Submitted:
28 May 2025
Posted:
30 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
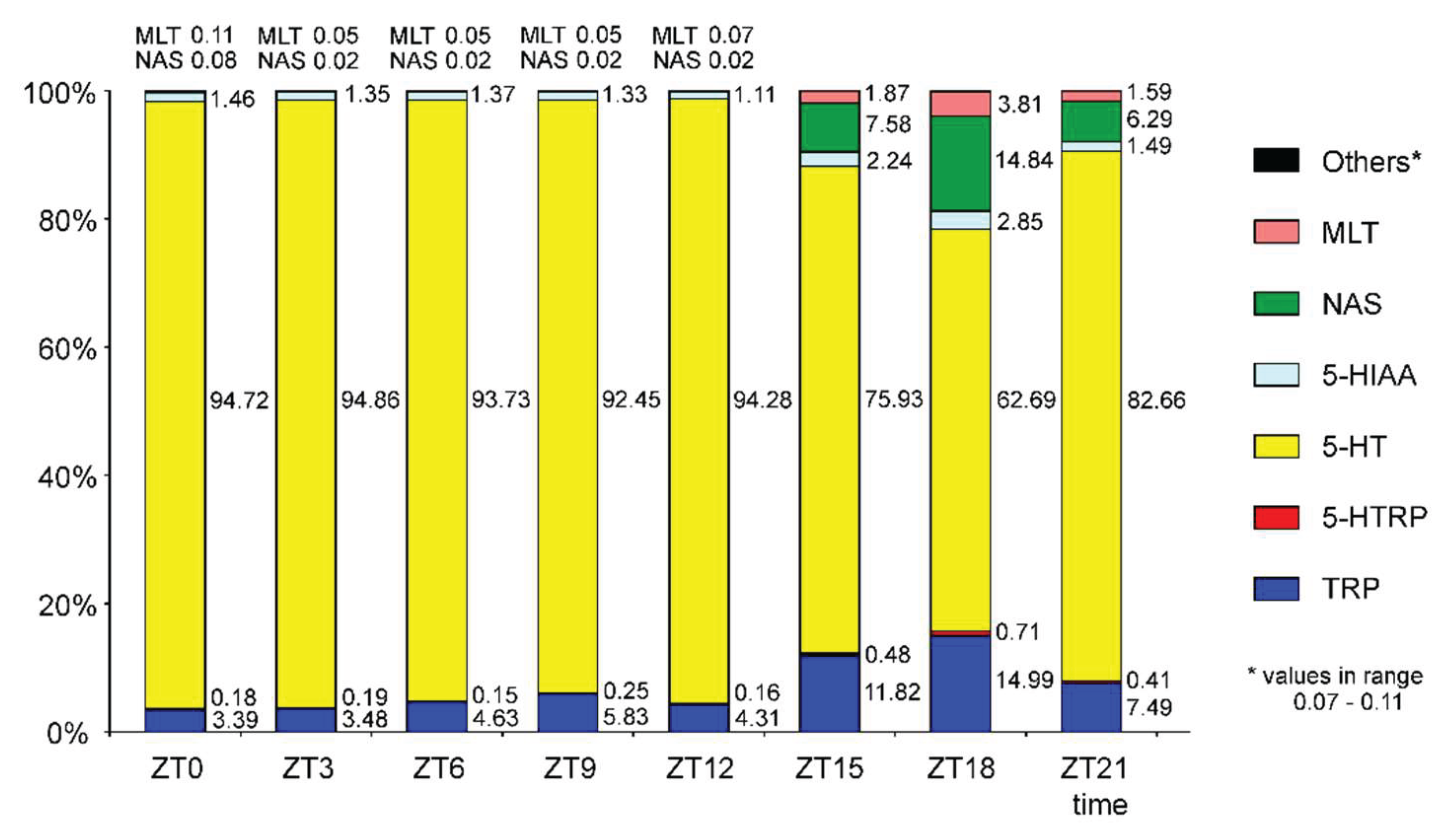
2. Results
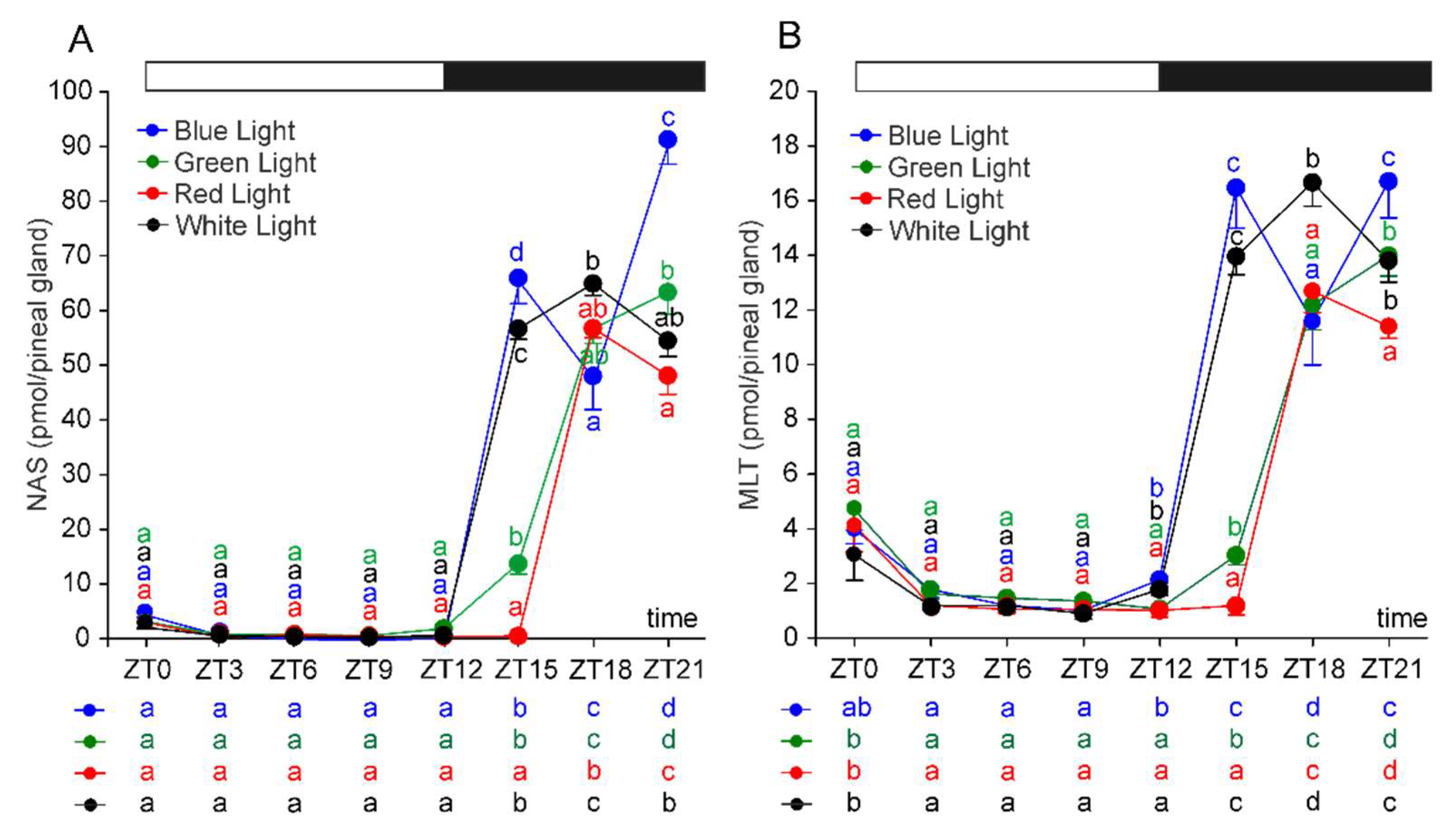
2.1. N-Acetylserotonin and Melatonin
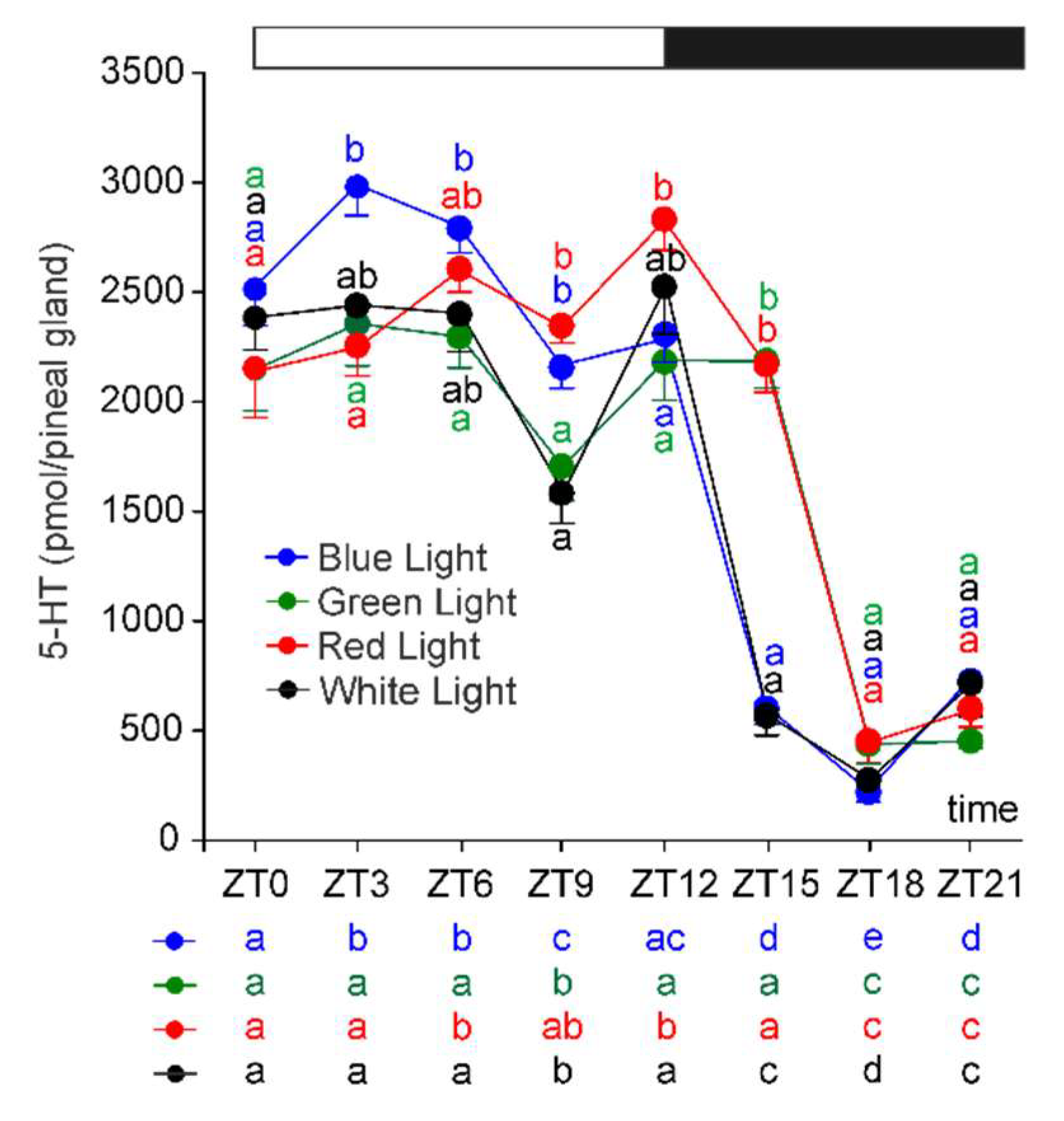
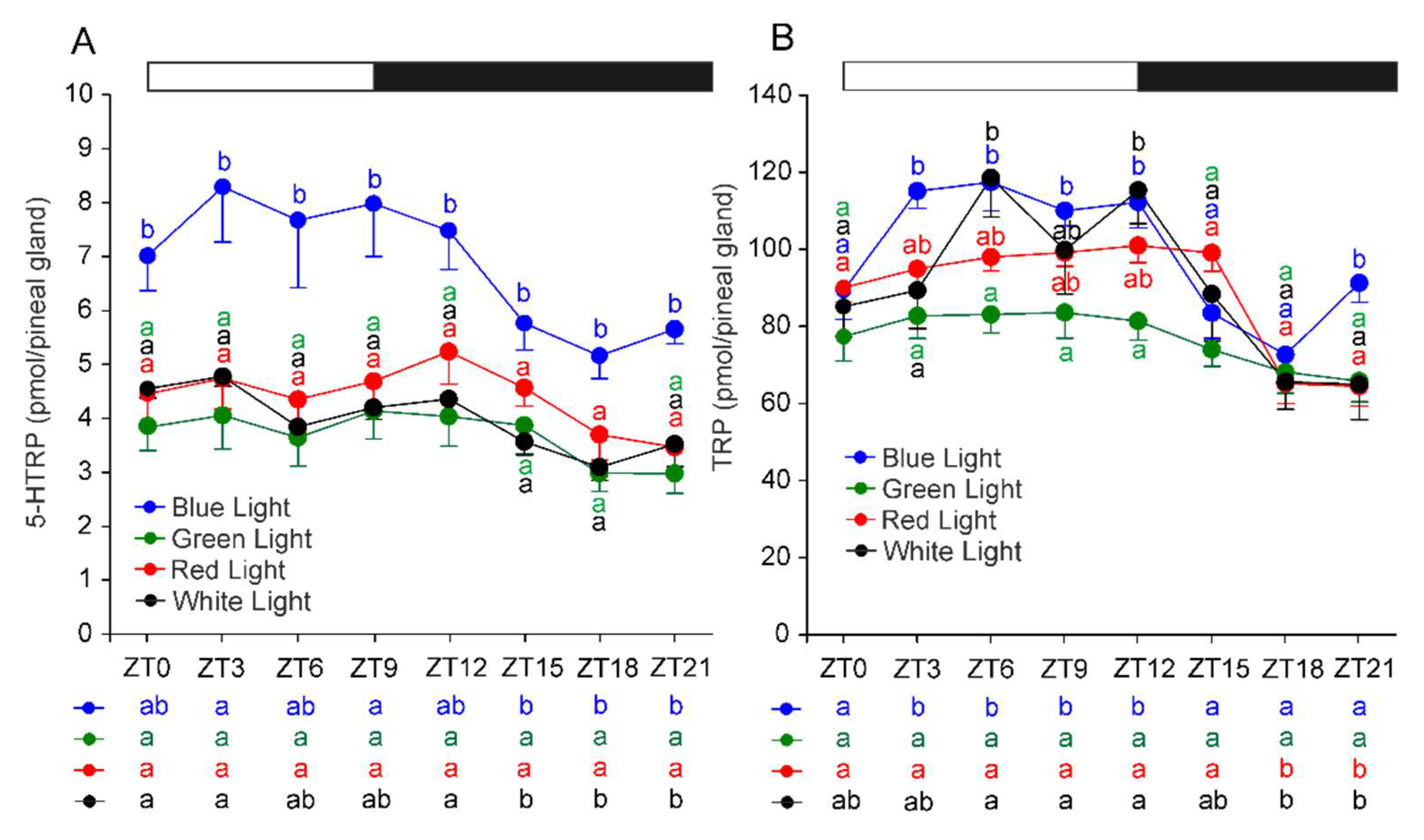
2.2. Serotonin and Its Precursors
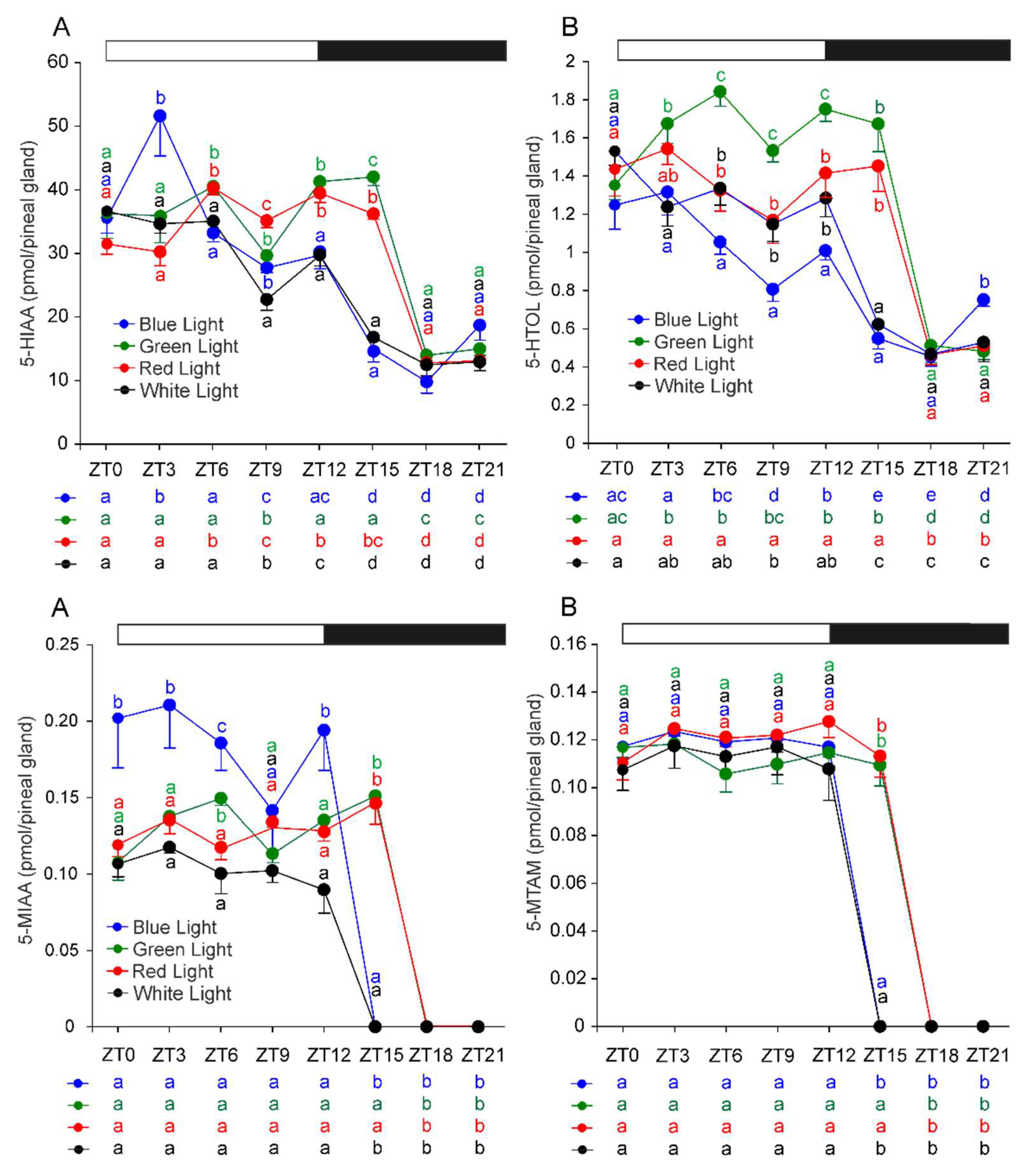
2.3. Metabolites of Serotonin Formed by Oxidative Deamination and Methylation
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Indole Assay
4.2.1. Chemicals
4.2.2. Sample Preparation
4.2.3. High-Pressure Liquid Chromatography
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | serotonin |
| 5-HTOL | 5-hydroxytryptophol |
| 5-HTRP | 5-hydroxytryptophan |
| LED | light-emitting diode |
| 5-MIAA | 5-methoxyindoleacetic acid |
| 5-MTAM | 5-methoxytryptamine |
| 5-MTOL | 5-methoxytryptophol |
| AA-NAT | arylalkylamine N-acetyltransferase |
| ASMT | N-acetylserotonin O-methyltransferase |
| HPLC | high pressure liquid chromatography |
| MLT | melatonin |
| NAS | N-acetylserotonin |
| TRP | tryptophan |
References
- LeGates, T.A.; Fernandez, D.C.; Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014, 15(7), 443-54. [CrossRef]
- Wahl, S.; Engelhardt, M.; Schaupp, P.; Lappe, C.; Ivanov, I.V. The inner clock - Blue light sets the human rhythm. J Biophotonics. 2019, 12(12):e201900102. [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 2010, 33 (5), 585–592. [CrossRef]
- Bhattarai, T.; Ebong, A.; Raja, M.Y.A. A review of light-emitting diodes and ultraviolet light-emitting diodes and their applications. Photonics 2024, 11, 491. [CrossRef]
- Lockley, S.W.; Brainard, G.C.; Czeisler, C.A. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J. Clin. Endocrinol. Metab. 2003, 88, 4502–4505. [CrossRef]
- Duda, M., Domagalik, A., Orlowska-Feuer, P., Krzysztynska-Kuleta, O., Beldzik, E., Smyk, M.K., Stachurska, A., Oginska, H,, Jęczmień-Lazur, J.S., Fafrowicz, M., Marek, T., Lewandowski, M.H., Sarna, T. Melanopsin: From a small molecule to brain functions. Neurosci Biobehav Rev. 2020, 113, 190-203. [CrossRef]
- Sato, K., Yamashita, T., Kojima, K., Sakai, K., Matsutani, Y., Yanagawa, M., Yamano, Y., Wada, A., Iwabe, N., Ohuchi, H., Shichida, Y. Pinopsin evolved as the ancestral dim-light visual opsin in vertebrates. Commun Biol. 2018, 1, 156. [CrossRef]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 2002, 295(5557), 1065-70. [CrossRef]
- Silvani, M.I.; Werder, R.; Perret, C. The influence of blue light on sleep, performance and wellbeing in young adults: A systematic review. Front Physiol. 2022, 13, 943108. [CrossRef]
- Ziółkowska, N.; Lewczuk, B.; Szyryńska, N.; Rawicka, A.; Vyniarska, A. Low-intensity blue light exposure reduces melanopsin expression in intrinsically photosensitive retinal ganglion cells and damages mitochondria in retinal ganglion cells in wistar rats. Cells 2023, 12, 1014. [CrossRef]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005, 90(3), 1311-6. [CrossRef]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151-180. [CrossRef]
- Klein, D.C.; Moore, R.Y.; Reppert, S.M. Suprachiasmatic nucleus: The mind's clock. Oxford University Press 1991, 5–456.
- Teclemariam-Mesbah, R.; Ter Horst, G.J.; Postema, F.; Wortel, J.; Buijs, R.M. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. J Comp Neurol. 1999, 406(2), 171-82.
- Mclntyre, I.M.; Norman, T.R.; Burrows, G.D.; Armstrong, S.M. Human melatonin suppression by light is intensity dependent. J. Pineal Res. 1989, 6( 2), 149-156. [CrossRef]
- Aoki, H.; Yamada, N.; Ozeki, Y.; Yamane, H.; Kato, N. Minimum light intensity required to suppress nocturnal melatonin concentration in human saliva. Neurosci. Lett. 1998, 252(2), 91-94. [CrossRef]
- Zeitzer, J.M.; Dijk, D.J.; Kronauer, R.E.; Brown, E.N.; Czeisler, C.A. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J. Physiol. 2000, 526( 3), 695-702. [CrossRef]
- Lynch, H.L.; Deng, M.H.; Wurtman, R.J. Light intensities required to suppress nocturnal melatonin secretion in albino rats. Life Sci. 1984, 35, 841-847. [CrossRef]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002; 295, 1070–1073. [CrossRef]
- Gooley J.J.; Lu J.; Fischer D.; Saper C.B. A broad role for melanopsin in nonvisual photoreception. J. Neurosci. 2003, 23, 7093–106. [CrossRef]
- Dacey, D.M.; Liao, H.-W.; Peterson, B.B.; Robinson, F.R.; Smith, V.C.; Pokorny, J.; Yau, K.-W.; Gamlin, P.D. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005, 433, 749-54. [CrossRef]
- Brainard, G.C.; Hanifin, J.P.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J. Neurosci. 2001, 21, 6405–12. [CrossRef]
- Walker, M.T.; Brown, R.L.; Cronin, T.W.; Robinson, P.R. Photochemistry of retinal chromophore in mouse melanopsin. Proc. Natl. Acad. Sci. USA 2008, 105, 8861-8865. [CrossRef]
- Thapan, K.; Arendt, J.; Skene, D.J. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J. Physiol. 2001, 535, 261-267. [CrossRef]
- Brainard, G.C.; Richardson, B.A.; King, T.S.; Reiter, R.J. The influence of different light spectra on the suppression of pineal melatonin content in the Syrian hamster. Brain Res. 1984, 294, 333-339. [CrossRef]
- Podolin, P.L.; Pangerl, A.; Brainard, G.C. The suppression of nocturnal pineal melatonin in the Syrian hamster: dose-response curves at 500 and 360 nm. Endocrinology 1987, 121, 266-270. [CrossRef]
- Honma, S.; Kanematsu, N.; Katsuno Y., Honma K.-I. Light suppression of nocturnal pineal and plasma melatonin in rats depends on wavelength and time of day. Neurosci. Lett. 1992, 147, 201-204. [CrossRef]
- Gooley, J.J.; Rajaratnam, S.M.W.; Brainard, G.C.; Kronauer, R.E.; Czeisler, C.A.; Lockley, S.W. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci. Transl. Med. 2010, 2, 31ra33. [CrossRef]
- Wright, H.R.; Lack, L.C.; Kennaway, D.J. Differential effects of light wavelength in phase advancing the melatonin rhythm. J. Pineal Res. 2004, 36, 140–144. [CrossRef]
- Hanifin, J.P.; Stewart, K.T.; Smith, P.; Tanner, R.; Rollag, M.; Brainard, G.C. High-intensity red light suppresses melatonin. Chronobiol. Int. 2006, 23, 251-268. [CrossRef]
- Zeitzer, J.M.; Kronauer, R.E.; Czeisler, C.A. Photopic transduction implicated in human circadian entrainment. Neurosci. Lett. 1997, 232, 135-138. [CrossRef]
- Martyniuk, K.; Hanuszewska, M.; Lewczuk, B. Metabolism of melatonin synthesis-related indoles in the turkey pineal organ and its modification by monochromatic light. Int. J. Mol. Sci. 2020, 21, 9750.. [CrossRef]
- Miguez, J.M.; Recio, J., Sanchez-Barceló, E.; Aldegunde, M. Changes with age in daytime and nighttime contents of melatonin, indoleamines, and catecholamines in the pineal gland: A comparative study in rat and Syrian hamster. J. Pineal Res. 1998, 25, 106-115. [CrossRef]
- Miguez, J.M.; Recio, J.; Vivien-Roels, B.; Pèvet, P. Diurnal changes in the content of indoleamines, catecholamines and methoxyindoles in the pineal gland of the Djungarian hamster (Phodopus sungorus): Effect of photoperiod. J. Pineal Res. 1996, 21, 7-14.. [CrossRef]
- Miguez, J.M.; Recio, J.; Vivien-Roels, B.; Pèvet, P. Daily variation in the content of indoleamines, catecholemines and related compounds in the pineal gland of Syrian hamsters kept under long and short photoperiods. J. Pineal Res. 1995, 19, 139-148. [CrossRef]
- Harumi, T.; Matsushima, S. Indoleamine Metabolism in the pineal gland of the Chinese hamster, Cricetulus griseus. Gen. Comp. Endocrinol. 1998, 109, 133–139. [CrossRef]
- McNulty, J.A.; Prechel, M.M.; Simmons, W.H. Correlations of serotonin and its metabolites in individual rat pineal glands over light: dark cycles and after acute light exposure. Life Sci. 1986, 39, 1-6. [CrossRef]
- Frese, T.; Bach, A. G.; Mühlbauer, E.; Pönicke, K.; Brömme, H. J.; Welp, A.; Peschke, E. Pineal melatonin synthesis is decreased in type 2 diabetic Goto-Kakizaki rats. Life Sci. 2009, 85(13-14), 526–533.. [CrossRef]
- Steinlechner, S.; Baumgartner, I.; Klante, G.; Reiter, R. J. Melatonin synthesis in the retina and pineal gland of Djungarian hamsters at different times of the year. Neurochem. Int. 1995, 27(3), 245–251. doi:/10.1016/0197-0186(95)00037-9.
- Pévet, P.; Vivien-Roels, B.; Masson-Pévet, M.; Steinlechner, S.; Skene, D.; Canguilhem, B. Melatonin, serotonin, 5-hydroxyindole-3-acetic acid and N-acetyltransferase in the pineal of the European hamster (Cricetus cricetus) kept under natural environmental conditions: lack of a day/night rhythm in melatonin formation in spring and early summer. J. Pineal Res. 1989, 6(3), 233–242. [CrossRef]
- Lewczuk, B.; Prusik, M.; Ziółkowska, N.; Dąbrowski, M.; Martniuk, K.; Hanuszewska, M.; Zielonka, Ł. Effects of streptozotocin-induced diabetes on the pineal gland in the domestic pig. Int. J. Mol. Sci, 2018, 19(10), 3077. [CrossRef]
- Young, S.N.; Anderson, G.M. Factors influencing melatonin, 5-hydroxytrytophol, 5-hydroxyindoleacetic acid, 5-hydroxytryptamine and tryptophan in rat pineal gland. Neuroendocrinology 1982, 35, 464-468. [CrossRef]
- Lewczuk, B.; Ziółkowska, N.; Prusik, M.; Przybylska-Gornowicz, B. Diurnal profiles of melatonin synthesis-related indoles, catecholamines and their metabolites in the duck pineal organ. Int. J. Mol. Sci 2014, 15(7), 12604–12630. [CrossRef]
- Adamska, I.; Lewczuk, B.; Markowska, M.; Majewski, P. M. Daily profiles of melatonin synthesis-related indoles in the pineal glands of young chickens (Gallus gallus domesticus L.). J. Photochem. Photobiol. B: Biol. 2016, 164, 335–343. [CrossRef]
- Ziółkowska, N.; Lewczuk, B.; Prusik, M. Diurnal and circadian variations in indole contents in the goose pineal gland. Chronobiol. Int. 2018, 35(11), 1560–1575. [CrossRef]
- Martyniuk, K.; Hanuszewska-Dominiak, M.; Lewczuk, B. Changes in the metabolic profile of melatonin synthesis-related indoles during post-embryonic development of the turkey pineal organ. Int. J. Mol. Sci. 2022, 23(18), 10872. [CrossRef]
- Tomaka, M.; Malz, M.; Lewczuk, B.; Turkowska, E.; Markowska, M. A.; Majewski, P. M.; Adamska, I. Seasonal differences in the influence of peritonitis on the biosynthetic activity of the pineal gland in young chickens. J. Physiol. Pharmacol. 2024, 75(6), 691-704. [CrossRef]
- Deguchi T. Tryptophan hydroxylase in pineal gland of rat: postsynaptic localization and absence of circadian change. J. Neurochem. 1977, 28(3), 667–668. [CrossRef]
- Steinlechner S, Steger RW, King TS, Reiter RJ. Diurnal variation in the serotonin content and turnover in the pineal gland of the Syrian hamster. Neurosci Lett. 1983 35(2), 167-72. [CrossRef] [PubMed]
- Mefford, I.N.; Chang, P.; Klein, D.C.; Namboodiri, M.A.A.; Sugden, D.; Barchas, J. Reciprocal day/night relationship between serotonin oxidation and N-acetylation products in the rat pineal gland. Endocrinology 1983, 113 (5), 1582. [CrossRef]
- Liu, T.; Borjigin, J. N-acetyltransferase is not the rate limiting enzyme of melatonin synthesis at night. J. Pineal Res. 2005, 39, 91–96. [CrossRef]
- Chattoraj, A.; Liu, T.; Zhang, L.S.; Huang, Z.; Borjigin J. Melatonin formation in mammals: in vivo perspectives. Rev. Endocr. Metab. Disord. 2009, 10, 237-43. [CrossRef]
- Beck, O. Analysis of melatonin, 5-methoxytryptophol and 5-methoxyindoleacetic acid in the pineal gland and retina of hamster by capillary column gas chromatography-mass spectrometry. J. Chromatogr. 1984, 311, 1-8. [CrossRef]
- Li, P.; Pang, S.F.; Chan, C.L.; Tsang, C.W. Identification and diurnal studies of pineal and serum 5-methoxytryptamine in the rat and quail. Neurosci. Lett. 1997, 228, 63-5. [CrossRef]
- Galzin, A.M.; Eon, M.T.; Esnaud, H.; Lee, C.R.; Pèvet, P.; Langer, S.Z. Day-night rhythm of 5-methoxytryptamine biosynthesis in the pineal gland of the golden hamster (Mesocricetus auratus). J. Endocr. 1988, 118, 389-397. [CrossRef]
- Hofman, M.A.; Skene, D.J.; Swaab, D.F. Effect of photoperiod on the diurnal melatonin and 5-methoxytryptophol rhythms in the human pineal gland. Brain Res. 1995, 671, 254–260. [CrossRef]
- Lakhdar-Ghazal, N.; Vivien-Roels, B.; Pevet, P. Seasonal variations in pineal 5-methoxytryptophol (5-ML) concentrations and in the daily pattern of pineal 5-ML and melatonin in the desert rodent Jaculus orientalis: effect of prolonged illumination during the night. J. Pineal Res. 1992, 13, 28–35. [CrossRef]
- Mustafi, D.; Engel, A.H.; Palczewski, K. Structure of cone photoreceptors. Prog Retin Eye Res. 2009, 28(4), 289-302. [CrossRef]
- Hofmann, K.P.; Lamb, T.D. Rhodopsin, light-sensor of vision. Prog Retin Eye Res. 2023 93, 101116. [CrossRef]
- Stritzel WJ, Levy C, Ravenel JR, Strnad HK, Osman M, Prévost ED, Root DH, Reuter JD, Sloan AM, Spencer RL. Red light sensitivity of non-image and image forming visual systems of laboratory rodents: circadian disruption and behavioral detection. J Neurosci. 2025, 45(20), e0157252025. [CrossRef]
- Sigulinsky, C.L.; Pfeiffer, R.L.; Jones, B.W. Retinal connectomics: A Review. Annu. Rev. Vis. Sci. 2024, 10(1), 263-291. [CrossRef]
- Rosenwasser, A.M.; Turek, F.W. Neurobiology of circadian rhythm regulation. Sleep Med Clin. 2015, 10(4), 403-12. [CrossRef]
- Patton AP, Hastings MH. The suprachiasmatic nucleus. Curr Biol. 2018, 28(15), R816-R822. [CrossRef]
- Challet. E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 2007, 148(12), 5648-55. [CrossRef]
- Tischkau, S.A.; Gallman, E.A.; Buchanan, G.F.; Gillette, M,U. Differential cAMP gating of glutamatergic signaling regulates long-term state changes in the suprachiasmatic circadian clock. J Neurosci. 2000, 20(20), 7830-7. [CrossRef]
- Ziółkowska, N.; Lewczuk, B. Profiles of Rho, Opn4, c-Fos, and Birc5 mRNA expression in Wistar rat retinas exposed to white or monochromatic light. Front. Neuroanat. 2022, 16, 956000. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




