1. Introduction
Microorganisms are among the most biologically active components of soil ecosystems, and play a pivotal role in maintaining ecosystems functionality [
1,
2,
3,
4]. As the main decomposers in farmland ecosystems, they are closely involved in key soil processes, including organic matter decomposition, nutrient cycling, and humus formation [
3,
5]. Currently, soil microbial community diversity is widely recognized as a critical indicator of soil ecological characteristics and serves as an essential metric for assessing soil quality. Soil microorganisms exhibit high sensitive to ecological perturbation, with their diversity and community composition being profoundly affected by field management practices including tillage, fertilization, and irrigation [
6,
7].
SOC is a key determinant of plant productivity and soil fertility, and its maintenance and enhancement essential for ensuring food security [
8,
9]. The active SOC fractions were prone to decomposition, volatilization, poor stability and easy to be absorbed and utilized by plants and microorganisms. As well as the main source of energy for soil microbes that could participate in SOC turnover, regulate crop nutrients availability [
10,
11]. Adopting methods such as straw returning to the field and conservation tillage to optimize the agricultural practice strategies, in order to increase the input of exogenous carbon and improve the soil microbial environment, and further enhance the crop yield [
12,
13].
The selection of appropriate tillage regimes is crucial for improving soil structure, enhancing crop cultivation suitability, optimizing resource utilization, maintaining soil fertility, and increasing crop yields [
14]. Different tillage practices demonstrate significant variations in their effects on soil nutrient content and microbial communities [
15]. Straw returning represent an environmentally sustainable agricultural production measure that enhance nutrient effectiveness, improves soil structure, and induces shifts in the diversity, structure, and composition of soil microbial communities [
16,
17,
18]. As a nutrient-rich resources, straw incorporation increases the input of SOC content. The straw decomposition process, mediated by soil microorganisms, converts stable organic carbon in straw into labile organic carbon fractions, thereby elevating the content of active organic carbon in the soil [
19]. Straw return is commonly implemented using different tillage practices. Studies have shown that straw return with conventional tillage increased SOC content by 3.9%–11.2% in the 10–40 cm soil layer [
20]. Compared to conventional tillage, no-tillage practices are more conducive to forming stable soil aggregates and reducing soil erosion. Straw return combined with NT reduced the soil disturbance frequency relative to the conventional tillage, effectively maintaining soil stability [
21]. Comminated of RT and straw returning has been widely recognized as a crucial sustainable management practice, which can effectively mitigate soil erosion and enhances SOC sequestration [
22]. Busar’s [
23] research confirmed that the deep tillage practice exhibit higher microbial diversity indices, dominance indices, and abundance indices. In addition, Studies have shown that the model of deep tillage with straw return increase soil microbial activity by changing soil structure of the lower plough layer [
24].
As one of the world’s three major staple crops, corn serves as a crucial position in the agricultural economy. However, uncertainty remains regarding the corn yield for straw return with appropriate tillage practices is one of the obstructive factors affecting its large-scale application. Therefore, revealing the impact of conservation tillage in black soil regions on corn yield and its key factors is of vital importance for protecting black soil and ensuring national food security [
25]. Specifically, this study aims to: (1) examine how the integration of straw return combined with three tillage practices to influences SOC, its labile fractions, microbial diversity, community structure, and corn yield; (2) evaluate the interrelationship among soil carbon fractions, microbial diversity, and corn yield. The conclusions of this study will provide a theoretical foundation for developing scientifically sound straw return strategies in agricultural production.
2. Materials and Methods
2.1. Site Description
The experiment was conducted on typical black soil at the Gongzhuling (
Figure 1), located in central Jilin Province, China. The region has cold-temperate continental monsoon climate characterized by synchronous precipitation and thermal regimes, with mean annual precipitation of 500-600 mm, a frost-free period of approximately 140 days, and an average annual temperature of 4.5 ℃.
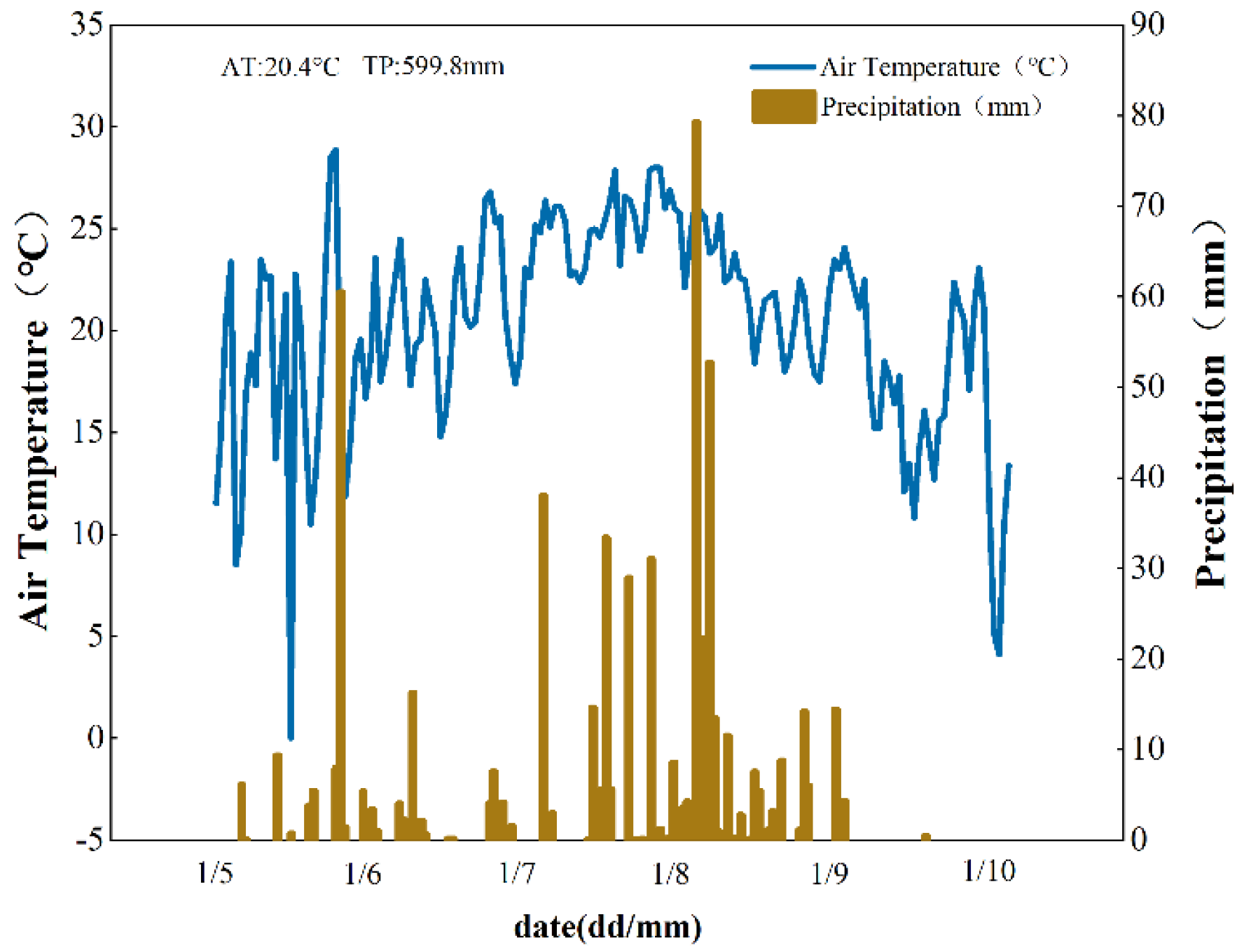
Meteorological data for the experimental period are presented in
Figure 2. The study site received 599.8 mm annual precipitation with a mean, temperature of 20.4 °C in 2019. The corn cropping system followed annual rainfed monoculture without supplemental irrigation.
2.2. Experimental Design
The soil is classified as Mollisols with loamy texture, according to the USDA soil Taxonomy. The in-situ straw mulching experiment was established in spring of 2017, comprising four treatments: (1) Control subjects (CK): Conventional tillage without straw return; (2) No-tillage with straw mulching (NTS): Straw coverage on soil surface using no-tillage planter for seeding; (3) Rotary tillage through straw incorporation (RTS): Mechanically crushed straw uniformly mixed into 0-20 cm soil layer using a rotary tiller; (4) Deep tillage with straw incorporation (PTS): Straw buried at approximately 25 cm depth in the soil. The nutrient content of applied straw is shown in
Table 1.
In this study, the experiment followed a randomized complete block design with three replications. Each plot measured 1040 m
2 in each region, resulting in a total experimental area of 12480 m
2 (12 plots). Fertilizer application was consistent across all treatments, including 220 kg hm
-2 of nitrogen fertilizer, 90kg hm
-2 of phosphorus fertilizer (P
2O
5), and 100 kg hm
-2 of potash fertilizer (K
2O). The corn cultivar Dika159 was sown in late April and harvested in early October. The soil initial physicochemical properties are shown in
Table 2.
2.3. Analysis Methods
Soil samples were collected from 0-20 cm and 20-40 cm depths in July 2019 using the “S” sampling method and trichotomy method. After passing through a 2 mm sieve, samples were stored at -80 °C for subsequent DNA extraction. Genomic DNA was extracted from soil samples using the Cetyltrimethylammonium Bromide (CTAB) method. The bacterial 16S rRNA gene was amplified using primers 27F (5’-AGAGTTTGATCMTGGCTCAG-3’) and 1492R (5’-GGTTACCTTGTTACGACTT-3’), while fungal ITS regions were amplified with primers. ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) [
26,
27,
28]. PCR amplification was performed in a 50 μL reaction system with the following condition: initial denaturation at 94°C for 4 min; denaturation at 94°C for 50 s, annealing at 52°C for 1 min, and extension at 72°C for 1.5 min; followed by a final extension at 72°C for 14 min. Fluorescence spectrophotometry was applied to quantify DNA, and the quality of electrophoresis completion of enriched fragments was assessed using an Agilent 2100 Biosnslyzer. The DNA library fragment size and distribution patterns were investigated and then sequenced in high throughput on the Illumina MiSeq platform [
29]. Soil organic carbon (SOC) concentration was determined using potassium dichromate heating method [
30]. Dissolved organic carbon (DOC) concentration was determined using a TOC analyzer [
31]. Readily oxidizable organic carbon (ROC) was determined by the potassium permanganate oxidation method [
32]. Microbial biomass carbon (MBC) concentration was analyzed by the chloroform fumigation and extraction method [
33]. In each plot, to avoid border effects, three random quadrates reach covering 9 m
2 were selected to determine maize grain yield, measured by adjusting the grain moisture content to 14% [
34].
2.4. Data Analysis
Microbial community alpha diversity indices (Chao1, Shannon, and Simpson) were calculated using QIIME software [
35]. Data processing was performed in Microsoft Excel, while statistical analysis and visualization were conducted using SPSS 22.0 (IBM, USA) and Origin 2022 (OriginLab, USA), respectively. Significant differences among treatments were determined using Duncan’s multiple range test at a 5% significance level (
p < 0.05). The correlation between the measured indicators were evaluated through Pearson correlation analysis [
36].
3. Results
3.1. Crop Yield
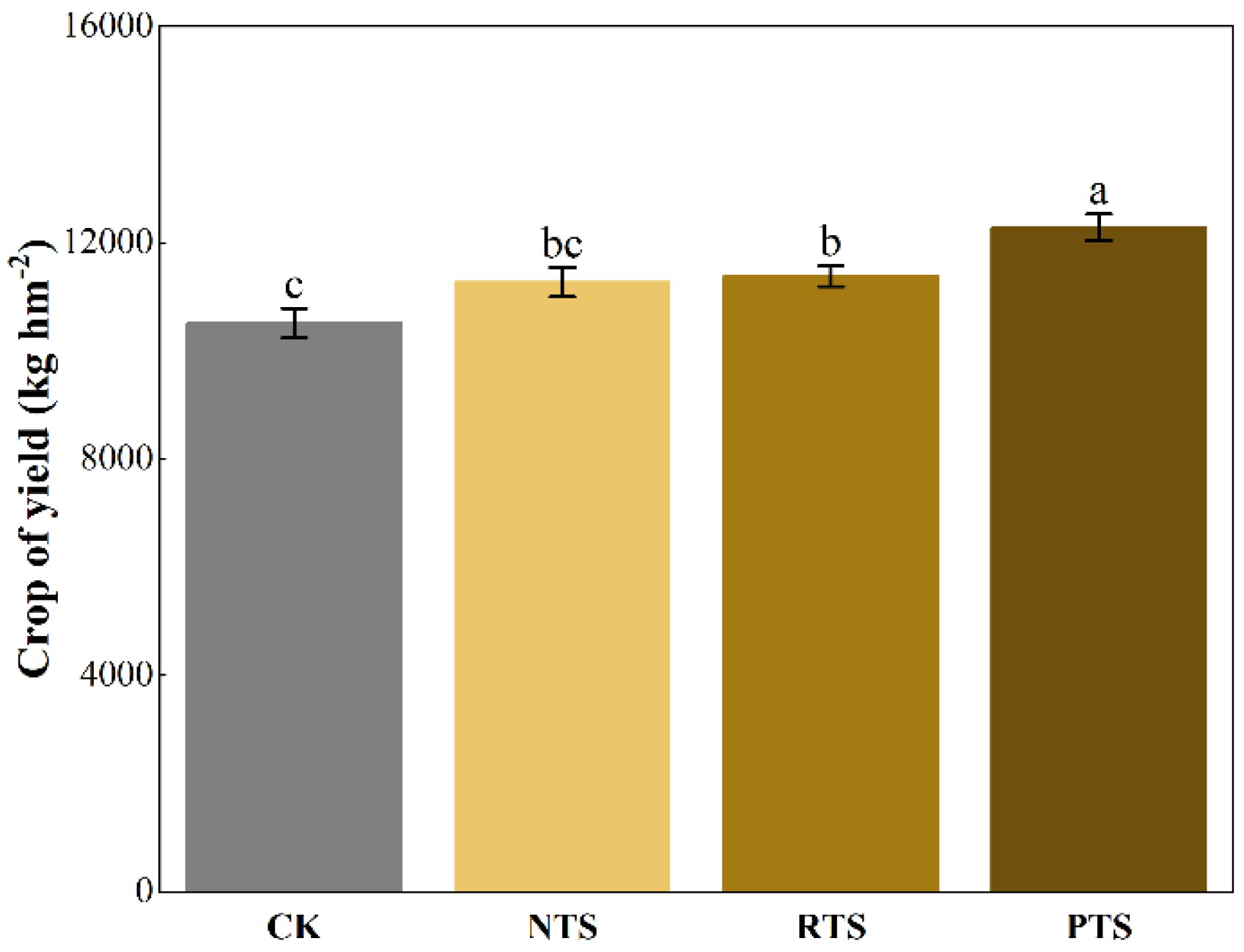
Compared with conventional tillage without straw return (CK), all straw-incorporated treatments (NTS, RTS, and PTS) increased corn yield. Notably, RTS and PTS treatments showed significant yield enhancements of 8.22% and 16.90%, respectively (
p < 0.05;
Figure 3). In addition, the physical and chemical properties of the soil are shown in
Figure S1.
3.2. Soil Organic Carbon and Its Labile Fractions
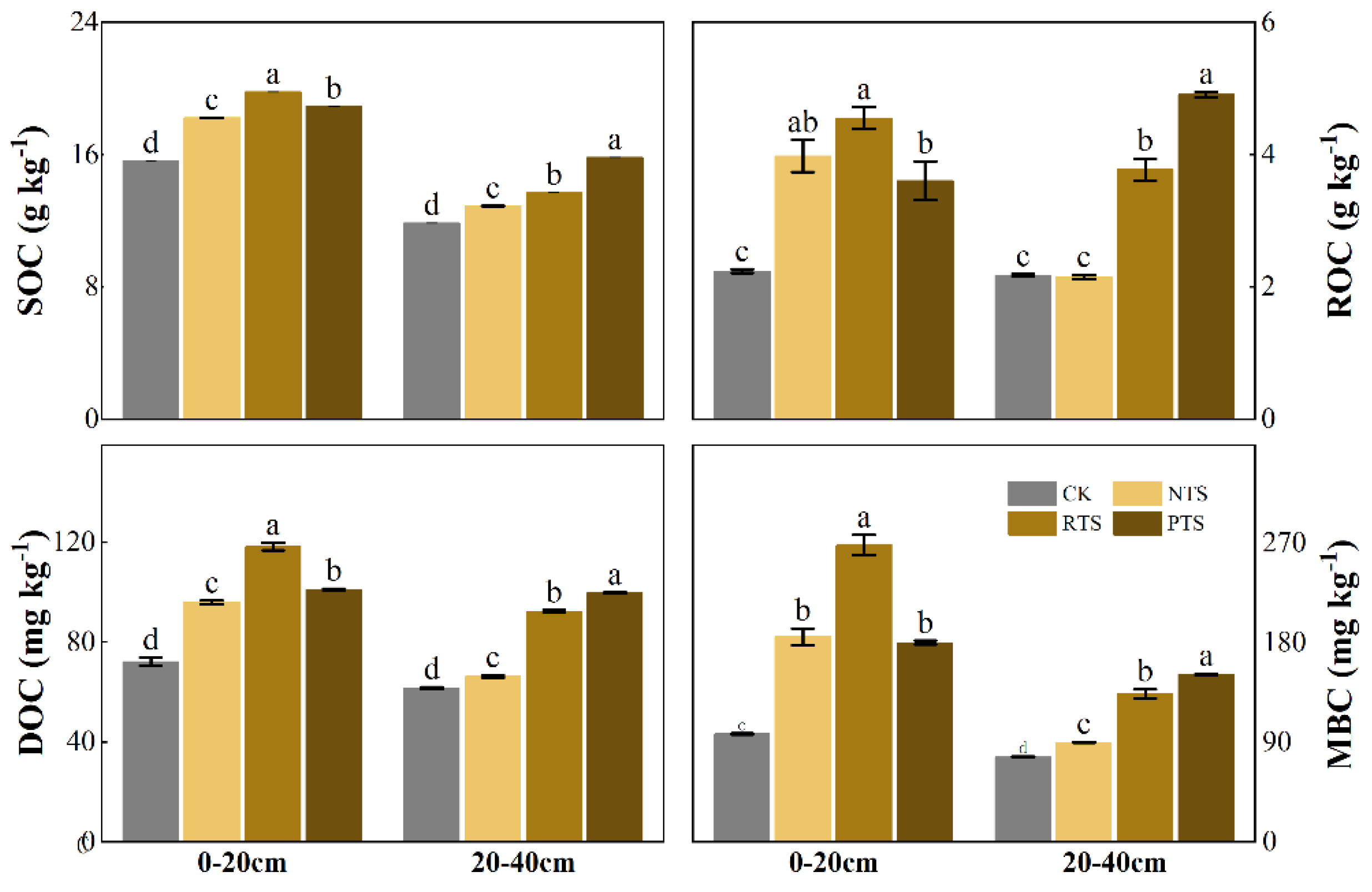
The variations in SOC, ROC, DOC, and MBC contents under different straw return practices are shown in
Figure 4. Generally, the contents of SOC and its labile fractions decreased with increasing soil depth. In the 0-20 cm layer, all straw return treatments (NTS, RTS, and PTS) significantly increased SOC, ROC, DOC, and MBC contents compared to CK (
p < 0.05), with the highest values observed in RTS treatment. In the 20-40 cm layer, these carbon fractions also showed varying degrees of enhancement across treatments, with PTS treatment exhibiting the highest content.
3.3. Diversity of Microbial Bacterial and Fungal Communities
The soil bacterial α-diversity indices showed different patterns among treatments (
Table 3). Overall, the Chao1 index decreased with soil depth, with the lowest values observed in CK and the highest values in RTS (0-20 cm soil layer) and PTS (20-40 cm soil layer) treatments. However, no significant differences were found for Shannon and Simpson indices across treatments.
For fungal communities, the Chao1 index also decreased with increasing soil depth, though this did not affect Shannon and Simpson indices. Compared to CK, all straw return treatments (NTS, RTS and PTS) increased the fungal Chao1 index. In the 0-20 cm layer, RTS treatment showed the highest fungal Chao1 index, while PTS treatment showed the highest fungal Chao1 index in the 20-40 cm layer. The Simpson index exhibited an opposite trend, with decreased values in all treatments. No statistically significant differences were observed for Shannon index among treatments.
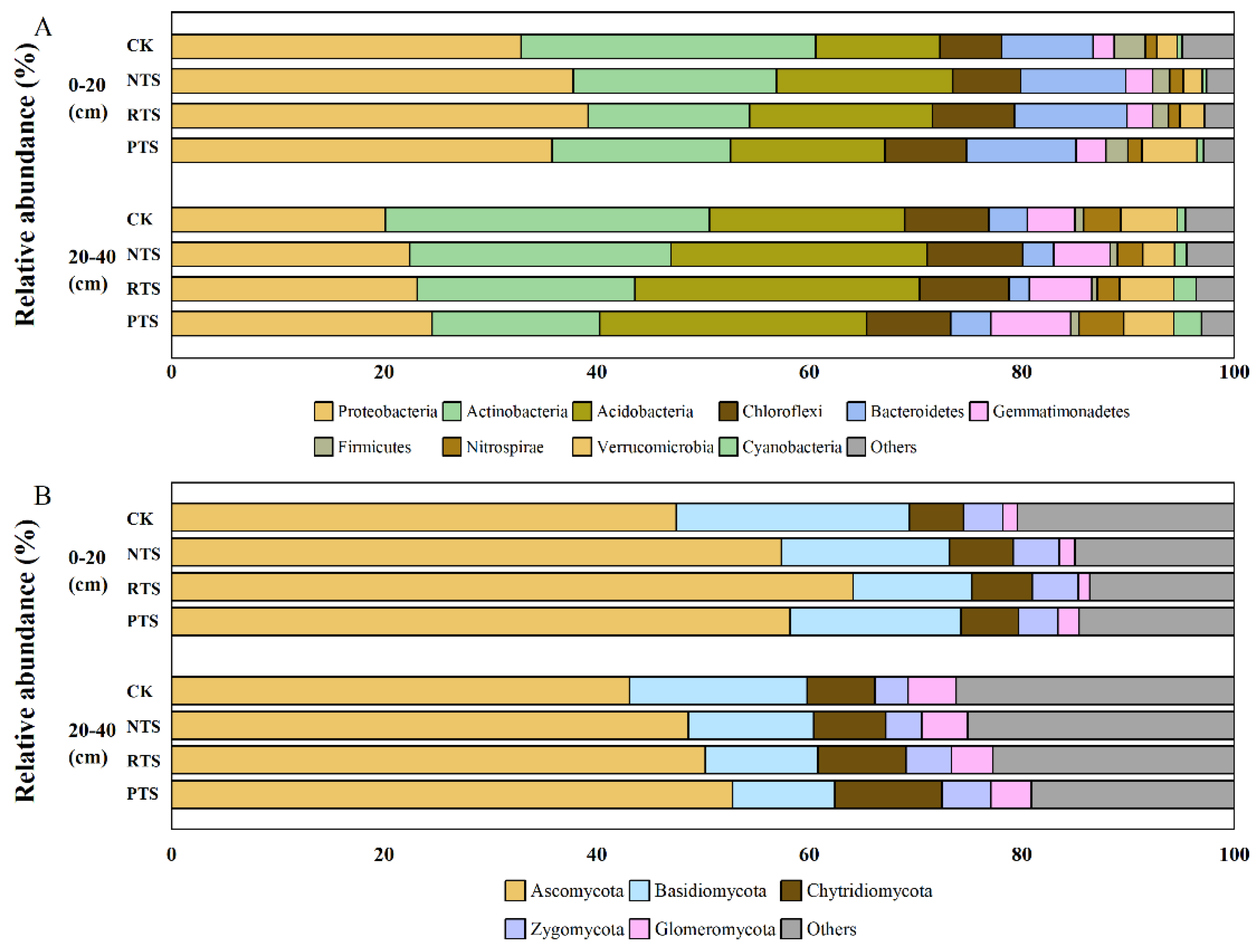
3.4. Composition of Bacterial and Fungal Communities at the Phylum Level
At 0-20 cm depth, straw return treatments significantly increased the relative abundance of
Proteobacteria, Acidobacteria, Chloroflexi and
Bacteroidetes compared to CK (
Figure 5, A). The RTS treatment showed the greatest enhancement, with increases of 19.14%, 47.00%, 32.76% and 23.26%, respectively. In contrast, straw return reduced the relative abundance of Actinobacteria and Firmicutes. At 20-40 cm depth, straw return increased the relative abundance of Proteobacteria,
Acidobacteria and
Gemmatimonadetes. The PTS treatment exhibited the highest increase in Proteobacteria (21.89%). On the contrary, straw return significantly decreased the relative abundance of
Actinobacteria with PTS showing the largest reduction (48.20%).
For fungal communities at 0-20 cm depth, straw return significantly increased the relative abundance of
Ascomycota compared to CK, with the RTS treatment showing the largest increase of 34.95% (
Figure 5, B). Straw return elevated the abundance of
Chytridiomycota and
Zygomycota while reducing that of
Basidiomycota. In deeper soil (20-40 cm), NTS and RTS treatments increased
Ascomycota and
Basidiomycota abundance with depth, whereas PTS boosted
Ascomycota (22.51%) and
Zygomycota (47.39%) while decreasing the abundance of B
asidiomycota and
Glomeromycota.
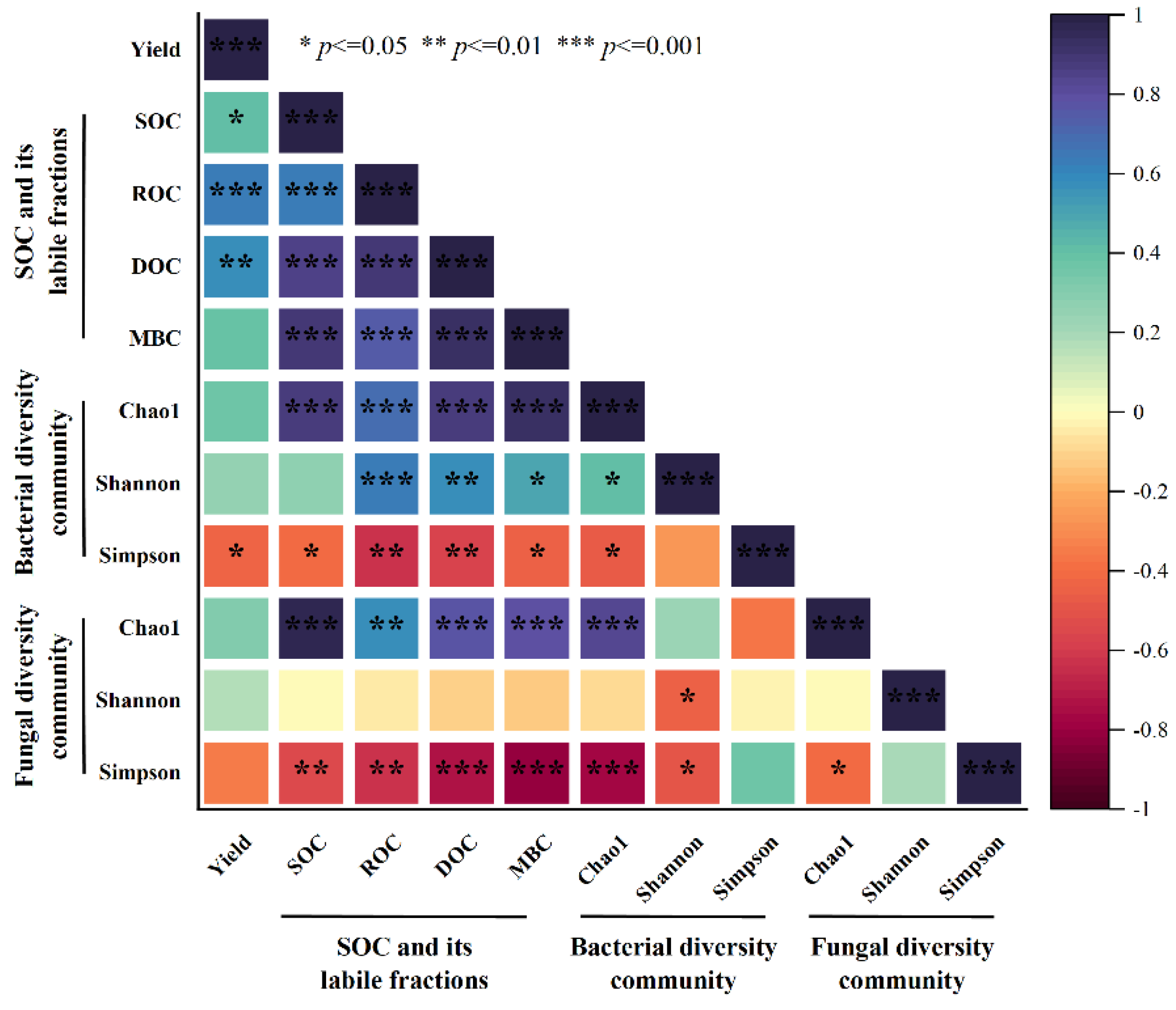
3.5. Correlation Analysis of Soil Microbial Community Composition, Organic Carbon Fractions and Crop Yield
The correlation analysis revealed that, among SOC and its labile fractions, SOC, ROC and DOC showed significant positive correlations with corn yield (p < 0.05). In contrast, the bacterial Simpson diversity index exhibited a significant negative correlation with yield (p < 0.05). Furthermore, soil carbon components (SOC and its labile fractions) were positively correlated with both bacterial and fungal Chao1 diversity indices, while showing negative correlations with Simpson indices (p < 0.05).
4. Discussion
4.1. Effects of Integrated Straw Return and Tillage Practices on Soil Organic Carbon and Its Labile Fractions
SOC serves as a critical component of the soil carbon pool, playing a pivotal role in soil nutrient cycling. Extensive studies have demonstrated that corn straw return increases organic matter input, significantly enhancing both total SOC and labile organic carbon fractions [
37]. The findings of this study align with these observations, showing elevated SOC content across both soil layers under straw incorporation treatments (
Figure 4). In the 0-20 cm layer, the RTS treatment exhibited particularly pronounced SOC accumulation. This enhancement may be attributed to improved straw-soil contact through rotary tillage, which optimizes soil aeration and water infiltration while stimulating microbial activity to accelerate straw decomposition. These mechanisms collectively favor SOC sequestration under RTS treatment [
38]. The PTS treatment facilitated SOC accumulation in deeper soil profiles (0-40 cm) through direct input of exogenous carbon to subsoil layers and enhanced root growth and rhizodeposition induced by deep tillage [
39].
As sensitive indicators of soil quality, labile SOC fractions (DOC and ROC) respond rapidly to agricultural management practices like straw return [
40]. DOC represents a readily available microbial carbon source, while ROC comprises oxidizable and mineralizable organic components [
10]. The findings of this study suggest two mechanisms for the increase in organic carbon content of labile SOC fractions under different straw return treatments: (1) Direct input of decomposable straw-derived carbon elevating DOC and ROC pools; (2) Straw decomposition enhanced microbial activity, driving the transformation of organic carbon [
41]. The RTS and PTS treatments had the highest DOC content in the 0-20 cm and 20-40 cm soil layers, respectively. This might be because in RTS, the straw was fully mixed with the soil, which promoted the decomposition rate of straw and facilitated the rapid release of dissolved substances [
42]; the PTS treatment promoted the migration of active organic carbon in the soil.
4.2. Effects of Integrated Straw Return and Tillage Practices on the Diversity of Microbial Communities
The RTS treatment significantly increased both the bacterial Chao 1 and Shannon indices of soil bacteria at 0-20 cm depth (Table.3). The highest Chao 1 index was observed in the PTS treatment at 20-40 cm depth, indicating that straw incorporation promotes the growth of deep soil bacteria microbial community diversity. However, the Simpson index remained unaffected by straw return. These findings align with Li et al. [
43], who reported inconsistent patterns in bacteria Simpson indices across different studies. The large amount of nutrients in straw promotes the growth and reproduction of microorganisms, thus increasing bacterial diversity in farmland soils.
For fungal communities, diversity and richness varied depending on the soil environment and farmland management practices [
44,
45]. Straw return also increased the Chao l index of soil fungi across treatments. The RTS treatments showed the highest fungal diversity in soil at 0-20 cm depth, likely due to straw-induced modifications in SOC content, soil bulk weight, and moisture conditions that collectively influenced fungal community development. Notably, the PTS treatment exhibited the highest mycorrhizal diversity in deeper soil layer [
46], suggesting that deep straw incorporation can modify soil physical properties and consequently enhance overall fungal community diversity in deeper soil profiles [
44,
46,
47].
4.3. Effects of Integrated Straw Return and Tillage Practices on Bacterial and Fungal Community Composition
Fierer et al. [
48] demonstrated that
Proteobacteria in soil primarily participate in organic matter transformation and soil structure formation, being closely associated with carbon utilization. In this study, straw incorporation increased the content of labile organic carbon (
Figure 4), which consequently led to an increase in the relative abundance of
Proteobacteria. Moreover, deep tilling of straw also further enhanced the abundance of
Proteobacteria in deeper soil [
47,
49]. Compared to CK, straw return also increased the relative abundance of
Bacteroidetes. As a beneficial bacteria phylum involved in carbon and nitrogen cycle,
Bacteroidetes with soil carbon availability, explaining their increased relative abundance. Certain microorganisms within
Bacteroidetes possess the capability utilize carbon sources, and straw return specifically promoted the enrichment of these microbial groups [
50]. Notably, B
acteroidetes can secrete
Carbohydrate-active enzymes in soil that facilitate the decomposition of polysaccharides into plant-available forms [
51].
In this study,
Ascomycota and
Basidiomycota were the dominant soil fungal phyla across different soil layer and treatments, with
Ascomycota showing the highest relative abundance.
Ascomycota are well adapted to soil environments and represent a dominant phylum in soils, consistent with previous studies [
1]. Exogenous input of organic matter has been shown to effectively increase the relative abundance of
Ascomycota in soil [
52]. The results of this study demonstrate that straw return effectively increased soil labile organic carbon content, thereby promoting the growth and multiplication of microorganisms of
Ascomycota. Studies have shown that
Ascomycetes respond faster to changes in soil labile organic carbon, while
Basidiomycota can decompose to produce specific enzymes to degrade decomposition-resistant carbon compounds in soil [
53]. In addition, NTS increased the relative abundance of
Ascomycetes and
Zygomycotain in the subsurface soil layer (0-20 cm), which may be attributed to improved soil physicochemical conditions under straw mulching. Liu et al. similarly demonstrated that straw return could promote soil moisture storage, increase soil surface temperature, and improve the topsoil environment, consequently increasing the relative abundance of
Ascomycetes and
Zygomycota [
54]. The changes in the relative abundance of each phylum differed between the two soil layers examined. In addition, straw decomposition also produces organic matter that contributes to the increase of fungal flora, creating favorable growth conditions for
Basidiomycota and supporting their rapid growth.
4.4. Responses of Corn Yield to Integrated Straw Return and Tillage Practices
Different tillage practices can affect corn yield by altering soil properties and hydrothermal conditions [
55]. The results of this study showed that all straw incorporation treatments increased corn yield regardless of tillage method. However, the NTS treatment did not achieve significant yield improvement because straw mulching reduces soil temperature, which may negatively affect early crop growth, particularly in the cold climate of Northeast China [
56]. In contrast, both RTS and PTS treatments significantly increased yield. This can be attributed to: (1) the abundant nutrients in straw that become available for microbial uptake after decomposition, especially carbon, thereby participating in nutrient cycling and increasing soil organic matter content; and (2) the slow release rate of straw-derived nutrients, which can continue to provide nutrients during the later growth stages of corn。
The integrated straw return and tillage practices affected corn yield through multiple pathways. Correlation analysis revealed that SOC, its labile fractions, and soil microbial diversity collectively influenced corn yield. Specifically, SOC, ROC and DOC contents were positive factors affecting yield, while the bacterial Simpson diversity index showed a significant negative correlation with yield (
Figure 6). These findings are consistent with Ma et al. [
57], who reported that straw return increases both total SOC and the stability of labile organic carbon, thereby enhancing soil carbon sequestration capacity and improving fundamental soil productivity. Previous studies have demonstrated a linear relationship between SOM content and crop yield [
58]. Tillage practices optimize microbial community composition by altering bacterial abundance and diversity. The negative effect of the Simpson index may be related to uneven distribution of microbial populations and competition among microorganisms.
In Northeast China, where corn accounts for 31% of the nation’s total planting area [
25], the experimental results demonstrate the importance of straw return for yield improvement. When combined with appropriate tillage practices, straw return can effectively enhance corn productivity.
5. Conclusions
This study examined three straw incorporation treatments (no-tillage, rotary tillage, and deep tillage) in Northeast black soils. All treatments promoted soil organic carbon (SOC) and its labile fractions, enhanced soil microbial diversity (Chao1 and Shannon indices), and improved bacterial and fungal community structures at the phylum level, and increased corn yield. Microbial communities and soil organic carbon fractions are sensitive indicators for monitoring soil quality and crop yield.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Soil physical and chemical properties in the different integrating straw return and tillage practices.
Author Contributions
Data Curation, Formal analysis, Investigation, Visualization, Writing - Original Draft Preparation, Writing - Review & Editing, L. F.; Writing - Review & Editing, G. C.; Conceptualization, Formal analysis, Visualization, Writing - Original Draft Preparation, Funding acquisition, Project administration Writing - Review & Editing, Y. S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [National Key Research and Development Program of China], grant number [2021YFD1500105], and [Jilin Province Science and Technology Development Plan], grant number [20240203007NC].
Institutional Review Board Statement
No applicable.
Informed Consent Statement
No applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, X.C.; Sun, L.Y.; Song, F.B.; Liu, S.Q.; Liu, F.L.; Li, X.N. Soil microbial community and activity are affected by integrated agricultural practices in China. European Journal Soil Science, 2018, 69, 924-935. [CrossRef]
- van der Heijden, M.G.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters,2008, 11, 296-310. [CrossRef]
- Zak, J.C.; Willig, M.R.; Moorhead, D.L.; Wildman, H.G. Functional diversity of microbial communities: A quantitative approach. Soil Biology and Biochemistry, 1994, 26, 1101-1108. [CrossRef]
- Finlay, B.J. Global dispersal of free-living microbial eukaryote species. Science, 2002, 296, 1061-1063. [CrossRef]
- Ma, J.Q.; Ma, Y.; Wei, Z.; Wu, J.; Sun, C.; Yang, J.; Liu, L.; Liao, H. Effects of arbuscular mycorrhizal fungi symbiosis on microbial diversity and enzyme activities in the rhizosphere soil of Artemisia annua. Soil Science Society of America Journal, 2021, 85, 703-716. [CrossRef]
- Tang, Z.; Zhang, L.; He, N.; Gong, D.; Gao, H.; Ma, Z.; Fu, L. Soil bacterial community as impacted by addition of rice straw and biochar. Scientific Reports, 2021, 11, 22185. [CrossRef]
- Mueller G. M., JP, S. Fungal biodiversity: what do we know? What can we predict? Biodiversity and Conservation, 2007, 1, 1-5. [CrossRef]
- Poeplau, C.; Dechow, R. The legacy of one hundred years of climate change for organic carbon stocks in global agricultural topsoils. Scientific Reports, 2023,13, 7483. [CrossRef]
- Mason, A.R.G.; Salomon, M.; Lowe, A.; Cavagnaro, T. Microbial solutions to soil carbon sequestration. Journal of Cleaner Production. 2023, 417, 137993. [CrossRef]
- Liu, C. Z.; Chang, D.; Li, B. Cao, W.; Lu, Y.; Pan, Z. Effects of planting and incorporation of Chinese Milk Vetch coupled with application of chemical fertilizer on active organic carbon and nitrogen in paddy soil. Acta Pedologica Sinica, 2017, 54(3): 657–669. [CrossRef]
- Cui, H. X.; Luo, Y.; Chen, J.; Jin, M.; Li, Y. Straw return strategies to improve soil properties and crop productivity in a winter wheat-summer maize cropping system. European Journal of Agronomy, 2022, 133, 126436. [CrossRef]
- Dikgwatlhe, S.B.; Chen, Z.-D.; Lal, R., Zhang, H.-L.; Chen, F. Changes in soil organic carbon and nitrogen as affected by tillage and residue management under wheat-maize cropping system in the North China Plain. Soil Tillage Research. 2014,144, 110–118. [CrossRef]
- Ding, J.L.; Wu, J.; Ding, D.; Yang, Y.; Gao, C. Effects of tillage and straw mulching on the crop productivity and hydrothermal resource utilization in a winter wheat-summer maize rotation system. Agricultural water management, 2021, 254, 106933. [CrossRef]
- Pan Y.W.; Fan, J.; Hao, M.; Chen, X. Effects of long-term tillage and mulching methods on properties of surface soil and maize yield in tableland region of the Loess Plateau. Journal of Plant Nutrition and Fertilizers, 2016, 22(6): 1558-1567. [CrossRef]
- Wang, Y. L.; Li, J. Study of tillage patterns suitable for soil physicochemical properties and crop yields in wheat/maize fields. Journal of Plant Nutrition and Fertilizers, 2014, 20(5): 1139–1150. [CrossRef]
- Li, H.D.; LI, j.; Jiao, X.; Jiang, H.; Liu, Y.; Wang, X. The fate and challenges of the main nutrients in returned straw: a basic review. Agronomy-Basel, 2024. 14(4), 698. [CrossRef]
- Liu, N; Li, Y.; Cong, P.; Wang, J.; Guo, W.; Pang, H. Depth of straw incorporation significantly alters crop yield, soil organic carbon and total nitrogen in the North China Plain. Soil & Tillage Research, 2021. 205, 104772. [CrossRef]
- Sun, X.D.; Men, X.; Huang, W.; Yi, S.; Wang, W.; Zhang, F.; Zhang, Z.; Wang, Z. Effects of exiguobacterium sp. DYS212, a saline-alkaline-tolerant P-solubilizing bacterium, on Suaeda salsa germination and growth. Sustainability, 2023. 15(7):6259. [CrossRef]
- Zhang, Z. M.; Yan, J.; Han, X.; Zou, W.; Chen, X.; Lu, X. Labile organic carbon fractions drive soil microbial communities after long-term fertilization. Global Ecology and Conservation, 2021, 32: e01867. [CrossRef]
- Wang, H.G.; Yu, Z.; Shi, Y.; Zhang, Y. Effects of tillage practices on grain yield formation of wheat and the physiological mechanism in rainfed areas. Soil Tillage Research, 2020, 202, 104675. [CrossRef]
- Dong, L.; Shi, X.; Xu, S.; Wang, M. Effects of different management measures on the organic carbon of farmland soil profile in China based on Meta analysis. Soils, 2021, 53 (6), 1290–1298. [CrossRef]
- Xiao, L.A.; Zhou, S.; Zhao, R.; Wei, C. The net and combined effects of minimum tillage and straw mulching on carbon accumulation in global croplands. European Journal Agron, 2023, 143, 126719. [CrossRef]
- Busari, M.A.; Kukal, S.; Kaur, A.; Bhatt, R.; Dulazi, A. Conservation tillage impacts on soil, crop and the environment. International Soil and Water Conservation Research. 2015, 3 (2), 119–129. [CrossRef]
- Essel E., 2019, Long-term tillage effects on soil microbial diversity, CO2 emission and the underlying mechanisms in wheat-pea rotation field in the Semi-arid Loess Plateau, China, Dissertation for Ph.D., Gansu Agricultural University, Supervisor: Li L.L., pp.16.
- Li, R. P.; Xie, R.; Luo, Y. Sui, P. Zheng, H.; Ming, B.; Wang, H. Effects of conservation tillage methods on maize growth and yields in a typical black soil region. Chinese Journal of Eco-Agriculture, 2024, 32(1): 71−82. [CrossRef]
- Liu, B. S.; Hu, Y.; Wang, Y.; Xue, H.; Li, Z. Effects of saline-alkali stress on bacterial and fungal community diversity in Leymus chinensis rhizosphere soil. Environment Science Pollution Research. 2022, 29(46), pp. 70000-700013. [CrossRef]
- Sun, T.; Miao, J.; Saleem, M.; Zhang, H.; Yang, Y. Bacterial compatibility and immobilization with biochar improved tebuconazole degradation, soil microbiome composition and functioning. Journal of Hazardous Materals.2020, 398, 122941. [CrossRef]
- Miao, T.; Gao, S.; Jiang, S.; Kan, G.; Liu, P.; Wu, X.; AN, Y.; Yao, S. A method suitable for DNA extraction from humus-rich soil. Biotechnology Letters. 2014, 36(11), 2223-2228. [CrossRef]
- Maguire, V.G.; Bordenave, C.; Nieva, A.; Llames, M.; Colavolpe, M. Soil bacterial and fungal community structure of a rice monoculture and rice-pasture rotation systems. Applied Soil Ecology, 2020, 151, 103535. [CrossRef]
- Lu, R. Analytical Methods for Soil Agricultural Chemistry. China Agricultural Science and Technology Press, Beijing.2000.
- Zhao, W. Q.; Walker, S.; Huang, Q.; Cai, P. Adhesion of bacterial pathogens to soil colloidal particles: influences of cell type, natural organic matter, and solution chemistry. Water Research, 2014, 53, 35-46. [CrossRef]
- Blair, G.J.; Lefroy, R.; Lise, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Australian Journal of Agricultural Research, 1995, 1459-1466. [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G. Jenjinson, D. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biology and Biochemistry, 1985, 17, 837-842. [CrossRef]
- Su, Y.; Yu, M.; Xi, H.; Lv, J.; Ma, Z.; Kou, C.; Shen, A. Soil microbial community shifts with long-term of different straw return in wheat-corn rotation system. Scientific Reports ,2020, 10, 6360. [CrossRef]
- Schloss, P.D.; Gevers, D.; Westcott, S.L. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. Plos One,2011, 6, e27310. [CrossRef]
- Zhang, Y.; Wu, C.; Deng, S. Zhang, J.; Hou, J. Wang, C.; Fu, Z. Effect of different washing solutions on soil enzyme activity and microbial community in agricultural soil severely contaminated with cadmium. Environmental Science and Pollution Research International, 2022, 29, 54641-54651. [CrossRef]
- Cong, Y.; H, J. Maize straw returning for three consecutive years: effects on soil physical and chemical properties and crop yield. Chinese Agricultural Science Bulletin. 2016, 34(17):95-98. [CrossRef]
- Tian, P.; Jiang, Y.; Sun, Y.; Ma, Q.; Sui, P. Effect of straw return methods on maize straw decomposition and soil nutrients contents. Chinese Journal of Eco-Agriculture, 2019, 27(1): 100-108. [CrossRef]
- Wei, B. H. Yield increasing and quality improving effects of smash-ridging method and its potential in benefiting the nation and the people. Agricultural Science and Technology, 2014, 15(10):1767-1769. [CrossRef]
- Lu, W. T.; Jia, Z.; Zhang, P.; Wang, W.; Hou, X.; Yang, B. Effects of straw returning on soil labile organic carbon and enzyme activity in Semi-arid areas of Southern Ningxia, China. Journal of Agro-Environment Science, 2011,30(3):522-528. https://doi.org/CNKI:SUN:NHBH.0.2011-03-020.
- Dhaliwal, S. S.; Naresh, R.; Gupta, R.; Panwar, A.; Mahajan, N. Effect of tillage and straw return on carbon footprints, soil organic carbon fractions and soil microbial community in different textured soils under rice–wheat rotation: a review. Reviews in Environmental Science and Bio/Technology, 2020, 19(1):103-115. [CrossRef]
- Johansson, J. F.; Paul, L.; Finlay, R. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiology Ecology, 2010, 48(1):1-13. [CrossRef]
- Li, Z. Q.; Li, D.; Ma, L.; Yu, Y.; Zhao, B. Effects of straw management and nitrogen application rate on soil organic matter fractions and microbial properties in North China Plain. Journal of Soil Sediment, 2019, 19, 618-628. [CrossRef]
- Brennan, E.B.; Acosta-Martinez, V. Cover cropping frequency is the main driver of soil microbial changes during six years of organic vegetable production. Soil Biology and Biochemistry, 2017, 109, 188-204. [CrossRef]
- Wang, Y.; Jin, Y.; Han, P.; Hao, J. Impact of soil disinfestation on fungal and bacterial communities in soil with cucumber cultivation. Front Microbiol, 2021, 12, 685111. [CrossRef]
- Zhu, F. N.; Lin, X.; Guan, S.; Dou, S. Deep incorporation of corn straw benefits soil organic carbon and microbial community composition in a black soil of Northeast China. Soil Use Manage, 2022, 38, 1266-1279. [CrossRef]
- Chen, H.; Yin, C.; Fan, X.; Ye, M.; Liang, Y. Effect of P availability on straw-induced priming effect was mainly regulated by fungi in croplands. Applied Microbiology and Biotechnology, 2021, 105, 9403-9418. [CrossRef]
- Fierer, N.; Mark, A.; Robert, B. Toward an ecological classification of soil bacteria. Ecology, 2007,88(6): 1354-1364. [CrossRef]
- Patkowska, E.; Blazewicz-Wozniak, M.; Konopinski, M. The effect of cover crops on the fungal and bacterial communities in the soil under carrot cultivation. Plant, Soil and Environment, 2016, 62, 237-242. [CrossRef]
- Liu, Y. L.; Gu, Y.; Wu, C.; Zhao, H.; Hu, W. Short-term straw returning improves quality and bacteria community of black soil in Northeast China. Polish Journal of Environmental Studies, 2022, 31, 1869-1883. [CrossRef]
- Larsbrink, J.; Tuveng, T.; Pope, P.; Bulone, P.; Eijsink, V. Proteomic insights into mannan degradation and protein secretion by the forest floor bacterium Chitinophaga pinensis. Journal of Proteomics, 2017, 56, 63-74. [CrossRef]
- Fan, W.; Wu, J.; Changes in soil fungal community on SOC and POM accumulation under different straw return modes in dryland farming. Ecosystem Health and Sustainability, 2021, 7,1935326. [CrossRef]
- Breulmann, M.; Masyutenko, N.; Kogut, B.; Schroll, R. Short-term bioavailability of carbon in soil organic matter fractions of different particle sizes and densities in grassland ecosystems. Science of the total Environment, 2014, 497, 29-37. [CrossRef]
- Liu, D.; Wang, H.; An, S.; Bhople, P.; Davlatbekov, F. Geographic distance and soil microbial biomass carbon drive biogeographical distribution of fungal communities in Chinese Loess Plateau soils. Science of the total Environment, 2019, 660, 1058-1069. [CrossRef]
- Zhang, L. H.; Xu, C.; Yu, J.; Yan, W.; Sun, N.; Tan, G.; Zhao, H.; Li, F.; Meng, X.; Bian, S. Effects of straw returning on soil moisture, temperature and maize yield in semi humid area. Journal of Soil and Water Conservation, 2021, 35(4):299−306. [CrossRef]
- Drury. C. F.; Tan, C.; Welacky, T.; Oloya, T.; Hamill, A. Red clover and tillage influence on soil temperature, water content, and corn emergence. Agronomy Journal, 1999, 91(1): 101−108. [CrossRef]
- Ma, P.; Zhang, Y.; Lin, C.; Lv, X. Effects of nitrogen fertilizer input and rice season nitrogen fertilizer application on soil nutrients,carbon pool and yield in rape-rice rotation. Jiangsu Journal of Agricultural Science, 2020,36 (4): 896-904. https://doi.org/CNKI:SUN:JSNB.0.2020-04-014.
- Zheng, J. S.; Hu, Y.; Wei, Y.; Shen, Z.; Meng, Y. Effects of Green Manure Returning on Soil Labile Organic Carbon in Paddy Field Under Smash Ridging. Soils, 2021, 53(2): 368–374. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).