Submitted:
25 May 2025
Posted:
26 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction to Bioinspired and Smart Nanocarrier Drug Delivery Systems
| Topic | Description |
| Advancement in Targeted Delivery | Development of bioinspired and smart nanocarriers enables site-specific drug delivery, reducing off-target effects and improving therapeutic efficacy. |
| Stimuli-Responsive Systems | Smart nanocarriers are designed to respond to physiological cues (e.g., pH, temperature, enzymes), allowing for controlled and personalized drug release. |
| Nanotechnology in Oncology | Nanocarriers help overcome biological barriers and improve drug accumulation in tumors, significantly enhancing outcomes in cancer treatment. |
2. Liposomal Innovations and Vesicular Drug Delivery Advances
| Subsection | Description |
| Versatility of Liposomal Carriers | Liposomal systems can encapsulate both hydrophilic and hydrophobic drugs, improving their stability and bioavailability while reducing systemic toxicity [4]. |
| Types of Liposomes | Formulations such as multilamellar and stealth liposomes are engineered to enhance circulation time and target specificity, playing a critical role in modern drug delivery [5]. |
| Surface Modification Techniques | Recent strategies involve ligand and polymer surface modifications for site-specific targeting and controlled release, thereby improving therapeutic outcomes [6]. |
| Clinical Potential of Liposomes | Advanced liposomal formulations have shown improved pharmacokinetics and biodistribution, supporting their use across various disease treatments and expanding clinical applicability [7]. |
3. Polymeric Nanoparticles and Programmable Lipid Systems
| Subsection | Description |
| Advantages of Polymeric Nanoparticles | Polymeric nanoparticles are valued for their tunable properties, high biocompatibility, and ability to provide sustained and controlled drug release, making them applicable across various therapeutic areas [8]. |
| Programmable Lipid Nanoparticles | A four-domain structural model enables precision in design and function of lipid nanoparticles, improving targeted delivery and cellular uptake [9]. |
| Diversification of Liposomal Systems | Continuous innovation in liposome design targets specific challenges such as drug loading efficiency, encapsulation stability, and delivery precision [10]. |
| Impact of Liposomal Composition | The pharmacokinetics and therapeutic performance of liposomal drugs are strongly influenced by their composition, highlighting the need for rational formulation and clinical validation [11]. |
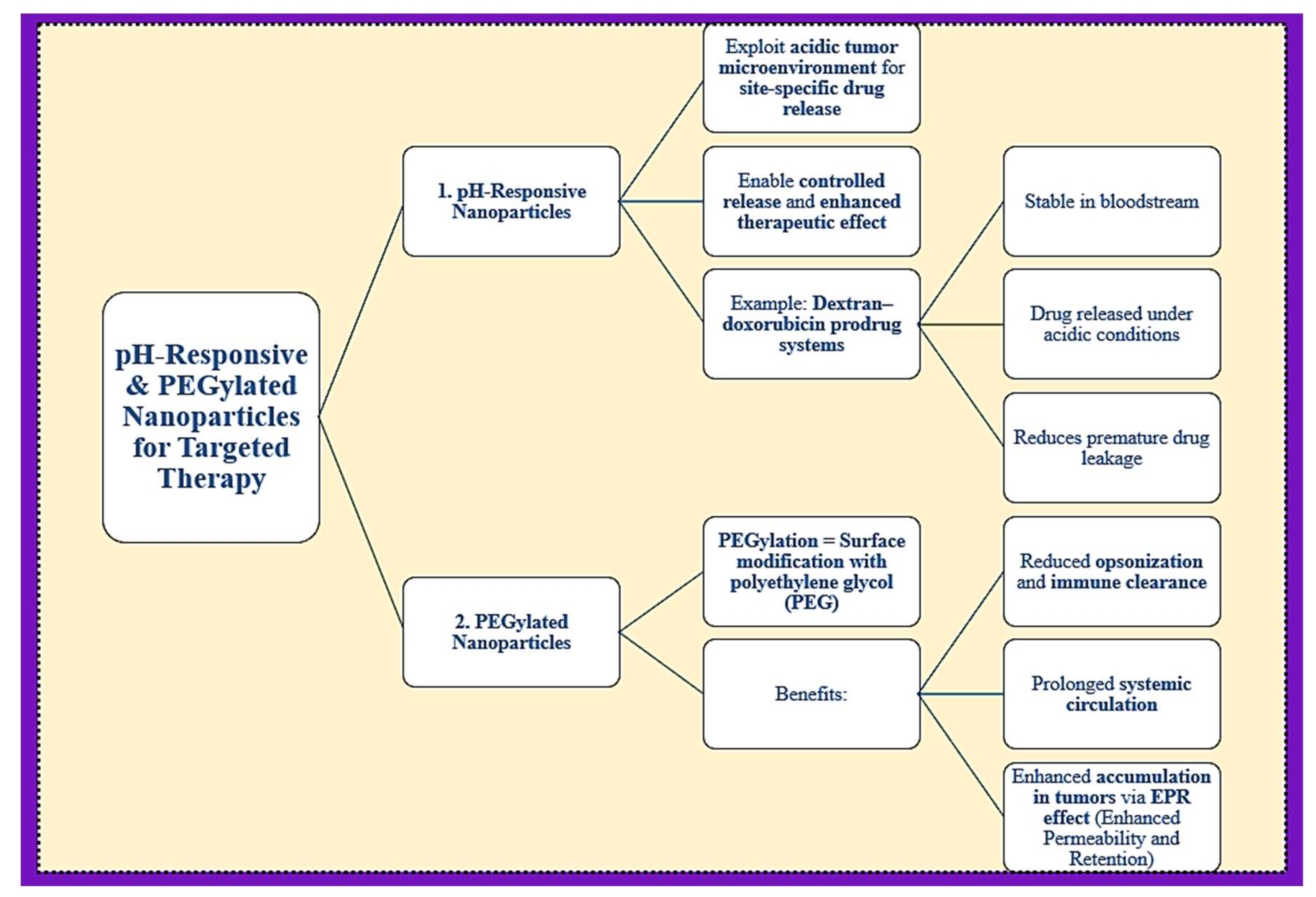
4. pH-Responsive and PEGylated Nanoparticles for Targeted Therapy
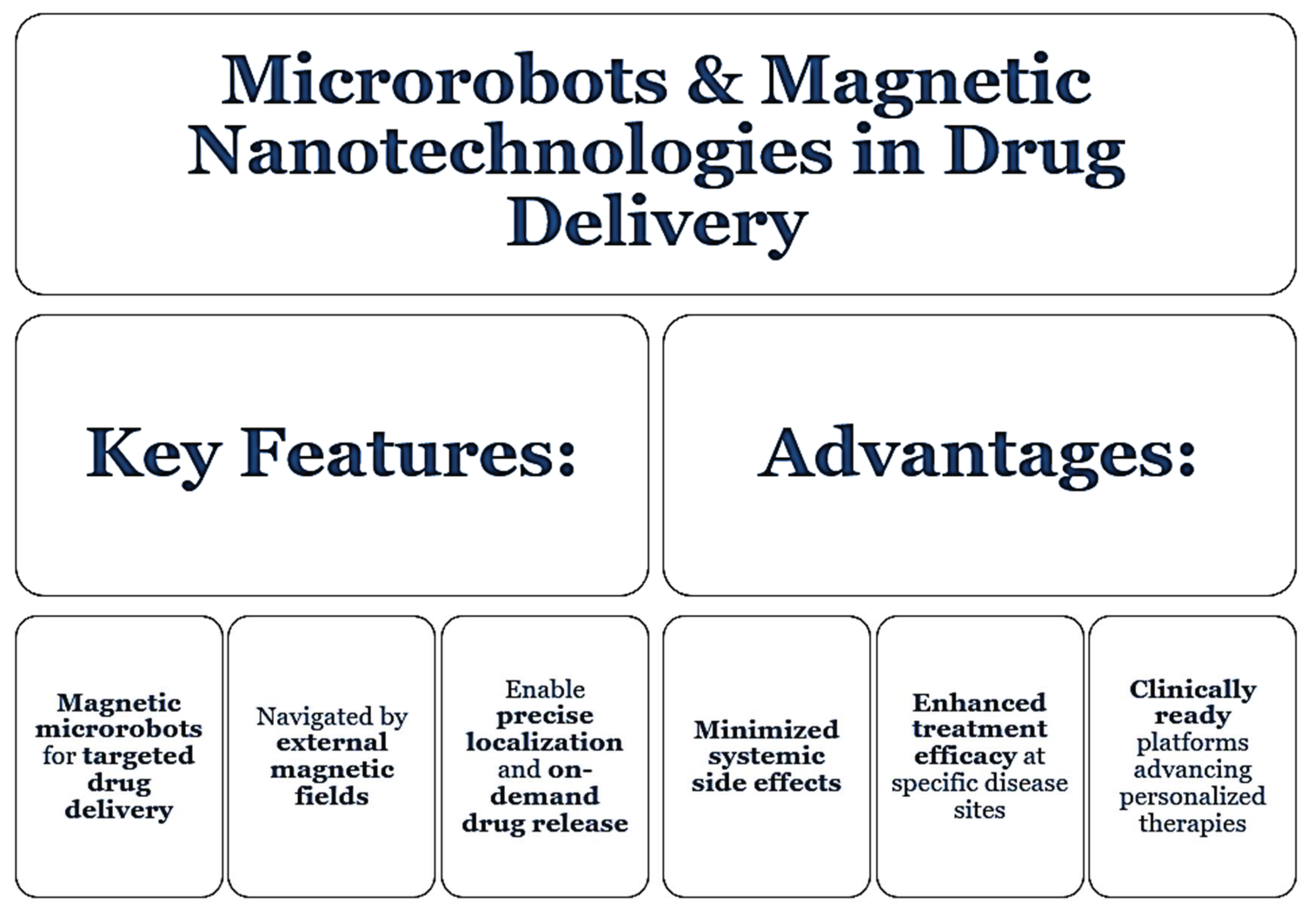
5. Microrobots and Magnetic Nanotechnologies in Drug Delivery
6. Proniosomes, Effervescent Systems, and Herbal-Based Approaches
7. Extracellular Vesicles, Safety, and Translational Considerations
| Subsection | Description |
| Role of Extracellular Vesicles (EVs) | EVs act as natural nanocarriers in intercellular communication and drug delivery due to their high biocompatibility and capability to traverse biological barriers [19]. |
| Interaction with Blood Proteins | Synthetic liposomes can interact with plasma proteins, altering their biodistribution and clearance. Strategic design is essential to improve circulation time and drug stability [20]. |
| Therapeutic Efficacy in Topical Applications | Liposomal vesicles are effective in delivering anti-inflammatory drugs, improving therapeutic outcomes in localized treatments [21]. |
| Historical Significance of Liposomes | Foundational research established liposomes as versatile drug delivery platforms, paving the way for future developments in nanomedicine [22]. |
| Mesoporous Silica Nanoparticles | These nanoparticles exhibit smart characteristics—high drug loading and responsive release—making them particularly useful in colorectal cancer treatment [23]. |
| Expanding Applications in Oncology | Nanotechnology enables improved tumor targeting and reduced toxicity, addressing limitations of conventional therapies [24]. |
| Integration of Liposomal and Nanoparticle Systems | Recent strategies merge liposomes and nanoparticles to optimize targeting, pharmacokinetics, and patient safety, showing promise for clinical translation [25]. |
Conclusions
References
- Sengar, A. (2025). Advancements in Targeted Drug Delivery: Innovations in Liposomal, Nanoparticle, and Vesicular Systems. Int J Bioinfor Intell Comput, 4(2), 1–9.
- Sengar, A. Precision in Practice: Nanotechnology and Targeted Therapies for Personalized Care. Int. J. Adv. Nano Comput. Anal. 2024, 3, 56–67. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A. (2025). Liposomes and Beyond: Pioneering Vesicular Systems for Drug Delivery. Journal of BioMed Research and Reports, 7(1), 1–6. [CrossRef]
- Alavi, M.; Karimi, N.; Safaei, M. Application of Various Types of Liposomes in Drug Delivery Systems. Adv. Pharm. Bull. 2017, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A. (2025). Innovations and Mechanisms in Liposomal Drug Delivery: A Comprehensive Intro-duction. J Emerg Med OA, 3(1), 1–5.
- Akhter, S. , Ahmad, M. Z., Ahmad, F. J., Storm, G., & Alvi, M. M. (2013). Advances in liposomal drug delivery: A review. Current Drug Delivery, 10(5), 546–561.
- Zielińska, A. , Carreiró, F., Oliveira, A. M., Neves, A., & Pires, B. (2020). Polymeric nanoparticles: Pro-duction, characterization, toxicology and ecotoxicology. Molecules, 25(16), 3731.
- Liu, Z. , Chen, J., Xu, M., Gracias, D. H., Yong, K.-T., Wei, Y., & Ho, H.-P. (2024). Advancements in pro-grammable lipid nanoparticles: Exploring the four-domain model for targeted drug delivery. arXiv:2408.05695.
- Tseu, G. Y. , & Kamaruzaman, K. A. (2023). A review of different types of liposomes and their applica-tions. Molecules, 28(3), 1498.
- Large, D. E. , Abdelmessih, R. G., Fink, E. A., & Auguste, D. T. (2021). Liposome composition in drug delivery: design, synthesis, characterization, and clinical application. Advanced Drug Delivery Reviews, 176, 113851.
- Song, J.; Xu, B.; Yao, H.; Lu, X.; Tan, Y.; Wang, B.; Wang, X.; Yang, Z. Schiff-Linked PEGylated Doxorubicin Prodrug Forming pH-Responsive Nanoparticles With High Drug Loading and Effective Anticancer Therapy. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, T.; Ma, X.; Wang, Y.; Lu, Y.; Jia, D.; Huang, X.; Chen, J.; Xu, Z.; Wen, F. The design and synthesis of dextran-doxorubicin prodrug-based pH-sensitive drug delivery system for improving chemotherapy efficacy. Asian J. Pharm. Sci. 2020, 15, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Landers, F. C. , Hertle, L., Pustovalov, V., Sivakumaran, D., Brinkmann, O., Meiners, K.,... & Nelson, B. J. (2025). Clinically ready magnetic microrobots for targeted therapies. arXiv:2501.11553.
- Sengar, A. , Saha, S., Sharma, L., Hemlata, Saindane, P. S., & Sagar, S. D. (2024). Fundamentals of proni-osomes: Structure & composition, and core principles. World Journal of Pharmaceutical Research, 13(21), 1063–1071.
- Sengar, A. , Tile, S. A., Sen, A., Malunjkar, S. P., Bhagat, D. T., & Thete, A. K. (2024). Effervescent tablets explored: Dosage form benefits, formulation strategies, and methodological insights. World Journal of Pharmaceutical Research, 13(18), 1424–1435.
- Sengar, A.; et al. (2025). Advancing urolithiasis treatment through herbal medicine: A focus on Tribulus terrestris fruits. World Journal of Pharmaceutical Research, 14(2), 91–105.
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Chonn, A.; Semple, S.; Cullis, P. Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes. J. Biol. Chem. 1992, 267, 18759–18765. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Trombetta, D.; Venuti, V.; Saija, A.; Bonina, F. Evaluation of in-vivo topical anti-inflammatory activity of indometacin from liposomal vesicles. J. Pharm. Pharmacol. 2004, 56, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G., & Florence, A. T. (1993). Liposomes in drug delivery. Drug Development and Industrial Pharmacy, 19(6), 785–804.
- Mann, R. A. , Hossen, M. E., Withrow, A. D. M., Burton, J. T., Blythe, S. M., & Evett, C. G. (2024). Mes-oporous silica nanoparticles-based smart nanocarriers for targeted drug delivery in colorectal cancer therapy. arXiv:2409.18809.
- Nie, S., Xing, Y., Kim, G. J., & Simons, J. W. (2007). Nanotechnology applications in cancer. Annual Review of Biomedical Engineering, 9, 257–288.
- Sengar, A. (2025). Advancements in Liposomal and Nanoparticle-Based Targeted Drug Delivery. Pharm Res J, 2(1), 1–5.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




