Introduction
Laryngeal squamous cell carcinoma (LSCC) represents the predominant malignancy of the larynx, accounting for approximately 95% of cases [
1]. Locally advanced LSCC (LA-LSCC) presents a significant clinical challenge characterized by a higher risk of treatment failure compared to earlier stages, increased functional morbidity affecting phonation, deglutition, and respiration, and a less favorable prognosis with diminished survival rates [
2,
3].
The strategy of employing induction chemotherapy followed by definitive chemoradiotherapy in locally advanced laryngeal squamous cell carcinoma (LA-LSCC) is predicated on several key rationales [
4]. Induction chemotherapy, typically administered prior to the main locoregional treatment, aims to achieve tumor downstaging, eradicate micrometastatic disease, and assess the tumor’s chemosensitivity [
5]. Potential advantages of this approach include an increased likelihood of organ preservation by reducing tumor bulk, potentially enhancing the efficacy of subsequent chemoradiotherapy by improving tumor oxygenation and reducing the burden of disease [
6]. Furthermore, by identifying chemosensitive tumors early, induction chemotherapy may help to select patients who are more likely to benefit from further aggressive treatment, while potentially sparing less responsive patients from unnecessary toxicities associated with definitive chemoradiotherapy alone [
7,
8]. While its impact on overall survival remains a subject of ongoing research, this sequential approach seeks to optimize locoregional control and improve functional outcomes in this challenging patient population [
7].
MicroRNA-449a (miR-449a) is a small non-coding RNA with a significant role in cancer. Primarily a tumor suppressor, it targets oncogenes, inhibiting cell proliferation, migration, invasion, and promoting apoptosis by modulating pathways involving genes like CDK6, E2F3, ZEB1, and Snail [
9]. Downregulation of miR-449a is common in cancers like prostate, lung, gastric, and laryngeal, correlating with aggressive behavior and poor prognosis [
10]. However, it can have oncogenic roles in some contexts, highlighting its context-dependent function [
11,
12,
13]. Understanding its specific targets and signaling pathways is crucial for its potential as a diagnostic and therapeutic tool.
The present study investigates the potential of microRNA-449a (miR-449a) as a clinically relevant biomarker in laryngeal squamous cell carcinoma (LSCC). Specifically, it evaluates the diagnostic utility of miR-449a by analyzing its expression profiles to discriminate between patients with LSCC and healthy control subjects. Furthermore, this research explores the potential prognostic significance of miR-449a expression levels, examining their correlation with pertinent clinicopathological features or patient outcomes. Through a comprehensive evaluation of miR-449a expression patterns, the study aims to validate its suitability as a non-invasive biomarker for LSCC diagnosis and to assess its capacity to yield valuable prognostic information that could contribute to refined risk stratification and personalized treatment strategies.
Results
This study enrolled eighty-one patients diagnosed with locally advanced laryngeal squamous cell carcinoma, and their baseline demographic and clinical characteristics are detailed in
Table 1.
Distribution of Nodal Involvement by Tumor Stage in Laryngeal Squamous Cell Carcinoma
The distribution of nodal stage (N-stage) in relation to tumor stage (T-stage) among patients with laryngeal squamous cell carcinoma (LSCC) reveals a clear pattern of increasing cervical lymph node involvement with advancing primary tumor stage. Notably, N2 disease is more frequently observed in T4 tumors, underscoring the association between greater tumor burden and regional metastasis (
Table 2).
Clinical Response to Induction Chemotherapy and Radiation Therapy in Locally Advanced Laryngeal Squamous Cell Carcinoma (LSCC)
Table 3 presents the clinical response to induction chemotherapy (IC) followed by radiation therapy (RT) in patients with locally advanced laryngeal squamous cell carcinoma. Induction chemotherapy resulted in a complete response (CR) in 31% of the 81 patients and a partial response (PR) in 49%, with 20% exhibiting stable disease (SD). Among the 65 patients who subsequently received RT, the complete response rate significantly increased to 74%, while 20% achieved PR and 6% SD. These data suggest that while IC provides initial tumor control in a notable proportion of patients, the addition of RT substantially enhances the rate of complete remission, indicating a synergistic effect of this sequential treatment strategy.
Surgical Salvage Strategies in Locally Advanced Laryngeal Squamous Cell Carcinoma Following Induction Chemotherapy or Chemo-Radiation Therapy
The surgical salvage strategies and the extent of neck dissection in patients with locally advanced laryngeal squamous cell carcinoma (LSCC) following initial treatment with either induction chemotherapy (IC) or chemo-radiation therapy (CRT) underscore the heterogeneity of disease presentation and response to non-surgical modalities (
Table 4).
In the cohort of 16 patients who underwent salvage surgery after induction chemotherapy, a range of procedures was employed. Total laryngectomy, with or without partial pharyngectomy, was performed in 9 patients (5 with unilateral neck dissection, 3 with bilateral neck dissection, and 2 without neck dissection). Organ-preserving hemi-laryngectomy was carried out in 7 patients, all of whom underwent either unilateral (n = 4) or bilateral (n = 3) neck dissection.
Among the 17 patients who underwent salvage surgery after chemo-radiation therapy, the surgical approach was more frequently directed toward nodal disease. Four patients underwent hemi-laryngectomy, while 13 were treated with neck dissection alone (8 unilateral and 5 bilateral).
miR-449a Expression and Diagnostic Accuracy in Laryngeal Squamous Cell Carcinoma: Insights from Tissue and Serum Analysis
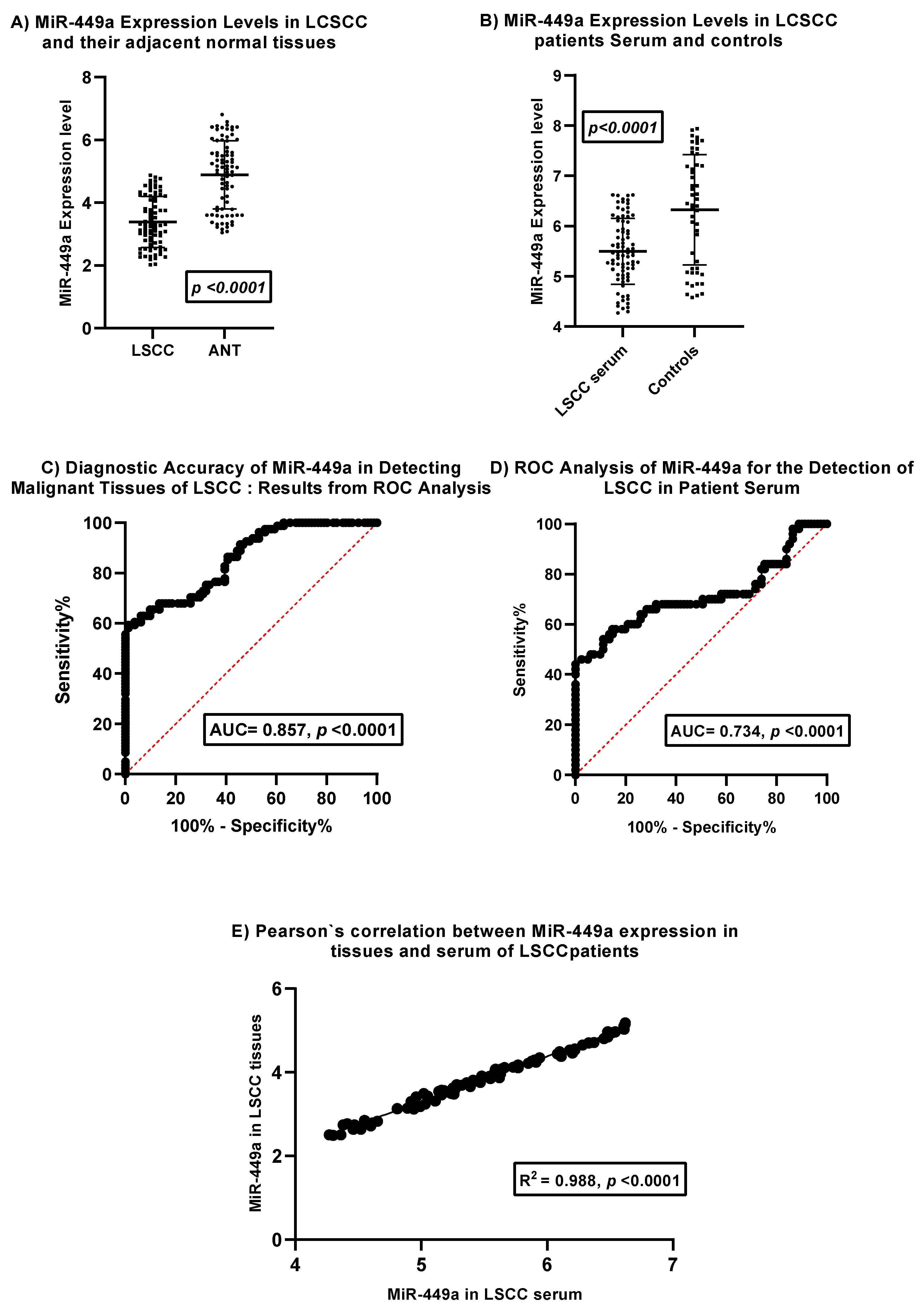
Figure 1 presents the analysis of miR-449a expression in laryngeal squamous cell carcinoma (LSCC) tissues, adjacent normal laryngeal tissues (ANT), and serum, along with its diagnostic potential.
Panel A compares miR-449a expression levels in LSCC tissues and their corresponding adjacent normal tissues. The dot plot with error bars indicates a statistically significant downregulation of miR-449a in LSCC tissues compared to ANT (p < 0.0001). This suggests a potential tumor-suppressive role for miR-449a in LSCC development.
Panel B compares serum miR-449a expression levels in LSCC patients and healthy controls. The dot plot with error bars reveals a statistically significant downregulation of miR-449a in the serum of LSCC patients compared to controls (p < 0.0001). This observation suggests the potential utility of serum miR-449a as a diagnostic biomarker.
Panel C displays the Receiver Operating Characteristic (ROC) curve for miR-449a in distinguishing malignant LSCC tissues from adjacent normal laryngeal tissues. The Area Under the Curve (AUC) of 0.857 with a highly significant p-value (< 0.0001) indicates good diagnostic accuracy of tissue miR-449a in identifying malignant tissue.
Panel D presents the ROC curve for serum miR-449a in detecting LSCC in patient serum. The AUC of 0.734 with a highly significant p-value (< 0.0001) demonstrates moderate diagnostic accuracy of serum miR-449a for LSCC detection. While lower than the AUC for tissue, it still suggests potential clinical utility as a less invasive biomarker.
Panel E investigates the correlation between miR-449a expression levels in tissues and serum of LSCC patients. The scatter plot shows a strong positive correlation (R² = 0.988, p < 0.0001) between miR-449a levels in LSCC tissues and their corresponding serum samples. This strong correlation suggests that serum miR-449a levels may reflect tissue expression levels in LSCC patients, supporting its potential as a surrogate biomarker.
In summary, this figure provides evidence for the significant downregulation of miR-449a in both LSCC tissues and patient serum compared to their respective controls. Both tissue and serum miR-449a demonstrate statistically significant diagnostic accuracy for LSCC, with tissue showing better performance. Importantly, the strong positive correlation between tissue and serum miR-449a levels in LSCC patients supports the potential of serum miR-449a as a non-invasive biomarker that reflects tumor expression. These findings warrant further investigation for clinical translation.
This figure illustrates the expression patterns and diagnostic utility of miR-449a in laryngeal squamous cell carcinoma (LSCC). (A) miR-449a expression levels are significantly downregulated in LSCC tissues compared to adjacent normal tissues (ANT). (B) Serum miR-449a levels are also significantly lower in LSCC patients compared to healthy controls. (C) ROC curve analysis demonstrates good diagnostic accuracy of tissue miR-449a in distinguishing malignant from normal laryngeal tissue (AUC = 0.857, p < 0.0001). (D) ROC curve analysis reveals moderate diagnostic accuracy of serum miR-449a for LSCC detection in patient serum (AUC = 0.734, p < 0.0001). (E) A strong positive correlation is observed between miR-449a expression levels in LSCC tissues and corresponding patient serum samples (R² = 0.988, p < 0.0001). Error bars in (A) and (B) represent standard deviation.
miR-449a as a Potential Biomarker in Locally Advanced Laryngeal Squamous Cell Carcinoma: Clinicopathological Insights
Analysis of miR-449a expression across various clinicopathological variables in patients with laryngeal squamous cell carcinoma (LSCC) revealed statistically significant associations across all tested parameters (all p < 0.0001) (
Table 5). Notably, the observed patterns suggest a potential tumor-suppressive role for miR-449a, as higher expression levels were associated with younger age (35–45 years), female sex, non-smoking status, and less aggressive disease features, including lower tumor grade (I–II), early T-stage (T1–T2), limited nodal involvement (N0–N1), and earlier TNM stage (Stage III). These findings indicate that miR-449a expression is inversely correlated with disease aggressiveness and may be linked to favorable clinical characteristics in LSCC. The consistent and highly significant differences across all categories support its potential utility as a biomarker for molecular stratification or diagnostic differentiation within distinct patient subgroups.
miR-449a Expression Dynamics as a Potential Indicator of Treatment Efficacy in Laryngeal Cancer
Table 6 presents the change in miR-449a expression levels in relation to treatment response following induction chemotherapy and radiation therapy in patients with laryngeal squamous cell carcinoma (LSCC). Paired t-tests were utilized to assess the significance of the change in miR-449a expression within each response group.
Following induction chemotherapy, patients who achieved a Complete Response (CR, n=25) exhibited a significant increase in miR-449a expression from a mean of 2.369 ± 0.416 to 3.948 ± 0.491 (p < 0.0001). Similarly, patients with a Partial Response (PR, n=40) to induction chemotherapy also showed a significant increase in miR-449a expression from 2.728 ± 0.360 to 3.984 ± 0.447 (p < 0.0001).
In the subsequent radiation therapy phase, patients who achieved a Complete Response (CR, n=48) demonstrated a significant increase in miR-449a expression from a mean of 2.809 ± 0.421 to 3.472 ± 0.401 (p < 0.0001). Likewise, patients with a Partial Response (PR, n=13) to radiation therapy also showed a significant increase in miR-449a expression from 2.170 ± 0.353 to 3.482 ± 0.740 (p < 0.0001).
In summary, this table indicates a consistent and statistically significant increase in miR-449a expression levels after both induction chemotherapy and radiation therapy in patients who achieved either a complete or partial response. This suggests a potential association between increased miR-449a expression and favorable tumor response to these treatment modalities in LSCC. Further investigation is warranted to elucidate the underlying mechanisms and the potential role of miR-449a as a predictive marker for treatment response.
Multivariate Analysis of Factors Influencing miR-449a Expression in Locally Advanced Laryngeal Squamous Cell Carcinoma (LSCC)
A multiple regression analysis examined the impact of clinical and pathological factors on miR-449a expression in locally advanced laryngeal squamous cell carcinoma (LA-LSCC) patients. The analysis revealed that female sex, older age, and more advanced T-stage, N-stage, and TNM stage were independently associated with higher miR-449a expression. Conversely, smoking was a significant negative predictor of miR-449a expression. Pathological grade and performance status did not show significant associations. Notably, higher miR-449a expression was also significantly associated with a positive response to treatment. These findings suggest that miR-449a expression is influenced by multiple clinical and pathological variables and may play a role in treatment response in LA-LSCC. Further investigation is warranted to elucidate the underlying biological mechanisms and the potential clinical utility of miR-449a as a biomarker and therapeutic target in this disease (
Table 7).
miR-449a and Clinical Outcome in LA-LSCC: Insights from Survival Analysis
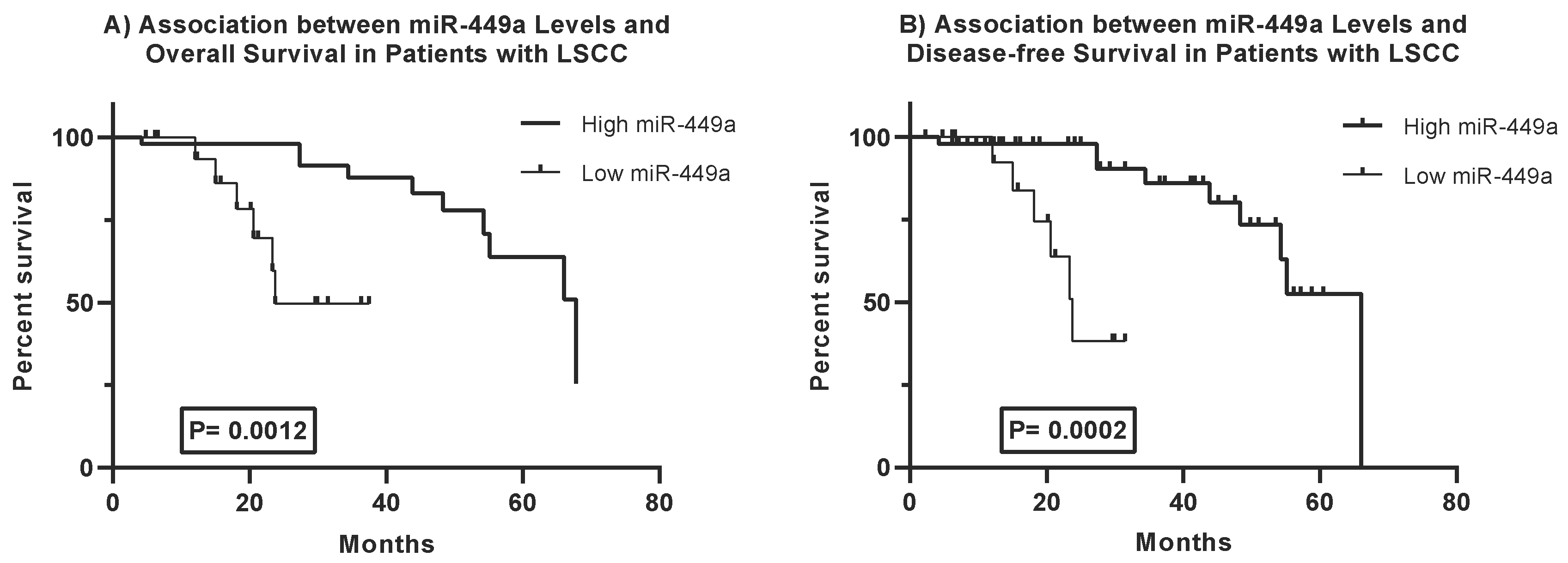
Kaplan-Meier survival analysis reveals a significant association between miR-449a expression levels and survival outcomes in Locally Advanced Laryngeal Squamous Cell Carcinoma (LA-LSCC) patients (
Figure 2).
Overall Survival: Patients with high miR-449a expression demonstrated a significantly longer overall survival compared to those with low expression (Log-rank test, p = 0.0012). The median overall survival was 67.82 months in the high miR-449a group versus 23.74 months in the low miR-449a group.
Disease-Free Survival: Similarly, high miR-449a expression was associated with significantly prolonged disease-free survival (Log-rank test, p = 0.0002). The median disease-free survival was 66.03 months for patients with high miR-449a expression, compared to 23.74 months for those with low expression.
These findings indicate that higher miR-449a expression is a strong predictor of both improved overall survival and prolonged disease-free survival in LA-LSCC patients, suggesting its potential as a favorable prognostic biomarker for this malignancy.
Prognostic Significance of miR-449a Expression in Locally Advanced Laryngeal Squamous Cell Carcinoma: A Multivariate Survival Analysis
The Cox proportional hazards regression analysis presented in
Table 8 identifies miR-449a expression and several clinicopathological variables as independent predictors of overall survival in patients with locally advanced laryngeal squamous cell carcinoma (LA-LSCC) (
Table 8). High miR-449a expression was significantly associated with a reduced risk of death (HR = 0.15, 95% CI: 0.055–0.410, p < 0.001), suggesting a protective role in survival outcomes. In contrast, older age, smoking status, higher tumor grade, advanced T-stage, N-stage, and higher TNM stage were all independently linked to an increased hazard of mortality. These findings highlight the prognostic value of miR-449a alongside established clinical factors in stratifying patient risk and guiding therapeutic decision-making in locally advanced laryngeal cancer.
Discussion
The management of locally advanced laryngeal squamous cell carcinoma (LA-LSCC) has evolved to favor combined treatment methods for better patient outcomes, often integrating surgery, radiotherapy, chemotherapy, targeted therapies, and immunotherapy [
14]. Concurrent chemoradiation is now standard for advanced-stage disease, aiming to reduce tumor size and eliminate micrometastatic disease. This approach has significantly improved laryngeal cancer management by enabling laryngeal preservation and enhancing quality of life [
15].
Our findings demonstrate the effectiveness of combining chemotherapy and radiotherapy in reducing tumor burden and achieving high laryngeal preservation rates in patients with LSCC, highlighting the synergistic effects of this treatment strategy. By preserving the larynx in most patients, this approach improves oncological outcomes and helps maintain essential functions such as speech and swallowing, enhancing overall quality of life. These results support the use of induction chemotherapy followed by radiotherapy as a valuable treatment option for LSCC and are consistent with findings from other studies. Specifically, our data shows that while induction chemotherapy (IC) alone achieved a 31% complete response (CR) and 49% partial response (PR) in 81 patients, the subsequent addition of radiation therapy (RT) in 65 patients dramatically increased the CR rate to 74% and maintained a 20% PR rate. This highlights the synergistic effects of this sequential treatment strategy. By preserving the larynx in most patients, this approach improves oncological outcomes and helps maintain essential functions such as speech and swallowing, enhancing the overall quality of life. These results support the use of induction chemotherapy followed by radiotherapy as a valuable treatment option for LA-LSCC and are consistent with findings from other studies [
16,
17,
18,
19].
In this context, the identification of novel, clinically relevant biomarkers for early diagnosis, accurate prognosis, and effective treatment response prediction remains a critical need for optimizing patient care in locally advanced laryngeal squamous cell carcinoma (LA-LSCC). The present study was specifically designed to investigate the potential of microRNA-449a (miR-449a) as such a biomarker. Our objectives were to evaluate its diagnostic utility by analyzing expression profiles to discriminate between patients with LA-LSCC and healthy control subjects, and to explore its prognostic significance by examining correlations with pertinent clinicopathological features and patient outcomes. Through a comprehensive evaluation of miR-449a expression patterns, this research aimed to validate its suitability as a non-invasive biomarker for LA-LSCC diagnosis and to assess its capacity to yield valuable prognostic information that could contribute to refined risk stratification and personalized treatment strategies.
Our findings establish miR-449a as a potential diagnostic biomarker for LA-LSCC. We observed a significant downregulation of miR-449a expression in LA-LSCC tissues compared to adjacent normal laryngeal tissues (p < 0.0001). This pattern aligns with the widely recognized role of miR-449a as a tumor suppressor in various cancers, where its diminished expression can promote uncontrolled cell proliferation and tumor progression [
12,
20,
21]. Extending these findings to a non-invasive context, we also detected a statistically significant downregulation of miR-449a in the serum of LA-LSCC patients compared to healthy controls (p < 0.0001), highlighting its potential as a circulating biomarker. Regarding diagnostic accuracy, ROC curve analysis demonstrated that tissue miR-449a possessed good discriminatory power, with an Area Under the Curve (AUC) of 0.857 (p < 0.0001) for distinguishing malignant LSCC tissues from normal tissues. While serum miR-449a showed a moderate diagnostic accuracy (AUC = 0.734, p < 0.0001) for detecting LA-LSCC in patient serum, its non-invasive nature makes it a highly attractive candidate for initial screening or disease monitoring. Importantly, a strong positive correlation (R² = 0.988, p < 0.0001) was observed between miR-449a levels in LA-LSCC tissues and their corresponding serum samples. This robust correlation suggests that serum miR-449a can reliably reflect the expression status within the tumor, further supporting its utility as a non-invasive surrogate biomarker for LA-LSCC. These results are consistent with a growing body of literature on microRNAs as promising non-invasive diagnostic tools in head and neck cancers. To the best of our knowledge, this is the first study to validate miR-449a’s diagnostic and prognostic role specifically in LSCC, particularly in locally advanced disease.
Our analysis explored the association of miR-449a expression with various clinicopathological characteristics. Univariate analysis indicated that higher miR-449a expression was significantly associated with younger age, female sex, non-smoking status, and less aggressive disease features (lower tumor grade, earlier T-stage, limited nodal involvement, and earlier TNM stage). However, the multivariate multiple regression analysis, which provides a more robust assessment of independent predictors by controlling for other variables, revealed nuanced insights into factors influencing miR-449a expression. In this model, female sex (p = 0.014), older age (p = 0.034), more advanced T-stage (p = 0.049), more advanced N-stage (p = 0.001), and higher TNM stage (p = 0.002) were all independently associated with higher miR-449a expression. Conversely, smoking status was a significant negative predictor (p < 0.0001), meaning smokers had lower miR-449a levels. Pathological grade and performance status did not show significant independent associations. The difference between univariate and multivariate findings underscores the complexity of biological systems and the importance of accounting for confounding factors. While the univariate analysis might suggest an inverse correlation with disease aggressiveness, the multivariate analysis implies that in the context of other variables, miR-449a expression can increase with certain aspects of disease progression, potentially reflecting a compensatory mechanism or involvement in complex cellular responses to advanced disease.
Beyond its diagnostic potential, our study firmly establishes miR-449a as a significant prognostic factor in LA-LSCC. Kaplan-Meier survival curves clearly demonstrated that patients with high miR-449a expression exhibited significantly prolonged overall survival (median 67.82 months vs. 23.74 months; Log-rank p = 0.0012) compared to those with low expression. A similar trend was observed for disease-free survival, where high miR-449a expression was associated with significantly better outcomes (median 66.03 months vs. 23.74 months; Log-rank p = 0.0002). The multivariate Cox proportional hazards regression analysis (
Table 8) further strengthened these findings by identifying high miR-449a expression as an independent predictor of improved overall survival (Hazard Ratio [HR] = 0.15, 95% CI: 0.055–0.410, p < 0.001). This indicates a strong protective effect of elevated miR-449a on survival outcomes in LA-LSCC. These results are consistent with previous research indicating that miR-449a can be a valuable tool to predict cancer-specific survival outcomes in patients with different types of cancer [
12,
22,
23]. Consistent with known prognostic factors in cancer, our analysis also confirmed that older age (p = 0.004), smoking status (p = 0.017), higher tumor grade (p = 0.003), and more advanced T-stage (p = 0.001), N-stage (0.006), and TNM stage (p < 0.001) were all independently associated with an increased hazard of mortality. The robust independent prognostic value of miR-449a suggests its utility in refining risk stratification for LA-LSCC patients, potentially guiding more personalized therapeutic strategies and follow-up schedules.
Our investigation into miR-449a expression dynamics during treatment provides valuable insights into its potential as a predictor of therapeutic response. We observed a consistent and statistically significant increase in miR-449a expression levels after both induction chemotherapy and subsequent radiation therapy in patients who achieved either a complete or partial response (all p < 0.0001). For instance, in patients achieving complete response to induction chemotherapy, miR-449a significantly increased from 2.369 ± 0.416 to 3.948 ± 0.491. Similarly, complete responders to radiation therapy showed an increase from 2.809 ± 0.421 to 3.472 ± 0.401. This suggests that miR-449a levels dynamically change in response to effective treatment, potentially reflecting tumor cell apoptosis, reduced tumor burden, or other favorable biological responses. This finding is particularly exciting as it positions miR-449a as a potential real-time biomarker for monitoring therapeutic efficacy, which could inform adaptive treatment protocols and improve patient management. Such dynamic changes in miR-449a expression in response to treatment have also been observed in other cancers; for example, a study in osteosarcoma patients found that miR-449a levels were significantly higher after surgery in responders, suggesting its role in assessing treatment success [
24].
Despite the compelling findings, our study has certain limitations. The cohort size of 81 patients, while sufficient for statistical significance in many analyses, may limit the generalizability to broader LA-LSCC populations. As a single-center study, our findings warrant validation in larger, multi-institutional, prospective cohorts to confirm their broader applicability and clinical utility. Further research should also focus on elucidating the precise molecular mechanisms by which miR-449a exerts its observed effects in LA-LSCC. This includes identifying its direct mRNA targets and the downstream signaling pathways involved in cell cycle regulation, apoptosis, and epithelial-mesenchymal transition, which could explain both its tumor-suppressive role and its dynamic changes with treatment. Functional studies in vitro and in vivo, such as overexpression or knockdown experiments of miR-449a in LSCC cell lines and animal models, are crucial to establish a causal relationship between miR-449a levels and tumor behavior or treatment response. Ultimately, combining miR-449a with other promising biomarkers or integrating it into multiparametric panels could further enhance its diagnostic and prognostic accuracy in LA-LSCC.
Patients and Methods
Study Population
This prospective study enrolled 81 patients diagnosed with locally advanced laryngeal squamous cell carcinoma (LA-LSCC) and 50 healthy control subjects between April 2016 and November 2023.
Eligibility Criteria
Patients were eligible for inclusion if they met the following criteria:
No prior treatment for LSCC.
Histopathological confirmation of LSCC via biopsy.
Disease classified as T3 or T4 with nodal status N0, N1, or N2, according to the American Joint Committee on Cancer (AJCC) Staging System [
25].
An Eastern Cooperative Oncology Group (ECOG) performance status less than 2 [
26].
Normal liver, kidney, cardiac, and bone marrow function, as assessed by standard clinical and laboratory parameters.
Adequate nutritional status and absence of significant hearing impairment.
Age below 70 years at the time of enrollment.
Pre-treatment Evaluation and Sample Collection
During the initial evaluation, a comprehensive diagnostic workup was performed to determine disease extent and severity. This included a thorough physical examination, complete blood cell count, routine serum chemistry panels, creatinine clearance assessment, chest X-ray, computed tomography (CT) or magnetic resonance imaging (MRI) of the head and neck, and a bone scan. Additionally, triple endoscopy was conducted to evaluate local tumor extent and regional metastases. During the endoscopic procedure, biopsies were obtained from both the tumor and adjacent normal tissues for histopathological and molecular analysis. Blood samples were collected from all enrolled patients and healthy control subjects, and the serum was preserved for subsequent investigation of miR-449a expression levels.
Treatment Regimen
The induction chemotherapy regimen consisted of three cycles of docetaxel (75 mg/m2 on day 1) combined with cisplatin (75 mg/m2 on day 1), along with a continuous intravenous infusion of fluorouracil (500 mg/m2 per day) from days 1 to 5. This cycle was repeated every four weeks.
Treatment Response Assessment and Subsequent Management
Patient response to induction chemotherapy was assessed prior to each subsequent cycle through clinical and radiological evaluations. Clinical assessments included endoscopic inspection and palpation of lymph nodes. Radiologically, CT or MRI scans were performed after the second chemotherapy cycle to evaluate tumor response. Patients achieving a complete response (CR) or partial response (PR) received a maximum of three chemotherapy cycles before proceeding to definitive radiation therapy. In cases of disease progression, surgical resection followed by radiation therapy was performed. Patients who achieved CR or PR after the third chemotherapy cycle advanced to radiation therapy.
Radiotherapy was administered to all patients, either immediately following chemotherapy for responders or after salvage surgery for non-responders. The treatment targeted both sides of the neck and the primary tumor site, delivered in daily fractions of 2 Gy, five days per week. Patients undergoing irradiation post-chemotherapy received a total dose of 5000 cGy, supplemented by an additional boost of 2000 cGy focused on the tumor site and any palpable lymph nodes. Following salvage surgery, a radiation dose of 5000 cGy was administered, with a booster dose of 14 Gy applied to areas with positive margins, extracapsular spread, or three or more involved lymph nodes.
Response to radiation therapy was re-evaluated twelve weeks after completion. Patients with persistent laryngeal disease underwent salvage laryngectomy. If the primary tumor was controlled but neck disease persisted, only neck dissection was performed. The scope of surgical resection was determined by the initial tumor size before chemotherapy. Classic wide-field total laryngectomy was performed for all primary tumors. Regional neck dissection was carried out on all surgical patients, with the exception of those with T3N0 or midline supraglottic T4N0 tumors where the risk of occult metastases to a specific neck side was unclear. The presence of residual primary tumors in patients undergoing salvage surgery was confirmed by biopsy. All patients were monitored with follow-up appointments every three months for the first-year post-treatment, and every six months thereafter. Complete or partial response was evaluated by tissue biopsy after neoadjuvant chemotherapy and again after radiation therapy.