > 0.05 |
| Right anterior thoracotomy | 64.7 ± 5.9 (n = 6) | — (n = 0) | — |
| Perceval prosthesis size | |||
| Small (19–21 mm) | 12 (17.9%) | N/A | — |
| Medium (21–23 mm) | 18 (26.9%) | N/A | — |
| Large (23–25 mm) | 28 (41.8%) | N/A | — |
| X-Large (25–27 mm) | 9 (13.4%) | N/A | — |
| Distal anastomoses (CABG) | |||
| 1 graft | 5 (6.8%) | 11 (6.9%) | > 0.05 |
| 2 grafts | 3 (4.0%) | 6 (3.8%) | > 0.05 |
| 3 grafts | 6 (8.1%) | 15 (9.4%) | > 0.05 |
| Complications | |||
| Paravalvular leak (significant) | 3 (4.0%) | 2 (1.3%) | > 0.05 |
| Neurological dysfunction | 2 (2.7%) | 2 (1.3%) | > 0.05 |
| Thrombocytopenia | 9 (12.2%) | 11 (6.9%) | > 0.05 |
| Re-exploration for bleeding | 4 (5.4%) | 11 (6.9%) | > 0.05 |
| Permanent pacemaker required | 4 (5.4%) | 5 (3.1%) | > 0.05 |
| 24-h chest tube output (mL) | 405 ± 93 | 494 ± 102 | > 0.05 |
| ICU stay (days) | 2.4 ± 1.9 | 2.7 ± 2.1 | > 0.05 |
| Hospital stays (days) | 7.6 ± 3.6 | 8.1 ± 2.3 | > 0.05 |
| 30-day (hospital) mortality | 4 (5.9%) | 10 (6.3%) | > 0.05 |
| Characteristic | Sutureless (N = 74) |
Stented (N = 159) |
p-value |
|---|---|---|---|
| Stroke, n (%) | 0 (0%) | 0 (0%) | > 0.05 |
| Endocarditis, n (%) | 0 (0%) | 3 (1.9%) | > 0.05 |
| Neurological event, n (%) * | 3 (4.0%) | 6 (3.8%) | > 0.05 |
| Thrombocytopenia, n (%) | 15 (20.3%) | 17 (10.7%) | < 0.05 |
| Paravalvular leak (trivial), n (%) | 4 (5.4%) | 5 (3.1%) | > 0.05 |
| Peak transvalvular gradient (postop, mmHg) | 22.5 ± 8.1 | 24.5 ± 8.7 | > 0.05 |
| Mean transvalvular gradient (postop, mmHg) | 11.2 ± 4.3 | 12.6 ± 5.3 | > 0.05 |
| Peak transvalvular gradient (follow-up, mmHg) | 19 ± 2 | 20 ± 2.1 | > 0.05 |
| Mean transvalvular gradient (follow-up, mmHg) | 9 ± 2 | 10 ± 2.2 | > 0.05 |
| NYHA class I (latest) | 48 (64.8%) | 92 (57.8%) | > 0.05 |
| NYHA class II (latest) | 26 (35.2%) | 67 (42.2%) | > 0.05 |
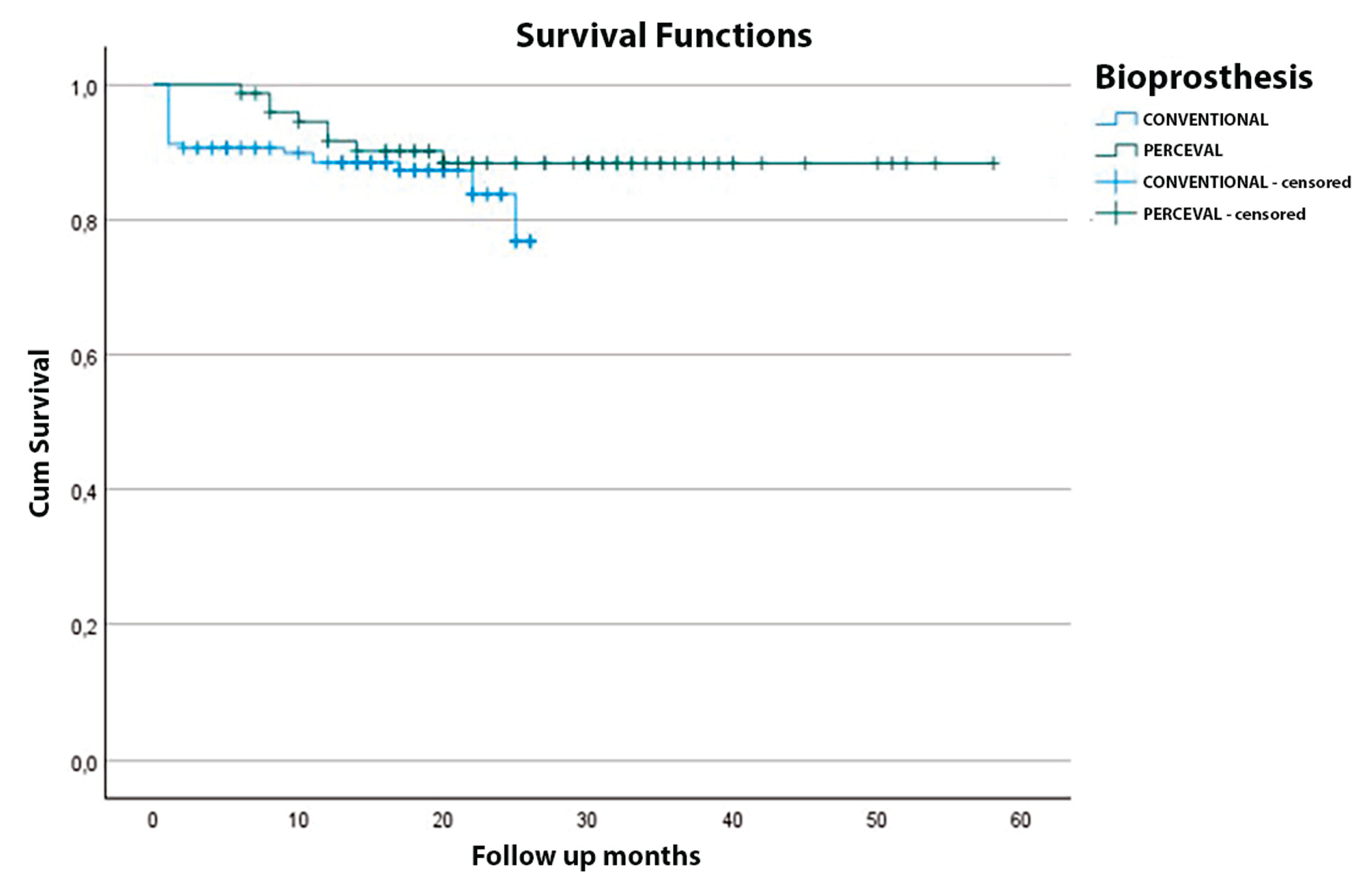
| Months after AVR | Overall survival (%) | Stented group (%) | Sutureless group (%) |
|---|---|---|---|
| 1 | 93.2 | 91.2 | 94.1 |
| 3 | 92.6 | 90.8 | 94.1 |
| 12 | 89.3 | 88.4 | 91.6 |
| 24 | 85.8 | 83.7 | 88.3 |
| 36 | 84.0 | 76.8 | 88.3 |
| 48 | 84.0 | — | 88.3 |
| 58 | 84.0 | — | 88.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





