1. Introduction
Zeolites, recognized as environmentally sustainable and economically viable hydrated aluminosilicate materials, inherently possess valuable ion-exchange and sorption properties. Their distinctive characteristics, including ordered microporous frameworks, high hydrothermal stability, extensive surface area, tunable acidity, and shape selectivity, render them promising catalysts with widespread applications in the petrochemical, petroleum refining, and natural gas processing sectors. However, the exclusive presence of microporous channels within zeolite structures inherently limits the diffusion of larger reactant and product molecules into and out of these networks, potentially leading to diminished reaction kinetics or catalyst deactivation [
1]. A significant strategy for enhancing zeolite performance involves chemical modification, particularly acid activation. It is generally hypothesized that acid treatment induces dealumination of the zeolite framework, resulting in an increased silica-to-alumina ratio (SAR) and consequently improved catalytic activity [
2].
Existing literature indicates that the ion-exchange capacity of natural zeolites can be enhanced through various modification techniques. Chemical activation, utilizing acids, bases, and other reagents, is considered a highly effective approach [
3,
4,
5]. For the synthesis of high-silica zeolites, a common method involves the modification of low-SAR zeolites via the selective removal of aluminum atoms from their crystalline framework, a process termed dealumination [
6]. This aluminum extraction can be achieved using a range of reagents, including inorganic acids, steam at temperatures exceeding 500 °C, ethylenediaminetetraacetic acid (EDTA) and other complexing agents (e.g., acetylacetone), volatile silicon compounds (SiCl₄), and aqueous solutions of (NH₄)₂SiF₆. The efficacy of a specific dealumination method is contingent upon the strength of the Al-O bonds within the framework and the inherent stability of the framework itself. A general trend suggests that zeolites with higher aluminum content tend to exhibit reduced framework stability when subjected to acidic and thermal treatments.
The chemical stability of zeolites represents a critical parameter that dictates their long-term performance and effectiveness across diverse industrial and environmental applications. This stability is intrinsically linked to the zeolite’s structural attributes, chemical composition, and operational conditions, such as temperature and exposure to corrosive chemical agents. Modification of zeolites through treatment with various substances offers a pathway to significantly enhance their properties and broaden their application scope, encompassing improvements in mechanical strength, ion-exchange capacity, and resistance to chemical degradation.
Acid treatment stands out as one of the most straightforward and widely adopted methods for modifying zeolites. This technique facilitates the enhancement of the acid-base properties of zeolites, alongside an increase in their porosity and sorption characteristics. The selection of the acid employed is dictated by the specific objective of the zeolite modification. The leaching of zeolites with acids leads to the exposure of the mineral framework while preserving the fundamental structural arrangement of the zeolite.
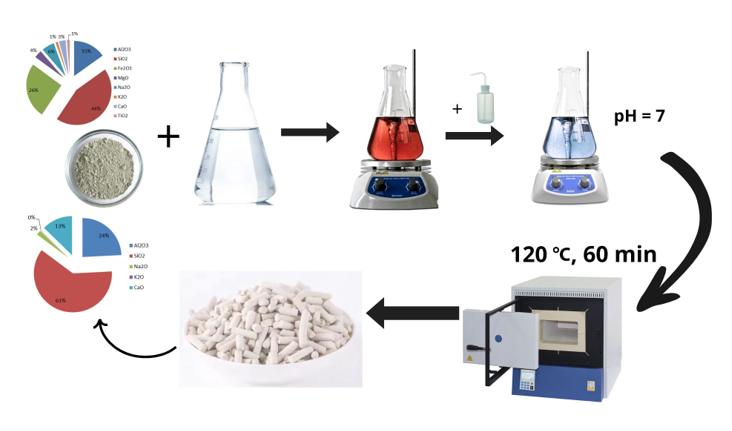
In this study, the impact of acid treatment (leaching in boiling acids) on the structural characteristics of zeolite originating from the Shankhanai deposit was investigated using a comprehensive suite of physicochemical analytical techniques.
The objective of this research is to elucidate the influence of acid treatment on the structure of natural zeolites.
2. Results and Discussion
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted. This study investigated a natural zeolite from the Shankhanai deposit in the Almaty region, characterized by the following chemical composition: Al₂O₃ – 15.1%; SiO₂ – 44.2%; Fe₂O₃ – 25.7%; CaO – 3.1%; MgO – 3.6%; Na₂O – 5.8%; K₂O – 1.1%; TiO₂ – 1.3%. The treatment of the natural zeolite with nitric acid and a mixture of acetic and nitric acids resulted in the leaching of alkali and alkaline earth metal cations from its structure.
The efficiency of zeolite treatment increased with increasing acid concentration, which is attributed to a more pronounced disruption of the zeolite structure and a more extensive ion exchange process. The observed increase in the silica-to-alumina ratio (Si/Al) indicates a reduction in aluminum content following acid treatment, potentially due to the leaching of more labile aluminosilicate phases. This, in turn, can lead to an improvement in the adsorption properties and ion exchange capacity of the zeolite.
Calculations revealed that the silica-to-alumina ratio of the pristine natural zeolite sample was approximately 7, which aligns well with literature values reported for the SAR of natural erionite [
7]. Upon increasing the acid concentration during treatment, this ratio increased to 10.
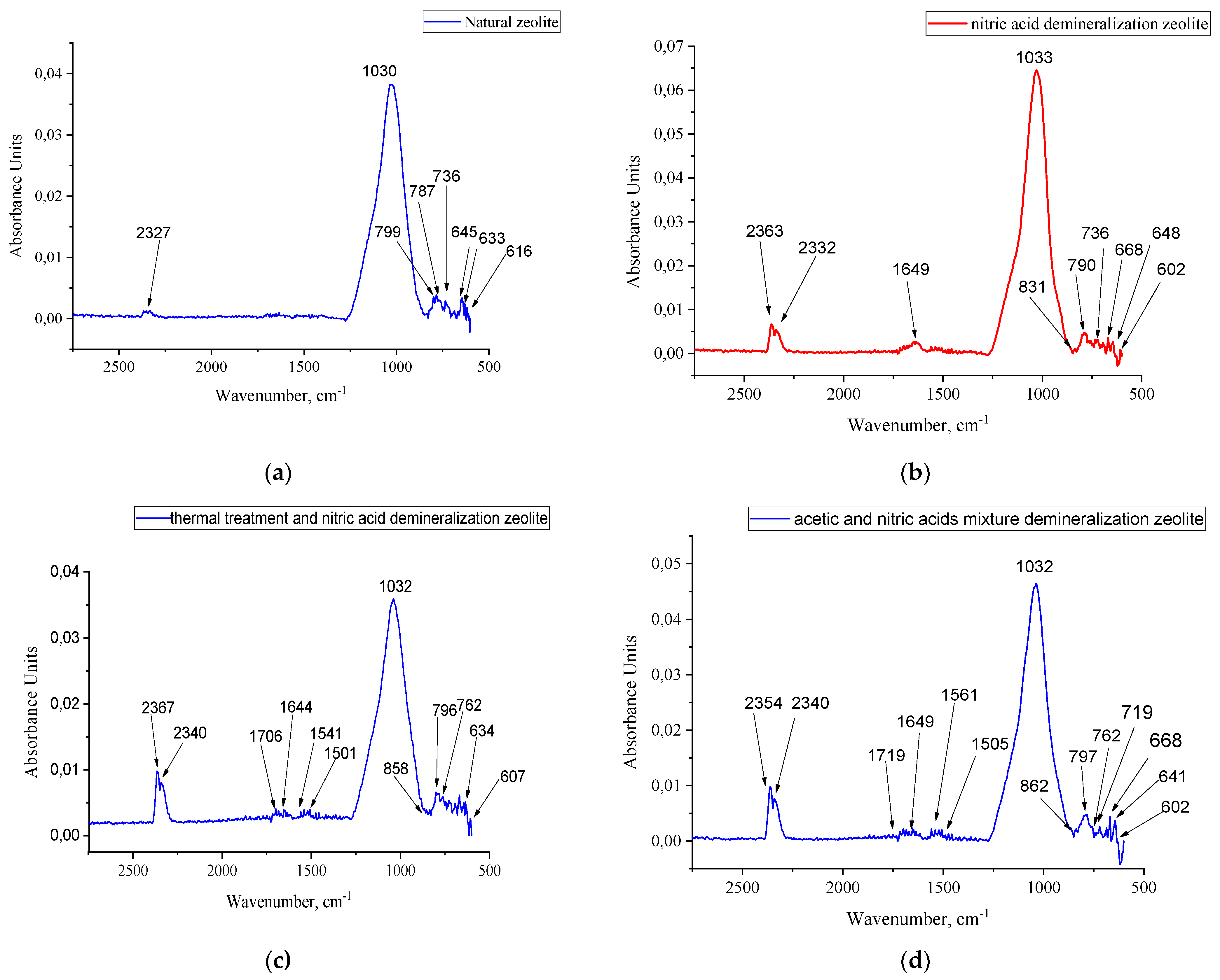
Analysis of the FTIR spectra of the natural zeolite after treatment with boiling acids provided insights into changes in its structural and functional characteristics. Specifically, alterations in the intensity and position of absorption peaks in the 1000-1200 cm⁻¹ region may indicate the disruption of the silicate framework and dehydration of the material. Furthermore, the appearance of new peaks suggests the formation of novel functional groups, indicating a transformation in the chemical composition of the zeolite.
The FTIR spectrum of the natural zeolite is presented in
Figure 1(a). As depicted, the spectrum exhibits all the characteristic absorption bands of the aluminosilicate framework of zeolites in the 400-1200 cm⁻¹ region. It is well-established that frequencies in the 800-1200 cm⁻¹ range correspond to the stretching vibrations of the (TO₄) tetrahedra that form the pores within the zeolite structure. Frequencies in the 700-800 cm⁻¹ range are attributed to the stretching vibrations of Si-O-Si bridges, while the 1020-1200 cm⁻¹ region shows intra-tetrahedral stretching vibrations of Si(Al)-O bonds [
6]. The spectrum of the original natural zeolite exhibits characteristic absorption bands at 616, 633, 645, 736, 787, 799, and 1030 cm⁻¹, corresponding to the vibrational modes of tetrahedral Si–O, Al–O, and Si–O–Al bonds in the aluminosilicate matrix. The prominent band at 1030 cm⁻¹ is indicative of the asymmetric stretching of Si–O–Si groups and serves as a fingerprint of the intact zeolite structure.
The absence of an absorption band in the 3720-3740 cm⁻¹ region, which is characteristic of amorphous SiO₂, further confirms the high crystallinity and phase purity of all samples.
Figure 1 (b), (c), (d) displays the FTIR spectra of the modified zeolite treated with boiling acids. Upon treatment with nitric acid, notable spectral changes are observed, including new absorption bands at 602, 648, 668, 736, 790, 831, 1033, and 1649 cm⁻¹. The shift of the main band from 1030 to 1033 cm⁻¹ suggests local structural reorganization, likely due to the partial leaching of aluminum ions. The appearance of the band at 831 cm⁻¹ may indicate the formation of new Al–O vibration modes associated with the modified surface. The band at 1649 cm⁻¹ corresponds to the bending vibrations of surface-bound hydroxyl groups formed during the acid-induced dissolution of framework cations. As evident from
Figure 1, in the frequency region corresponding to the aluminosilicate framework vibrations, the intensity of bands in the 602-787 cm⁻¹ range decreases with increasing acid concentration. It is known that stretching vibrations involving TO₄ tetrahedra correspond to absorption bands in the 600-820 cm⁻¹ region. The position of these bands is dependent on the Si/Al ratio within the zeolite framework: a shift to lower frequencies occurs with an increase in the content of tetrahedrally coordinated aluminum cations [10]. In
Figure 1(c), the reduction in the intensity of the band at 990-1000 cm⁻¹ suggests the leaching of aluminum from the zeolite framework. Additional thermal treatment following acid modification leads to further changes in the vibrational profile, including bands at 607, 634, 762, 796, 858, 1032 cm⁻¹ and a complex pattern within 1501–1706 cm⁻¹. These features reflect increased surface functionalization and the formation of oxygen-containing groups, which act as acid sites while maintaining the integrity of the crystalline zeolite structure. The enhancement of the O-H stretching band at approximately 3600 cm⁻¹ in
Figure 1(d) confirms the formation of Brønsted acid sites. The most pronounced spectral variations are observed for samples treated with a mixture of nitric and acetic acids. The corresponding FTIR spectrum displays absorption bands at 602, 641, 668, 719, 762, 797, 862, 1032, 1505, 1561, 1649, and 1719 cm⁻¹. The presence of multiple peaks in the 1500–1750 cm⁻¹ range is attributed to the incorporation of carboxyl-containing species or other surface-bound functional groups resulting from acid interaction with the aluminosilicate framework. Changes in the chemical bonds of all synthesized samples are clearly indicated by the band in the 600-800 cm⁻¹ range, which is attributed to the stretching vibrations of TO₄-TO₄ linkages. As previously mentioned, these bands are specifically associated with the tetrahedra of the zeolite crystalline lattice.
Concurrently, the intensity of the bands in the 1032-1038 cm⁻¹ region, characteristic of internal tetrahedral stretching vibrations, remains virtually unchanged upon acid treatment. Based on the IR spectroscopy analysis, it was established that acid activation facilitates the extraction of cations without compromising the structural framework of the zeolite [11].
The process involves the ion exchange of native cations (Na⁺, K⁺, Ca²⁺, Mg²⁺) with protons (H⁺), leading to the formation of Brønsted acid sites (
Figure 2). Under controlled conditions, selective leaching of aluminum species occurs without disrupting the crystalline framework. However, exposure to stronger acidic environments can induce partial SiO₂ removal, resulting in degradation of the zeolite structure and reduced mechanical integrity. The scheme highlights both the preservation of crystallinity under moderate treatment and the transition toward amorphization under harsh conditions, enabling tailored modification of zeolite properties for catalytic and adsorptive applications.
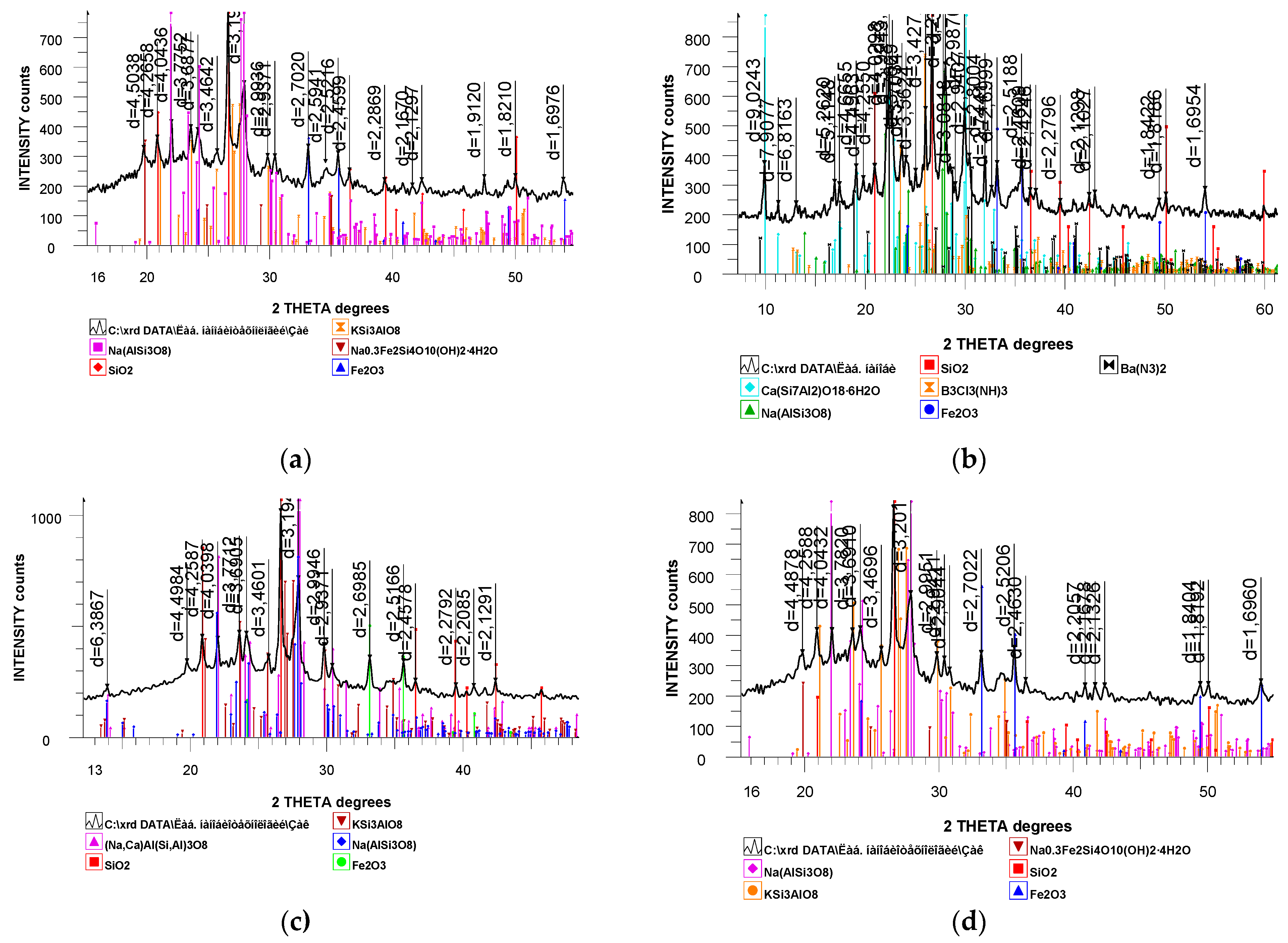
X-ray Diffraction (XRD) analysis corroborates the findings obtained through Infrared (IR) spectroscopy. The diffractograms of the pristine natural zeolite and the zeolite treated with boiling acids are presented in
Figure 3.
The XRD data revealed that despite minor alterations in the chemical composition, the crystalline lattice of the zeolite remained largely intact after acid treatment. The diffractograms of the natural and boiling acid-treated zeolite exhibit a similar overall pattern to that of the untreated zeolite.
Comparison of the spectra in
Figure 3 with the diffractogram of the initial natural zeolite showed no shifts in the interference maxima or distortions in their shape. The observed differences in the diffractograms are related to changes in the intensity of certain basal reflections. Notably, in all cases (at various acid concentrations), the intensity of the strongest basal reflection of the zeolite at d = 13.56 Å remained constant.
The conducted XRD analysis of the initial zeolite samples before and after acid treatment indicated that the scattering angles did not change, and the effect of acid treatment was manifested only in variations in the intensity of characteristic lines. Furthermore, the decrease in the intensity of different characteristic lines occurred unevenly, suggesting a partial disruption of the crystalline structure and an increase in the defect density within the internal pores of the zeolite. The changes in the intensity of lines in the diffractograms of the acid-treated zeolite are likely a consequence of the leaching of certain phases.
XRD analysis also established that the limit of thermal stability of the natural zeolite increased as a result of the acid treatment. However, minor changes in the crystalline lattice structure could already be observed in the IR spectrum of the sample treated with 1 N acid, whereas the X-ray diffractogram did not reveal these changes. This indicates a higher sensitivity of IR spectroscopy to structural alterations in zeolites compared to X-ray diffraction [
8].
As shown in
Figure 3, the zeolitic structure of the natural sample is preserved, indicating that the treatment of the zeolite with boiling acids (nitric and acetic) up to a certain concentration does not lead to the destruction of the zeolite framework. This is also supported by the X-ray diffractogram of the examined sample, which lacks sharp lines characteristic of the collapse of the initial zeolite structure.
The results of the XRD analysis established that upon treatment of the natural zeolite with a mixture of acids, its degree of crystallinity, initially 86%, decreased and varied within the range of 75%. Based on the absorption bands of the IR spectra obtained after treatment with each reagent, the degree of crystallinity of the zeolite was calculated, which showed good agreement with the data obtained from XRD analysis.
In summary, XRD confirms the preservation of the crystalline structure. The reduction in the intensity of characteristic reflections indicates partial dealumination and structural defects. The absence of new phases suggests that the fundamental zeolite structure is maintained.
Table 1.
Elemental Composition (wt. %) of Natural and Modified Zeolite Treated with Boiling Acids.
Table 1.
Elemental Composition (wt. %) of Natural and Modified Zeolite Treated with Boiling Acids.
| Sample |
phase composition, mass.% |
| Al2O3
|
SiO2
|
Fe2O3
|
MgO |
Na2O |
K2O |
CaO |
TiO2
|
| Natural Zeolite |
15,1 |
44,2 |
25,7 |
3,6 |
5,8 |
1,1 |
3,1 |
1,3 |
| Zeolite treated with nitric acid |
16,7 |
57,5 |
3,5 |
15,1 |
2,3 |
1,5 |
3,4 |
- |
| Zeolite treated with nitric acid and thermal treatment |
18,2 |
69,9 |
- |
2,2 |
1,6 |
0,3 |
7,8 |
- |
| Zeolite treated with a mixture of acetic and nitric acids |
24,2 |
61,0 |
- |
- |
2,0 |
0,1 |
12,7 |
- |
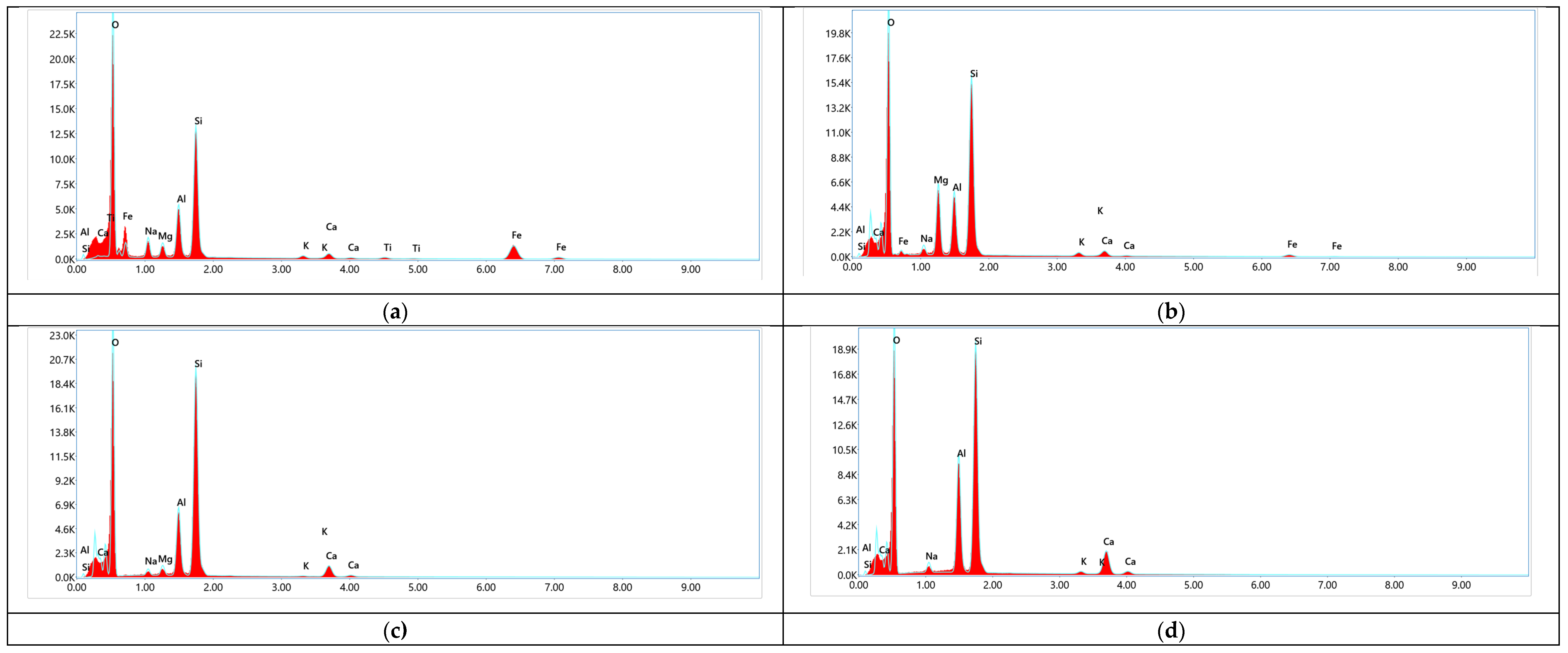
Figure 4 presents the Energy-Dispersive X-ray Spectroscopy (EDAX) results of the initial and acid-modified zeolite samples.
In
Figure 4a, the EDAX spectrum of the natural zeolite shows a typical composition dominated by silicon (Si) and aluminum (Al), with minor amounts of exchangeable cations such as sodium (Na), potassium (K), calcium (Ca), and magnesium (Mg). The Si/Al atomic ratio indicates a relatively low-silica zeolite framework.
Upon treatment with nitric acid (
Figure 4b), a noticeable decrease in the concentrations of exchangeable cations (Na⁺, K⁺, Ca²⁺, Mg²⁺) is observed, confirming effective ion removal without significant alteration of the primary Si–Al framework. The slight increase in the Si/Al ratio suggests partial dealumination.
Figure 3c (after subsequent thermal treatment) shows further depletion of extra-framework cations and a more pronounced increase in the Si/Al ratio, indicative of enhanced framework stability and surface enrichment with silicon atoms.
The sample treated with a mixture of nitric and acetic acids (
Figure 4d) exhibits the most significant changes: complete removal of exchangeable cations and a notable increase in the Si/Al ratio. This suggests that acid treatment with a mixed system not only promotes ion extraction but also induces partial framework reorganization, while preserving the crystalline integrity. Overall, the EDAX results support the FTIR findings, confirming that acid activation facilitates selective cation removal and enhances the surface properties of the natural zeolite without extensive structural degradation.
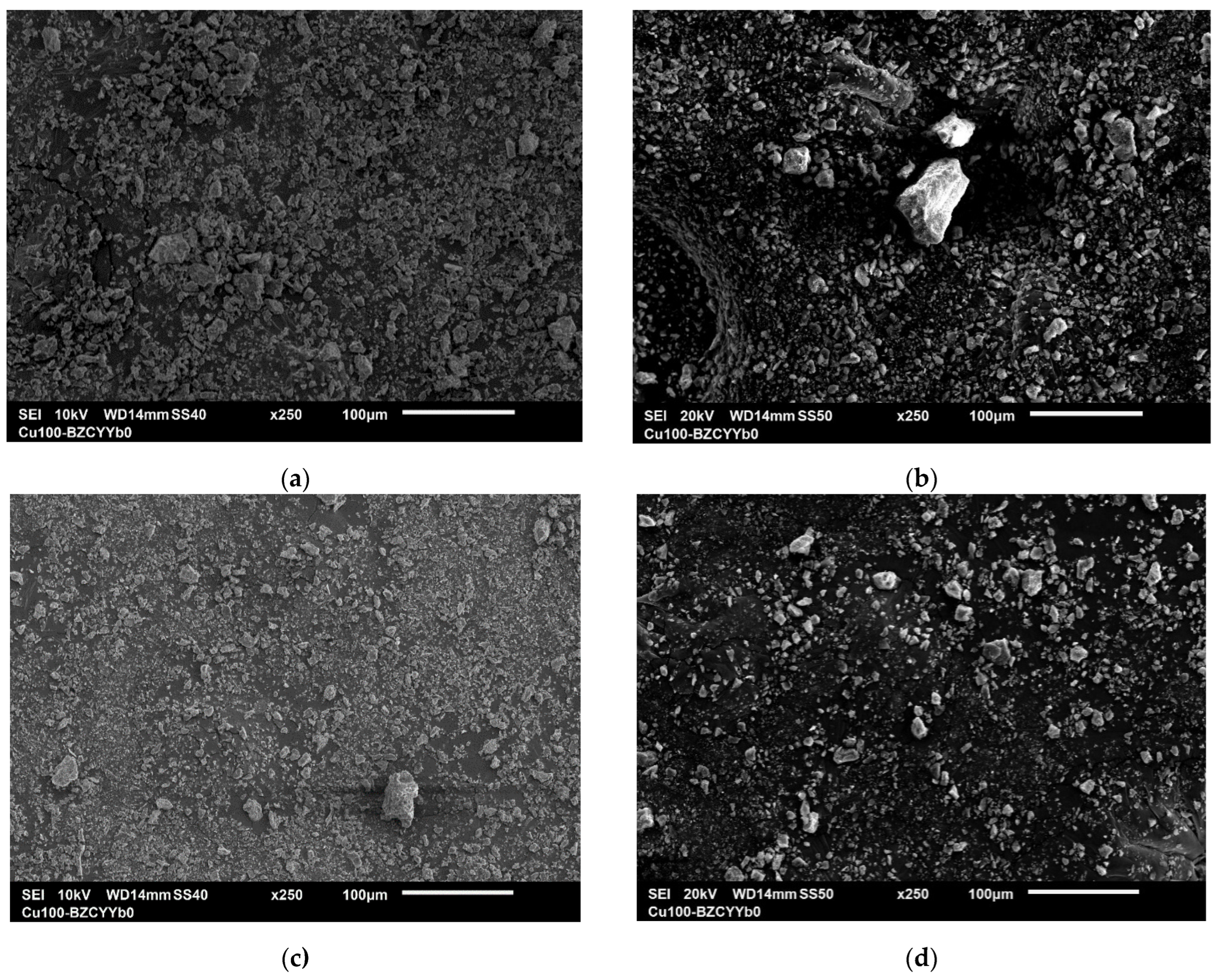
Figure 5 shows micrographs of natural and modified zeolite samples treated with boiling acids. As observed in the SEM images (
Figure 5), the treatment with boiling acids does not cause significant changes in the external structure of the zeolite. However, the surface reveals the opening of internal pores, which enhances the catalytic properties of the samples. The effect of the acids primarily influences the ratio of metals in the composition of the natural zeolite. The phase composition analysis of the samples indicates the removal of alkali and alkaline earth metals, which are replaced by hydrogen ions.
According to SEM data, the morphology of the zeolite particles is preserved before and after acid treatment. The particles retain their initial structure, and no aggregation is observed.
Thus, based on the obtained results from FTIR spectroscopy, XRD, SEM, and elemental analysis, it can be concluded that the crystalline lattice of the natural zeolite remains unchanged, confirming its resistance to aggressive environments.