1. Introduction
Escherichia coli (
E. coli) is a facultative anaerobic bacterium commonly found in the intestinal microbiota [
1] and involved in maintaining gut microbiome homeostasis [
2]. Advances in genomic sequencing and phylotyping have led to the progressive classification of
E. coli into increasingly refined phylogenetic groups, initially categorized into four [
3], then five [
4], seven [
5,
6], eight [
7,
8,
9,
10], and most recently, twelve phylogroups [
11]. For intraspecific classification, two main approaches have been widely used: multilocus sequence typing (MLST) [
5,
7] and the PCR-based method developed by Clermont and colleagues [
3,
4,
6,
8], which targets virulence-associated genes. These studies have demonstrated that pathogenic
E. coli strains are not randomly distributed across phylogroups [
12], leading to the general assumption that pathogenicity emerging in some phylotypes due to horizontal transfer of virulent genes [
13] requires a specific genetic background for their expression [
14]. Consequently, phylotyping has gained significant importance in epidemiological surveillance and pathogenicity studies.
In 2018 in silico PCR-based classification and the MASH clustering using marker
k-mers [
15] were combined in a new tool, ClermonTyping [
6]. By analyzing a dataset of over 300
E. coli genomes, the authors observed only a few discrepancies between the two approaches: one relying on virulence genes detection and the other assessing genomic background. In our previous study [
10], we compared MLST-based typing of 124 genomes with
k-mer-based clustering and found that both methods generated topologically congruent trees, consistently identifying the eight established
E. coli phylogroups (A, B1, B2, D, E, F, C and G). However, a subsequent MASH-based analysis of a much larger dataset, containing 10,667
E. coli and
Shigella genomes, divided them into 12 clusters [
11]. While phylogroups A, B1, F, C and G remained distinct, B2 was split into clusters B2-1 and B2-2; D diversified into subgroups D1, D2 and D3, while E separated into E1 and E2. Thus, despite the significantly expanded dataset, no entirely new phylogroups were discovered, suggesting that the eight major phylogroups represent the core genetic structure of
E. coli.
While all phylogroups include strains prone to pathogenicity, epidemiological evi-dence indicates that ancient groups F and D harbor more pathogenic bacteria compared to groups A and B1 [
9,
16], whereas recently diverged lineages are frequently associated with severe infections [
8,
12,
14]. A striking example is the 2011 German outbreak of
E. coli infections, caused by the enteroaggregative hemorrhagic strain O104:H4 C227-11 [
17,
18,
19], belonging to group B1. This strain exhibited exceptional virulence, attributed to the accu-mulation of horizontally acquired genetic determinants of pathogenicity [
17,
19,
20,
21]. These virulence factors included enhanced adhesion mediated by AAF plasmid-encoded fim-briae [
19], potent cytotoxicity due to prophage-derived Shiga toxin (Stx-2) [
17], and multi-drug resistance mainly conferred by extended-spectrum β-lactamases.
Large phylogroup B1, which comprises about 30% of known
E. coli genomes, includes only a small portion (2.4%) of potentially virulent strains, such as those of serotypes O104:H4 and O121:H19 [
11]. In contrast, group E, currently comprising just 10.9% of known genomes, harbors a disproportionally high fraction (10.2%) of highly pathogenic
E. coli strains of serotype O157:H7 [
11]. The remarkable virulence of O157:H7 stems from extensive horizontal gene transfer, having incorporated genetic material from 53 species [
22] and over 460 prophages [
23] (compared to just 29 in non-pathogenic
E. coli K-12 [
24]). Major chromosomal pathogenicity factors include Shiga toxin-encoding prophages (similar to those in O104:H4) [
25] and 5 LEE (locus of enterocyte effacement) pathogenicity islands, encoding a type III secretion system and adhesins [
26]. Over a thousand genes are absent from non-pathogenic
E. coli K-12 [
27,
28], forming about 180 O157:H7-specific O-islands [
29], and including 131 potentially virulent genes [
28]. Given these distinctive genomic features, phylogroup E strains are readily identifiable through both PCR-based typing and
k-mer-based clustering [
6,
10,
11].
The pathogenicity of B2 strains is strongly associated with 54-kb
pks genomic island, which encodes enzymes for colibactin biosynthesis [
30,
31]. However, the presence of such islands is not exclusive to pathogenic bacteria. Even the probiotic strain
E. coli Nissle 1917, which is widely used to treat various intestinal disorders [
32], has
pks island in the genome [
33,
34]. Nevertheless, neither live bacteria of this strain nor the spent culture supernatant had a genotoxic effects [
33], exemplifying epistatic suppression of virulence genes. While B2 is often considered as the most pathogenic phylogroup, its clinical prevalence may reflect enhanced ecological fitness rather than intrinsic virulence [
35]. This is exemplified by CTX-M-15-producing B2 strains (serotypes O16:H5 and O25b:H4) that caused major outbreaks of infectious diseases in the early 2000s through their extended-spectrum β-lactamase-mediated antibiotic resistance [
36,
37]. Additional B2 virulence factors include: cytolethal distending toxins (CDTs) causing DNA damage in infected cells [
30] and cytotoxic necrotizing factor (CNF) [
38]. Both types of genes are frequently plasmid-encoded but many are located within genomic islands [
30,
38,
39], contributing to the genomic background traced by virtual
k-mer-based screening of chromosomes.
The effectiveness of PCR-based phylotyping indicates that phylogroups have a specific combination of marker genes. This was recently validated in a comprehensive study of 844 uropathogenic
E. coli strains, which revealed certain associations between phylogroups and specific virulence factors, including genes of antimicrobial resistance, motility and biofilm formation [
40]. According to the efficiency of PCR typing, 111 strains of phylogroup G, showed no isolates carrying genes for the adhesin Air, toxin Sat and the transcription factor EilA [
9], of which Air and EilA are specific to groups D and F, while Sat is produced by groups D and B2 bacteria [
41]. Phylogroup A virulence is most often associated with the adhesive fimbriae FimA and YfcV, as well as receptors for yersiniabactin (FyuA) and ferric aerobactin (IutA) [
42]. Isolates from the less studied group C, encode the enzyme HlyF [
43], which triggers eukaryotic autophagy due to toxin release via outer membrane vesicles [
44]. In combination with Shiga toxin of
E. coli O80:H2, this caused bacteremia in Europe [
45]. Therefore, not all but many virulence genes are distributed across most phylogroups (Shiga toxins Stx-1/2 so far have not found only in group G genomes [
46,
47]). However, the mere presence of virulence genes does not guarantee pathogenicity. As proposed two decades ago, their functional integration requires a compatible genetic background [
14].
Current understanding suggests that this genetic background emerges through complex interactions between cellular regulatory networks and horizontally acquired genes via epistatic relations. The enhanced recombination efficiency within phylogroups likely promotes the preferentially maintenance of beneficial and/or virulence genes among phylogenetically related strains. Yet, even phylogroups harboring multiple virulence determinants show unpredictable expansion patterns, implying the existence of more complex, higher-order epistatic interactions within microbial communities. To investigate this phenomenon at the level of E. coli phylogroup homeostasis, we employed k-mer-based profiling of natural microbiomes. The study specifically examined how endogenous and exogenous factors triggering adaptive responses in the human gut microbiome influence intraspecific equilibrium within E. coli populations.
3. Results
In our previous study [
10], we performed
E. coli phylotyping using
k-mer-based approach and MLST. For MLST, a combined set of 27 marker genes proposed in [
4,
63,
64,
65] was implemented. The individually aligned sequences of these genes were concatenated and a phylogenetic tree was constructed using the IQ-TREE [
66]. For the
k-mer-based approach, phylogenetic trees were inferred from a pairwise distance matrix of
Escherichia coli/
Shigella-specific 18- and 22-mers (124 genomes total), identified using the UniSeq algorithm [
10]. Both methods produced topologically identical trees. Their clustering precisely matched ClermonTyping [
6], though 14 strains showed discrepancies compared to the MASH-based clustering used in [
11]. These included 12 strains classified as C, which in [
11] were assigned to group B1, and 2 strains, classified in our study as B1 bacteria were assigned to group C. Since the MASH algorithm, originally developed to assess intraspecific polymorphism in bacteria, estimates genomic distances based on “mutation rates” using representative 21-mers [
15], the observed discrepancies between our classification and MASH-based typing might be due to difference in the marker
k-mer sets, used for clustering. Given that the accuracy of taxonomic analysis critically depends on the quality of
k-mer barcodes, we re-evaluated the phylotyping of our 124 genomes using the option of UniSeq software analogous to MASH.
3.1. Validation of Intraspecific E. сoli Phylotyping Using MASH-Like Option of UniSeq Pipeline
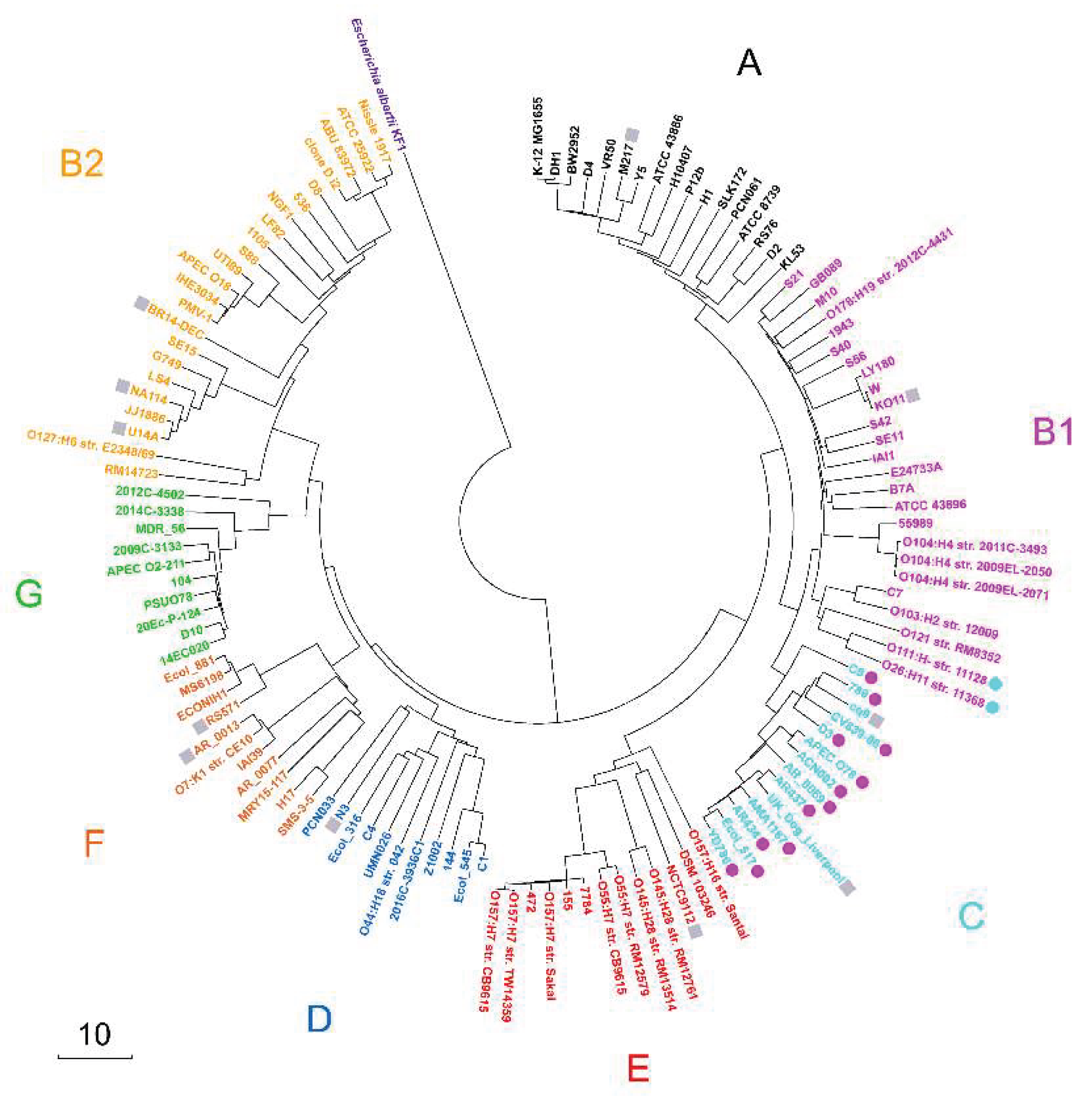
Unlike our previous analysis, which used k-mers unique for E. coli/Shigella, this implementation considered all non-redundant 18-mers present in each genome. Then, pairwise Sørensen similarity indices [
54] were computed for all 124 18-mer sets and the phylogenetic tree was constructed from the pairwise distance matrix using neighbor-joining method [
55]. As a result, the constructed tree (
Figure 1) showed identical topology to our earlier phylogeny based on unique 18-mers (Figure 3 in [
10]).
Thus, five distinct approaches, including MLST analysis using 27 marker genes [
10], in silico PCR and MASH via ClermonTyping resource [
6], and two UniSeq-based techniques ([
10] and
Figure 1) consistently yielded congruent phylotyping results. Thus, the same 124 genomes as in our previous study [
10] were used for phylotyping with their distribution across phylogroups detailed in
Table 2 (Set 1). Strains O111:H- str. 11128 and O26:H11 str. 11368 were assigned to phylogroup B1, while strains C8, 789, cq9, CV839-06, D3, APEC O78, ACN002, AR_0069, AR437, UK_dog_Liverpool, AM1167, AR434, Ecol_517 and YD786 were considered as members of phylogroup C.
3.2. Assessment of Barcoding Specificity
While both UniSeq- and MASH-based typing methods are equally effective for phylogenetic analysis, only UniSeq, which relies on unique marker k-mers absent in other bacterial genomes, enables intraspecific taxonomic analysis of natural microbial communities. To estimate the abundance of bacterial groups in metagenomes, the algorithm quantifies reads containing their specific 18-mer markers. The accuracy of these estimates depends on both the number and specificity of the 18-mer markers used. Increasing the number of known genomes within each phylogroup improves barcode specificity. However, expanding the reference database for filtering reduces the cumulative barcode size. Since natural microbiomes contain many bacteria not represented in reference database, uncharacterized microorganisms will inevitably generate reads misassigned to known groups. To account for this bias, two complementary approaches were applied.
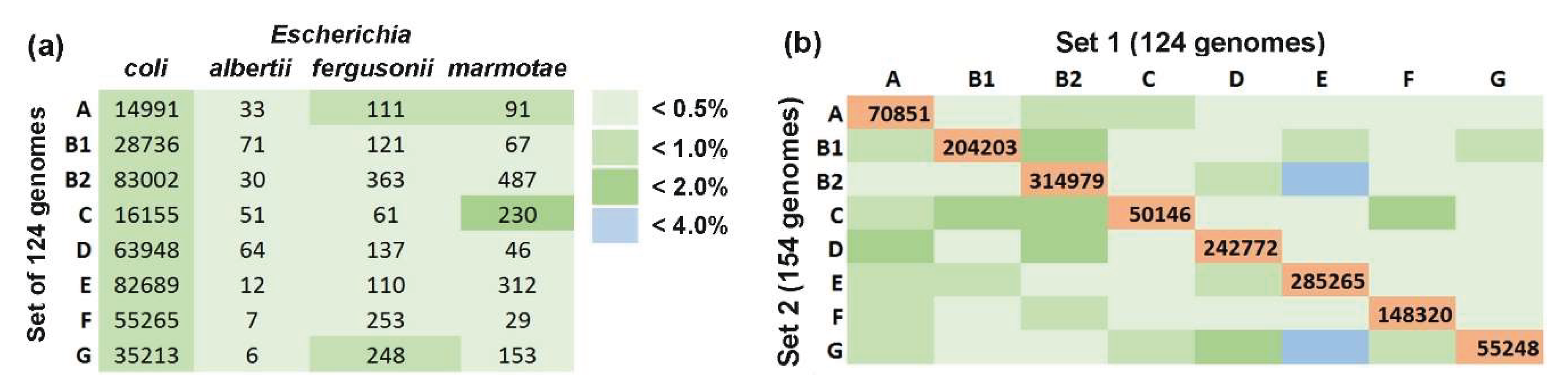
To assess the species specificity of marker k-mers, we estimated their average number in the 124 genomes used for barcoding. These values were then compared with the
k-mer content of other
Escherichia species (
E. albertii,
E. fergusonii, and
E. marmotae) (
Figure 2a). Genome selection was based on two criteria: (1) absence from the reference dataset of their close homologs to prevent 18-mers filtering during barcode construction, and (2) mutual evolutionary dissimilarity as determined by the phylogenetic tree topology (constructed similarly to
Figure 1).
Three genomes of each species appeared to be sufficiently representative and the highest overlap was observed between E. coli group C barcodes and 18-mers of E. marmotae RHB35-E2-C08 (1.4%). The average overlap across other combinations was significantly lower (0.34 ± 0.07%). These estimates suggest that the presence of uncharacterized Escherichia is unlikely to substantially affect the accuracy of E. coli phylogroup quantification in natural microbiomes.
For the second trial, we constructed another training set containing 154 genomes with phylogroup identities assigned via ClermonTyping and UniSeq approach (Set 2). These genomes were selected from NCBI GenBank using the same criteria as stated above with additional requirement of no close homologs present in Set 1. Several iterations of phylogenetic analysis were required to choose suitable genomes from 6300 completed genomes of
E. coli deposited in the GenBank database to create a phylogenetically balanced subset. Set 2 (
Table 2) was independently barcoded using the same reference database as for Set 1. Phylogenetic reconstruction confirmed clear separation of genomes into eight phylogroups with no discordance against expected identities (
Supplementary Figure S1), although all groups except E exhibited a reduction in the number of marker 18-mers (
Table 2). This is at least partly due to the more stringent interphylogroup filtering in Set 2, which was difficult to avoid during genome selection.
We next quantified the overlap between the two sets for each phylogroup and revealed that 20-52% of 18-mers from the smaller set (usually Set 2) were shared with Set 1 barcodes. Overlaps between different groups ranged from 0.049% to 3.950% (
Figure 2b) with the highest cross-phylogroup similarity observed between group E of Set 1 with groups B1 (2.89%) and G (3.95%) from Set 2. The average overlap across other phylogroup combinations was substantially lower (0.55 ± 0.08%), only marginally exceeding typical interspecific overlap levels (0.34 ± 0.07%) (
Figure 2b and
Figure 2a). Therefore, we proceeded with Set 1 barcodes (experimentally verified by multiple methods) for all subsequent intraspecific taxonomic analyses.
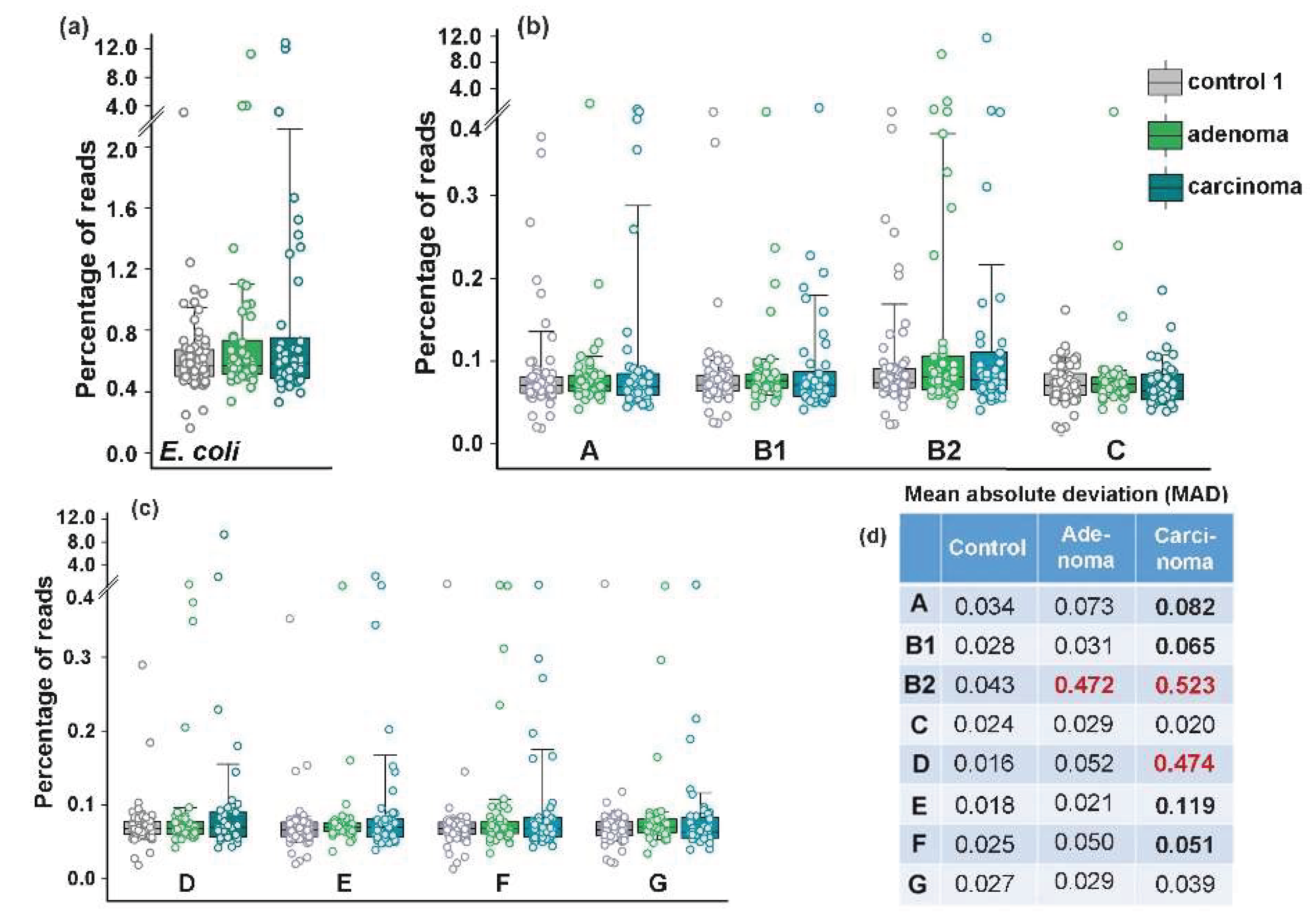
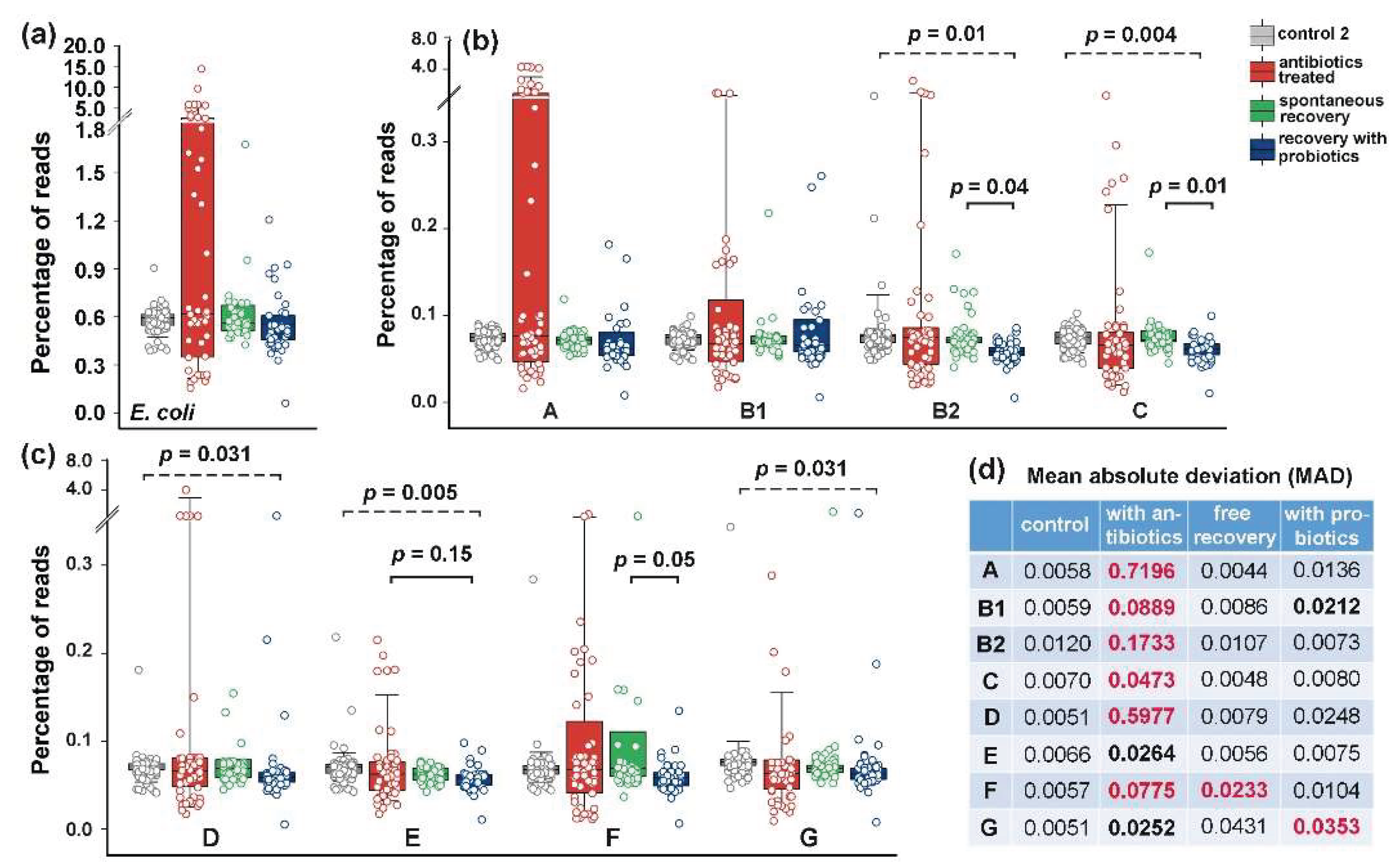
3.3. Colorectal Adenoma and Carcinoma Had Different Impact on the Distribution of E. coli Phylogroups in the Intestinal Microflora
Consistent with previous reports [
35], we anticipated that at least carcinoma would either promote greater persistence of phylogroup B2 in the gut or otherwise affect E. coli phylogroup homeostasis. The average abundance of this phylogroup was indeed higher in the samples obtained from patients with adenoma and carcinoma (0.349% and 0.362, respectively) than in the control group (0.097%) (
Figure 3).
Only group D bacteria known to produce Air adhesin and Sat toxin [
41] increased their presence to almost the same level (from 0.173% to 0,319%) in microbiomes associated with carcinoma, but all changes, along with those in total
E. coli abundance were not statistically significant (
Figure 3a–c). Nevertheless, we observed a priori unexpected increase in the variability of
E. coli phylogroups abundance across different biological samples. In particular, adenoma increased the mean absolute deviation (MAD) in the presence of B2 bacteria, while carcinoma promoted significant variability for six phylogroups, with the greatest impact on B2 and D group bacteria (
Figure 3d).
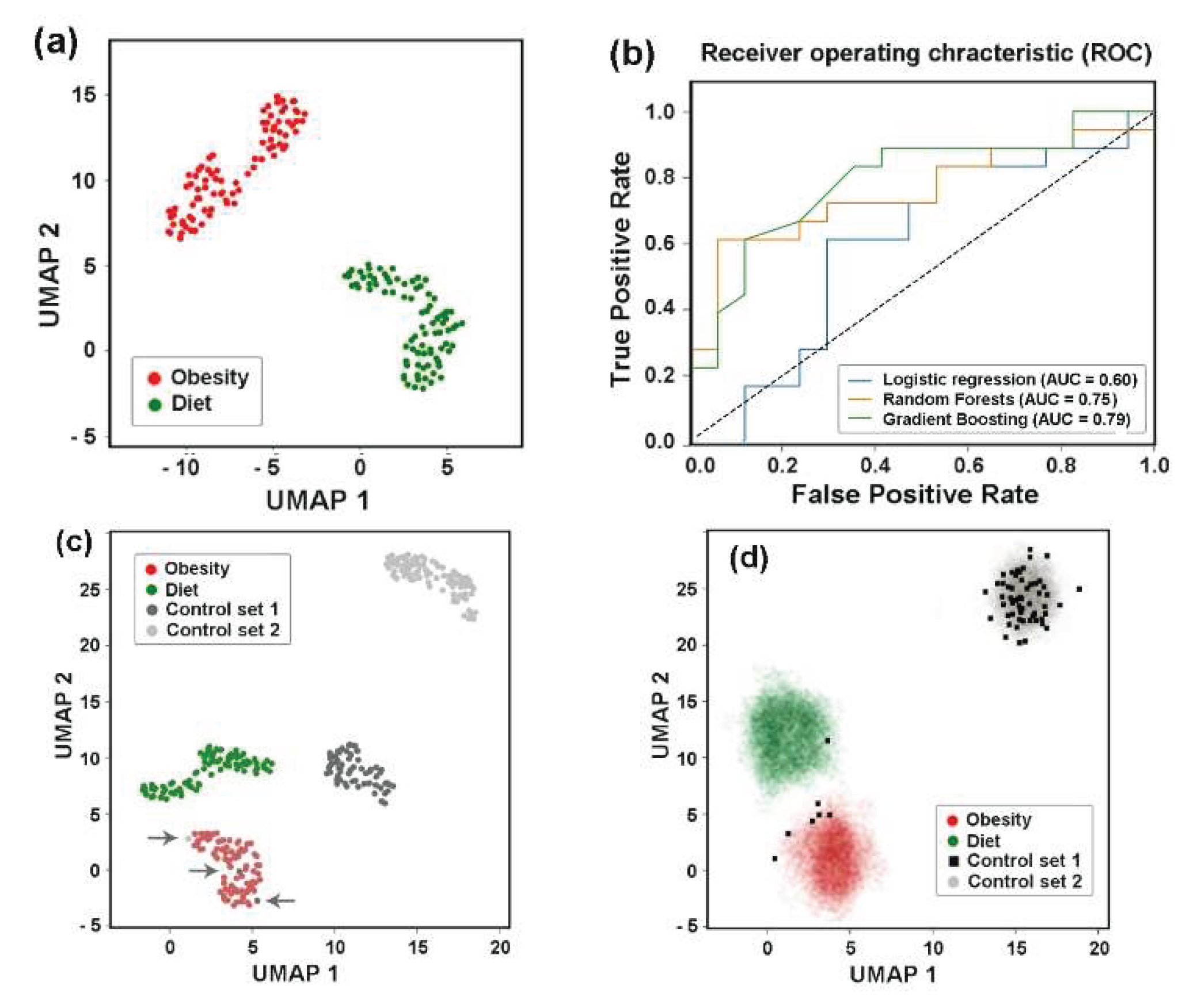
Assuming that the difference in
E. coli homeostasis between the usually harmless adenoma and the dangerous carcinoma reflects variable ways in which
E. coli adapts to the specific environment created in the gut, we used machine-learning algorithms to assess whether the frequency profile of phylogroups can distinguish between healthy and pathological state. Normalized percentages of the eight phylogroups in control and pathologi-cal/treatment conditions were used as features defining the target variable Y in machine learning models for each type of samples. Binary classification was performed using Lo-gistic Regression (LR), Random Forest (RF), and Gradient Boosting (GB) models (
Figure 4a–c). The best hyperparameters for each model were obtained as described in Materials and Methods section. Their performance was evaluated using the Area Under the Curve (AUC) as a distance-based metric for the Receiver Operating Characteristics (ROC) curves.
The resulting ROC-AUC scores of 0.57 (LR), 0.58 (RF) and 0.61 (GB) were close to 0.5, indicating a random distribution of the control samples and samples from patients with colorectal adenoma (
Figure 4a). However, when the control set was compared with the carcinoma patient samples (
Figure 4b), the AUC scores were higher and, at least for the LR model, separated the two sets with 73% accuracy. The discriminatory power of this model also distinguished between E. coli populations in the microbiomes of people with colorectal carcinoma and adenoma with approximately the same accuracy (
Figure 4c). An algorithm based on nonlinear dimensionality reduction (Uniform Manifold Approxima-tion and Projection, UMAP [
59]) clearly separated all three sets of biological samples (
Figure 4d), reflecting distinct phylogroup compositions.
Based on this observation, we next evaluated intraspecific correlations in the abundance of E. coli phylogroups within the microbiomes (
Supplementary Table S3). In the control set (
Figure 4e) and in the microbiota associated with adenoma (
Figure 4f) all phylogroups exhibited positive correlation with each other. Although not all correlations were statistically significant, this likely suggests that bacteria from different groups do not specialize in entirely independent functions within E. coli populations. The number of correlated groups in samples obtained from the microbiomes of patients with carcinoma, on the contrary, was much smaller, and B2 group showed non relationships with A, B1, D and E phylogroups (
Figure 4g). Thus, while the abundance of E. coli phylogroups did not differ significantly among microbiomes adapted to different host physiological states (
Figure 3b,c), their interaction patterns appeared sensitive to the chronic alterations induced by carcinoma.
3.4. Bimodal Response of E. coli to Antibiotics and Recovery with Probiotics
Despite being inherently susceptible to most clinical antimicrobials, E. coli has a remarkable capacity to acquire resistance genes through horizontal gene transfer [
40,
67]. They are distributed across all phylogroups and may function collectively to counteract antimicrobial drags. Using data from the PRJEB28097 project [
49,
50] we aimed to characterize the individual responses of E. coli groups to ciprofloxacin, resistance to which was observed among isolates of all phylotypes [
43,
68,
69]. The selected dataset contained stool samples from healthy volunteers receiving ciprofloxacin (500 mkg, twice daily) and metronidazole (500 mkg, three times daily) [
49,
50]. As metronidazole exhibits activity against E. coli only in the presence of other susceptible bacteria [
70], this antibiotic combination enabled detection of community-dependent response. While the abundance of E. coli in these microbiomes was comparable to that observed in the PRJEB7774 project (
Figure 2a and
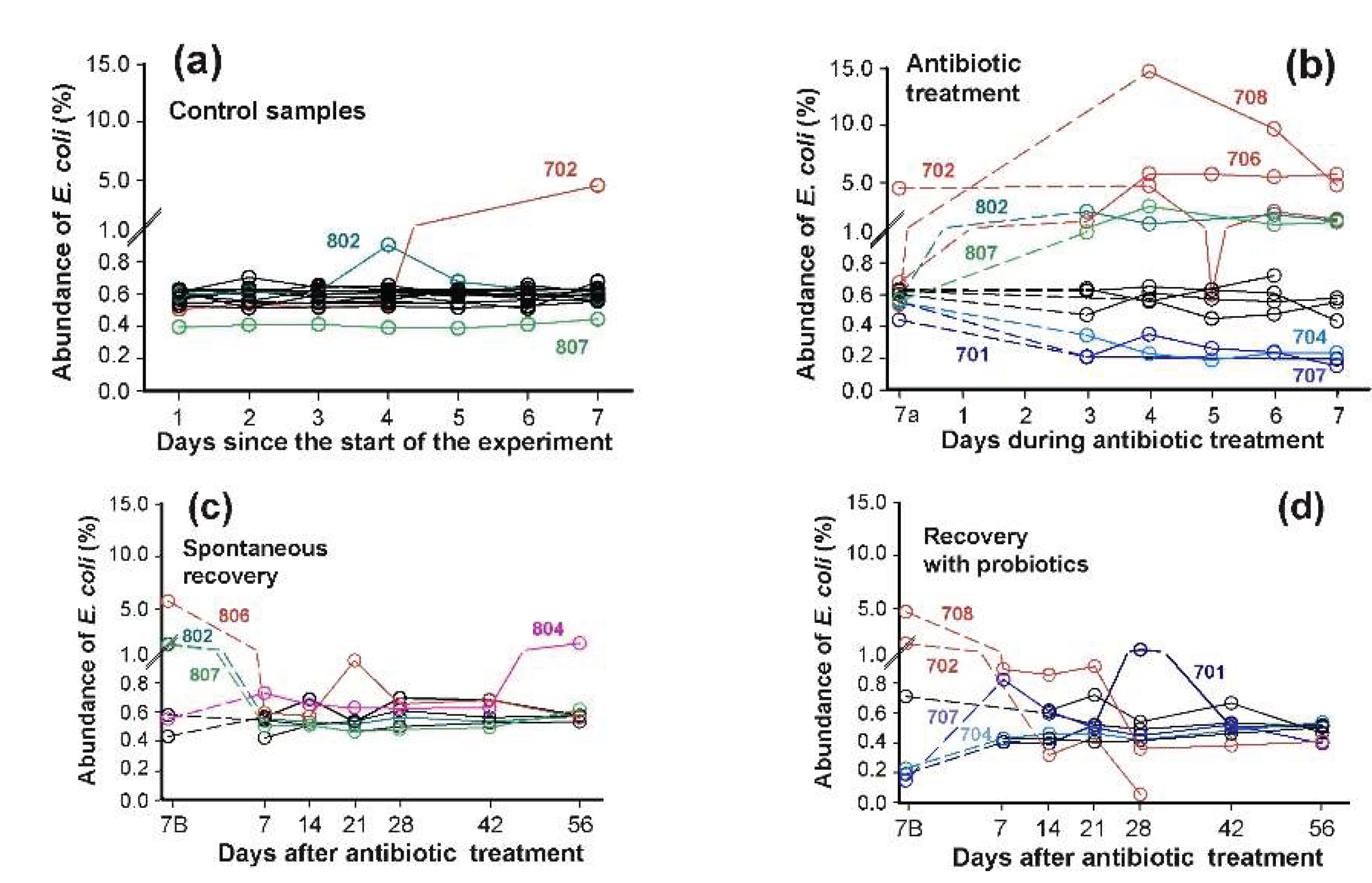
Figure 5a), this set allowed for longitudinal tracking of antibiotic-induced microbiome alterations (Figure 5b).
Prior to antibiotic administration,
E. coli abundance was stable in 13 out of 15 microbiomes (
Figure 5a). Two exceptions were observed: sample 802 showed only a transient increase, while in sample 702
E. coli exhibited significant spontaneous expansion beginning on day 5 of the pre-testing phase. This elevated level persisted until day 5 of antibiotic treatment, when a temporarily decline occurred (
Figure 5b). As expected, antibiotics exposure caused divergent effects on
E. coli abundance across individuals. Among the twelve samples analyzed dynamically, four (703, 802-804) demonstrated complete antibiotic insensitivity. Conversely, five microbiomes showed significant
E. coli proliferation, including sample 807, which had minimal baseline abundance (
Figure 5a). Samples 701, 704 and 707 displayed the opposite pattern, with notable
E. coli reduction.
Following seven days of spontaneous recovery, most microbiomes returned to near-baseline
E. coli levels. Only sample 804, previously identified as antibiotic-insensitive, displayed unexpected
E. coli expansion at the end of experiment (
Figure 5c). Spontaneous recovery of
E. coli in microbiomes took less than 7 days (
Figure 5c). However, supplementation with an 11-strain probiotic mixture [
49,
50], containing four strains of the genus
Bifidobacterium, this period was longer (
Figure 5d). This lag is not surprising, as administration of only
B. longum and
L. paracasei to laboratory rats significantly suppressed
Escherichia in at least one enterotype of their gut microbiomes [
71]. Thus, the abundance of
E. coli in fecal microbiomes is sensitive to the presence of both antibiotics and probiotics.
3.5. The Response of E. coli to Antibiotics Was Not Uniform Among Phylogroups
The lack of strong time dependence in samples from the same donor across particular experimental stages (days 1-7 pre-treatment, days 3-7 during treatment and days 7-56 post-treatment) (
Figure 5) allowed us to use the mean phylogroup abundance values in each microbiome as independent variables for statistical assessment (
Figure 6).
The bidirectional response of
E. coli to antibiotics (
Figure 5b and
Figure 6a) significantly increased the MAD of all phylogroups without statistically significant changes of their abundance (
Figure 6b–d). The main contribution to the variability of
E. coli was made by group A (
Figure 6a,b), the variance of which was less pronounced in microbiomes associated with colon diseases (
Figure 3d). Consistent with the dynamic plots (
Figure 5c) one week of spontaneous recovery following antibiotic treatment significantly reduced
E. coli variance and abundance to near-baseline levels (
Figure 6d). However, this effect was not similar for all groups. Phylogroup F, in particular, retained significantly higher variability compared to controls (
Figure 6c,d). The influence of probiotics tested on independent group of volunteers was also apparent: most groups exhibited reduced persistence in microbiomes, though group G remained variable throughout the extended recovery period (
Figure 6c,d). Therefore, the response of
E. coli to antibiotics and probiotics was not uniform for all of its phylogroups.
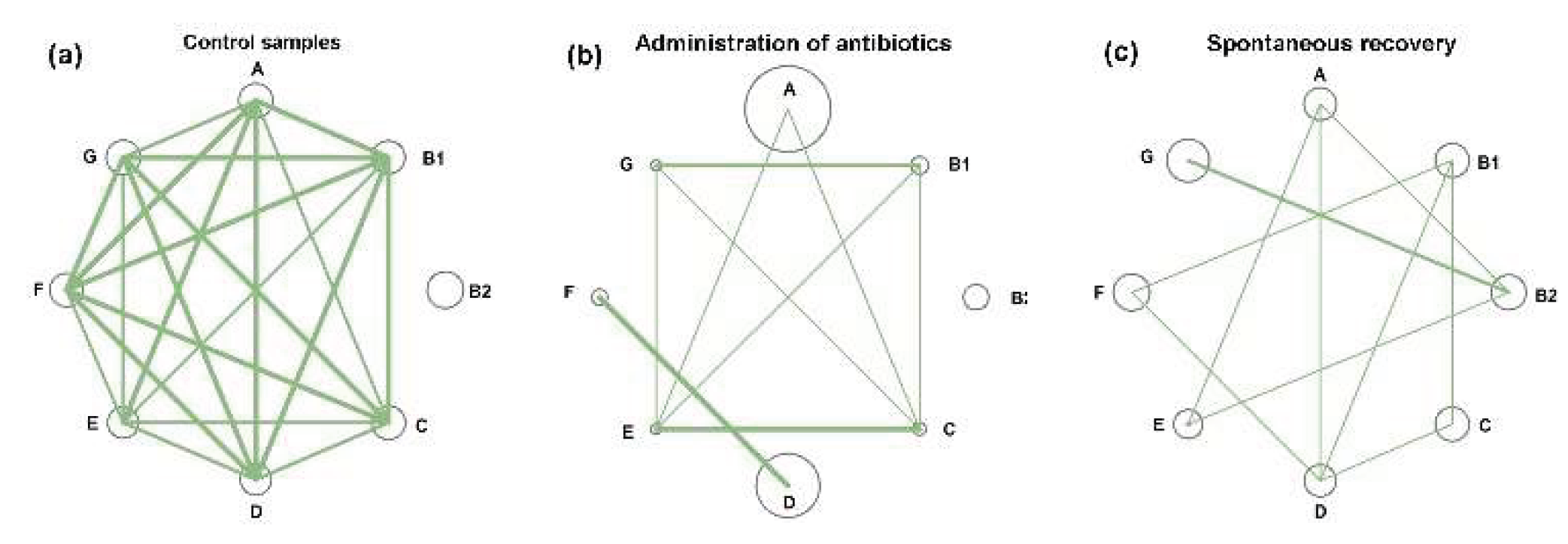
3.6. Post-Treatment Recovery Partially Restored the Intra- and Interspecies Balance Disrupted by Antibiotics, But Not the Original Correlation Between Phylogroups and UMAP Cluster
In control microbiomes, all
E. coli phylogroups were present at nearly equal proportions, and their percentages were highly correlated (
Figure 7a). Only group B2 showed no statistically significant intraspecific associations (
Supplementary Table S3). By inducing divergent changes in all groups, antibiotics disrupted this equilibrium. Consequently, more than half of the intraspecific correlations were lost (
Figure 7b).
Within eight weeks after antibiotic exposure, the relative abundance of all phylogroups had nearly returned to baseline levels (circles in
Figure 7a,c). This recovery was particularly evident for groups A and D, which had expanded during antibiotic treatment, as well as for the antibiotic-suppressed groups B1, C, E, F and G, which rebounded close to their original abundances. However, the number of intraspecific links remained reduced compared to control samples, and the network structure differed from both baseline and antibiotic-perturbed states. For instance, group B2 bacteria, which showed no significant correlations with other groups in control and antibiotic-treated samples, developed significant connections with groups A, E and G (
Figure 7c). Probiotic-assisted restoration, which suppressed the expansion of groups A and D established statistically significant associations between B2 bacteria and groups B1, C, E and F, but disrupted all intraspecific links of group G, which “survived” antibiotic exposure.
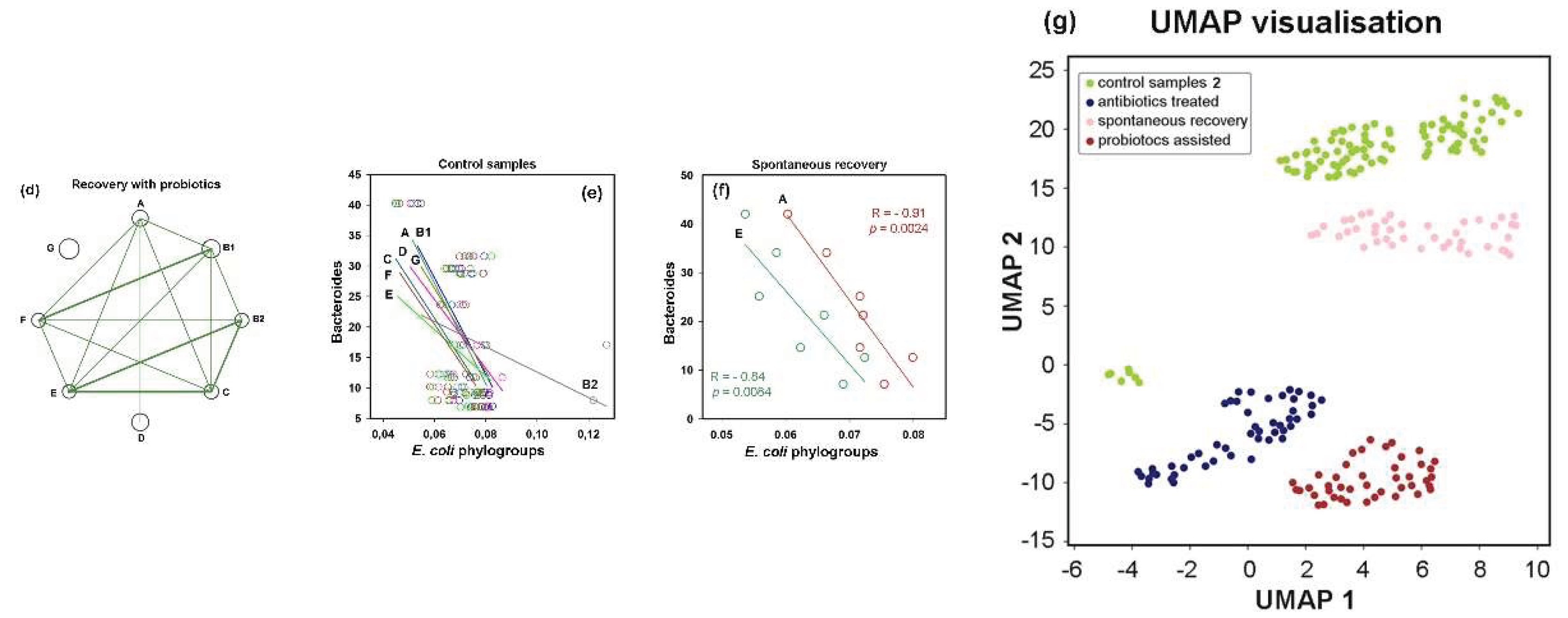
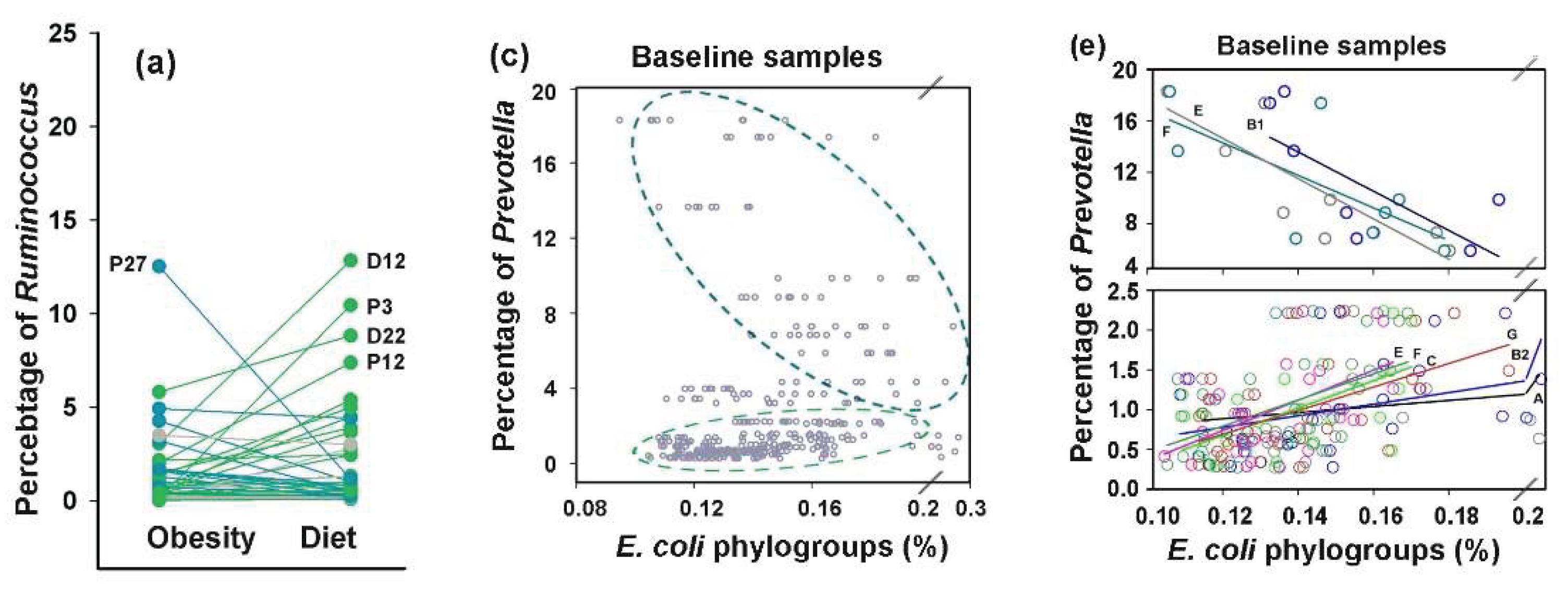
Probiotic-induced rearrangements in
E. coli intraspecific networks suggested that phylogroup homeostasis is dependent on interspecific relationships. To investigate this, we estimated the abundance of dominant enterotype-associated genera (
Bacteroides,
Prevotella and
Ruminococcus) [
1] in all samples using the metagenomic classifier Centrifuge [
57]. In control samples, we observed consistent negative correlations between all
E. coli phylogroups and
Bacteroides, with the strongest association for phylogroup D (R = –0.5) and weakest for group B1 (R = –0.21) (exemplified in
Figure 7e).
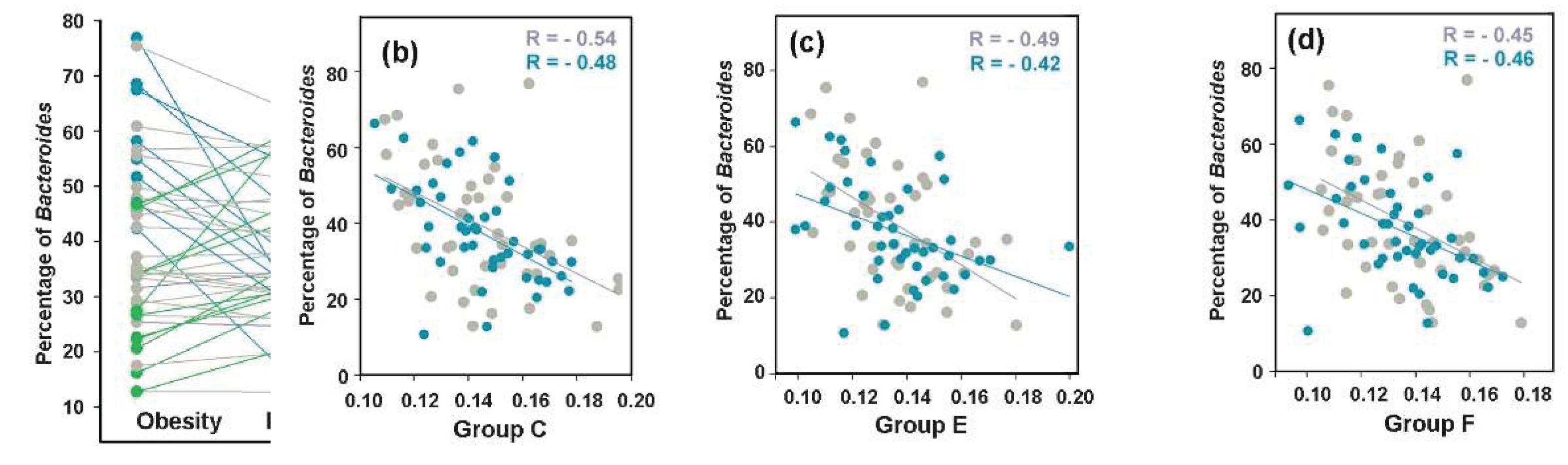
Prevotella showed positive correlations with all
E. coli phylogroups (statistically significant associations for groups A, B1 and D in the range 0.52 < R < 0.61).
Ruminococcus, on the contrary, exhibited weak negative correlations with all groups, except B2. Antibiotics preserved the negative correlations between
Bacteroides and three
E. coli phylogroups but inverted the relationship with group B2, resulting in a stable positive correlation with R = 0.40 ± 0.04 in jackknife analysis. During spontaneous recovery,
Bacteroides re-established their negative link with B2 and strengthened negative associations with groups A and E (
Figure 7f). In the presence of probiotics, only group G maintained its original connections with
Bacteroides, while all other groups showed an inverse trend (
Supplementary Table S3).
Antibiotics converted the positive correlations between E. coli groups and Prevotella into weak negative connections, while simultaneously strengthening positive links with Ruminococcus. During spontaneous recovery, Prevotella re-established positive links with groups A, B2, E and G; whereas probiotics restored positive associations only with groups B2 and C. Interactions with Ruminococcus spontaneously reverted to negative correlations for all E. coli phylogroups. However, probiotic intervention not only re-established their positive links with group B2 but also created new positive association with group C.
Therefore, although interspecific connections were weaker than intraspecific ones, our analysis indicated that phylogroups tend to explore similar connections with dominant taxa (
Supplementary Table S3). However, we also found several examples, revealing individual interspecific links. These distinctive connections may play a crucial role in shaping the networks of intraspecific connections (
Figure 7a-d), which difference was confirmed by UMAP clustering (
Figure 7g). It has become clear that therapeutic antibiotic doses induce significant adaptive changes, leading to intraspecific reorganization and affecting interspecific interactions of
E. coli phylogroups.
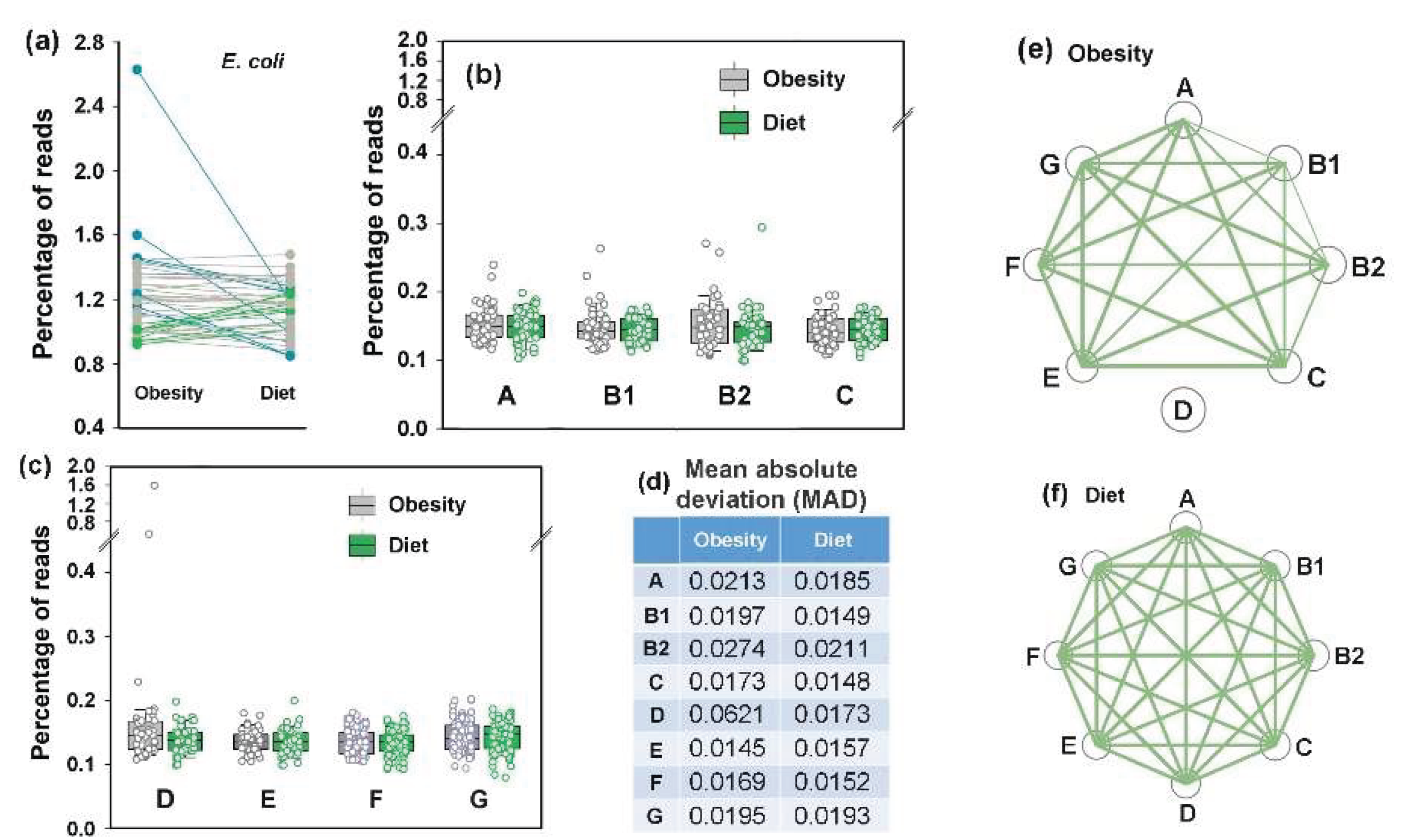
3.7. Idealizing E. coli Intraspecific Balance, the Mediterranean Diet Intensified Its Negative Link to Bacteroides and Unlocks Bidirectional Connections with Prevotella
The PRJEB33500 project dataset [
52] comprises duplicate fecal samples from 43 overweight/obese volunteers collected before and after an 8-week of Mediterranean diet (MD) restriction. Both intra- and interspecific
E. coli relationships were assessed using the mean values of duplicate samples (
Figure 8). The average abundance of
E. coli in the microbiomes of overweight individuals (
Figure 8a) was higher than in healthy donors from the other two analyzed projects (
Figure 3a and
Figure 6a). Although the adaptive response of microbiomes to dietary restriction was also bidirectional (
Figure 8a–c), the divergence was much less pronounced than that observed under antibiotic exposure. Only six microbiomes exhibited a 10 – 30% increase in
E. coli persistence, while in the biota of seven donors, its abundance decreased by 10 – 55%, including samples, whose elevated
E. coli levels were primarily driven by phylogroup D bacteria (two outliers in
Figure 8c). Notably that dietary restriction led to a reduction in mean absolute deviation (
Figure 8d), possibly reflecting the stabilizing effect of a balanced diet. While no significant changes were observed in the average abundance of
E. coli phylogroups (
Figure 8b,c), intraspecific balance improved markedly. The equilibration abundance of all phylogroups (nodes in
Figure 8f) and the reduced persistence of phylogroup D bacteria in microbiomes with their initial overabundance resulted in strong correlations among all groups (
Figure 8f). This observation suggest that the Mediterranean diet may be used to restore
E. coli intraspecific homeostasis.
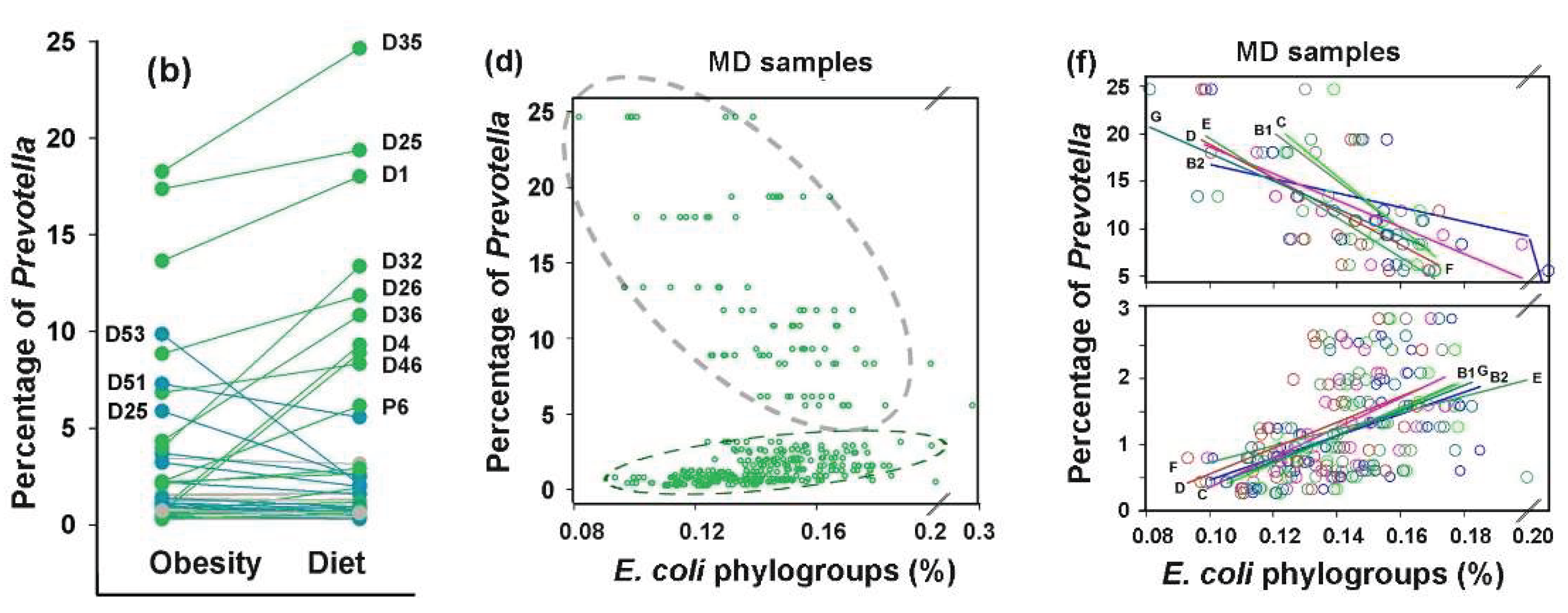
Based on the previous studies, demonstrated significant underrepresentation of
Bacteroides in the gut microbiomes of obese individuals [
72], we anticipated to detect a difference in their presence in response to MD or resulting changes in their relationships with
E. coli. The expected increase of at least 10% in
Bacteroides abundance relative to baseline was indeed observed in 18 out of 43 model microbiomes, but in 14 samples, the percentage of
Bacteroides decreased (
Figure 9a).
Therefore, even dominant bacterial genera employ bimodal adaptive response to dietary restriction. Prior to dietary intervention, only
E. coli groups B1, C, E and F (
Figure 9b–d) exhibited statistically significant negative links with
Bacteroides (0.00044 ≤
p ≤ 0.036), but following 8 weeks of diet, these antagonistic relationships became significant for all
E. coli phylogroups (
Supplementary Table S3). This connection aligns with observations from the PRJEB28097 project datasets (
Figure 7e,f), suggesting that the antagonistic interaction with
Bacteroides may represent a fundamental property of
E. coli.
While the plant-based diet increased
Ruminococcus abundance in 25 of 43 samples, it had little effect on the average percentage of bacteria from this genus (
Figure 10a). Furthermore, we found no robust evidence of interspecific associations between
Ruminococcus and
E. coli (
Supplementary Table S3).
However, correlation with
Prevotella, anticipated based on its symbiosis with
E. coli [
1] and the relationships found in the PRJEB28097 project datasets (
Supplementary Table S3) emerged in unexpected manner (
Figure 10c–f). As a dominant genus in the second human enterotype [
1],
Prevotella, was detected in all microbiomes, with a mean abundance approximately twice as high as that of
Ruminococcus (
Figure 10a,b). The high fruit/vegetable, low meat Mediterranean diet is beneficial for
Prevotella consuming complex carbohydrates and resulted in bidirectional adaptive changes in the persistence of this genus (
Figure 10b). As a result, its weak positive correlations across the entire baseline dataset with groups C (R = 0.31,
p = 0.021) and D (R = 0.46,
p = 0.0009) disappeared (
Supplementary Table S3). However, the scatter plots between abundances of
Prevotella and
E. coli phylogroups (
Figure 10c,d) were far from both correlative and random, suggesting a potential bimodal relationship governing
E. coli phylogroup abundance as a function of
Prevotella levels. When the control set containing samples from overweight individuals was divided into two subsets (
Figure 10e), the estimated R-values for correlation in the eight
Prevotella-rich microbiomes (>5%) ranged from -0.53 to -0.84, with significant negative relationships observed for groups B1, D, E and F (0.009 < P ≤ 0.050). In contrast, significant positive correlations were detected in the 30 samples with low
Prevotella abundance (<3%) for groups A, B2, C, E, F and G (0.42 < R < 0.54, 0.0019 < P ≤ 0.022). Thus, at least groups E and F may employ distinct communication modes with
Prevotella. Following dietary intervention, when intraspecific homeostasis became balanced (
Figure 8f), the 11
Prevotella-rich microbiomes exhibited R-values ranging from -0.48 to -0.77 with statistically significant correlations in all groups except A (0.003 <
p ≤ 0.019,
Figure 10f), and all groups in 31
Prevotella-low samples demonstrated significant positive correlations (
Supplementary Table S3). These findings are among the most important in our study, as they highlighted adaptive capacity of
E. coli to modulate interactions with
Prevotella based on its abundance and phylogroup-specific connections with dominant taxa, suggesting divergent ecological strategies among
E. coli lineages.
3.8. Assessing Difference Between Samples, Machine-Learning Approaches May Also Be Implemented to Reveal Individual Similarity
UMAP clustering clearly segregated pre- and post-MD microbiome samples into two distinct groups (
Figure 11a) and binary classification using at least two models (RF and GB) reliable distinguished between them (
Figure 11b).
However, when these two sets were jointly classified with control samples from the PRJEB7774 and PRJEB28097 projects, we observed three samples, in which the
E. coli populations closely resembled those of overweight individuals (indicated by arrows in
Figure 11c). It was not surprising that both sets of the last project differed from the control sets of PRJEB7774 and PRJEB28097, as the PRJEB33500 samples were obtained from apparently healthy individuals with a clear physiological peculiarity. The divergence of the two sets with control samples obtained from healthy donors (
Figure 11c) likely reflects a batch effect – a phenomenon when non-biological experimental factors introduce artefactual changes. Known contributors to batch effects include differences in laboratory conditions, reagents, and sequencing instrumentation [
73,
74]. As the PRJEB7774 and PRJEB28097 samples were originated from different sources (Beijing Genome Institute and Weizmann Institute of Science, respectively), their national/geographical characteristics may have driven their separation more strongly than shared
E. coli phylogroup distributions could combine them. The most important observation made in this part of the study, was the presence of three control samples located outside their primary clusters (
Figure 11c), suggesting their similarity to the samples of overweight people.
To evaluate, how samples from Control set 1 would be distributed across the other three clusters when added individually, we performed a “virtual diagnostic” experiment (
Figure 11d). This ignored the influence of Control set 1’s specific features, while emphasizing the intraspecific similarities inherent to “healthy microbiomes”. The joint classification was performed 65 times with 265 samples, of which 92 belonged to Control set 2, 172 were from dietary experiment sets, and 1 test sample was from Control set 1 in each iteration. Because each substitution altered the UMAP projection, we overlaid only clusters rather than entire images (
Figure 11d). Due to variations in cluster configurations in the individual UMAP images, the overlapping regions appeared as diffuse clouds. Black squares mark the locations of all Control set 1 samples in the UMAP images. The majority (89.2%) of them clustered with samples obtained from healthy donors who had not received antibiotics or probiotics prior to sampling (Control set 2). Six samples grouped with microbiomes from overweight individuals, while one joined to samples from Mediterranean diet followers. Even if six healthy donors from Control set 1 were not clinically obese and one did not prefer a plant-rich diet, this non-random distribution of Control set 1 samples among health-associated categories demonstrated the predictive power of intraspecific
E. coli characteristics. Therefore, the distribution patterns of
E. coli phylogroups or the intraspecific homeostasis of some other gut bacteria may reflect host physiological state, potentially serving as a basis for diagnostic applications.
4. Discussion
Based on the assumption that the genetic background establishing epistatic interactions with horizontally acquired genes also shapes phylogroup homeostasis of E. coli, which in turn governs both intraspecific and interspecific relationships within microbiomes, we investigated how E. coli phylogroups distribution in the human gut correlates with host physiological state. Our study addressed two key questions: (1) Are there any phylogroup-specific responses of E. coli to environmental changes caused by either chronic intestinal disorders or acute temporary disturbances? (2) How sensitive is intraspecific balance of E. coli to such disruptions? While seemingly straightforward, these questions led us to discover unexpectedly profound rearrangements in both intraspecific relationships and in interspecific connections.
When assessing differences in the abundance of
E. coli phylogroups between the control samples and microbiomes from individuals formed under different physiological conditions, we observed a statistically significant decrease only in phylogroups B2, C and F following probiotic-mediated recovery after antibiotic treatment (
Figure 6b,c). In most other cases, changes in mean abundance were not statistically significant. However, a phylogroup-specific response was evident in their variability. Even in microbiomes adapted to chronical colon diseases, phylogroups B2 and D exhibited a significant increase in mean absolute deviation (
Figure 3d). More pronounced individual changes were observed in response to antibiotic treatment, with groups A, B2, D and F contributing most to adaptive variability of
E. coli (
Figure 6b,d).
Bidirectional changes in isogenic bacterial populations have long been recognized as “bistability” [
75] or “bimodality” [
76]. This phenomenon enables bacteria to adopt alternative survival strategies in adverse environments [
75,
76] or enhance virulence [
77]. A classic example of such diversification is the emergence of persisters, i.e. subpopulations of cells that develop resistance to toxic agents or achieve antimicrobial tolerance by entering a dormant, slow-growing state [
78]. Several mechanisms drive population bifurcation, including structural rearrangements and mutations in the genome [
79], epigenetic modifications [
77] and “transcriptional noise” [
80,
81], which, due to stochastic gene expression and feedback regulation [
76] can cause segregation into two or more sub-populations. Some of the most compelling evidences for behavioral variability comes from single-cell studies [
82,
83]. Particularly relevant to our work are findings on species variability in natural microbiomes. For instance, using a mouse model of chronic colonization, W. Elhenawy and coauthors showed that Crohn’s disease-associated adherent-invasive
E. coli (AIEC) isolates undergo host-specific adaptive diversification [
84]. The authors identified two lineages that outcompeted the ancestral strain by enhancing invasion or improving acetate utilization in the gut. Although AIEC bacteria are distributed across all
E. coli phylogroups, they are predominantly associated with group B2 [
85], which displayed significantly increased variability in the gut microbiota of patients with chronic colorectal disorders and following antibiotic exposure (
Figure 3d and
Figure 6d). Consequently, phylogroups B2 and D formed the fewest intraspecific connections in the analyzed datasets (
Figure 4e–g,
Figure 7b–e,
Figure 8e,f).
Phylogroup E, on the contrary, demonstrated the highest stability in baseline samples (0.008 ≤ MAD ≤ 0.0145) and along with the group F formed the most extensive network of intraspecific correlations. By incorporating over a thousand alien genes [
27,
28] into the chromosomes of
E. coli serotype O157:H7 alone [
22] and domesticating more than 460 prophages [
23], group E bacteria are forced to control expression of a larger number of genes than bacteria with smaller genomes of other groups. Thus, a weaker correlation between them was intuitively expected. Yet our analysis revealed the opposite pattern. It is therefore possible that the genetic background of group E bacteria, evolutionarily tuned to integrate alien genes, was also tuned to maintain the balance of
E. coli phylogroups.
In evaluating interspecific relationships between
E. coli phylogroups and dominant enterotype taxa, we observed predominantly negative correlations with
Bacteroides (
Figure 7 and
Figure 9 and
Supplementary Table S3). This is in line with several publications describing competitive relationships between these genera [
86,
87,
88]. Following complete disruption of these links by antibiotics, the negative correlations with
Bacteroides were spontaneously restored by group A bacteria and increased from an insignificant level for group E bacteria. By the end of the experiment, the interspecies connectivity network with
Bacteroides had nearly returned to baseline levels. However, probiotic-mediated restoration converted phylogroups A and D links with
Bacteroides from significantly negative to weakly positive (R = 0.46,
p = 0.12). That means that the type of interspecific relations between
E. coli phylogroups and dominant gut taxa may change depending on the presence of probiotic bacteria, which are only minor components in the human gut biota.
Interspecific interactions with
Ruminococcus were mostly non-significant. Surprisingly, however, significant positive correlations were observed with phylogroups B1, C, E and G under antibiotic exposure, i.e. a condition when most microbial connections were disrupted (
Supplementary Table S3). Interestingly, phylogroups B1, C, and G formed similar associations in the microbiomes of overweight individuals with high
Prevotella levels. After adherence to the Mediterranean diet, positive associations with
Ruminococcus expanded to all groups, though statistically significant links shifted to groups D and E.
An even more unexpected observation emerged from our analysis of interspecific relationships between
E. coli phylogroups and
Prevotella. While the MetaHIT consortium’s human gut microbiome analysis predicted negative correlations between these taxa [
1], our evaluation of the PRJEB28097 project control dataset revealed positive correlations between
Prevotella and three
E. coli groups (A, B1 and D). The same links persisted in samples from overweight individuals with low
Prevotella abundance (positive correlation with all
E. coli phylogroups except B1). Only when
Prevotella abundance exceeded 5%, we observed the predicted negative correlations with groups B1, E, and F (
Figure 10e). Following dietary restriction, antagonistic relationships in
Prevotella-rich biota displayed all phylogroups except A, while positive correlations in
Prevotella-depleted microbiomes were enhanced and maitained by all phylogroups except E. Thus, both interaction types became significantly stronger and cannot be ignored. To our knowledge, this represent the first documented evidence of
E. coli phylogroups switching between interspecific correlation types based on dominant taxa abundance. Unfortunately, we were unable to validate this phenomenon using the other analyzed datasets. In the colorectal disease project (PRJEB774),
Prevotella abundance in all samples was very low (< 1.2%) and no significant correlations with
E. coli were observed, while the small number of independent variables in PRJEB28097 project prevented their meaningful stratification into two categories.
Figure 1.
Phylogenetic tree of 124
E. coli strains constructed using the neighbor-joining method [
55] in MEGA X [
56] based on a pairwise distance matrix derived from sets of represetative 18-mers. The scale bar shows the Sørensen distance (percentage). The eight
E. coli phylogroups are color-coded. A set of
Escherichia albertii KF1 representative 18-mers was used as an outgroup. Strains with discordant phylotyping (B1 vs. C) relative to [
11] are highlighted with colored circles, while strains not analyzed in [
11] are denoted as gray squares.
Figure 1.
Phylogenetic tree of 124
E. coli strains constructed using the neighbor-joining method [
55] in MEGA X [
56] based on a pairwise distance matrix derived from sets of represetative 18-mers. The scale bar shows the Sørensen distance (percentage). The eight
E. coli phylogroups are color-coded. A set of
Escherichia albertii KF1 representative 18-mers was used as an outgroup. Strains with discordant phylotyping (B1 vs. C) relative to [
11] are highlighted with colored circles, while strains not analyzed in [
11] are denoted as gray squares.
Figure 2.
Intraspecific barcoding of
E. coli using 124 genomes with verified phylogroup identity provided marker 18-mer sets, demonstrated
(a) limited cross-species similarity and
(b) acceptable for taxonomic analysis specificity. (
a) Average percentage of marker 18-mers detected in 124
E. coli genomes (
Figure 1 and
Table 1) compared to chromosomes of
E. albertii (CP130156.1, CP141901.1, CP157789.1),
E. fergusonii (CP099328.1, CP125351.1, CP137855.1) and
E. marmotae (CP099344.1, CP099351.1, CP173213.1).
(b) Overlap of barcodes derived from 124- and 154-genome sets, illustrating shared markers within the same phylogroup (magenta) and unintended cross-phylogroup overlaps (shades of green and blue). The percentage of matches was assessed relative to the size of the smaller barcode in the pair. A unified color scheme denotes overlaps in both panels.
Figure 2.
Intraspecific barcoding of
E. coli using 124 genomes with verified phylogroup identity provided marker 18-mer sets, demonstrated
(a) limited cross-species similarity and
(b) acceptable for taxonomic analysis specificity. (
a) Average percentage of marker 18-mers detected in 124
E. coli genomes (
Figure 1 and
Table 1) compared to chromosomes of
E. albertii (CP130156.1, CP141901.1, CP157789.1),
E. fergusonii (CP099328.1, CP125351.1, CP137855.1) and
E. marmotae (CP099344.1, CP099351.1, CP173213.1).
(b) Overlap of barcodes derived from 124- and 154-genome sets, illustrating shared markers within the same phylogroup (magenta) and unintended cross-phylogroup overlaps (shades of green and blue). The percentage of matches was assessed relative to the size of the smaller barcode in the pair. A unified color scheme denotes overlaps in both panels.
Figure 3.
The diversity of E. coli (
a) and its phylogroups (
b,c) increased in the microbiomes associated with colon diseases (
d). The box plots show the percentages of reads with phylogroup-specific 18-mers (
b,
c) or E. coli/Shigella-specific 18-mers (
a). Significance of MAD alterations was assessed using Mann-Whitney-Wilcoxon test [
60] and Interquartile Interval Range (IQR). MADs with statistically significant changes (p < 0.001) and IQR increased for at least 1.5 times are shown in bold. An increase in IQR by more than 2 times is indicated in red.
Figure 3.
The diversity of E. coli (
a) and its phylogroups (
b,c) increased in the microbiomes associated with colon diseases (
d). The box plots show the percentages of reads with phylogroup-specific 18-mers (
b,
c) or E. coli/Shigella-specific 18-mers (
a). Significance of MAD alterations was assessed using Mann-Whitney-Wilcoxon test [
60] and Interquartile Interval Range (IQR). MADs with statistically significant changes (p < 0.001) and IQR increased for at least 1.5 times are shown in bold. An increase in IQR by more than 2 times is indicated in red.
Figure 4.
While binary clustering failed to differentiate
E. coli phylogroup composition between control samples and adenoma-associated microbiomes (
a), RF and LR models (
b,c), UMAP dimensionality reduction algorithm [
59] (
d) and intraspecific correlations (
e-g) effectively detected changes in
E. coli homeostasis. The C parameters used for binary classification with LR were 0.1 (
a) and 1.0 (
b,
c). The parameters for clustering with RF were: max_depth = 5, n_estimator 200 (
a,
b) and 50 (
c). The best learning_rate/n_estimator combinations for GB were: 0.1/100 (
a), 0.05/200 (
b) and 0.1/200 (
c). UMAP clustering (
d) was performed using parameters n_neighbors = 35, min_dist = 0.7. (
e–
g) Network visualization of intraspecific correlations among
E. coli phylogroups based on Pearson’s correlation coefficient (R). Node size reflects mean phylogroup abundance in samples. Statistically significant correlations are indicated by lines with thickness reflecting their strength:
p < 0.00001 (thick),
p < 0.001 (medium),
p < 0.05 (thin).
Figure 4.
While binary clustering failed to differentiate
E. coli phylogroup composition between control samples and adenoma-associated microbiomes (
a), RF and LR models (
b,c), UMAP dimensionality reduction algorithm [
59] (
d) and intraspecific correlations (
e-g) effectively detected changes in
E. coli homeostasis. The C parameters used for binary classification with LR were 0.1 (
a) and 1.0 (
b,
c). The parameters for clustering with RF were: max_depth = 5, n_estimator 200 (
a,
b) and 50 (
c). The best learning_rate/n_estimator combinations for GB were: 0.1/100 (
a), 0.05/200 (
b) and 0.1/200 (
c). UMAP clustering (
d) was performed using parameters n_neighbors = 35, min_dist = 0.7. (
e–
g) Network visualization of intraspecific correlations among
E. coli phylogroups based on Pearson’s correlation coefficient (R). Node size reflects mean phylogroup abundance in samples. Statistically significant correlations are indicated by lines with thickness reflecting their strength:
p < 0.00001 (thick),
p < 0.001 (medium),
p < 0.05 (thin).
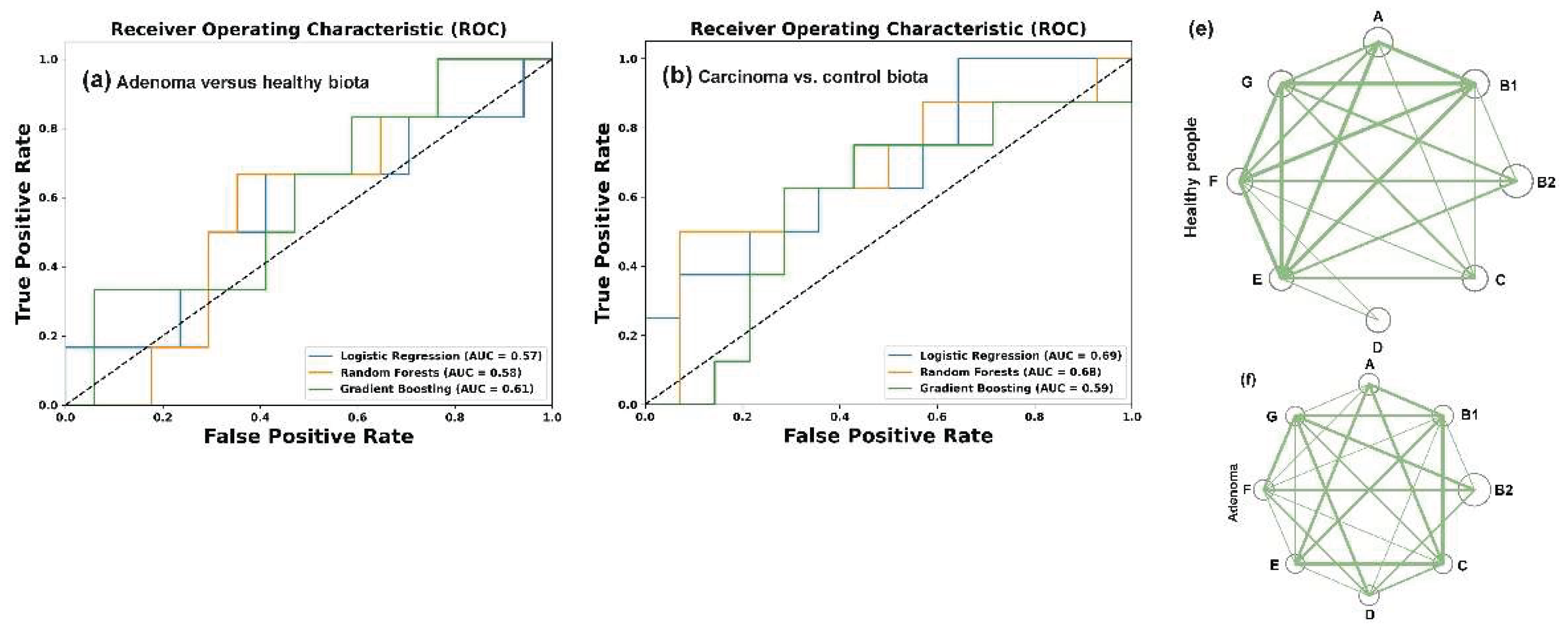
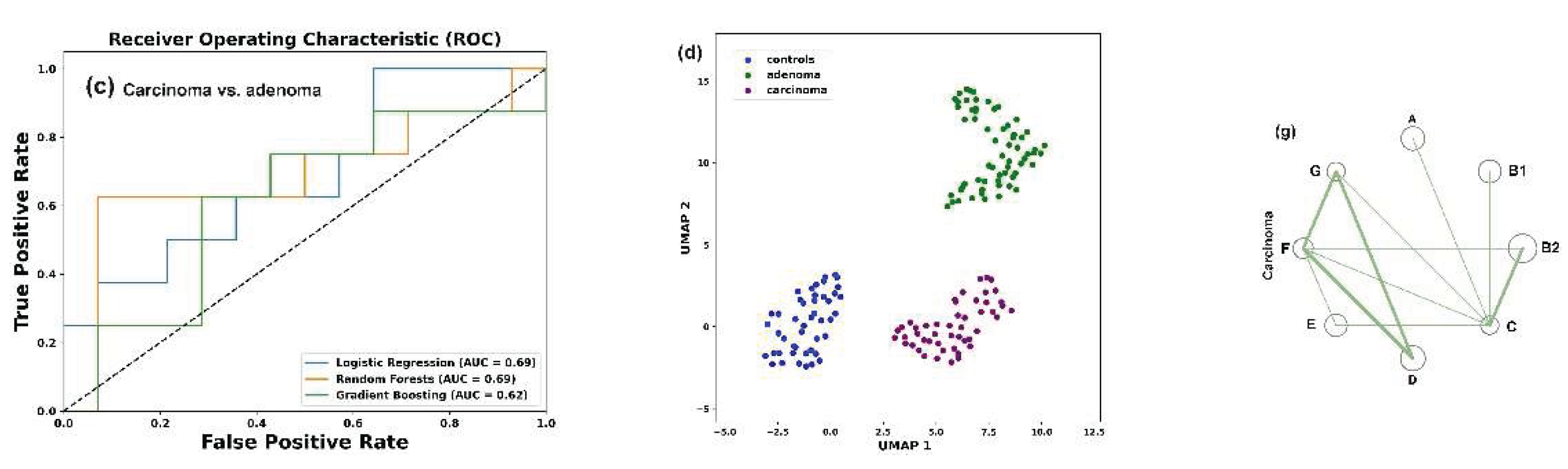
Figure 5.
Dynamics of E. coli abundance (sum of all phylogroups) in healthy human microbiomes. (
a): Stable baseline levels during 7-day pretreatment period. (
b): Differential response patterns following antibiotic administration. (
c,
d): Recovery patterns in the absence (
c) or presence (
d) of probiotic supplementation. Numerals indicate sample IDs (
Supplementary Table S2). Left time points in
b–d represent endpoints of preceding stages. Whenever possible, dashed lines connect longitudinal measurements from the same microbiome. Colored samples are discussed in the text.
Figure 5.
Dynamics of E. coli abundance (sum of all phylogroups) in healthy human microbiomes. (
a): Stable baseline levels during 7-day pretreatment period. (
b): Differential response patterns following antibiotic administration. (
c,
d): Recovery patterns in the absence (
c) or presence (
d) of probiotic supplementation. Numerals indicate sample IDs (
Supplementary Table S2). Left time points in
b–d represent endpoints of preceding stages. Whenever possible, dashed lines connect longitudinal measurements from the same microbiome. Colored samples are discussed in the text.
Figure 6.
Phylogroup-dependent response of
E. coli to antibiotics and probiotic supplementation. Scatter plots show the percentages of reads containing (
a)
E. coli-specific or (
b,
c) phylogroup-specific 18-mers. (
b–
c) Symbols show percentage of phylogroups in microbiomes, while box plots display their mean values averaged across samples from individual donors. (
d) Statistical significance of MAD alterations was assessed using Mann-Whitney-Wilcoxon test [
60] and Interquartile Interval Range (IQR). MADs with IQR increase of more than 3-fold and statistically significant changes (
p < 0.05 vs. controls) are bolded. Changes with
p ≤ 0.001 are highlighted in red.
Figure 6.
Phylogroup-dependent response of
E. coli to antibiotics and probiotic supplementation. Scatter plots show the percentages of reads containing (
a)
E. coli-specific or (
b,
c) phylogroup-specific 18-mers. (
b–
c) Symbols show percentage of phylogroups in microbiomes, while box plots display their mean values averaged across samples from individual donors. (
d) Statistical significance of MAD alterations was assessed using Mann-Whitney-Wilcoxon test [
60] and Interquartile Interval Range (IQR). MADs with IQR increase of more than 3-fold and statistically significant changes (
p < 0.05 vs. controls) are bolded. Changes with
p ≤ 0.001 are highlighted in red.
Figure 7.
Networks of intraspecific E. coli correlations and their connections with other taxa of (a–d) Network visualization of intraspecific correlations among E. coli phylogroups, which were disrupted by antibiotics (b) and partly restored in a probiotic-dependent manner (c,d). Node size represents mean phylogroup abundance across samples. Lines depict statistically significant correlations, with thickness reflecting their strength: p ≤ 0.00001 (thick), p < 0.001 (medium), p < 0.05 (thin). (e,f) A trend toward negative interspecific correlations with Bacteroides in control samples (e) became statistically significant for two phylogroups during spontaneous recovery (f). (g) UMAP clustering (parameters: n_neighbors = 35, min_dist = 1.0) visualize differences in E. coli homeostasis among four gut microbiota states.
Figure 7.
Networks of intraspecific E. coli correlations and their connections with other taxa of (a–d) Network visualization of intraspecific correlations among E. coli phylogroups, which were disrupted by antibiotics (b) and partly restored in a probiotic-dependent manner (c,d). Node size represents mean phylogroup abundance across samples. Lines depict statistically significant correlations, with thickness reflecting their strength: p ≤ 0.00001 (thick), p < 0.001 (medium), p < 0.05 (thin). (e,f) A trend toward negative interspecific correlations with Bacteroides in control samples (e) became statistically significant for two phylogroups during spontaneous recovery (f). (g) UMAP clustering (parameters: n_neighbors = 35, min_dist = 1.0) visualize differences in E. coli homeostasis among four gut microbiota states.
Figure 8.
The Mediterranean diet did not significantly alter the overall abundance of E. coli or its phylogroups in the gut microbiomes, but improved intraspecific balance without induced diversification. (a–d) Box plots display the percentages of reads containing either all E. coli-specific 18-mers (a) or phylogroup-specific 18-mers (b,c) in the metagenomes. (d) Mean absolute deviations estimated from the mean values of 43 paired samples. (e,f) Network visualization of intraspecific correlations among E. coli phylogroups before and after MD. Node sizes correspond to mean phylogroup abundances, while the connecting lines represent statistically significant correlations, with thickness reflecting their strength: p ≤ 0.00001 (thick), p < 0.001 (medium), p < 0.05 (thin).
Figure 8.
The Mediterranean diet did not significantly alter the overall abundance of E. coli or its phylogroups in the gut microbiomes, but improved intraspecific balance without induced diversification. (a–d) Box plots display the percentages of reads containing either all E. coli-specific 18-mers (a) or phylogroup-specific 18-mers (b,c) in the metagenomes. (d) Mean absolute deviations estimated from the mean values of 43 paired samples. (e,f) Network visualization of intraspecific correlations among E. coli phylogroups before and after MD. Node sizes correspond to mean phylogroup abundances, while the connecting lines represent statistically significant correlations, with thickness reflecting their strength: p ≤ 0.00001 (thick), p < 0.001 (medium), p < 0.05 (thin).
Figure 9.
Bidirectional changes in Bacteroides abundance induced by Mediterranean diet adherence retained negative correlations with E. coli phylogroups. (a) Changes in Bacteroides prevalence were calculated from mean percentages in replicate fecal samples collected from 43 overweight individuals before and after 8 weeks of a diet. Plots are color-coded to indicate ≥10% increase (green) or decrease (blue) from baseline. Changes within 10% of baseline are shown in gray. (b-d): Representative negative correlation patterns between Bacteroides and E. coli phylogroups C (b), E (c), and F (d). Baseline and post-intervention samples are shown in gray and blue, respectively.
Figure 9.
Bidirectional changes in Bacteroides abundance induced by Mediterranean diet adherence retained negative correlations with E. coli phylogroups. (a) Changes in Bacteroides prevalence were calculated from mean percentages in replicate fecal samples collected from 43 overweight individuals before and after 8 weeks of a diet. Plots are color-coded to indicate ≥10% increase (green) or decrease (blue) from baseline. Changes within 10% of baseline are shown in gray. (b-d): Representative negative correlation patterns between Bacteroides and E. coli phylogroups C (b), E (c), and F (d). Baseline and post-intervention samples are shown in gray and blue, respectively.
Figure 10.
While inducing bidirectional changes in Ruminococcus and Prevotella abundance, MD did not significantly alter their presence in the gut, but revealed a bimodal relationship between the abundance of E. coli and the persistence of Prevotella. (a,b) Changes in Ruminococcus (a) and Prevatella prevalence were calculated from mean percentages in replicate fecal samples collected from 43 overweight individuals before and after 8 weeks of a diet. Plots are color-coded to indicate ≥10% increase (green) or decrease (blue) from baseline. (c–f) Scatter plots showing either the entire set of samples (c,d), or samples divided into two categories based on the percentage of Prevotella in the microbiomes (e,f). Ovals outline symbols with different correlation modes.
Figure 10.
While inducing bidirectional changes in Ruminococcus and Prevotella abundance, MD did not significantly alter their presence in the gut, but revealed a bimodal relationship between the abundance of E. coli and the persistence of Prevotella. (a,b) Changes in Ruminococcus (a) and Prevatella prevalence were calculated from mean percentages in replicate fecal samples collected from 43 overweight individuals before and after 8 weeks of a diet. Plots are color-coded to indicate ≥10% increase (green) or decrease (blue) from baseline. (c–f) Scatter plots showing either the entire set of samples (c,d), or samples divided into two categories based on the percentage of Prevotella in the microbiomes (e,f). Ovals outline symbols with different correlation modes.
Figure 11.
Visualization and statistical assessment of differences between E. coli populations (a,b) and optimization of UMAP for individual identity testing (c,d). (a) UMAP clustering of all 172 samples from the PRJEB33500 project (parameters: n_neighbors = 35, min_dist = 0.7). (b) ROC curves and AUC values for the same dataset, calculated using mean values of paired samples. (c) Joint UMAP clustering of Control set 1 and 2 (baseline samples from PRJEB7774 and PRJEB28097) with PRJEB33500 project samples. Arrows highlight samples with altered clustering in the combined classification. (d) Superimposed clusters from 65 images obtained in a “virtual diagnostics” experiment, when each sample from Control set 1 (black squares) was individually added to a combined set of 264 samples from three other datasets. Clustering parameters for (c,d): n_neighbors=20, min_dist=1.
Figure 11.
Visualization and statistical assessment of differences between E. coli populations (a,b) and optimization of UMAP for individual identity testing (c,d). (a) UMAP clustering of all 172 samples from the PRJEB33500 project (parameters: n_neighbors = 35, min_dist = 0.7). (b) ROC curves and AUC values for the same dataset, calculated using mean values of paired samples. (c) Joint UMAP clustering of Control set 1 and 2 (baseline samples from PRJEB7774 and PRJEB28097) with PRJEB33500 project samples. Arrows highlight samples with altered clustering in the combined classification. (d) Superimposed clusters from 65 images obtained in a “virtual diagnostics” experiment, when each sample from Control set 1 (black squares) was individually added to a combined set of 264 samples from three other datasets. Clustering parameters for (c,d): n_neighbors=20, min_dist=1.
Table 1.
Datasets used for intraspecific taxonomic analysis.
Table 1.
Datasets used for intraspecific taxonomic analysis.
|
Type ofdataset
|
Donor types and number of samples |
Bioproject |
| Types |
Number |
| Inflammatory bowel diseases and cancer |
Healthy individuals |
65 |
PRJEB7774 [48] |
| Patients with adenoma |
49 |
| Patients with carcinoma |
46 |
| Antibiotic treatment with or without probiotic recovery |
Fifteen healthy donors before antibiotic treatment |
92 |
PRJEB28097[49,50] |
| Twelve donors of the same group during antibiotic treatment |
49 |
| Seven donors of the same group during self-recovery |
42 |
| Eight donors of the same goup during recovery with probiotics |
43 |
| Overweight donors before and after diet |
Samples from 43 overweight or obese individuals |
86 |
PRJEB33500[52] |
| Samples from the same 43 persons after Mediterranean diet |
86 |
Table 2.
Number of genomes in phylogroups and size of sets with unique 18-mers (barcodes).
Table 2.
Number of genomes in phylogroups and size of sets with unique 18-mers (barcodes).
| Phylogroups |
Number of genomes |
Number of 18-mers |
| Set 1 |
Set 2 |
Set 1 |
Set 2 |
| A |
17 |
21 |
415335 |
354997 |
| B1 |
25 |
25 |
710784 |
524927 |
| B2 |
23 |
29 |
1014716 |
783899 |
| C |
14 |
17 |
242272 |
170224 |
| D |
11 |
15 |
673338 |
524936 |
| E |
13 |
19 |
680604 |
802163 |
| F |
11 |
15 |
445835 |
313383 |
| G |
10 |
13 |
254624 |
171176 |