Submitted:
15 May 2025
Posted:
16 May 2025
Read the latest preprint version here
Abstract
Keywords:
Introduction
Results
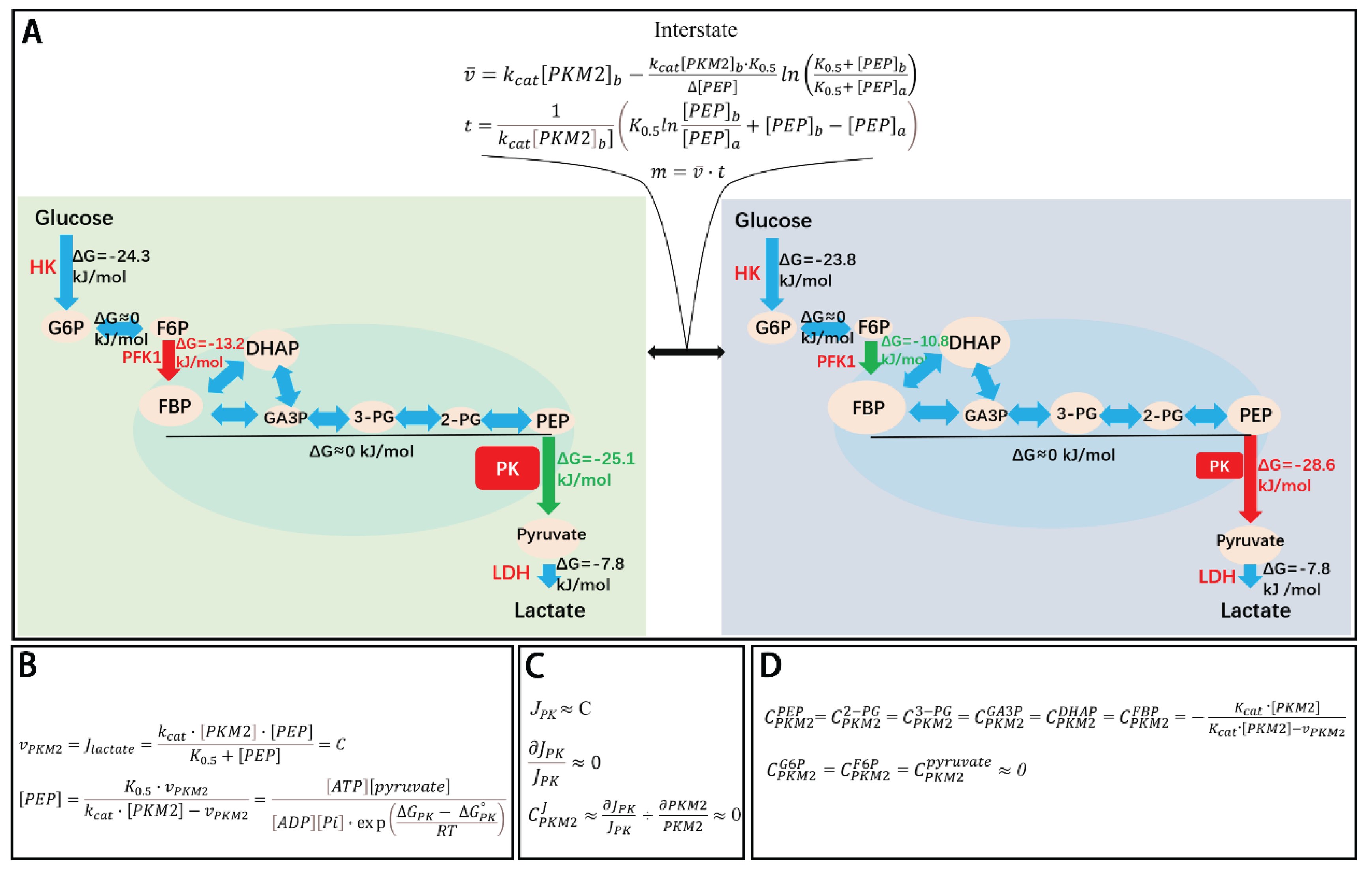
Kinetic-Thermodynamic Coupling in the Glycolytic Pathway
- The steady state, in which intermediate metabolite levels and reaction rates remain constant over time; and
- The interstate, a transient phase during transitions between steady states.
Steady-State Glycolysis: A Question of Equal Flux
- the enzyme’s total concentration or activity, and
- the concentration of its substrate.
Thermodynamic Determinants of Intermediate Distribution
- the enzyme’s total activity, and
- the thermodynamic state of its substrate.
Pathway-Level Conservation of Thermodynamic Features
Interstate Between Any Two Steady States
Summary 1
Application of Kinetic-Thermodynamic Coupling to the Regulation of Glycolysis by PKM2
Thermodynamic and Kinetic Reasoning
- Steady state a, where PKM2t is high, and
- Steady state b, where PKM2t is low.
Stabilization of Glycolytic Input
Intermediate Metabolite Redistribution and Thermodynamic equilibration
Thermodynamic Buffering of PKM2 Activity
Theoretical Prediction and Experimental Validation
Summary 2
- m denotes the total amount of substrate processed by PKM2 during the transition
- v̅ denotes the average catalytic velocity of PKM2 during the transition
- t denotes the time required to complete the transition
- Instantaneous changes in PKM2t, or
- Stepwise changes across n discrete steps.
Summary 3
Quantitative Coupling of PKM2 Kinetics with Thermodynamics in the Glycolytic Pathway
- Cell lysates containing all glycolytic enzymes
- Substrates such as glucose, ATP, ADP, and NAD⁺
- Minimal diversion into branch pathways (e.g., PPP, SSP, mitochondrial metabolism)
- KFBP ≈ 25.5 ± 148.1 nM (40)
- [FBP] ranges from ~35 to 61 μM in cell-free glycolysis, and from ~210 to 1510 μM in cells (4-7)
Linking Thermodynamics to Kinetics
- When [PKM2] or PKM2t decreases, ΔGPK becomes more negative, and [PEP] increases.
- When [PKM2] or PKM2t increases, ΔGPK becomes less negative, and [PEP] decreases.
Summary 4
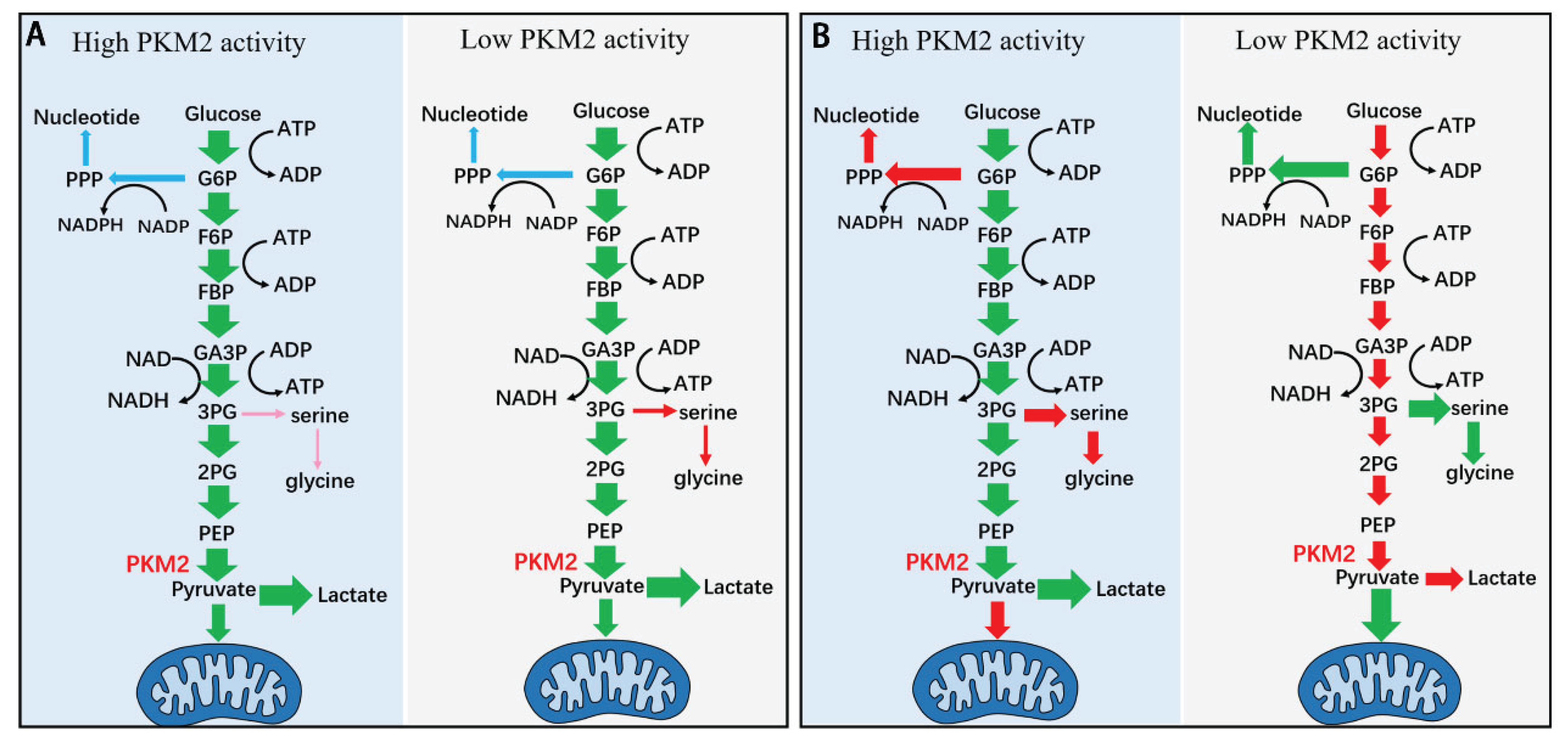
Relevance to Living Cells
PKM2’s Flux Control Coefficient and Intermediate Concentration Control Coefficient
- FCC quantifies an enzyme's influence on the flux through the pathway.
- CCC quantifies its influence on the concentration of a given intermediate.
Flux Control Coefficient (FCC) of PKM2
Concentration Control Coefficient (CCC) of PKM2
- The ΔG of the PFK1 reaction insulating upstream segments.
- The final step (LDH) maintaining pyruvate homeostasis
Summary 5
Discussion
Flux Stability Through Thermodynamic Buffering
The Interstate between 2 steady states
Flux and Concentration Control
Pathway-Level Thermodynamic Organization
Feedforward Saturation
A Diagnostic Framework for Enzyme Regulation
The flux to lactate and to side branches
Beyond PKM2
Methods
The Average Velocity for the System to Transition Between Steady States
The Time (t) Required for the System to Transition Between Steady States
The Data Used to Calculate v̅, t, and Mass Transfer (m) for the System to Transition Between Steady States
|
Kcat[PKM2]a (μmol/min∙mg protein) (before PKM2 KD) |
3387 |
|
Kcat[PKM2]b (μmol/min∙mg protein) (after PKM2 KD) |
1012 |
| mg protein/l cells | 282000 |
| Kcat[PKM2]a (μmol/min∙l cells) | 955134000 |
| Kcat[PKM2]b (μmol/min∙l cells) | 285666000 |
| K0.5 (μM) | 74 |
| [PEP]a (μM) (before PKM2 KD) | 67 |
| [PEP]b (μM) (after PKM2 KD) | 215 |
| ∆[PEP] (μM) | 148 |
| v̅ (μmol/min∙l cells) | 183159000 |
| t (ms) | 0.049 |
| m (μmol/l cells) | 150 |
Calculation of
Calculation of CCC of PKM2 for Other Glycolytic Intermediates
Funding and Additional Information
References
- Jia, D., Lu, M., Jung, K. H., Park, J. H., Yu, L., Onuchic, J. N., Kaipparettu, B. A., and Levine, H. Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc Natl Acad Sci U S A 2019, 116, 3909–3918. [Google Scholar] [CrossRef] [PubMed]
- Fendt, S. M., Frezza, C., and Erez, A. Targeting Metabolic Plasticity and Flexibility Dynamics for Cancer Therapy. Cancer Discov 2020, 10, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Parlani, M., Jorgez, C., and Friedl, P. (2023) Plasticity of cancer invasion and energy metabolism. Trends Cell Biol 33, 388-402.
- Jin, C., Zhu, X., Wu, H., Wang, Y., and Hu, X. Perturbation of phosphoglycerate kinase 1 (PGK1) only marginally affects glycolysis in cancer cells. J Biol Chem 2020, 295, 6425–6446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X., Jin, C., Pan, Q., and Hu, X. (2021) Determining the quantitative relationship between glycolysis and GAPDH in cancer cells exhibiting the Warburg effect. J Biol Chem 296, 100369.
- Jin, C., Hu, W., Wang, Y., Wu, H., Zeng, S., Ying, M., and Hu, X. (2024) Deciphering the interaction between PKM2 and the built-in thermodynamic properties of the glycolytic pathway in cancer cells. J Biol Chem 300, 107648.
- Zeng, S., Wang, Y., Ying, M., Jin, C., Ying, C., Wang, D., Wu, H., and Hu, X. (2024) Elucidating the kinetic and thermodynamic insight into regulation of glycolysis by lactate dehydrogenase and its impact on tricarboxylic acid cycle and oxidative phosphorylation in cancer cells. eLife Sciences Publications, Ltd.
- de Wit, R. H., Mujic-Delic, A., van Senten, J. R., Fraile-Ramos, A., Siderius, M., and Smit, M. J. (2016) Human cytomegalovirus encoded chemokine receptor US28 activates the HIF-1alpha/PKM2 axis in glioblastoma cells. Oncotarget 7, 67966-67985.
- Yang, L., Hou, Y., Yuan, J., Tang, S., Zhang, H., Zhu, Q., Du, Y. E., Zhou, M., Wen, S., Xu, L., Tang, X., Cui, X., and Liu, M. (2015) Twist promotes reprogramming of glucose metabolism in breast cancer cells through PI3K/AKT and p53 signaling pathways. Oncotarget 6, 25755-25769.
- Sun, Q., Chen, X., Ma, J., Peng, H., Wang, F., Zha, X., Wang, Y., Jing, Y., Yang, H., Chen, R., Chang, L., Zhang, Y., Goto, J., Onda, H., Chen, T., Wang, M. R., Lu, Y., You, H., Kwiatkowski, D., and Zhang, H. (2011) Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci U S A 108, 4129-4134.
- Panasyuk, G., Espeillac, C., Chauvin, C., Pradelli, L. A., Horie, Y., Suzuki, A., Annicotte, J. S., Fajas, L., Foretz, M., Verdeguer, F., Pontoglio, M., Ferre, P., Scoazec, J. Y., Birnbaum, M. J., Ricci, J. E., and Pende, M. (2012) PPARgamma contributes to PKM2 and HK2 expression in fatty liver. Nat Commun 3, 672.
- Wu, M., An, J., Zheng, Q., Xin, X., Lin, Z., Li, X., Li, H., and Lu, D. (2016) Double mutant P53 (N340Q/L344R) promotes hepatocarcinogenesis through upregulation of Pim1 mediated by PKM2 and LncRNA CUDR. Oncotarget 7, 66525-66539.
- Shang, Y., He, J., Wang, Y., Feng, Q., Zhang, Y., Guo, J., Li, J., Li, S., Wang, Y., Yan, G., Ren, F., Shi, Y., Xu, J., Zeps, N., Zhai, Y., He, D., and Chang, Z. CHIP/Stub1 regulates the Warburg effect by promoting degradation of PKM2 in ovarian carcinoma. Oncogene 2017, 36, 4191–4200. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q., Liu, L. Z., Yin, Y., He, J., Li, Q., Qian, X., You, Y., Lu, Z., Peiper, S. C., Shu, Y., and Jiang, B. H. Regulatory circuit of PKM2/NF-kappaB/miR-148a/152-modulated tumor angiogenesis and cancer progression. Oncogene 2015, 34, 5482–5493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. F., Lou, J. T., Lu, M. H., Gao, C., Zhao, S., Li, B., Liang, S., Li, Y., Li, D., and Liu, M. F. (2015) Suppression of miR-199a maturation by HuR is crucial for hypoxia-induced glycolytic switch in hepatocellular carcinoma. EMBO J 34, 2671-2685.
- Guo, M., Zhao, X., Yuan, X., Jiang, J., and Li, P. (2017) MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in cervical cancer. Oncotarget 8, 28226-28236.
- Li, H., Li, J., Jia, S., Wu, M., An, J., Zheng, Q., Zhang, W., and Lu, D. (2015) miR675 upregulates long noncoding RNA H19 through activating EGR1 in human liver cancer. Oncotarget 6, 31958-31984.
- Morgan, H. P., O'Reilly, F. J., Wear, M. A., O'Neill, J. R., Fothergill-Gilmore, L. A., Hupp, T., and Walkinshaw, M. D. (2013) M2 pyruvate kinase provides a mechanism for nutrient sensing and regulation of cell proliferation. Proc Natl Acad Sci U S A 110, 5881-5886.
- Sparmann, G., Schulz, J., and Hofmann, E. (1973) Effects of L-alanine and fructose (1,6-diphosphate) on pyruvate kinase from ehrlich ascites tumour cells. FEBS Lett 36, 305-308.
- Yuan, M., McNae, I. W., Chen, Y., Blackburn, E. A., Wear, M. A., Michels, P. A. M., Fothergill-Gilmore, L. A., Hupp, T., and Walkinshaw, M. D. (2018) An allostatic mechanism for M2 pyruvate kinase as an amino-acid sensor. Biochem J 475, 1821-1837.
- Dombrauckas, J. D., Santarsiero, B. D., and Mesecar, A. D. (2005) Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry 44, 9417-9429.
- Chaneton, B., Hillmann, P., Zheng, L., Martin, A. C. L., Maddocks, O. D. K., Chokkathukalam, A., Coyle, J. E., Jankevics, A., Holding, F. P., Vousden, K. H., Frezza, C., O'Reilly, M., and Gottlieb, E. (2012) Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 491, 458-462.
- Keller, K. E., Tan, I. S., and Lee, Y. S. (2012) SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Science 338, 1069-1072.
- Hitosugi, T., Kang, S., Vander Heiden, M. G., Chung, T. W., Elf, S., Lythgoe, K., Dong, S., Lonial, S., Wang, X., Chen, G. Z., Xie, J., Gu, T. L., Polakiewicz, R. D., Roesel, J. L., Boggon, T. J., Khuri, F. R., Gilliland, D. G., Cantley, L. C., Kaufman, J., and Chen, J. (2009) Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal 2, ra73.
- Presek, P., Reinacher, M., and Eigenbrodt, E. (1988) Pyruvate kinase type M2 is phosphorylated at tyrosine residues in cells transformed by Rous sarcoma virus. FEBS Lett 242, 194-198.
- Bettaieb, A., Bakke, J., Nagata, N., Matsuo, K., Xi, Y., Liu, S., AbouBechara, D., Melhem, R., Stanhope, K., Cummings, B., Graham, J., Bremer, A., Zhang, S., Lyssiotis, C. A., Zhang, Z. Y., Cantley, L. C., Havel, P. J., and Haj, F. G. (2013) Protein tyrosine phosphatase 1B regulates pyruvate kinase M2 tyrosine phosphorylation. J Biol Chem 288, 17360-17371.
- Christofk, H. R., Vander Heiden, M. G., Wu, N., Asara, J. M., and Cantley, L. C. (2008) Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452, 181-186.
- Yang, W., Zheng, Y., Xia, Y., Ji, H., Chen, X., Guo, F., Lyssiotis, C. A., Aldape, K., Cantley, L. C., and Lu, Z. (2012) ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol 14, 1295-1304.
- Park, Y. S., Kim, D. J., Koo, H., Jang, S. H., You, Y. M., Cho, J. H., Yang, S. J., Yu, E. S., Jung, Y., Lee, D. C., Kim, J. A., Park, Z. Y., Park, K. C., and Yeom, Y. I. (2016) AKT-induced PKM2 phosphorylation signals for IGF-1-stimulated cancer cell growth. Oncotarget 7, 48155-48167.
- Lee, K. M., Nam, K., Oh, S., Lim, J., Lee, T., and Shin, I. (2015) ECM1 promotes the Warburg effect through EGF-mediated activation of PKM2. Cell Signal 27, 228-235.
- Lv, L., Li, D., Zhao, D., Lin, R., Chu, Y., Zhang, H., Zha, Z., Liu, Y., Li, Z., Xu, Y., Wang, G., Huang, Y., Xiong, Y., Guan, K. L., and Lei, Q. Y. (2011) Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol Cell 42, 719-730.
- Lv, L., Xu, Y. P., Zhao, D., Li, F. L., Wang, W., Sasaki, N., Jiang, Y., Zhou, X., Li, T. T., Guan, K. L., Lei, Q. Y., and Xiong, Y. (2013) Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization. Mol Cell 52, 340-352.
- Park, S. H., Ozden, O., Liu, G., Song, H. Y., Zhu, Y., Yan, Y., Zou, X., Kang, H. J., Jiang, H., Principe, D. R., Cha, Y. I., Roh, M., Vassilopoulos, A., and Gius, D. (2016) SIRT2-Mediated Deacetylation and Tetramerization of Pyruvate Kinase Directs Glycolysis and Tumor Growth. Cancer Res 76, 3802-3812.
- Luo, W., Hu, H., Chang, R., Zhong, J., Knabel, M., O'Meally, R., Cole, R. N., Pandey, A., and Semenza, G. L. (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732-744.
- Wang, J., Yang, P., Yu, T., Gao, M., Liu, D., Zhang, J., Lu, C., Chen, X., Zhang, X., and Liu, Y. (2022) Lactylation of PKM2 Suppresses Inflammatory Metabolic Adaptation in Pro-inflammatory Macrophages. Int J Biol Sci 18, 6210-6225.
- Iqbal, M. A., Siddiqui, F. A., Gupta, V., Chattopadhyay, S., Gopinath, P., Kumar, B., Manvati, S., Chaman, N., and Bamezai, R. N. (2013) Insulin enhances metabolic capacities of cancer cells by dual regulation of glycolytic enzyme pyruvate kinase M2. Mol Cancer 12, 72.
- Anastasiou, D., Poulogiannis, G., Asara, J. M., Boxer, M. B., Jiang, J. K., Shen, M., Bellinger, G., Sasaki, A. T., Locasale, J. W., Auld, D. S., Thomas, C. J., Vander Heiden, M. G., and Cantley, L. C. (2011) Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278-1283.
- Liu, F., Ma, F., Wang, Y., Hao, L., Zeng, H., Jia, C., Wang, Y., Liu, P., Ong, I. M., Li, B., Chen, G., Jiang, J., Gong, S., Li, L., and Xu, W. (2017) PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. Nat Cell Biol 19, 1358-1370.
- Wang, Y., Liu, J., Jin, X., Zhang, D., Li, D., Hao, F., Feng, Y., Gu, S., Meng, F., Tian, M., Zheng, Y., Xin, L., Zhang, X., Han, X., Aravind, L., and Wei, M. (2017) O-GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect. Proc Natl Acad Sci U S A 114, 13732-13737.
- Macpherson, J. A., Theisen, A., Masino, L., Fets, L., Driscoll, P. C., Encheva, V., Snijders, A. P., Martin, S. R., Kleinjung, J., Barran, P. E., Fraternali, F., and Anastasiou, D. (2019) Functional cross-talk between allosteric effects of activating and inhibiting ligands underlies PKM2 regulation. Elife 8.
- Wang, Y., Wu, H., and Hu, X. (2025) Quantification of the inputs and outputs of serine and glycine metabolism in cancer cells. Arch Biochem Biophys 768, 110367.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




